Elaboration of Natural Hydroxyapatite Coating by Plasma Spraying
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroxyapatite Powder Preparation
2.2. Coating Elaboration
2.3. Characterization
3. Results and Discussion
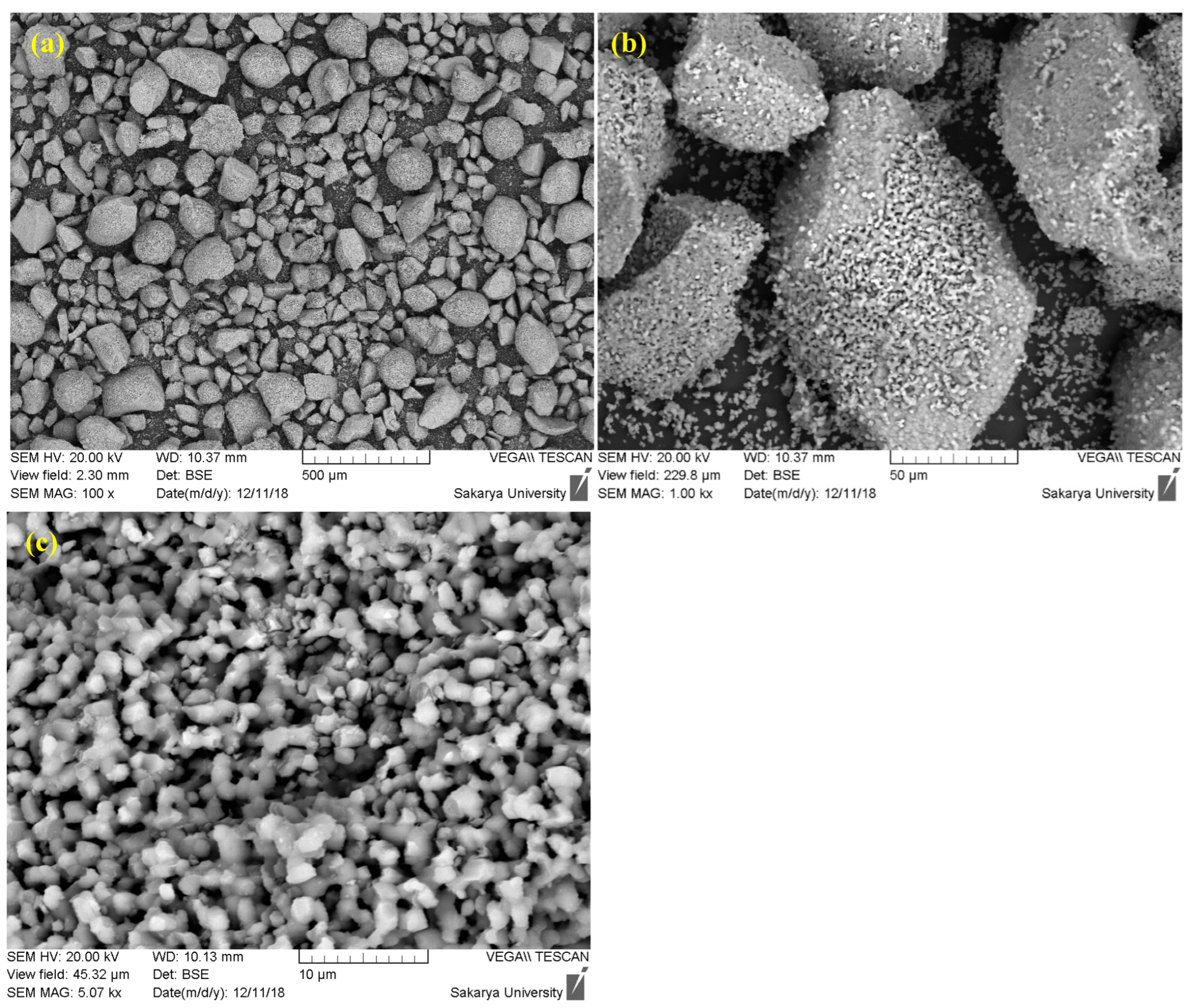
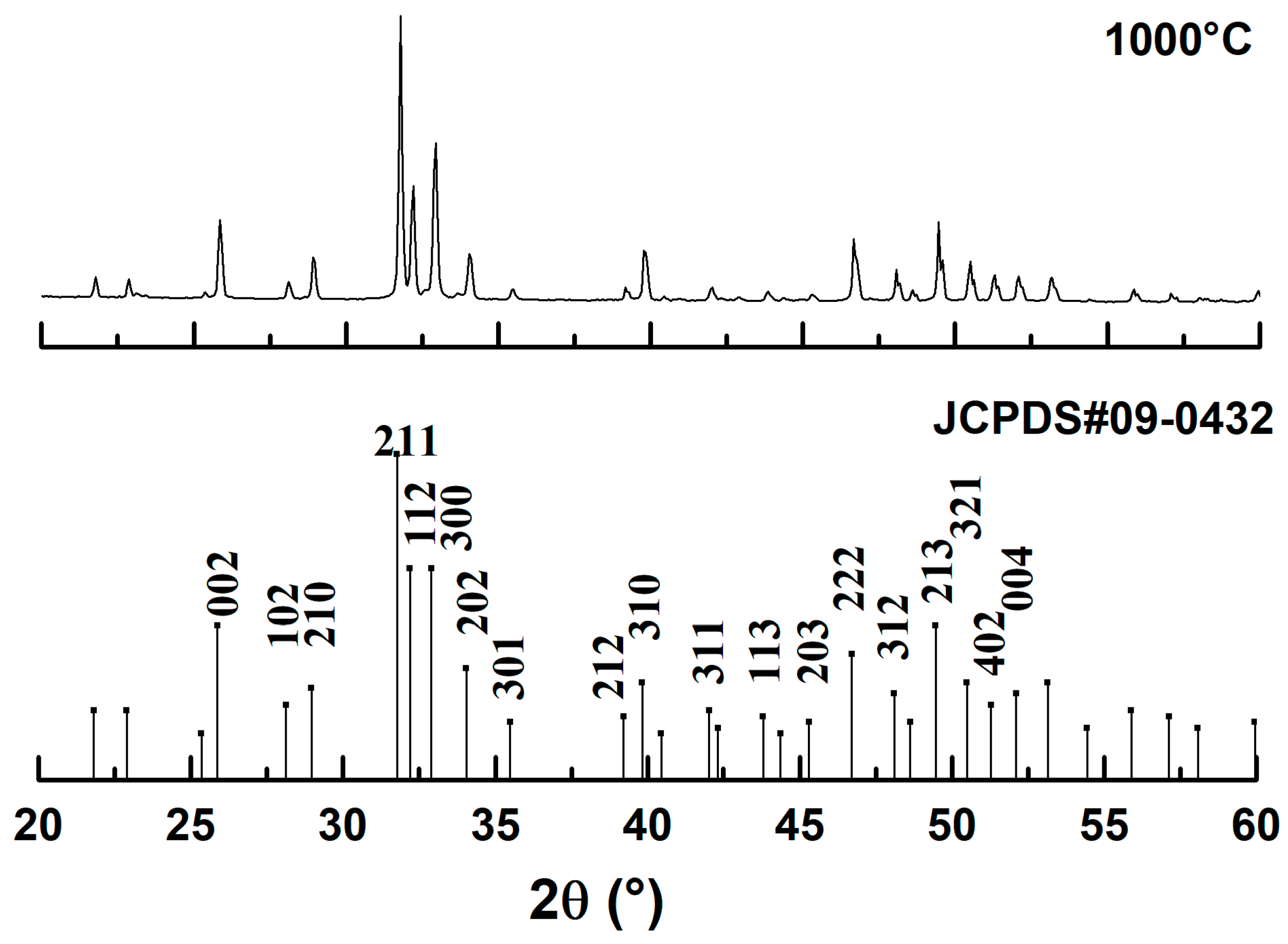
3.1. Characterization of the Hydroxyaapatite Powder
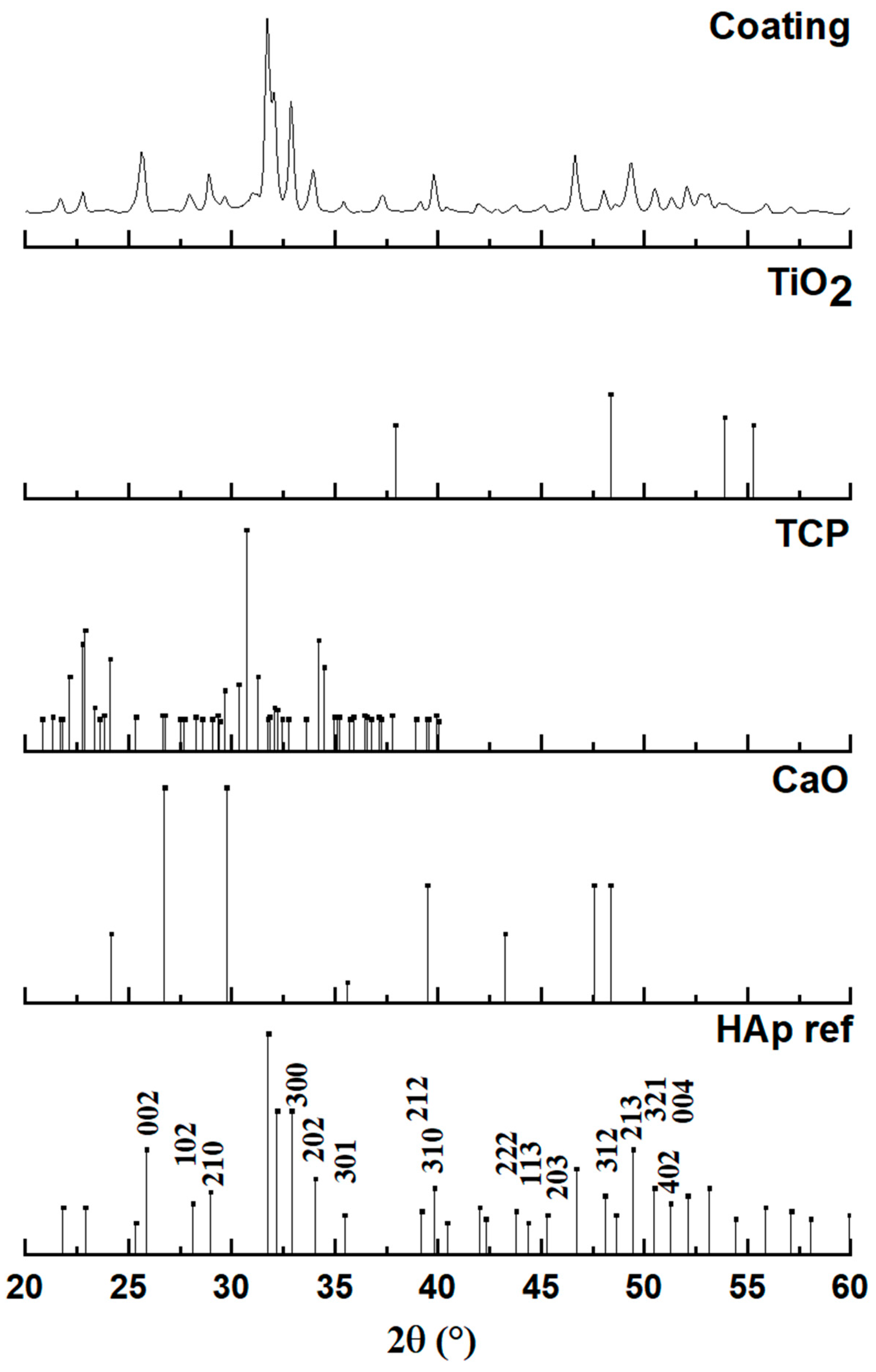
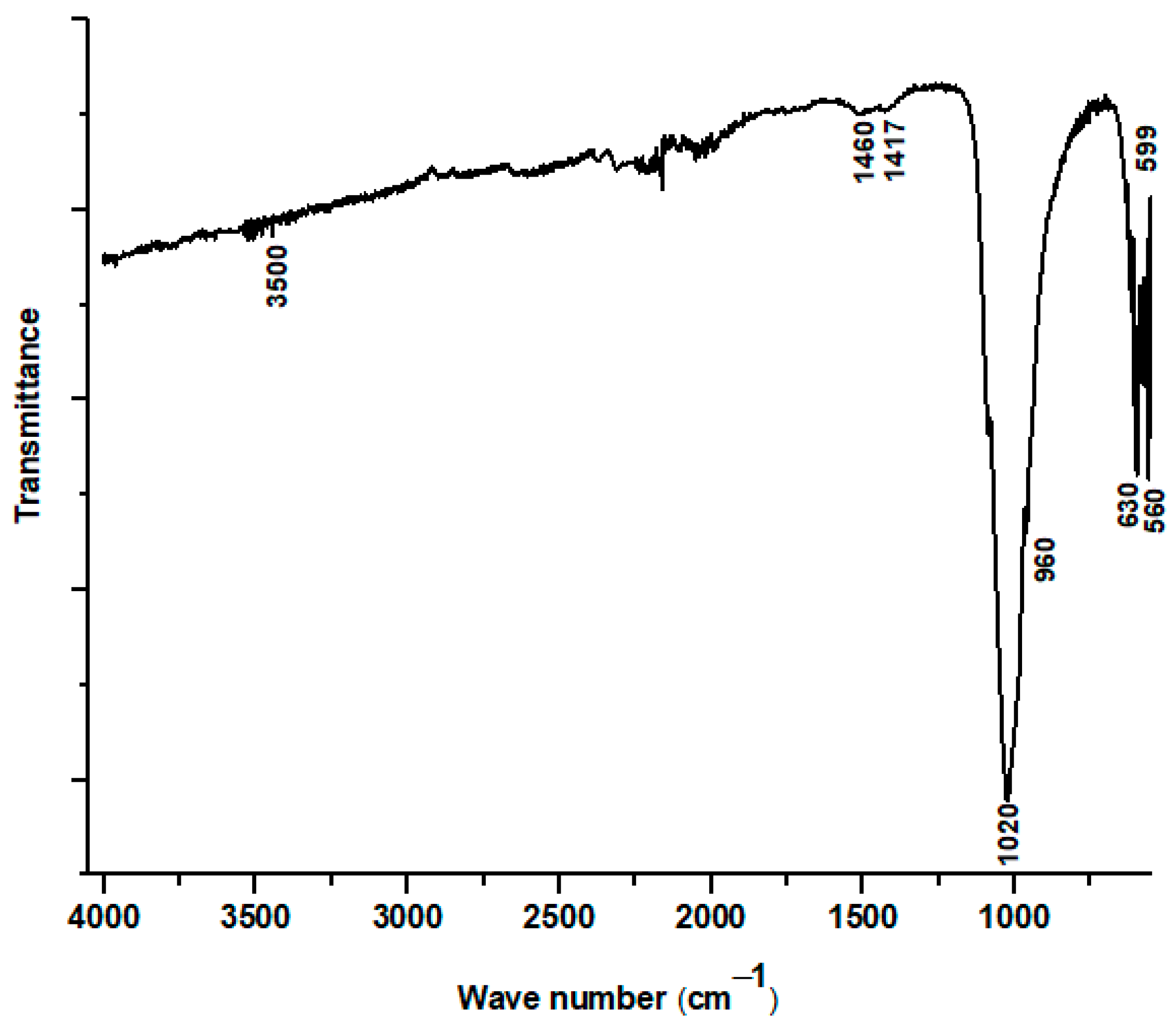
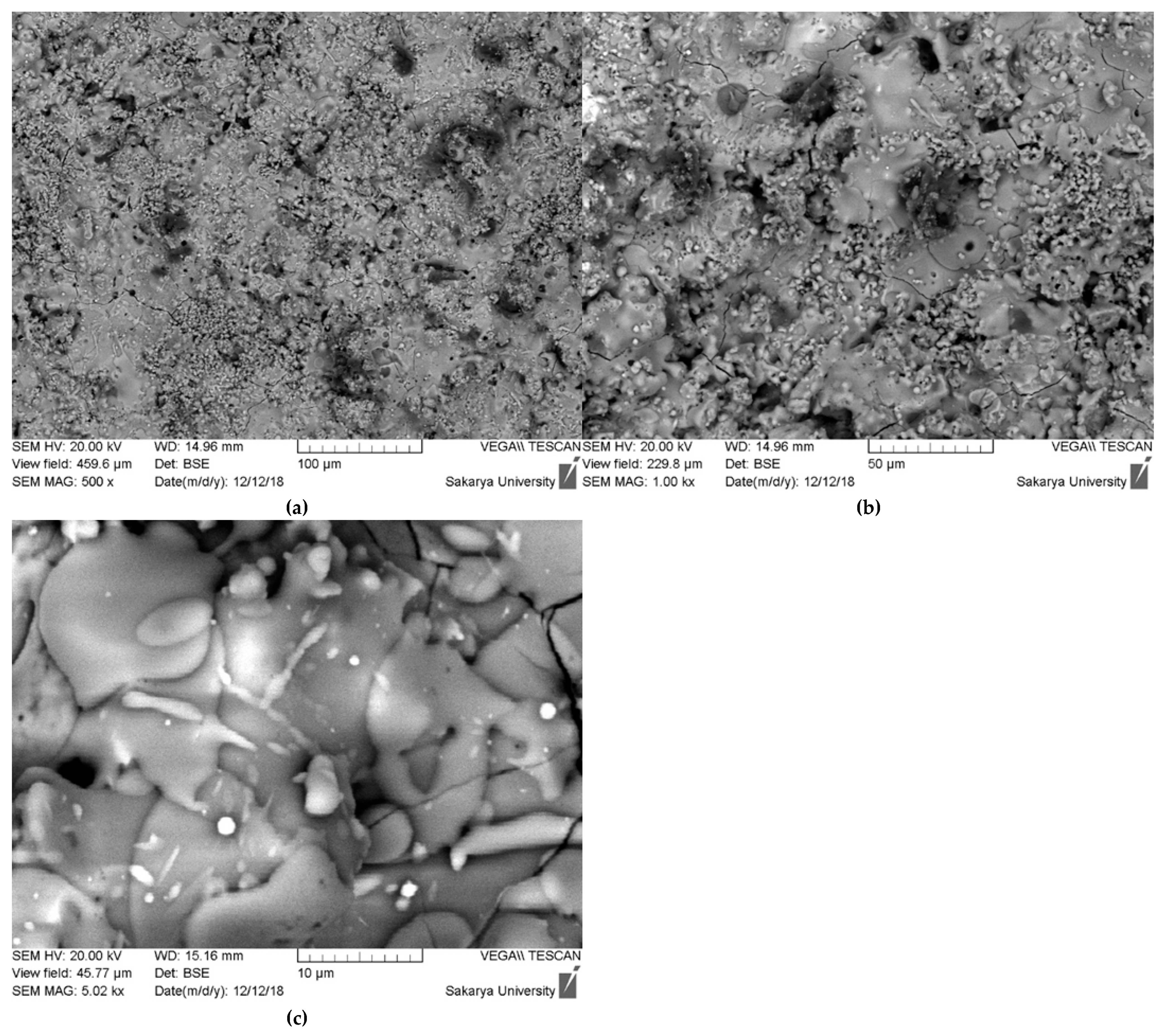
