Depolymerization to Decontamination: Transforming PET Waste into Tailored MOFs for Advanced Pollutant Adsorption
Abstract
1. Introduction
2. PET: Presentation and Recycling
2.1. PET: Presentation
2.2. Recycling Strategies of PET
2.2.1. Mechanical Recycling
2.2.2. Energetic Recycling
2.2.3. Chemical Recycling
2.3. Chemical Recycling of PET for Metal–Organic Framework Ligand Recovery
2.3.1. Glycolysis
2.3.2. Alcoholysis
2.3.3. Hydrolysis
Neutral Hydrolysis
Alkaline Hydrolysis
Acid Hydrolysis of PET
2.3.4. Aminolysis
3. MOF from Waste PET
3.1. Early Development of PET Waste-Derived MOFs
3.2. Method of Preparation of MOF Derived PET
3.3. Techniques of Synthesis
3.3.1. Conventional Synthesis Method (Solvothermal and Hydrothermal Methods)
3.3.2. Unconventional Methods of Synthesis
Mechanochemical Synthesis
Sonochemical Synthesis
Electrochemical Synthesis
3.4. Recent Synthesis and Applications Studies in PET-Derived MOF (2024–2025)
3.5. Ligand Variants from PET in MOF Synthesis
3.6. Sustainable Synthesis of MOFs from PET and Metallic Waste Precursors
3.7. General Considerations on the Quality of PET-Derived MOFs
4. Application for Water Treatment
4.1. Heavy Metal Removal
4.2. Pharmaceutical Contaminants
4.3. Dye Removal
4.4. Pesticides
4.5. Phosphate Pollution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Joseph, T.M.; Azat, S.; Ahmadi, Z.; Jazani, O.M.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethyleneterephthalate (PET) recycling: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Shanmugam, M.; Chuaicham, C.; Augustin, A.; Sasaki, K.; Sagayaraja, P.J.J.; Sekar, K. Upcycling hazardous metals and PET waste-derived metal–organic frameworks: A review on recent progresses and prospects. New J. Chem. 2022, 33. [Google Scholar] [CrossRef]
- Cherian, R.; Binish, C.J.; Vijayasankar, A.V. Eco-frameworks for a cleaner planet: Harnessing next-gen MOFs for pollution and plastic waste remediation. Polym. Degrad. Stab. 2025, 238, 111349. [Google Scholar] [CrossRef]
- Dhaka, V.; Singh, S.; Anil, A.G.; Naik, T.S.S.K.; Garg, S.; Samuel, J.; Kumar, M.; Ramamurthy, P.C.; Singh, J. toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef] [PubMed]
- Ashby, R. Migration from polyethylene terephthalate under all conditions of use. Food Addit. Contam. 1988, 5, 485–492. [Google Scholar] [CrossRef]
- Achilias, D. Material Recycling: Trends and Perspectives; BoD—Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-51-0327-1. [Google Scholar]
- Çeven, E.K.; Karakan Günaydin, G. Global Trends for Fibre Production and Marketing. Int. Conf. Trends Adv. Res. 2023, 1, 255–262. [Google Scholar]
- Pudack, C.; Stepanski, M.; Fassler, P. PET recycling—Contributions of crystallization to sustainability. Chem. Ing. Tech. 2020, 92, 452–458. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Frounchi, M. Studies on degradation of PET in mechanical recycling. Macromol. Symp. 1999, 144, 465–469. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Motoki, T.; Okuwaki, A. Kinetics of hydrolysis of poly(ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 2001, 40, 75–79. [Google Scholar] [CrossRef]
- Yoshioka, T.; Okayama, N.; Okuwaki, A. Kinetics of hydrolysis of PET powder in nitric acid by a modified shrinking-core model. Ind. Eng. Chem. Res. 1998, 37, 336–340. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene terephthalate (PET) bottle-to-bottle recycling for the beverage industry: A review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Abedsoltan, H. A focused review on recycling and hydrolysis techniques of polyethylene terephthalate. Polym. Eng. Sci. 2023, 63, 2651–2674. [Google Scholar] [CrossRef]
- Mohamed, R.M.S.R.; Wurochekke, A.A.; Misbah, G.S.; Kassim, A.H.M. Energy recovery from polyethylene terephthalate (PET) recycling process. GSTF Int. J. Eng. Technol. (JET) 2014, 2, 39–44. [Google Scholar] [CrossRef]
- Campanelli, J.R.; Cooper, D.G.; Kamal, M.R. Catalyzed Hydrolysis of Polyethylene Terephthalate Melts. J. Appl. Polym. Sci. 1994, 53, 985–991. [Google Scholar] [CrossRef]
- Lozano-Martinez, P.; Torres-Zapata, T.; Martin-Sanchez, N. Directing Depolymerization of PET with Subcritical and Supercritical Ethanol to Different Monomers through Changes in Operation Conditions. ACS Sustain. Chem. Eng. 2021, 9, 9846–9853. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ohshima, M.-A.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Gruschke, H.; Hammerschick, W.; Medem, H. Process for Depolymerizing Polyethylene-Terephthalate to Terephthalic Acid Dimethyl Ester. US Patent 3,403,115, 24 September 1968. [Google Scholar]
