Phosphatidylinositol-4,5-biphosphate (PIP2)-Dependent Thermoring Basis for Cold-Sensing of the Transient Receptor Potential Melastatin-8 (TRPM8) Biothermometer
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Mining Resources
2.2. The Definition of the Necessary Gating Pathway
2.3. Preparation of the Systematic Fluidic Noncovalent Interaction Mesh Network Maps
2.4. Equations
3. Results
3.1. Identification of a Phosphatidylinositol-4,5-biphosphate (PIP2)-Dependent Cooling Switch at 32–37 °C in Mouse Transient Receptor Potential Melastatin-8 (mTRPM8)
3.2. Addition of Ca2+ and Cooling Agent cryosim-3 (C3) Decreased the Activation Threshold Range of Mouse Transient Receptor Potential Melastatin-8 (mTRPM8) to 22–27 °C
3.3. Further Addition of allyl isothiocyanate (AITC) Opened Mouse Transient Receptor Potential Melastatin-8 (mTRPM8) at the Matched Threshold of 20 °C
4. Discussion
4.1. Interaction of Transient Receptor Potential Melastatin-8 (TRPM8) with Phosphatidylinositol-4,5-biphosphate (PIP2) Determined the Temperature Threshold Range for Channel Opening
4.2. Implication of Transient Receptor Potential Melastatin-8 (TRPM8) Activation by Lysophospholipids (LPLs) and Menthol
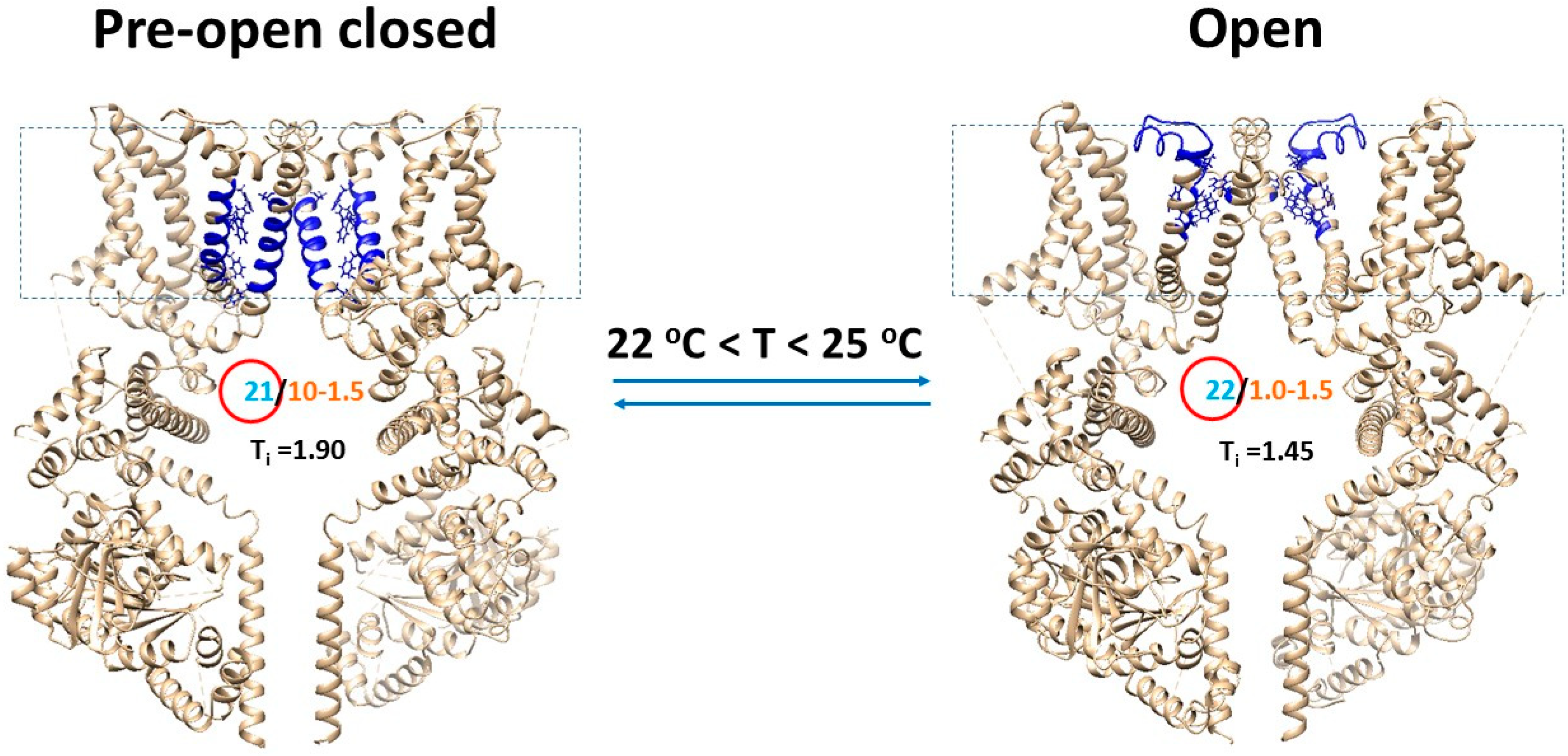
4.3. Overlapped Temperature Thresholds between Open and Pre-Open Closed States Were Required for Cold-Sensing
4.4. Thermostable F874–Y908 π Interaction in the Pore Domain Was Needed for the Active Selectivity Filter
4.5. Thermostable Anchor Grid7 at the S5/S6 interface Was Necessary for the Active Lower S6 Gate
4.6. Transient Receptor Potential Melastatin-8 (TRPM8) Opened with the Matched Thermosensitivity and the Decrease in the Systematic Thermal Instability
5. Closing Remark
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Reid, G.; Babes, A.; Pluteanu, F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: Properties and role in cold transduction. J. Physiol. 2002, 545, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-H.; Neuhausser, W.M.; Julius, D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 2004, 43, 859–869. [Google Scholar] [CrossRef]
