Reversible Thermochemical Routes for Carbon Neutrality: A Review of CO2 Methanation and Steam Methane Reforming
Abstract
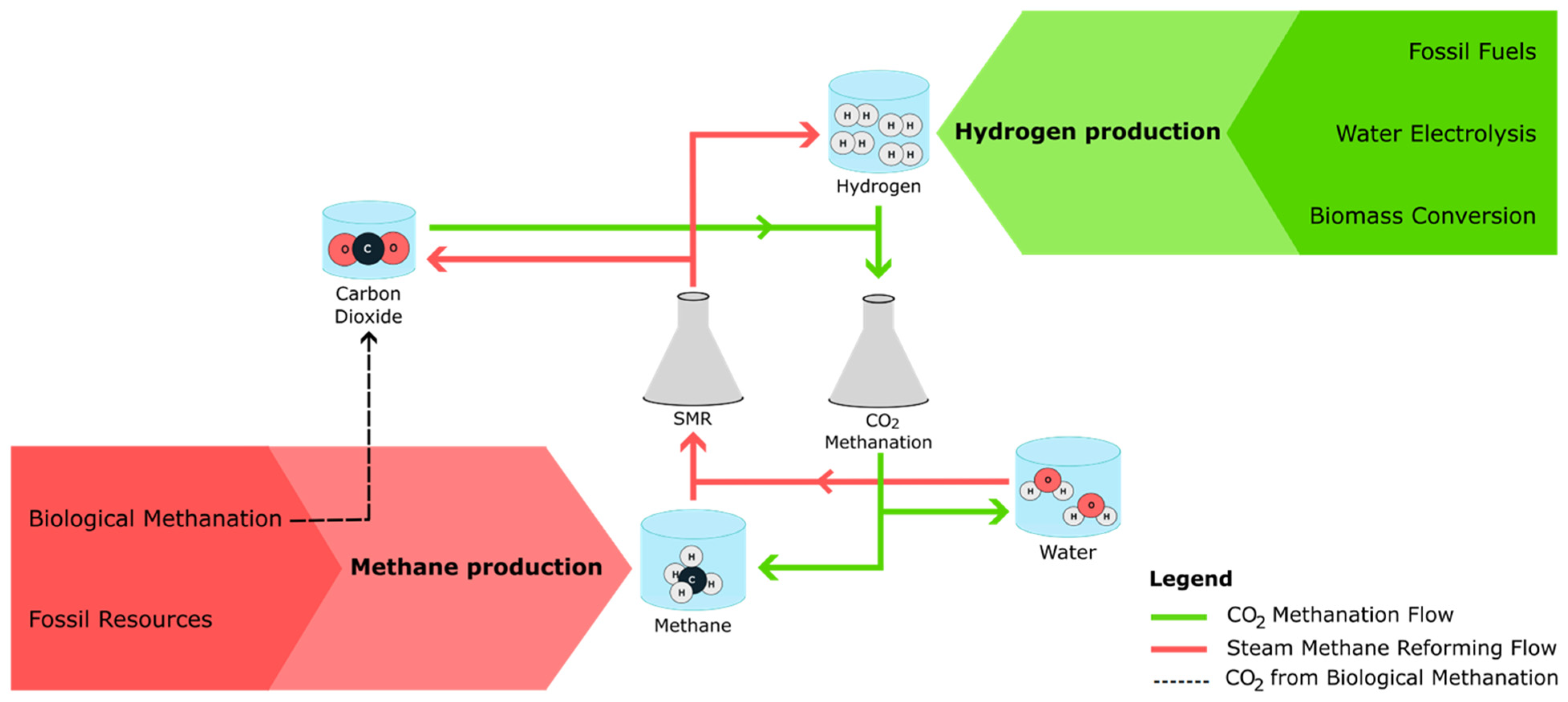
1. Introduction
1.1. Hydrogen Production Processes
1.2. Methane Production Processes
2. Methane Production from Carbon Dioxide Methanation
2.1. Operational Conditions Effects
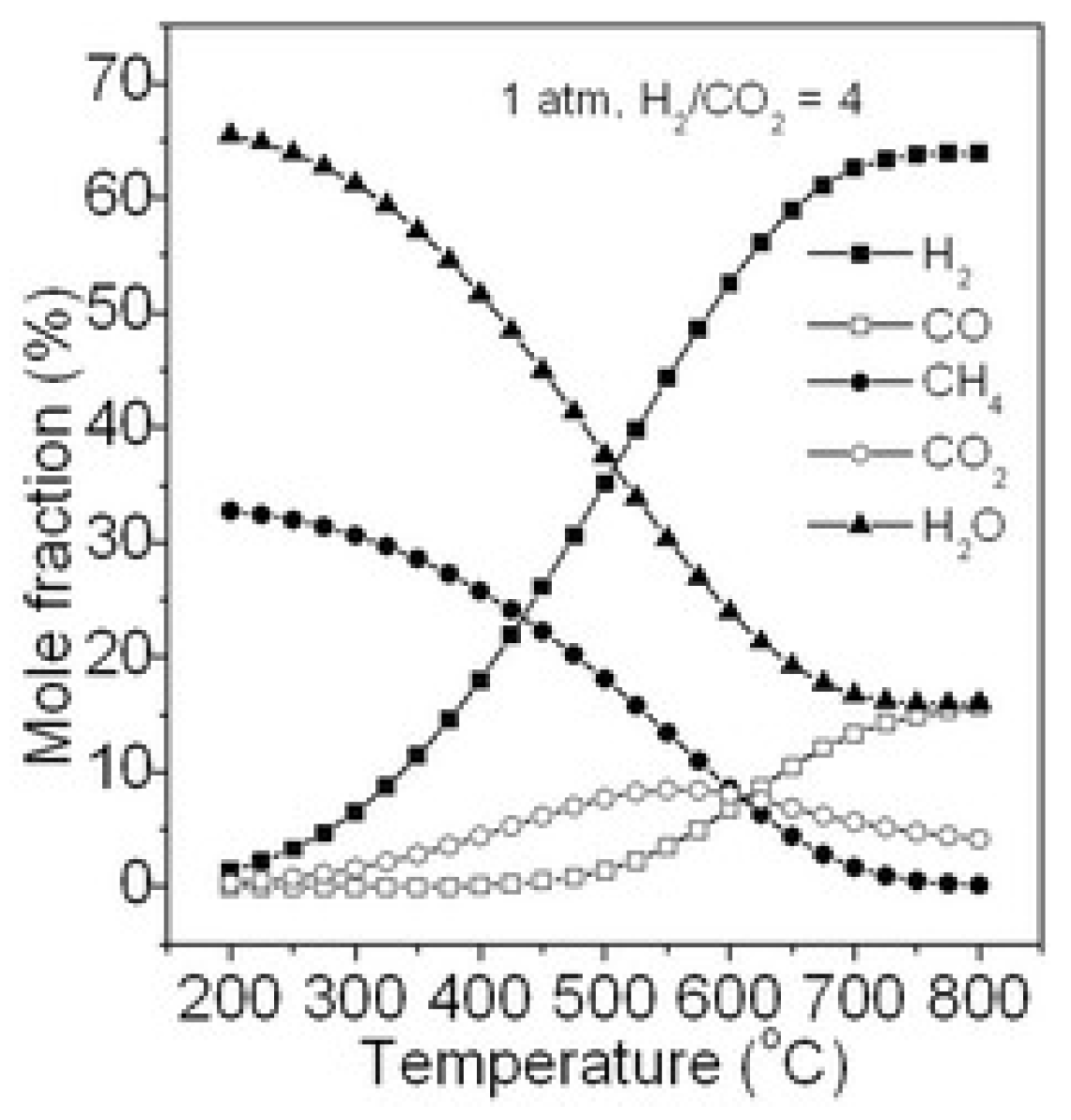
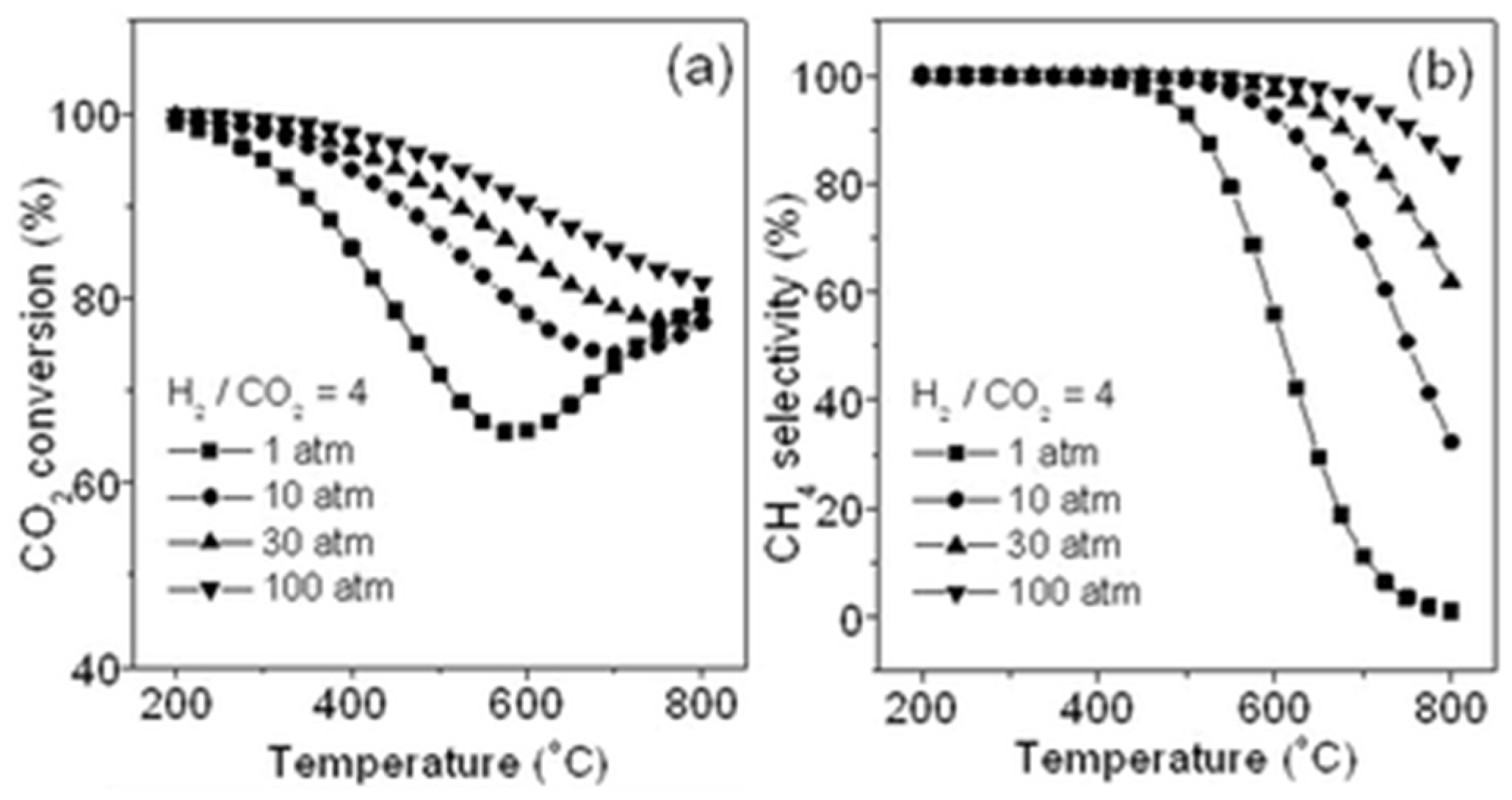
2.1.1. Effect of Temperature and Pressure
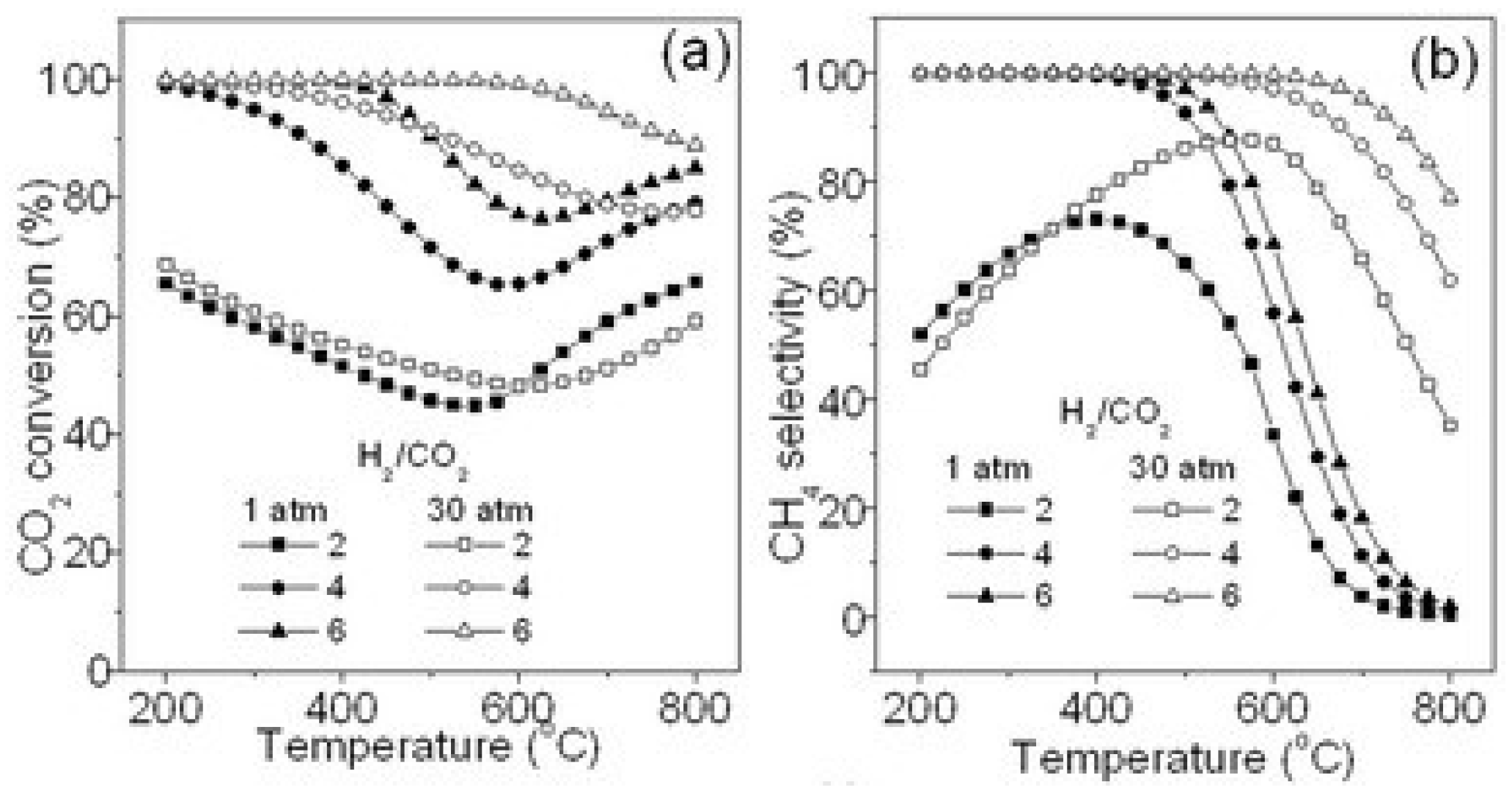
2.1.2. Effect of the H2/CO2 Ratio
2.1.3. Effect of Adding H2O
2.2. CO2 Methanation Catalysts
2.2.1. Comparative Performance of Metal Catalysts for CO2 Methanation
2.2.2. Ru-Based Catalysis
2.2.3. Ni-Based Catalysts
2.3. CO2 Methanation Environmental Impact
2.4. CO2 Methanation Process Costs
2.5. Discussion on CO2 Methanation
3. Hydrogen Production from Steam Methane Reforming Process
3.1. Steam Methane Reforming Catalysts
3.2. Steam Methane Reforming Technologies
3.2.1. Sorption-Enhanced Steam Methane Reforming
3.2.2. Membrane Reactors
3.2.3. Wall Coating Steam Methane Reformers
3.2.4. Electrified Methane Reforming
3.2.5. Chemical Looping Reforming
3.2.6. Solar-Assisted Steam Methane Reforming
3.3. Steam Methane Reforming Environmental Impact
3.4. Steam Methane Reforming Process Costs
3.5. Discussion on Steam Methane Reforming
4. Comparative Analysis of CO2 Methanation and SMR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Parliament Climate Change in Europe: Facts and Figures. Available online: https://www.europarl.europa.eu/topics/en/article/20180703STO07123/climate-change-in-europe-facts-and-figures (accessed on 19 May 2025).
