Process Optimization Using Experimental and Statistical Modeling in Biodiesel Production from Palm Oil
Abstract
1. Introduction
1.1. Experimental Design and Statistical Modeling Techniques
- Response Surface Methodology (RSM): RSM remains a cornerstone in optimizing biodiesel production. Techniques such as Central Composite Design (CCD) and Box–Behnken Design (BBD) are extensively used to model the effects of variables such as catalyst concentration, methanol-to-oil ratio, and reaction time. Studies have demonstrated that RSM can achieve biodiesel yields exceeding 90% from palm oil feedstocks [32,33,34].
- Machine Learning Approaches: Machine Learning (ML) models, including Artificial Neural Networks (ANNs), Adaptive Neuro-Fuzzy Inference Systems (ANFISs), and ensemble methods like AdaBoost, have been employed to predict and optimize biodiesel production. For instance, AdaBoost regression has shown high accuracy in modeling biodiesel yield and free fatty acid conversion, offering a promising alternative to traditional methods [35,36].
1.2. Feedstock Considerations and Process Optimization Strategies
1.3. Future Directions
- Integration of Computational Tools: Utilizing software like Design-Expert and Statistica for data analysis and model validation.
- Advanced Hybrid Models: Developing more sophisticated hybrid models combining RSM, ML, and optimization algorithms to further enhance prediction accuracy.
- Sustainability Metrics: Incorporating environmental and economic assessments into optimization models to promote sustainable biodiesel production practices.
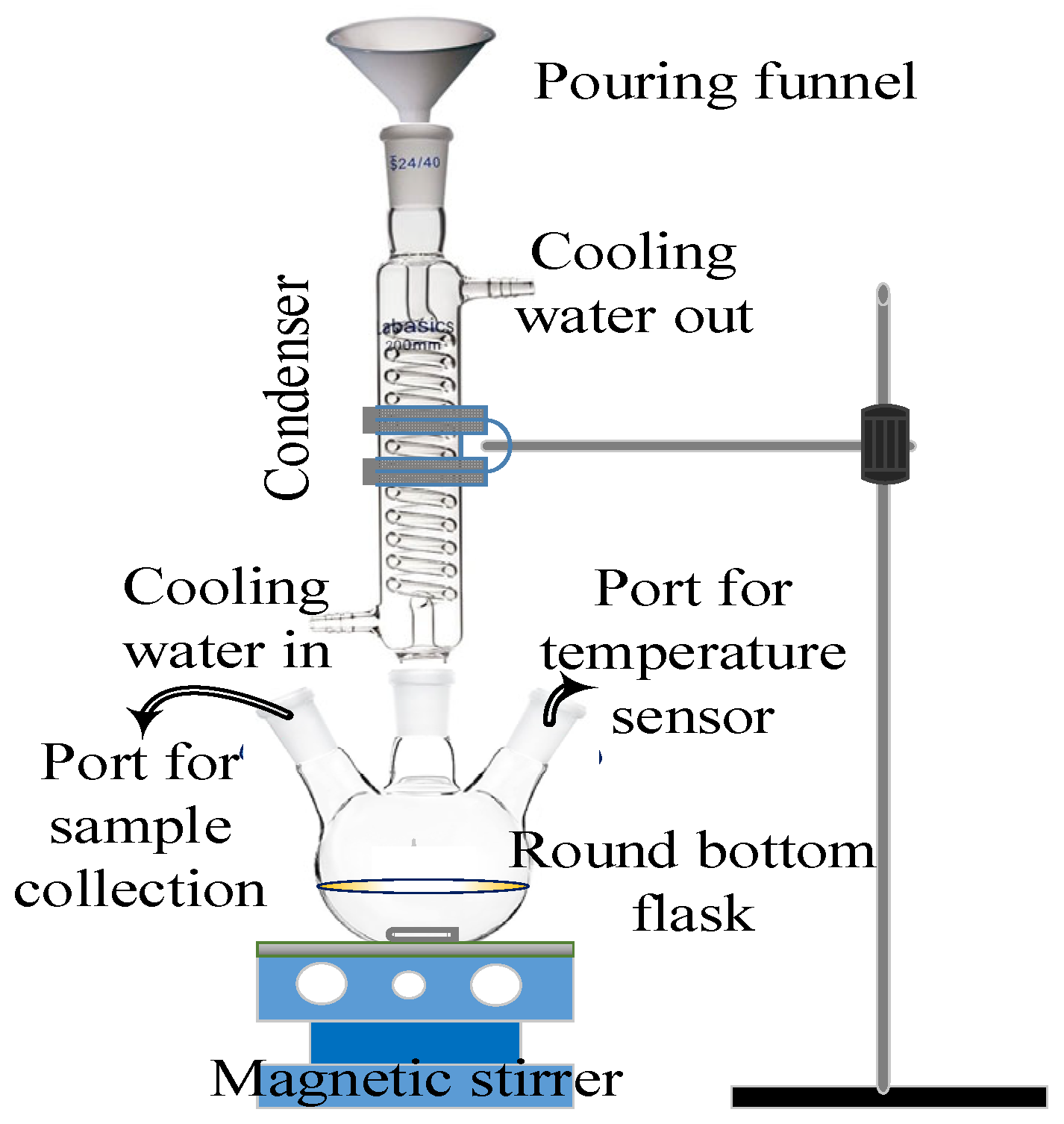
2. Experimental Configuration and Procedures
2.1. Design Computation of the Model
2.2. Nomenclatures/Interpretations of Statistical Terminologies
- F-value is the ratio of variance between group means to the variance within the groups. value is a key statistic in Analysis of Variance (ANOVA), used to determine whether there are significant differences between the means of three or more groups. A large F-value suggests that at least one group mean significantly differs from the others. A small F-value implies the group means are similar, and observed differences are likely due to chance.
- p-value is a statistical metric used to evaluate the strength of evidence against the null hypothesis in hypothesis testing. It reflects the probability of obtaining results as extreme as those observed, assuming the null hypothesis is true. A p-value < 0.05 typically indicates statistical significance, leading to rejection of the null hypothesis. On the other hand, p-value > 0.05 suggests insufficient evidence to reject the null hypothesis (example: a p-value of 0.03 in a comparison between two treatments implies a 3% chance that the observed difference is due to random variation, indicating a statistically significant effect).
