Sustainable Fabrication of Reddish Silk Fabric with Enhanced Color Intensity and Fastness Using Lycopene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV–Vis Absorption Spectroscopy
2.3. Fabric Treatment
2.4. CCD Experiment
2.5. Measurement
2.5.1. Adsorption Quantity
2.5.2. Color Characteristics
3. Results
3.1. Properties of LYC in Various Solvents
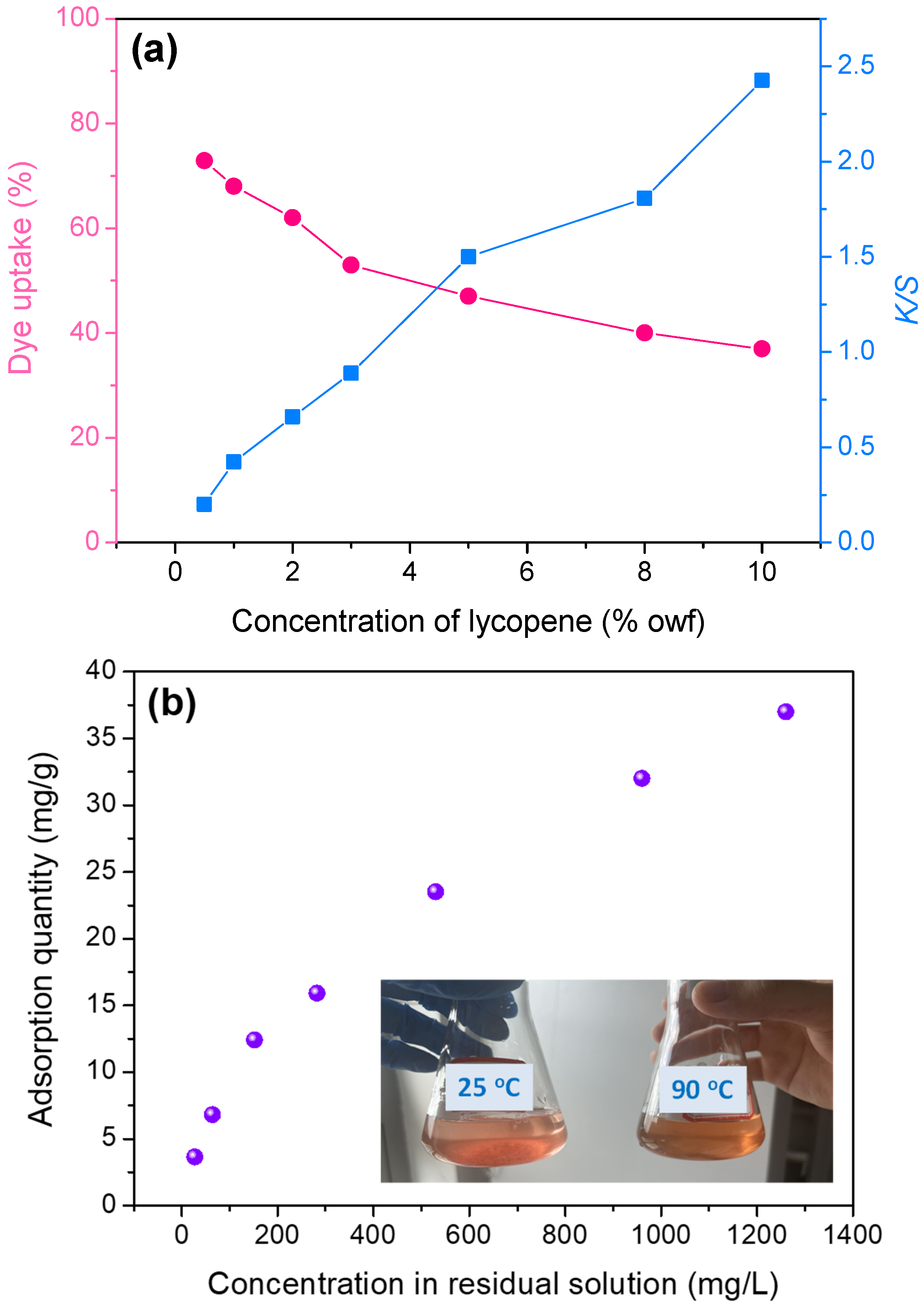
3.2. Building-Up Property
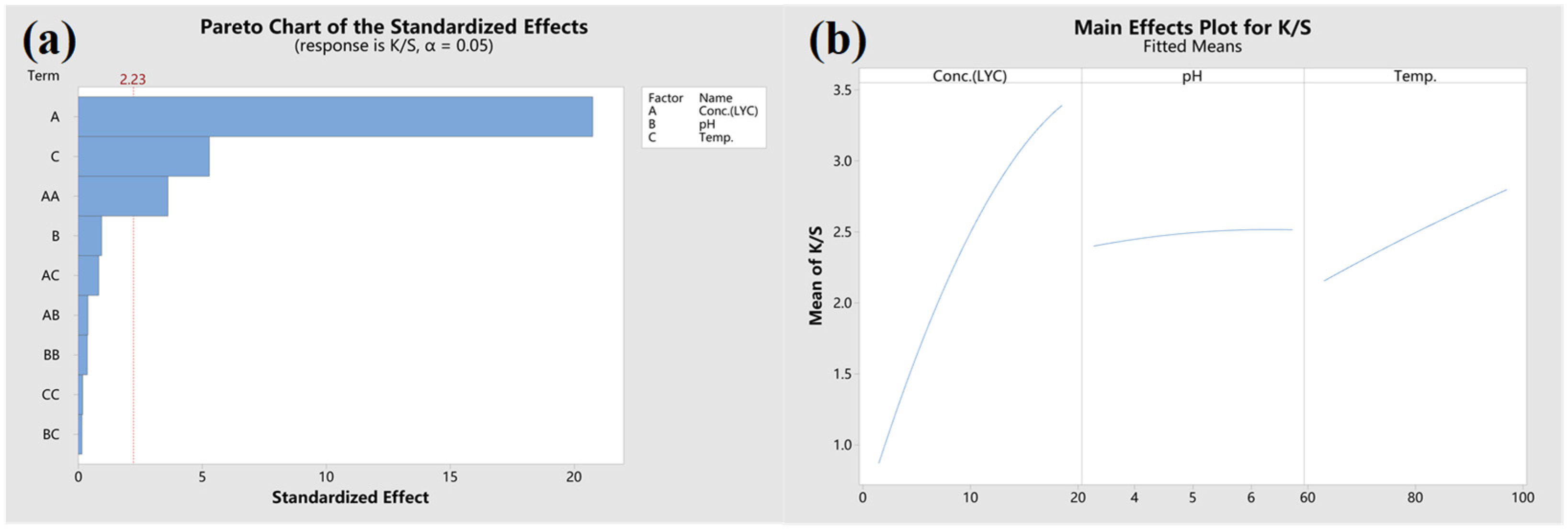
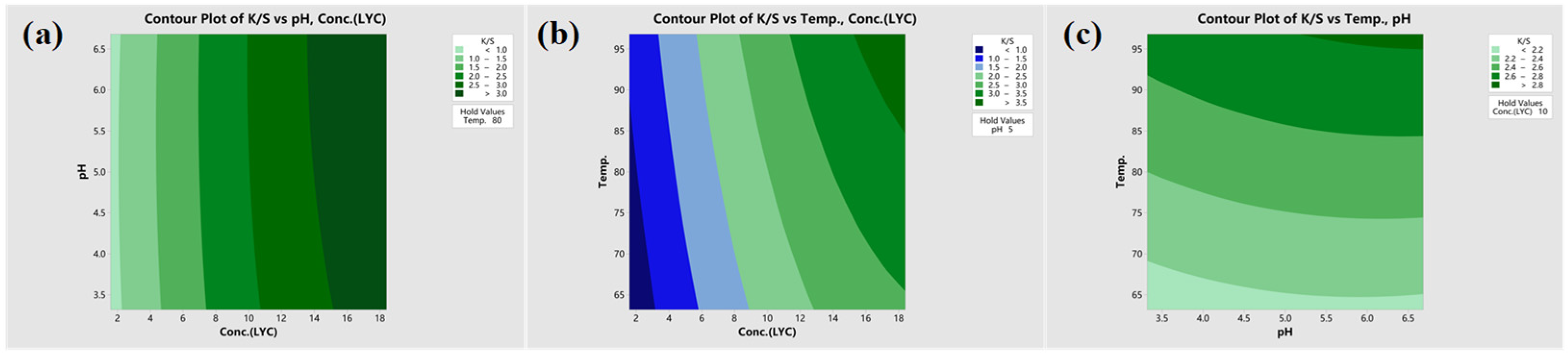
3.3. Analysis of CCD Experiment
3.4. Color Characterization and Fastness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid Profile, Antioxidant Capacity, and Chromoplasts of Pink Guava (Psidium guajava L. Cv. ‘Criolla’) during Fruit Ripening. J. Agric. Food Chem. 2017, 65, 3737–3747. [Google Scholar] [CrossRef] [PubMed]
- Skibsted, L.H. Carotenoids in Antioxidant Networks. Colorants or Radical Scavengers. J. Agric. Food Chem. 2012, 60, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Ahirwar, A.; Singh, S.; Lodhi, R.; Lodhi, A.; Rai, A.; Jadhav, D.A.; Harish; Varjani, S.; Singh, G.; et al. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Mar. Drugs 2023, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Baysal, T. Color and lycopene content of tomato puree affected by electroplasmolysis. Int. J. Food Prop. 2007, 10, 489–495. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction(UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, J.Z.; Zhao, L.C. The optimization of response surface method on capsicum red dyestuff extracting by ultrasonic method. Chin. Agric. Sci. Bull. 2008, 24, 96–101. [Google Scholar] [CrossRef]