- European Parliament Green Deal: Key to Climate-Neutral and Sustainable EU. Available online: https://www.europarl.europa.eu/topics/en/article/20200618STO81513/green-deal-key-to-a-climate-neutral-and-sustainable-eu (accessed on 13 May 2025).
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent Trend in Thermal Catalytic Low Temperature CO2 Methanation: A Critical Review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, W.; Song, M.; Wu, X.; Li, G.; Hu, C. Research Progress and Reaction Mechanism of CO2 Methanation over Ni-Based Catalysts at Low Temperature: A Review. Catalysts 2022, 12, 244. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.-Z. Power-to-Methane: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Advances in Hydrogen Production from Natural Gas Reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Dutta, S. Review and Outlook of Hydrogen Production through Catalytic Processes. Energy Fuels 2024, 38, 2601–2629. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Umar, M.; Kotob, E.; Abdulhakam, A.; Taialla, O.A.; Awad, M.M.; Hussain, I.; Alhooshani, K.R.; Ganiyu, S.A. Recent Advances in Bimetallic Catalysts for Methane Steam Reforming in Hydrogen Production: Current Trends, Challenges, and Future Prospects. Chem. Asian J. 2024, 19, 202300641. [Google Scholar] [CrossRef] [PubMed]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on Methane Steam Reforming to Produce Hydrogen through Membrane Reactors Technology: A Review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Habib, M.A.; Harale, A.; Paglieri, S.; Alrashed, F.S.; Al-Sayoud, A.; Rao, M.V.; Nemitallah, M.A.; Hossain, S.; Hussien, M.; Ali, A.; et al. Palladium-Alloy Membrane Reactors for Fuel Reforming and Hydrogen Production: A Review. Energy Fuels 2021, 35, 5558–5593. [Google Scholar] [CrossRef]
- Trimm, D.L. Coke Formation and Minimisation during Steam Reforming Reactions. Catal. Today 1997, 37, 233–238. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.-H.; Lee, H.; Seo, J.-C.; Lee, K. Effect of Mg Contents on Catalytic Activity and Coke Formation of Mesoporous Ni/Mg-Aluminate Spinel Catalyst for Steam Methane Reforming. Catalysts 2020, 10, 828. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Abate, S.; Perathoner, S.; Centi, G.; Palkovits, R. CO2 Methanation: Principles and Challenges. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–103. [Google Scholar]
- Djaidja, A.; Messaoudi, H.; Kaddeche, D.; Barama, A. Study of Ni–M/MgO and Ni–M–Mg/Al (M=Fe or Cu) Catalysts in the CH4–CO2 and CH4–H2O Reforming. Int. J. Hydrogen Energy 2015, 40, 4989–4995. [Google Scholar] [CrossRef]
- Lopez Ortiz, A.; Harrison, D.P. Hydrogen Production Using Sorption-Enhanced Reaction. Ind. Eng. Chem. Res. 2001, 40, 5102–5109. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent Advancement and Assessment of Green Hydrogen Production Technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Tahmasbi, M.; Siavashi, M.; Ahmadi, R. A Comprehensive Review of Hydrogen Production and Storage Methods: Fundamentals, Advances, and SWOT Analysis. Energy Convers. Manag. X 2025, 26, 101005. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Aravindan, M.; Madhan Kumar, V.; Hariharan, V.S.; Narahari, T.; Arun Kumar, P.; Madhesh, K.; Praveen Kumar, G.; Prabakaran, R. Fuelling the Future: A Review of Non-Renewable Hydrogen Production and Storage Techniques. Renew. Sustain. Energy Rev. 2023, 188, 113791. [Google Scholar] [CrossRef]
- Usman, M.; Podila, S.; Alamoudi, M.A.; Al-Zahrani, A.A. Current Research Status and Future Perspective of Ni- and Ru-Based Catalysts for CO2 Methanation. Catalysts 2025, 15, 203. [Google Scholar] [CrossRef]
- Chatzis, A.; Gkotsis, P.; Zouboulis, A. Biological Methanation (BM): A State-of-the-Art Review on Recent Research Advancements and Practical Implementation in Full-Scale BM Units. Energy Convers. Manag. 2024, 314, 118733. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A Technological and Economic Review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Ghaib, K.; Nitz, K.; Ben-Fares, F. Chemical Methanation of CO2: A Review. ChemBioEng Rev. 2016, 3, 266–275. [Google Scholar] [CrossRef]
- Miguel, C.V.; Soria, M.A.; Mendes, A.; Madeira, L.M. Direct CO2 Hydrogenation to Methane or Methanol from Post-Combustion Exhaust Streams—A Thermodynamic Study. J. Nat. Gas Sci. Eng. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A Thermodynamic Analysis of Methanation Reactions of Carbon Oxides for the Production of Synthetic Natural Gas. RSC Adv. 2012, 2, 2358. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Roger, A.-C. Methanation of Carbon Dioxide over Nickel-Based Ce0.72Zr0.28O2 Mixed Oxide Catalysts Prepared by Sol–Gel Method. Appl. Catal. A Gen. 2009, 369, 90–96. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further Insight into the Mechanism over Rh/γ-Al2O3 Catalyst. Appl. Catal. B 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Gaikwad, R.; Villadsen, S.N.B.; Rasmussen, J.P.; Grumsen, F.B.; Nielsen, L.P.; Gildert, G.; Møller, P.; Fosbøl, P.L. Container-Sized CO2 to Methane: Design, Construction and Catalytic Tests Using Raw Biogas to Biomethane. Catalysts 2020, 10, 1428. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Dynamic Biogas Upgrading Based on the Sabatier Process: Thermodynamic and Dynamic Process Simulation. Bioresour. Technol. 2015, 178, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Varandas, B.; Oliveira, M.; Andrade, C.; Borges, A. Thermodynamic Equilibrium Analysis of CO2 Methanation through Equilibrium Constants: A Comparative Simulation Study. Physchem 2024, 4, 258–271. [Google Scholar] [CrossRef]