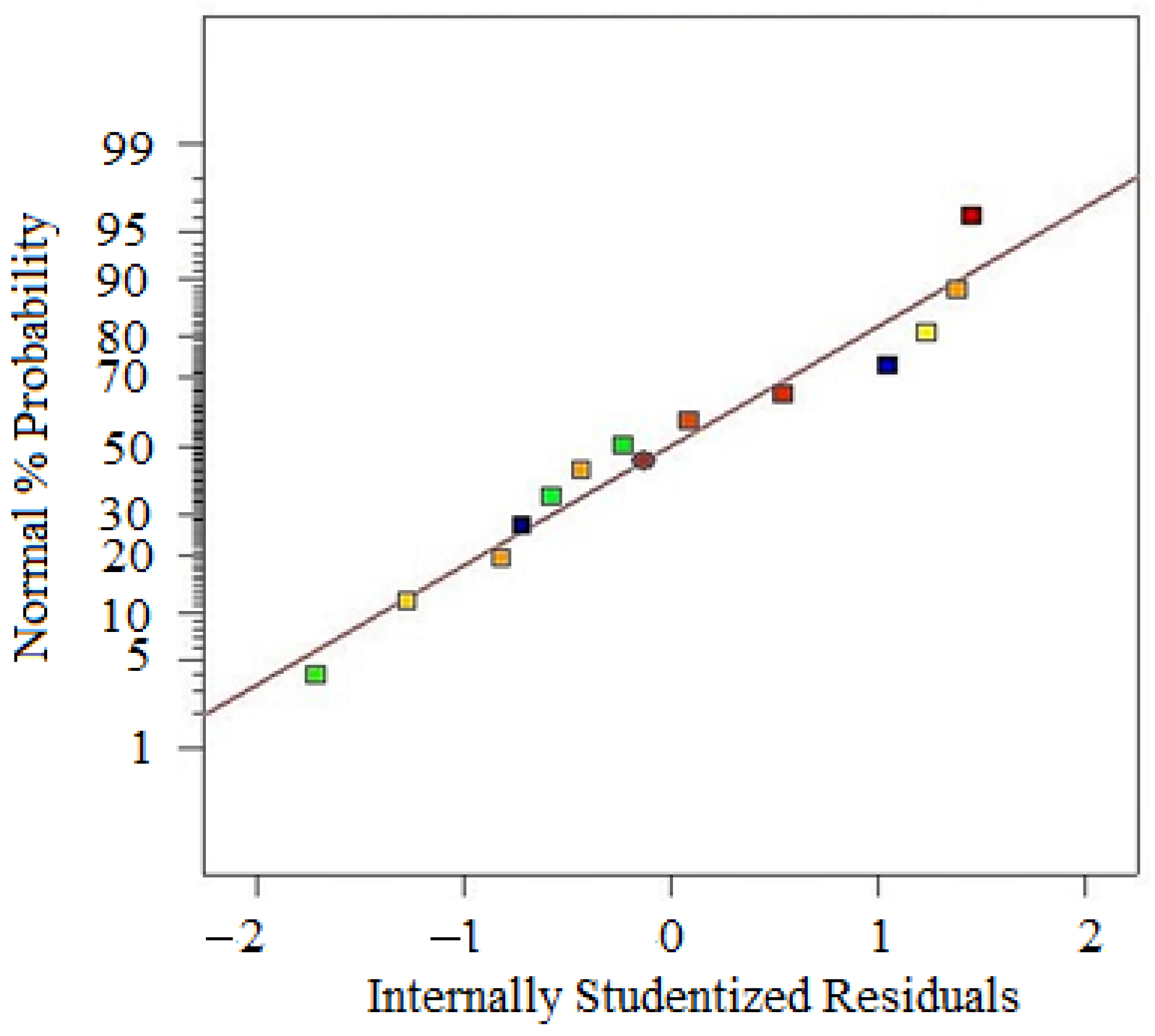
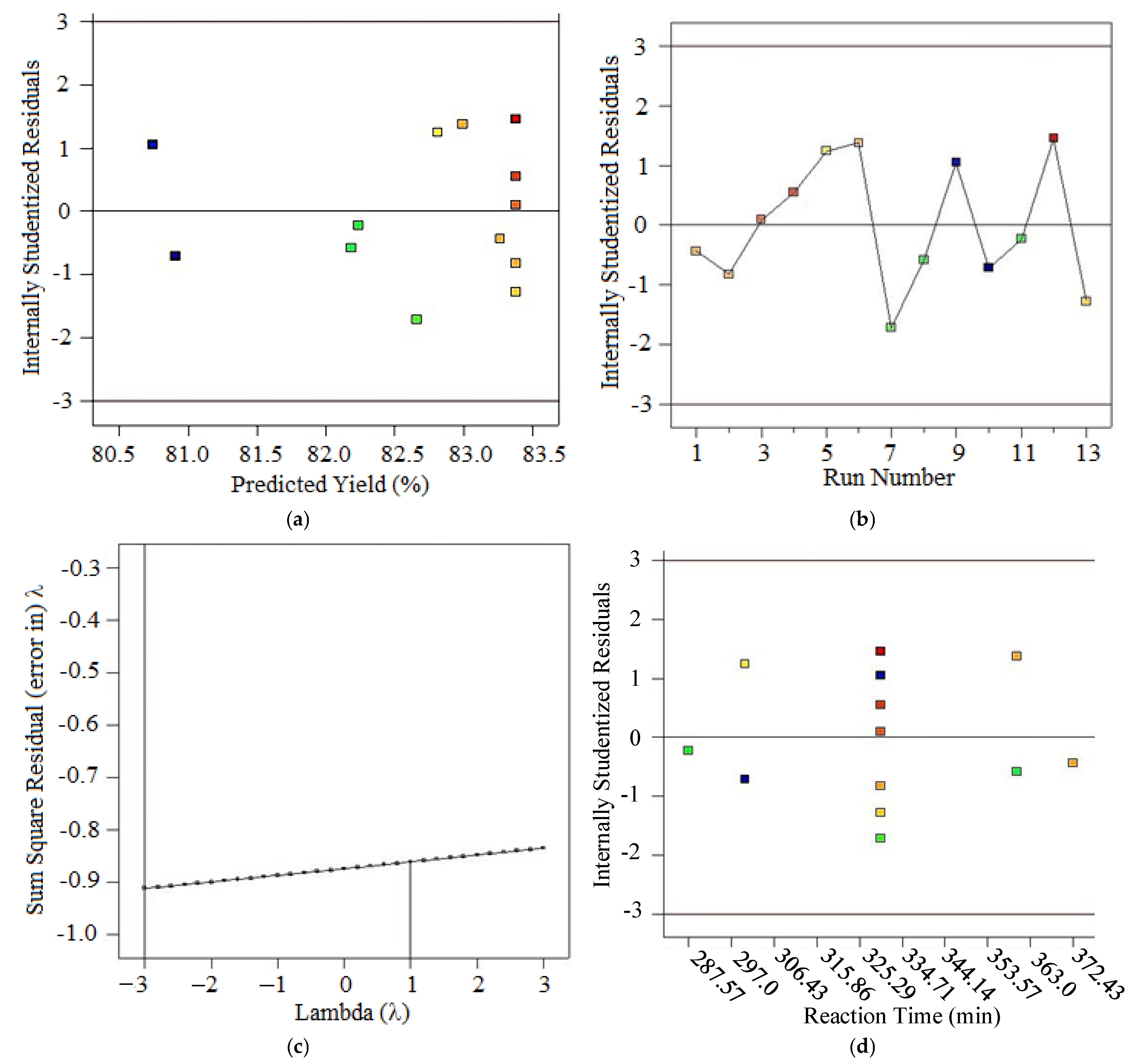
- Box–Cox Plot: A graphical tool to identify the optimal λ that best normalizes the data by maximizing the log-likelihood function. The Box–Cox transformation is a technique used to stabilize variance and make data more normally distributed.
