Effect of Vibration on Open-Cathode Direct Methanol Fuel Cell Stack Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Assembly of the OC-DMFC Stack
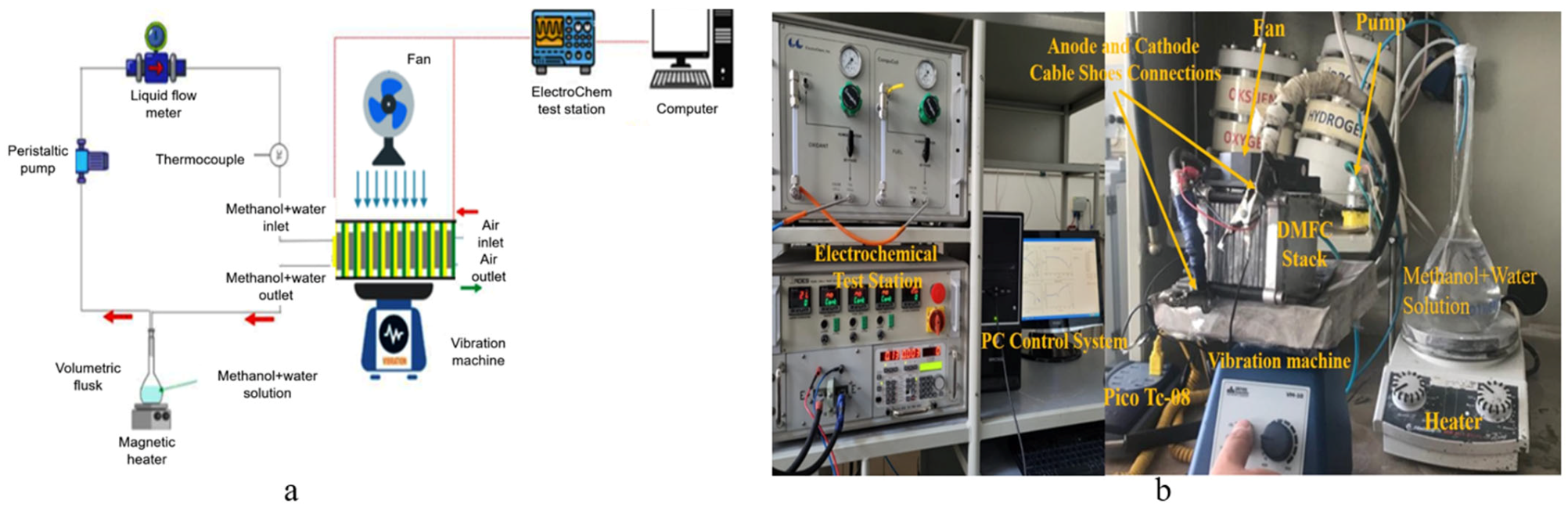
2.2. Experimental Setup and Test Conditions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OC-DMFC | Open cathode-direct methanol fuel cell |
| M | Molar concentration |
| Hz | Hertz |
| DEFC | Direct ethanol fuel cells |
| DMFC | Direct methanol fuel cells |
| FD | Frictional force |
| FSL | Shear force |
| BPs | Bipolar plates |
| MEA | Membrane electrode assembly |
| PEMFC | Proton exchange membrane fuel cells |
References
- Gray, P.G.; Petch, M.I. Advances with HotSpot fuel processing. Efficient hydrogen production for use with solid polymer fuel cells. Platin. Met. Rev. 2000, 44, 108–111. [Google Scholar] [CrossRef]
- Golunski, S. HotSpot™ fuel processor. Platin. Met. Rev. 1998, 42, 2–7. [Google Scholar] [CrossRef]
- Edwards, N.; Ellis, S.R.; Frost, J.C.; Golunski, S.E.; van Keulen, A.N.; Lindewald, N.G.; Reinkingh, J.G. On-board hydrogen generation for transport applications: The HotSpot™ methanol processor. J. Power Sources 1998, 71, 123–128. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells-fundamentals and applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Hogarth By, M.P.; Hards, G.A. Direct methanol fuel cells technological advances and further requirements. Platin. Met Rev. 1996, 40, 151–159. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Khanna, V.; Prakash, C. Energising the Future—Graphene in Fuel Cells and Beyond. In Graphene for Electrochemical Energy Storage: Energizing the Future; Springer: Cham, Switzerland, 2025; pp. 83–102. [Google Scholar]
- Divya, K.; Liu, H.; Zhang, W.; Xu, Q.; Su, H. Sulfonated poly (ether ether ketone)/MOF hybrid polymer electrolyte membrane with ultra--low methanol permeability for enhanced direct methanol fuel cell performance. J. Appl. Polym. Sci. 2024, 141, e55749. [Google Scholar] [CrossRef]
- Tariq, A.H.; Kazmi, S.A.A.; Hassan, M.; Ali, S.M.; Anwar, M. Analysis of fuel cell integration with hybrid microgrid systems for clean energy: A comparative review. Int. J. Hydrogen Energy 2024, 52, 1005–1034. [Google Scholar] [CrossRef]
- Xue, Y.; Chan, S. Layer-by-layer self-assembly of CHI/PVS–Nafion composite membrane for reduced methanol crossover and enhanced DMFC performance. Int. J. Hydrogen Energy 2015, 40, 1877–1885. [Google Scholar] [CrossRef]
- Das, H.S.; Mishra, S.; Roymahapatra, G. Advanced Nano-Structured Materials for Energy Storage Devices. In Design, Fabrication, and Significance of Advanced Nanostructured Materials; IGI Global: New York, NY, USA, 2025; pp. 1–34. [Google Scholar]
- Tong, Y.-C.; Wang, Q.-Y.; Hu, Y.-J.; Shi, Z.-J.; Zhang, K. The Size and Charge Effect of Pt Cluster on the Electrocatalytic Activity Toward the First Step of Dehydrogenation of Methanol. J. Electrochem. Energy Convers. Storage 2025, 22, 011006. [Google Scholar] [CrossRef]
- Kamarudin, S.; Daud, W.; Ho, S.; Hasran, U. Overview on the challenges and developments of micro-direct methanol fuel cells (DMFC). J. Power Sources 2007, 163, 743–754. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Basri, S.; Kamarudin, S.K.; Daud, W.R.W.; Yaakub, Z. Nanocatalyst for direct methanol fuel cell (DMFC). Int. J. Hydrogen Energy 2010, 35, 7957–7970. [Google Scholar] [CrossRef]
