Fiber-Based Masks and Respirators: Using Decontamination Methods and Antimicrobial Treatment to Improve Its Reusability during Pandemic
Abstract
:1. Introduction
2. Materials and Methods
- What are the available RPDs in the market and their corresponding testing standards?
- What are the possible decontamination methods for the reuse of the RPDs in the literature study?
- What are the cutting-edge antimicrobial treatments on masks in research?
3. Results and Discussion
3.1. Testing Standards
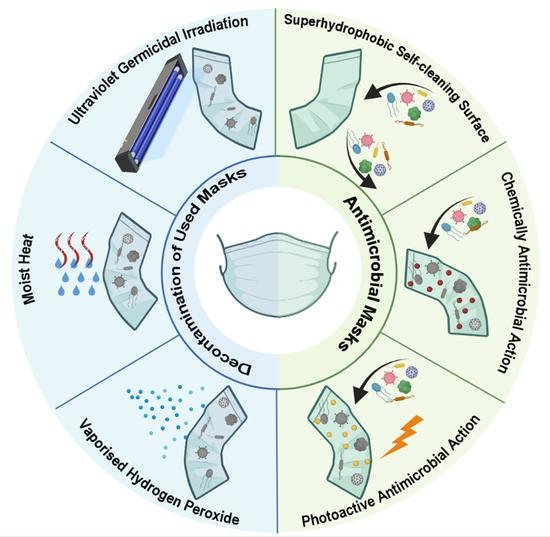
3.2. Decontamination Methods
3.3. Antimicrobial Masks
4. Conclusions and Perspectives
- Limited microbial contaminants used in the assay (H1N1, H5N1, MS2 phage, S. aureus and biological indicators);
- Lack of comprehensive testing of the microbial removal, FFRs structural integrity and function change after decontamination procedure;
- Results vary based on the FFR models, materials, and designs, as well as testing protocols.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OSHA. Personal Protective Equipment; US Department of Labor, Ed.; Occupational Safety and Health Administration: Washington, DC, USA, 2004.
- University of Washington. Guidelines For Personal Protective Equipment (PPE); EH&S Occupational Safety and Health Office: Washington, DC, USA, 2017; p. 35.
- HSE. The Health and Safety Toolbox: How to Control Risks at Work; Health and Safety Executive: Merseyside, UK. Available online: https://www.hse.gov.uk/toolbox/index.htm (accessed on 2 June 2022).
- OSHC. Guidelines for the Use of Personal Protective Equipment; National Council for Occupational Safety and Health, Ed.; Occupational Safety & Health Council: Hongkong, China, 2001; p. 17.
- NIOSH. Workplace Safety & Health Topics; The National Institute for Occupational Safety and Health: Washington, DC, USA, 2005.
- The European Parliament; The Council of the European Union. REGULATION (EU) 2016/425 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on personal protective equipment and repealing Council Directive 89/686/EEC. Off. J. Eur. Union 2016, OJ L 81, 51–98. [Google Scholar]
- Taylor, N.A.S.; Lewis, M.C.; Notley, S.R.; Peoples, G.E. A fractionation of the physiological burden of the personal protective equipment worn by firefighters. Eur. J. Appl. Physiol. 2011, 112, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Akduman, D.; Kim, L.E.; Parks, R.L.; L’Ecuyer, P.B.; Mutha, S.; Jeffe, D.B.; Fraser, V.J. Use of Personal Protective Equipment and Operating Room Behaviors in Four Surgical Subspecialties: Personal Protective Equipment and Behaviors in Surgery; The Official Journal of the Society of Hospital Epidemiologists of America, Washington University School of Medicine: Washington, DC, USA, 1999; pp. 110–114. [Google Scholar]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control. 2007, 35, S65–S164. [Google Scholar] [CrossRef] [PubMed]
- Newman, M. COVID-19: Doctors’ leaders warn that staff could quit and may die over lack of protective equipment. BMJ 2020, 368, m1257. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- bin-Reza, F.; Lopez Chavarrias, V.; Nicoll, A.; Chamberland, M.E. The use of masks and respirators to prevent transmission of influenza: A systematic review of the scientific evidence. Influenza Other Respir. Viruses 2012, 6, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Seale, H.; Dwyer, D.E.; Cowling, B.J.; Wang, Q.; Yang, P.; MacIntyre, C.R. A review of medical masks and respirators for use during an influenza pandemic. Influenza Other Respir. Viruses 2009, 3, 205–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klompas, M.; Morris, C.A.; Sinclair, J.; Pearson, M.; Shenoy, E.S. Universal Masking in Hospitals in the COVID-19 Era. N. Engl. J. Med. 2020, 382, e63. [Google Scholar] [CrossRef] [PubMed]