- López-Fonseca, R.; González-Velasco, J.; Gutiérrez-Ortiz, J. A shrinking core model for the alkaline hydrolysis of PET assisted by tributylhexadecylphosphonium bromide. Chem. Eng. J. 2009, 146, 287–294. [Google Scholar] [CrossRef]
- Ikenaga, K.; Inoue, T.; Kusakabe, K. Hydrolysis of PET by Combining Direct Microwave Heating with High Pressure. Procedia Eng. 2016, 148, 314–318. [Google Scholar] [CrossRef]
- Stanica-Ezeanu, D.; Matei, D. Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water. Sci. Rep. 2021, 11, 4431. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.J.; Zeronian, S.H. The molecular weight distribution and oligomers of sodium hydroxide hydrolyzedpoly(ethylene terephthalate). J. Appl. Polym. Sci. 1992, 45, 797–804. [Google Scholar] [CrossRef]
- Pitat, J.; Holcik, V.; Bacak, M. Method of Processing Waste of Polyethylene Terephthalate by Hydrolysis. GB Patent 822 834, 1959. [Google Scholar]
- Lazarus, S.D.; Twilley, J.C.; Snider, O.E. Simultaneous Depolymerization of Polycaproamide and Polyester with Recovery of Caprolactam. US Patent 3,317,519, 2 May 1967. [Google Scholar]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Paliwal, N.R.; Mungray, A.K. Ultrasound assisted alkaline hydrolysis of poly(ethylene terephthalate) in presence of phase transfer catalyst. Polym. Degrad. Stab. 2013, 98, 2094–2101. [Google Scholar] [CrossRef]
- Pusztaseri, S.F. Method for Recovery of Terephthalic Acid from Polyester Scrap. US Patent 4,355,175, 19 October 1982. [Google Scholar]
- Vinitha, V.; Preeyanghaa, M.; Anbarasu, M.; Jeya, G.; Neppolian, B.; Sivamurugan, V. Aminolytic depolymerization of polyethylene terephthalate wastes using Sn-doped ZnO nanoparticles. J. Polym. Environ. 2022, 30, 3566–3581. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Sodium carbonate catalyzed aminolytic degradation of PET. Prog. Rubber, Plast. Recycl. Technol. 2015, 32, 153–168. [Google Scholar] [CrossRef]
- Parab, Y.S.; Pingale, N.D.; Shukla, S.R. Aminolytic depolymerization of poly(ethylene terephthalate) bottle waste by conventional and microwave irradiation heating. J. Appl. Polym. Sci. 2011, 127, 323–328. [Google Scholar] [CrossRef]
- Tawfik, M.E.; Ahmed, N.M.; Eskander, S.B. Aminolysis of poly(ethylene terephthalate) wastes based on sunlight and utilization of the end product [bis(2-hydroxyethylene) terephthalamide] as an ingredient in the anticorrosive paints for the protection of steel structures. J. Appl. Polym. Sci. 2011, 116, 2658–2667. [Google Scholar] [CrossRef]
- Musale, R.M.; Shukla, S.R. Deep eutectic solvent as effective catalyst for aminolysis of polyethylene terephthalate (PET) waste. Int. J. Plast. Technol. 2016, 20, 106–120. [Google Scholar] [CrossRef]
- Aslzadeh, M.M.; Sadeghi, G.M.M.; Abdouss, M. Synthesis and characterization of BHETA-based new polyurethanes. Mater. Werkst. 2010, 41, 682–688. [Google Scholar] [CrossRef]
- More, A.P.; Kokate, S.R.; Rane, P.C.; Mhaske, S.T. Studies of different techniques of aminolysis of poly(ethylene terephthalate) with ethylenediamine. Polym. Bull. 2016, 74, 3269–3282. [Google Scholar] [CrossRef]
- Heo, D.Y.; Do, H.H.; Ahn, S.H.; Kim, S.Y. Metal-Organic Framework Materials for Perovskite Solar Cells. Polymers 2020, 12, 2061. [Google Scholar] [CrossRef] [PubMed]
- Deleu, W.P.R.; Stassen, I.; Jonckheere, D.; Ameloot, R.; De Vos, D.E. Waste PET (bottles) as a resource or substrate for MOF synthesis. J. Mater. Chem. A 2016, 4, 9519–9525. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Lai, Y.-L.; Lin, C.-H.; Wang, S.-L. Direct use of waste PET as unfailing source of organic reagents in the synthesis of intrinsic white/yellow luminescent nanoporous zincophosphates. Green Chem. 2011, 13, 2000–2003. [Google Scholar] [CrossRef]
- Lo, S.-H.; Raja, D.S.; Chen, C.-W.; Kang, Y.-H.; Chen, J.-J.; Lin, C.-H. Waste polyethylene terephthalate (PET) materials as sustainable precursors for the synthesis of nanoporous MOFs, MIL-47, MIL-53(Cr, Al, Ga) and MIL-101(Cr). Dalton Trans. 2016, 45, 9565–9573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, S.J.; Serre, C. Direct synthesis of robust hcp UiO-66(Zr) MOF using poly(ethylene terephthalate) waste as ligand source. Microporous Mesoporous Mater. 2019, 290, 109675. [Google Scholar] [CrossRef]
- Waribam, P.; Katugampalage, T.R.; Sreearunothai, P. Upcycling plastic waste: Rapid aqueous depolymerization of PET and simultaneous growth of defective UiO-66. Chem. Eng. J. 2023, 473, 145291. [Google Scholar] [CrossRef]
