Preparation of Poly(vinylidene fluoride-co-hexafluoropropylene) Doped Cellulose Acetate Films for the Treatment of Calcium-Based Hardness from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PVDF-HFP Film
2.1.1. Preparation of CA Film
2.1.2. Preparation of PVDF-HFP and 3 wt.% PVDF-HFP/CA Film
2.1.3. Characterisation Techniques
2.1.4. Adsorption Studies of Ca2+ Ions in Synthetic Water Samples
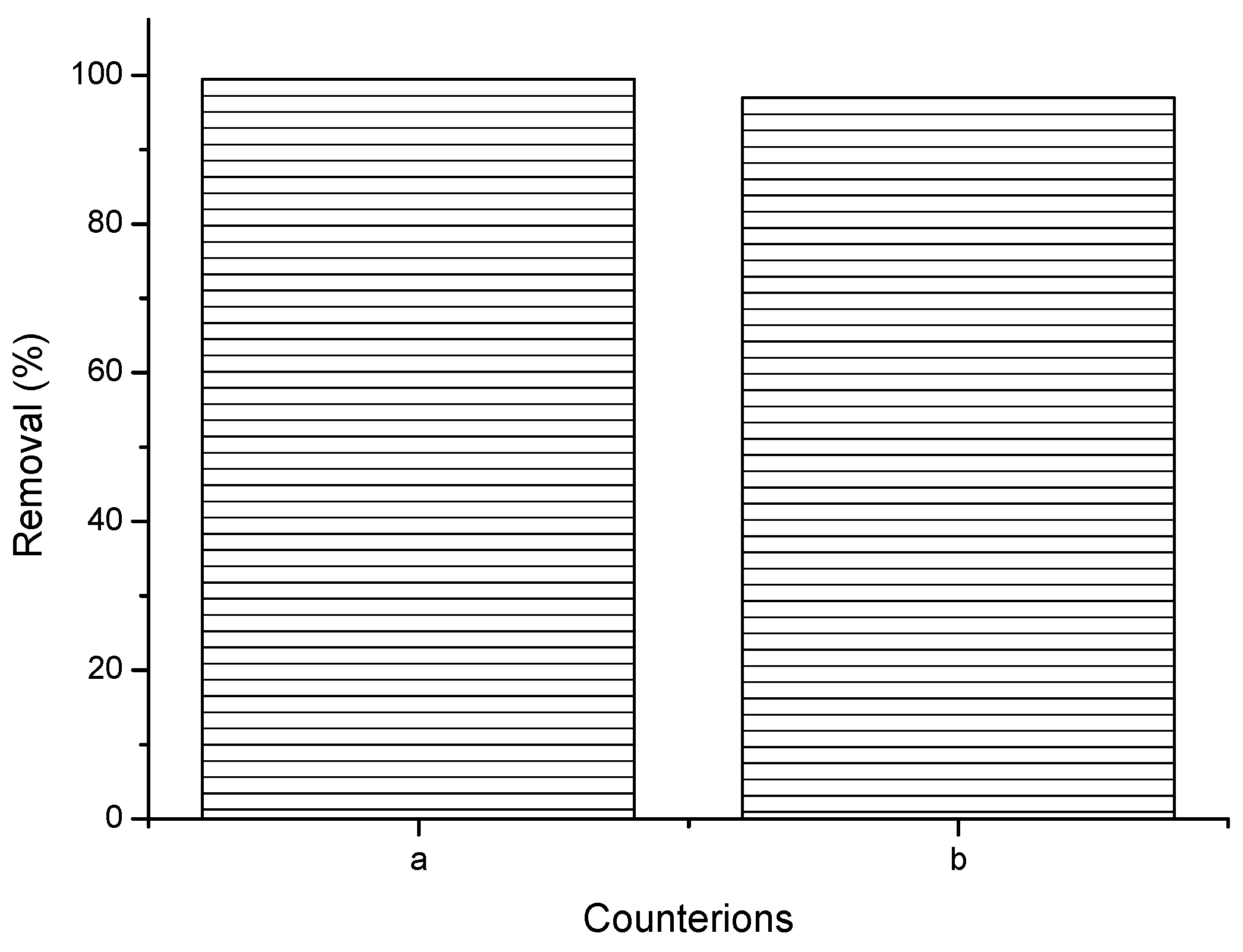
2.1.5. Effect of Counterions
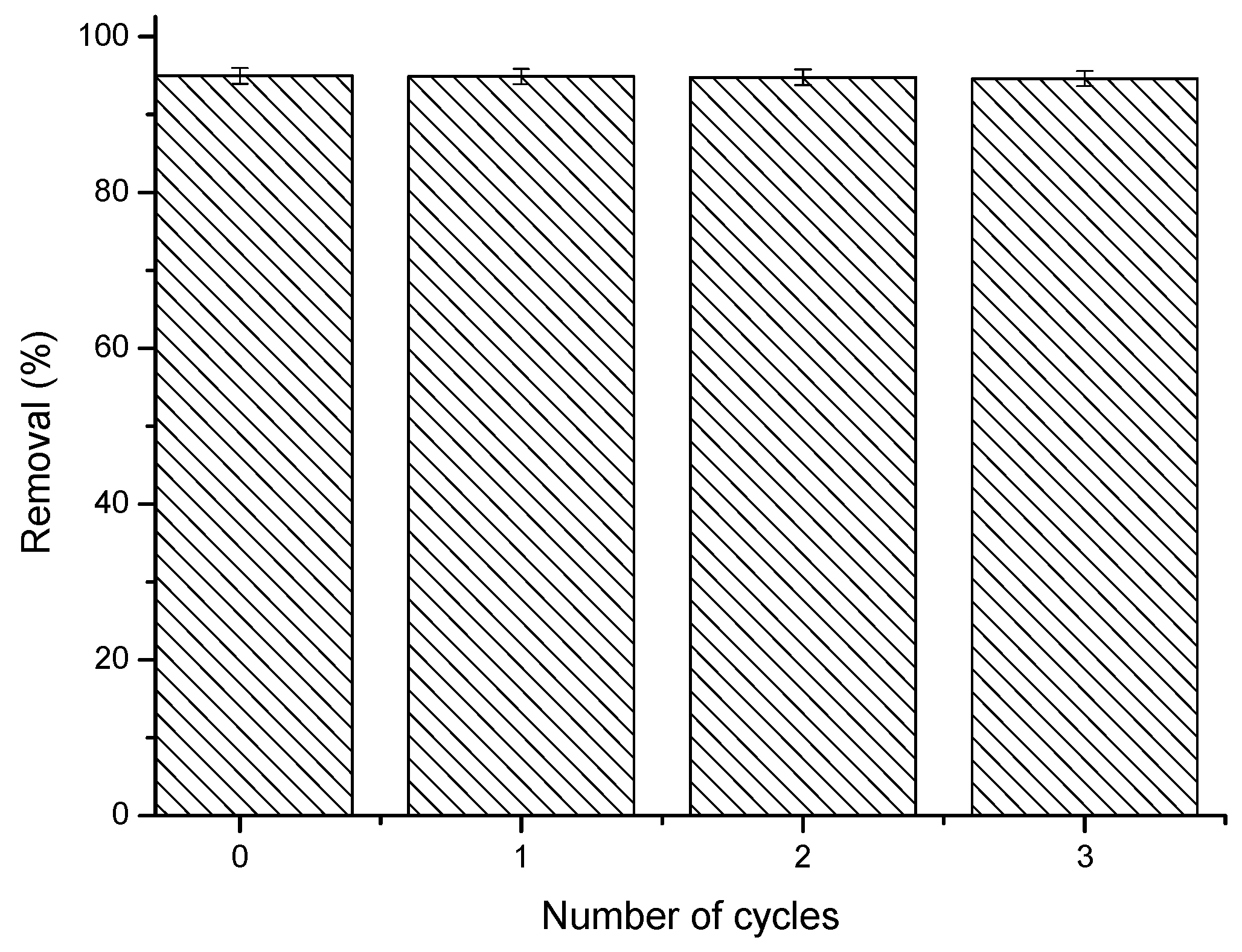
2.1.6. Reusability Studies
3. Results
3.1. Characterisation of Films
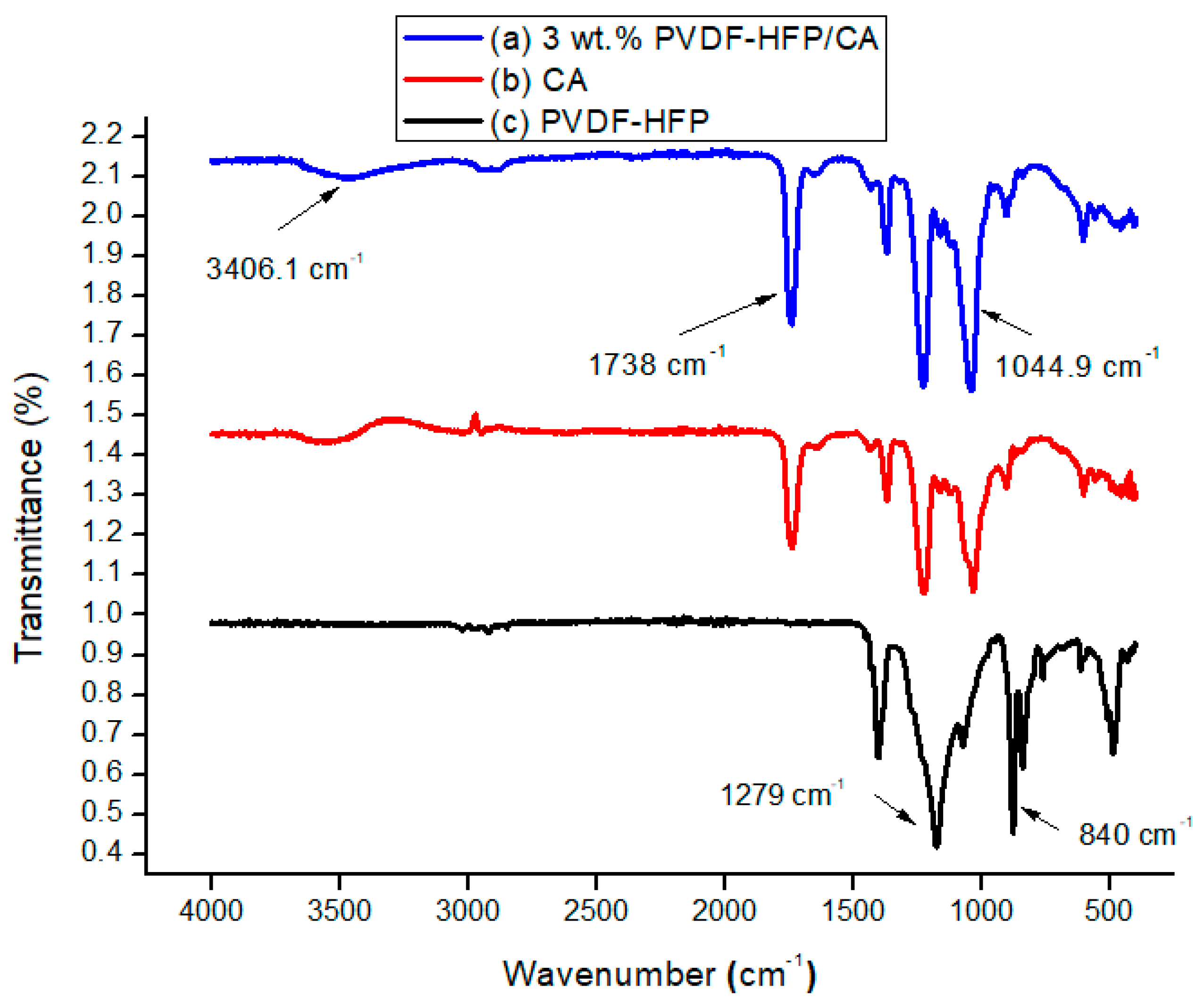
3.1.1. The FTIR Profiles of PVDF-HFP, CA, and 3 wt.% PVDF-HFP/CA Films
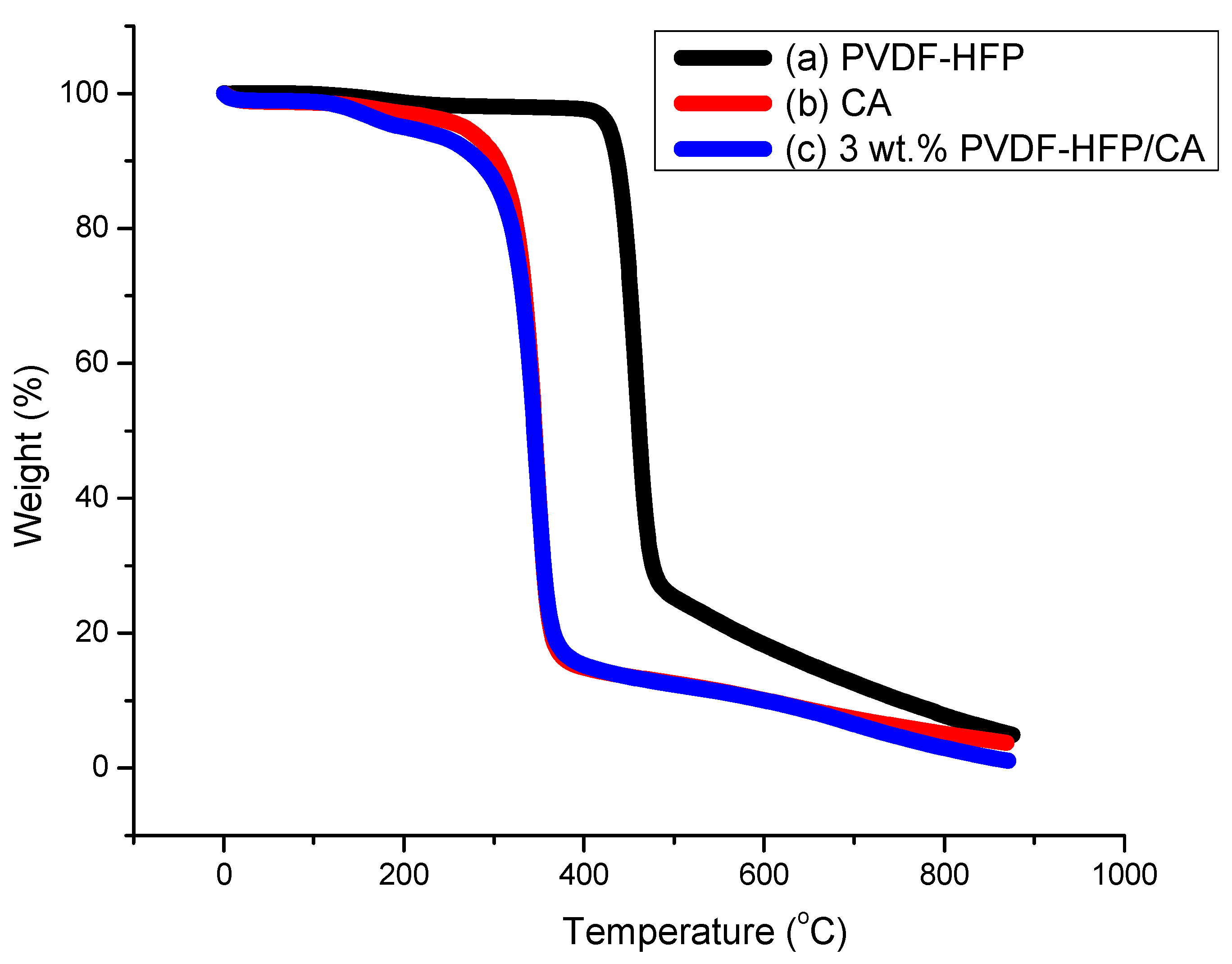
3.1.2. TGA Profiles of PVDF-HFP, CA, and 3 wt.% PVDF-HFP/CA Films
3.1.3. SEM Images of PVDF-HFP, CA, and 3 wt.% PVDF-HFP/CA Films
3.2. Adsorption Studies
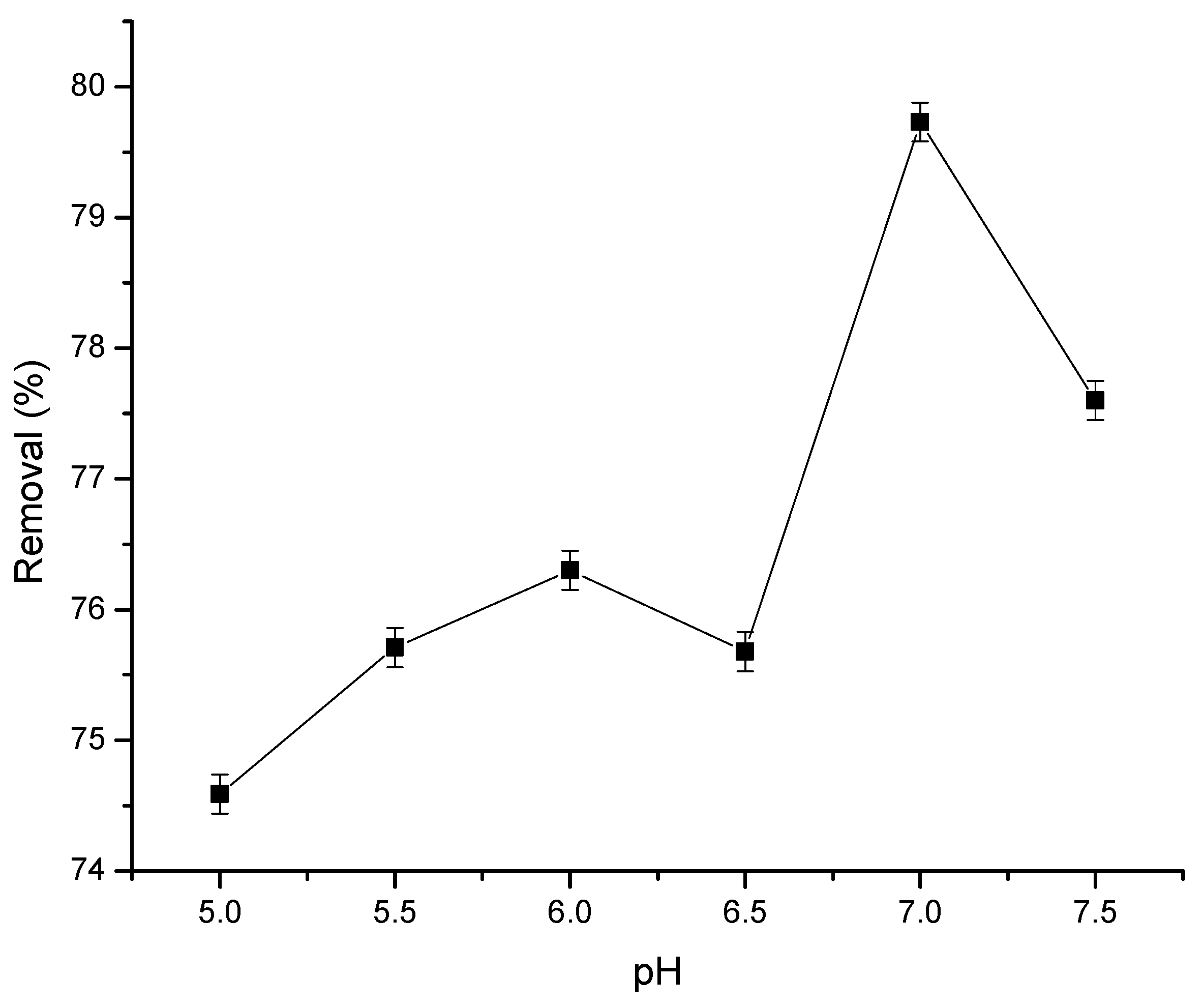
3.2.1. Impact of pH on Ca2+ Ions Removal by PVDF-HFP Film
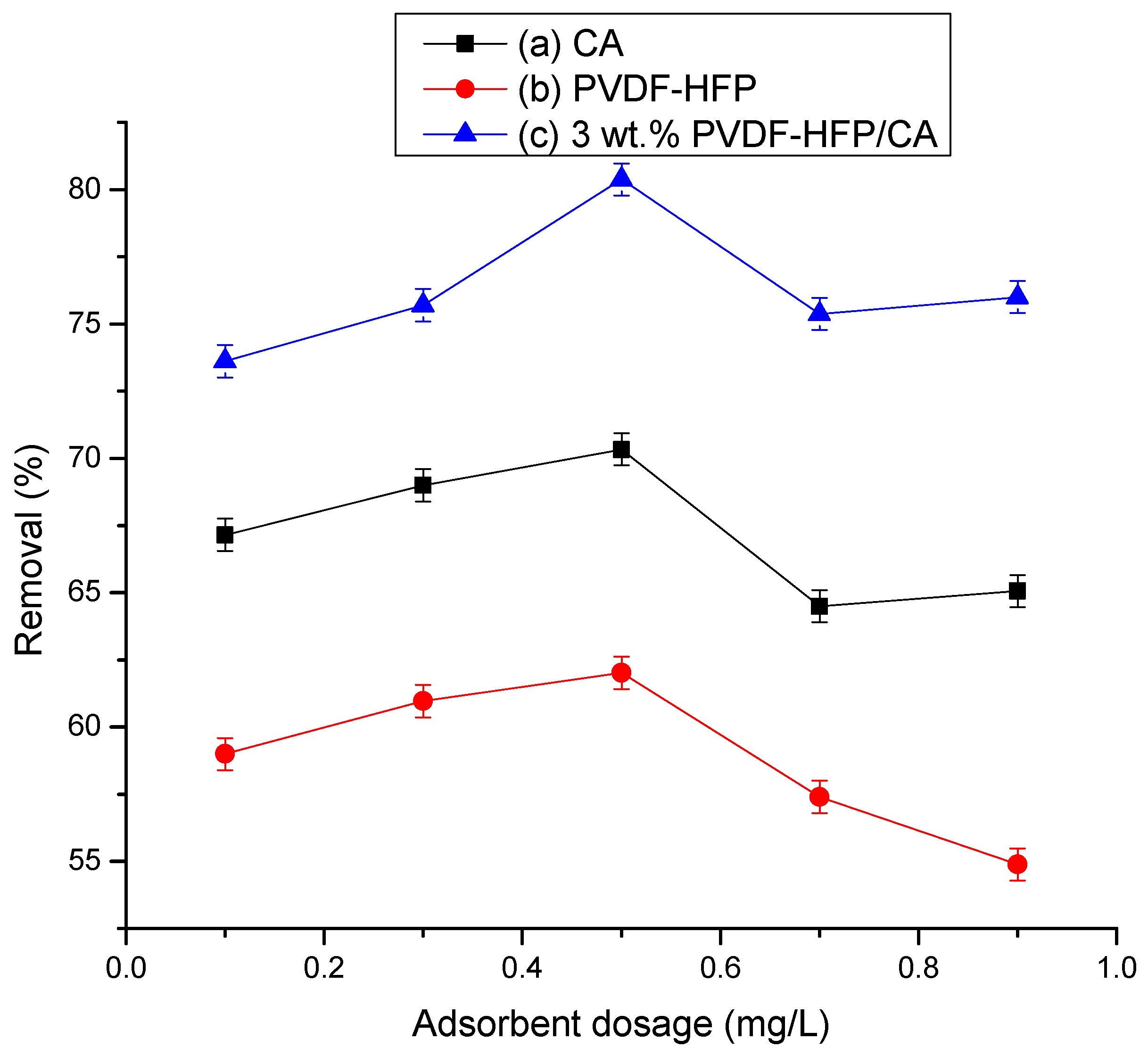
3.2.2. Effect of Film Adsorbent Dosage
3.2.3. Influence of Contact Duration on the Adsorption of Ca2+ Ions onto Polymeric Films
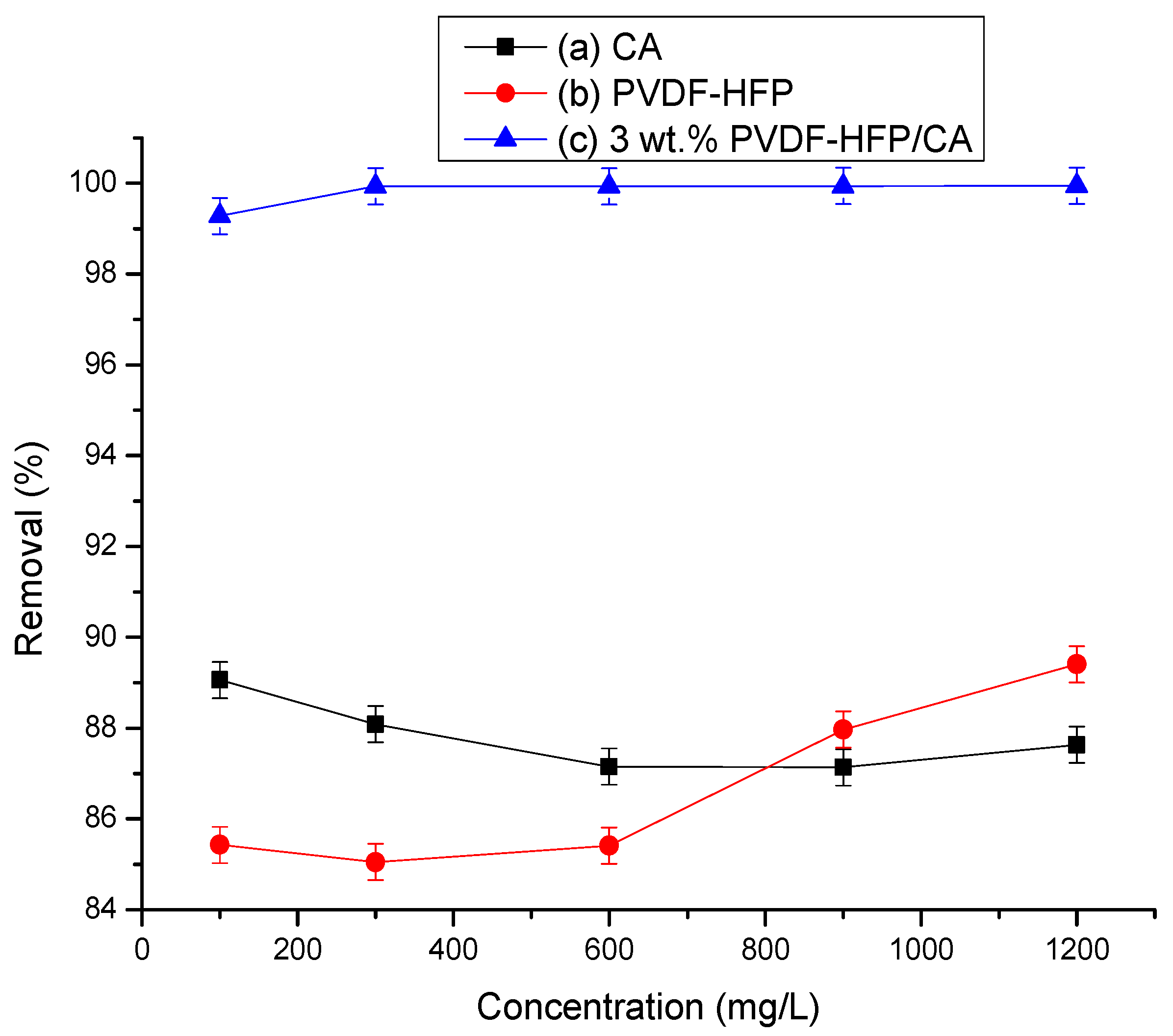
3.2.4. Influence of Ca2+ Ion Concentration on Their Adsorption by Different Polymeric Films
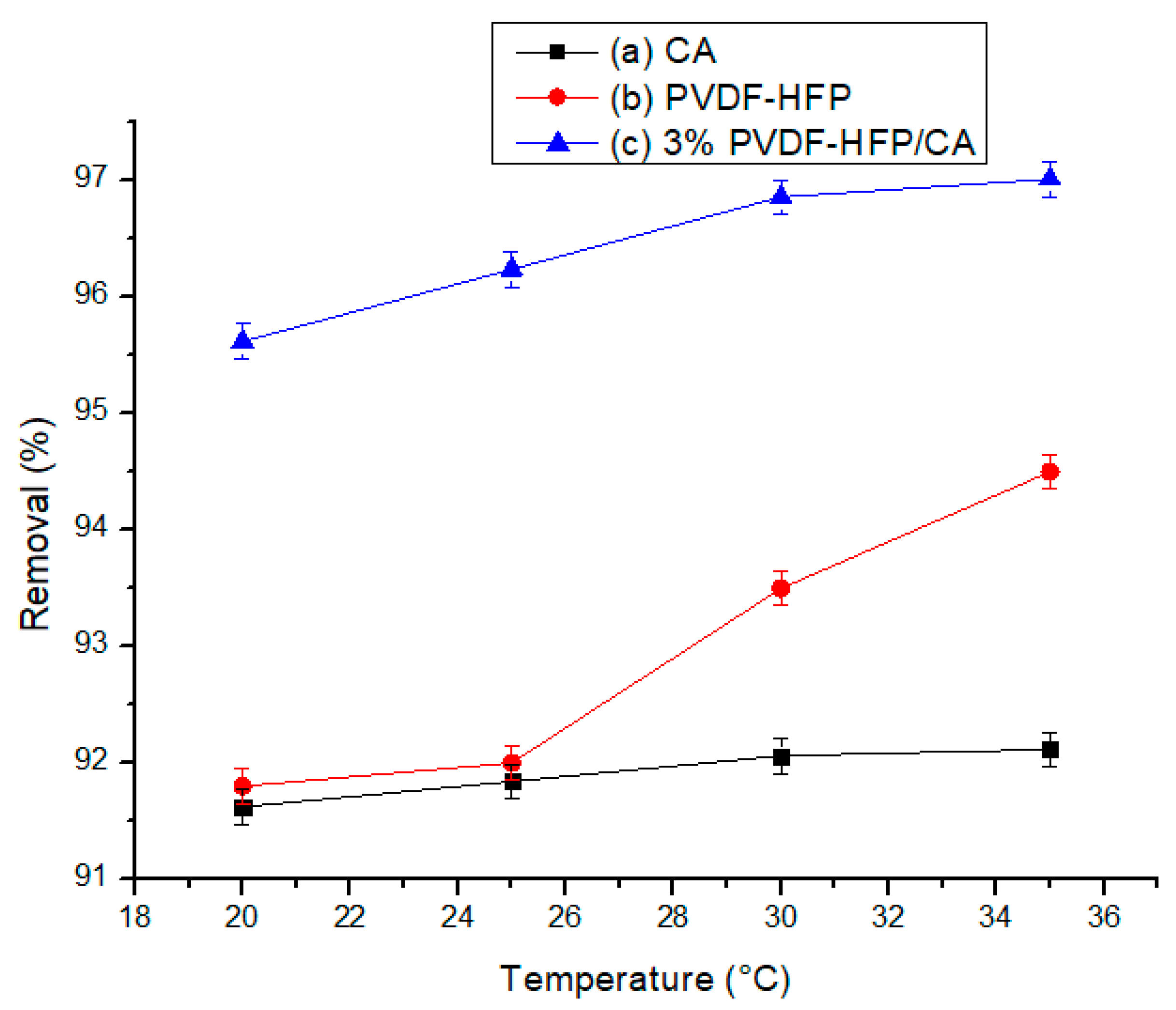
3.2.5. Effect of Temperature
3.3. Adsorption Kinetics of Ca2+ Ions Using Polymer-Based Films
3.4. Models for Adsorption Isotherms
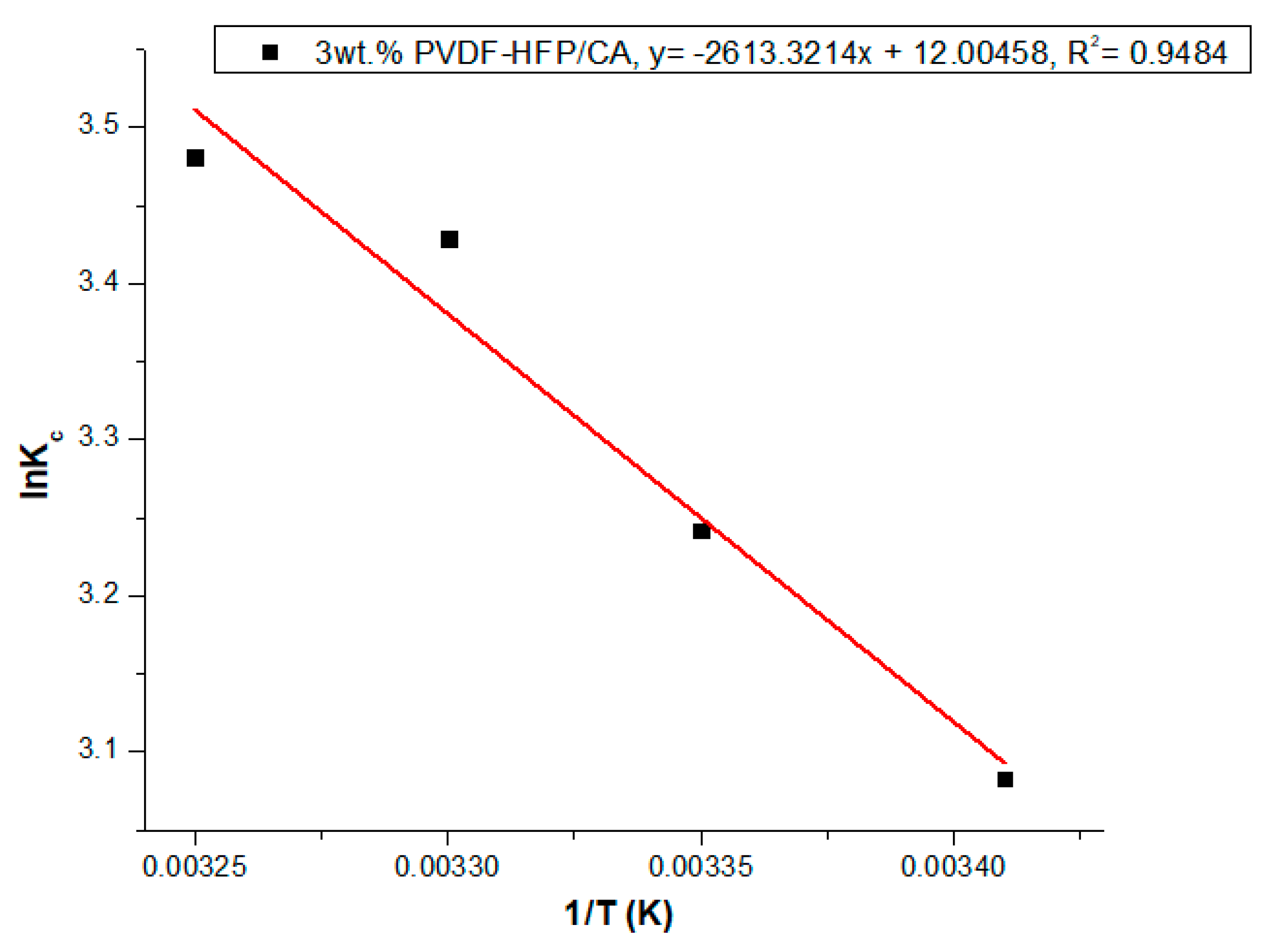
3.5. Thermodynamics Studies of Ca2+ Ions Adsorption on 3 wt.% PVDF-HFP/CA
3.6. Impact of Counterions
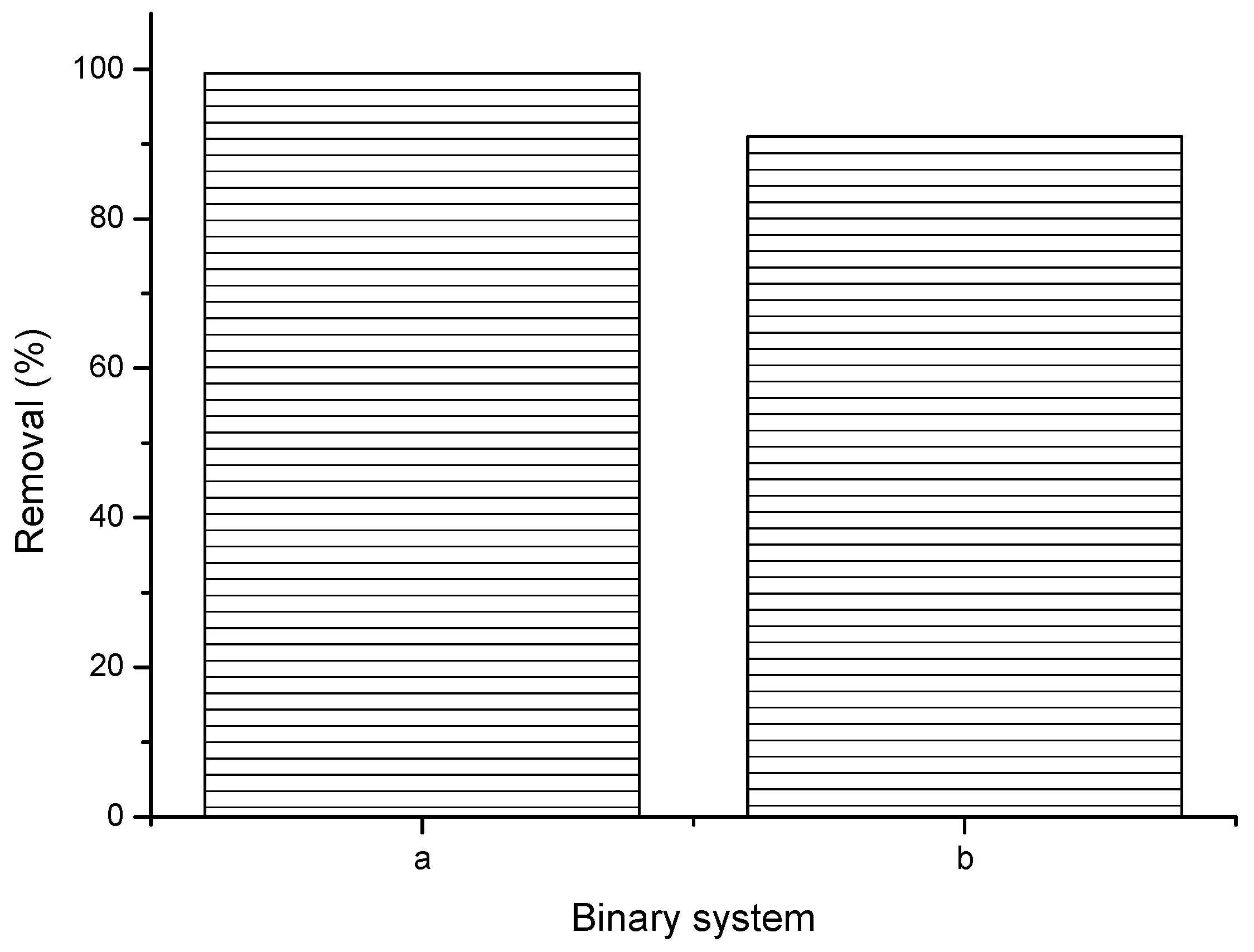
3.7. Influence of Binary System on the Uptake of Ca2+ Ions
3.8. Reutilisation of Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TGA | Thermogravimetric analysis |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared |
| PVDF-HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| CA | Cellulose acetate |
References
- Hailu, Y.; Tilahun, E.; Brhane, A.; Resky, H.; Sahu, O. Ion Exchanges Process for Calcium, Magnesium and Total Hardness from Ground Water with Natural Zeolite. Groundw. Sustain. Dev. 2019, 8, 457–467. [Google Scholar] [CrossRef]
