Biotextiles for Biomedical Applications: A Review
Abstract
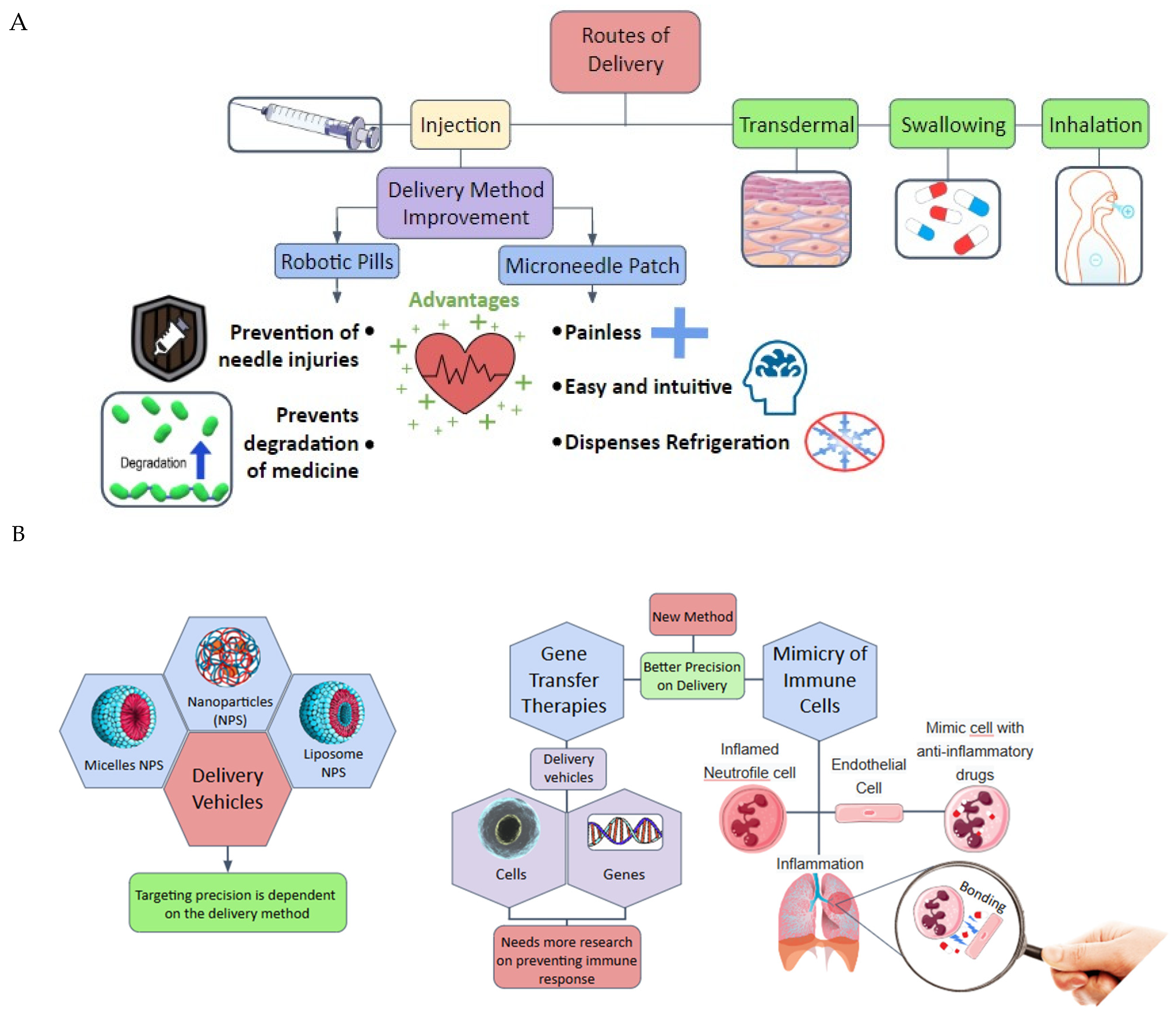
1. Introduction
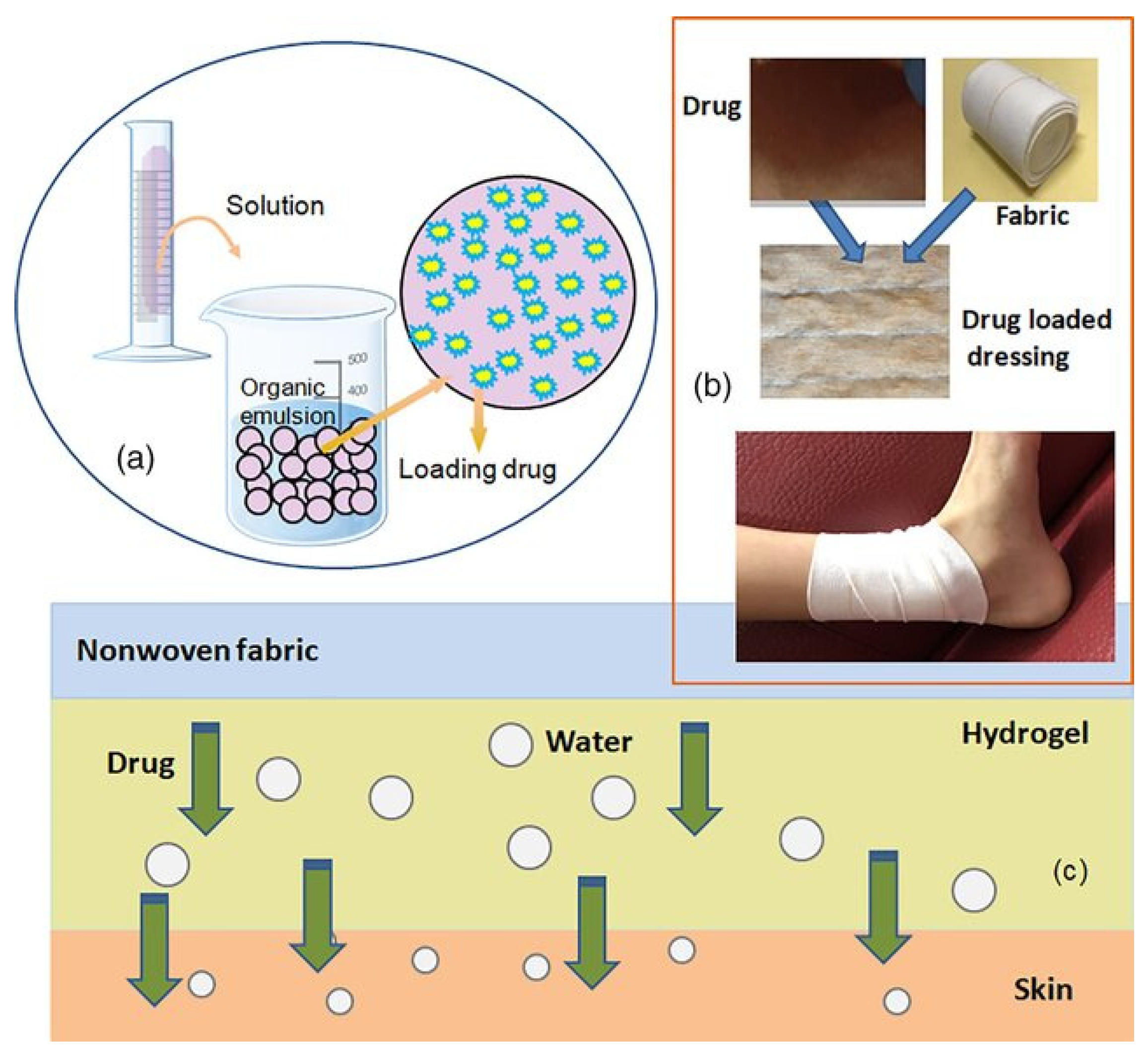
2. Drug Delivery Systems
- ✓
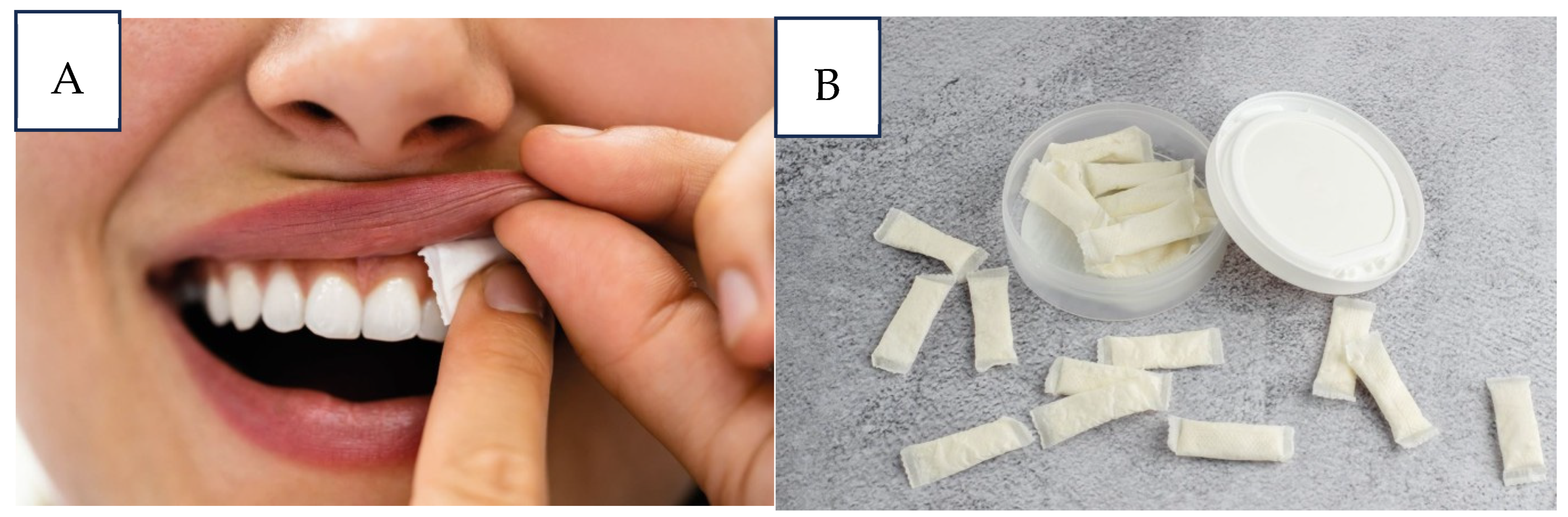
- Buccal administration can resolve some problems involving conventional drug administration, such as presystemic metabolism and drug depletion in the digestive tract. The structures come into direct contact with both the absorbent surface and the mucus membrane, enhancing the therapeutic effects of the medication administered. Certain drawbacks must be taken into account, such as the substantial loss of medication through salivation and swallowing, as well as the limited delivery areas [23].
- ✓
- Sublingual—used when a rapid onset of action is required owing to its greater bioavailability and better patient compliance [24].
- ✓
- ✓
- Rectal—used in case of gastric irritation, when low enzymatic activity and localized activity are required [28].
- ✓
- ✓
- Ocular—indicated for the treatment of anterior segment diseases. It is considered the most challenging type of DDSs by experts [31].
- ✓
- Gastrointestinal—this mode is the most convenient for patients. However, the absorption of drugs is limited by the individual characteristics of each patient [32].
2.1. Applications
2.2. Future Trends
3. Biotextiles for Medical Implants and Regenerative Medicine
3.1. Applications
3.2. Future Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cherenack, K.; van Pieterson, L. Smart textiles: Challenges and opportunities. J. Appl. Phys. 2012, 112, 091301. [Google Scholar] [CrossRef]
- van Langenhove, L.; Hertleer, C. Smart clothing: A new life. Int. J. Cloth. Sci. Technol. 2004, 16, 63–72. [Google Scholar] [CrossRef]
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Adi, D.S.; Damayanti, R.; Subiyanto, B.; Fatriasari, W.; Fudholi, A. A review on natural fibers for development of eco-friendly bio-composite: Characteristics, and utilizations. J. Mater. Rese. Technol. 2021, 13, 2442–2458. [Google Scholar] [CrossRef]
- Xing, T.; He, A.; Huang, Z.; Luo, Y.; Zhang, Y.; Wang, M.; Shi, Z.; Ke, G.; Bai, J.; Zhao, S.; et al. Silk-based flexible electronics and smart wearable Textiles: Progress and beyond. Chem. Eng. J. 2023, 474, 145534. [Google Scholar] [CrossRef]
- Williams, D.F. Definitions in Biomaterials: Proceedings of a Consensus Conference of the European Society for Biomaterials; Elsevier Science Limited: London, UK, 1987; pp. 3–5. [Google Scholar]
- Rostamitabar, M.; Abdelgawad, A.M.; Jockenhoevel, S.; Ghazanfari, S. Drug-eluting medical textiles: From fiber production and textile fabrication to drug loading and delivery. Macromol. Biosci. 2021, 21, 2100021. [Google Scholar] [CrossRef]
- Libanori, A.; Chen, G.; Zhao, X.; Zhou, Y.; Chen, J. Smart textiles for personalized healthcare. Nat. Electron. 2022, 5, 142–156. [Google Scholar] [CrossRef]