- Drabek, J.; Zatloukal, M. Meltblown technology for production of polymeric microfibers/nanofibers: A review. Phys. Fluids 2019, 31, 091301. [Google Scholar] [CrossRef]
- Institute of Medicine. Reusability of Facemasks During an Influenza Pandemic; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Farzaneh, S.; Shirinbayan, M. Processing and Quality Control of Masks: A Review. Polymers 2022, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Huang, V.J. Evaluation of the Efficiency of Medical Masks and the Creation of New Medical Masks. J. Int. Med. Res. 2016, 35, 213–223. [Google Scholar] [CrossRef]
- Baig, A.S.; Knapp, C.; Eagan, A.E.; Radonovich, L.J. Health care workers’ views about respirator use and features that should be included in the next generation of respirators. Am. J. Infect. Control. 2010, 38, 18–25. [Google Scholar] [CrossRef]
- Litchfield, S.M. Respiratory Protection—Preparing for H1N1 Influenza. AAOHN J. 2009, 57, 483–484. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Cloth Masks and Mask Sterilisation as Options in Case of Shortage of Surgical Masks and Respirators. Stockholm, Sweden. Available online: https://www.ecdc.europa.eu/en/publications-data/cloth-masks-sterilisation-options-shortage-surgical-masks-respirators (accessed on 8 April 2020).
- Centers for Disease Control and Prevention. Decontamination and Reuse of Filtering Facepiece Respirators. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html (accessed on 9 April 2020).
- CDC. Interim Guidance for the Use of Masks to Control Seasonal Influenza Virus Transmission; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- European Centre for Disease Prevention and Control. Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients with Suspected or Confirmed COVID-19; European Centre for Disease Prevention and Control: Solna, Sweden, 2020; p. 13. [Google Scholar]
- COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 Concerning Medical Devices, in: E.P.a.t. Council (Ed.) 1993L0042—EN—11.10.2007—005.001, 14 June 1993, p. 60. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:en:PDF (accessed on 2 June 2022).
- CEN, EN 14683:2019; Medical Face Masks–Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2019; p. 23. Available online: http://www.shanghaijifa.com/uploadfile/file/20200318/1584515148111951.pdf (accessed on 2 June 2022).
- CEN, EN 149:2001+A1:2009; Respiratory Protective Devices—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking. European Committee for Standardization: Brussels, Belgium, 2009.
- Long, Y.; Hu, T.; Liu, L.; Chen, R.; Guo, Q.; Yang, L.; Cheng, Y.; Huang, J.; Du, L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J. Evid.-Based Med. 2020, 13, 93–101. [Google Scholar] [CrossRef]
- Lawrence, R.B.; Duling, M.G.; Calvert, C.A.; Coffey, C.C. Comparison of Performance of Three Different Types of Respiratory Protection Devices. J. Occup. Environ. Hyg. 2006, 3, 465–474. [Google Scholar] [CrossRef]
- Gralton, J.; McLaws, M.-L. Protecting healthcare workers from pandemic influenza: N95 or surgical masks? Crit. Care Med. 2010, 38, 657–667. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.K.; Lee, S.; Lee, J.; Kim, J.; Tsai, P.; Yoon, C. Comparison of Filtration Efficiency and Pressure Drop in Anti-Yellow Sand Masks, Quarantine Masks, Medical Masks, General Masks, and Handkerchiefs. Aerosol Air Qual. Res. 2014, 14, 991–1002. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Wang, Q.; Cauchemez, S.; Seale, H.; Dwyer, D.E.; Yang, P.; Shi, W.; Gao, Z.; Pang, X.; Zhang, Y.; et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir. Viruses 2011, 5, 170–179. