Green Synthesis and Characterization of Fe3O4 and ε-Fe2O3 Nanoparticles Using Apricot Kernel Shell Extract and Study of Their Optical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection and Preparation of Plant Material for Apricot Kernel Shell Extraction
2.3. Synthesis of ε-Fe2O3 NPs
2.4. Synthesis of Fe3O4 NPs
3. Characterizations
4. Results
4.1. HPLC Analysis of Apricot Kernel Peel Extract
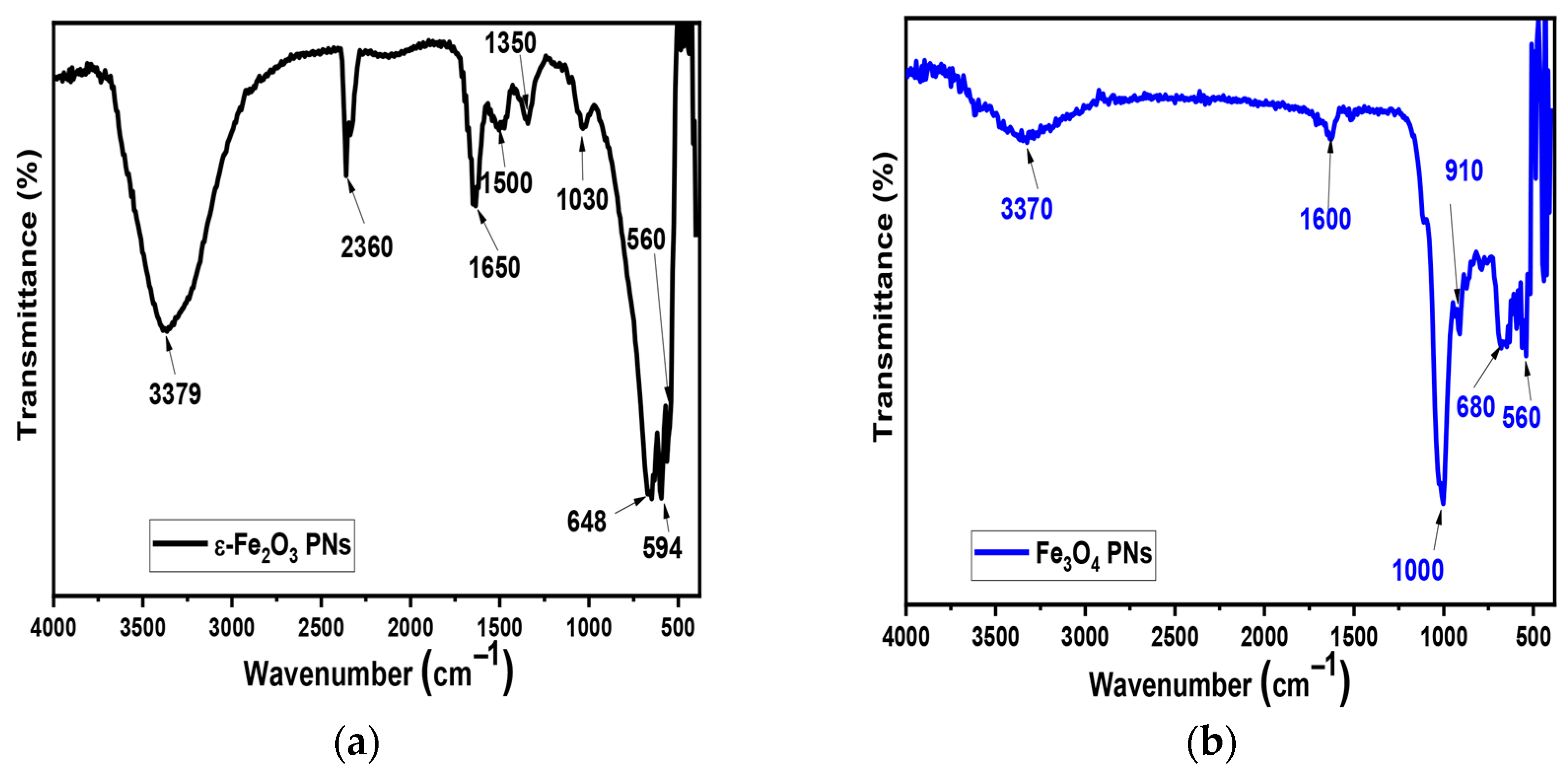
4.2. FTIR Analysis
4.3. XRD Analysis
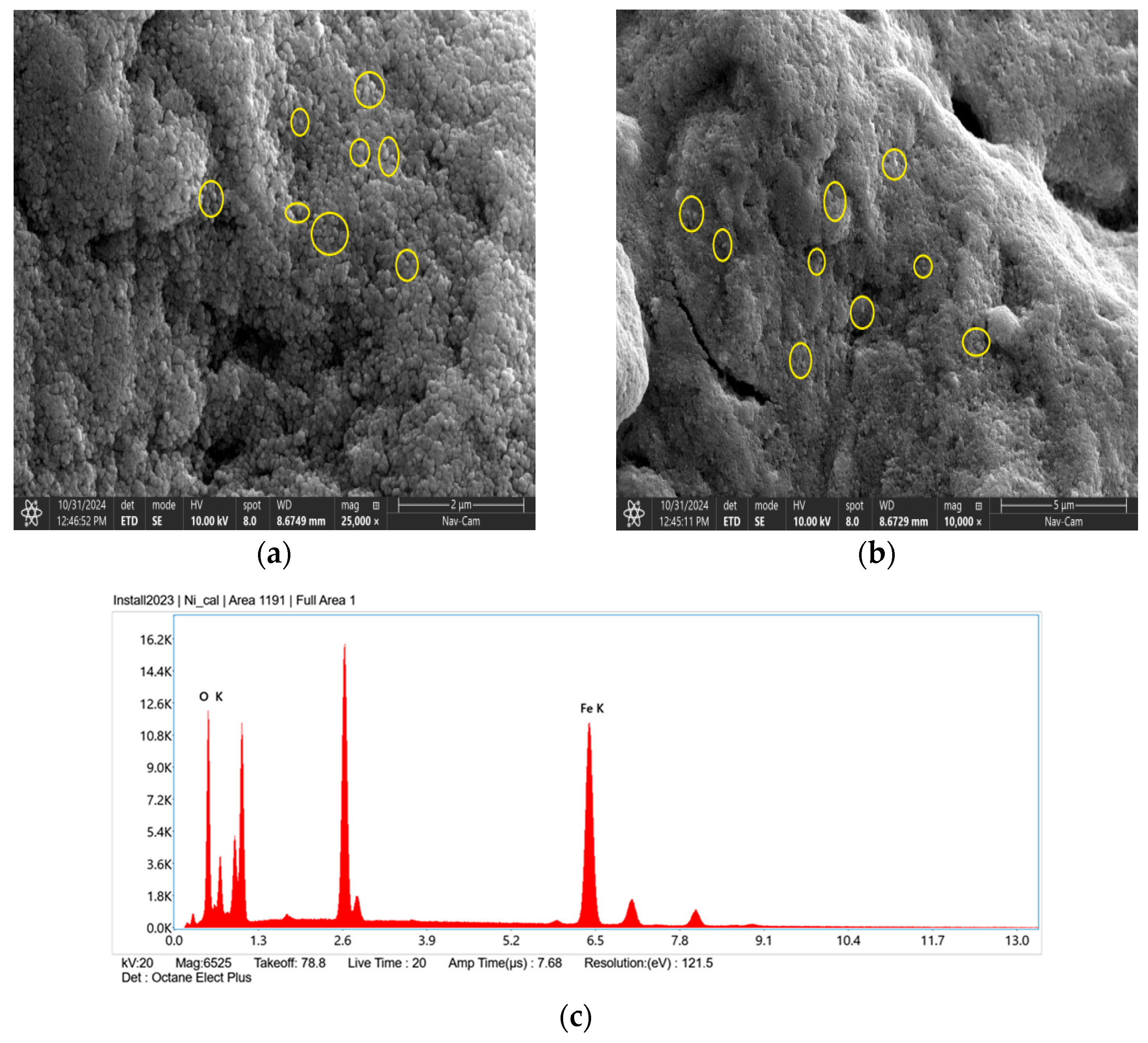
4.4. Morphological Characteristics and Elemental Composition
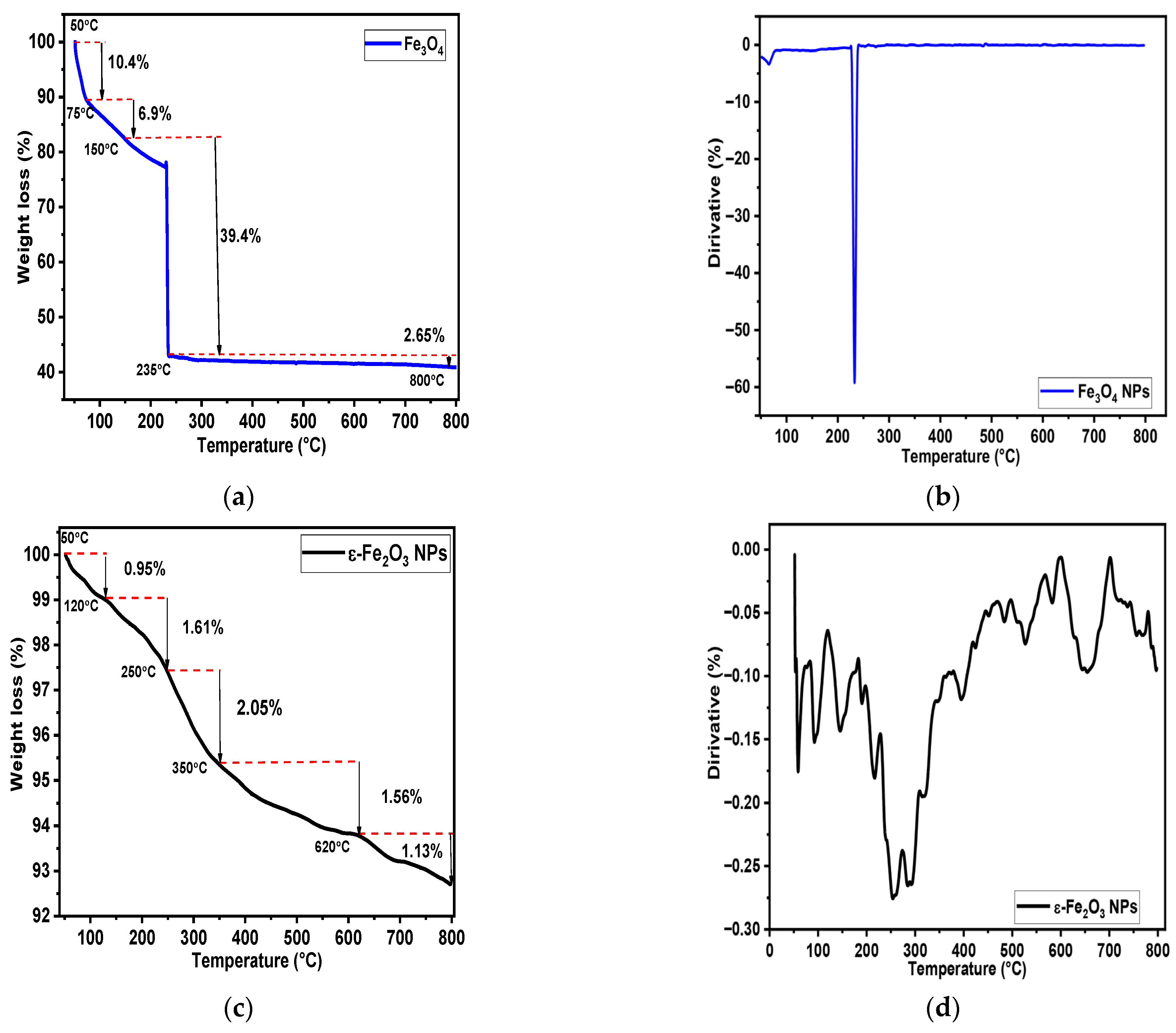
4.5. Thermal Properties
5. Optical Properties
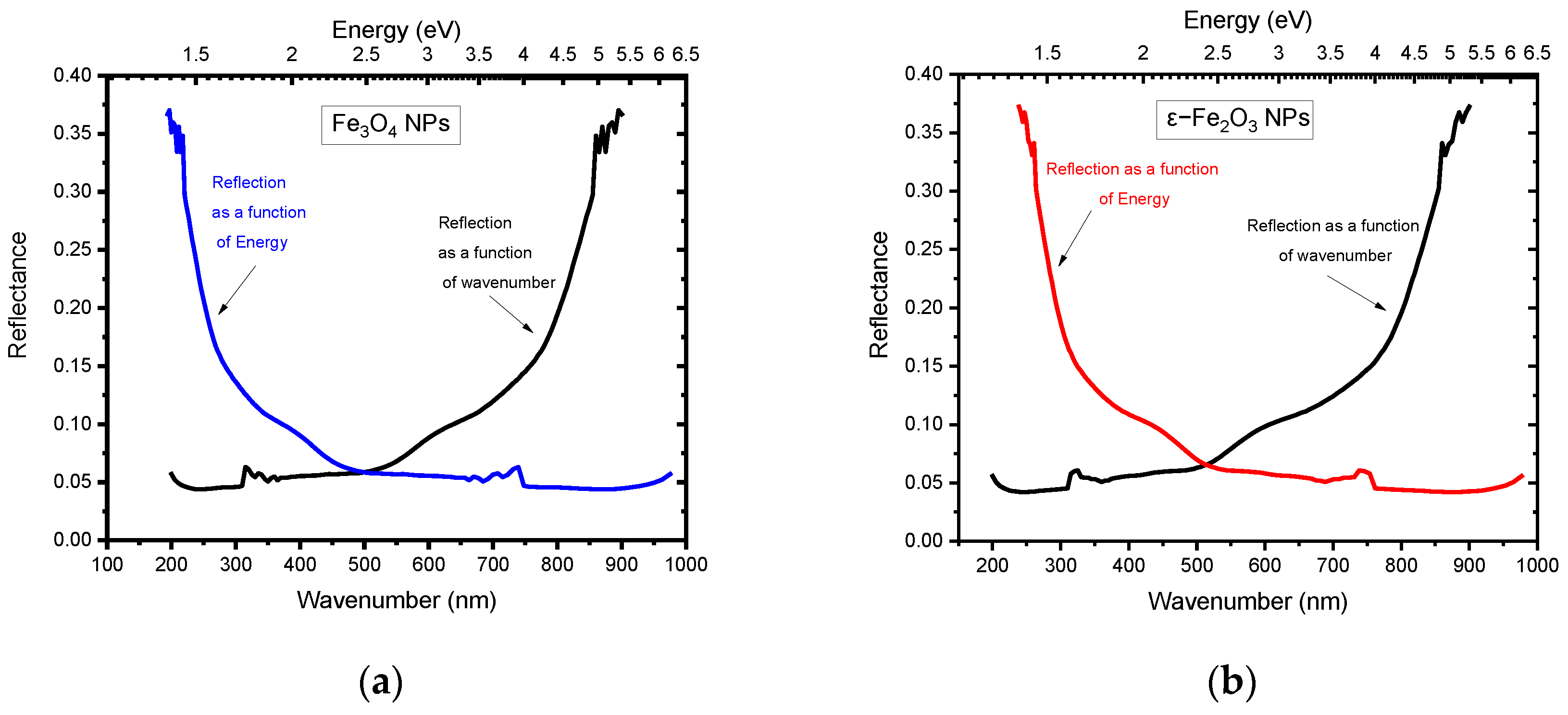
5.1. Reflectance
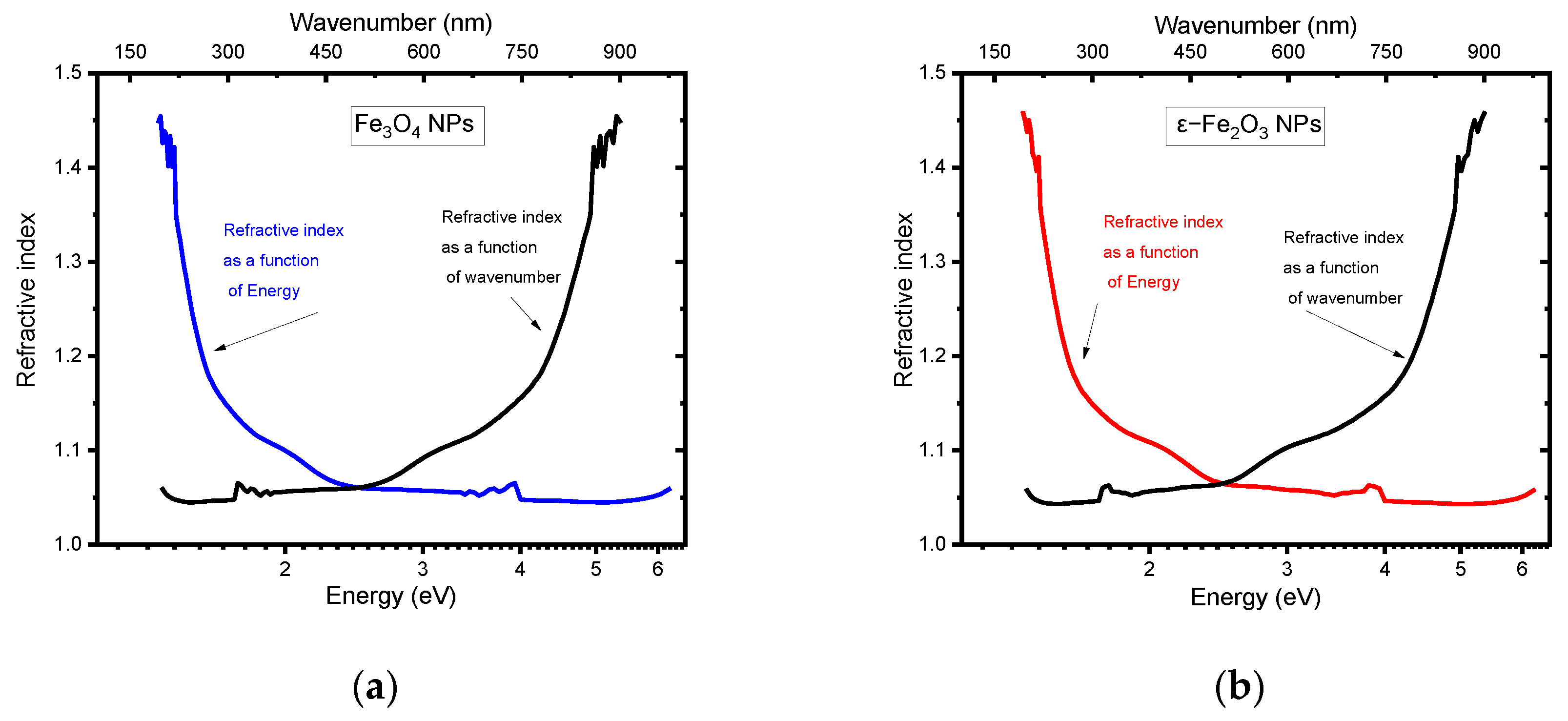
5.2. Refractive Index
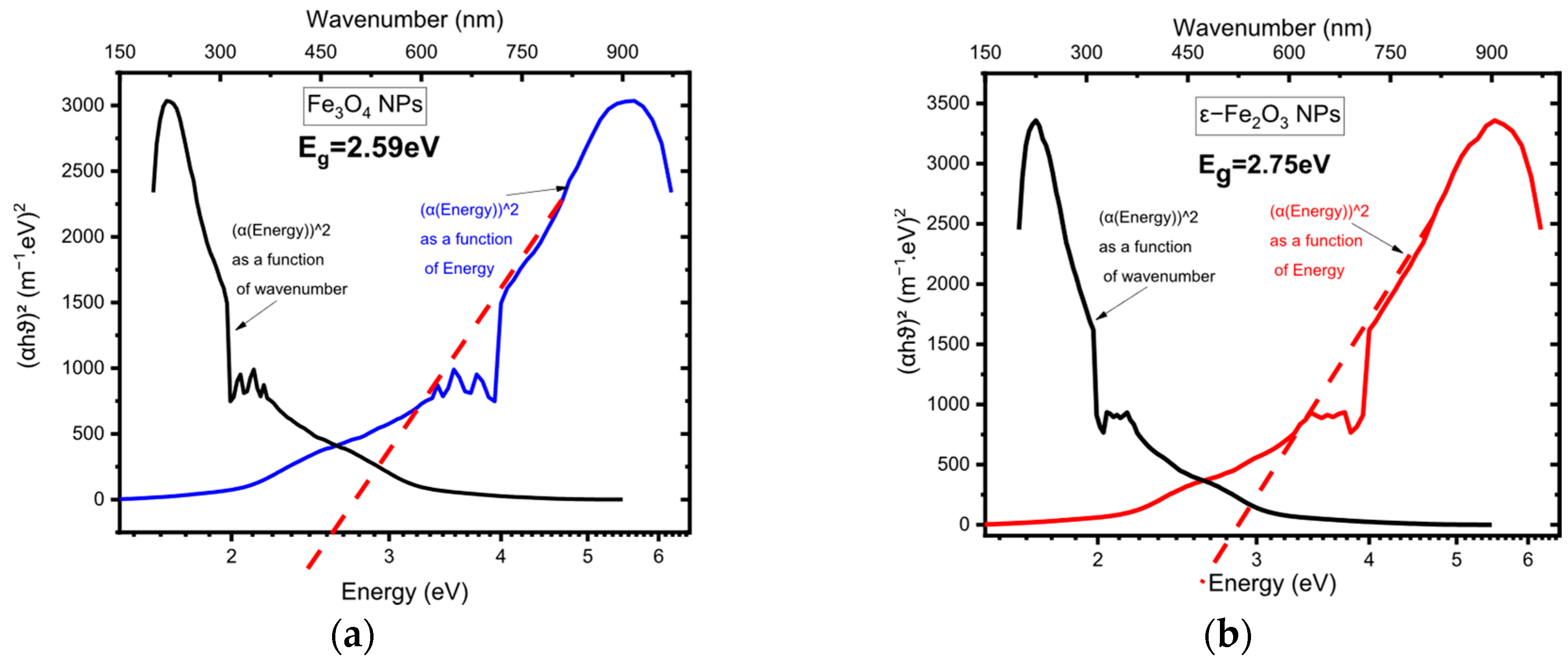
5.3. Band Gap
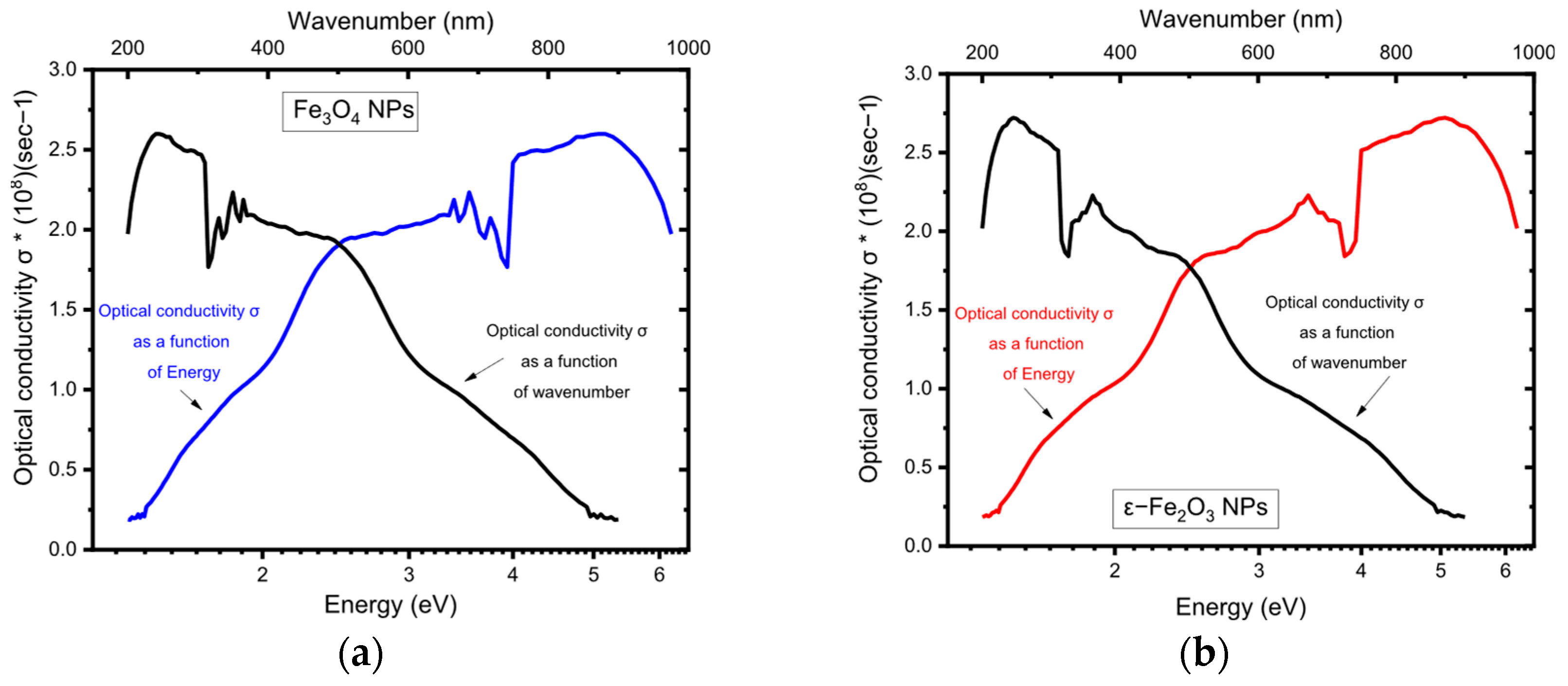
5.4. Optical Conductivity
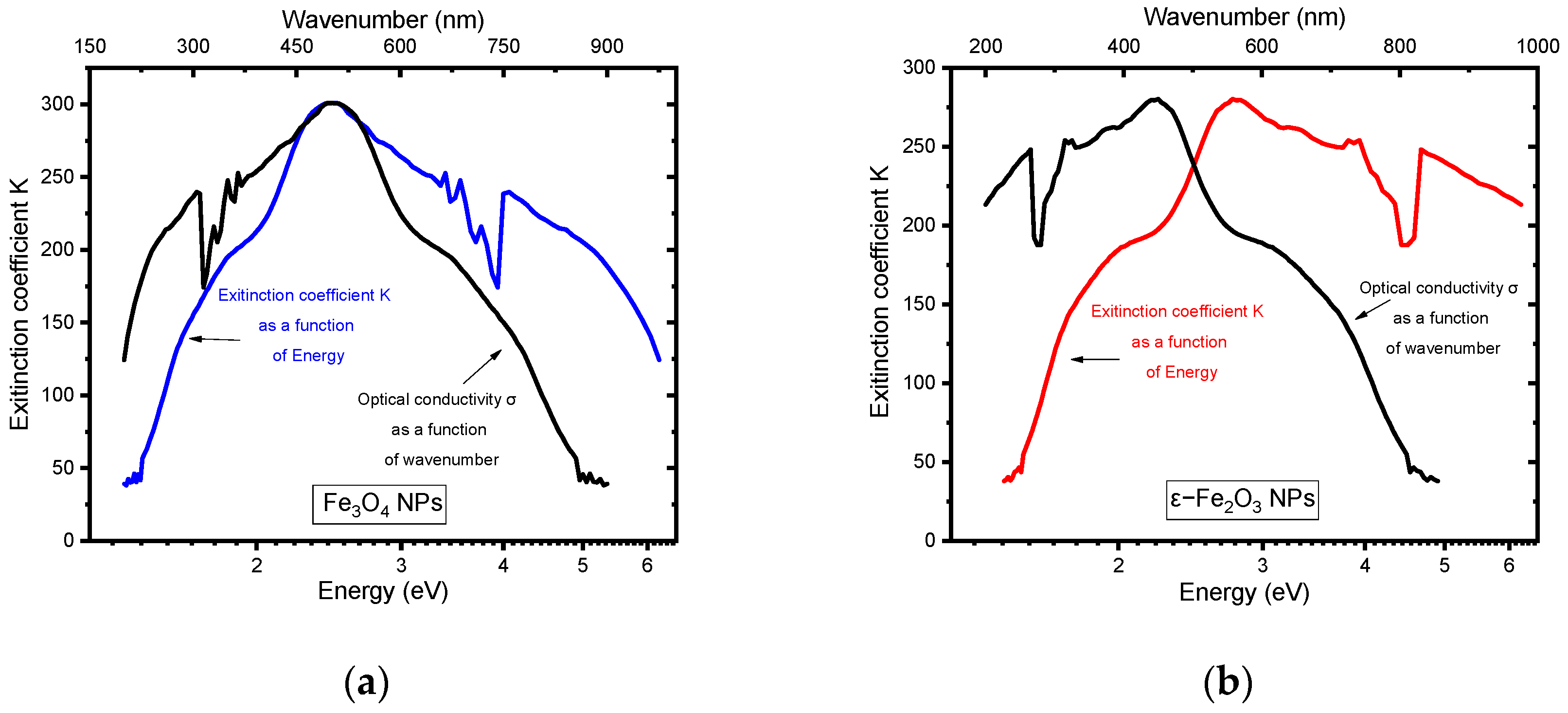
5.5. The Extinction Coefficient
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Fe3O4 NPs | Iron oxide nanoparticles |
| ε-Fe2O3 NPs | Iron oxide nanoparticles |
| Akse | Apricot kernel shell extract |
References