- Esa, Y.A.M.; Sapawe, N. A Short Review on Carbon Dioxide (CO2) Methanation Process. Mater. Today Proc. 2020, 31, 394–397. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over Supported Noble Metal Catalysts. Appl. Catal. A Gen. 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on Methanation—From Fundamentals to Current Projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of Metal Loading on the CO2 Methanation: A Comparison between Alumina Supported Ni and Ru Catalysts. Catal. Today 2020, 356, 419–432. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.Y.; Li, H.; Yu, Z. Active and Stable Ni Based Catalysts and Processes for Biogas Upgrading: The Effect of Temperature and Initial Methane Concentration on CO2 Methanation. Appl. Energy 2018, 227, 206–212. [Google Scholar] [CrossRef]
- Ullah, S.; Yang, X.; Khan, Z.U.; Aziz, S.; Haider, W.; Ben, H. Relationship between Catalyst Structure and Activity in CO2 Methanation of Ru-Based Catalysts: Recent Progress and Future Prospects. New J. Chem. 2025, 49, 7097–7125. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Su, X.; Duan, H.; Geng, H.; Huang, Y. CO2 Methanation over TiO2–Al2O3 Binary Oxides Supported Ru Catalysts. Chin. J. Chem. Eng. 2016, 24, 140–145. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of Carbon Dioxide on Ru/Al2O3 and Ni/Al2O3 Catalysts at Atmospheric Pressure: Catalysts Activation, Behaviour and Stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Liang, L.; Gu, W.; Jiang, J.; Miao, C.; Krasilin, A.A.; Ouyang, J. Effective CO2 Methanation over Site-Specified Ruthenium Nanoparticles Loaded on TiO2/Palygorskite Nanocomposite. J. Colloid Interface Sci. 2022, 623, 703–709. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Liu, X.; Jin, X.; Feng, Y.; Shi, H.; Wang, D.; Zhang, Y.; Wang, Y.; Yan, Z. Low-Temperature CO2 Methanation over Ru/CeO2: Investigation into Ru Loadings. Fuel 2023, 345, 128238. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass Derived N-Doped Biochar as Efficient Catalyst Supports for CO2 Methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Roldán, L.; Marco, Y.; García-Bordejé, E. Origin of the Excellent Performance of Ru on Nitrogen-Doped Carbon Nanofibers for CO2 Hydrogenation to CH4. ChemSusChem 2017, 10, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Lippi, R.; Howard, S.C.; Barron, H.; Easton, C.D.; Madsen, I.C.; Waddington, L.J.; Vogt, C.; Hill, M.R.; Sumby, C.J.; Doonan, C.J.; et al. Highly Active Catalyst for CO2 Methanation Derived from a Metal Organic Framework Template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent Trends in Developments of Active Metals and Heterogenous Materials for Catalytic CO2 Hydrogenation to Renewable Methane: A Review. J. Environ. Chem. Eng. 2021, 9, 105460. [Google Scholar] [CrossRef]
- Halder, A.; Lenardi, C.; Timoshenko, J.; Mravak, A.; Yang, B.; Kolipaka, L.K.; Piazzoni, C.; Seifert, S.; Bonačić-Koutecký, V.; Frenkel, A.I.; et al. CO2 Methanation on Cu-Cluster Decorated Zirconia Supports with Different Morphology: A Combined Experimental In Situ GIXANES/GISAXS, Ex Situ XPS and Theoretical DFT Study. ACS Catal. 2021, 11, 6210–6224. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon Dioxide Methanation over Ni Catalysts Supported on Various Metal Oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 Methanation over Ni Catalysts Supported on CeO2, Al2O3 and Y2O3 Oxides. Appl. Catal. B 2020, 264, 118494. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, Y.C.; Wu, J.H.; Lin, J.H.; Lin, I.K.; Chen, C.S. Methanation of CO2 and Reverse Water Gas Shift Reactions on Ni/SiO2 Catalysts: The Influence of Particle Size on Selectivity and Reaction Pathway. Catal. Sci. Technol. 2015, 5, 4154–4163. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 Methanation over Supported Ni Catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of Surface Ni and Ce Species of Ni/CeO2 Catalyst in CO2 Methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C. Structural Effect of Ni/ZrO2 Catalyst on CO2 Methanation with Enhanced Activity. Appl. Catal. B 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández, E.; Alfaro, S.; López Medina, R.; Valverde Aguilar, G.; Albiter, E.; Valenzuela, M.A. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts 2018, 9, 24. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Luo, J.; Kong, X.; Xu, Y.; Ma, G.; Wang, J.; Zhang, C.; Li, Z.; Ding, M. Preparation of Ni Based Mesoporous Al2O3 Catalyst with Enhanced CO2 Methanation Performance. RSC Adv. 2019, 9, 8684–8694. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced Low-Temperature Performance of CO2 Methanation over Mesoporous Ni/Al2O3-ZrO2 Catalysts. Appl. Catal. B 2019, 243, 262–272. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Rajput, Y.B.; Osman, A.I.; Alreshaidan, S.B.; Ahmed, H.; Fakeeha, A.H.; Al-Awadi, A.S.; El-Salamony, R.A.; Kumar, R. Impact of Sr Addition on Zirconia–Alumina-Supported Ni Catalyst for COx-Free CH4 Production via CO2 Methanation. ACS Omega 2024, 9, 9309–9320. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wen, X.; Yang, H.; Lu, T.; Lian, L.; Wu, C.; Cao, Z.; Wang, S.; Xu, L.; Chen, M. Development of Efficient Ni-Based Catalysts for CO2 Methanation: Unraveling the Impact of Support Properties on Catalytic Performance. J. Environ. Chem. Eng. 2025, 13, 117117. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 Addition on Ni/Al2O3 Catalysts for Methanation of Carbon Dioxide with Hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A Study of Ni/La-Al2O3 Catalysts: A Competitive System for CO2 Methanation. Appl. Catal. B 2019, 248, 286–297. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Zhang, Z.; Ma, Z.; Wang, L.; Liu, Y. Highly Dispersed and Stable Ni Nanoparticles Confined by MgO on ZrO2 for CO2 Methanation. Appl. Surf. Sci. 2019, 481, 1538–1548. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, P.; Cao, X.; Chen, J.; Pu, T.; Shi, B.; Xu, J.; Moon, J.; Wu, Z.; Han, Y.-F. Vacancy Engineering of the Nickel-Based Catalysts for Enhanced CO2 Methanation. Appl. Catal. B Environ. 2021, 282, 119561. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; AlKhoori, S.; AlKhoori, A.A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Highly Selective and Stable Nickel Catalysts Supported on Ceria Promoted with Sm2O3, Pr2O3 and MgO for the CO2 Methanation Reaction. Appl. Catal. B Environ. 2021, 282, 119562. [Google Scholar] [CrossRef]