- Kadir, N.N.A.; Shahadat, M.; Ismail, S. Formulation Study for Softening of Hard Water Using Surfactant Modified Bentonite Adsorbent Coating. Appl. Clay Sci. 2017, 137, 168–175. [Google Scholar] [CrossRef]
- Pourshadlou, S.; Mobasherpour, I.; Majidian, H.; Salahi, E.; Shirani Bidabadi, F.; Mei, C.T.; Ebrahimi, M. Adsorption System for Mg2+ Removal from Aqueous Solutions Using Bentonite/γ-Alumina Nanocomposite. J. Colloid Interface Sci. 2020, 568, 245–254. [Google Scholar] [CrossRef]
- Helte, E.; Säve-Söderbergh, M.; Larsson, S.C.; Åkesson, A. Calcium and Magnesium in Drinking Water and Risk of Myocardial Infarction and Stroke—A Population-Based Cohort Study. Am. J. Clin. Nutr. 2022, 116, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Zhou, A. Smartphone Colorimetric Detection of Calcium and Magnesium in Water Samples Using a Flow Injection System. Microchem. J. 2019, 147, 215–223. [Google Scholar] [CrossRef]
- Lee, P.X.; Liu, B.L.; Show, P.L.; Ooi, C.W.; Chai, W.S.; Munawaroh, H.S.H.; Chang, Y.K. Removal of Calcium Ions from Aqueous Solution by Bovine Serum Albumin (BSA)-Modified Nanofiber Membrane: Dynamic Adsorption Performance and Breakthrough Analysis. Biochem. Eng. J. 2021, 171, 108016. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Cao, Z.; Shan, M.; Bing, Y. Effect and Mechanism of Induced Crystallization Softening Treatment on Water Quality in Drinking Water Distribution System with High Hardness Water Source. J. Environ. Chem. Eng. 2023, 11, 110474. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, T.; Hua, X.; Cheng, P.; Zhang, L.; Wang, S.; Du, W. The Future Prospect of China’s Independent R&D Technology (ITK) in Water Resources Utilization and Wastewater Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128183397. [Google Scholar]
- Voojodi, S.; Rastgoo, M.; Izadi-Darbandi, E.; Hajmohammadnia Ghalibaf, K.; Hasanfard, A. Tank-Mixing 2,4-D Amine and Sulfosulfuron Can Help Alleviate the Adverse Effects of Water Hardness on Controlling Flixweed [Descurainia sophia (L.) Webb Ex Prantl]. Crop Prot. 2023, 173, 106377. [Google Scholar] [CrossRef]
- Rastgoo, M.; Mirzaei, M.; Gherekhloo, J.; Hasanfard, A. Effect of Water Hardness Induced by Bicarbonate and Chloride Forms of Magnesium and Sodium on the Performance of Herbicides for Littleseed Canarygrass Control. J. Crop Prot. 2022, 11, 315–327. [Google Scholar]