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Wang, Q.; Seale, H.; Yang, P.; Shi, W.; Gao, Z.; Rahman, B.; Zhang, Y.; Wang, X.; Newall, A.T.; et al. A Randomized Clinical Trial of Three Options for N95 Respirators and Medical Masks in Health Workers. Am. J. Respir. Crit. Care Med. 2013, 187, 960–966. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Seale, H.; Dung, T.C.; Hien, N.T.; Nga, P.T.; Chughtai, A.A.; Rahman, B.; Dwyer, D.E.; Wang, Q. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open 2015, 5, e006577. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.D.; MacDougall, C.C.; Johnstone, J.; Copes, R.A.; Schwartz, B.; Garber, G.E. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: A systematic review and meta-analysis. Can. Med. Assoc. J. 2016, 188, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.F.; Druce, J.D.; Birch, C.; Grayson, M.L. A Quantitative Assessment of the Efficacy of Surgical and N95 Masks to Filter Influenza Virus in Patients with Acute Influenza Infection. Clin. Infect. Dis. 2009, 49, 275–277. [Google Scholar] [CrossRef] [Green Version]
- Loeb, M.; Dafoe, N.; Mahony, J.; John, M.; Sarabia, A.; Glavin, V.; Webby, R.; Smieja, M.; Earn, D.J.D.; Chong, S.; et al. Surgical Mask vs N95 Respirator for Preventing Influenza Among Health Care Workers. JAMA 2009, 302, 1865–1871. [Google Scholar] [CrossRef] [Green Version]
- Atrie, D.A. Worster, Surgical mask versus N95 respirator for preventing influenza among health care workers: A randomized trial. Can. J. Emerg. Med. 2015, 14, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Radonovich, L.J.; Simberkoff, M.S.; Bessesen, M.T.; Brown, A.C.; Cummings, D.A.; Gaydos, C.A.; Perl, T.M. N95 Respirators vs Medical Masks for Preventing Influenza Among Health Care Personnel. JAMA 2019, 322, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Casanova, L.; Alfano-Sobsey, E.; Rutala, W.A.; Weber, D.J.; Sobsey, M. Virus Transfer from Personal Protective Equipment to Healthcare Employees’ Skin and Clothing. Emerg. Infect. Dis. 2008, 14, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Division, P.S. Comparison of FFP2, KN95, and N95 and Other Filtering Facepiece Respirator Classes; 3M: St. Paul, MN, USA, 2020. [Google Scholar]
- Chughtai, A.A.; Seale, H.; MacIntyre, C.R. Availability, consistency and evidence-base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: A global analysis. BMC Res. Notes 2013, 6, 216. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.M.; Weiss, P.D.; Weiss, D.E.; Weiss, J.B. Disrupting the Transmission of Influenza A: Face Masks and Ultraviolet Light as Control Measures. Am. J. Public Health 2007, 97 (Suppl. 1), S32–S37. [Google Scholar] [CrossRef]
- Lawrence, C.; Harnish, D.A.; Sandoval-Powers, M.; Mills, D.; Bergman, M.; Heimbuch, B.K. Assessment of half-mask elastomeric respirator and powered air-purifying respirator reprocessing for an influenza pandemic. Am. J. Infect. Control. 2017, 45, 1324–1330. [Google Scholar] [CrossRef]
- Subhash, S.S.; Cavaiuolo, M.; Radonovich, L.J.; Eagan, A.; Lee, M.L.; Campbell, S.; Martinello, R.A. Effectiveness of Common Healthcare Disinfectants against H1N1 Influenza Virus on Reusable Elastomeric Respirators. Infect. Control. Hosp. Epidemiol. 2016, 35, 894–897. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration. Respiratory Protection. In OSHA Technical Manual Section VIII: Chapter 2; National Institute for Occupational Safety & Health: Washington, DC, USA. Available online: https://www.osha.gov/otm/section-8-ppe/chapter-2 (accessed on 2 June 2022).