- Rohács, T.; Lopes, C.M.B.; Michailidis, I.; E Logothetis, D. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005, 8, 626–634. [Google Scholar] [CrossRef]
- Liu, B.; Qin, F. Functional control of cold- and menthol-sensitive TRPM8 Ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005, 25, 1674–1681. [Google Scholar] [CrossRef]
- Voets, T.; Owsianik, G.; Janssens, A.; Talavera, K.; Nilius, B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007, 3, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Bödding, M.; Wissenbach, U.; Flockerzi, V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium 2007, 42, 618–628. [Google Scholar] [CrossRef]
- Zakharian, E.; Cao, C.; Rohacs, T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 2010, 30, 12526–12534. [Google Scholar] [CrossRef]
- Abeele, F.V.; Zholos, A.; Bidaux, G.; Shuba, Y.; Thebault, S.; Beck, B.; Flourakis, M.; Panchin, Y.; Skryma, R.; Prevarskaya, N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J. Biol. Chem. 2006, 281, 40174–40182. [Google Scholar] [CrossRef]
- Andersson, D.A.; Nash, M.; Bevan, S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J. Neurosci. 2007, 27, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Chase, H.W.N.; Bevan, S. TRPM8 activation by menthol, icilin, and cold Is differentially modulated by intracellular pH. J. Neurosci. 2004, 24, 5364–5369. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.-E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J.; Wang, Y., Jr.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef]
- Wang, S.; Lee, J.; Ro, J.Y.; Chung, M.-K. Warmth suppresses and desensitizes damage-sensing ion channel TRPA1. Mol. Pain 2012, 8, 22. [Google Scholar] [CrossRef]
- Bandell, M.; Dubin, A.E.; Petrus, M.J.; Orth, A.; Mathur, J.; Hwang, S.W.; Patapoutian, A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 2006, 9, 493–500. [Google Scholar] [CrossRef]