- Abdullah, A.D. Modelling Approaches to Understand Salinity Variations in a Highly Dynamic Tidal River: The Case of the Shatt Al-Arab River; CRC Press: London, UK, 2017; pp. 1–188. [Google Scholar] [CrossRef]
- Elumalai, V.; Nethononda, V.G.; Manivannan, V.; Rajmohan, N.; Li, P.; Elango, L. Groundwater Quality Assessment and Application of Multivariate Statistical Analysis in Luvuvhu Catchment, Limpopo, South Africa. J. Afr. Earth Sci. 2020, 171, 103967. [Google Scholar] [CrossRef]
- Rao, G.P.; Lu, C.; Su, F. Sorption of Divalent Metal Ions from Aqueous Solution by Carbon Nanotubes: A Review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Pandiyan, R.; Dharmaraj, S.; Ayyaru, S.; Sugumaran, A.; Somasundaram, J.; Kazi, A.S.; Samiappan, S.C.; Ashokkumar, V.; Ngamcharussrivichai, C. Ameliorative Photocatalytic Dye Degradation of Hydrothermally Synthesized Bimetallic Ag-Sn Hybrid Nanocomposite Treated upon Domestic Wastewater under Visible Light Irradiation. J. Hazard. Mater. 2022, 421, 126734. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, A. Drinking Water Contamination and Treatment Techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Macevele, L.E.; Moganedi, K.L.M.; Magadzu, T. Effects of Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) Nanocomposite Membrane on Reduction in Microbial Load and Heavy Metals in Surface Water Samples. J. Compos. Sci. 2024, 8, 119. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Zarrabi, M.; Kazemian, H.; Amrane, A.; Yaghmaian, K.; Ghaffari, H.R. Removal of Hardness Agents, Calcium and Magnesium, by Natural and Alkaline Modified Pumice Stones in Single and Binary Systems. Appl. Surf. Sci. 2013, 274, 295–305. [Google Scholar] [CrossRef]
- Ayaliew Werkneh, A. Removal of Water Hardness Causing Constituents Using Alkali Modified Sugarcane Bagasse and Coffee Husk at Jigjiga City, Ethiopia: A Comparative Study. Int. J. Environ. Monit. Anal. 2015, 3, 7. [Google Scholar] [CrossRef][Green Version]