- Immunization and Respiratory Diseases (NCIRD). Decontamination and Reuse of Filtering Facepiece Respirators; CDC Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- van Straten, B.; Ligtelijn, S.; Droog, L.; Putman, E.; Dankelman, J.; Weiland, N.H.; Horeman, T. A life cycle assessment of reprocessing face masks during the COVID-19 pandemic. Sci. Rep. 2021, 11, 17680. [Google Scholar] [CrossRef]
- Lee, A.W.L.; Neo, E.R.K.; Khoo, Z.Y.; Yeo, Z.; Tan, Y.S.; Chng, S.; Low, J.S.C. Life cycle assessment of single-use surgical and embedded filtration layer (EFL) reusable face mask, Resources. Conserv. Recycl. 2021, 170, 105580. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Face mask decontamination and reuse: Is it ok? Am. J. Infect. Control. 2011, 39, 615. [Google Scholar] [CrossRef]
- Salter, W.B.; Kinney, K.; Wallace, W.H.; Lumley, A.E.; Heimbuch, B.K.; Wander, J.D. Analysis of Residual Chemicals on Filtering Facepiece Respirators After Decontamination. J. Occup. Environ. Hyg. 2010, 7, 437–445. [Google Scholar] [CrossRef]
- Derraik, J.G.; Anderson, W.A.; Connelly, E.A.; Anderson, Y.C. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.M.; Donzanti, M.J.; Minahan, D.J.; Shirazi, J.; Hatem, C.L.; Hayward-Piatkovskyi, B.; Gleghorn, J.P. Mask Reuse in the COVID-19 Pandemic: Creating an Inexpensive and Scalable Ultraviolet System for Filtering Facepiece Respirator Decontamination. Glob. Health Sci. Pract. 2020, 8, 582–595. [Google Scholar] [CrossRef]
- Rubio-Romero, J.C.; del Carmen Pardo-Ferreira, M.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef]
- Ma, Q.X.; Shan, H.; Zhang, C.M.; Zhang, H.L.; Li, G.M.; Yang, R.M.; Chen, J.M. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: Experimental supports. J. Med. Virol. 2020, 92, 1971–1974. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltran-Partida, E.; Nelson Cheng, J.S.C.; Valdez-Salas, E.A.; Curiel-Alvarez, M.; Ibarra-Wiley, R. Promotion of Surgical Masks Antimicrobial Activity by Disinfection and Impregnation with Disinfectant Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 2689–2702. [Google Scholar] [CrossRef]
- Bergman, M.; Zhuang, Z.; Brochu, E.; Palmiero, A. Fit Assessment of N95 Filtering-Facepiece Respirators in the U.S. Centers for Disease Control and Prevention Strategic National Stockpile. J. Int. Soc. Respir. Prot. 2015, 32, 50–64. [Google Scholar] [PubMed]
- Lore, M.B.; Heimbuch, B.K.; Brown, T.L.; Wander, J.D.; Hinrichs, S.H. Effectiveness of Three Decontamination Treatments against Influenza Virus Applied to Filtering Facepiece Respirators. Ann. Occup. Hyg. 2011, 56, 92–101. [Google Scholar]
- Kumar, A.; Kasloff, S.B.; Leung, A.; Cutts, T.; Strong, J.E.; Hills, K.; Krishnan, J. N95 Mask Decontamination using Standard Hospital Sterilization Technologies. MedRxiv 2020. [Google Scholar] [CrossRef]
- Schwartz, A.; Stiegel, M.; Greeson, N.; Vogel, A.; Thomann, W.; Brown, M.; Lewis, S. Decontamination and Reuse of N95 Respirators with Hydrogen Peroxide Vapor to Address Worldwide Personal Protective Equipment Shortages During the SARS-CoV-2 (COVID-19) Pandemic. Appl. Biosaf. 2020, 25, 67–70. [Google Scholar] [CrossRef]
- Heimbuch, B.K.; Kinney, K.; Lumley, A.E.; Harnish, D.A.; Bergman, M.; Wander, J.D. Cleaning of filtering facepiece respirators contaminated with mucin and Staphylococcus aureus. Am. J. Infect. Control. 2014, 42, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, E.M.; Williams, J.L.; Shaffer, R.E. Evaluation of Microwave Steam Bags for the Decontamination of Filtering Facepiece Respirators. PLoS ONE 2011, 6, e18585. [Google Scholar] [CrossRef] [Green Version]
- Heimbuch, B.K.; Wallace, W.H.; Kinney, K.; Lumley, A.E.; Wu, C.Y.; Woo, M.H.; Wander, J.D. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control. 2011, 39, e1–e9. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Wong, S.C.; Kwan, G.S.W.; Hui, W.T.; Yuen, K.Y. Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J. Hosp. Infect. 2020, 105, 358–359. [Google Scholar] [CrossRef]
