Green Silver Nanoparticles: Plant-Extract-Mediated Synthesis, Optical and Electrochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aqueous Plant Extracts
2.3. Determination of Plant Extract AOA
2.4. Phytosynthesis of AgNPs
2.5. Characterization of Phyto-AgNPs
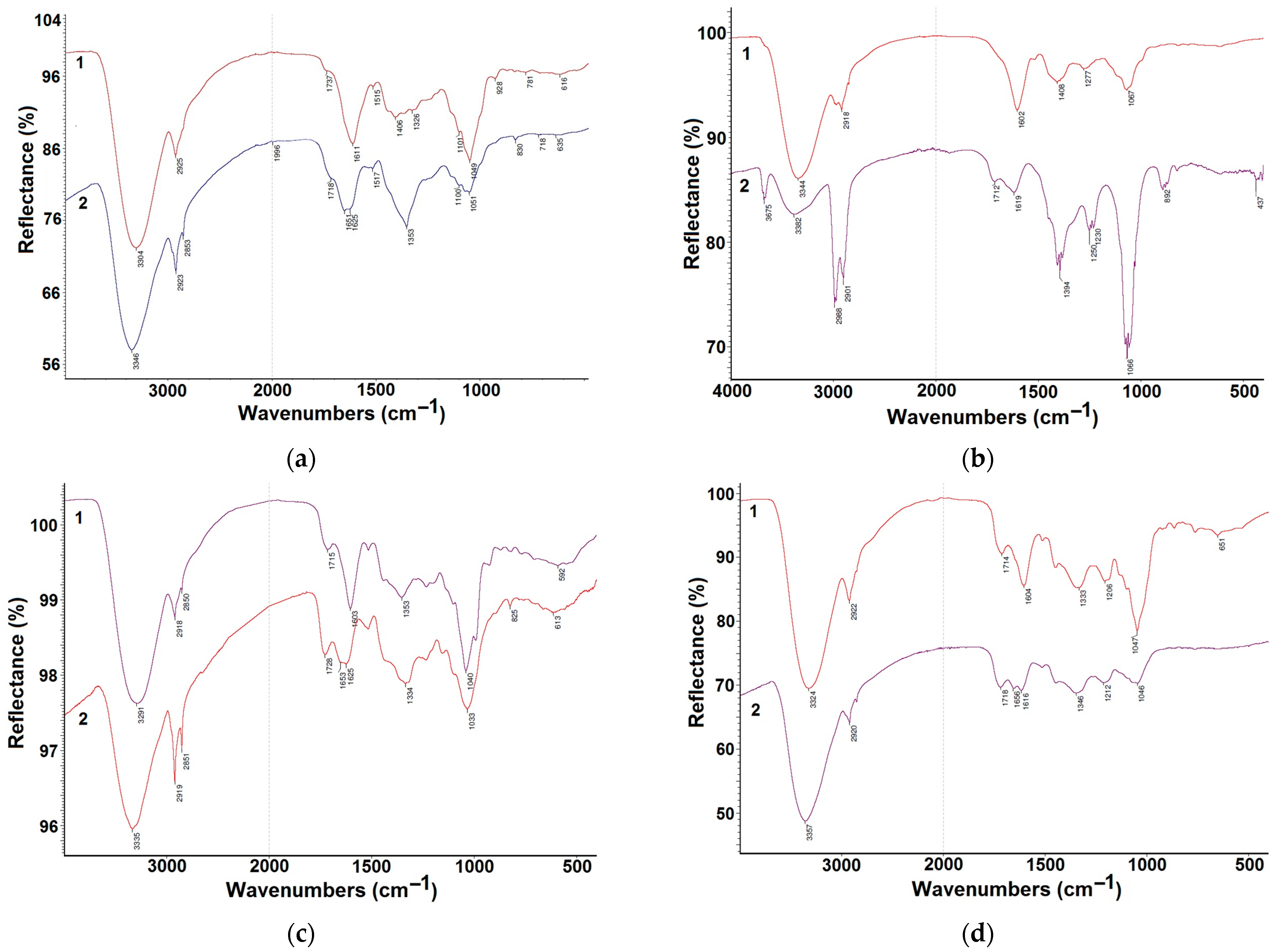
2.5.1. FTIR
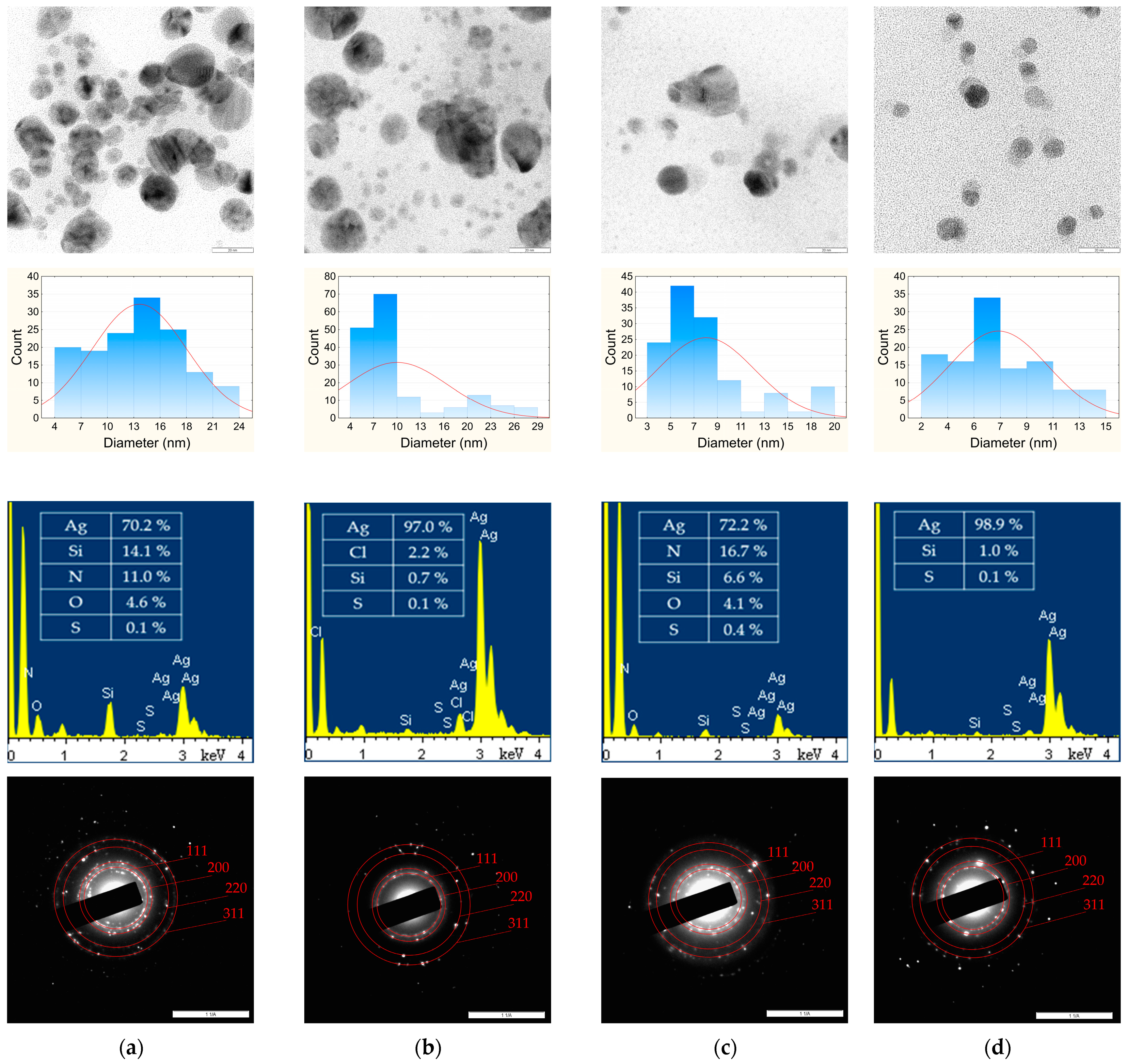
2.5.2. TEM
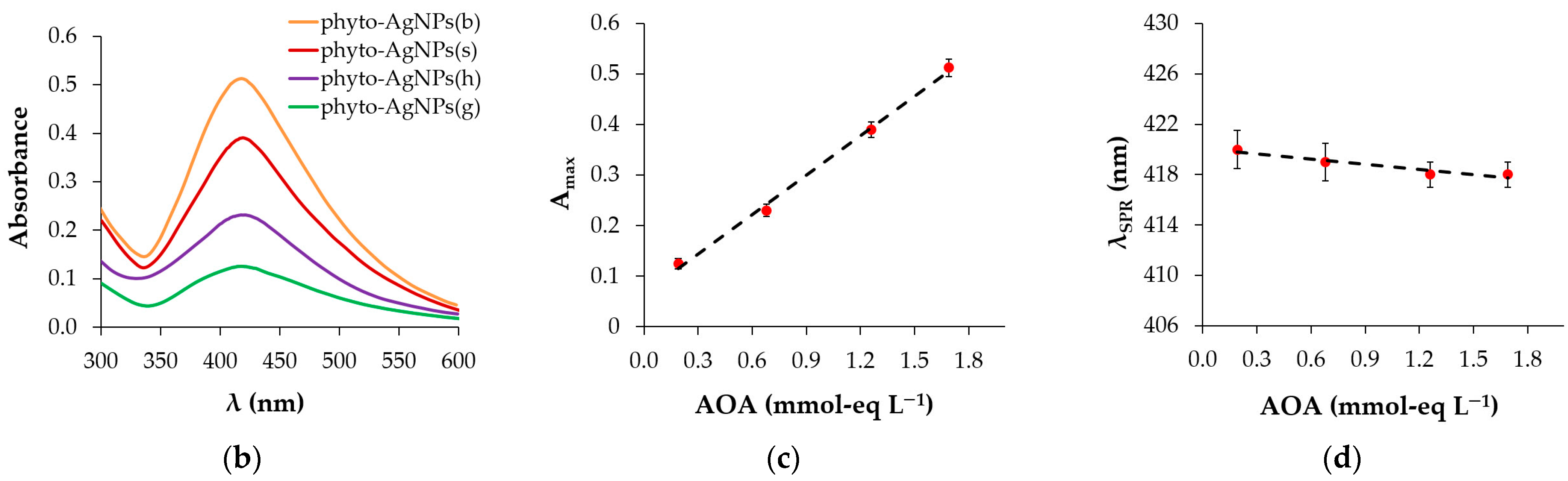
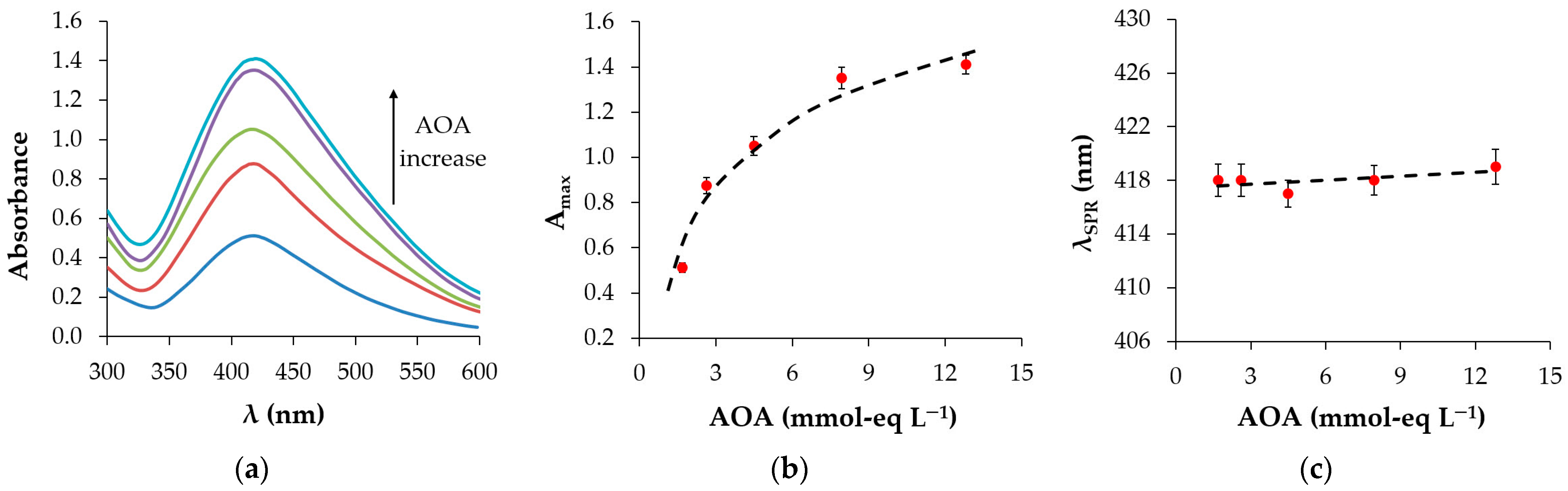
2.5.3. UV–Vis Spectroscopy
2.5.4. Electrochemical Techniques
2.6. Data Treatment
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. Transmission Electron Microscopy (TEM)
3.3. UV–Vis Spectroscopy
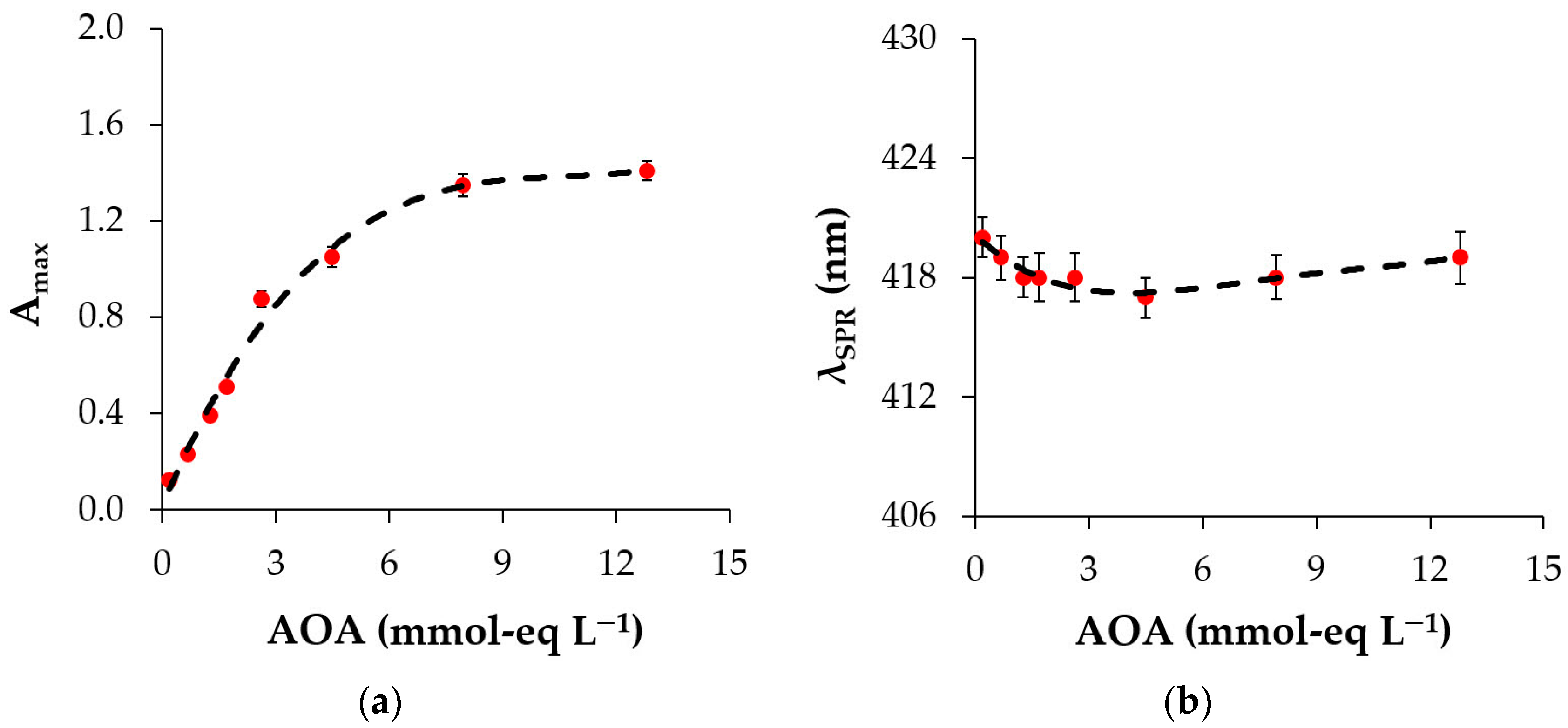
3.3.1. Effect of Plant Extract AOA
3.3.2. Maturation of Silver Sols
3.4. Electrochemical Investigations
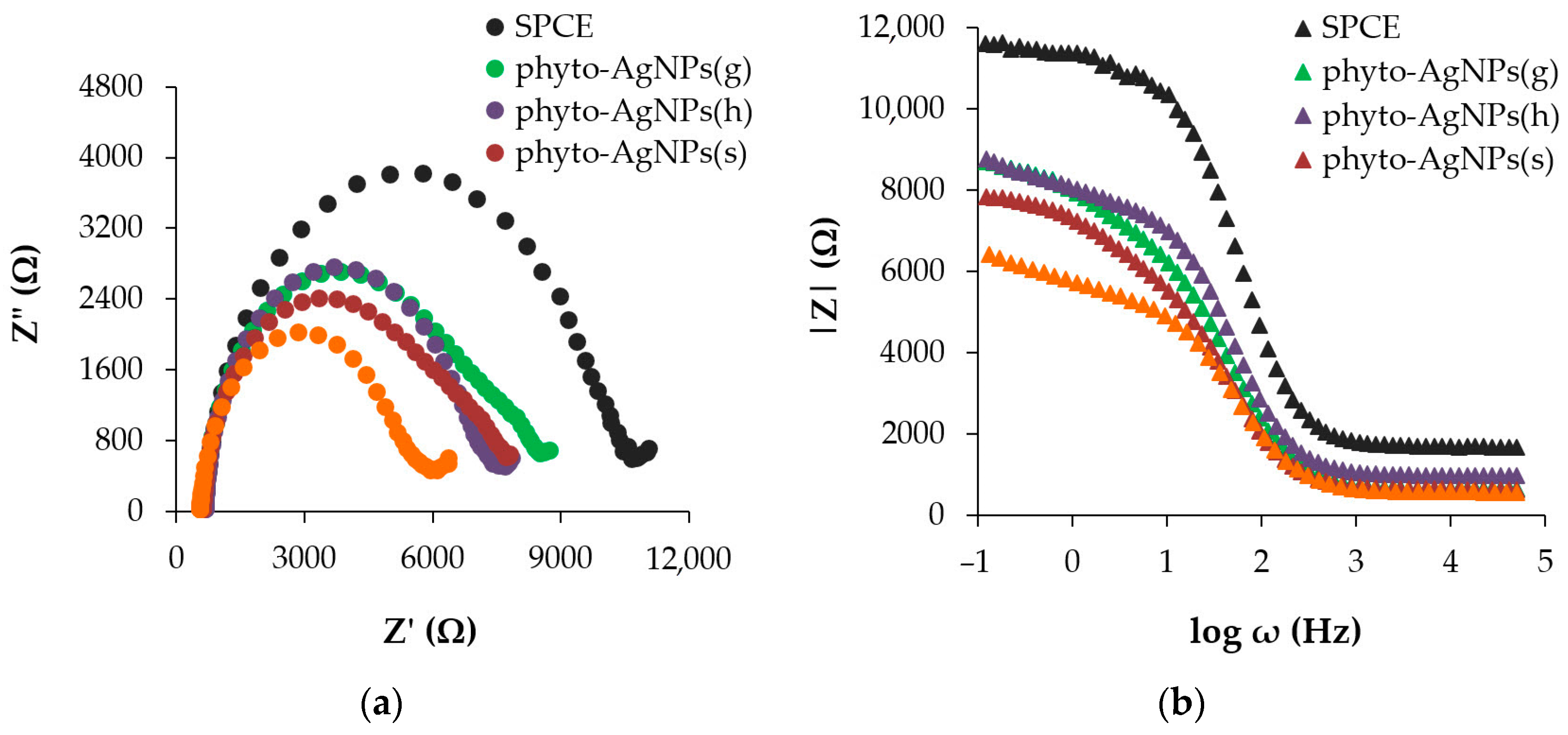
3.4.1. Electrochemical Impedance Spectroscopy (EIS)
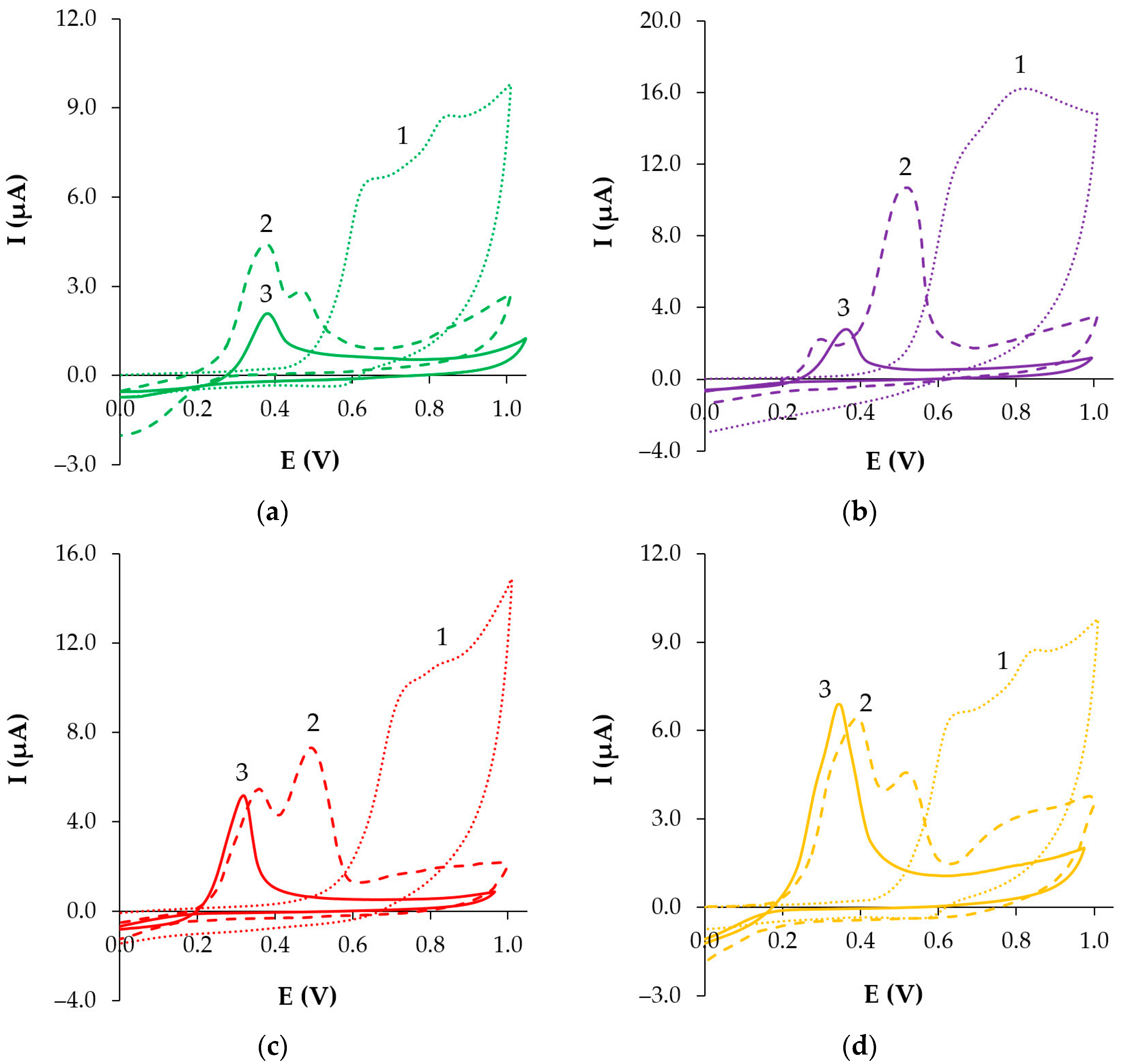
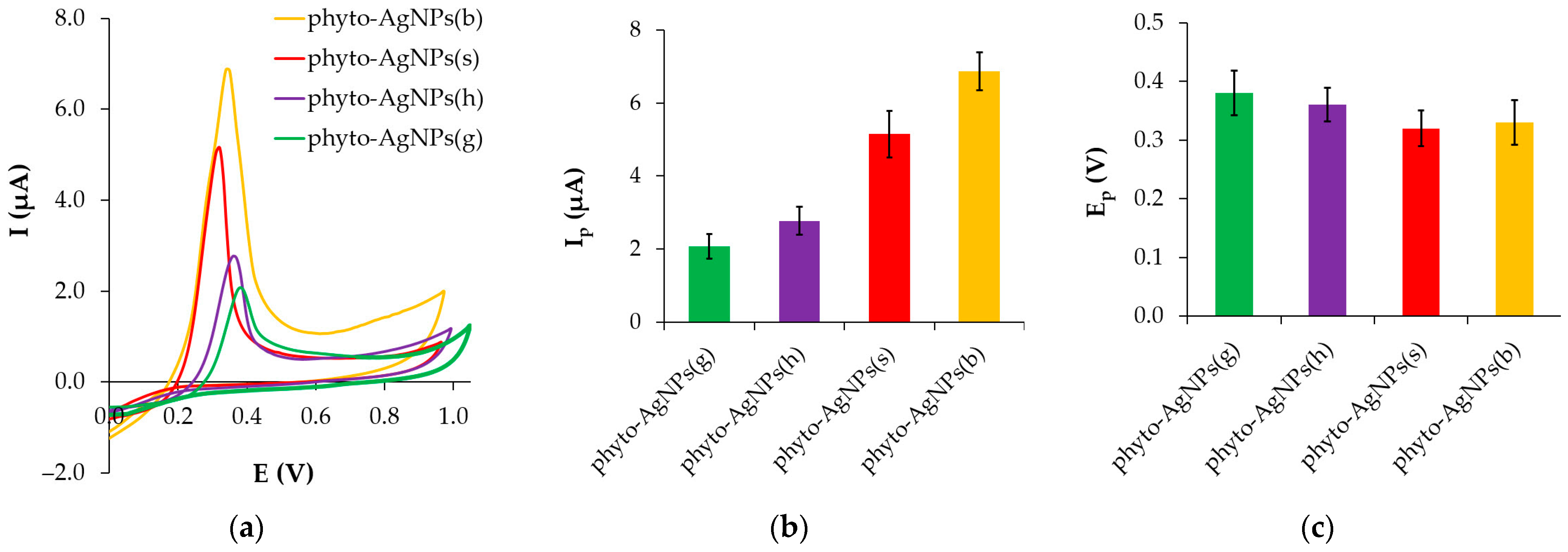
3.4.2. Cyclic Voltammetry (CV)
3.5. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magdy, G.; Aboelkassim, E.; Elhaleem, S.M.A.; Belal, F. A comprehensive review on silver nanoparticles: Synthesis approaches, characterization techniques, and recent pharmaceutical, environmental, and antimicrobial applications. Microchem. J. 2024, 196, 109615. [Google Scholar] [CrossRef]