- Indrayana, I.; Tuny, M.; Putra, R.; Istiqomah, N.; Suharyadi, E. Optical Properties of Fe3O4/Chitosan and Its Applications for Signal Amplifier in Surface Plasmon Resonance Sensor. In Proceedings of the 2nd International Conference on Science, Technology, and Modern Society (ICSTMS 2020), Langsa, Indonesia, 25–26 November 2021; Atlantis Press: Dordrecht, The Netherlands; pp. 424–429. [Google Scholar]
- Zheng, Y.-H.; Cheng, Y.; Bao, F.; Wang, Y.-S. Synthesis and magnetic properties of Fe3O4 nanoparticles. Mater. Res. Bull. 2006, 41, 525–529. [Google Scholar] [CrossRef]
- Kind, H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire ultraviolet photodetectors and optical switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, A.; Gupta, S.K.; Rajput, P.; Singh, A. A facile route to synthesis of ferromagnetic and antiferromagnetic phases of iron oxide nanoparticles by controlled heat treatment of ferritin. J. Supercond. Novel Magn. 2020, 33, 3841–3852. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Sirivat, A.; Paradee, N. Facile synthesis of gelatin-coated Fe3O4 nanoparticles: Effect of pH in single-step co-precipitation for cancer drug loading. Mater. Des. 2019, 181, 107942. [Google Scholar] [CrossRef]