- Jiang, S.J.; Wu, J.B. Study on the extracting process of orange peel stuff by ultrasonic technology. J. Anhui. Sic. 2008, 36, 3934–3935, 3940. [Google Scholar]
- Carvalho, C.; Santos, G. Global communities, biotechnology and sustainable design–natural/bio dyes in textiles. Procedia Manuf. 2015, 3, 6557–6564. [Google Scholar] [CrossRef]
- Baaka, N.; El Ksibi, I.; Mhenni, M.F. Optimisation of the recovery of carotenoids from tomato processing wastes: Application on textile dyeing and assessment of its antioxidant activity. Nat. Prod. Res. 2017, 31, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.A.; Leite, B.S.; Assuncao, L.S.; de Jesus Freitas, T.; Colauto, N.B.; Linde, G.A.; Otero, D.M.; Machado, B.A.S.; Ferreira Ribeiro, C.D. Red Tomato Products as an Alternative to Reduce Synthetic Dyes in the Food Industry: A Review. Molecules 2021, 26, 7125. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.S.; Räisänen, R. Waterless Dyeing with Plant-Based Natural Dyes as Colorants; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2023; pp. 141–148. [Google Scholar]
- Wu, M.; Zhou, Y.; Tang, R.-C. Bridging phycocyanin onto silk by genipin towards durable colouristic, antioxidant and UV protective properties: A sustainable strategy for fully bio-based textile. Chem. Eng. J. 2023, 477, 146808. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.Y.; Tang, R.C. Bioactive and UV protective silk materials containing baicalin—The multifunctional plant extract from Scutellaria baicalensis Georgi. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 336–344. [Google Scholar] [CrossRef] [PubMed]