- Wang, G. Ligand-stereoselective allosteric activation of cold-sensing TRPM8 channels by an H-bonded homochiral menthol dimer with head-to-head or head-to-tail. Chirality 2021, 33, 783–796. [Google Scholar] [CrossRef]
- Brauchi, S.; Orio, P.; Latorre, R. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc. Natl. Acad. Sci. USA 2004, 101, 15494–15499. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, F.; Feng, S.; Butay, K.J.; Borgnia, M.J.; Im, W.; Lee, S.-Y. Activation mechanism of the mouse cold-sensing TRPM8 channel by cooling agonist and PIP2. Science 2022, 378, eadd1268. [Google Scholar] [CrossRef] [PubMed]
- Jonstrup, A.T.; Fredsøe, J.; Andersen, A.H. DNA hairpins as temperature switches, thermometers and ionic detectors. Sensors 2013, 13, 5937–5944. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. The network basis for the structural thermostability and the functional thermoactivity of aldolase B. Molecules 2023, 28, 1850. [Google Scholar] [CrossRef]
- Wang, G. Network basis for the heat-adapted structural thermostability of bacterial class II fructose bisphosphate aldolase. ACS Omega 2023, 8, 17731–17739. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Thermal ring-based heat switches in hyperthermophilic class II bacterial fructose aldolase. ACS Omega 2023, 8, 24624–24634. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Thermoring-Based Heat Activation Switches in the TRPV1 Biothermometer. Int. J. Biol. Macromol. 2023, 248, 125915. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Thermoring basis for the TRPV3 bio-thermometer. Sci. Rep. 2023, 13, 21594. [Google Scholar] [CrossRef]
- Floyd, R.W. Algorithm-97: Shortest Path. Commun. Acm 1962, 5, 345. [Google Scholar] [CrossRef]
- Kiehna, S.E.; Waters, M.L. Sequence dependence of β-hairpin structure: Comparison of a salt bridge and an aromatic interaction. Protein Sci. 2003, 12, 2657–2667. [Google Scholar] [CrossRef]
