Electrochemical Oxidation of Pollutants in Textile Wastewaters Using BDD and Ti-Based Anode Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Textile Wastewater
2.2. Electrochemical Oxidation Experiments
2.3. Analytical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vajnhandl, S.; Valh, J.V. The Status of Water Reuse in European Textile Sector. J. Environ. Manag. 2014, 141, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Volmajer Valh, J.; Majcen Le Marechal, A.; Vajnhandl, S.; Jerič, T.; Šimon, E. Water in the Textile Industry. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 685–706. ISBN 978-0-444-53199-5. [Google Scholar]
- Mohan, N.; Balasubramanian, N.; Basha, C. Electrochemical Oxidation of Textile Wastewater and Its Reuse. J. Hazard. Mater. 2007, 147, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; Crespi, M. A Review of Electrochemical Treatments for Colour Elimination. Color. Technol. 1999, 115, 342–345. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.-S.; Jiang, Y.-X.; Sun, L.; Sun, X.-H. Wastewater Treatment by Anodic Oxidation in Electrochemical Advanced Oxidation Process: Advance in Mechanism, Direct and Indirect Oxidation Detection Methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.; Soni, B.; Shah, M. A Comprehensive Review on Electro-Oxidation and Its Types for Wastewater Treatment. Groundw. Sustain. Dev. 2023, 23, 100980. [Google Scholar] [CrossRef]
- Zier, T.; Bouafia-Chergui, S.; Chabani, M. Anodic Oxidation of Synthetic Refinery Effluent on Lead Anode: Mass Transport and Charge Rate Balance. Water Sci. Technol. 2021, 84, 2422–2431. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the Electrochemical Conversion/Combustion of Organic Pollutants for Waste Water Treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Sillanpää, M.; Shestakova, M. Electrochemical Water Treatment Methods; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-811463-6. [Google Scholar]