- Shi, L.; Wang, R.; Cao, Y.; Feng, C.; Liang, D.T.; Tay, J.H. Fabrication of Poly(Vinylidene Fluoride-Co-Hexafluropropylene) (PVDF-HFP) Asymmetric Microporous Hollow Fiber Membranes. J. Memb. Sci. 2007, 305, 215–225. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, S.G.; Renganathan, N.G.; Kulandainathan, M.A. Characterization of Poly(Vinylidene Fluoride-Hexafluoropropylene) (PVdF-HFP) Electrolytes Complexed with Different Lithium Salts. Eur. Polym. J. 2005, 41, 15–21. [Google Scholar] [CrossRef]
- Macevele, L.E.; Moganedi, K.L.M.; Magadzu, T. Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-Co-Hexafluoropropylene) Composite Membrane. Membranes 2017, 7, 35. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Hao, J.; Fu, H. Poly(Vinylidene Fluoride-Hexafluoropropylene) Porous Membrane with Controllable Structure and Applications in Efficient Oil/Water Separation. Materials 2018, 11, 443. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Wei, G.; Wang, S.; Zhao, X.; Kong, F. Cellulose Acetate Propionate Incorporated PVDF-HFP Based Polymer Electrolyte Membrane for Lithium Batteries. Compos. Commun. 2022, 33, 101226. [Google Scholar] [CrossRef]
- Macevele, L.E.; Lydia, K.; Moganedi, M.; Magadzu, T. Adsorption of Cadmium (II) Ions from Aqueous Solutions Using Poly (Amidoamine)/Multi-Walled Carbon Nanotubes Doped Poly (Vinylidene Fluoride-Co-Hexafluoropropene) Composite Membrane. J. Membr. Sci. Res. 2021, 7, 152–165. [Google Scholar] [CrossRef]
- Rajesh, S.; Maheswari, P.; Senthilkumar, S.; Jayalakshmi, A.; Mohan, D. Preparation and Characterisation of Poly (Amide-Imide) Incorporated Cellulose Acetate Membranes for Polymer Enhanced Ultrafiltration of Metal Ions. Chem. Eng. J. 2011, 171, 33–44. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, Y.; Wu, A.; Yan, X.; Shen, S.; Ke, C.; Zhang, J. Preparation of Microporous Cellulose/Poly(Vinylidene Fluoride-Hexafluoropropylene) Membrane for Lithium Ion Batteries by Phase Inversion Method. J. Power Sources 2018, 379, 197–205. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-Based Li-Ion Batteries: A Review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Kuribayashi, I. Characterization of Composite Cellulosic Separators for Rechargeable Lithium-Ion Batteries. J. Power Sources 1996, 63, 87–91. [Google Scholar] [CrossRef]
- Muqeet, M.; Khalique, A.; Qureshi, U.A.; Mahar, R.B.; Ahmed, F.; Khatri, Z.; Kim, I.S.; Brohi, K.M. Aqueous Hardness Removal by Anionic Functionalized Electrospun Cellulose Nanofibers. Cellulose 2018, 25, 5985–5997. [Google Scholar] [CrossRef]