- Scott, K.; Shukla, A.K. Direct methanol fuel cells: Fundamentals, problems and perspectives. Mod. Asp. Electro-Chem. 2007, 40, 127–227. [Google Scholar]
- Boscolo, M. Analytical solution for free vibration analysis of composite plates with layer-wise displacement assumptions. Compos. Struct. 2013, 100, 493–510. [Google Scholar] [CrossRef]
- Ahmed, H.; Banan, R.; Zu, J.; Bazylak, A. Free vibration analysis of a polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 5520–5525. [Google Scholar] [CrossRef]
- Tseng, J.G.; Hsiao, D.R.; Huang, B.W. Dynamic Analysis of the Proton Exchange Membrane Fuel Cell. Appl. Mech. Mater. 2013, 284–287, 718. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, M.; Wu, S.; Tongsh, C.; Sun, X.; Wang, B.; Park, J.W.; Jiao, K. Investigation of mechanical vibration effect on proton exchange membrane fuel cell cold start. Int. J. Hydrogen Energy 2020, 45, 14528–14538. [Google Scholar] [CrossRef]
- Hosseinloo, A.H.; Ehteshami, M.M. Shock and vibration effects on performance reliability and mechanical integrity of proton exchange membrane fuel cells: A critical review and discussion. J. Power Sources 2017, 364, 367–373. [Google Scholar] [CrossRef]
- El-Emam, S.H.; Mousa, A.A.; Awad, M.M. Effects of stack orientation and vibration on the performance of PEM fuel cell. Int. J. Hydrogen Energy Res. 2015, 39, 75–83. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Wei, M.; Wu, C. Vibration mode analysis of the proton exchange membrane fuel cell stack. J. Power Sources 2016, 331, 299–307. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Pandian, S.; Dhathathreyan, K.S. Vibration tests on a PEM fuel cell stack usable in transportation application. Int. J. Hydrogen Energy 2009, 34, 3833–3837. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Nessim, G.D.; Kader, M.A. Introduction to direct methanol fuel cells. In Direct Methanol Fuel Cell Technology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 1–12. [Google Scholar]
- Charoen, K.; Prapainainar, C.; Sureeyatanapas, P.; Suwannaphisit, T.; Wongamornpitak, K.; Kongkachuichay, P.; Holmes, S.M.; Prapainainar, P. Application of response surface methodology to optimize direct alcohol fuel cell power density for greener energy production. J. Clean. Prod. 2017, 142, 1309–1320. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, H.; Tian, Z.; Zhu, J.; Zhu, J. Analysis of CO2 bubble growth detachment kinetics in direct methanol fuel cell flow channels. J. Power Sources 2024, 628, 235880. [Google Scholar] [CrossRef]
- Vasu, V.; Srinivasulu, G.; Rao, K.N. Investigation of CO2 bubble behavior and performance in air-breathing direct methanol fuel cells with spiral-patterned anode flow field. Therm. Sci. Eng. Prog. 2025, 59, 103346. [Google Scholar] [CrossRef]
- Chi, X.; Chen, F.; Mo, T.; Li, Y.; Wei, W. Improve methanol efficiency for direct methanol fuel cell system via investigation and control of optimal operating methanol concentration. Energy 2023, 290, 130147. [Google Scholar] [CrossRef]
- Zoheiry, E.; Radwan, M.; Ookawara, S.; Ahmed, M. Efficient fuel utilization by enhancing the under-rib mass transport using new serpentine flow field designs of direct methanol fuel cells. Energy Convers. Manag. 2017, 144, 88–103. [Google Scholar] [CrossRef]
- Çelik, S.; Yagız, M.; Atalmis, G. Experimental improvement of the performance of the open cathode-direct methanol fuel cell stack by magnetic field effect. Int. J. Hydrogen Energy 2024, 50, 32–40. [Google Scholar] [CrossRef]