- He, P.; Hu, Z.; Dai, Z.; Bai, H.; Fan, Z.; Niu, R.; Gong, J.; Zhao, Q.; Tang, T. Mechanochemistry milling of waste poly(ethylene terephthalate) into metal-organic frameworks. ChemSusChem 2023, 16, e202201935. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on metal–organic framework classification, synthetic approaches, and influencing factors: Applications in energy, drug delivery, and wastewater treatment. ACS Omega 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.P.; Singh, G.P.; Singha, S.; Roymahapatra, G. Synthesis of metal-organic frameworks (MOFs) and their biological, catalytic and energetic application: A mini review. Eng. Sci. 2020, 13, 1–10. [Google Scholar]
- Yeskendir, B.; de Souza, P.M.; Simon, P.; Wojcieszak, R.; Courtois, C.; Lorgouilloux, Y.; Royer, S.; Dacquin, J.P.; Dhainaut, J. Water-based synthesis of Zr6-based metal–organic framework nanocrystals with sulfonate functions: Structural features and application to fructose dehydration. ACS Appl. Nano Mater. 2022, 28, 5. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Vallejo, J.A.; Muñoz, J.P.; León, C.A. Sustainable synthesis of MOF-5 using terephthalic acid from recycled PET and evaluation of CO2 adsorption capacity. Mater. Chem. Phys. 2022, 274, 125241. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous metal–organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- D’aMato, R.; Bondi, R.; Moghdad, I.; Marmottini, F.; McPherson, M.J.; Naïli, H.; Taddei, M.; Costantino, F. “Shake ‘n Bake” route to functionalized Zr-UiO-66 metal–organic frameworks. Inorg. Chem. 2021, 60, 14294–14301. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Varsha, M.; Nageswaran, G. Direct electrochemical synthesis of metal-organic frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar]
- Ghoorchian, A.; Afkhami, A.; Madrakian, T.; Ahmadi, M. Metal-Organic Frameworks for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–195. ISBN 9780128190425. [Google Scholar]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Yeganeh, M.; Hatefi-Mehrjardi, A.; Esrafili, A.; Sobhi, H.R. Recycling of Polyethylene Terephthalate Waste Bottles and Zinc-Carbon Used Batteries for Preparation of a MOF-Based Catalyst: Application in Photodegradation of Organophosphorus Pesticides. J. Photochem. Photobiol. A 2025, 466, 116388. [Google Scholar] [CrossRef]
- Kim, D.; Kalimuthu, P.; Lee, S.-M.; Jung, J.; Elanchezhiyan, S. Utilization of Waste PET-Derived Metal-Organic Framework Grafted Polyaniline Composite for Heavy Metal Adsorption from Aqueous Solution. J. Ind. Eng. Chem. 2025, 144, 663–671. [Google Scholar] [CrossRef]
- Das, S.; Zhang, T.; Clarkson, G.J.; Zhao, Y.; Walton, R.I. Selective Electrocatalytic Production of Formic Acid from Plastic Waste Using a Nickel Metal-Organic Framework Constructed from a Biomass-Derived Ligand. ChemSusChem 2025, 18, e202402319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, D.; Ni, X.; Sun, Z.; Yuan, H. Waste PET Plastic-Mediated Synthesis of Manganese- and Cobalt-Doped RuO2 Catalyst for Electro-Oxidation of Water with Robust Stability. Resour. Conserv. Recycl. 2025, 215, 108056. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Xu, J.; Xiang, W.; Zhang, Y. Upcycling of Organic and Inorganic Waste into MIL-88B(Fe) at Room Temperature for Tetracycline Degradation. Inorg. Chem. 2025, 64, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Waribam, P.; Katugampalage, T.R.; Ogawa, M.; Puathawee, P.; Sreearunothai, P. Magnetic Metal-Organic Frameworks (MOFs) from Waste: A Solvent-Free Rapid Synthesis of Green Catalyst for Environmental Cleanup. ACS Sustain. Chem. Eng. 2025, 13, 9576–9587. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, M.; Ga, L.; Ai, J. Terbium-Doped Cobalt-Based Metal-Organic Frameworks for Electrocatalytic Hydrogen Production and Polyethylene Terephthalate Plastic Upcycling. Chem. Eng. J. 2024, 496, 154062. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Y.; Yu, Z.; Wu, W.; Su, Y. Waste PET-Derived MOF-5 for High-Efficiency Removal of Tetracycline. Sep. Purif. Technol. 2024, 339, 126490. [Google Scholar] [CrossRef]
- Karamat, S.; Akhter, T.; Ul Hassan, S.; Han, S.-K.; Park, C.H. Recycling of Polyethylene Terephthalate to Bismuth-Embedded Bimetallic MOFs as Photocatalysts Toward Removal of Cationic Dye in Water. J. Ind. Eng. Chem. 2024, 137, 503–513. [Google Scholar] [CrossRef]
- Nouira, A.; Bekri-Abbes, I.; Cansado, I.P.P.; Mourão, P.A.M. Taguchi Robust Design of Phase Transfer Catalytic Hydrolysis of Polyethylene Terephthalate (PET) Waste in Mild Conditions: Application for the Preparation of Metal-Organic Frameworks. Solids 2025, 6, 10. [Google Scholar] [CrossRef]
- Agarwal, M.; Pal, N.; Kushwaha, P.; Dohare, R.K. Waste to Value Added: Cu-Ni MOF Catalyst Synthesized from Waste Plastic for Enhanced Hydrogen Generation and Electrochemical Energy Storage. Chem. Pap. 2025, 79. [Google Scholar] [CrossRef]