- Tohdee, K.; Kaewsichan, L.; Asadullah. Enhancement of Adsorption Efficiency of Heavy Metal Cu(II) and Zn(II) onto Cationic Surfactant Modified Bentonite. J. Environ. Chem. Eng. 2018, 6, 2821–2828. [Google Scholar] [CrossRef]
- Ahmad, A.; Razali, M.H.; Mamat, M.; Mehamod, F.S.B.; Anuar Mat Amin, K. Adsorption of Methyl Orange by Synthesized and Functionalized-CNTs with 3-Aminopropyltriethoxysilane Loaded TiO2 Nanocomposites. Chemosphere 2017, 168, 474–482. [Google Scholar] [CrossRef]
- Ren, X.; Yang, L.; Liu, M. Kinetic and Thermodynamic Studies of Acid Scarlet 3r Adsorption onto Low-Cost Adsorbent Developed from Sludge and Straw. Chin. J. Chem. Eng. 2014, 22, 208–213. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Wu, Y.; Lu, G.; Cao, Z.; Cheng, J.; Wang, K.; Yang, H.; Xia, Y.; Wen, X.; et al. Facile Preparation of Polyacrylamide/Chitosan/Fe3O4 Composite Hydrogels for Effective Removal of Methylene Blue from Aqueous Solution. Carbohydr. Polym. 2020, 234, 115882. [Google Scholar] [CrossRef]
- Kahu, S.S.; Shekhawat, A.; Saravanan, D.; Jugade, R.M. Two Fold Modified Chitosan for Enhanced Adsorption of Hexavalent Chromium from Simulated Wastewater and Industrial Effluents. Carbohydr. Polym. 2016, 146, 264–273. [Google Scholar] [CrossRef]
- Alghamdi, M.M.; El-Zahhar, A.A.; Idris, A.M.; Said, T.O.; Sahlabji, T.; El Nemr, A. Synthesis, Characterization, and Application of a Novel Polymeric-Bentonite-Magnetite Composite Resin for Water Softening. Sep. Purif. Technol. 2019, 224, 356–365. [Google Scholar] [CrossRef]
- Le, T.T.N.; Le, V.T.; Dao, M.U.; Nguyen, Q.V.; Vu, T.T.; Nguyen, M.H.; Tran, D.L.; Le, H.S. Preparation of Magnetic Graphene Oxide/Chitosan Composite Beads for Effective Removal of Heavy Metals and Dyes from Aqueous Solutions. Chem. Eng. Commun. 2019, 206, 1337–1352. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.O.; Ndamitso, M.M.; Abdulkareem, S.A.; Shuaib, D.T.; Mohammed, A.K.; Sumaila, A. The Role of Kaolin and Kaolin/ZnO Nanoadsorbents in Adsorption Studies for Tannery Wastewater Treatment. Sci. Rep. 2020, 10, 13068. [Google Scholar] [CrossRef]