- Tipsawat, P.; Wongpratat, U.; Phumying, S.; Chanlek, N.; Chokprasombat, K.; Maensiri, S. Magnetite (Fe3O4) nanoparticles: Synthesis, characterization and electrochemical properties. Appl. Surf. Sci. 2018, 446, 287–292. [Google Scholar] [CrossRef]
- Oktivina, M.; Nurrohman, D.; Rinto, A.; Suharyadi, E.; Abraha, K. Effect of Fe3O4 magnetic nanoparticle concentration on the signal of surface plasmon resonance (SPR) spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012032. [Google Scholar] [CrossRef]
- Thu, N.T.A.; Duc, H.V.; Hai Phong, N.; Cuong, N.D.; Hoan, N.T.V.; Quang Khieu, D. Electrochemical determination of paracetamol using Fe3O4/reduced graphene-oxide-based electrode. J. Nanomater. 2018, 2018, 7619419. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Singh, A. Synthesis and characterization of iron oxide nanoparticles (Fe2O3, Fe3O4): A brief review. Contemp. Phys. 2021, 62, 144–164. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Huang, W.; Yao, A.; Kamitakahara, M.; Ioku, K. Preparation of magnetite nanoparticles coated with silica via a sol-gel approach. J. Ceram. Soc. Jpn. 2007, 115, 877–881. [Google Scholar] [CrossRef]