- Çavuşoğlu, F.C.; Bayazit, Ş.S. Evaluation of Waste Polyethylene Terephthalate Bottles as Ligands for the Synthesis of Manganese-Based Metal-Organic Framework and Removal of Tetracycline Antibiotics from Aqueous Solutions. J. Environ. Chem. Eng. 2025, 13, 116402. [Google Scholar] [CrossRef]
- Berehe, B.A.; Desalew, A.A.; Derbe, G.W.; Chang, J.-Y.; Girma, W.M. Enhanced Photocatalytic Degradation of Methylene Blue Dye via Valorization of a Polyethylene Terephthalate Plastic Waste-Derived Metal-Organic Framework-Based ZnO@Co-BDC Composite Catalyst. Nanoscale Adv. 2025, 7, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Nbl, H.L.; Le, T.T.; Cao, V.D.; Nguyen, M.L.; Nguyen, T.T. Conversion of Polyethylene Terephthalate Plastic into Metal-Organic Framework Materials for the Adsorption of Organic Dyes. Environ. Eng. Sci. 2025, 42, 126–136. [Google Scholar] [CrossRef]
- Ko, Y.; Uyar, T.; Hinestroza, J.P. UiO-66 Inspired Superhydrophobic Coatings Fabricated from Discarded Polyester/Spandex Textiles. ACS Appl. Mater. Interfaces 2024, 16, 53163–53176. [Google Scholar] [CrossRef] [PubMed]
- Jindakaew, J.; Ratanatawanate, C.; Erwann, J.; Yang, R.-X.; Elaissari, A. Upcycling of Post-Consumer Polyethylene Terephthalate Bottles into Aluminum-Based Metal-Organic Framework Adsorbents for Efficient Orthophosphate Removal. Sci. Total. Environ. 2024, 935, 173394. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.-X.; Qiao, M.; Zhang, B.; Zhang, H.-T.; Wang, J.-X. Upcycling Plastic Wastes into High-Performance Nano-MOFs by Efficient Neutral Hydrolysis for Water Adsorption and Photocatalysis. J. Mater. Chem. A 2024, 12, 19452–19461. [Google Scholar] [CrossRef]
- Loganathan, M.; Rajendraprasad, M.; Murugesan, A.; Arun, T. Recovery of bis(2-hydroxyethyl) terephthalate and terephthalic acid from waste PET bottles for synthesis of cerium-based metal-organic frameworks: A study towards supercapacitor applications. React. Funct. Polym. 2024, 205, 106101. [Google Scholar] [CrossRef]
- Moumen, E.; Boukayouht, K.; Elmoutchou, S.; Kounbach, S.; El Hankari, S. Sustainable and shaped synthesis of MOF composites using PET waste for efficient phosphate removal. New J. Chem. 2024, 48, 2226–2235. [Google Scholar] [CrossRef]
- Al-Busafi, S.N.; Al-Shafouri, Y.A. Green preparation of aluminum-based metal-organic framework (Al-MOF) from waste plastic bottles and waste aluminum scraps. SQU J. Sci. 2021, 26, 98–106. [Google Scholar] [CrossRef]
- Boukayouht, K.; Bazzi, L.; Daouli, A.; Maurin, G.; El Hankari, S. Ultrarapid and sustainable synthesis of trimetallic-based MOF (CrNiFe-MOF) from stainless steel and disodium terephthalate-derived PET wastes. ACS Appl. Mater. Interfaces 2024, 16, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Qiu, X.; Han, B.; Liang, S.; Lin, Z. Efficient upcycling of electroplating sludge and waste PET into Ni-MOF nanocrystals for the effective photoreduction of CO2. Environ. Sci. Nano 2021, 8, 390–398. [Google Scholar] [CrossRef]
- Nason, A.K.; Phamonpon, W.; Pitt, T.A.; Rodthongkum, N.; Suntivich, J. Reactive depolymerization of polyethylene terephthalate textiles into metal-organic framework intermediates produces additive-free monomers. Chem. Mater. 2024, 36, 10319–10326. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, Z.; Zhou, X.; Ma, N.; Dai, W. Defective iron-based metal–organic framework derived from discarded plastics for rapid and efficient adsorptive removal of methylmercury. Environ. Sci. Pollut. Res. 2025, 32, 14730–14742. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. The United Nations World Water Development Report 2021: Valuing Water. 2021. Available online: https://www.unesco.org/reports/wwdr/2021/en (accessed on 17 March 2025).
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.—Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.-S.; Bundschuh, J.; Bhattacharya, P. (Eds.) Arsenic in Geosphere and Human Diseases; Arsenic 2010: Proceedings of the Third International Congress on Arsenic in the Environment (As-2010), 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Kalimuthu, P.; Kim, Y.; Jung, J. Comparative evaluation of Fe-, Zr-, and La-based metal-organic frameworks derived from recycled PET plastic bottles for arsenate removal. Chemosphere 2022, 294, 133735. [Google Scholar] [CrossRef] [PubMed]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated carbons for arsenic removal from natural waters and wastewaters: A review. Water 2021, 13, 2982. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Beat Plastic Pollution. 2022. Available online: https://www.unep.org/interactives/beat-plastic-pollution/ (accessed on 17 March 2025).