- λ (Lambda): Determines the power to which data is transformed, with λ = 1 for No transformation, λ = 0 for Log transformation, λ = 0.5 for Square root transformation, and λ = −1 for Reciprocal transformation. If the confidence interval for λ includes 1, transformation is not required. Or else if λ significantly differs from 1, transformation is recommended to improve normality.
3. Results and Discussions
4. Statistical Modeling on the Experimental Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Karimi, M.S.; Weerasinghe, N.M.; Bilan, Y.; Shahzad, U. Interplay between economic progress, carbon emissions and energy prices on green energy adoption: Evidence from USA and Germany in context of sustainability. Renew. Energy 2024, 232, 121038. [Google Scholar] [CrossRef]
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Al-Jiboory, A.K.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. The renewable energy role in the global energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Avesh, M.; Hossain, I.; Sharma, R.C. Revolutionizing Transportation: The Future Impact of Green Energy. In Dynamics of Transportation Ecosystem, Modeling, and Control; Springer: Berlin/Heidelberg, Germany, 2024; pp. 261–293. [Google Scholar]
- Bahani, N.; El Kourdi, S.; Abderafi, S. Argan cake oil transesterification kinetics and an optimized choice of a high-performance catalyst for biodiesel production. Waste Biomass Valorization 2024, 15, 2591–2610. [Google Scholar] [CrossRef]
- Mansoorsamaei, Z.; Mowla, D.; Esmaeilzadeh, F.; Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 2024, 357, 129821. [Google Scholar] [CrossRef]
- Chatterjee, S. An experimental investigation on preparation and performances of two diesel blends of karanj oil methyl ester. Int. J. Oil Gas Coal Technol. 2024, 35, 442–475. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Thompson-Yusuff, K.A.; Igbafe, A.I. Synthesis of biodiesel via methanolysis of waste frying oil by biowaste-derived catalyst: Process optimization and biodiesel blends characterization. Biomass Convers. Biorefinery 2024, 14, 1781–1792. [Google Scholar] [CrossRef]