- Nthumbi, R.M.; Adelodun, A.A.; Ngila, J.C. Electrospun and Functionalized PVDF/PAN Composite for the Removal of Trace Metals in Contaminated Water. Phys. Chem. Earth 2017, 100, 225–235. [Google Scholar] [CrossRef]
- Istiningrum, R.B.; Tiwow, C.D.; Nuryono; Narsito. Au(III) Selective Adsorption of Quaternary Ammonium-Silica Hybrid in Au/Cu System. Procedia Chem. 2015, 17, 132–138. [Google Scholar] [CrossRef]
- El-Gendi, A.; Abdallah, H.; Amin, A.; Amin, S.K. Investigation of Polyvinylchloride and Cellulose Acetate Blend Membranes for Desalination. J. Mol. Struct. 2017, 1146, 14–22. [Google Scholar] [CrossRef]
- Sagar, S.; Iqbal, N.; Maqsood, A. Dielectric, Electric and Thermal Properties of Carboxylic Functionalized Multiwalled Carbon Nanotubes Impregnated Polydimethylsiloxane Nanocomposite. J. Phys. Conf. Ser. 2013, 439, 012024. [Google Scholar] [CrossRef]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and Characterization of Cellulose Acetate from Rice Husk: Eco-Friendly Condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef]
- Asghar, M.R.; Anwar, M.T.; Rasheed, T.; Naveed, A.; Yan, X.; Zhang, J. Lithium Salt Doped Poly(Vinylidene Fluoride)/Cellulose Acetate Composite Gel Electrolyte Membrane for Lithium Ion Battery. IOP Conf. Ser. Mater. Sci. Eng. 2019, 654. [Google Scholar] [CrossRef]
- Zi, X.; Wu, H.; Song, J.; He, W.; Xia, L.; Guo, J.; Luo, S.; Yan, W. Electrospun Sandwich-like Structure of PVDF-HFP/Cellulose/PVDF-HFP Membrane for Lithium-Ion Batteries. Molecules 2023, 28, 4998. [Google Scholar] [CrossRef]
- Shi, R.J.; Wang, T.; Lang, J.Q.; Zhou, N.; Ma, M.G. Multifunctional Cellulose and Cellulose-Based (Nano) Composite Adsorbents. Front. Bioeng. Biotechnol. 2022, 10, 891034. [Google Scholar] [CrossRef]
- Rolence, C.; Machunda, R.L.; Njau, K.N. Water Hardness Removal by Coconut Shell Activated Carbon. Int. J. Sci. Technol. Soc. 2014, 2, 97–102. [Google Scholar] [CrossRef]
- Bibiano-Cruz, L.; Garfias, J.; Salas-García, J.; Martel, R.; Llanos, H. Batch and Column Test Analyses for Hardness Removal Using Natural and Homoionic Clinoptilolite: Breakthrough Experiments and Modeling. Sustain. Water Resour. Manag. 2016, 2, 183–197. [Google Scholar] [CrossRef]