| Variables | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| A: Conc. (LYC) (% owf) | 1.6 | 5 | 10 | 15 | 18.4 |
| B: pH | 3.3 | 4 | 5 | 6 | 6.7 |
| C: Temp. (°C) | 63.2 | 70 | 80 | 90 | 96.8 |
| Run | Conc.(LYC) | pH | Temp. | K/S | |
|---|---|---|---|---|---|
| Predicted | Actual | ||||
| 1 | 5.00 | 4.00 | 70.00 | 1.45 | 1.43 |
| 2 | 15.00 | 4.00 | 70.00 | 2.82 | 2.83 |
| 3 | 5.00 | 6.00 | 70.00 | 1.47 | 1.43 |
| 4 | 15.00 | 6.00 | 70.00 | 2.91 | 2.95 |
| 5 | 5.00 | 4.00 | 90.00 | 1.74 | 1.61 |
| 6 | 15.00 | 4.00 | 90.00 | 3.26 | 3.21 |
| 7 | 5.00 | 6.00 | 90.00 | 1.78 | 1.69 |
| 8 | 15.00 | 6.00 | 90.00 | 3.38 | 3.31 |
| 9 | 1.59 | 5.00 | 80.00 | 0.89 | 1.01 |
| 10 | 18.41 | 5.00 | 80.00 | 3.38 | 3.38 |
| 11 | 10.00 | 3.32 | 80.00 | 2.40 | 2.47 |
| 12 | 10.00 | 6.68 | 80.00 | 2.51 | 2.56 |
| 13 | 10.00 | 5.00 | 63.18 | 2.16 | 2.12 |
| 14 | 10.00 | 5.00 | 96.82 | 2.79 | 2.95 |
| 15 | 10.00 | 5.00 | 80.00 | 2.49 | 2.65 |
| 16 | 10.00 | 5.00 | 80.00 | 2.49 | 2.37 |
| 17 | 10.00 | 5.00 | 80.00 | 2.49 | 2.40 |
| 18 | 10.00 | 5.00 | 80.00 | 2.49 | 2.64 |
| 19 | 10.00 | 5.00 | 80.00 | 2.49 | 2.53 |
| 20 | 10.00 | 5.00 | 80.00 | 2.49 | 2.35 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 8.24191 | 0.91577 | 52.59 | <0.001 |
| Linear | 3 | 7.99803 | 2.66601 | 153.11 | <0.001 |
| Conc.(LYC) | 1 | 7.49490 | 7.49490 | 430.42 | <0.001 |
| pH | 1 | 0.01560 | 0.01560 | 0.90 | 0.366 |
| Temp. | 1 | 0.48753 | 0.48753 | 28.00 | <0.001 |