- Kenney, P.A.; Chan, B.K.; Kortright, K.; Cintron, M.; Havill, N.; Russi, M.; Martinello, R.A. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. MedRxiv 2020. [Google Scholar] [CrossRef]
- Mills, D.; Harnish, D.A.; Lawrence, C.; Sandoval-Powers, M.; Heimbuch, B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control. 2018, 46, e49–e55. [Google Scholar] [CrossRef] [Green Version]
- Lindsley, W.G.; Martin, S.B., Jr.; Thewlis, R.E.; Sarkisian, K.; Nwoko, J.O.; Mead, K.R.; Noti, J.D. Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 Respirator Filtration Performance and Structural Integrity. J. Occup. Environ. Hyg. 2015, 12, 509–517. [Google Scholar] [CrossRef]
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar]
- Viscusi, D.J.; King, W.P.; Shaffer, R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. Int. Soc. Respir. Prot. 2007, 24, 93. [Google Scholar]
- Viscusi, D.J.; Bergman, M.S.; Novak, D.A.; Faulkner, K.A.; Palmiero, A.; Powell, J.; Shaffer, R.E. Impact of Three Biological Decontamination Methods on Filtering Facepiece Respirator Fit, Odor, Comfort, and Donning Ease. J. Occup. Environ. Hyg. 2011, 8, 426–436. [Google Scholar] [CrossRef]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.D.; Sambol, A.R.; Shaffer, R.E. Evaluation of Multiple (3-Cycle) Decontamination Processing for Filtering Facepiece Respirators. J. Eng. Fibers Fabr. 2018, 5, 155892501000500405. [Google Scholar] [CrossRef] [Green Version]
- Lowe, J.J.; Paladino, K.D.; Farke, J.D.; Boulter, K.; Cawcutt, K.; Emodi, M.; Rupp, M.E. N95 Filtering Facepiece Respirator Ultraviolet Germicidal Irradiation (UVGI) Process for Decontamination and Reuse; University of Nebraska Medical Center: Omaha, NE, USA, 2020. [Google Scholar]
- Nebraska Medicine COVID-19 PPE Guidance, Extended Use and Limited Reuse of Disposable Facemasks, Respirators and Protective Eyewear, Nebraska Medicine, Omaha, 19 March 2020. Available online: https://www.nebraskamed.com/sites/default/files/documents/covid-19/COVID-Extended-Use-Reuse-of-PPE-and-N95.pdf?date03212020 (accessed on 2 June 2022).
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef]
- Fisher, E.M.; Shaffer, R.E. Considerations for Recommending Extended Use and Limited Reuse of Filtering Facepiece Respirators in Health Care Settings. J. Occup. Environ. Hyg. 2014, 11, D115–D128. [Google Scholar] [CrossRef]
- Feldmann, F.; Shupert, W.L.; Haddock, E.; Twardoski, B.; Feldmann, H. Gamma Irradiation as an Effective Method for Inactivation of Emerging Viral Pathogens. Am. J. Trop. Med. Hyg. 2019, 100, 1275–1277. [Google Scholar] [CrossRef]
- da Silva Aquino, K.A. Sterilization by gamma irradiation. Gamma Radiat. 2012, 9, 172–202. [Google Scholar]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, T.H.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef] [Green Version]
- Selvaranjan, K.; Navaratnam, S.; Rajeev, P.; Ravintherakumaran, N. Environmental challenges induced by extensive use of face masks during COVID-19: A review and potential solutions. Environ. Chall. 2021, 3, 100039. [Google Scholar] [CrossRef]
- Liang, Y.; Tan, Q.; Song, Q.; Li, J. An analysis of the plastic waste trade and management in Asia. Waste Manag. 2021, 119, 242–253. [Google Scholar] [CrossRef]
- Tavis, J.E.; Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar]
- Rengasamy, S.; Fisher, E.; Shaffer, R.E. Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies. Am. J. Infect. Control. 2010, 38, 9–17. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, e2104298. [Google Scholar] [CrossRef]
- Seidi, F.; Deng, C.; Zhong, Y.; Liu, Y.; Huang, Y.; Li, C.; Xiao, H. Functionalized Masks: Powerful Materials against COVID-19 and Future Pandemics. Small 2021, 17, e2102453. [Google Scholar] [CrossRef] [PubMed]
- Blosi, M.; Costa, A.L.; Ortelli, S.; Belosi, F.; Ravegnani, F.; Varesano, A.; Tonetti, C.; Zanoni, I.; Vineis, C. Polyvinyl alcohol/silver electrospun nanofibers: Biocidal filter media capturing virus-size particles. J. Appl. Polym. Sci. 2021, 138, 51380. [Google Scholar] [CrossRef]