- Everett, O.E.; Zonetti, P.C.; Alves, O.C.; de Avillez, R.R.; Appel, L.G. The Role of Oxygen Vacancies in the CO2 Methanation Employing Ni/ZrO2 Doped with Ca. Int. J. Hydrogen Energy 2020, 45, 6352–6359. [Google Scholar] [CrossRef]
- Le, M.C.; Van, K.L.; Nguyen, T.H.T.; Nguyen, N.H. The Impact of Ce-Zr Addition on Nickel Dispersion and Catalytic Behavior for CO2 Methanation of Ni/AC Catalyst at Low Temperature. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced Activity of CO2 Methanation over Ni/CeO2-ZrO2 Catalysts: Influence of Preparation Methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Wang, X.; Yang, M.; Zhu, X.; Zhu, L.; Wang, S. Experimental Study and Life Cycle Assessment of CO2 Methanation over Biochar Supported Catalysts. Appl. Energy 2020, 280, 115919. [Google Scholar] [CrossRef]
- Sholeha, N.A.; Jannah, L.; Rohma, H.N.; Widiastuti, N.; Prasetyoko, D.; Jalil, A.A.; Bahruji, H. Synthesis of Zeolite Nay From Dealuminated Metakaolin as Ni Support for CO2 Hydrogenation to Methane. Clays Clay Miner. 2020, 68, 513–523. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Motak, M.; Gálvez, M.E.; Da Costa, P. Ni/Zeolite X Derived from Fly Ash as Catalysts for CO2 Methanation. Fuel 2020, 267, 117139. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Dongil, A.B.; Benito, N.; Espinoza-González, R.; Escalona, N.; Gracia, F. CO2 Methanation over Nickel-ZrO2 Catalyst Supported on Carbon Nanotubes: A Comparison between Two Impregnation Strategies. Appl. Catal. B Environ. 2018, 237, 817–825. [Google Scholar] [CrossRef]
- Wang, W.; Chu, W.; Wang, N.; Yang, W.; Jiang, C. Mesoporous Nickel Catalyst Supported on Multi-Walled Carbon Nanotubes for Carbon Dioxide Methanation. Int. J. Hydrogen Energy 2016, 41, 967–975. [Google Scholar] [CrossRef]
- Zhi, G.; Guo, X.; Wang, Y.; Jin, G.; Guo, X. Effect of La2O3 Modification on the Catalytic Performance of Ni/SiC for Methanation of Carbon Dioxide. Catal. Commun. 2011, 16, 56–59. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing Catalytic Activity and Stability for CO2 Methanation on Ni@MOF-5 via Control of Active Species Dispersion. Chem. Commun. 2015, 51, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Michaelis, V.K.; Ong, T.-C.; Bellarosa, L.; López, N.; Griffin, R.G.; Dincă, M. Dynamic DMF Binding in MOF-5 Enables the Formation of Metastable Cobalt-Substituted MOF-5 Analogues. ACS Cent. Sci. 2015, 1, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Ozbilen, A.; Dincer, I.; Rosen, M.A. Comparative Environmental Impact and Efficiency Assessment of Selected Hydrogen Production Methods. Environ. Impact Assess. Rev. 2013, 42, 1–9. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Global Warming Potential of Hydrogen and Methane Production from Renewable Electricity via Power-to-Gas Technology. Int. J. Life Cycle Assess. 2015, 20, 477–489. [Google Scholar] [CrossRef]
- Meylan, F.D.; Piguet, F.-P.; Erkman, S. Power-to-Gas through CO2 Methanation: Assessment of the Carbon Balance Regarding EU Directives. J. Energy Storage 2017, 11, 16–24. [Google Scholar] [CrossRef]
- Navajas, A.; Mendiara, T.; Gandía, L.M.; Abad, A.; García-Labiano, F.; de Diego, L.F. Life Cycle Assessment of Power-to-Methane Systems with CO2 Supplied by the Chemical Looping Combustion of Biomass. Energy Convers. Manag. 2022, 267, 115866. [Google Scholar] [CrossRef]
- Leeuwen, C.V.; Andreas, Z. Innovative Large-Scale Energy Storage Technologies and Power-to-Gas Concepts after Optimisation. In Report on the Costs Involved with PtG Technologies and Their Potentials Across the EU; STORE&GO Project; European Research Institute for Gas and Energy Innovation (ERIG): Kaiserslautern, Germany, 2018. [Google Scholar]
- Pakarinen, O.M.; Tähti, H.P.; Rintala, J.A. One-Stage H2 and CH4 and Two-Stage H2+ CH4 Production from Grass Silage and from Solid and Liquid Fractions of NaOH Pre-Treated Grass Silage. Biomass Bioenergy 2009, 33, 1419–1427. [Google Scholar] [CrossRef]
- Baier, J.; Schneider, G.; Heel, A. A Cost Estimation for CO2 Reduction and Reuse by Methanation from Cement Industry Sources in Switzerland. Front. Energy Res. 2018, 6, 5. [Google Scholar] [CrossRef]
- Gorre, J.; Ortloff, F.; van Leeuwen, C. Production Costs for Synthetic Methane in 2030 and 2050 of an Optimized Power-to-Gas Plant with Intermediate Hydrogen Storage. Appl. Energy 2019, 253, 113594. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.; Kang, S.; Lim, H. Stochastic Techno-Economic Analysis of Power-to-Gas Technology for Synthetic Natural Gas Production Based on Renewable H2 Cost and CO2 Tax Credit. J. Energy Storage 2019, 24, 100791. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The Progress in Water Gas Shift and Steam Reforming Hydrogen Production Technologies—A Review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Okere, C.J.; Sheng, J.J. Review on Clean Hydrogen Generation from Petroleum Reservoirs: Fundamentals, Mechanisms, and Field Applications. Int. J. Hydrogen Energy 2023, 48, 38188–38222. [Google Scholar] [CrossRef]
- Pashchenko, D.; Makarov, I. Carbon Deposition in Steam Methane Reforming over a Ni-Based Catalyst: Experimental and Thermodynamic Analysis. Energy 2021, 222, 119993. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Liu, X.; Wang, S.; Li, Y.; Zhang, L.; et al. Industrial Status, Technological Progress, Challenges, and Prospects of Hydrogen Energy. Nat. Gas Ind. B 2022, 9, 427–447. [Google Scholar] [CrossRef]