- Xu, H.; Cui, L.; Tong, N.; Gu, H. Development of high magnetization Fe3O4/polystyrene/silica nanospheres via combined miniemulsion/emulsion polymerization. J. Am. Chem. Soc. 2006, 128, 15582–15583. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Kamada, K.; Enomoto, N.; Hojo, J.; Enpuku, K. Sonochemical synthesis of the magnetite nanoparticles in aqueous solution. J. Ceram. Soc. Jpn. 2007, 115, 867–872. [Google Scholar] [CrossRef][Green Version]
- Suh, W.H.; Suslick, K.S. Magnetic and porous nanospheres from ultrasonic spray pyrolysis. J. Am. Chem. Soc. 2005, 127, 12007–12010. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Z.; Hong, Y.C.; Uhm, H.S.; Li, Z.K. Synthesis of nanocrystalline iron oxide particles by microwave plasma jet at atmospheric pressure. Jpn. J. Appl. Phys. 2004, 43, 7714. [Google Scholar] [CrossRef]
- Grabis, J.; Heidemane, G.; Rašmane, D. Preparation of Fe3O4 and γ-Fe2O3 nanoparticles by liquid and gas phase processes. Mater. Sci. 2008, 14, 292–295. [Google Scholar]
- Mohammed, A.M.; Saud, W.; Ali, M. Green synthesis of Fe2O3 nanoparticles using Olea europaea leaf extract and their antibacterial activity. Dig. J. Nanomater. Biostruct 2020, 15, 175–183. [Google Scholar] [CrossRef]
- Abd, A.N.; Latif, D.M.; Wasna’a, M.A. Synthesis and some physical properties of magnetite (Fe3O4) NPs. Synthesis 2016, 2, 341–345. [Google Scholar]