- Cesarelli, G.; Donisi, L.; Coccia, A.; Amitrano, F.; D’Addio, G.; Ricciardi, C. The E-textile for biomedical applications: A systematic review of literature. Diagnostics 2021, 11, 2263. [Google Scholar] [CrossRef]
- Ornaghi, H.L., Jr.; Neves, R.M.; Monticelli, F.M.; Dall Agnol, L. Smart Fabric Textiles: Recent advances and challenges. Textiles 2022, 2, 582–605. [Google Scholar] [CrossRef]
- Ornaghi, H.L., Jr.; Bianchi, O. Temperature-dependent shape-memory textiles: Physical principles and applications. Textiles 2023, 3, 257–274. [Google Scholar] [CrossRef]
- Kubley, A.; Chitranshi, M.; Hou, X.; Schulz, M. Manufacturing and characterization of customizable flexible carbon nanotube fabrics for smart wearable applications. Textiles 2021, 1, 534–546. [Google Scholar] [CrossRef]
- Islam, M.R.; Golovin, K.; Dolez, P.I. Clothing thermophysiological Comfort: A textile science perspective. Textiles 2023, 3, 353–407. [Google Scholar] [CrossRef]
- Dolez, P.I.; Marsha, S.; McQueen, R.H. Fibers and textiles for personal protective equipment: Review of recent progress and perspectives on future developments. Textiles 2022, 2, 349–381. [Google Scholar] [CrossRef]
- Mondal, M.I.H. Medical Textiles from Natural Resources, 1st ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2022; p. 934. [Google Scholar]
- da Silva, C.J.G.; de Medeiros, A.D.M.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial celulose biotextiles for the future of sustainable fashion: A review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Biomedical Textiles Market Worth $20.7 Billion by 2027—Exclusive Report by Markets and Markets™. Available online: https://www.prnewswire.com/news-releases/biomedical-textiles-market-worth-20-7-billion-by-2027--exclusive-report-by-marketsandmarkets-301698086.html (accessed on 11 November 2024).
- Indira, M.; Sudarsini, B.; Sumalatha, B. Advancements in Implantable Medical Textile Materials. In Textile Materials for Good Health and Wellbeing: Design and Applications; Springer Nature: Singapore, 2024; pp. 197–229. [Google Scholar]
- Baptista-Silva, S.; Borges, S.; Brassesco, M.E.; Ezequiel, R.C.; Oliveira, A.L.; Pintado, M. Research, development and future trends for medical textile products. In Medical Textiles from Natural Resources; Woodhead Publishing: Cambridge, UK, 2022; pp. 795–828. [Google Scholar]
- Bazaka, K.; Jacob, M.V. Implantable devices: Issues and challenges. Electronics 2012, 2, 1–34. [Google Scholar] [CrossRef]
- Drug Delivery Systems Market Size, Share & Industry Analysis, By Type (Inhalation, Transdermal, Injectable, and Others), By Device Type (Conventional and Advanced), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and Regional Forecast, 2024–2032. Available online: https://www.fortunebusinessinsights.com/drug-delivery-systems-market-103070 (accessed on 1 July 2024).