- Chua, S.Y.; Goh, C.M.H.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Walvekar, R.; Abdullah, E. Biodiesel synthesis using natural solid catalyst derived from biomass waste—A review. J. Ind. Eng. Chem. 2020, 81, 41–60. [Google Scholar] [CrossRef]
- Dhivya Priya, N.; Thirumarimurugan, M. Biodiesel—A review on recent advancements in production. In Bioresource Utilization and Bioprocess; Springer: Berlin/Heidelberg, Germany, 2020; pp. 117–129. [Google Scholar]
- Mahlia, T.; Syazmi, Z.; Mofijur, M.; Abas, A.P.; Bilad, M.; Ong, H.C.; Silitonga, A. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- McCarthy, S.M.; Melman, J.H.; Reffell, O.K.; Gordon-Wylie, S.W. Synthesis and partial characterization of biodiesel via base-catalyzed transesterification. In Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 519–524. [Google Scholar]
- Galusnyak, S.C.; Petrescu, L.; Cosprundan, A.M.; Cormos, C.-C. Biodiesel production using various methanol sources and catalytic routes: A techno-environmental analysis. Renew. Energy 2024, 232, 121051. [Google Scholar] [CrossRef]
- Farouk, S.M.; Tayeb, A.M.; Abdel-Hamid, S.M.; Osman, R.M. Recent advances in transesterification for sustainable biodiesel production, challenges, and prospects: A comprehensive review. Environ. Sci. Pollut. Res. 2024, 31, 12722–12747. [Google Scholar] [CrossRef]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Kivevele, T.; Kichonge, B. Techno-economic evaluation of transesterification processes for biodiesel production from low quality non-edible feedstocks: Process design and simulation. Energy 2024, 297, 131302. [Google Scholar] [CrossRef]
- Pasha, M.K.; Rahim, M.; Dai, L.; Liu, D.; Du, W.; Guo, M. Comparative study of a two-step enzymatic process and conventional chemical methods for biodiesel production: Economic and environmental perspectives. Chem. Eng. J. 2024, 489, 151254. [Google Scholar] [CrossRef]
- Xiao, G.; Gao, L. First generation biodiesel. In Biofuel Production-Recent Developments and Prospects; Intechopen: London, UK, 2011. [Google Scholar]