- Sharma, M.; Anshika; Sharma, P.; Janu, V.C.; Gupta, R. Harnessing waste PET bottles for sustainable Ca-MOF synthesis: A high-efficiency adsorbent for uranium and thorium. J. Mater. Chem. A 2024, 12, 26833–26847. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Farahani, S.D. Experimental design optimization and isotherm modeling for removal of copper(II) by calcium-terephthalate MOF synthesized from recycled PET waste. J. Chemom. 2022, 36, e3396. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, G. Facile synthesis of Sn(II)-MOF using waste PET bottles as an organic precursor and its derivative SnO2 NPs: Role of surface charge reversal in adsorption of toxic ions. J. Environ. Chem. Eng. 2021, 9, 105288. [Google Scholar] [CrossRef]
- Price, R. O’Neill report on antimicrobial resistance: Funding for antimicrobial specialists should be improved. Eur. J. Hosp. Pharm. 2016, 23, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kim, J.H.; Choi, J.W. Synthesis of magnetic porous carbon composite derived from MOF using recovered terephthalic acid from polyethylene terephthalate (PET) waste bottles as organic ligand and its potential as adsorbent for antibiotic tetracycline hydrochloride. Compos. Part B Eng. 2020, 187, 107857. [Google Scholar] [CrossRef]
- Heng, Y.; Fang, Z.; Li, J.; Luo, L.; Zheng, M.; Huang, H. Defective metal–organic framework derived from waste plastic bottles for rapid and efficient nitroimidazole antibiotics removal. J. Colloid Interface Sci. 2023, 650, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Wang, L.; Liu, G.; Boczkaj, G. Valorization of waste plastics to a novel metal-organic framework derived cobalt/carbon nanocatalyst as peroxymonosulfate activator for antibiotics degradation. J. Clean. Prod. 2025, 486, 144539. [Google Scholar] [CrossRef]
- Cho, E.; Lee, S.Y.; Jung, K.W. Direct upcycling of polyethylene terephthalate (PET) waste bottles into α-Fe2O3 incorporated MIL-53(Al) for the synthesis of Al2O3/Fe3O4-encapsulated magnetic carbon composite and efficient removal of non-steroidal anti-inflammatory drugs. Sep. Purif. Technol. 2021, 279, 119758. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Y.; Yu, Z.; Su, Y. Efficient adsorption removal of anionic dyes by waste PET-derived MIL-101(Cr). Sep. Purif. Technol. 2025, 354, 128985. [Google Scholar] [CrossRef]
- Keshta, B.E.; Yu, H.; Wang, L. Cutting-edge in the green synthesis of MIL-101(Cr) MOF based on organic and inorganic waste recycling with extraordinary removal for anionic dye. Sep. Purif. Technol. 2024, 332, 125891. [Google Scholar] [CrossRef]
- Farahani, S.D.; Zolgharnein, J. Removal of Alizarin red S by calcium-terephthalate MOF synthesized from recycled PET-waste using Box-Behnken and Taguchi designs optimization approaches. J. Solid State Chem. 2022, 316, 123560. [Google Scholar] [CrossRef]
- Yarahmadi, H.; Salamah, S.K.; Kheimi, M. Synthesis of an efficient MOF catalyst for the degradation of OPDs using TPA derived from PET waste bottles. Sci. Rep. 2023, 13, 19136. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Assessment of Pesticide Pollution in Water Systems. Food and Agriculture Organization of the United Nations. 2023. Available online: https://openknowledge.fao.org/items/f364bfc0-5526-4290-894a-3bfb65c6e3b2 (accessed on 17 March 2025).