- Neel, A.J.; Hilton, M.J.; Sigman, M.S.; Toste, F.D. Exploiting non-covalent π interactions for catalyst design. Nature 2017, 543, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Fujita, F.; Uchida, K.; Takaishi, M.; Sokabe, T.; Tominaga, M. Ambient temperature affects the temperature threshold for TRPM8 activation through interaction of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2013, 33, 6154–6159. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Neuberger, A.; Trofimov, Y.A.; Krylov, N.A.; Sinica, V.; Kupko, N.; Vlachova, V.; Zakharian, E.; Efremov, R.G.; Sobolevsky, A.I. Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nat. Struct. Mol. Biol. 2021, 28, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Zhang, F.; Suo, Y.; Bouvette, J.; Borgnia, M.J.; Lee, S.-Y. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat. Struct. Mol. Biol. 2021, 28, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Yudin, Y.; Lukacs, V.; Cao, C.; Rohacs, T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J Physiol. 2011, 589, 6007–6027. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Han, Y.; Chen, X.; Aierken, A.; Wen, H.; Zheng, W.; Wang, H.; Lu, X.; Zhao, Z.; Ma, C.; et al. Molecular mechanisms underlying menthol binding and activation of TRPM8 ion channel. Nat. Commun. 2020, 11, 3790. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gisselmann, G.; Vogt-Eisele, A.K.; Doerner, J.F.; Hatt, H. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak. J. Pharm. Sci. 2008, 21, 370–378. [Google Scholar]
- Chen, X.; Xu, L.; Zhang, H.; Wen, H.; Yang, F. Differential Activation of TRPM8 by the Stereoisomers of Menthol. Front. Pharmacol. 2022, 13, 898670. [Google Scholar] [CrossRef]
- Gamper, N.; Shapiro, M.S. Target-specific PIP2 signalling: How might it work? J. Physiol. 2007, 582, 967–975. [Google Scholar] [CrossRef]
- Matos-Cruz, V.; Schneider, E.R.; Mastrotto, M.; Merriman, D.K.; Bagriantsev, S.N.; Gracheva, E.O. Molecular Prerequisites for Diminished Cold Sensitivity in Ground Squirrels and Hamsters. Cell Rep. 2017, 21, 3329–3337. [Google Scholar] [CrossRef]