- Yuan, W.; Yang, F. Chemical conversion process for biodiesel production. In Biomass to Renewable Energy Processes; CRC Press: Boca Raton, FL, USA, 2017; pp. 299–374. [Google Scholar]
- Knothe, G. History of vegetable oil-based diesel fuels. In The Biodiesel Handbook; Elsevier: Amsterdam, The Netherlands, 2010; pp. 5–19. [Google Scholar]
- Garg, R.; Sabouni, R.; Ahmadipour, M. From waste to fuel: Challenging aspects in sustainable biodiesel production from lignocellulosic biomass feedstocks and role of metal organic framework as innovative heterogeneous catalysts. Ind. Crops Prod. 2023, 206, 117554. [Google Scholar] [CrossRef]
- Muhammad, M.; Wibert Victor Hasudungan, H. Analyzing the market conditions and long-term dynamics in the biodiesel industry. Biofuels 2025, 16, 32–41. [Google Scholar] [CrossRef]
- Miftah, F.K.; Mutta, D. Potential markets and policies for sustainable liquid biofuel production with emphasis to Eastern Africa countries: A review. Energy Sustain. Soc. 2024, 14, 1. [Google Scholar] [CrossRef]
- Shafiq, F.; Mumtaz, M.W.; Mukhtar, H.; Touqeer, T.; Raza, S.A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Response surface methodology approach for optimized biodiesel production from waste chicken fat oil. Catalysts 2020, 10, 633. [Google Scholar] [CrossRef]
- Asaad, S.M.; Inayat, A.; Ghenai, C.; Shanableh, A. Response surface methodology in biodiesel production and engine performance assessment. Int. J. Thermofluids 2024, 21, 100551. [Google Scholar] [CrossRef]
- Veza, I.; Spraggon, M.; Fattah, I.R.; Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Hasan, M.; Rasul, M.; Jahirul, M.; Khan, M. Fast pyrolysis of macadamia nutshell in an auger reactor: Process optimization using response surface methodology (RSM) and oil characterization. Fuel 2023, 333, 126490. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Aslan, V.; Eryilmaz, T. Polynomial regression method for optimization of biodiesel production from black mustard (Brassica nigra L.) seed oil using methanol, ethanol, NaOH, and KOH. Energy 2020, 209, 118386. [Google Scholar] [CrossRef]