- Corbett, D.B.; Kohan, N.; Machado, G.; Jing, C.; Nagardeolekar, A.; Bujanovic, B.M. Chemical composition of apricot pit shells and effect of hot-water extraction. Energies 2015, 8, 9640–9654. [Google Scholar] [CrossRef]
- Amidon, T.E.; Bujanovic, B.; Liu, S.; Howard, J.R. Commercializing biorefinery technology: A case for the multi-product pathway to a viable biorefinery. Forests 2011, 2, 929–947. [Google Scholar] [CrossRef]
- Lin, H. The Role of Green Chemistry in Biomass Processing and Conversion; Xie, H., Gathergood, N., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gong, C.; Bujanović, B.M. Impact of hot-water extraction on acetone–water oxygen delignification of Paulownia spp. and lignin recovery. Energies 2014, 7, 857–873. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Soflaee, F. Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-precipitation method. Phys. Chem. Res. 2015, 3, 191–196. [Google Scholar]
- Bertolucci, E.; Galletti, A.M.R.; Antonetti, C.; Marracci, M.; Tellini, B.; Piccinelli, F.; Visone, C. Chemical and magnetic properties characterization of magnetic nanoparticles. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings, Pisa, Italy, 11–14 May 2015; pp. 1492–1496. [Google Scholar]