- Drug Delivery Systems. Available online: https://www.nibib.nih.gov/science-education/science-topics/drug-delivery-systems-getting-drugs-their-targets-controlled-manner (accessed on 2 July 2024).
- Pei, J.; Yan, Y.; Palanisamy, C.P.; Jayaraman, S.; Natarajan, P.M.; Umapathy, V.R.; Gopathy, S.; Roy, J.R.; Sadagopan, J.C.; Thalamati, D.; et al. Materials-based drug delivery approaches: Recent advances and future perspectives. Green Process. Synth. 2024, 13, 20230094–20230123. [Google Scholar] [CrossRef]
- Hussain, M.; Hussain, M.S. A brief review on buccal drug delivery system: Advantages, limitations, and impact on healthcare system. World J. Pharm. Res. 2021, 10, 558–576. [Google Scholar]
- Nayak, B.S.; Sourajit, S.; Palo, M.; Behera, S. Sublingual drug delivery system: A novel approach. Int. J. Pharm. Drug Anal. 2017, 5, 399–405. [Google Scholar]
- Osmalek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barzyńska, J.; et al. Recent advances in polymer-based vaginal drug delivery systems. Pharmaceutics 2021, 13, 884. [Google Scholar] [CrossRef]
- Das Neves, J.; Sarmento, B. Precise engineering of dapivirine-loaded nanoparticles for the development of anti-HIV vaginal microbicides. Acta Biomater. 2015, 18, 77–87. [Google Scholar] [CrossRef]
- Gu, J.; Yang, S.; Ho, E.A. Biodegradable film for the targeted delivery of siRNA-loaded nanoparticles to vaginal immune cells. Mol. Pharm. 2015, 12, 2889–2903. [Google Scholar] [CrossRef]
- Rathi, R.; Sanshita, M.; Kumar, A.; Vishvakarma, V.; Huanbutta, K.; Singh, I.; Sangnim, T. Advancements in rectal drug delivery systems: Clinical trials, and patents perspective. Pharmaceutics 2022, 17, 2210. [Google Scholar] [CrossRef] [PubMed]
- Katare, P.; Medhe, T.P.; Nadkarni, A.; Deshpande, M.; Tekade, R.T.; Benival, D.; Jain, A. Nasal drug delivery system and devices: An overview on health effects. ACS Chem. Health Saf. 2024, 31, 127–143. [Google Scholar] [CrossRef]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Chu, J.N.; Traverso, G. Foundations of gastrointestinal-based drug delivery and future developments. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 219–238. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, M.; et al. Nanoparticles in drug delivery: From history to therapeutic applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Couvreur, P.; Vauthier, C. Poly alkyl cyanoacrylate nanoparticles as drug carrier: Present state and perspectives. J. Control. Release 1991, 17, 187–198. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Chauvierre, C.; Brigger, I.; Couvreur, P. Drug delivery to resistant tumors: The potential of poly (alkyl cyanoacrylate) nanoparticles. J. Control. Release 2003, 93, 151–160. [Google Scholar] [CrossRef]
- Shinto, Y.; Uchida, A.; Korkusuz, F.; Araki, N.; Ono, K. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J. Bone Jt. Surg. Br. 1992, 74, 600–604. [Google Scholar] [CrossRef]
- Esterhai, J.L., Jr.; Bednar, J.; Kimmelman, C.P. Gentamicin-induced ototoxicity complicating treatment of chronic osteomyelitis. Clin. Orthop. 1986, 209, 185–188. [Google Scholar] [CrossRef]
- Couvreur, P.; Puisieux, F. Nano-and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993, 10, 141–162. [Google Scholar] [CrossRef]
- Hwang, S.R.; Byun, Y. Advances in oral macromolecular drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.J.; Lukowski, G.; Kröber, R.; Damaschun, G.; Dittgen, M. Acrylic acid copolymer nanoparticles for drug delivery: Structural characterization of nanoparticles by small-angle X-ray scattering. Colloid Polym. Sci. 1994, 272, 755–769. [Google Scholar] [CrossRef]
- Lukowski, G.; Müller, R.H.; Müller, B.W.; Dittgen, M. Acrylic acid copolymer nanoparticles for drug delivery. Part II: Characterization of nanoparticles surface-modified by adsorption of ethoxylated surfactants. Colloid Polym. Sci. 1993, 271, 100–105. [Google Scholar] [CrossRef]
- Fresta, M.; Puglisi, G.; Giammona, G.; Cavallaro, G.; Micali, N.; Furneri, P.M. Pefloxacine mesilate-and ofloxacin-loaded poly ethyl cyanoacrylate nanoparticles: Characterization of the colloidal drug carrier formulation. J. Pharm. Sci. 1995, 74, 895–902. [Google Scholar] [CrossRef]
- Cavallaro, G.; Fresta, M.; Giammona, G.; Puglisi, G.; Villari, A. Entrapment of β-lactams antibiotics in polyethylcyanoacrylate nanoparticles: Studies on the possible in vivo application of this colloidal delivery system. Int. J. Pharm. 1994, 111, 31–41. [Google Scholar] [CrossRef]
- Pardridge, W.M. Physiologic-based strategies for protein drug delivery to the brain. J. Control. Release 1996, 39, 281–286. [Google Scholar] [CrossRef]