- Sai, B.A.; Subramaniapillai, N.; Mohamed, M.S.B.K.; Narayanan, A. Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. J. Environ. Chem. Eng. 2020, 8, 103603. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Lu, C.; Gong, Z.; Hu, X. Catalytic performance of NaAlO2/γ-Al2O3 as heterogeneous nanocatalyst for biodiesel production: Optimization using response surface methodology. Energy Convers. Manag. 2020, 203, 112263. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V.V. Application of machine learning technologies in biodiesel production process—A review. Front. Energy Res. 2023, 11, 1122638. [Google Scholar] [CrossRef]
- Rajali, N.A.; Radzi, S.M.; Rehan, M.M.; Amin, N.A.M. Optimization of the biodiesel production via transesterification reaction of palm oil using response surface methodology (RSM): A review. Malays. J. Sci. Health Technol. 2022, 8, 58–67. [Google Scholar] [CrossRef]
- Anjaneya, G.; Sunil, S.; Manjunatha, N.; Giri, J.; Al-Lohedan, H.A.; Sathish, T.; Prasad, C.D. Performance analysis and optimization of thermal barrier coated piston diesel engine fuelled with biodiesel using RSM. Case Stud. Therm. Eng. 2024, 57, 104351. [Google Scholar] [CrossRef]
- Kolakoti, A.; Tadros, M.; Ambati, V.K.; Gudlavalleti, V.N.S. Optimization of biodiesel production, engine exhaust emissions, and vibration diagnosis using a combined approach of definitive screening design (DSD) and artificial neural network (ANN). Environ. Sci. Pollut. Res. 2023, 30, 87260–87273. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Kalita, K.; Ghadai, R.K.; Ramachandran, M.; Gao, X.-Z. Machine learning-based predictive modelling of biodiesel production—A comparative perspective. Energies 2021, 14, 1122. [Google Scholar] [CrossRef]
- Ajao, J.; Akanbi, O.; Akinwole, I.; Adedokun, A.; Ogunsakin, O.; Ige, A. Research Advances on Machine Learning Technologies for Enhanced Biodiesel Production: A Comprehensive Review. Pet. Coal 2024, 66, 1–29. [Google Scholar]
- Ishola, N.B.; Epelle, E.I.; Betiku, E. Machine learning approaches to modeling and optimization of biodiesel production systems: State of art and future outlook. Energy Convers. Manag. X 2024, 23, 100669. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; Lostado-Lorza, R.; Sabando-Fraile, C.; Íñiguez-Macedo, S. An artificial intelligence approach to model and optimize biodiesel production from waste cooking oil using life cycle assessment and market dynamics analysis. Energy 2024, 307, 132712. [Google Scholar] [CrossRef]