- Faris, V.M.; Barzinjy, A.A.; Hamad, S.M. The Applications of Green Synthesized Silver Nanoparticles: A Review. Eurasian J. Sci. Eng. 2022, 8, 16–34. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Ong, W.T.J.; Nyam, K.L. Evaluation of silver nanoparticles in cosmeceutical and potential biosafety complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef]
- Angelopoulou, P.; Giaouris, E.; Gardikis, K. Applications and Prospects of Nanotechnology in Food and Cosmetics Preservation. Nanomaterials 2022, 12, 1196. [Google Scholar] [CrossRef]
- Satsangi, N. Synthesis and Characterization of Biocompatible Silver Nanoparticles for Anticancer Application. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1907–1914. [Google Scholar] [CrossRef]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Arroyo, G.V.; Madrid, A.T.; Gavilanes, A.F.; Naranjo, B.; Debut, A.; Arias, M.T.; Angulo, Y. Green synthesis of silver nanoparticles for application in cosmetics. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 55, 1304–1320. [Google Scholar] [CrossRef]
- Wani, I.A. Review—Recent Advances in Biogenic Silver Nanoparticles & NanoComposite Based Plasmonic-Colorimetric and Electrochemical Sensors. ECS J. Solid State Sci. Technol. 2021, 10, 047003. [Google Scholar] [CrossRef]
- Desalegn, T.; Ravikumar, C.R.; Murthy, H.C.A. Eco-friendly synthesis of silver nanostructures using medicinal plant Vernonia amygdalina Del. leaf extract for multifunctional applications. Appl. Nanosci. 2021, 11, 535–551. [Google Scholar] [CrossRef]