- Rodríguez-Narváez, O.M.; Picos, A.R.; Bravo-Yumi, N.; Pacheco-Alvarez, M.; Martínez-Huitle, C.A.; Peralta-Hernández, J.M. Electrochemical Oxidation Technology to Treat Textile Wastewaters. Curr. Opin. Electrochem. 2021, 29, 100806. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of Wastewaters Containing Synthetic Organic Dyes by Electrochemical Methods. An Updated Review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Qiao, J.; Xiong, Y. Electrochemical Oxidation Technology: A Review of Its Application in High-Efficiency Treatment of Wastewater Containing Persistent Organic Pollutants. J. Water Process Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Zarei, M.; Babaei, T.; Ebratkhahan, M.; Nasiri Khoshnevis, T.; Zoghmand, M.; Gheshlaghi, A.; Hosseini, M.G. Application of Ti-Based Mixed Metal Oxide Electrodes towards Electrochemical Mineralization of Metronidazole: Evaluation of Influencing Factors and Removal by-Products. J. Taiwan Inst. Chem. Eng. 2023, 149, 104940. [Google Scholar] [CrossRef]
- Jaimes-López, R.; Jiménez-Vázquez, A.; Pérez-Rodríguez, S.; Estudillo-Wong, L.A.; Alonso-Vante, N. Catalyst for the Generation of OH Radicals in Advanced Electrochemical Oxidation Processes: Present and Future Perspectives. Catalysts 2024, 14, 703. [Google Scholar] [CrossRef]
- American Public Health Association. 5220 Chemical Oxygen Demand (COD). In Standard Methods For the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-299-8. [Google Scholar]
- Fernandes, A.; Pastorinho, M.R.; Sousa, A.C.; Silva, W.; Silva, R.; Nunes, M.J.; Rodrigues, A.S.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Ecotoxicological Evaluation of Electrochemical Oxidation for the Treatment of Sanitary Landfill Leachates. Environ. Sci. Pollut. Res. 2019, 26, 24–33. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. 5310 Total Organic Carbon. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-299-8. [Google Scholar]