- Buasri, A.; Sirikoom, P.; Pattane, S.; Buachum, O.; Loryuenyong, V. RSM Process Optimization of Biodiesel Production from Waste Cooking Palm Oil in the Presence of SO3H-PSC Catalysts. Eng. Proc. 2023, 37, 73. [Google Scholar]
- Díaz-García, J.A.; González-Farías, G. A note on the Cook’s distance. J. Stat. Plan. Inference 2004, 120, 119–136. [Google Scholar] [CrossRef]
- Easton, P.D.; Sommers, G.A. Scale and the scale effect in market-based accounting research. J. Bus. Financ. Account. 2003, 30, 25–56. [Google Scholar] [CrossRef]
- Choudhary, S.; Satija, R. Comparison and evaluation of statistical error models for scRNA-seq. Genome Biol. 2022, 23, 27. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.; Salar-García, M.; Palacios-Nereo, F.; Olivares-Carrillo, P.; Quesada-Medina, J.; De Los Ríos, A.P.; Hernández-Fernández, F. In-depth study of the transesterification reaction of Pongamia pinnata oil for biodiesel production using catalyst-free supercritical methanol process. J. Supercrit. Fluids 2016, 113, 23–30. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Singh, M.V.P.; Fransila, B.; Kumar, R.P.; Devi, G.K. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Ogunkunle, O.; Oniya, O.O.; Adebayo, A.O. Yield response of biodiesel production from heterogeneous and homogeneous catalysis of milk bush seed (Thevetia peruviana) oil. Energy Policy Res. 2017, 4, 21–28. [Google Scholar] [CrossRef]
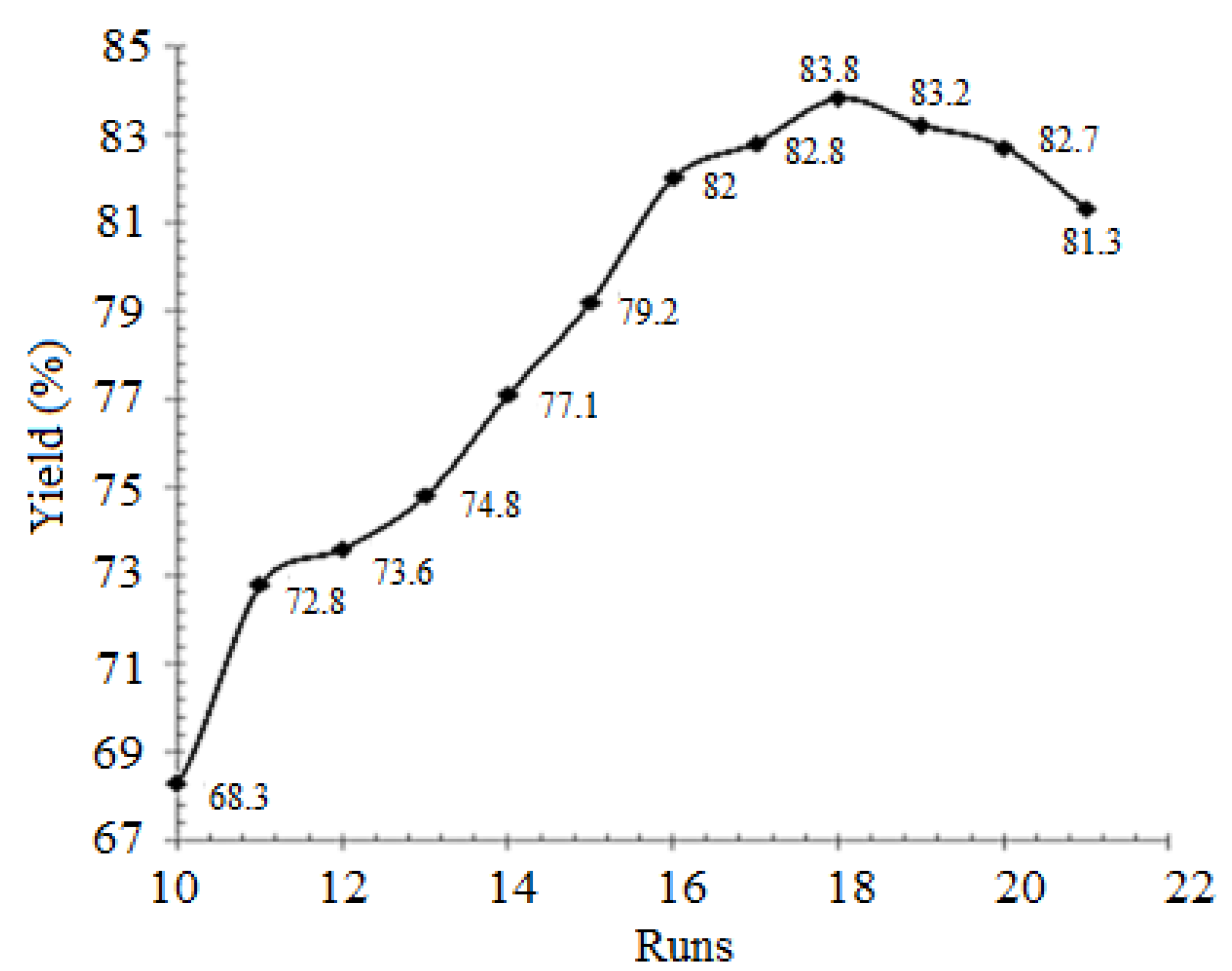
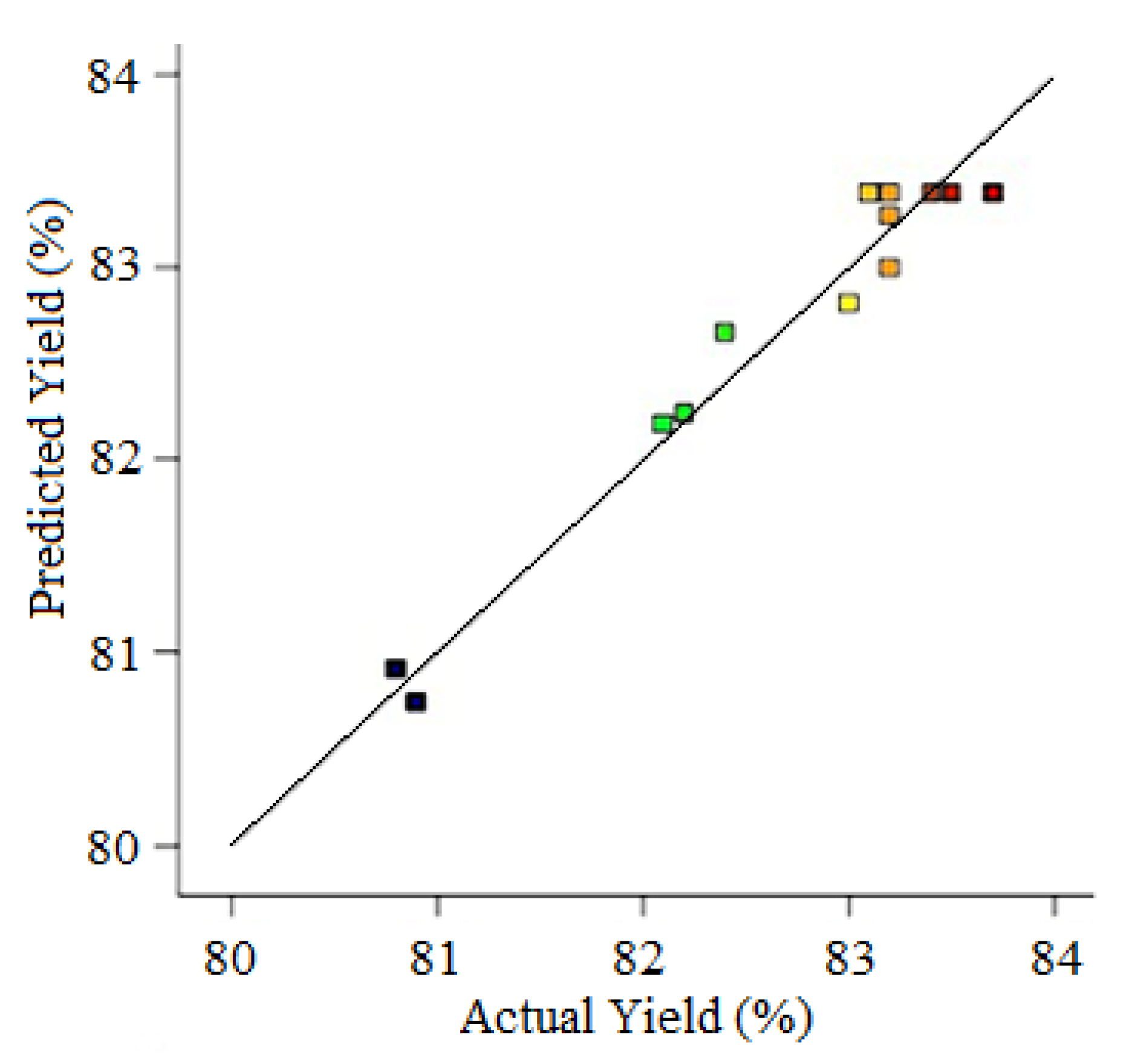
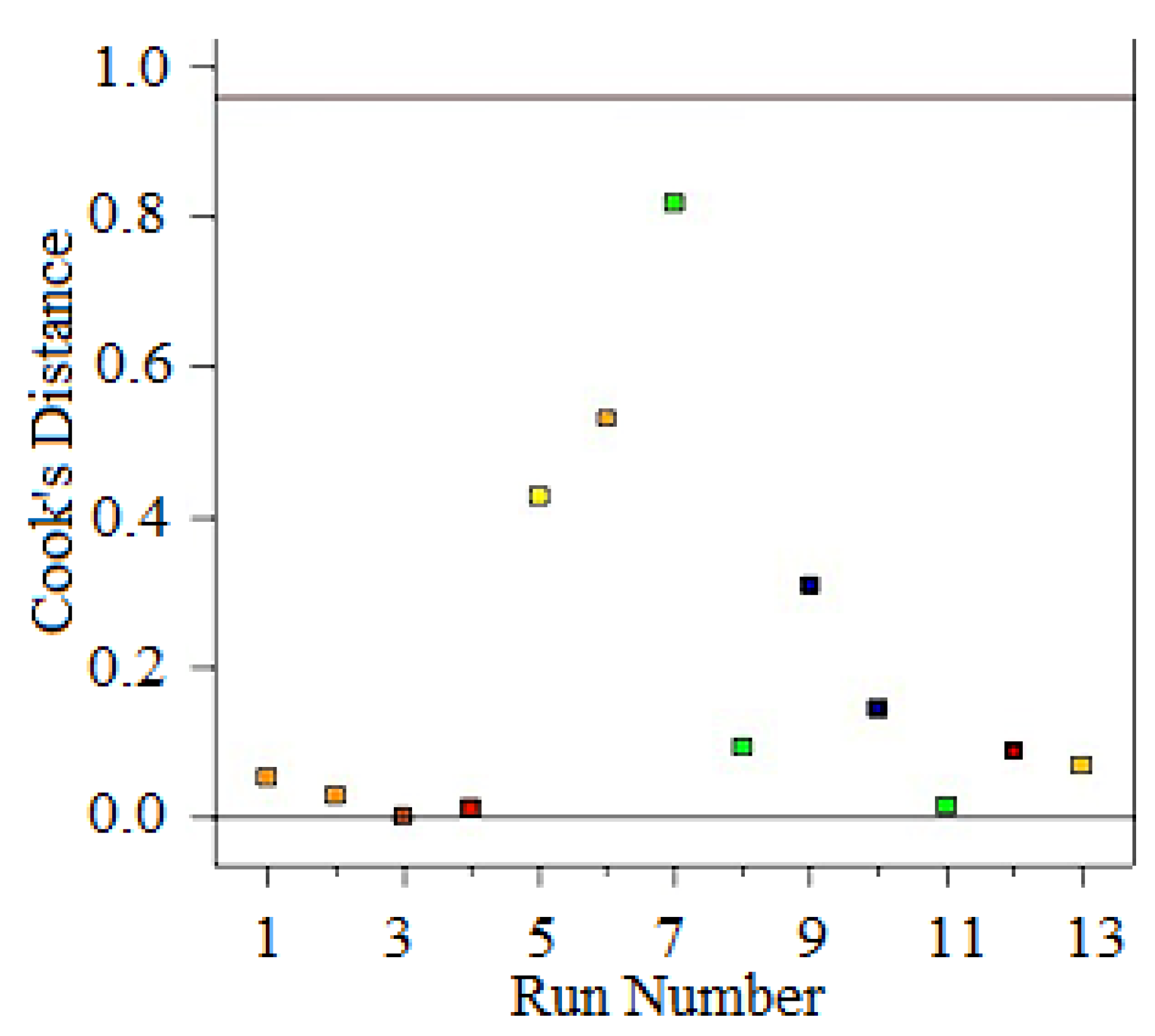
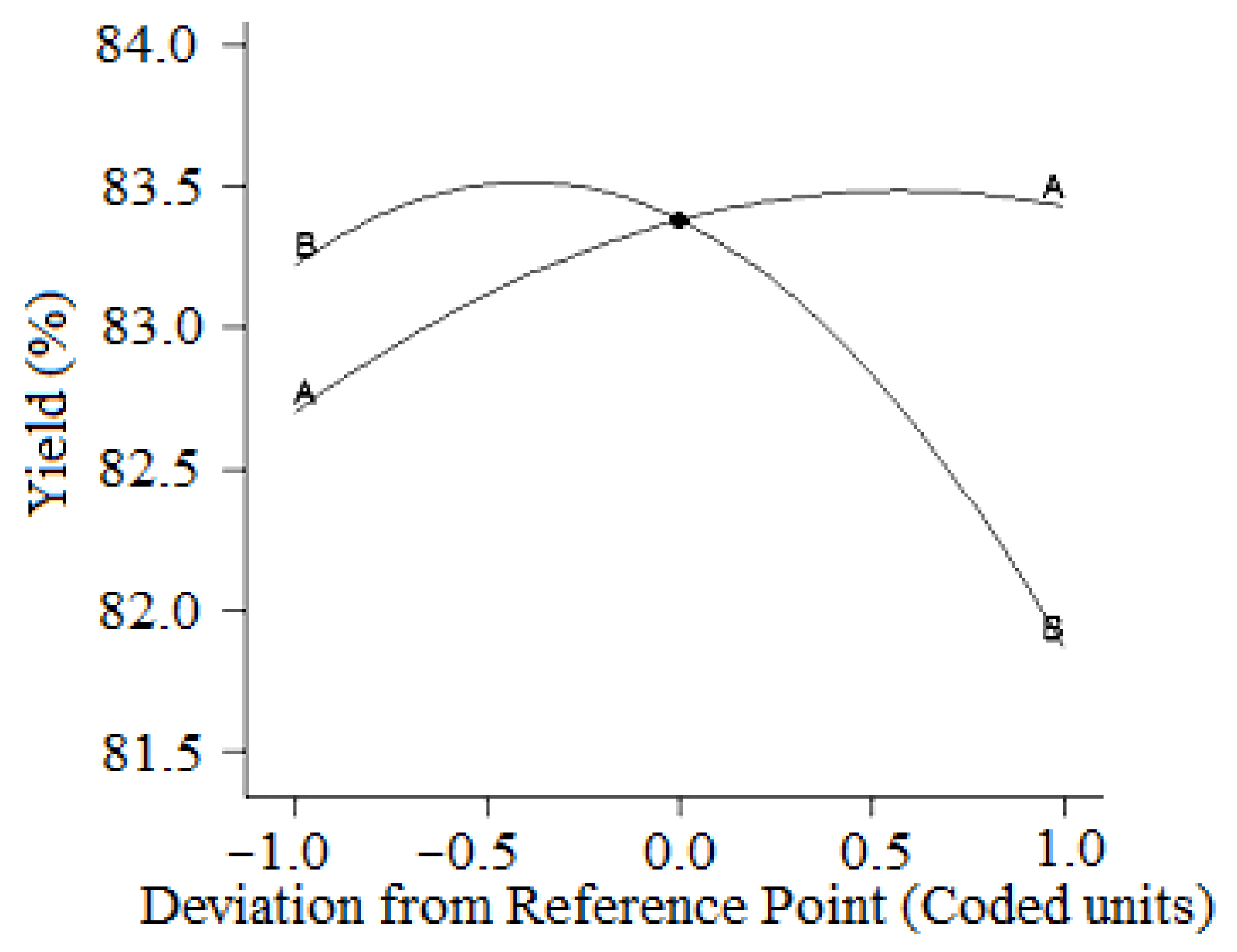
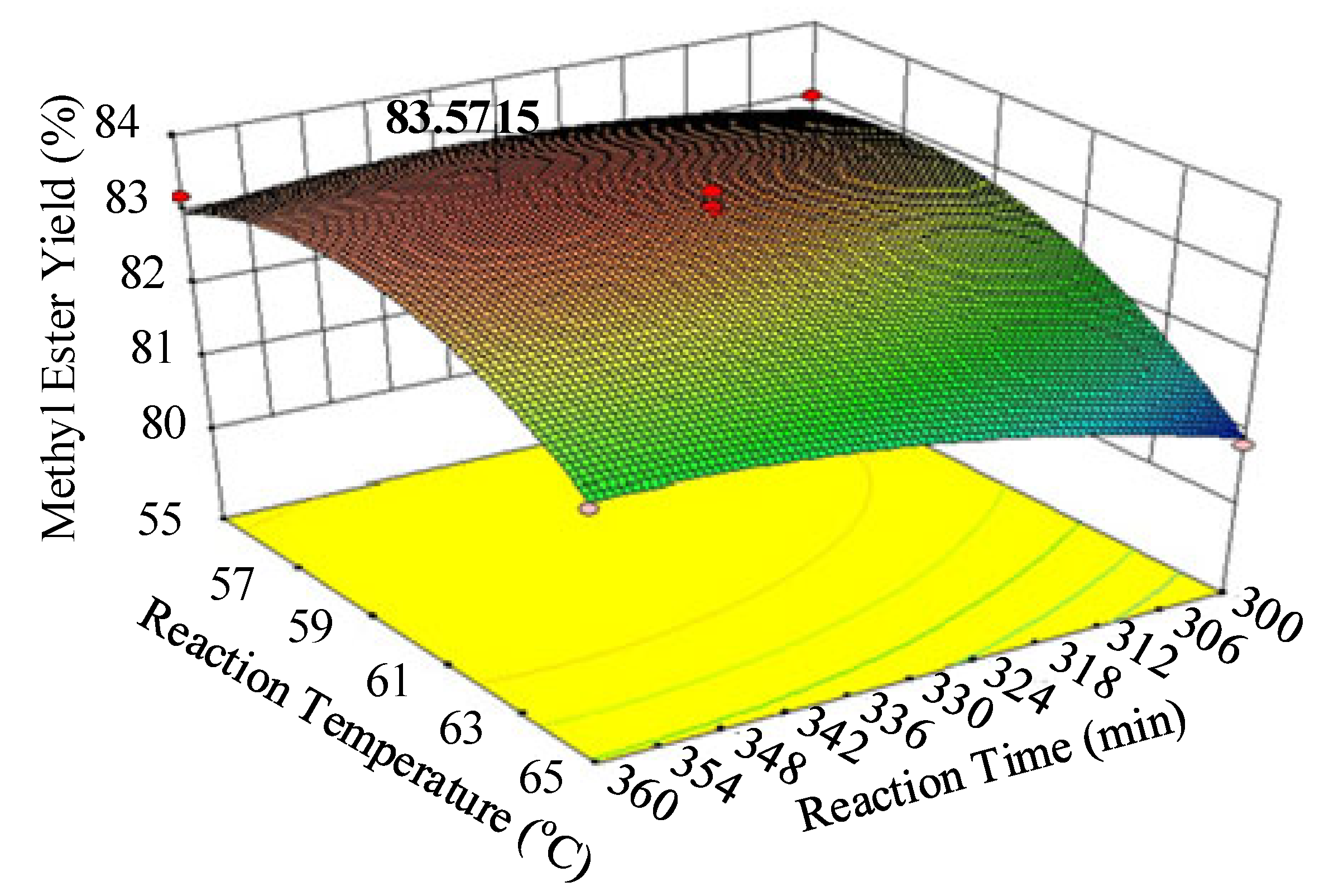
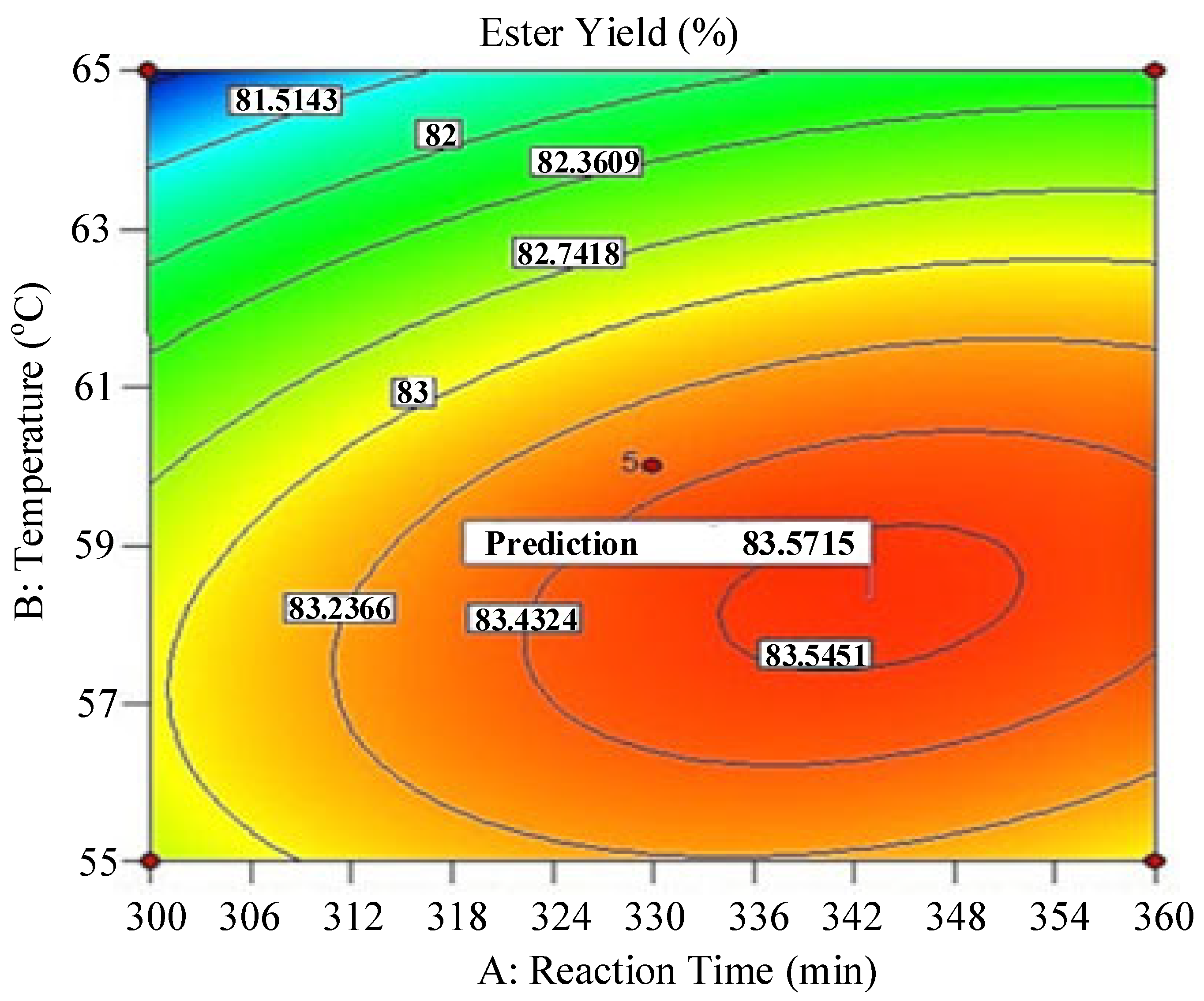
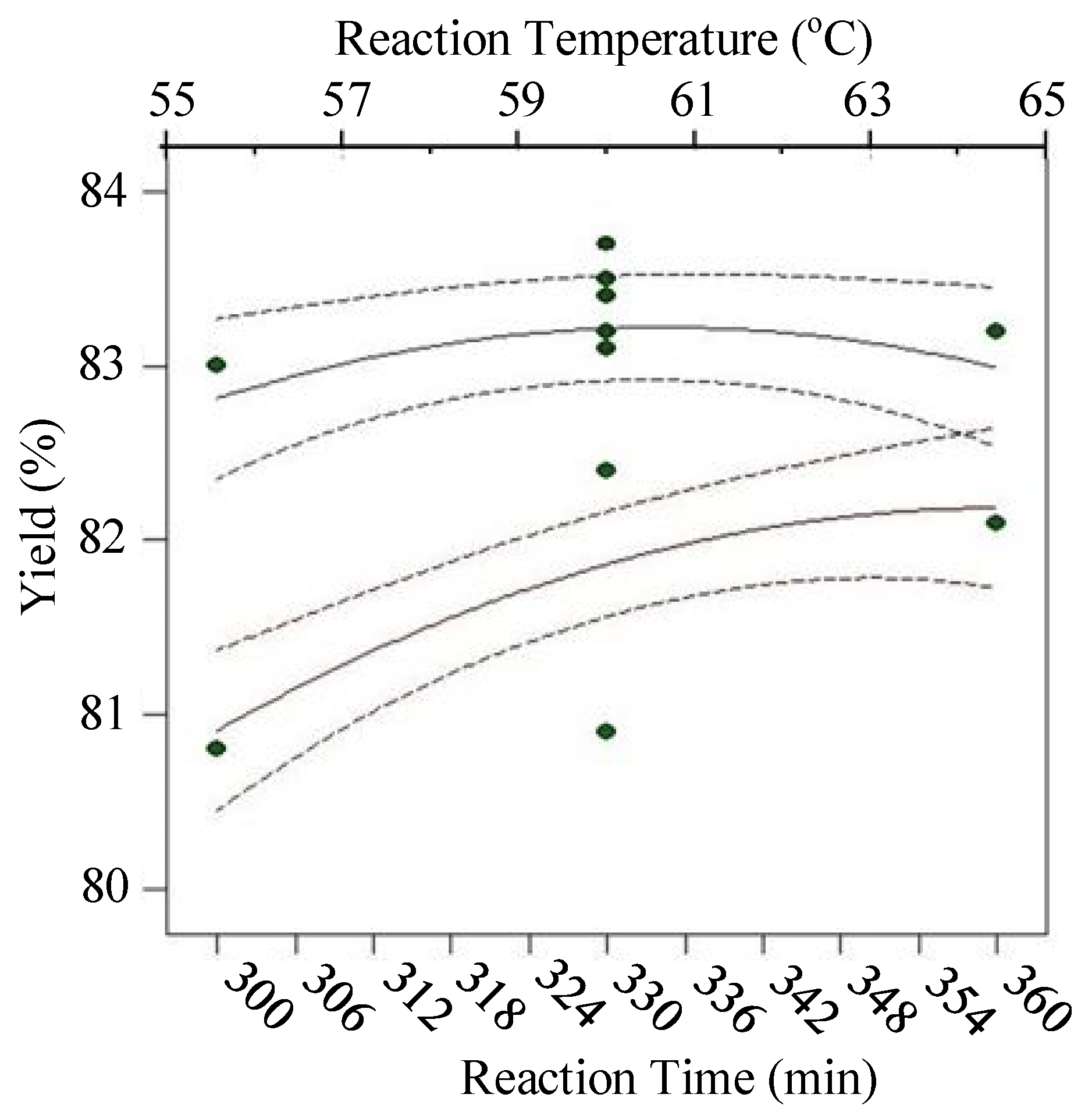
| Runs | Actual Yield (%) | Predicted Yield (%) | Relative Deviation (%) | Cook’s Distance | Studentized Residuals |
|---|---|---|---|---|---|