- Umair Raza, M.; Abasi, F.; Shahbaz, M.; Ehsan, M.; Seerat, W.; Akram, A.; Raja, N.I.; Mashwani, Z.u.-R.; Hassan, H.U.; Proćków, J. Phytomediated Silver Nanoparticles (AgNPs) Embellish Antioxidant Defense System, Ameliorating HLB-Diseased ‘Kinnow’ Mandarin Plants. Molecules 2023, 28, 2044. [Google Scholar] [CrossRef]
- Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.T.; Van Mai, B.; Van Nguyen, D.; Nguyen, N.Q.T.; Van Pham, V.; Pham, T.L.M.; Le, H.T. Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines. Green Process. Synth. 2023, 12, 20230024. [Google Scholar] [CrossRef]
- Javed, B.; Nadhman, A.; Mashwani, Z.R. Phytosynthesis of Ag nanoparticles from Mentha longifolia: Their structural evaluation and therapeutic potential against HCT116 colon cancer, Leishmanial and bacterial cells. Appl. Nanosci. 2020, 10, 3503–3515. [Google Scholar] [CrossRef]
- Murthy, H.C.A.; Zeleke, T.D.; Ravikumar, C.R.; Kumar, M.R.A.; Nagaswarupa, H.P. Electrochemical properties of biogenic silver nanoparticles synthesized using Hagenia abyssinica (Brace) JF. Gmel. medicinal plant leaf extract. Mater. Res. Express 2020, 7, 055016. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Obaid, A.Y.; Al-Thabaiti, S.A.; El-Mossalamy, E.H.; Al-Harbi, L.M.; Khan, Z. Extracellular bio-synthesis of silver nanoparticles. Arab. J. Chem. 2017, 10, 226–231. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver Nanoparticles Mediated by Costus afer Leaf Extract: Synthesis, Antibacterial, Antioxidant and Electrochemical Properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef]