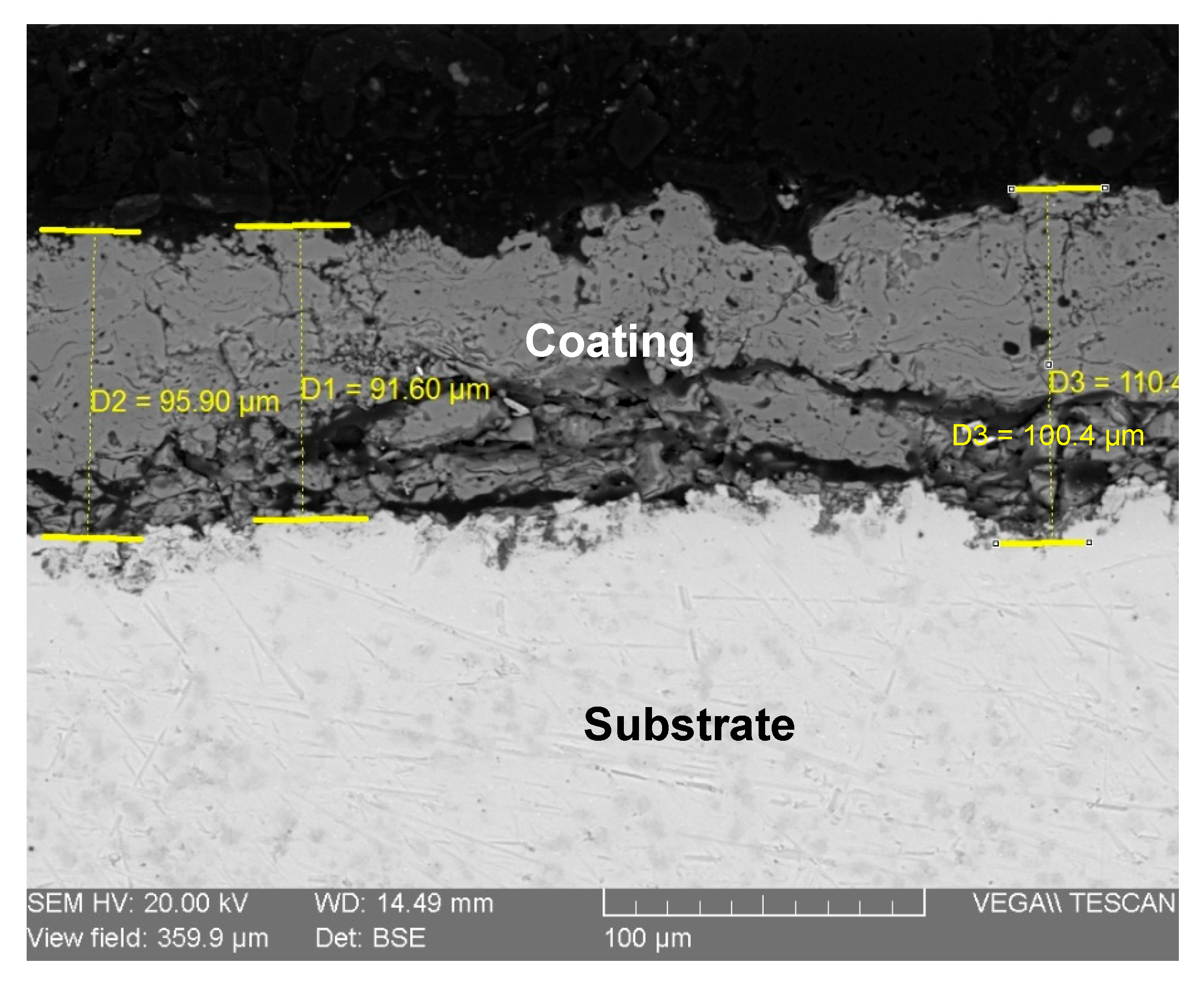
3.2. Coating Characterization
4. Conclusions
- HAp Powder Characteristics: The heat treatment process at 1000 °C for 36 h effectively produced a pure HAp powder from dromedary bone, as confirmed by XRD showing no additional phases. FTIR analysis confirmed the successful elimination of organic matter, with only carbonate groups remaining, which can influence biological behavior. SEM revealed that prolonged sintering at 1000 °C for 24 h significantly modified particle morphology to a more spherical shape and increased particle size, optimizing it for the plasma spraying process.
- Coating Homogeneity and Adhesion: Macroscopic observations indicated a good distribution and homogeneity of the natural HAp particles across the metallic substrate surface. Furthermore, the notable difficulty in manually scraping the deposit provided qualitative evidence of good adhesion of the coating to the titanium substrate.