- Partridge, W.M. Drug and gene targeting to the brain via blood–brain barrier receptor-mediated transport systems. Int. Congr. Ser. 2005, 1277, 49–62. [Google Scholar] [CrossRef]
- Labhasetwar, V.; Song, C.; Levy, R.J. Nanoparticle drug delivery system for restenosis. Adv. Drug Deliv. Rev. 1997, 24, 63–85. [Google Scholar] [CrossRef]
- Labhasetwar, V.; Underwood, T.; Schwendeman, S.P.; Levy, R.J. Iontophoresis for modulation of cardiac drug delivery in dogs. Proc. Natl. Acad. Sci. USA 1995, 92, 2612–2616. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S. Diblock copolymer nanoparticles for drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1998, 5, 481–512. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Fernández-Urrusuno, R.; Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- May, J.P.; Li, S.-D. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013, 10, 511–527. [Google Scholar] [CrossRef]
- Calvo, P. PEGylated poly cyanoacrylate nanoparticles as vector for drug delivery in prion diseases. J. Neurosci. Methods 2001, 111, 151–155. [Google Scholar] [CrossRef]
- Collinge, J. Molecular neurology of prion disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 906–919. [Google Scholar] [CrossRef]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin-and transferrin-receptor-antibody6modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bio reduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Targeting intracellular targets. Curr. Drug Deliv. 2004, 1, 235–247. [Google Scholar] [CrossRef]
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2005, 9, 1864–7336. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Kingston, D.G.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Hattori, Y.; Maitani, Y. Folate-linked lipid-based nanoparticle for targeted gene delivery. Curr. Drug Deliv. 2005, 2, 243–252. [Google Scholar] [CrossRef]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Xiao, S. Preparation of folate-conjugated starch nanoparticles and its application to tumor-targeted drug delivery vector. Chin. Sci. Bull. 2006, 51, 1693–1697. [Google Scholar] [CrossRef]
- Yu, D.; Xiao, S.; Tong, C.; Chen, L.; Liu, X. Dialdehyde starch nanoparticles: Preparation and application in drug carrier. Chin. Sci. Bull. 2007, 52, 2913–2918. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Multi-Functional Gold Nanoparticles for Drug Delivery. In Bio-Applications of Nanoparticles; Chan, W.C.W., Ed.; Springer: New York, NY, USA, 2007; pp. 48–56. [Google Scholar]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [PubMed]
- Gazori, T.; Khoshayand, M.R.; Azizi, E.; Yazdizade, P.; Nomani, A.; Haririan, I. Evaluation of Alginate/Chitosan nanoparticles as antisense delivery vector: Formulation, optimization and in vitro characterization. Carbohydr. Polym. 2009, 77, 599–606. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D.C.; Neufeld, R.J. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2847. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Cancer-Cell-Specific Induction of Apoptosis Using Mesoporous Silica Nanoparticles as Drug-Delivery Vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef]
- Kohler, N.; Sun, C.; Wang, J.; Zhang, M. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir 2005, 21, 8858–8864. [Google Scholar] [CrossRef]
- Alhaddad, A. Nanodiamond as a vector for siRNA delivery to Ewing sarcoma cells. Small 2011, 21, 3087–3095. [Google Scholar] [CrossRef]
- Mengesha, A.E.; Youan, B.C. Nano diamonds for drug delivery systems. In Diamond-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 186–205. [Google Scholar]
- Arjunan, N.K.; Murugan, K.; Rejeeth, C.; Madhiyazhagan, P.; Barnard, D.R. Green Synthesis of Silver Nanoparticles for the Control of Mosquito Vectors of Malaria, Filariasis, and Dengue. Vector-Borne Zoonotic Dis. 2012, 12, 262–268. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Brown, P.K.; Qureshi, A.T.; Moll, A.N.; Hayes, D.J.; Monroe, W.T. Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing. ACS Nano 2013, 7, 2948–2959. [Google Scholar] [CrossRef]
- Minelli, C.; Lowe, S.B.; Stevens, M.M. Engineering nanocomposite materials for cancer therapy. Small 2010, 21, 2336–2357. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Synthesis of silver nanoparticles using fresh bark of Pongamia pinnata and characterization of its antibacterial activity against gram positive and gram negative pathogens. Resour.-Effic. Technol. 2016, 2, 30–35. [Google Scholar]
- Beg, M. Green synthesis of silver nanoparticles using Pongamia pinnata seed: Characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J. Mol. Recognit. 2017, 30, e2565. [Google Scholar] [CrossRef] [PubMed]