- Ajaykumar, A.P.; Mathew, A.; Chandni, A.P.; Varma, S.R.; Jayaraj, K.N.; Sabira, O.; Rasheed, V.A.; Binitha, V.S.; Swaminathan, T.R.; Basheer, V.S.; et al. Green Synthesis of Silver Nanoparticles Using the Leaf Extract of the Medicinal Plant, Uvaria narum and Its Antibacterial, Antiangiogenic, Anticancer and Catalytic Properties. Antibiotics 2023, 12, 564. [Google Scholar] [CrossRef]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Farnad, N.; Farhadi, K. Introducing potato starch-ecofriendly silver nanoparticles as a novel binary system for nanoencapsulation of riboflavin. Food Chem. 2023, 398, 133910. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Chang, L.; Satti, U.Q.; Rehman, S.u.; Arshad, H.; Mustafa, G.; Shaukat, U.; Wang, F.; Tong, C. Phyto-Synthesis, Characterization, and In Vitro Antibacterial Activity of Silver Nanoparticles Using Various Plant Extracts. Bioengineering 2022, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, B.N.; Harlapur, S.F.; Avinash, B.; Ravikumar, C.R.; Nagaswarupa, H.P.; Anil Kumar, M.R.; Gurushantha, K.; Santosh, M.S. Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorg. Chem. Commun. 2020, 111, 107580. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, S336–S343. [Google Scholar] [CrossRef]
- Waiezi, S.; Nik Malek, N.A.N.; Asraf, M.H.; Sani, N.S. Preparation, Characterization, and Antibacterial Activity of Green-Biosynthesised Silver Nanoparticles using Clinacanthus nutans Extract. Biointerface Res. Appl. Chem. 2023, 13, 171. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Fayemi, O.E.; Botha, T.L. Green synthesis and electrochemistry of Ag, Au, and Ag–Au bimetallic nanoparticles using golden rod (Solidago canadensis) leaf extract. Appl. Phys. A 2019, 125, 42. [Google Scholar] [CrossRef]
- Phuong, T.N.M.; Thanh Hai, N.T.; Thu Thuy, N.T.; Phu, N.V.; Huong, N.T.; Hang Nga, D.T.; Quoc Huy, H.X.; Hoa, T.T. Factors affecting synthesis of silver-nanoparticles and antimicrobial applications. Hue Univ. J. Sci. Nat. Sci. 2020, 129, 25–31. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2020, 13, 1011–1019. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta Part A 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Abada, E.; Mashraqi, A.; Modafer, Y.; Al Abboud, M.A.; El-Shabasy, A. Review green synthesis of silver nanoparticles by using plant extracts and their antimicrobial activity. Saudi J. Biol. Sci. 2024, 31, 103877. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Olugbeko, S.C.; Folorunso, A.S.; Oyebamiji, A.K.; Folorunso, F.A. Potentials of phytosynthesized silver nanoparticles in biomedical fields: A review. Int. Nano Lett. 2021, 11, 273–293. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Chakraborty, S.; Owens, G.; Islam, R.U. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): The wonder particle of the past decade. Appl. Nanosci. 2021, 11, 2625–2660. [Google Scholar] [CrossRef] [PubMed]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Vidrevich, M.B.; Brainina, K.Z. The Effect of the Antioxidant Activity of Plant Extracts on the Properties of Gold Nanoparticles. Nanomaterials 2019, 9, 1655. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V. Electrochemical Properties of Phytosynthesized Gold Nanoparticles for Electrosensing. Sensors 2022, 22, 311. [Google Scholar] [CrossRef]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Kazakov, Y.; Stozhko, N. Disposable Potentiometric Sensory System for Skin Antioxidant Activity Evaluation. Sensors 2019, 19, 2586. [Google Scholar] [CrossRef]
- Mohanaparameswari, S.; Balachandramohan, M.; Sasikumar, P.; Rajeevgandhi, C.; Vimalan, M.; Pugazhendhi, S.; Ganesh Kumar, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; et al. Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method. Green Process. Synth. 2023, 12, 20230080. [Google Scholar] [CrossRef]
- Lateef, A.; Akande, M.A.; Azeez, M.A.; Ojo, S.A.; Folarin, B.I.; Gueguim-Kana, E.B.; Beukes, L.S. Phytosynthesis of silver nanoparticles (AgNPs) using miracle fruit plant (Synsepalum dulcificum) for antimicrobial, catalytic, anticoagulant, and thrombolytic applications. Nanotechnol. Rev. 2016, 5, 507–520. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Manzoor, N.; Ahmed, J.; Asiri, A.M. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C. The Basics of Crystallography and Diffraction, 3rd ed.; Oxford University Press: New York, NY, USA, 2009; pp. 281–282. [Google Scholar]