- Safe Drinking Water Foundation. Pesticides and Water Pollution [Fact Sheet]. Available online: https://www.safewater.org/fact-sheets-1/2017/1/23/pesticides (accessed on 23 January 2017).
- EPA. Pesticide Environmental Fate. United States Environmental Protection Agency. 2022. Available online: https://www.epa.gov/pesticides (accessed on 17 March 2025).
- Prabhakar, N.; Isloor, A.M.; Farnood, R. Effective removal of hazardous atrazine and chlorpyrifos by waste PET bottles-derived linker having novel MIL-53(Al)/PMMA-nanofiber incorporated poly(vinylidene) fluoride membranes. J. Environ. Chem. Eng. 2025, 13, 115351. [Google Scholar] [CrossRef]
- Isloor, A.M.; Prabhakar, N.; Farnood, R. Field performance of PET-derived MOF membranes for agricultural runoff treatment. Environ. Sci. Water Res. Technol. 2024, 10, 456–468. [Google Scholar]
- Semyonov, O.; Chaemchuen, S.; Ivanov, A.; Verpoort, F.; Kolska, Z.; Syrtanov, M.; Svorcik, V.; Yusubov, M.S.; Lyutakov, O.; Guselnikova, O.; et al. Smart recycling of PET to sorbents for insecticides through in situ MOF growth. Appl. Mater. Today 2021, 22, 100910. [Google Scholar] [CrossRef]

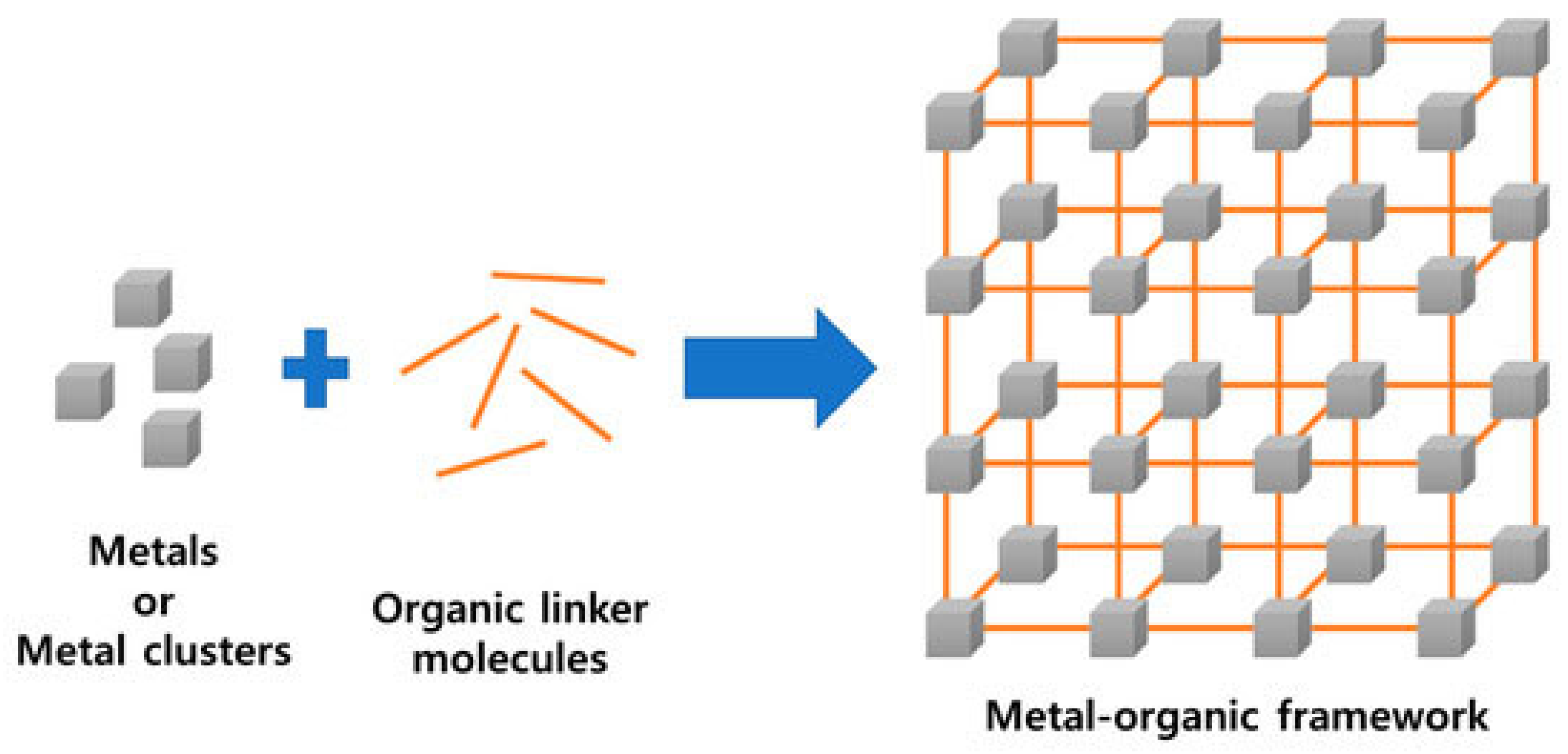
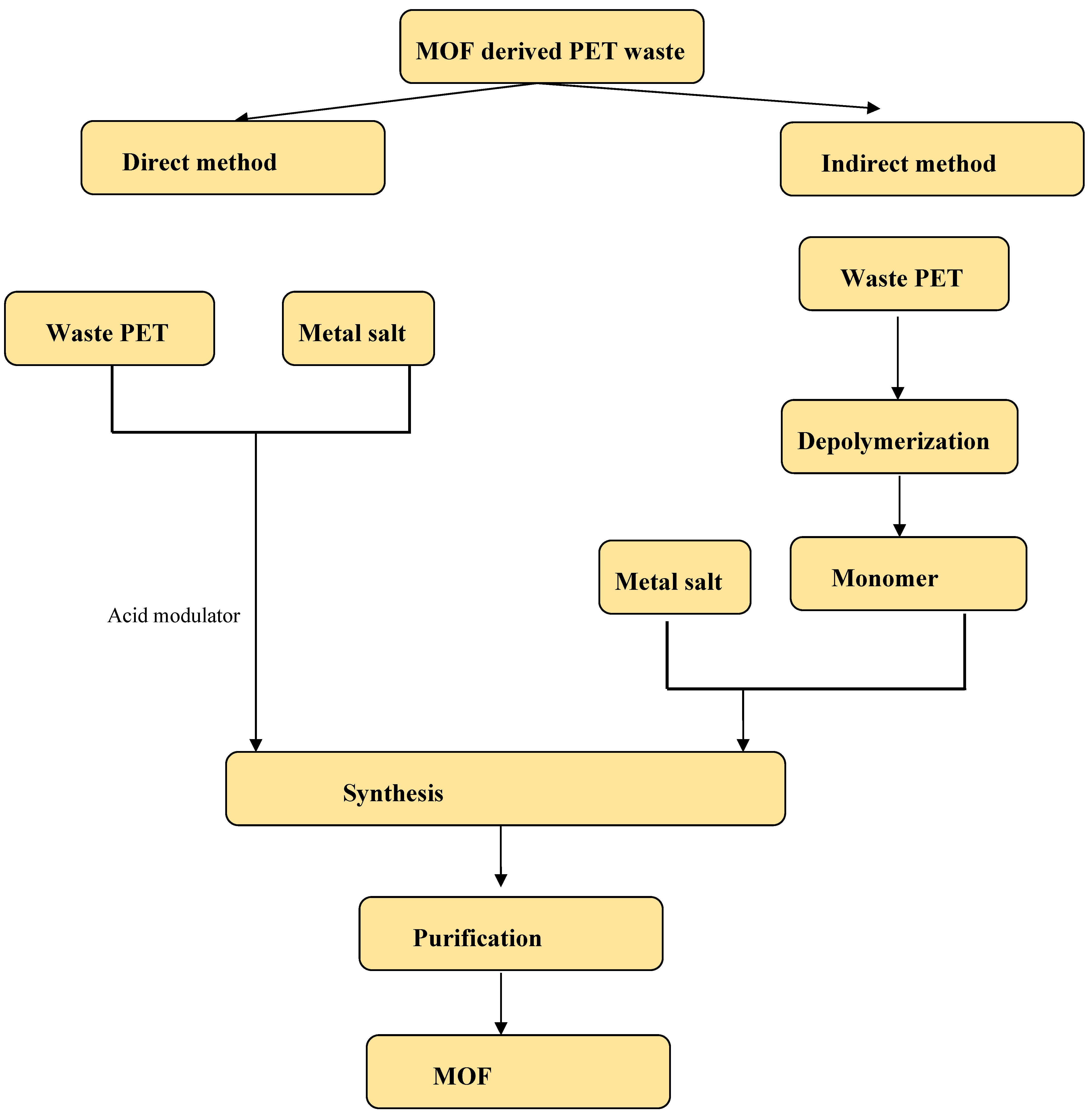

| Method | Operation Conditions | Advantages | Disadvantages | Chemical Mechanism |
|---|---|---|---|---|
| Glycolysis | Ethylene glycol (primary) Temp: 110–270 °C | Direct repolymerization Closed-loop recycling | High energy input Requires catalyst (e.g., Zn acetate) Purification needed | Nucleophilic acyl substitution: Ethylene glycol hydroxyl groups attack electron-deficient carbonyl carbons in PET ester bonds, facilitated by metal catalysts that polarize the C=O bond, forms BHET through a tetrahedral intermediate that collapses, releasing polymer fragments. |
| Alcoholysis | Methanol/ethanol Temp: 160–350 °C | Low solvent cost High DMT/DET purity (>90%) Scalable industrially | Extreme conditions Methanol toxicity Safety risks | Transesterification cascade: Alcohol nucleophiles (methanol/ethanol) attack proton-activated ester linkages. Smaller alcohol molecules penetrate polymer crystallites more effectively, but require metal catalysts to stabilize the transition state. |
| Neutral Hydrolysis | Water (5:1 mass ratio) Temp: 200–250 °C | No solvents Eco-friendly TPA suitable for MOFs | Slow (Energy-intensive TPA purification costly | Hydrolysis: Water molecules act as nucleophiles under subcritical conditions (200–250 °C). The rate-determining step involves water penetration into PET amorphous regions, with proton transfer stabilizing the carboxylate intermediate. |
| Alkaline Hydrolysis | NaOH/KOH (4–20%) Temp: 100–225 °C | Fast, Mild conditions High TPA yield | Corrosive | Base-catalyzed saponification: Hydroxide ions initiate chain scission through direct nucleophilic attack on ester groups. The reaction proceeds via a concerted mechanism where C-O bond cleavage coincides with carboxylate formation. |
| Acid Hydrolysis | H2SO4/HNO3 (7–14 M) 60–100 °C | No pressure needed TPA purity | Reactor corrosion Toxic byproducts Acid disposal | Strong acids protonate ester oxygens, making carbonyl carbons more susceptible to nucleophilic attack. The reaction proceeds through an acid-stabilized carbocation intermediate that undergoes nucleophilic capture by water. |
| Parameter | Direct Method | Indirect Method (Depolymerization First) |
|---|---|---|