- Coating Phase Composition: X-ray diffraction of the coating confirmed that the main deposited phase is hydroxyapatite, exhibiting a nanocrystalline hexagonal crystal structure. However, the presence of minor additional phases, specifically calcium oxide (CaO) and tricalcium phosphate (TCP), was noted, suggesting partial decomposition of HAp during the high-temperature plasma spraying process. FTIR analysis of the coating also indicated the absence of carbonate bands and partial dehydroxylation, further supporting HAp decomposition and the formation of CaO.
- Coating Microstructure and Thickness: SEM morphological analysis revealed a characteristic lamellar microstructure of thermal spray coatings, comprising fully melted (splat) particles alongside some unmelted or semi-fused globular particles, resulting in a rough, porous surface. This porous structure, with an average deposition thickness of approximately 95 µm (as measured from cross-sectional SEM), is potentially beneficial for enhancing osteointegration in biomedical applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.; Harun, W.; Hassan, M.; Ghani, S.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Duan, K.; Wang, R. Surface modifications of bone implants through wet chemistry. J. Mater. Chem. 2006, 16, 2309–2321. [Google Scholar] [CrossRef]
- Rizzi, G.; Scrivani, A.; Fini, M.; Giardino, R. Biomedical coatings to improve the tissue-biomaterial interface. Int. J. Artif. Organs 2004, 27, 649–657. [Google Scholar] [CrossRef]
- Tang, L.; Thevenot, P.; Hu, W. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Nadaraia, K.; Mashtalyar, D.; Piatkova, M.; Pleshkova, A.; Imshinetskiy, I.; Gerasimenko, M.; Belov, E.; Kumeiko, V.; Kozyrev, D.; Fomenko, K. Antibacterial HA-coatings on bioresorbable Mg alloy. J. Magnes. Alloys 2024, 12, 1965–1985. [Google Scholar] [CrossRef]
- Kuroda, K.; Okido, M. Hydroxyapatite coating of titanium implants using hydroprocessing and evaluation of their osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012, 730693. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Lizundia, E.; Luzi, F.; Puglia, D. Organic waste valorisation towards circular and sustainable biocomposites. Green Chem. 2022, 24, 5429–5459. [Google Scholar] [CrossRef]
- Hussin, M.S.F.; Abdullah, H.Z.; Idris, M.I.; Wahap, M.A.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef] [PubMed]
- Swarup, J.S.; Thomas, R.; Rucharitha, J.; Arunkumar, V.R.; Vasanthi, V. Eggshell-derived hydroxyapatite as a biomaterial in dentistry: A scoping review of synthesis, properties and applications. Evid.-Based Dent. 2025, 26, 153. [Google Scholar] [CrossRef]
- Hosseini, B.; Mirhadi, S.M.; Mehrazin, M.; Yazdanian, M.; Motamedi, M.R.K. Synthesis of Nanocrystalline Hydroxyapatite Using Eggshell and Trimethyl Phosphate. Trauma Mon. 2017, 22, e36139. [Google Scholar] [CrossRef]
- Ghedjemis, A.; Benouadah, A.; Fenineche, N.; Ayeche, R.; Hatim, Z.; Drouiche, N.; Lounici, H. Preparation of Hydroxyapatite from Dromedary Bone by Heat Treatment. Int. J. Environ. Res. 2019, 13, 547–555. [Google Scholar] [CrossRef]
- Ghedjemis, A.; Ayeche, R.; Benouadah, A.; Fenineche, N. A new application of Hydroxyapatite extracted from dromedary bone: Adsorptive removal of Congo red from aqueous solution. Int. J. Appl. Ceram. Technol. 2021, 18, 590–597. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Hamdi, M.; Ramesh, S. Properties of hydroxyapatite produced by annealing of bovine bone. Ceram. Int. 2007, 33, 1171–1177. [Google Scholar] [CrossRef]