- Joshi, P.; Manocha, S. Sorption of Cadmium Ions onto Synthetic Hydroxyapatite Nanoparticles. Mater. Today Proc. 2017, 4, 10460–10464. [Google Scholar] [CrossRef]
- Rahman, M.; Gul, S.; Ajmal, M.; Iqbal, A.; Achakzai, A. Removal of Cadmium from Aqueous Solutions Using Excised Leaves of Quetta Pine (Pinus halepensis mill.). Bangladesh J. Bot. 2014, 43, 277–281. [Google Scholar] [CrossRef]
- Lestari, A.Y.D.; Malik, A.; Sukirman; Ilmi, M.I.; Sidiq, M. Removal of Calcium and Magnesium Ions from Hard Water Using Modified Amorphophallus Campanulatus Skin as a Low Cost Adsorbent. MATEC Web Conf. 2018, 154, 4–7. [Google Scholar] [CrossRef][Green Version]
- Calgan, E.; Ozmetin, E. Optimization of Hardness Removal Using Response Surface Methodology from Wastewater Containing High Boron by Bigadiç Clinoptilolite. Desalination Water Treat. 2019, 172, 24907. [Google Scholar] [CrossRef]
- Snoussi, Y.; Abderrabba, M.; Sayari, A. Removal of Cadmium from Aqueous Solutions by Adsorption onto Polyethylenimine-Functionalized Mesocellular Silica Foam: Equilibrium Properties. J. Taiwan Inst. Chem. Eng. 2016, 66, 372–378. [Google Scholar] [CrossRef]
- Ramollo, K.V. Preparation of Poly(vinylidene fluoride-co-hexafluoropropylene) Composite Membranes for Treatment of Water Hardness. Master’s Thesis, University of Limpopo, Polokwane, South Africa, 2022. [Google Scholar]



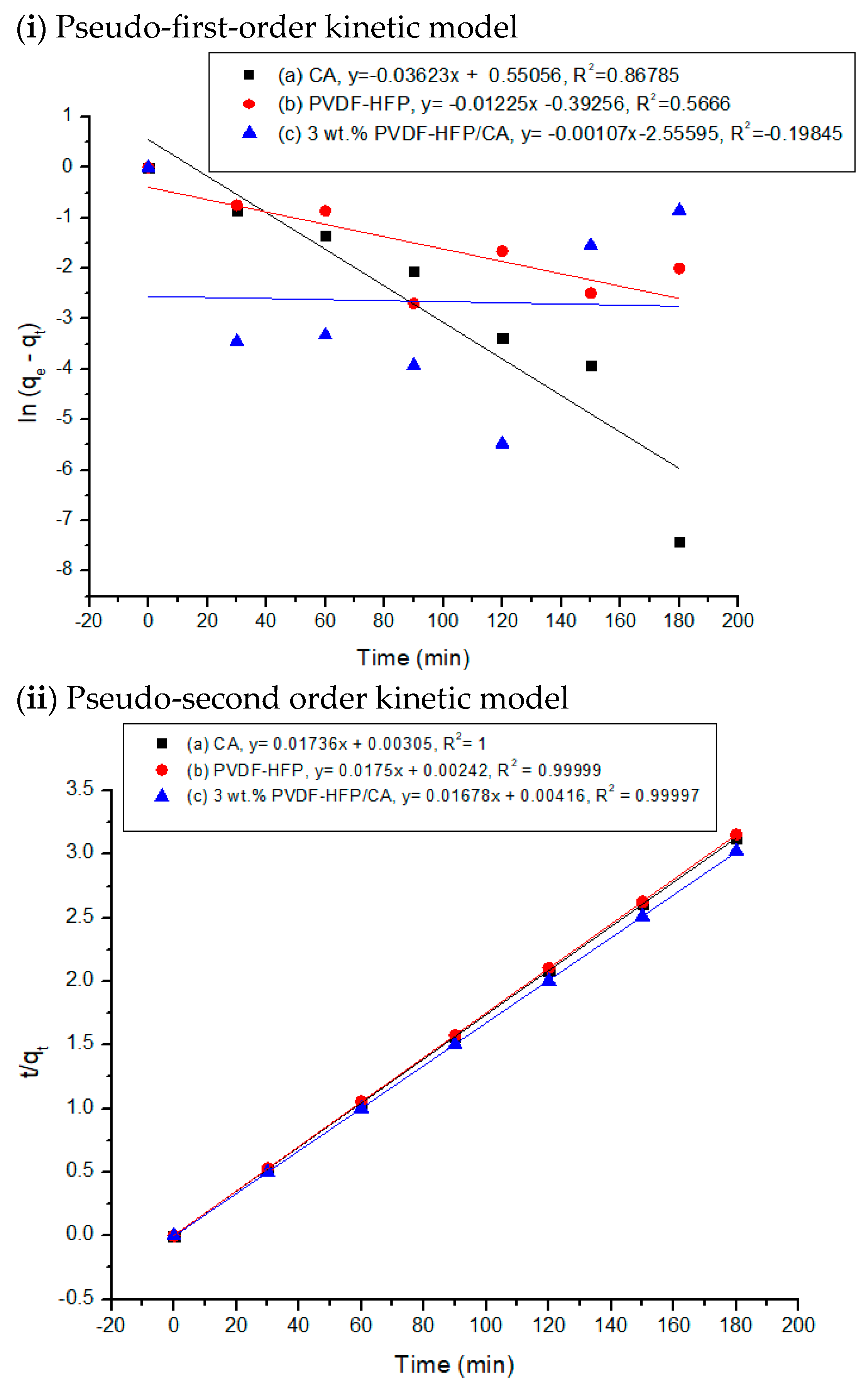


| Film | Experimental qe (mg/g) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg·g−1) | R2 | K2 (g/mg·min) | qe (mg·g−1) | R2 | ||
| CA | 57.57 | 0.04 | 1.73 | 0.87 | 0.10 | 57.60 | 1.00 |
| PVDF-HFP | 57.21 | 0.01 | 0.68 | 0.57 | 0.13 | 57.14 | 0.99 |
| 3 wt.% PVDF-HFP/CA | 59.94 | 0.00 | 0.08 | −0.20 | 0.07 | 59.60 | 0.99 |
| Film | qmax (mg/g) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| b (L/mg) | R2 | RL | Kf (mg/g) | n | R2 | ||
| CA | 50.63 | −1.26 | 0.99 | −0.01 | 16,268.22 | −11.29 | 0.99 |
| PVDF-HFP | 51.71 | −1.66 | 0.96 | −0.01 | 15,000.30 | −13.70 | 0.99 |
| 3 wt.% PVDF-HFP/CA | 55.71 | −6.46 | 0.99 | −0.001 | 12,944.94 | −26.66 | 0.99 |
| Adsorbent | Conditions of the Experiment | Adsorption Capacity (mg/g) | % Removal | Reference(s) |
|---|---|---|---|---|
| 3 wt.% PVDF-HFP/CA | pH = 7, Temp = 298 K, Dosage = 0.5 mg/L | 56 | 99 | This study |