- Manikandan, A.; Vijaya, J.J.; Mary, J.A.; Kennedy, L.J.; Dinesh, A. Structural, optical and magnetic properties of Fe3O4 nanoparticles prepared by a facile microwave combustion method. J. Ind. Eng. Chem. 2014, 20, 2077–2085. [Google Scholar] [CrossRef]
- Horia, F.; Easawi, K.; Khalil, R.; Abdallah, S.; El-Mansy, M.; Negm, S. Optical and thermophysical characterization of Fe3O4 nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 956, 012016. [Google Scholar] [CrossRef]
- Pandey, B.K.; Shahi, A.K.; Shah, J.; Kotnala, R.K.; Gopal, R. Optical and magnetic properties of Fe2O3 nanoparticles synthesized by laser ablation/fragmentation technique in different liquid media. Appl. Surf. Sci. 2014, 2, 462–471. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Tsedenbal, B.; Hussain, I.; Anwar, M.S.; Koo, B.H. Morphological, magnetic and optical properties of α-Fe2O3 nanoflowers. J. Nanosci. Nanotechnol. 2018, 18, 6127–6132. [Google Scholar] [CrossRef]


| Chemical Compounds of the Apricot Kernel Shells | Percentage |
|---|---|
| ascorbic acid (C6H8O6) | 0.50% |
| pyrocatechol (C6H6O2) | 0.36% |
| salicin (C13H18O7) | 0.34% |
| esculin (C15H16O9) | 0.30% |
| caffeine (C8H10N4O2) | 0.36% |