- Haberko, K.; Bućko, M.M.; Brzezińska-Miecznik, J.; Haberko, M.; Mozgawa, W.; Panz, T.; Pyda, A.; Zarębski, J. Natural hydroxyapatite—Its behaviour during heat treatment. J. Eur. Ceram. Soc. 2006, 26, 537–542. [Google Scholar] [CrossRef]
- Karamian, E.; Khandan, A.; Eslami, M.; Gheisari, H.; Rafiaei, N. Investigation of HA Nanocrystallite Size Crystallographic Characterizations in NHA, BHA and HA Pure Powders and their Influence on Biodegradation of HA. Adv. Mater. Res. 2013, 829, 314–318. [Google Scholar] [CrossRef]
- Kannan, S.; Vieira, S.; Olhero, S.; Pina, S.; da Cruz e Silva, O.; Ferreira, J. Synthesis, mechanical and biological characterization of ionic doped carbonated hydroxyapatite/β-tricalcium phosphate mixtures. Acta Biomater. 2011, 7, 1835–1843. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Liao, Z.; Wang, Q.; Tyagi, R.; Liu, W. Tribological behaviour of titanium alloy modified by carbon–DLC composite film. Surf. Eng. 2015, 31, 934–941. [Google Scholar] [CrossRef]
- Demnati, I. Développement et Caractérisation de Revêtements Bioactifs D’apatite Obtenus par Projection Plasma à Basse Énergie: Application Aux Implants Biomédicaux. Ph.D. Thesis, Institut National Polytechnique de Toulouse-INPT, Toulouse, France, 2011. [Google Scholar]
- Derradji, F.Z.; Labaïz, M.; Bououdina, M.; Ourdjini, A.; Montagne, A.; Iost, A. Porous plasma sprayed bioceramic (Ca10 (PO4). 6 (OH)2) coated Ti6Al4V: Morphological, adhesion and tribological studies. Mater. Res. Express 2019, 6, 095401. [Google Scholar] [CrossRef]
- Tomaszek, R.; Pawlowski, L.; Gengembre, L.; Laureyns, J.; Le Maguer, A. Microstructure of suspension plasma sprayed multilayer coatings of hydroxyapatite and titanium oxide. Surf. Coat. Technol. 2007, 201, 7432–7440. [Google Scholar] [CrossRef]
- Ghorbel, H.F.; Guidara, A.; Danlos, Y.; Bouaziz, J.; Coddet, C. Synthesis and characterization of alumina-fluorapatite coatings deposited by atmospheric plasma spraying. Mater. Lett. 2016, 185, 268–271. [Google Scholar] [CrossRef]




| Parameters | Value |
|---|---|
| Ar Plasma gas flow (L/min) | 55 |
| He flow (L/min) | 10 |
| Current intensity (A) | 540 |
| Powder flow (g/min) | 1.5 |
| Projection distance (cm) | 12 |
| Number of passes | 20 |
| Plasma arc voltage (V) | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebaili, M.; Ghedjemis, A.; Benchikh, L.; Aitferhat, Y.; Abacha, I.; Hebbache, K.; Belebchouche, C.; Kadri, E.H. Elaboration of Natural Hydroxyapatite Coating by Plasma Spraying. Physchem 2025, 5, 57. https://doi.org/10.3390/physchem5040057
Kebaili M, Ghedjemis A, Benchikh L, Aitferhat Y, Abacha I, Hebbache K, Belebchouche C, Kadri EH. Elaboration of Natural Hydroxyapatite Coating by Plasma Spraying. Physchem. 2025; 5(4):57. https://doi.org/10.3390/physchem5040057
Chicago/Turabian StyleKebaili, Maya, Amina Ghedjemis, Lilia Benchikh, Yazid Aitferhat, Ilyes Abacha, Kamel Hebbache, Cherif Belebchouche, and El Hadj Kadri. 2025. "Elaboration of Natural Hydroxyapatite Coating by Plasma Spraying" Physchem 5, no. 4: 57. https://doi.org/10.3390/physchem5040057
APA StyleKebaili, M., Ghedjemis, A., Benchikh, L., Aitferhat, Y., Abacha, I., Hebbache, K., Belebchouche, C., & Kadri, E. H. (2025). Elaboration of Natural Hydroxyapatite Coating by Plasma Spraying. Physchem, 5(4), 57. https://doi.org/10.3390/physchem5040057