- Oliveira, V.; Rangel, C.; Pinto, A. Effect of anode and cathode flow field design on the performance of a direct methanol fuel cell. Chem. Eng. J. 2010, 157, 174–180. [Google Scholar] [CrossRef]
- Yuan, Z.; Fu, W.; Zhao, Y.; Li, Z.; Zhang, Y.; Liu, X. Investigation of μDMFC (micro direct methanol fuel cell) with self-adaptive flow rate. Energy 2013, 55, 1152–1158. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, T.S., Q.; Ye, Q. In situ visualization study of CO2 gas bubble behavior in DMFC anode flow fields. J. Power Sources 2005, 139, 79–90. [Google Scholar] [CrossRef]
- Liu, J.X.; Guo, H.; Ye, F.; Qiu, D.C.; Ma, C.-F. Interfacial phenomena and heat transfer in proton exchange membrane fuel cells. Interfacial Phenom. Heat Transf. 2015, 3, 259–301. [Google Scholar] [CrossRef]
- Pei, F.; Ouyang, Y. Fuel cell electric performance and gas tightness attenuation under influence of enhanced road vibration spectrum. Energy Storage Sci. Technol. 2021, 10, 714. [Google Scholar]
- Hou, Y.; Hao, D.; Shen, C.; Shao, Z. Experimental investigation of the steady-state efficiency of fuel cell stack under strengthened road vibrating condition. Int. J. Hydrogen Energy 2013, 38, 3767–3772. [Google Scholar] [CrossRef]
- Zhang, L.J.; Si, Y.; Yu, Z. Investigation into road simulation experiment of powertrain and its key components of a fuel cell passenger car. J. Tongji Univ. 2009, 37, 244–248. [Google Scholar]
- Breziner, L.; Strahs, P.; Hutapea, P. Effect of vibration on the liquid water transport of pem fuel cel In ASME. Int. Mech. Eng. Congr. Expo. 2009, 43796, 17–22. [Google Scholar]
- Popovici, O.D.; Tataru, M.B.; Hathazi, F.I.; Popovici, D.M. The behaviour of the Direct Methanol Fuel Cell under low frequency acoustic vibrations. In Proceedings of the 13th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 11–12 June 2015; pp. 1–4. [Google Scholar]
- Zhang, Y.; Hao, D.; Wang, R.; Hou, Y. Research on torque characteristics of clamping bolts for PEMFC Stack under strengthened durability vibration. J. Phys. Conf. Ser. 2020, 1549, 032149. [Google Scholar] [CrossRef]




| Operational Parameters | Results |
|---|---|
| Methanol flow rate | 1, 5, 25, 50 mL min−1 |
| Vibration frequency | 15, 30, and 60 Hz |
| Methanol fuel test temperature | 70 °C |
| Methanol concentration | 1 M |
| Air flow rate | 2.4, 4.8, and 7.2 m/s |
| Air temperature | 21 °C (Ambient air) |
| Vibration power | 0.160–1 mW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, S.; Atalmis Sari, G.; Yagiz, M.; Özcan, H.; Amini Horri, B. Effect of Vibration on Open-Cathode Direct Methanol Fuel Cell Stack Performance. Physchem 2025, 5, 44. https://doi.org/10.3390/physchem5040044
Celik S, Atalmis Sari G, Yagiz M, Özcan H, Amini Horri B. Effect of Vibration on Open-Cathode Direct Methanol Fuel Cell Stack Performance. Physchem. 2025; 5(4):44. https://doi.org/10.3390/physchem5040044
Chicago/Turabian StyleCelik, Selahattin, Gamze Atalmis Sari, Mikail Yagiz, Hasan Özcan, and Bahman Amini Horri. 2025. "Effect of Vibration on Open-Cathode Direct Methanol Fuel Cell Stack Performance" Physchem 5, no. 4: 44. https://doi.org/10.3390/physchem5040044
APA StyleCelik, S., Atalmis Sari, G., Yagiz, M., Özcan, H., & Amini Horri, B. (2025). Effect of Vibration on Open-Cathode Direct Methanol Fuel Cell Stack Performance. Physchem, 5(4), 44. https://doi.org/10.3390/physchem5040044