- Shanmugam, V.; Babu, K.; Garrison, T.F.; Capezza, A.J.; Olsson, R.T.; Ramakrishna, S.; Hedenqvist, M.S.; Singha, S.; Bartoli, M.; Giorcelli, M.; et al. Potential natural polymer-based nanofibres for the development of facemasks in countering viral outbreaks. J. Appl. Polym. Sci. 2021, 138, 50658. [Google Scholar] [CrossRef]
- Elena, P.; Miri, K. Formation of contact active antimicrobial surfaces by covalent grafting of quaternary ammonium compounds. Colloids Surf. B Biointerfaces 2018, 169, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bureš, F. Quaternary Ammonium Compounds: Simple in Structure, Complex in Application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef] [PubMed]
- Tuñón-Molina, A.; Martí, M.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Antimicrobial Face Shield: Next Generation of Facial Protective Equipment against SARS-CoV-2 and Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2021, 22, 9518. [Google Scholar] [CrossRef]
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS-CoV-2, and Methicillin-Resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, S.; Oh, E.; Han, S.; Choi, H.-J. Photopolymerizable, Universal Antimicrobial Coating to Produce High-Performing, Multifunctional Face Masks. Nano Lett. 2021, 21, 5422–5429. [Google Scholar] [CrossRef]
- Pollard, Z.A.; Karod, M.; Goldfarb, J.L. Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. Sci. Rep. 2021, 11, 19216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.M.; Vanyo, S.T.; Li, C.; Visser, M.B.; Mahajan, S.D.; Sharma, R.K.; Swihart, M.T. Copper@ZIF-8 Core-Shell Nanowires for Reusable Antimicrobial Face Masks. Adv. Funct. Mater. 2020, 31, 2008054. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Ngo-Minh, X.; Tang-Le-Quynh, T.; Tang-Thi-Thao, T.; Nguyen-Quoc, B.; Le-Quang, K.; Tran-Thanh, D.; Doan-Thi-Quynh, N.; Canty, E.; Do, T.; et al. The use of exhaled nitric oxide and peak expiratory flow to demonstrate improved breathability and antimicrobial properties of novel face mask made with sustainable filter paper and Folium Plectranthii amboinicii oil: Additional option for mask shortage during COVID-19 pandemic. Multidiscip. Respir. Med. 2020, 15, 664. [Google Scholar] [PubMed]
- Son, B.C.; Park, C.H.; Kim, C.S. Fabrication of Antimicrobial Nanofiber Air Filter Using Activated Carbon and Cinnamon Essential Oil. J. Nanosci. Nanotechnol. 2020, 20, 4376–4380. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Shuvho, M.B.A.; Shahid, M.A.; Haque, A.K.M.M.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, M.A.; Mofijur, M. Prospect of biobased antiviral face mask to limit the coronavirus outbreak. Environ. Res. 2021, 192, 110294. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Gianfranceschi, G.; Romano Spica, V.; D’Ermo, G.; Refi, C.; Podico, M.; Vitali, M.; Romano, F.; Valeriani, F. Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2. Int. J. Environ. Res. Public Health 2021, 18, 8662. [Google Scholar] [CrossRef]
- Wu, F.; He, P.; Chang, X.; Jiao, W.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Visible-Light-Driven and Self-Hydrogen-Donated Nanofibers Enable Rapid-Deployable Antimicrobial Bioprotection. Small 2021, 17, e2100139. [Google Scholar] [CrossRef] [PubMed]
- Monmaturapoj, N.; Sri-on, A.; Klinsukhon, W.; Boonnak, K.; Prahsarn, C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater. Sci. Eng. C 2018, 92, 96–102. [Google Scholar] [CrossRef]
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.-H.; Cho, Y.-K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nano Lett. 2020, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ganczak, M.; Szych, Z. Surgical nurses and compliance with personal protective equipment. J. Hosp. Infect. 2007, 66, 346–351. [Google Scholar] [CrossRef] [PubMed]


| Method | FFRs Type | Tested Virus | Other Tests | Comments | Ref. |
|---|---|---|---|---|---|