- Bader, A.C.; Hussein, H.J.; Jabar, M.T. BOD: COD Ratio as Indicator for Wastewater and Industrial Water Pollution. Int. J. Spec. Educ. 2022, 37, 2164–2171. [Google Scholar]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of Wastewater Determines Microbial Assembly Mechanisms in Full-Scale Wastewater Treatment Plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef]
- Abdallah, K.Z.; Hammam, G. Correlation between Biochemical Oxygen Demand and Chemical Oxygen Demand for Various Wastewater Treatment Plants in Egypt to Obtain the Biodegradability Indices. Int. J. Sci. Basic Appl. Res. 2014, 13, 42–48. [Google Scholar]
- Dbira, S.; Bensalah, N.; Bedoui, A.; Cañizares, P.; Rodrigo, M.A. Treatment of Synthetic Urine by Electrochemical Oxidation Using Conductive-Diamond Anodes. Environ. Sci. Pollut. Res. 2015, 22, 6176–6184. [Google Scholar] [CrossRef]
- Fernandes, A.; Santos, D.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Electrochemical Oxidation of Humic Acid and Sanitary Landfill Leachate: Influence of Anode Material, Chloride Concentration and Current Density. Sci. Total Environ. 2016, 541, 282–291. [Google Scholar] [CrossRef]
- Candia-Onfray, C.; Espinoza, N.; Sabino Da Silva, E.B.; Toledo-Neira, C.; Espinoza, L.C.; Santander, R.; García, V.; Salazar, R. Treatment of Winery Wastewater by Anodic Oxidation Using BDD Electrode. Chemosphere 2018, 206, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Labiadh, L.; Barbucci, A.; Carpanese, M.P.; Gadri, A.; Ammar, S.; Panizza, M. Comparative Depollution of Methyl Orange Aqueous Solutions by Electrochemical Incineration Using TiRuSnO2, BDD and PbO2 as High Oxidation Power Anodes. J. Electroanal. Chem. 2016, 766, 94–99. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Souiad, F.; Fernandes, A.; Baía, A.; Pacheco, M.J.; Ciríaco, L.; Bendaoud-Boulahlib, Y.; Lopes, A. Treatment of Fruit Processing Wastewater by Electrochemical and Activated Persulfate Processes: Toxicological and Energetic Evaluation. Environ. Res. 2022, 209, 112868. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Kariman, A.; Marshall, A.T. Improving the Stability of DSA Electrodes by the Addition of TiO2 Nanoparticles. J. Electrochem. Soc. 2019, 166, E248–E251. [Google Scholar] [CrossRef]
- Fernandes, A.; Santos, D.; Pacheco, M.J.; Ciríaco, L.; Simões, R.; Gomes, A.C.; Lopes, A. Electrochemical Treatment of Cork Boiling Wastewater with a Boron-Doped Diamond Anode. Environ. Technol. 2015, 36, 26–35. [Google Scholar] [CrossRef]
- Ridgwell, A.; Zeebe, R. The Role of the Global Carbonate Cycle in the Regulation and Evolution of the Earth System. Earth Planet. Sci. Lett. 2005, 234, 299–315. [Google Scholar] [CrossRef]
- Souli, I.; Afonso, C.; Lopes, A.; Pacheco, M.J.; Ciríaco, L.; Labiadh, L.; Ammar, S.; Fernandes, A. Treatment of Fish Canning Wastewater by Electrochemical Oxidation Process. J. Water Process Eng. 2023, 56, 104423. [Google Scholar] [CrossRef]
- Fernandes, A.; Pereira, C.; Kozioł, V.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Emerging Contaminants Removal from Effluents with Complex Matrices by Electrooxidation. Sci. Total Environ. 2020, 740, 140153. [Google Scholar] [CrossRef]
- Santacruz, W.; Faria, J.; De Mello, R.; Boldrin, M.V.; Motheo, A.D.J. Comparative Study of MMO and BDD Anodes for Electrochemical Degradation of Diuron in Methanol Medium. Chemosphere 2024, 366, 143517. [Google Scholar] [CrossRef]

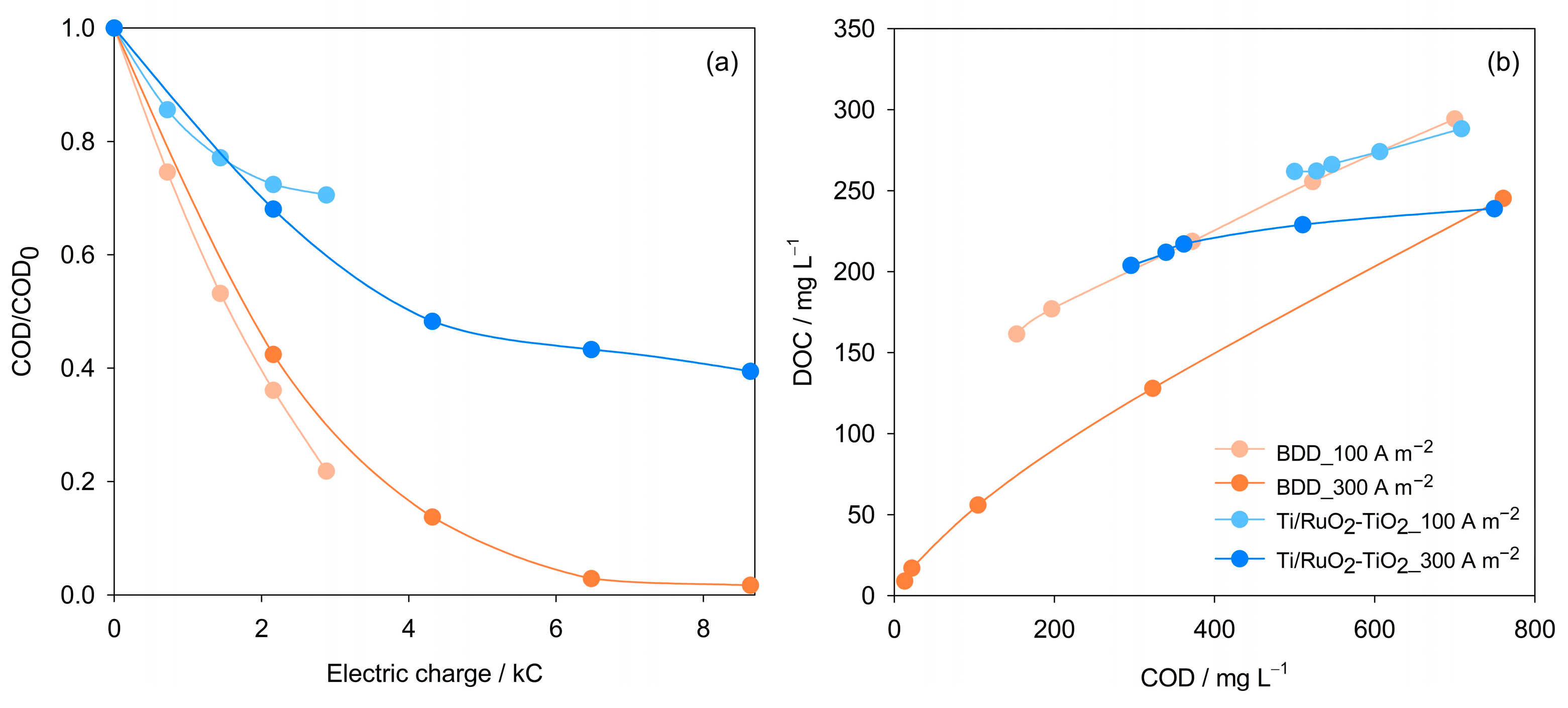
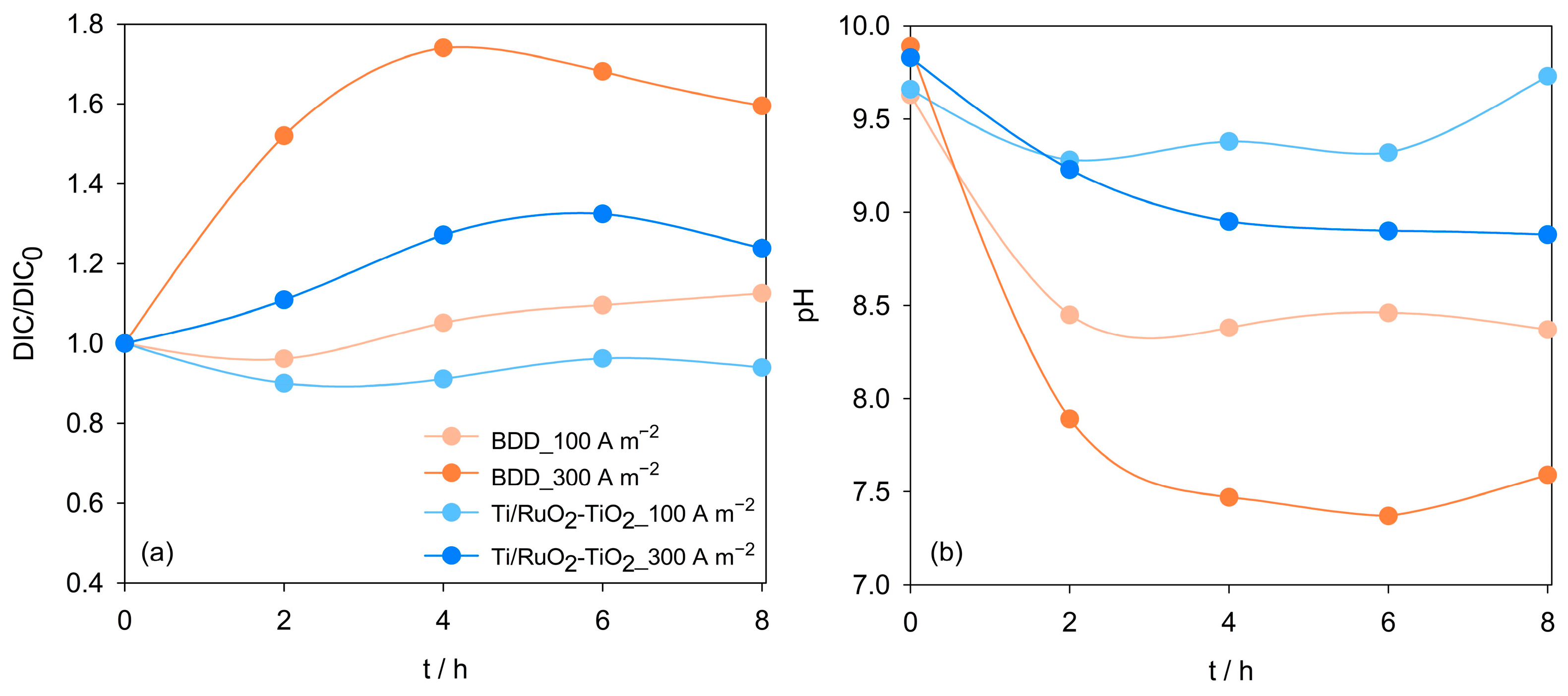
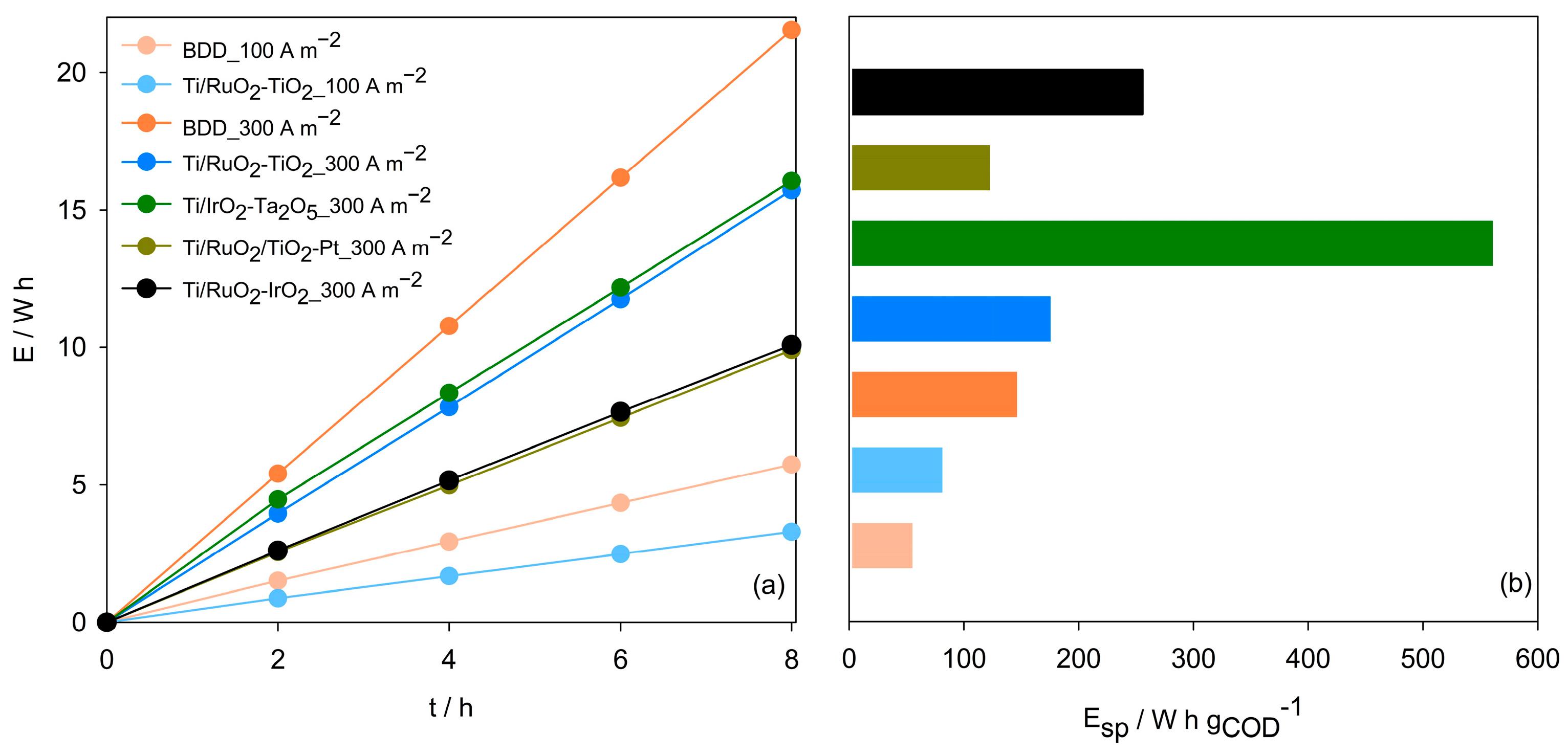
| Parameter | Mean Value (±SD) |
|---|---|