- Urbán, P.; Ranucci, E.; Fernàndez-Busquets, X. Polyamidoamine nanoparticles as nanocarriers for the drug delivery to malaria parasite stages in the mosquito vector. Nanomed 2015, 10, 3401–3414. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Kulbacka, J. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef]
- Ju, Z.; Sun, W. Drug delivery vectors based on filamentous bacteriophages and phage-mimetic nanoparticles. Drug Deliv. 2017, 24, 1898–1908. [Google Scholar] [CrossRef]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar]
- Aljabali, A.A. Innovative Applications of Plant Viruses in Drug Targeting and Molecular Imaging—A Review. Curr. Med. Imaging 2021, 17, 491–506. [Google Scholar] [CrossRef]
- Slita, A.; Egorova, A.; Casals, E.; Kiselev, A.; Rosenholm, J.M. Characterization of modified mesoporous silica nanoparticles as vectors for siRNA delivery. Asian J. Pharm. Sci. 2018, 13, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.; Vaidya, F.U.; Pandey, S.M. Mechanism for development of nanobased drug delivery system. Appl. Target. Nano Drugs Deliv. Syst. 2019, 1, 35–67. [Google Scholar]
- Ghaz-Jahanian, M.A.; Abbaspour-Aghdam, F.; Anarjan, N.; Berenjian, A.; Jafarizadeh-Malmiri, H. Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Mol. Biotechnol. 2015, 157, 201–218. [Google Scholar] [CrossRef]
- Assa, F. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, F.X.; Zhang, L.; Yang, W.; Wang, H.X. A pH-sensitive drug delivery system based on folic acid-targeted HBP-modified mesoporous silica nanoparticles for cancer therapy. Colloids Surf. Physicochem. Eng. Asp. 2020, 590, 124470. [Google Scholar] [CrossRef]
- Shafiei, N.; Nasrollahzadeh, M.; Iravani, S. Green Synthesis of Silica and Silicon Nanoparticles and Their Biomedical and Catalytic Applications. Comments Inorg. Chem. 2021, 41, 317–372. [Google Scholar] [CrossRef]
- Shariatinia, Z. Inorganic Material-Based Nanocarriers for Delivery of Biomolecules. Nanoeng. Biomater. Biomed. Appl. 2022, 2, 245–293. [Google Scholar]
- Gao, Y.; Gu, S.; Zhang, Y.; Xie, X.; Yu, T.; Lu, Y.; Zhu, Y.; Chen, W.; Zhang, H.; Dong, H.; et al. The architecture and function of monoclonal antibody-functionalized mesoporous silica nanoparticles loaded with mifepristone: Repurposing abortifacient for cancer metastatic chemoprevention. Small 2016, 12, 2595–2608. [Google Scholar] [CrossRef]
- Gencturk, A.; Kahraman, E.; Güngör, S.; Özhan, G.; Özsoy, Y.; Sarac, A.S. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: Characterization studies and in vitro assays. Artif. Cells Nanomed. Biotechnol. 2017, 45, 655–664. [Google Scholar] [CrossRef]
- Youseff, N.A.H.A.; Kassem, A.A.; El-Massik, M.A.E.; Boraie, N.A. Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int. J. Pharm. 2015, 486, 297–305. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; White, K.; Li, X.-L.; Zhu, L.-M. Dissolution improvement of electrospun nanofiber-based solid dispersions for acetaminophen. AAPS PharmSciTech 2010, 11, 809–817. [Google Scholar] [CrossRef]
- Zhu, L.-M.; Yu, D.G. Drug delivery systems using biotextiles. In Biotextiles as Medical Implants; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2013; pp. 213–231. [Google Scholar]
- Zilberman, M. Novel composite fiber structures to provide drug/protein delivery for medical implants and tissue regeneration. Acta Biomat. 2007, 3, 51–57. [Google Scholar] [CrossRef]
- Sikareepaisan, P.; Suksamrarn, A.; Supaphol, P. Electrospun gelatin fiber mats containing a herbal—Centella asiatica—Extract and release characteristic of asiaticoside. Nanotechnology 2007, 19, 015102. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
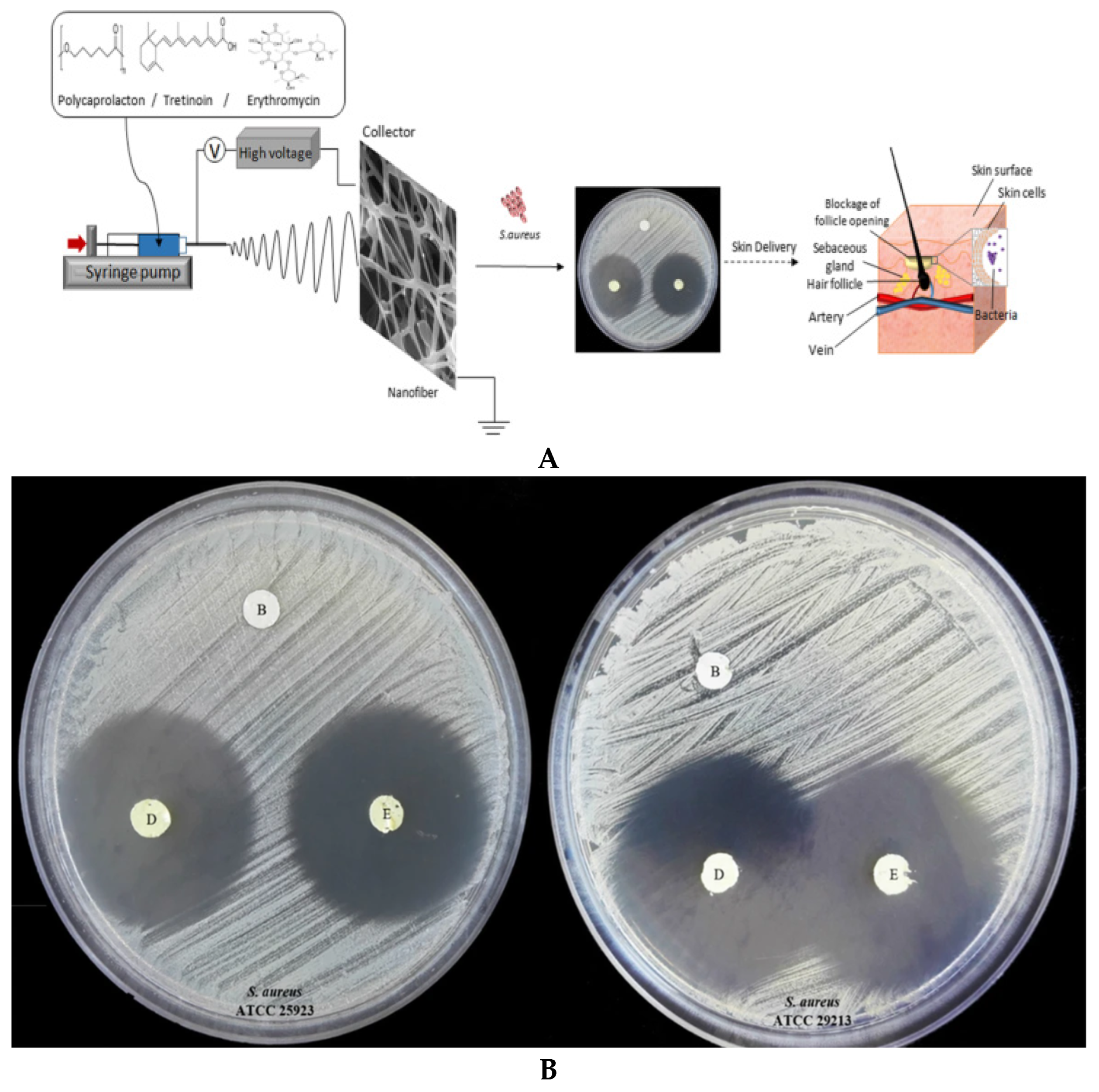
- Khoshbakht, S.; Asghari-Sana, F.; Fathi-Azarbayjani, A.; Sharifi, Y. Fabrication and characterization of tretinoin-loaded nanofiber for topical skin delivery. Biomat. Res. 2020, 24, 8. [Google Scholar] [CrossRef]
- Garg, A.; Alfatease, A.; Hani, U.; Haider, N.; Akbar, M.J.; Talath, S.; Angolkar, M.; Paramshetti, S.; Osmani, R.A.M.; Gundawar, R. Drug eluting protein and polysaccharides-based biofunctionalized fabric textiles- pioneering a new frontier in tissue engineering: An extensive review. Int. J. Biol. Macromol. 2024, 268, 131605. [Google Scholar] [CrossRef]
- Arik, B. Smart bio-textiles for medicine and healthcare applications. In Smart Textiles from Natural Resources; Woodhead Publishing: Cambridge, UK, 2024; pp. 495–537. [Google Scholar]
- Kim, Y.E.; Jung, H.Y.; Park, N.; Kim, J. Adhesive composite hydrogel patch for sustained transdermal drug delivery to treat atopic dermatitis. Chem. Mater. 2023, 35, 1209–1217. [Google Scholar] [CrossRef]