| Process Steps | 1-step: PET → MOF | 2-step: PET → Terephthalic acid → MOF |
| Reaction Conditions | Precence of acid modulator for depolymerization | Technique of depolymerization |
| Yield | Low | moderate |
| Time Required | Depolymerization and MOF synthesis at the same time | Time of depolymerization + time of MOF synthesis |
| Purity Control | Moderate (impurities from additives) | High if terephthalic acid or BHET pure |
| Crystallinity | Lower (defect-rich) | Higher (more ordered structures) |
| Energy Consumption | Lower (one-pot synthesis) | Higher (multiple steps) |
| Metal Source | MOF Synthesis Method | MOF Type | Key Synthesis Conditions | Yield/Purity | Application | Study |
|---|---|---|---|---|---|---|
| Zn from used batteries | Solvothermal + GO modification | GO-MOF | 120 °C, 24 h | High-purity TPA | Photodegradation of pesticides | [59] |
| Mn salts | Solvothermal | Mn-PET-MOF | Standard conditions | Comparable to commercial (8.27 mg/g) | Tetracycline adsorption | [70] |
| Co(NO3)2·6H2O | Solvothermal | ZnO@Co-BDC | 180 °C, 6 h | 87.5% MB degradation | Dye photocatalysis | [71] |
| Fe salts | Solvothermal + PANI composite | Fe-MOF@PANI | 100 °C, 12 h | 258 mg/g Pb(II) | Heavy metal adsorption | [60] |
| Ni salts | Electrochemical | Ni-MOF | 1.47 V, RT | 94% Faradaic efficiency | Formic acid production | [61] |
| Al salts | One-step hydrothermal | MIL-53(Al) | 200 °C, water solvent | 71.25% conversion | Dye adsorption | [72] |
| Fe salts | Hydrothermal | MIL-101(Fe)/MOF-235 | 80 °C, 24 h | 93.3% efficiency | Material synthesis | [68] |
| Fe from galvanizing waste | Room-temperature | MIL-88B(Fe) | RT, 1 h | 87% TC degradation | Photocatalysis | [63] |
| Fe from wastewater | Microwave-assisted | MagMOF | 30 min, 300 W | 94% purity | Azo dye degradation | [64] |
| Cu/Ni salts | Solvothermal | Cu-Ni-PET | Standard conditions | 806 F/g capacitance | Hydrogen generation | [69] |
| Ag salts | Solvothermal | Ag-MIL-101 | Standard conditions | 93% in 8 min | Dye degradation | [62] |
| Cu/Ni salts | Solvothermal + BiOI | Ni/Cu-MOF@BiOI | Sunlight 4 h | 99% MB degradation | Dye photocatalysis | [67] |
| Co/Tb salts | Electrodeposition | TbCo-MOF/NF | 1.55 V cell | 161 mV HER | H2 production | [65] |
| Zr | Alkaline depolymerization | UiO-66 | Ethanol modulation Room-temperature coating | - | Superhydrophobic textile coatings | [73] |
| Al salts | Hydrothermal | MIL-53(Al) | Standard conditions | 826 mg/g | Phosphate removal | [74] |
| Cr/Al/Fe salts | One/two-pot | MIL-101(Cr) etc. | 200 °C, 60 min | 95.6% yield | Water adsorption | [75] |
| Zn salts | Solvothermal | MOF-5 | 120 °C, 24 h | 2325 mg/g TC | Tetracycline adsorption | [66] |
| MOF Type | Heavy Metal Source | Key Characteristics | Performance Metrics | Mechanistic Insights and Performance | Reference |
|---|---|---|---|---|---|
| CrNiFe-MOF | Stainless steel waste (Cr, Ni, Fe) | Mesoporous, defect-rich structure; pH-stable (2–10) | Phosphate adsorption: 126 mg/g; STY: 5760 g m−3 day−1 | The trimetallic oxo-clusters (Cr/Ni/Fe) provide high-density Lewis acid sites for selective phosphate binding via ligand exchange. The mesoporous defective structure enhances diffusion kinetics, achieving 98% removal from eutrophic water. | [79] |