| Modified pumice adsorbents | pH = 6, Temp = 293 K, Dosage = 10 g/L | 62.34 | 96 | [18] |
| Coconut shell activated carbon | pH = 6.30, Temp = 303 K, Dosage = 0.16 g/cm3 | 48.50 | 60 | [47] |
| Alkali-modified sugarcane bagasse | pH = 7.50, Temp = 298 K, Dosage = 2.5 g/L | 52.9 | 78 | [19] |
| Natural and homoionic clinoptilolite | Not specified | 10.50 | Not specified | [48] |
| Surfactant-modified bentonite adsorbent coating | Not specified | 29.27 | 66.67 | [2] |
| Modified Amorphophallus campanulate skin as a low-cost adsorbent | Not specified | 10.85 | 85 | [51] |
| Bigadic clinoptilolite | Temp = 299 K, Dosage = 20 g/L, Time = 93 min | 12.30 | 99 | [52] |
| Natural zeolite | pH = 6.9, Dosage = 50 g/L | - | 80.2 | [1] |
| Film | Temperature (K) | Thermodynamic Variables | ||
|---|---|---|---|---|
| ∆G (KJ/mol) | ∆H (KJ/mol) | ∆S (J.mol/K) | ||
| 3 wt.% PVDF-HFP/CA | 293.15 | −7514.85 | 314.33 | 1.44 |
| 298.15 | −8037.39 | |||
| 303.15 | −8642.53 | |||
| 308.15 | −8918.86 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramollo, K.V.; Macevele, L.E.; Ambushe, A.A.; Magadzu, T. Preparation of Poly(vinylidene fluoride-co-hexafluoropropylene) Doped Cellulose Acetate Films for the Treatment of Calcium-Based Hardness from Aqueous Solution. Physchem 2025, 5, 45. https://doi.org/10.3390/physchem5040045
Ramollo KV, Macevele LE, Ambushe AA, Magadzu T. Preparation of Poly(vinylidene fluoride-co-hexafluoropropylene) Doped Cellulose Acetate Films for the Treatment of Calcium-Based Hardness from Aqueous Solution. Physchem. 2025; 5(4):45. https://doi.org/10.3390/physchem5040045
Chicago/Turabian StyleRamollo, Khaleke Veronicah, Lutendo Evelyn Macevele, Abayneh Ataro Ambushe, and Takalani Magadzu. 2025. "Preparation of Poly(vinylidene fluoride-co-hexafluoropropylene) Doped Cellulose Acetate Films for the Treatment of Calcium-Based Hardness from Aqueous Solution" Physchem 5, no. 4: 45. https://doi.org/10.3390/physchem5040045
APA StyleRamollo, K. V., Macevele, L. E., Ambushe, A. A., & Magadzu, T. (2025). Preparation of Poly(vinylidene fluoride-co-hexafluoropropylene) Doped Cellulose Acetate Films for the Treatment of Calcium-Based Hardness from Aqueous Solution. Physchem, 5(4), 45. https://doi.org/10.3390/physchem5040045