- Chen, G.; Au, C.; Chen, J. Textile triboelectric nanogenerators for wearable pulse wave monitoring. Trends Biotechnol. 2021, 39, 1078–1092. [Google Scholar] [CrossRef]
- Wu, X.; Liu, R.; Lao, T.T. Therapeutic compression materials and wound dressings for chronic venous insufficiency: A comprehensive review. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 892–909. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Heat: A highly efficient skin enhancer for transdermal drug delivery. Front. Bioeng. Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef]
- Jackson, J.M.; Weke, A.; Holliday, R. Nicotine pouches: A review for the dental team. Br. Dent. J. 2023, 235, 643–646. [Google Scholar] [CrossRef]
- Bhutto, M.A.; Wu, T.; Sun, B.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Fabrication and characterization of vitamin B5 loaded poly (l-lactide-co-caprolactone)/silk fiber aligned electrospun nanofibers for schwann cell proliferation. Colloids Surf. B Biointerfaces 2016, 144, 108–117. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, J.E.; Balikov, D.A.; Bae, M.S.; Heo, D.N.; Lee, D.; Rim, H.J.; Lee, D.-W.; Sung, H.-J.; Kwon, I.K. Poly (l-lactic acid)/gelatin fibrous scaffold loaded with simvastatin/beta-cyclodextrin-modified hydroxyapatite inclusion complex for bone tissue regeneration. Macromol. Biosci. 2016, 16, 1027–1038. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked hyaluronan electrospun nanofibers for ferulic acid ocular delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer e review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Hemamalini, T.; Giri Dev, V.R. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Kuwabara, M.; Sato, Y.; Ishihara, M.; Takayama, T.; Nakamura, S.; Fukuda, K.; Murakami, K.; Yokoe, H.; Kiyosawa, T. Healing of Pseudomonas aeruginosa-infected wounds in diabetic db/db mice by weakly acidic hypochlorous acid cleansing and silver nanoparticle/chitin-nanofiber sheet covering. Wound Med. 2020, 28, 100183. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in tissue engineering and biomolecules delivery systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef]
- Xie, Y.; Guan, Y.; Kim, S.H.; King, M.W. The mechanical performance of weft-knitted/ electrospun bilayer small diameter vascular prostheses. J. Mech. Behav. Biomed. Mater. 2016, 61, 410–418. [Google Scholar] [CrossRef]
- Akram, S.N.B.; Jahangir, M.U.; Mondal, M.I.H.; Arafat, M.T. Biotextiles for medical implants and regenerative medicine. In Medical Textiles from Natural Resources; Woodhead Publishing: Cambridge, UK, 2022; pp. 169–211. [Google Scholar]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-month results of biodegradable poly-I-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Asano, A.; Nakazawa, C.T.; Tsukatani, T.; Asakura, T. Structural characterization of silk-polyurethane composite material for biomaterials using solid-state NMR. Polym. J. 2012, 44, 802–807. [Google Scholar] [CrossRef]
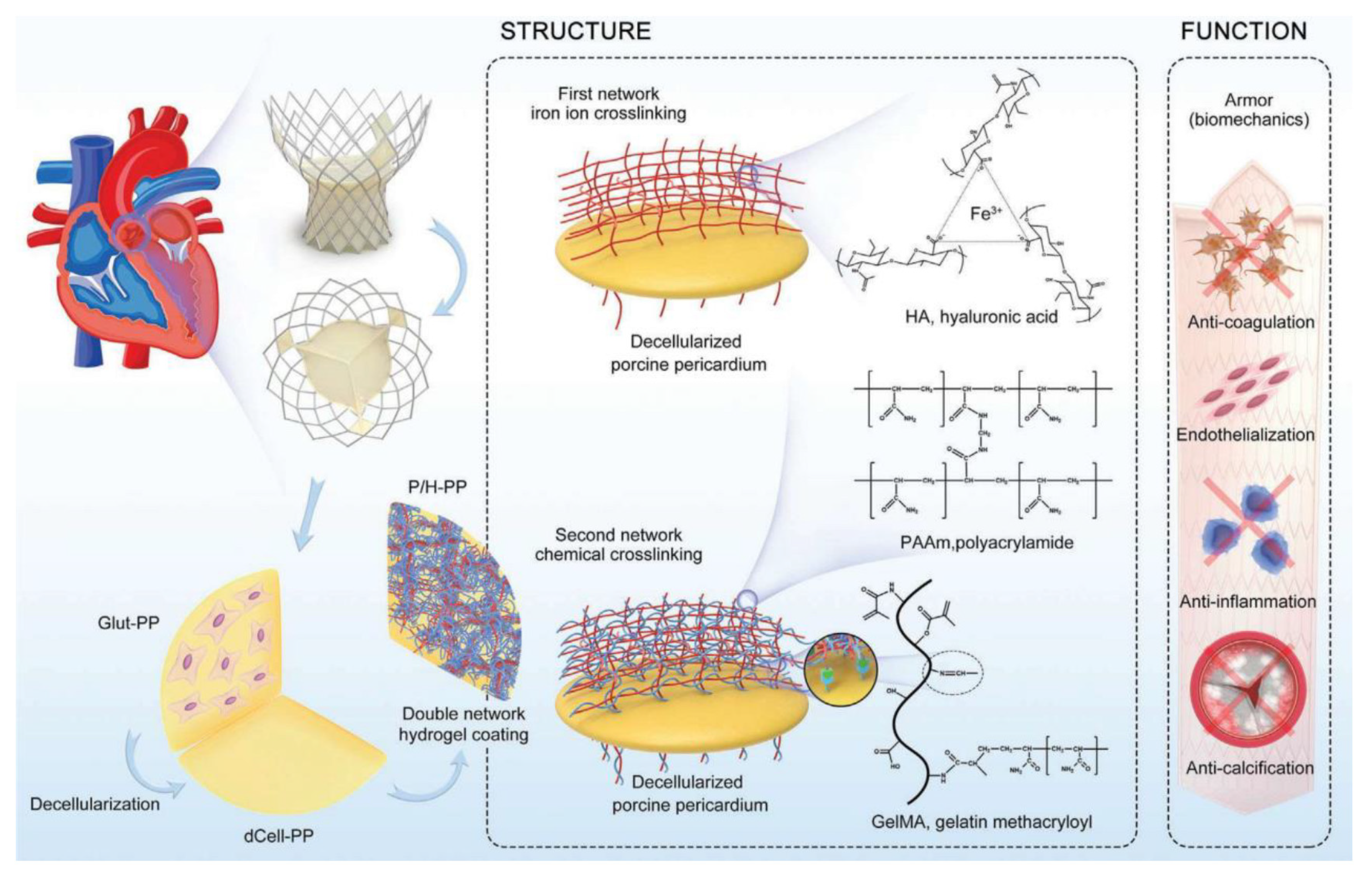
- Cheng, S.; Liu, X.; Qian, Y.; Maitusong, M.; Yu, K.; Cao, N.; Fang, J.; Liu, F.; Chen, J.; Xu, D.; et al. Double-Network Hydrogel Armored Decellularized Porcine Pericardium as Durable Bioprosthetic Heart Valves. Adv. Healthc. Mater. 2022, 11, 2102059. [Google Scholar] [CrossRef]
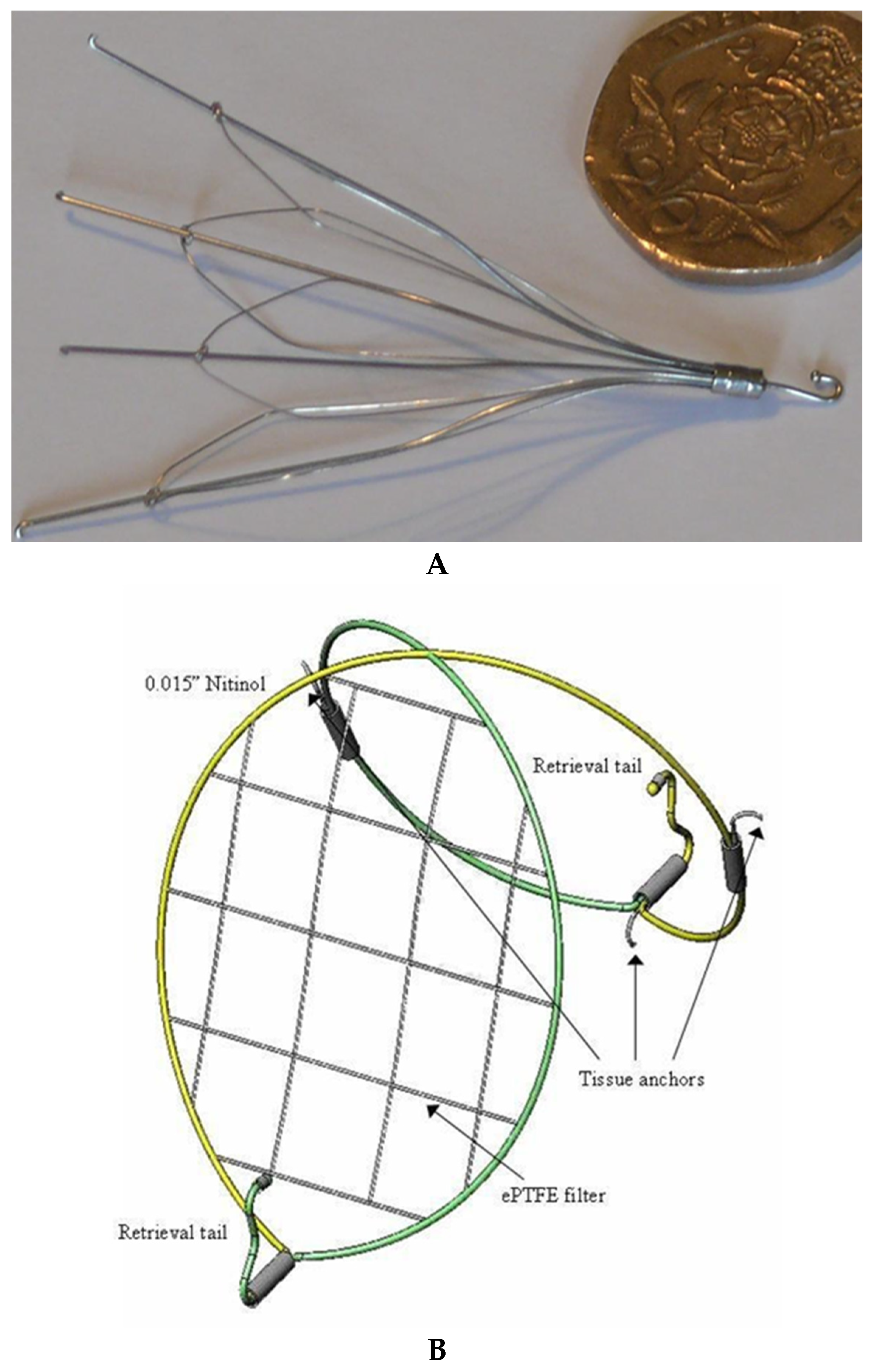
- Yoon, J.; King, M.W.; Johnson, E. Designing vena cava filters with textile structures. Medical and Healthcare Textiles. In Medical Textiles from Natural Resources; Woodhead Publishing: Cambridge, UK, 2010; pp. 334–341. [Google Scholar]
- Dacron e Patch na Anastomose Vascular. Available online: https://biomedical.com.br/home-en/ (accessed on 17 August 2024).
- Russel, S.J.; Mao, N. Anisotropic fluid transmission in nonwoven wound dressings. In Medical Textiles; Woodhead Publishing: Cambridge, UK, 2001; pp. 156–163. [Google Scholar]
- Devlin, J.J.; Kircher, S.; Kozen, B.G.; Littlejohn, L.F.; Johnson, A.S. Comparison of ChitoFlex®, CELOX™, and QuikClot® in control of hemorrhage. J. Emerg. Med. 2011, 41, 237–245. [Google Scholar] [CrossRef]
- PELNACTM. Available online: https://www.gunze.co.jp/medical/e/products/item_pn.html (accessed on 27 August 2024).
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Biomedical Textiles. Available online: https://confluentmedical.com/capabilities/biomedical-textiles (accessed on 25 August 2024).
- Deltamed Products. Available online: https://www.deltamed.com.tr/en/urunler/nerve-regeneration-grafts-012670478267976604/neuragen-08106615366558876 (accessed on 27 August 2024).