| vanillic acid (C8H8O4) | 2.67% |
| rutin (C27H30O16) | 0.56% |
| vanillin (C8H8O3) | 0.30% |
| caffeic acid (C9H8O4) | 1.10% |
| salicylic acid (C7H6O3) | 0.34% |
| enamic acid (C9H8O2) | 1.60% |
| Property | ε-Fe2O3 NPs | Fe3O4 NPs | ||
|---|---|---|---|---|
| T (°C) | Mass Loss (%) | T (°C) | Mass Loss (%) | |
| 1st weight loss | 50−120 | 0.95 | 50−75 | 10.4 |
| 2nd weight loss | 120−250 | 1.61 | 75−150 | 6.9 |
| 3rd weight loss | 250−350 | 2.05 | 150−235 | 39.4 |
| 4rd weight loss | 350−620 | 1.56 | 235−800 | 2.65 |
| 5rd weight loss | 620−800 | 1.13 | / | / |
| Residual at 800 °C | 800 | 92.7 | 800 | 40.65 |
| Optical Properties | ε-Fe2O3 NPs | Fe3O4 NPs | Literature References |
|---|---|---|---|
| Constant reflectance (R) | 0.05 to a maximum of 0.37 | 0.05 To a maximum of 0.37 | Fe3O4 Rmax = 0.2 [20] |
| Refractive index (n) | 1.05 to a maximum of 1.45 | 1.05 to a maximum of 1.45 | Fe3O4 nmax = 1.5 [20] |
| Band gap Eg (eV) | 2.75 | 2.56 | Fe3O4 2.2 [20] Fe3O4 2.51−3.01 [30] α-Fe2O3 1.94−2.27 [31] |
| Optical conductivity σ (eV) × 108 | 1.35 to a maximum of 6.22 | 1.37 to a maximum of 6.2 | Fe3O4 σ (eV)max = 60 [20] |