- Mageed, A.K.; Ali Alsaffar, M.; Abdel Ghany, M.A.R.; Sukkar, K.A.; Ayodele, B.V. Advances in Synthesis and Application of Cobalt and Nickel-Based Nanomaterials for Catalytic Reforming of Hydrocarbons and Oxygenates to Hydrogen-Rich Syngas. J. Ind. Eng. Chem. 2025, 144, 1–17. [Google Scholar] [CrossRef]
- Morales-Cano, F.; Lundegaard, L.F.; Tiruvalam, R.R.; Falsig, H.; Skjøth-Rasmussen, M.S. Improving the Sintering Resistance of Ni/Al2O3Steam-Reforming Catalysts by Promotion with Noble Metals. Appl. Catal. A Gen. 2015, 498, 117–125. [Google Scholar] [CrossRef]
- Nawfal, M.; Gennequin, C.; Labaki, M.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Hydrogen Production by Methane Steam Reforming over Ru Supported on Ni–Mg–Al Mixed Oxides Prepared via Hydrotalcite Route. Int. J. Hydrogen Energy 2015, 40, 1269–1277. [Google Scholar] [CrossRef]
- Katheria, S.; Deo, G.; Kunzru, D. Rh-Ni/MgAl2O4 Catalyst for Steam Reforming of Methane: Effect of Rh Doping, Calcination Temperature and Its Application on Metal Monoliths. Appl. Catal. A Gen. 2019, 570, 308–318. [Google Scholar] [CrossRef]
- Jaiswar, V.K.; Katheria, S.; Deo, G.; Kunzru, D. Effect of Pt Doping on Activity and Stability of Ni/MgAl2O4 Catalyst for Steam Reforming of Methane at Ambient and High Pressure Condition. Int. J. Hydrogen Energy 2017, 42, 18968–18976. [Google Scholar] [CrossRef]
- Lazar, M.D.; Dan, M.; Mihet, M.; Almasan, V.; Rednic, V.; Borodi, G. Hydrogen Production by Low Temperature Methane Steam Reforming Using Ag and Au Modified Alumina Supported Nickel Catalysts. Rev. Roum. Chim. 2011, 56, 637–642. [Google Scholar]
- Dan, M.; Mihet, M.; Biris, A.R.; Marginean, P.; Almasan, V.; Borodi, G.; Watanabe, F.; Biris, A.S.; Lazar, M.D. Supported Nickel Catalysts for Low Temperature Methane Steam Reforming: Comparison between Metal Additives and Support Modification. React. Kinet. Mech. Catal. 2012, 105, 173–193. [Google Scholar] [CrossRef]
- Parizotto, N.V.; Rocha, K.O.; Damyanova, S.; Passos, F.B.; Zanchet, D.; Marques, C.M.P.; Bueno, J.M.C. Alumina-Supported Ni Catalysts Modified with Silver for the Steam Reforming of Methane: Effect of Ag on the Control of Coke Formation. Appl. Catal. A Gen. 2007, 330, 12–22. [Google Scholar] [CrossRef]
- Zhai, X.; Ding, S.; Liu, Z.; Jin, Y.; Cheng, Y. Catalytic Performance of Ni Catalysts for Steam Reforming of Methane at High Space Velocity. Int. J. Hydrogen Energy 2011, 36, 482–489. [Google Scholar] [CrossRef]
- Bej, B.; Pradhan, N.C.; Neogi, S. Production of Hydrogen by Steam Reforming of Methane over Alumina Supported Nano-NiO/SiO2 Catalyst. Catal. Today 2013, 207, 28–35. [Google Scholar] [CrossRef]
- Boudjeloud, M.; Boulahouache, A.; Rabia, C.; Salhi, N. La-Doped Supported Ni Catalysts for Steam Reforming of Methane. Int. J. Hydrogen Energy 2019, 44, 9906–9913. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, X.; Guo, Y.; Chen, M.; Liu, W.; Xu, X.; Peng, H.; Gao, Z.; Wang, X.; Li, C. Highly Active and Stable Ni/Y2Zr2O7 Catalysts for Methane Steam Reforming: On the Nature and Effective Preparation Method of the Pyrochlore Support. Int. J. Hydrogen Energy 2016, 41, 11141–11153. [Google Scholar] [CrossRef]
- Garbarino, G.; Pugliese, F.; Cavattoni, T.; Busca, G.; Costamagna, P. A Study on CO2 Methanation and Steam Methane Reforming over Commercial Ni/Calcium Aluminate Catalysts. Energies 2020, 13, 2792. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Ochoa-Fernández, E.; Rusten, H.K.; Jakobsen, H.A.; Rønning, M.; Holmen, A.; Chen, D. Sorption Enhanced Hydrogen Production by Steam Methane Reforming Using Li2ZrO3 as Sorbent: Sorption Kinetics and Reactor Simulation. Catal. Today 2005, 106, 41–46. [Google Scholar] [CrossRef]
- Settar, A.; Mansouri, Z.; Nebbali, R.; Madani, B.; Abboudi, S. Impact of Ni-Based Catalyst Patterning on Hydrogen Production from MSR: External Steam Reformer Modelling. Int. J. Hydrogen Energy 2019, 44, 11346–11354. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbæk, J.S.; Vendelbo, S.B.; Eriksen, W.L.; Frandsen, C.; Mortensen, P.M.; Chorkendorff, I. Electrified Methane Reforming: Elucidating Transient Phenomena. Chem. Eng. J. 2021, 425, 131509. [Google Scholar] [CrossRef]
- Zheng, L.; Ambrosetti, M.; Marangoni, D.; Beretta, A.; Groppi, G.; Tronconi, E. Electrified Methane Steam Reforming on a Washcoated SiSiC Foam for Low-carbon Hydrogen Production. AIChE J. 2023, 69, e17620. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lim, H.S.; Kim, Y.; Lee, J.W. Enhancement of Highly-Concentrated Hydrogen Productivity in Chemical Looping Steam Methane Reforming Using Fe-Substituted LaCoO3. Energy Convers. Manag. 2020, 207, 112507. [Google Scholar] [CrossRef]
- Antzaras, A.N.; Heracleous, E.; Lemonidou, A.A. Sorption Enhanced–Chemical Looping Steam Methane Reforming: Optimizing the Thermal Coupling of Regeneration in a Fixed Bed Reactor. Fuel Process. Technol. 2020, 208, 106513. [Google Scholar] [CrossRef]
- Yu, X.; Yang, L.; Xuan, Y.; Liu, X.L.; Zhang, K. Solar-Driven Low-Temperature Reforming of Methanol into Hydrogen via Synergetic Photo- and Thermocatalysis. Nano Energy 2021, 84, 105953. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.; Dai, Y.; Rong, C. Advancing Solar-Powered Hydrogen Generation: A Comparative Analysis of Efficiency, Emissions, and Economic Viability. Int. J. Hydrogen Energy 2025, 131, 255–272. [Google Scholar] [CrossRef]
- Yan, Y.; Manovic, V.; Anthony, E.J.; Clough, P.T. Techno-Economic Analysis of Low-Carbon Hydrogen Production by Sorption Enhanced Steam Methane Reforming (SE-SMR) Processes. Energy Convers. Manag. 2020, 226, 113530. [Google Scholar] [CrossRef]
- Alrashed, F.; Zahid, U. Comparative Analysis of Conventional Steam Methane Reforming and PdAu Membrane Reactor for the Hydrogen Production. Comput. Chem. Eng. 2021, 154, 107497. [Google Scholar] [CrossRef]