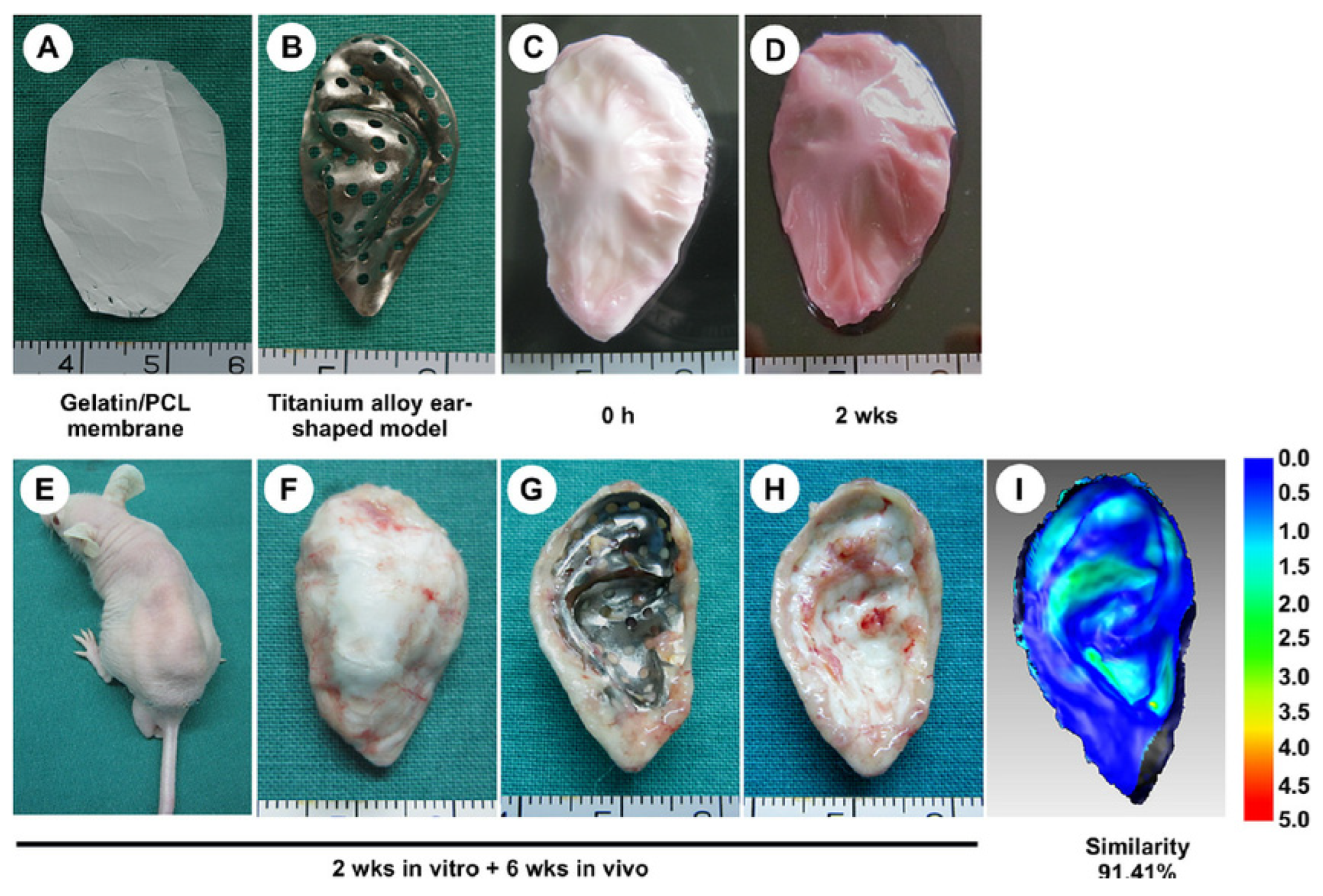
- Xue, j.; Feng, B.; Zheng, R.; Lu, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, Y.; Zhang, W.J. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials 2013, 34, 2624–2631. [Google Scholar] [CrossRef]
- Zhi, Y.; Jiang, J.; Zhang, P.; Chen, S. Silk enhances the ligamentization of the polyethylene terephthalate artificial ligament in a canine anterior cruciate ligament reconstruction model. Artif. Organs 2018, 43, E94–E108. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Biocomposites reinforced by fibers or tubes as scaffolds for tissue engineering or regenerative medicine. J. Biomed. Mater. Res. Part A 2014, 102, 1580–1594. [Google Scholar] [CrossRef]
- Dehari, D.; Chaudhuri, A.; Kumar, D.N.; Nath, G.; Agrawal, A.K. Fiber and textile in drug delivery to combat multidrug resistance microbial infection. In Fiber and Textile Engineering in Drug Delivery Systems; Woodhead Publishing: Cambridge, UK, 2023; pp. 359–387. [Google Scholar]
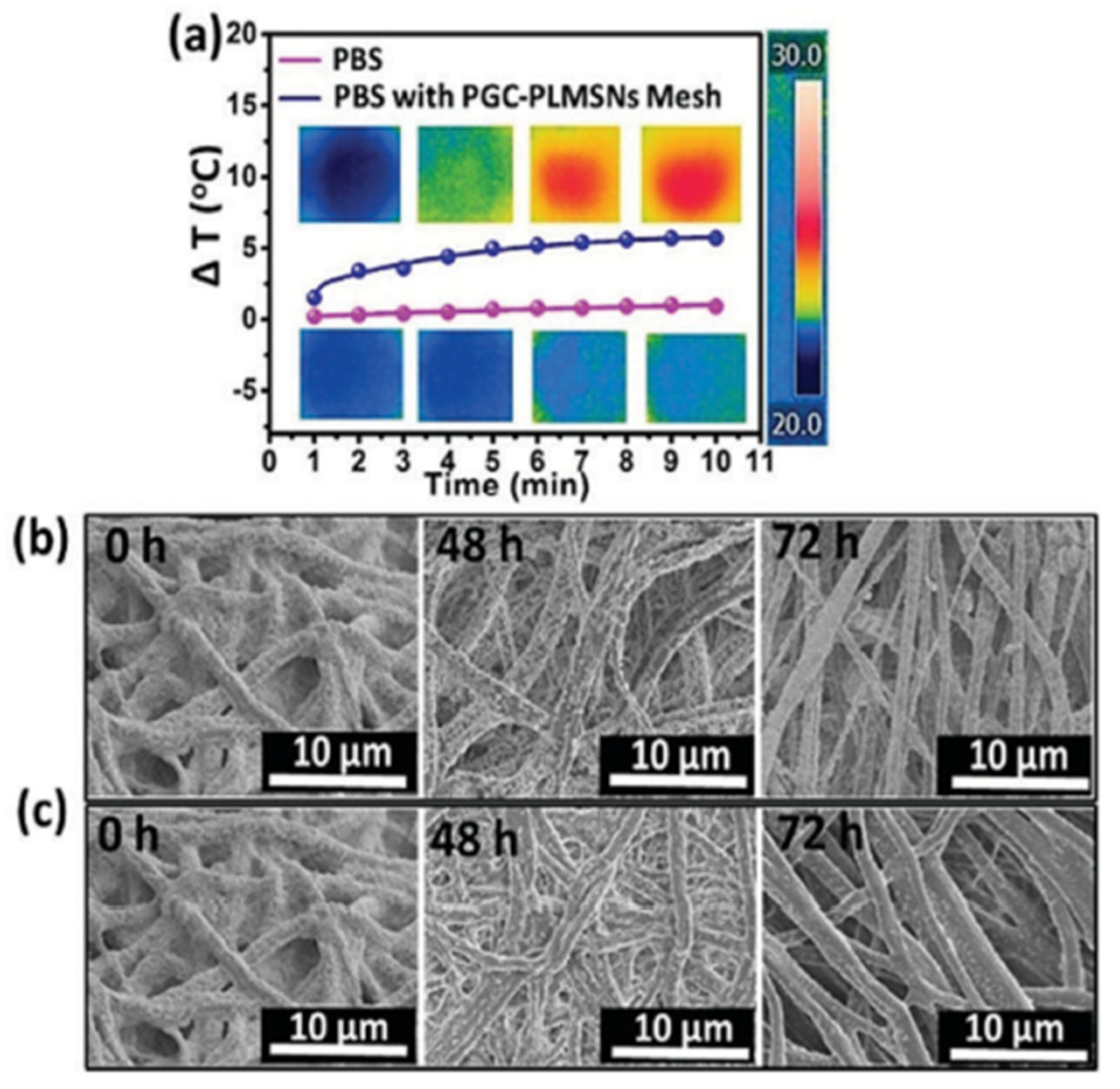
- Li, Y.; Fu, Y.; Ren, Z.; Li, X.; Mao, C.; Han, G. Enhanced cell uptake of fluorescent drug-loaded nanoparticles via an implantable photothermal fibrous patch for more effective cancer cell killing. J. Mater. Chem. B 2017, 5, 7504–7511. [Google Scholar] [CrossRef]








| Year | Types of NPs | Drug Delivery Approaches | Diseases | Applications | Characterization |
|---|---|---|---|---|---|
| 1991 [34,35] | Poly-alkyl-cyanoacrylate nanoparticles | A carrier that delivers drugs to target specific site. | Cancer | Cancer chemotherapy and intracellular antibiotherapy | Scanning electron microscope (SEM) |
| 1992 [36,37] | Calcium hydroxyapatite ceramic (CHC) | Drug gentamicin placed in porous blocks of calcium hydroxyapatite antibiotics (CHA). | Chronic osteomyelitis (animal model) | Bactericidal activity retained, with effective results | No in vivo experiments performed |
| 1993 [38,39] | Nano- and microparticles | Microparticulate system used for drug administration. | Enhancement of oral immune system (immunization) | In vitro experiments performed | No characterization provided |
| 1994 [40,41] | Acrylic acid copolymer NPs | Not specified | Not specified | Not specified | Not specified |
| 1995 [42,43] | Poly-alkyl-cyanoacrylate (PECA) nanoparticles | Acrylic acid, acrylic amide, acrylic-butyl ester, and methacrylic methyl ester are used as copolymers in drug delivery. OFX and PFX systems compared. | Bacterial diseases | OFX system showing greater efficient than the PFX system | No |
| 1996 [44,45] | Protein and peptide-based NPs | Monoclonal antibodies, recombinant proteins transported to the BBB by the chimeric Alzheimer’s disease peptide approach. | Brain diseases | Fluoroquinolone-loaded nanoparticles, enhanced antimicrobial activity. Avidin conjugated with BBB vector for protein transport across BBB | In vitro experiments performed; small angle X-ray scattering, freeze fracture electron microscopy, physicochemical characterization |
| 1997 [46,47] | Nanoparticles | Nanoparticles as carriers to deliver the drug to the intra-arterial localization system. | Restenosis (arterial reobstruction) | Catheter-based delivery | No |
| 1998 [48,49] | Diblock copolymer nanoparticles | Not specified | Not specified | Not specified | Not specified |
| 1999 [50,51] | Chitosan nanoparticles | Micelles and nanosphere carry genes and hydrophobic drugs to target site. | Diabetes | Potential of chitosan nanoparticles to improve insulin absorption through nasal cavity | MicroAB assay to determine insulin loading and release |
| 2000 [52,53] | Liposome with hyperthermia nanoparticles | Increased drug delivery to tumors. Hyperthermia helps liposomes work properly. | Ovarian carcinoma a | Enhanced drug delivery to tumor | Not specified |
| 2001 [54,55] | PEGylated poly-cyano-acrylate nanoparticles | Efficient drug carrier for therapeutic molecules. | Prion diseases | Effective drug delivery in prion disease test | Not specified |
| 2002 [56,57] | Transferrin-mediated receptor endocytosis | Transferrin and transferrin receptor in drug and gene transference via the BBB. | Cancer and hepatitis B | Efficient drug and gene delivery through BBB | Not specified |
| 2003 [58,59] | L-nanoparticles | Not specified | Not specified | Not specified | Not specified |
| 2004 [60,61] | Colloidal gold nanoparticles | Not specified | Not specified | Used as a vector to carry tumor necrosis factor (TNF) toward specific parts of tumor in mice | Not specified |
| 2005 [62,63] | Liposomes, nanoparticles | Not specified | Not specified | Not specified | Not specified |
| 2006 [64,65] | Folate-conjugated starch nanoparticles (StNP’s) | Vitamin folic acid inside cationic liposomes and conjugate liposomes as a carrier. | Cancer (human nasopharyngeal and prostate tumors) | Folate ligand as carrier and chemotherapeutic agent; DNA attached to receptor-bearing cancer cells in vitro | Not specified |