- Arblaster, J.W. Selected Values of the Crystallographic Properties of the Elements; ASM International: Materials Park, OH, USA, 2018; p. 363. [Google Scholar]
- Hung, N.D.; Nam, V.N.; ThiNhan, T.; Dung, T.T.N. Quantitative concentration determination of silver nanoparticles prepared by DC high voltage electrochemical method. Vietnam J. Chem. 2018, 56, 553–558. [Google Scholar] [CrossRef]
- Mekuye, B. The Impact of Size on the Optical Properties of Silver Nanoparticles Based on Dielectric Function. In Nanotechnology and Nanomaterials-Annual; Jiménez-Suárez, A., Seisdedos, G., Eds.; IntechOpen: London, UK, 2024; Volume 2024, pp. 1–20. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Kiryuhina, T.Y.; Galperin, A.L.; Stozhko, N.Y.; Murzakaev, A.M.; Timoshenkova, O.R. Silver nanoparticles electrooxidation: Theory and experiment. J. Solid State Electrochem. 2012, 16, 2365–2372. [Google Scholar] [CrossRef]


| Plant Extract | AOA, mmol-eq L−1 |
|---|---|
| Gooseberry (Ribes uva-crispa) | 1.8 ± 0.3 |
| Blue honeysuckle (Lonicera caerulea) | 6.4 ± 1.1 |
| Strawberry (Fragaria vesca) | 11.8 ± 0.9 |
| Sea buckthorn (Hippophae rhamnoides) | 15.7 ± 1.2 |
| AgNPs | Diameter Range (nm) | Diameter Value (nm) | PI | Lattice Parameter (nm) |
|---|---|---|---|---|
| phyto-AgNPs(g) | 4–24 | 13 ± 5 | 0.15 | 0.4216 |
| phyto-AgNPs(h) | 4–29 | 10 ± 7 | 0.49 | 0.4201 |
| phyto-AgNPs(s) | 3–20 | 8 ± 4 | 0.25 | 0.4195 |
| phyto-AgNPs(b) | 2–15 | 7 ± 3 | 0.18 | 0.4123 |
| Parameters | AOA | PI | Amax | λSPR | Rct | Ip | Ep | |
|---|---|---|---|---|---|---|---|---|