- Wang, W.; Olguin, G.; Hotza, D.; Seelro, M.A.; Fu, W.; Gao, Y.; Ji, G. Inorganic Membranes for In-Situ Separation of Hydrogen and Enhancement of Hydrogen Production from Thermochemical Reactions. Renew. Sustain. Energy Rev. 2022, 160, 112124. [Google Scholar] [CrossRef]
- Johannessen, E.; Jordal, K. Study of a H2 Separating Membrane Reactor for Methane Steam Reforming at Conditions Relevant for Power Processes with CO2 Capture. Energy Convers. Manag. 2005, 46, 1059–1071. [Google Scholar] [CrossRef]
- Pinsky, R.; Sabharwall, P.; Hartvigsen, J.; O’Brien, J. Comparative Review of Hydrogen Production Technologies for Nuclear Hybrid Energy Systems. Prog. Nucl. Energy 2020, 123, 103317. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Xie, H.; Ni, T.; Ouyang, C. Tech-Integrated Paradigm Based Approaches towards Carbon-Free Hydrogen Production. Renew. Sustain. Energy Rev. 2018, 82, 4279–4295. [Google Scholar] [CrossRef]
- Muradov, N.; Veziroglu, T. From Hydrocarbon to Hydrogen-Carbon to Hydrogen Economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Subramanian, B.; Sun, J.; Ge, J.; Guo, H.; Radhamani, A.V.; Ramakrishna, S.; Liu, Y. Techno-Economic Analysis of Different Shades of Renewable and Non-Renewable Energy-Based Hydrogen for Fuel Cell Electric Vehicles. Renew. Sustain. Energy Rev. 2023, 174, 113153. [Google Scholar] [CrossRef]
- Santos, M.; Nadaleti, W.C.; Gomes, J.; Wilsen, C.; Mello, N.; Przybyla, G. Biogas, Hydrogen, Green Ammonia and Electricity Production from Canned Peach Processing Residues: Aspects of the Circular Economy for the Brazilian Agroindustry. Int. J. Hydrogen Energy 2025, 105, 45–55. [Google Scholar] [CrossRef]
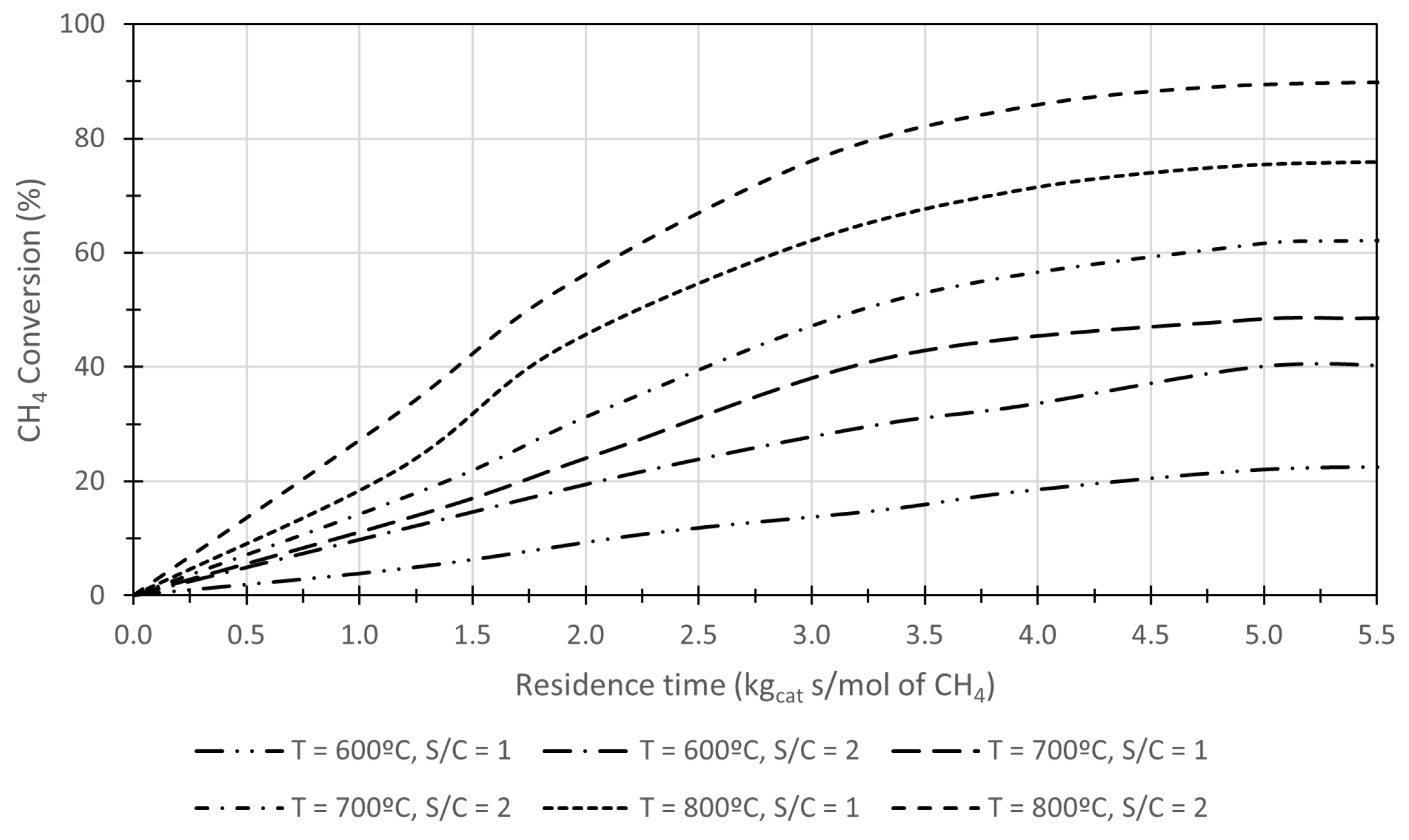
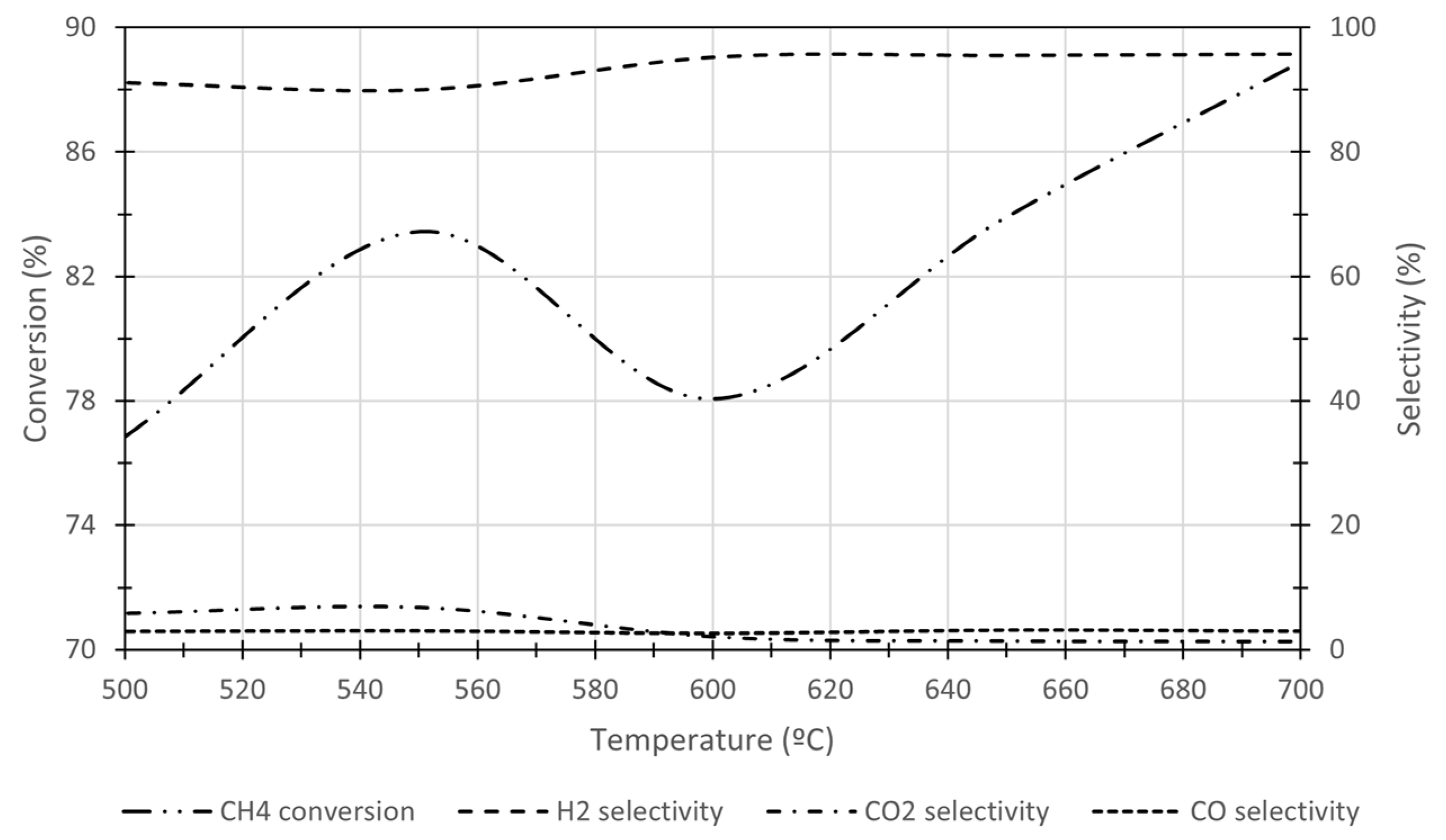
| Process | Topic | Authors | Year | Ref. |
|---|---|---|---|---|
| CO2 Methanation | Review of catalysts used over the past five decades | Frontera et al. | 2017 | [3] |
| Overview of low-temperature CO2 methanation | Lee et al. | 2021 | [4] | |
| Bimetallic Ni-based catalysts | Tsiotsias et al. | 2020 | [5] | |
| Nickel-based catalysts for low-temperature applications | Li et al. | 2022 | [6] | |
| Thermodynamic analysis and assessment of catalysts and reactors | Ghaib et al. | 2018 | [7] | |
| SMR | Steam reforming of natural gas for hydrogen production | Boretti et al. | 2021 | [8] |
| Reforming and partial oxidation of hydrocarbons for hydrogen production | Sengodan et al. | 2018 | [9] | |
| Catalytic processes for hydrogen production | Kumar et al. | 2024 | [10] | |
| Bimetallic catalysts for steam methane reforming | Yusuf et al. | 2024 | [11] | |
| Membrane reactor technology | Iulianelli et al. | 2016 | [12] | |
| Habib et al. | 2021 | [13] |
| Mechanism | Description | Ref. |
|---|---|---|
| Coke (or carbon) Deposition | Deposition of carbonaceous materials on the catalyst surface | [14,15] |
| Sintering | Loss of active surface area due to migration and growth of metals particles on the catalyst support | [16] |
| Metal segregation or agglomerate | Material particles may segregate or agglomerate, leading to reduced dispersion and catalyst deactivation | [17] |
| Poisoning | Irreversible chemical deactivation caused by deposition of impurities (e.g., sulfur, ammonia, etc.) on the active sites | [16,18] |
| Oxidation | Catalyst degradation due to exposure to oxidative environments | [8,11,16] |
| Hydrogen Color | Feedstock | Energy Used | Processes |
|---|---|---|---|
| Green | Water | Renewable sources | Electrolysis |
| Blue | Natural gas/methane | Fossil fuel | Reforming |
| Grey | Renewable natural gas/methane | Fossil fuel | Reforming |
| Brown/black | Coal | Fossil fuel | Gasification |
| Turquoise | Natural gas | Renewable sources | Reforming with carbon solidification or Pyrolysis |
| Purple | Water | Nuclear energy | Electrolysis |
| Yellow | Water | Grid energy | Electrolysis |
| Orange | Waste plastic | Fossil fuel | Gasification in carbon capture |
| White | Natural | Natural | Fracking |
| Reaction Formula | (kJ/mol) | Reaction Type | Equation No. |
|---|---|---|---|
| −165 | CO2 methanation | (1) | |
| −206 | CO methanation | (2) | |
| 41 | RWGS | (3) | |
| −247 | RDM | (4) | |
| −172 | CO disproportionation | (5) | |
| 75 | CH4 cracking | (6) | |
| −131 | CO reduction | (7) | |
| −90 | CO2 reduction | (8) |
| Catalyst | Active Metal, wt.% | T (°C) | X CO2 (%) | S CH4 (%) | Ref. |
|---|---|---|---|---|---|
| Ru/TiO2 | 2 | 250 | 20 | 100 | [38] |
| Ru/Al2O3 | 3 | 300 | 96 | 96 | [44] |
| Ru/Al2O3 | 4 | 375 | 85 | 98 | [40] |
| Ru/Ni/Al2O3 | 0.5 (Ru)–20 (Ni) | 350 | 82 | 100 | [41] |
| Ru/TiO2/Pal | 4 | 450 | 88.7 | 100 | [45] |
| Ru/CeO2 | 0.5 | 300 | 76 | 100 | [46] |
| Ru/N-ABC-600 | 1.7 | 380 | 94 | 100 | [47] |
| Ru/NCNF | 5 | 350 | 66 | 99 | [48] |
| Ru/ZrO2 | 1 | - | 96 | 99 | [49] |
| Catalyst | Active Metal, wt.% | T (°C) | X CO2 (%) | S CH4 (%) | Ref. |
|---|---|---|---|---|---|
| Ni/Y2O3 | 10 | 300 | 77 | 99.5 | [52] |
| Ni/Y2O3 | 35 | 350 | 83.5 | 90.3 | [53] |
| Ni/SiO2 | 10 | 350 | 10 | 90 | [54] |
| Ni/CeO2 | 10 | 340 | 31.1 | 100 | [56] |
| Ni/ZrO2 | 10 | 350 | 80 | 97 | [57] |
| Ni/ZrO2 | 20 | 400 | 50 | 100 | [58] |
| Ni/Al2O3-ZrO2 | 20 | 300 | 77 | 100 | [60] |
| Ni4Sr/10ZrO2-Al2O3 | 5 | - | 80 | 70 | [61] |
| Ni/CeO2 and Ni/NS-MFI | - | 400 | 80 | 98 | [62] |
| Ni/CeO2-Al2O3 | 15 | 350 | 85 | 100 | [63] |
| Ni/MgO-ZrO2 | 6 | 300 | 95 | 100 | [65] |
| Ni/Ce0.2Zr0.8O2/AC | 7 | 350 | 85 | 100 | [69] |
| Ni/CexZr1−xO2 | 10 | 275 | 55 | 99.8 | [70] |
| Ni/Ce-ABC | 15 | 360 | 88.6 | 92.3 | [71] |
| Ni4.5Ce/CNT | 12 | 350 | 83.8 | 98.8 | [75] |
| Authors | H2 Production | CO2 Origin | GWP (kg CO2 eq/MWh CH4) | Ref. |
|---|---|---|---|---|
| Reiter and Lindorfer | Wind | Residue | 22 | [80] |