- Pertusa, M.; Rivera, B.; González, A.; Ugarte, G.; Madrid, R. Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J. Biol. Chem. 2018, 293, 12454–12471. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lu, X.; Wang, Y.; Xu, L.; Chen, X.; Yang, F.; Lai, R. A paradigm of thermal adaptation in penguins and elephants by tuning cold activation in TRPM8. Proc. Natl. Acad. Sci. USA 2020, 117, 8633–8638. [Google Scholar] [CrossRef] [PubMed]
- Bidaux, G.; Sgobba, M.; Lemonnier, L.; Borowiec, A.-S.; Noyer, L.; Jovanovic, S.; Zholos, A.V.; Haider, S. Functional and modeling studies of the transmembrane region of the TRPM8 channel. Biophys. J. 2015, 109, 1840–1851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, C.; Xie, Y.; Xu, L.; Ye, F.; Xu, X.; Yang, W.; Yang, F.; Guo, J. Structures of a mammalian TRPM8 in closed state. Nat. Commun. 2022, 13, 3113. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Kusakizako, T.; Nguyen, T.H.D.; Nishizawa, T.; Hin, T.; Tominaga, M.; Nureki, O. The structure of lipid nanodisc-reconstituted TRPV3 reveals the gating mechanism. Nat. Struct. Mol. Biol. 2020, 27, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Taberner, F.J.; López-Córdoba, A.; Fernández-Ballester, G.; Korchev, Y.; Ferrer-Montiel, A. The region adjacent to the C-end of the inner gate in transient receptor potential melastatin 8 (TRPM8) channels plays a central role in allosteric channel activation. J. Biol. Chem. 2014, 289, 28579–28594. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.A.; Skryma, R.; Bidaux, G.; Magleby, K.L.; Scholfield, C.N.; McGeown, J.G.; Prevarskaya, N.; Zholos, A.V. Voltage- and cold-dependent gating of single TRPM8 ion channels. J. Gen. Physiol. 2011, 137, 173–195. [Google Scholar] [CrossRef]
- Sisco, N.J.; Helsell, C.V.M.; Van Horn, W.D. Competitive Interactions between PIRT, the Cold Sensing Ion Channel TRPM8, and PIP2 Suggest a Mechanism for Regulation. Sci. Rep. 2019, 9, 14128. [Google Scholar] [CrossRef]
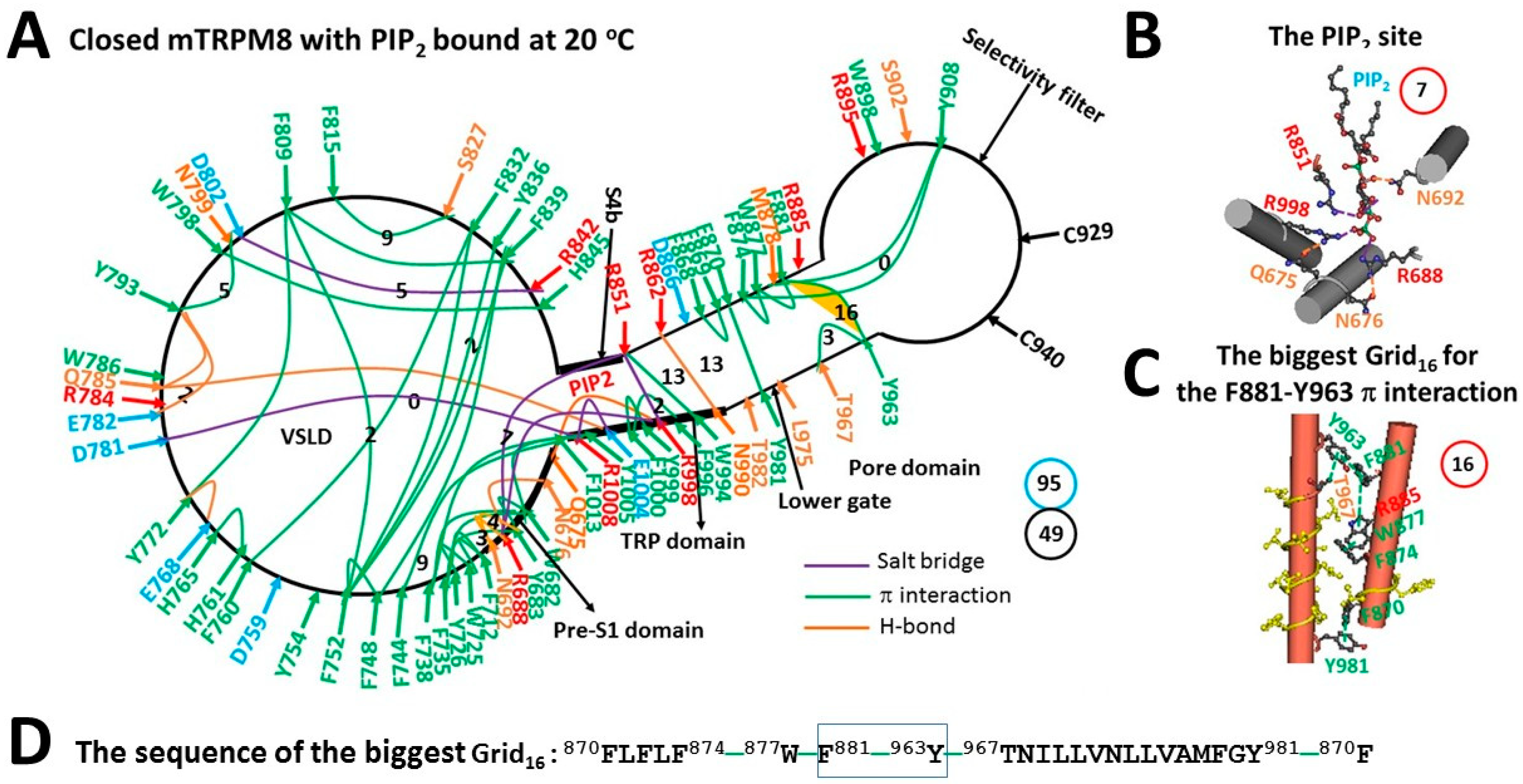