| Ultraviolet germicidal irradiation (UVGI), microwave-generated steam (MGS) and moist heat (MH) | N95 FFRs | H5N1 | Molecular amplification assay, filter performance | Three methods effectively reduce viral laden on the N95 and also do not dramatically affect the filtration performance. Other tests regarding structural integrity are needed for further investigation | [58] |
| Autoclave treatment, ethylene oxide (EtO) gassing, ionized hydrogen peroxide (iHP) fogging and vaporized hydrogen peroxide (VHP) | 4 different N95 FFR models | SARS-CoV-2 or vesicular stomatitis virus (as a surrogate) | Physical examination of structural and functional integrity, quantitative fit testing | All methods, which are commonly available in healthcare institutions, yielded effective decontamination performance. The response of structural and functional integrity change varies depending on the FFR models | [59] |
| Hydrogen peroxide vapor (HPV) | 3M (St. Paul, MN, USA) 1860 N95 | Biological indicator (Geobacillus stearothermophilus spores) | Off-gassing, odour, physical and performance degradation assessment, fitting test, facial structure check | HPV decontamination protocol is validated to effectively ensure the safe reuse of FFRs in real-world environments | [60] |
| Disinfecting wipes | Surgical N95 FFRs | Mucin and S. aureus | Particle penetration | 3–5 log reduction after cleaning FFRs with disinfecting wipes but not considered as effectively decontamination, particle penetration fulfills the standard (<5%) after cleaning despites the one with QACs wipe | [61] |
| MGS bags | FFR models pass the predefined quality standards | Bacteriophage MS2 (a surrogate for a pathogenic virus) | Filtration efficiency | 99.9% effective for inactivating MS2 and filtration efficacy remain above 95% after treatment | [62] |
| MGS, warm moist heat (WMH), UVGI at 254 nm | 6 commercially available FFR models | H1N1 influenza virus as aerosols or droplets | - | All three methods can reduce >4 log of viable H1N1 virus, and in 93% of the experiment, virus was reduced to undetectable level; no assessment of the integrity structural change of the FFR models | [63] |
| H2O2 iHP | N95 FFRs | Influenza A virus (subtype H1N1) | - | iHP could kill influenza A virus at moderate to high levels of inoculum; Residual of H2O2 in the inner surface of N95 should be monitored; The integrity structural change of N95 was not assessed | [64] |
| HPV | N95 | 3 aerosolized bacteriophages (proxy for SARS-CoV-2) | - | One HPV cycle can eliminate phage to undetectable level; 5 cycles post decontamination result in no deformation | [65] |
| UVGI | 15 N95 FFR models | H1N1 influenza | - | Significant reduction in influenza viability under soiled conditions in post-decontamination | [66] |
| UVGI (doses from 120–950 J cm−2 | Four models of N95 FFRs | - | Flow resistance, bursting strengths of the individual respirator coupon layers and the breaking strength of the respirator straps | UVGI has a minor effect on filtration performance but noticeable decrease in the structural integrity; maximum limited disinfection cycles depend on the FFRs models | [67] |
| UVGI, (EtO), VHP, microwave oven irradiation (MWI), and bleach | N95 FFRs, surgical N95 respirators, and P100 FFRs | - | Physical appearance, odour, laboratory performance (filter aerosol penetration and filter airflow resistance), dry heat laboratory oven exposures, off-gassing and FFR hydrophobicity | UVGI, EtO and VHP were found to be the most promising decontamination methods; the efficiency of the decontamination methods to inactivate viable microorganisms was not evaluated; infectious pathogen eradication was not assessed | [68] |
| Autoclave (160 °C dry heat), 70% isopropyl alcohol, soap and water (20-min soak), bleach, EtO, microwave oven, hydrogen peroxide (vaporized and liquid forms) and UV radiation | N95 and P100 FFRs | - | Filtration performance | Vaporized and liquid hydrogen peroxide and UV radiation appeared to have least effect on particle penetration performance; infectious pathogen eradication was not assessed | [69] |