| Extinction coefficient (k) | 37 to a maximum of 280 | 39 to a maximum of 300 | 0.5 to a maximum of 0.65 [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Kouider, T.; Souli, L.; Derouiche, Y.; Soltani, T.; Maschke, U. Green Synthesis and Characterization of Fe3O4 and ε-Fe2O3 Nanoparticles Using Apricot Kernel Shell Extract and Study of Their Optical Properties. Physchem 2025, 5, 33. https://doi.org/10.3390/physchem5030033
Ben Kouider T, Souli L, Derouiche Y, Soltani T, Maschke U. Green Synthesis and Characterization of Fe3O4 and ε-Fe2O3 Nanoparticles Using Apricot Kernel Shell Extract and Study of Their Optical Properties. Physchem. 2025; 5(3):33. https://doi.org/10.3390/physchem5030033
Chicago/Turabian StyleBen Kouider, Tayeb, Lahcene Souli, Yazid Derouiche, Taoufik Soltani, and Ulrich Maschke. 2025. "Green Synthesis and Characterization of Fe3O4 and ε-Fe2O3 Nanoparticles Using Apricot Kernel Shell Extract and Study of Their Optical Properties" Physchem 5, no. 3: 33. https://doi.org/10.3390/physchem5030033
APA StyleBen Kouider, T., Souli, L., Derouiche, Y., Soltani, T., & Maschke, U. (2025). Green Synthesis and Characterization of Fe3O4 and ε-Fe2O3 Nanoparticles Using Apricot Kernel Shell Extract and Study of Their Optical Properties. Physchem, 5(3), 33. https://doi.org/10.3390/physchem5030033