| Ni-MOF | Electroplating sludge (Ni2+, Fe3+, Cu2+) | Tolerant to impurity ions; nanocrystalline morphology | CO2 photoreduction: 9.68 × 103 μmol h−1 g−1 CO (96.7% selectivity); AQY: 1.36% (420 nm) | Visible light excitation generates electrons that migrate to Ni2+ nodes, reducing adsorbed CO2 through a proton-coupled electron transfer pathway. The reaction proceeds via CO2•− and formate intermediates, ultimately yielding CO with 96.7% selectivity. Fe3+/Cu2+ impurities from the sludge optimize the Ni sites’ electronic structure, suppressing H2 evolution and stabilizing key intermediates. The MOF’s high surface area and defect-rich framework enhance charge separation, contributing to its superior activity (9.68 × 103 μmol h−1 g−1 CO) and stability. | [80] |
| Zn-MOF (W-MOF) | Zn-C batteries (Zn2+) | GO-modified composite; visible-light active | Pesticide degradation: >95% (chlorpyrifos/profenofos); stable for multiple cycles | Zn2+ nodes and terephthalate linkers form a photoactive framework. Under visible light, charge separation generates •OH radicals, which cleave P−O bonds GO integration reduces electron–hole recombination, boosting degradation efficiency to 95% in 60 min (pH 5). | [59] |
| Heavy Metal | MOF Type | Source of Metal Node | Adsorption Capacity (mg/g) | Optimal pH | Removal Efficiency | Regeneration Cycles | Key Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Arsenic (As) | La-MOF | Lanthanum salts | 114.28 | 4–10 | >90% (500→10 μg/L) | 4 | Ligand exchange, oxophilicity | [87] |
| Uranium (U) | Ca-BDC | Marble waste | 829.18 | 5 | 98–99% | 5 | Monolayer chemisorption | [90] |
| Thorium (Th) | Ca-BDC | Marble waste | 273.16 | 5 | 98–99% | 5 | Monolayer chemisorption | [90] |
| Copper (Cu) | Ca-BDC | Calcium salts | 204.2 | 5–7 | >95% | 4 | Chemical interaction, RSM-optimized | [91] |
| Arsenate (AsO43−) | Sn(II)-MOF | Tin salts | 90.90 | 3–7 | >99% | 5 | Electrostatic attraction | [92] |
| MOF Type | Dye Type | Adsorption Capacity (mg/g) | Regeneration Efficiency | Mechanism | Reference |
|---|---|---|---|---|---|
| MIL-101(Cr) | Reactive Red 2 | 662.87 | >90% | Electrostatic, π-π stacking | [100] |
| MIL-101(Cr) | Reactive Blue 19 | 863.67 | >90% | Electrostatic, π-π stacking | [100] |
| MIL-101(Cr) | Acid Blue 92 | 2176 | >85% | Electrostatic interactions | [101] |
| Ni/Cu-MOF@BiOI | Methylene Blue | 99% degradation | High | Photocatalytic degradation | [67] |
| Ca-TPA-MOF | Alizarin Red S | 979.0 | High | Chemisorption | [102] |
| Cu-Zn-MOF | Multiple Dyes | >95% degradation | - | Catalytic degradation | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouira, A.; Bekri-Abbes, I. Depolymerization to Decontamination: Transforming PET Waste into Tailored MOFs for Advanced Pollutant Adsorption. Physchem 2025, 5, 28. https://doi.org/10.3390/physchem5030028
Nouira A, Bekri-Abbes I. Depolymerization to Decontamination: Transforming PET Waste into Tailored MOFs for Advanced Pollutant Adsorption. Physchem. 2025; 5(3):28. https://doi.org/10.3390/physchem5030028
Chicago/Turabian StyleNouira, Asma, and Imene Bekri-Abbes. 2025. "Depolymerization to Decontamination: Transforming PET Waste into Tailored MOFs for Advanced Pollutant Adsorption" Physchem 5, no. 3: 28. https://doi.org/10.3390/physchem5030028
APA StyleNouira, A., & Bekri-Abbes, I. (2025). Depolymerization to Decontamination: Transforming PET Waste into Tailored MOFs for Advanced Pollutant Adsorption. Physchem, 5(3), 28. https://doi.org/10.3390/physchem5030028