| Reiter and Lindorfer | PV | Residue | 108 | [80] |
| Reiter and Lindorfer | Mix | Residue | 994 | [80] |
| Reiter and Lindorfer | Wind | Fossil | 104 | [80] |
| Reiter and Lindorfer | PV | Fossil | 191 | [80] |
| Reiter and Lindorfer | Mix | Fossil | 1076 | [80] |
| Meylan et al. | Wind | DAC | 54 | [81] |
| Meylan et al. | PV | DAC | 134 | [81] |
| Navajas et al. (CLOU) | Wind and PV | Biomass | −341/−10 (a) | [82] |
| Navajas et al. (iG-CLC_sOC) | Wind and PV | Biomass | −418/−9 (a) | [82] |
| Navajas et al. (iG-CLC_mOC | Wind and PV | Biomass | −471/−8.5 (a) | [82] |
| Costs per kg CH4 | Costs per kWh CH4 | Costs per MWh CH4 | Ref. |
|---|---|---|---|
| EUR 1.30 | EUR 0.09 | EUR 93.53 | [83,84] |
| - | CHF 0.30 | CHF 300 | [85] |
| - | - | EUR 33.60 and EUR 204.62 (a) | [86] |
| - | USD 0.094 | USD 94 | [87] |
| Catalyst | Support | NiO wt.% | K2O wt.% | SiO2 wt.% | Al2O3 wt.% |
|---|---|---|---|---|---|
| 57-4Q | CaAl2O4 | 18 | - | - | - |
| 25-4Q | CaAl2O4 | 18 | 1.8 | - | - |
| HMMC | SiO2-Al2O3 | 33.3 | - | 3.3 | 63.3 |
| Catalyst | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Ni/Al2O3 | Low carbon deposition | Deactivates easily | [8,91] |
| Ni/MgAl2O4 | Maximum efficacy | - | [8] |
| Ni/SiO2 | - | Lowest efficacy Deactivates easily Carbon deposition | [8] |
| Ni/SiO2Al2O3 | Thermal stability Hydrogen selectivity Coke resistance | - | [106] |
| Ni2/Al2O5 | High and stable activity | - | [106] |
| Ru/Ni6Al2O9 | High CH4 conversion rate at high temperature | - | [95] |
| (Ni0.5M0.05Mg0.9)2Al (M = Fe or Cu) | High catalyst stability | - | [17] |
| Rh-Ni/Al2O4 | High catalytic stability and activity High hydrogen yield | - | [96] |
| Ni-Au/Al2O3 | Good catalytic stability and activity | - | [98] |
| Ni/La2O3-Al2O3 and Ni/CeO2-Al2O3 (at low temperature) | Improvement in methane conversion Improvement in hydrogen production Improvement in CO2 selectivity | - | [99] |
| Ni-Ag/Al2O3 | High resistance to coke formation | Lower catalytic stability and activity | [98,99,100] |
| Technologies | Process Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| SESMR | Use of a solid sorbent to remove carbon dioxide gases in hydrogen production from SMR | Lower GHG emissions | - | [18,107] |
| MR | Uses a membrane to separate the hydrogen from other composts | Low energy consumption Continuous separation Rigorous process conditions Integration with other separation technologies | Shorter lifetime Low flux or selectivity Fouling tendency | [13] |
| WC-SMR | Uses wall coating for the fuel processing in hydrogen production | Higher conversion rate Higher hydrogen production rate Increases residence time | - | [108] |
| eSMR | Uses electrified systems to produce the necessary heat for SMR | Operation flexibility Lower GHG emissions | - | [20,109,110] |
| CL-SMR | Uses oxidation and reduction reactions to produce hydrogen from SMR | Pure hydrogen at lower temperatures Reduces CO2 emissions | - | [20,111] |
| CL-SESMR | Combines both chemical lopping reforming and sorption-enhanced to produce hydrogen from SMR | Lower GHG emissions | - | [20,112] |
| SASMR | Uses solar thermal energy to provide heat for SMR | Lower GHG emissions | - | [20,113,114] |
| GWP (kg CO2-eq/MWh H2) | AP (g SO2-eq/MWh H2) | Ref. |
|---|---|---|
| ≈357.06 | ≈436.04 | [79,123] |
| ≈141.14 (or 318.32) | - | [119,123] |
| ≈750.75 | - | [23,123] |
| ≈357.15 | ≈435.92 | [120,123] |
| ≈285.29 to 345.35 | - | [114,123] |
| ≈411.41 | - | [121,123] |
| ≈90.90 to 210.21 | - | [122,123] |
| Costs (USD/kg H2) | Costs (USD/GJ H2) | Costs (USD/MWh H2) | Ref. |
|---|---|---|---|
| 1.54 to 2.30 | - | ≈46.25 to 69.07 | [119,123] |
| 1.50 | - | ≈45.05 | [23,123] |
| - | 11.44 | ≈41.19 | [120,123] |
| 1.00 to 2.00 | - | ≈30.03 to 60.06 | [114,123] |
| 0.70 to 2.10 | - | ≈21.02 to 63.06 | [122,123] |
| GWP CO2 Methanation (kg CO2 eq/MWh CH4) | Ref. | GWP SMR (kg CO2 eq/MWh H2) | Ref. | |
|---|---|---|---|---|
| H2 production from wind | 22 (a)/104 (b) | [80] | ≈357.06 | [79,123] |
| H2 production from PV | 108 (a)/191 (b) | [80] | ≈141.14 (or 318.32) | [119,123] |
| H2 production from mix | 994 (a)/1076 (b) | [80] | ≈750.75 | [23,123] |
| H2 production from wind | 54 | [81] | ≈357.15 | [120,123] |
| H2 production from mix | 134 | [81] | ≈285.29 to 345.35 | [114,123] |
| H2 production from wind and PV | −341(c,e)/−10 (d,e) (CLOU) | [82] | ≈411.41 | [121,123] |
| −471(c,e)/−8.5 (d,e) (iG-CLC_mOC) | [82] | ≈90.90 to 210.21 | [122,123] | |
| Costs of CO2 Methanation (per MWh CH4) | Ref. | Costs of SMR (per MWh H2) | Ref. |
|---|---|---|---|
| EUR 93.53 | [83,84] | USD 46.23 to 69.07 | [119,123] |
| CHF 300 | [85] | USD 45.05 | [23,123] |
| EUR 33.60 (a) | [86] | USD 41.19 | [120,123] |
| EUR 204.62 (b) | [86] | USD 30.03 to 60.06 | [114,123] |
| USD 94 | [87] | USD 21.02 to 63.06 | [122,123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, M.; Andrade, C.; Borges, A.D.S. Reversible Thermochemical Routes for Carbon Neutrality: A Review of CO2 Methanation and Steam Methane Reforming. Physchem 2025, 5, 29. https://doi.org/10.3390/physchem5030029
Martins M, Andrade C, Borges ADS. Reversible Thermochemical Routes for Carbon Neutrality: A Review of CO2 Methanation and Steam Methane Reforming. Physchem. 2025; 5(3):29. https://doi.org/10.3390/physchem5030029
Chicago/Turabian StyleMartins, Marisa, Carlos Andrade, and Amadeu D. S. Borges. 2025. "Reversible Thermochemical Routes for Carbon Neutrality: A Review of CO2 Methanation and Steam Methane Reforming" Physchem 5, no. 3: 29. https://doi.org/10.3390/physchem5030029
APA StyleMartins, M., Andrade, C., & Borges, A. D. S. (2025). Reversible Thermochemical Routes for Carbon Neutrality: A Review of CO2 Methanation and Steam Methane Reforming. Physchem, 5(3), 29. https://doi.org/10.3390/physchem5030029