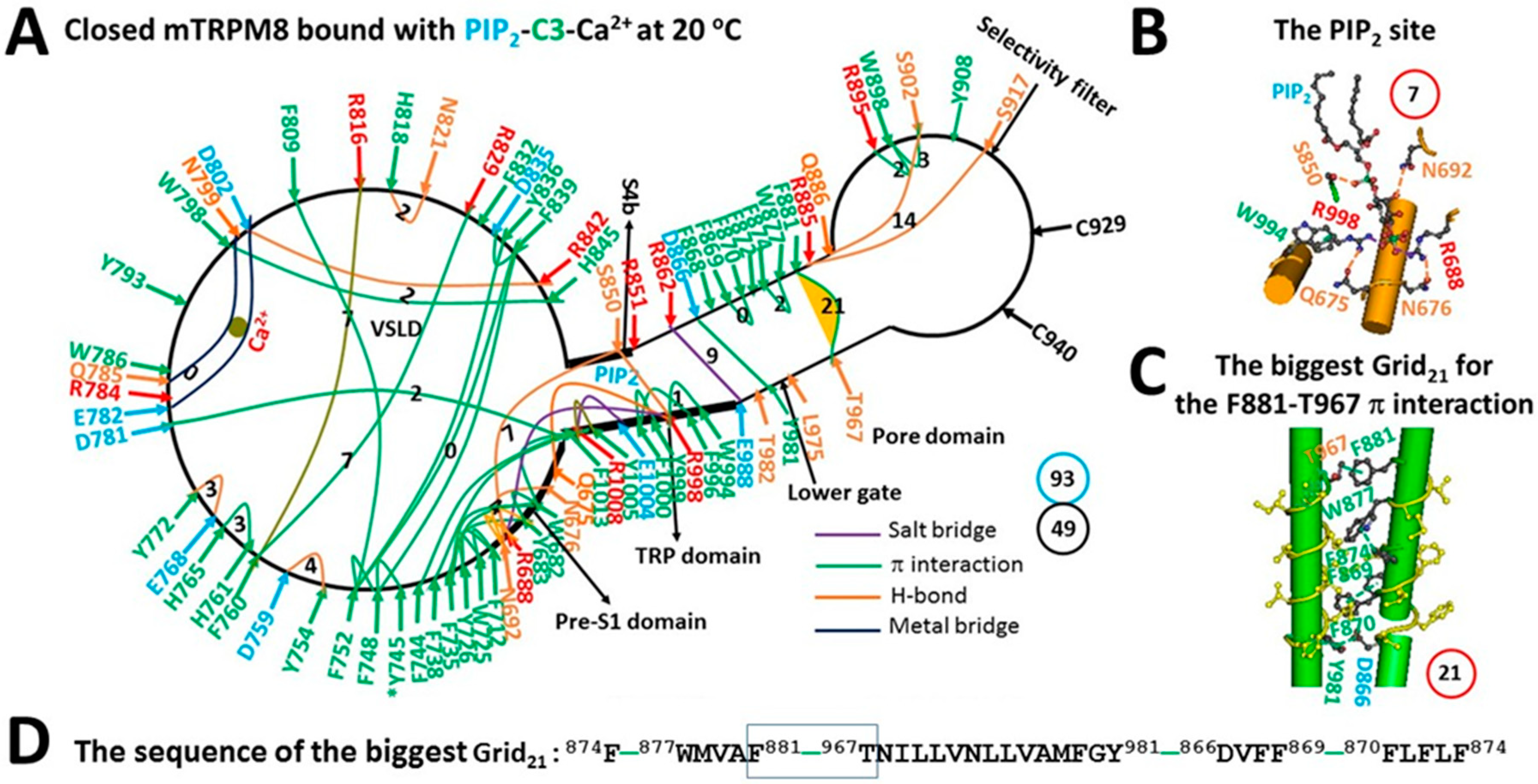

| Construct | mTRPM8 | ||
|---|---|---|---|
| PDB ID | 8E4L | 8E4M | 8E4N |
| Ca2+ | bound | bound | free |
| PIP2 | bound | bound | bound |
| Sampling temperature, °C | 20 | 20 | 20 |
| Gating state | open | closed | closed |
| Name of the biggest grid | Grid22 | Grid21 | Grid16 |
| Biggest grid size (Smax) | 22 | 21 | 16 |
| Equivalent basic H-bonds to control Smax | 1.0–1.5 | 1.0–1.5 | 1.0–1.5 |
| Total noncovalent interactions | 51 | 49 | 49 |
| Total grid sizes, a. a./atoms | 74 | 93 | 95 |
| Systematic thermal instability (Ti) | 1.45 | 1.90 | 1.94 |
| Calculated Tm, °C | 20–25 | 22–27 | 32–37 |
| Measured threshold Tth, °C | 23–29 | 23–29 | 31–37 |
| Calculated Ω10, min at E = 0.5 kcal/mol | −4.46 | ||
| Calculated Ω10, mean at E = 1.0 kcal/mol | −8.68 | ||
| Calculated Ω10, max at E = 3.0 kcal/mol | −24.9 | ||
| Measured Q10 | −9.16 | ||
| Ref. for measured Tth and Q10 | [5] | [5] | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G. Phosphatidylinositol-4,5-biphosphate (PIP2)-Dependent Thermoring Basis for Cold-Sensing of the Transient Receptor Potential Melastatin-8 (TRPM8) Biothermometer. Physchem 2024, 4, 106-119. https://doi.org/10.3390/physchem4020008
Wang G. Phosphatidylinositol-4,5-biphosphate (PIP2)-Dependent Thermoring Basis for Cold-Sensing of the Transient Receptor Potential Melastatin-8 (TRPM8) Biothermometer. Physchem. 2024; 4(2):106-119. https://doi.org/10.3390/physchem4020008
Chicago/Turabian StyleWang, Guangyu. 2024. "Phosphatidylinositol-4,5-biphosphate (PIP2)-Dependent Thermoring Basis for Cold-Sensing of the Transient Receptor Potential Melastatin-8 (TRPM8) Biothermometer" Physchem 4, no. 2: 106-119. https://doi.org/10.3390/physchem4020008
APA StyleWang, G. (2024). Phosphatidylinositol-4,5-biphosphate (PIP2)-Dependent Thermoring Basis for Cold-Sensing of the Transient Receptor Potential Melastatin-8 (TRPM8) Biothermometer. Physchem, 4(2), 106-119. https://doi.org/10.3390/physchem4020008