| Square | 3 | 0.22886 | 0.07629 | 4.38 | 0.033 |
| Conc.(LYC)∗Conc.(LYC) | 1 | 0.22832 | 0.22832 | 13.11 | 0.005 |
| pH∗pH | 1 | 0.00237 | 0.00237 | 0.14 | 0.720 |
| Temp.∗Temp. | 1 | 0.00060 | 0.00060 | 0.03 | 0.856 |
| 2-Way Interaction | 3 | 0.01503 | 0.00501 | 0.29 | 0.833 |
| Conc.(LYC)∗pH | 1 | 0.00269 | 0.00269 | 0.15 | 0.703 |
| Conc.(LYC)∗Temp. | 1 | 0.01193 | 0.01193 | 0.69 | 0.427 |
| pH∗Temp. | 1 | 0.00041 | 0.00041 | 0.02 | 0.881 |
| Error | 10 | 0.17413 | 0.01741 | ||
| Lack-of-Fit | 5 | 0.08281 | 0.01656 | 0.91 | 0.541 |
| Pure Error | 5 | 0.09132 | 0.01826 | ||
| Total | 19 | 8.41604 | |||
| Model Summary | |||||
| S | R-sq | R-sq(adj) | R-sq(pred) | ||
| 0.131958 | 97.93% | 96.07% | 90.97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Zhou, Y. Sustainable Fabrication of Reddish Silk Fabric with Enhanced Color Intensity and Fastness Using Lycopene. Textiles 2025, 5, 14. https://doi.org/10.3390/textiles5020014
Zuo J, Zhou Y. Sustainable Fabrication of Reddish Silk Fabric with Enhanced Color Intensity and Fastness Using Lycopene. Textiles. 2025; 5(2):14. https://doi.org/10.3390/textiles5020014
Chicago/Turabian StyleZuo, Jiahong, and Yuyang Zhou. 2025. "Sustainable Fabrication of Reddish Silk Fabric with Enhanced Color Intensity and Fastness Using Lycopene" Textiles 5, no. 2: 14. https://doi.org/10.3390/textiles5020014
APA StyleZuo, J., & Zhou, Y. (2025). Sustainable Fabrication of Reddish Silk Fabric with Enhanced Color Intensity and Fastness Using Lycopene. Textiles, 5(2), 14. https://doi.org/10.3390/textiles5020014