| Chemical oxygen demand (mg L−1) | 738 ± 7 |
| Biochemical oxygen demand (mg L−1) | 214 ± 1 |
| Biodegradability index | 0.29 |
| Total dissolved carbon (mg L−1) | 323 ± 5 |
| Dissolved organic carbon (mg L−1) | 233 ± 8 |
| Dissolved inorganic carbon (mg L−1) | 90 ± 5 |
| Total dissolved nitrogen (mg L−1) | 12 ± 3 |
| Na+ (mg L−1) | 829 ± 7 |
| Cl− (mg L−1) | 756 ± 1 |
| SO42− (mg L−1) | 107 ± 3 |
| pH | 9.6 ± 0.4 |
| Electrical conductivity (mS cm−1) | 4.9 ± 0.1 |
| Turbidity (NTU) | 18.6 ± 0.4 |
| Parameter | BDD | Ti/RuO2-TiO2 | Ti/RuO2/IrO2-Pt | Ti/IrO2-RuO2 | Ti/IrO2-Ta2O5 |
|---|---|---|---|---|---|
| Electrode cost | − | + + | + | + + + | + + |
| COD removal | + + + + | + + | + + | − | − |
| Mineralization degree | + + + + | + | − | − | − |
| Color removal | + + + + | + + + + | + | − | − |
| Esp | + + | + + | + + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, C.; Sousa, C.Y.; Farinon, D.M.; Lopes, A.; Fernandes, A. Electrochemical Oxidation of Pollutants in Textile Wastewaters Using BDD and Ti-Based Anode Materials. Textiles 2024, 4, 521-529. https://doi.org/10.3390/textiles4040030
Afonso C, Sousa CY, Farinon DM, Lopes A, Fernandes A. Electrochemical Oxidation of Pollutants in Textile Wastewaters Using BDD and Ti-Based Anode Materials. Textiles. 2024; 4(4):521-529. https://doi.org/10.3390/textiles4040030
Chicago/Turabian StyleAfonso, César, Carlos Y. Sousa, Daliany M. Farinon, Ana Lopes, and Annabel Fernandes. 2024. "Electrochemical Oxidation of Pollutants in Textile Wastewaters Using BDD and Ti-Based Anode Materials" Textiles 4, no. 4: 521-529. https://doi.org/10.3390/textiles4040030
APA StyleAfonso, C., Sousa, C. Y., Farinon, D. M., Lopes, A., & Fernandes, A. (2024). Electrochemical Oxidation of Pollutants in Textile Wastewaters Using BDD and Ti-Based Anode Materials. Textiles, 4(4), 521-529. https://doi.org/10.3390/textiles4040030