| UVGI, MGS and moist heat incubation (MHI) | 6 N95 FFR models | - | Fitting characteristics, odour, comfort and donning ease | No significant change in fitting, odour, comfort or donning ease with the six FFRs after UVGI, MHI or MGS decontamination | [70] |
| UVGI, EtO, hydrogen peroxide gas plasma (HPGP), HPV, MGS, bleach, liquid hydrogen peroxide (LHP) and MHI (pasteurization) | 6 N95 FFR models | - | Physical appearance, odour and laboratory filtration performance | HPGP decontamination methods failed the filter penetration test requested by N95; FFR filtration efficiency of actual bioaerosols, as well as fitting tests after decontamination treatment, was not evaluated; decontamination method regarding its ability to inactivate infectious biological organisms is not tested | [71] |
| Coating Method | Antimicrobial Agents | Mode of Action | Tested Microorganisms | Ref. |
|---|---|---|---|---|
| Polyethylene terephthalate transparent masks dip-coated with benzalkonium chloride | The positively charged nitrogen atoms of BAK | Chemically antimicrobial action | MRSA and MRSE | [90] |
| Cross-linked to form permanent antimicrobial coating on the surface of the face masks | 2,2′,4′-terpyridine methylammonium chloride (LTMAC) and lignin adenine hexyl ammonium chloride (LAHAC) | Chemically antimicrobial action | Alpha coronavirus: HCoV-229E, beta coronavirus: HCoV-OC43 and K. pneumoniae | [92] |
| The deposition of liquid metal copper alloy (LMCu) particles on the fabric by spontaneous galvanic replacement reaction | The reduction of Cu ions into metallic Cu by galvanic replacement | Chemically antimicrobial action | S. aureus, E. coli, C. albicans and prototype human coronavirus (HCoV 229E) | [84] |
| The surface of the blown polypropylene filtration media was dip coated with copper@ZIF-8 core-shell nanowires | The synergistic antimicrobial effects between the Cu nucleus and the ZIF-8 shell | Chemically antimicrobial action | S. mutans and E. coli | [94] |
| Glycyrrhetinic acid was added to polyvinyl alcohol solution to fabricate the biobased filtration mask by electrospinning | Glycyrrhetinic acid | Chemically antimicrobial action | N.a. | [97] |
| BC-BPTCD-RF nanofiber was loaded through the high-pressure airflow onto the surface of the nonwoven fibers | BC-BPTCD-RF nanofibers | Photoactive antiviral (combined photocatalytic and photothermal properties) | E. coli, S. aureus and simulated virus T7 bacteriophage | [99] |
| Shellac/copper nanoparticles (CuNPs) were coated on the polypropylene masks by dual-channel spray method | The generation of free radicals by the rapid rising of the mask surface temperature over 70 °C under the sunlight | Light-induced inactivation upon irradiation with near-UV and visible light | E. coli and extracellular vesicles | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Liu, P.; Yu, L.; Zille, A. Fiber-Based Masks and Respirators: Using Decontamination Methods and Antimicrobial Treatment to Improve Its Reusability during Pandemic. Textiles 2022, 2, 318-335. https://doi.org/10.3390/textiles2020018
Song X, Liu P, Yu L, Zille A. Fiber-Based Masks and Respirators: Using Decontamination Methods and Antimicrobial Treatment to Improve Its Reusability during Pandemic. Textiles. 2022; 2(2):318-335. https://doi.org/10.3390/textiles2020018
Chicago/Turabian StyleSong, Xinyu, Pengyan Liu, Liangmin Yu, and Andrea Zille. 2022. "Fiber-Based Masks and Respirators: Using Decontamination Methods and Antimicrobial Treatment to Improve Its Reusability during Pandemic" Textiles 2, no. 2: 318-335. https://doi.org/10.3390/textiles2020018
APA StyleSong, X., Liu, P., Yu, L., & Zille, A. (2022). Fiber-Based Masks and Respirators: Using Decontamination Methods and Antimicrobial Treatment to Improve Its Reusability during Pandemic. Textiles, 2(2), 318-335. https://doi.org/10.3390/textiles2020018