| 1 | 83.00 | 82.81 | 0.232 | 0.427 | 1.240 |
| 2 | 83.20 | 82.99 | 0.252 | 0.531 | 1.383 |
| 3 | 80.80 | 80.91 | −0.136 | 0.143 | −0.718 |
| 4 | 82.10 | 82.19 | −0.109 | 0.092 | −0.576 |
| 5 | 82.20 | 82.23 | −0.036 | 0.015 | −0.232 |
| 6 | 83.80 | 83.83 | −0.039 | 0.052 | −0.433 |
| 7 | 82.40 | 82.66 | −0.315 | 0.819 | −1.717 |
| 8 | 80.90 | 80.74 | 0.197 | 0.308 | 1.053 |
| 9 | 83.10 | 83.38 | −0.337 | 0.068 | −1.274 |
| 10 | 83.20 | 83.38 | −0.216 | 0.028 | −0.819 |
| 11 | 83.70 | 83.38 | 0.382 | 0.088 | 1.456 |
| 12 | 83.50 | 83.38 | 0.144 | 0.012 | 0.546 |
| 13 | 83.40 | 83.38 | 0.024 | 0.000 | 0.091 |
| Time (t-Min) | Temperature (T-°C) | Predicted Value (Ester Yield-%) |
|---|---|---|
| 343 | 58.34 | 83.83 |
| Feedstock | Yield (%) | Reaction Time | Temperature (°C) | Reference |
|---|---|---|---|---|
| Waste cooking oil with methanol | 88.83 | 180 min | 60 | [5] |
| Waste frying oil (WFO) | 90.81 | 180 min | 50 | [7] |
| Castor oil | 94.2 | 480 min | 35 | [8] |
| Ceiba pentandra | 95.42 | 388 s (microwave assisted) | ~300 | [27] |
| Black mustard (Brassica nigra L.) seed oil | 96.229–97.335 | 56–54.1 min | 35.4–57.1 | [28] |
| Rubber seed oil (RSO) | 97.84 | 240 min | 65 | [29] |
| Karanja oil | 81.15 | 90 min | 300 | [44] |
| Palm oil | 83.57 | 343 min | 58.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Roy, S. Process Optimization Using Experimental and Statistical Modeling in Biodiesel Production from Palm Oil. Physchem 2025, 5, 52. https://doi.org/10.3390/physchem5040052
Chatterjee S, Roy S. Process Optimization Using Experimental and Statistical Modeling in Biodiesel Production from Palm Oil. Physchem. 2025; 5(4):52. https://doi.org/10.3390/physchem5040052
Chicago/Turabian StyleChatterjee, Sushovan, and Sagar Roy. 2025. "Process Optimization Using Experimental and Statistical Modeling in Biodiesel Production from Palm Oil" Physchem 5, no. 4: 52. https://doi.org/10.3390/physchem5040052
APA StyleChatterjee, S., & Roy, S. (2025). Process Optimization Using Experimental and Statistical Modeling in Biodiesel Production from Palm Oil. Physchem, 5(4), 52. https://doi.org/10.3390/physchem5040052