| AOA | 1 | −0.98 * | −0.13 | 0.99 * | −0.96 * | −0.95 | 0.98 * | −0.92 |
| 1 | −0.04 | −0.96 * | 0.99 * | 0.88 | −0.93 | 0.94 | ||
| PI | 1 | −0.20 | −0.03 | 0.26 | −0.31 | 0.08 | ||
| Amax | 1 | −0.94 | −0.96 * | 0.99 * | −0.90 | |||
| λSPR | 1 | 0.82 | −0.90 | 0.98 * | ||||
| Rct | 1 | −0.97 * | 0.75 | |||||
| Ip | 1 | −0.88 | ||||||
| Ep | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stozhko, N.; Tarasov, A.; Tamoshenko, V.; Bukharinova, M.; Khamzina, E.; Kolotygina, V. Green Silver Nanoparticles: Plant-Extract-Mediated Synthesis, Optical and Electrochemical Properties. Physchem 2024, 4, 402-419. https://doi.org/10.3390/physchem4040028
Stozhko N, Tarasov A, Tamoshenko V, Bukharinova M, Khamzina E, Kolotygina V. Green Silver Nanoparticles: Plant-Extract-Mediated Synthesis, Optical and Electrochemical Properties. Physchem. 2024; 4(4):402-419. https://doi.org/10.3390/physchem4040028
Chicago/Turabian StyleStozhko, Natalia, Aleksey Tarasov, Viktoria Tamoshenko, Maria Bukharinova, Ekaterina Khamzina, and Veronika Kolotygina. 2024. "Green Silver Nanoparticles: Plant-Extract-Mediated Synthesis, Optical and Electrochemical Properties" Physchem 4, no. 4: 402-419. https://doi.org/10.3390/physchem4040028
APA StyleStozhko, N., Tarasov, A., Tamoshenko, V., Bukharinova, M., Khamzina, E., & Kolotygina, V. (2024). Green Silver Nanoparticles: Plant-Extract-Mediated Synthesis, Optical and Electrochemical Properties. Physchem, 4(4), 402-419. https://doi.org/10.3390/physchem4040028