| 2007 [66,67] | Gold nanoparticles (AuNPs) | Drug and gene delivery approach. | Liver cancer | Drug and gene delivery using gold nanoparticles; transfection efficacy for beta-galactosidase with various MMPCs | Not specified |
| 2008 [68,69] | PEGylated gold nanoparticles | Very effective drug transfers with AuNPs vector for in vivo photodynamic treatment. | Cancer | Enhanced drug transfer with gold nanoparticles for photodynamic cancer treatment | Not specified |
| 2009 [70,71] | Alginate/chitosan (Alg/Chi) nanoparticles | Not specified | Not specified | Targeted drug delivery using alginate/chitosan polymers | Optimization of Alg/Chi NPs preparation; no characterization provided |
| 2010 [72,73] | Mesoporous silica nanoparticles | Targeted carriage of methotrexate (MTX) to tumor cells. | Cancer | Poly (ethylenimine)-functionalized mesoporous silica small units as vectors for drug delivery | Not specified |
| 2011 [74,75] | Nanodiamond (ND) nanoparticles | Transport of small interfering RNA into sarcoma (Ewing) cells. | Ewing sarcoma cells (cancer) | Evaluation of route for in vivo anticancer nucleic acid drug transfer | Not specified |
| 2012 [76,77] | Silver nanoparticles | Not specified | Malaria, dengue fever, filariasis | Designed as larvicides and photoactivated vectors for drug delivery and photoactivated gene silencing | Zeta potential, laser Doppler anemometry, photon correlation spectroscopy |
| 2013 [78,79] | Silver nanoparticles | Designed for photoactivated gene silencing and drug delivery. | Cancer | Effective in human cancer treatment; long retention time in blood | No |
| 2014 [80,81] | Silver nanoparticles | Synthesized from the plant Pongamia pinnata using the green method. | Malaria | Medically active; larvicidal action against Aedes aegypti | UV-visible absorption spectrum, TEM, XRD, FTIR |
| 2015 [82,83] | Polyamidoamine nanoparticles | Nanocarrier for antimalarial drug delivery. | Dengue | Delivery of antimalarial drug as nanomedicine | No |
| 2016 [84,85] | Solid lipid nanoparticles (SLNP) | Electroporation and nanocarrier delivery, loaded with cyanine-type IR-780 and flavonoid derivatives. | Colon cancer | Delivery of chemotherapeutic agents | No |
| 2017 [86,87] | Filamentous bacteriophage and phage-mimetic nanoparticles | Delivery of drugs and genes through phage particles. | Bacterial and viral diseases | Virus-based delivery system; phages chemically altered or genetically designed | Fluorescence-assisted cell sorting, transmission electron microscopy, confocal immunofluorescence |
| 2018 [88,89] | Mesoporous silica nanoparticles (MSNs) | Electrostatic absorption; loading with surface-hyperbranching polymerized poly(ethyleneimine). | Ocular drug delivery, vaccine delivery, mucosal and nasal drug transfer, gene carriage | Non-viral vectors for various drug delivery methods | Transmission electron microscopy, dynamic light scattering (DLS), zeta determination |
| 2019 [90,91] | Chitosan nanoparticles | Drug loaded onto chitosan nanoparticles for targeting various delivery sites. | Not specified | Buccal medicine distribution, vaccine transfer, cancer treatment | No |
| 2020 [92,93] | Mesoporous silica NPs with folic acid (MSN-COOH-Tet-HBP-FA) | pH-sensitive drug delivery system; folic-acid-targeted HBP. | Cancer | Enhanced drug delivery and stability; zero pre-release within 20 h in physiological conditions | XRD, TEM, HNMR spectra, SEM, UV-analysis, TGA |
| 2021 [94] | Novel silver nanoparticles | Nucleic acid sequences for protein production. | SARS-CoV-2, COVID-19 | DNA or mRNA transport for immune response; COVID-19 treatment and prevention | No |
| 2021 [95,96,97] | Lipid-based nanoparticles, metal and metal oxide NPs | Drug delivery and immuno-anti-inflammatory responses. | COVID-19 | Vaccine success rate; used in face masks, immune sensors, and coatings | No |
| 2022 [98] | Resveratrol–zinc NPs | Immuno-anti-inflammatory response. | Cancer, nervous breakdown | Drug delivery for COVID-19 and cancer treatment; neuroprotective mediators | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Júnior, H.L.O.; Garavatti, J.P. Biotextiles for Biomedical Applications: A Review. Textiles 2025, 5, 19. https://doi.org/10.3390/textiles5020019
Júnior HLO, Garavatti JP. Biotextiles for Biomedical Applications: A Review. Textiles. 2025; 5(2):19. https://doi.org/10.3390/textiles5020019
Chicago/Turabian StyleJúnior, Heitor Luiz Ornaghi, and Julia Pradella Garavatti. 2025. "Biotextiles for Biomedical Applications: A Review" Textiles 5, no. 2: 19. https://doi.org/10.3390/textiles5020019
APA StyleJúnior, H. L. O., & Garavatti, J. P. (2025). Biotextiles for Biomedical Applications: A Review. Textiles, 5(2), 19. https://doi.org/10.3390/textiles5020019








