An Investigation of Antimicrobial Activity for Plant Pathogens by Green-Synthesized Silver Nanoparticles Using Azadirachta indica and Mangifera indica
Abstract
1. Introduction
2. Methods
2.1. Chemicals and Plants
| Azadirachta indica | Mangifera indica |
| Family: Meliaceae | Family: Anacardiaceae |
| Common name: Neem | Common name: Mango |
| Species: A. indica | Species: M. indica |
| Genus: Azadirachta | Genus: Magnifera |
2.2. Pathogens
2.3. Preparation of Plant Extract
2.4. Synthesis of Silver Nanoparticles
2.5. Preparation of PDA Media
2.6. Isolation of Fungus and Bacteria
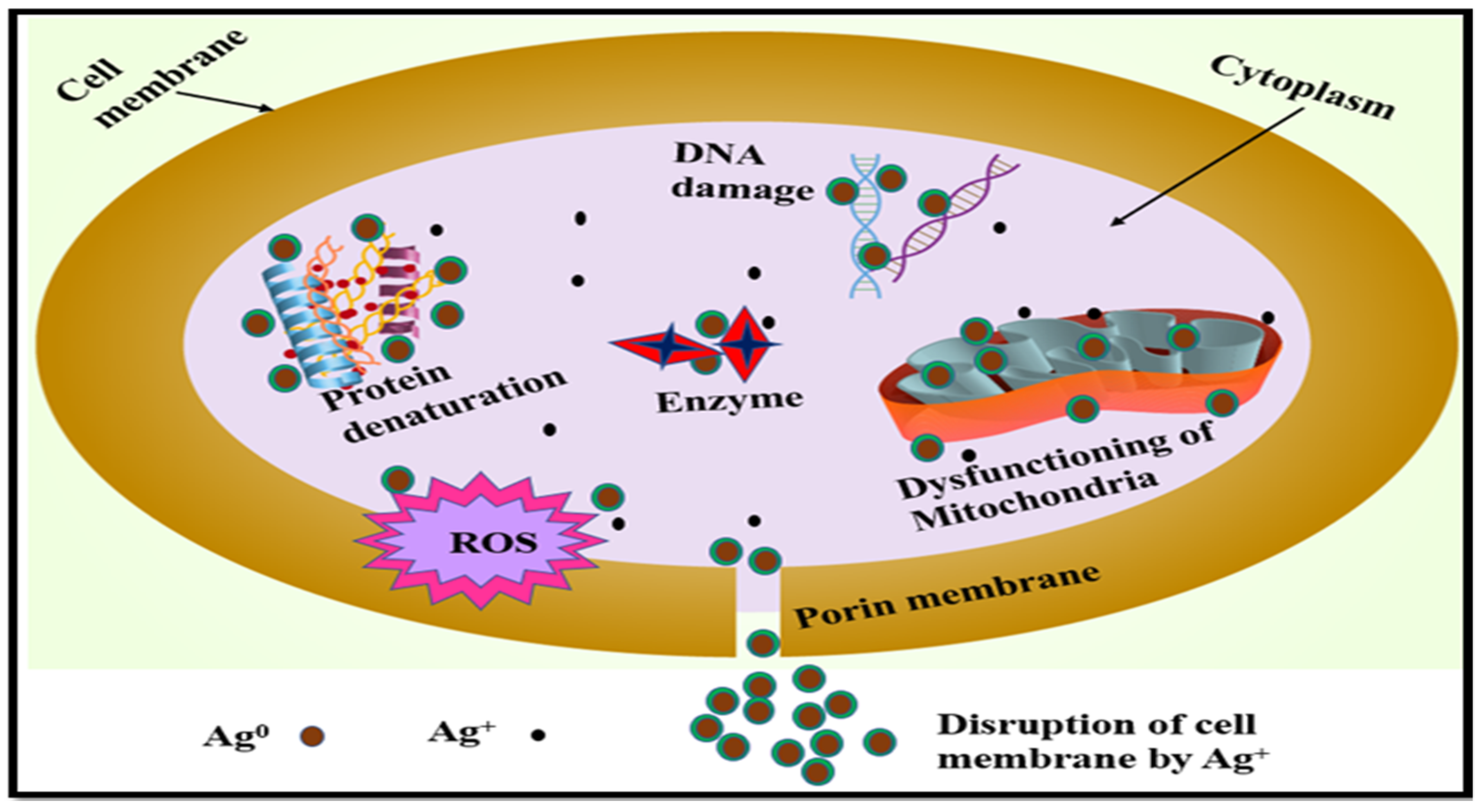
2.7. Mechanism of Silver Nanoparticles
2.8. Antioxidant Assay
2.8.1. Free Radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH) Scavenger
2.8.2. Phytochemical Presence in Different Plants
3. Characterization of the Synthesized AgNPs
4. Antimicrobial Activity
4.1. Anti-Fungal Activity
4.2. Anti-Bacterial Activity
5. Results
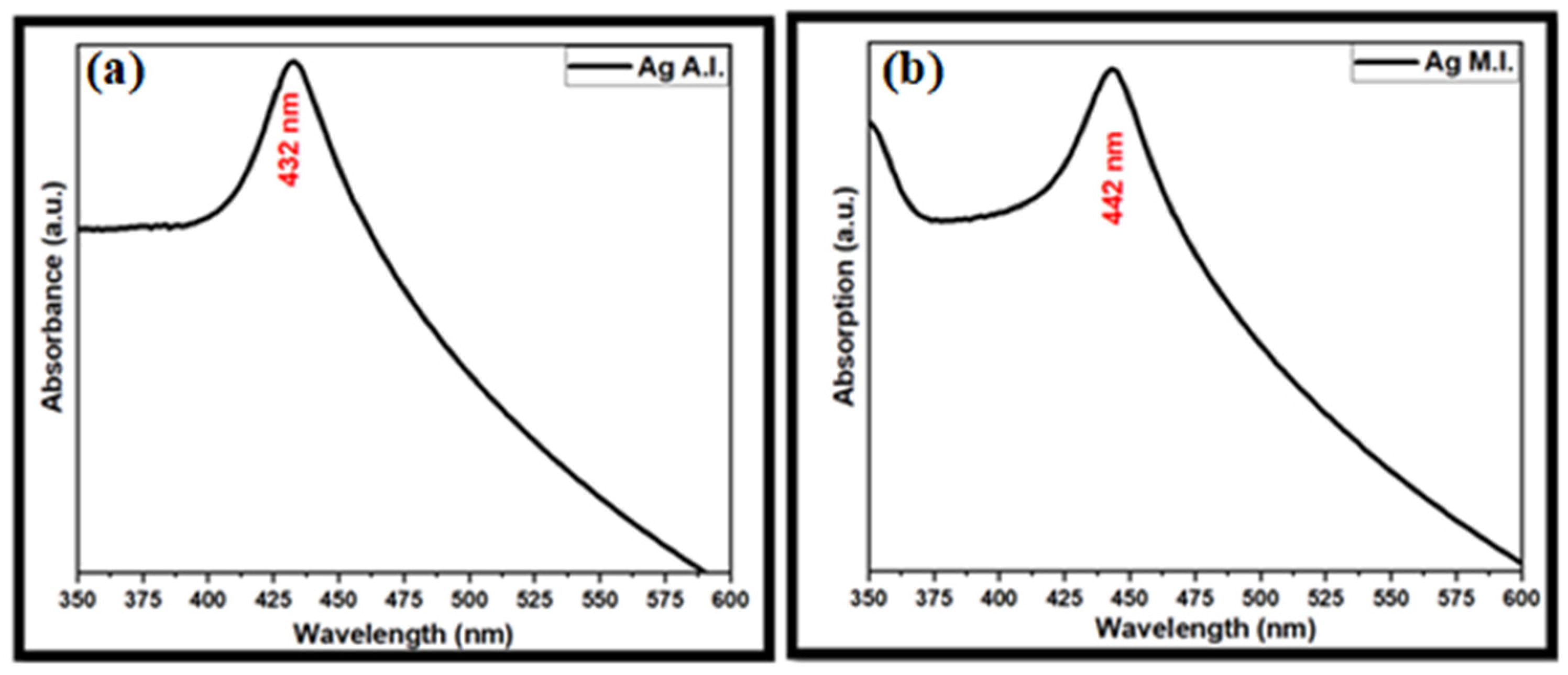
5.1. Optical Analysis
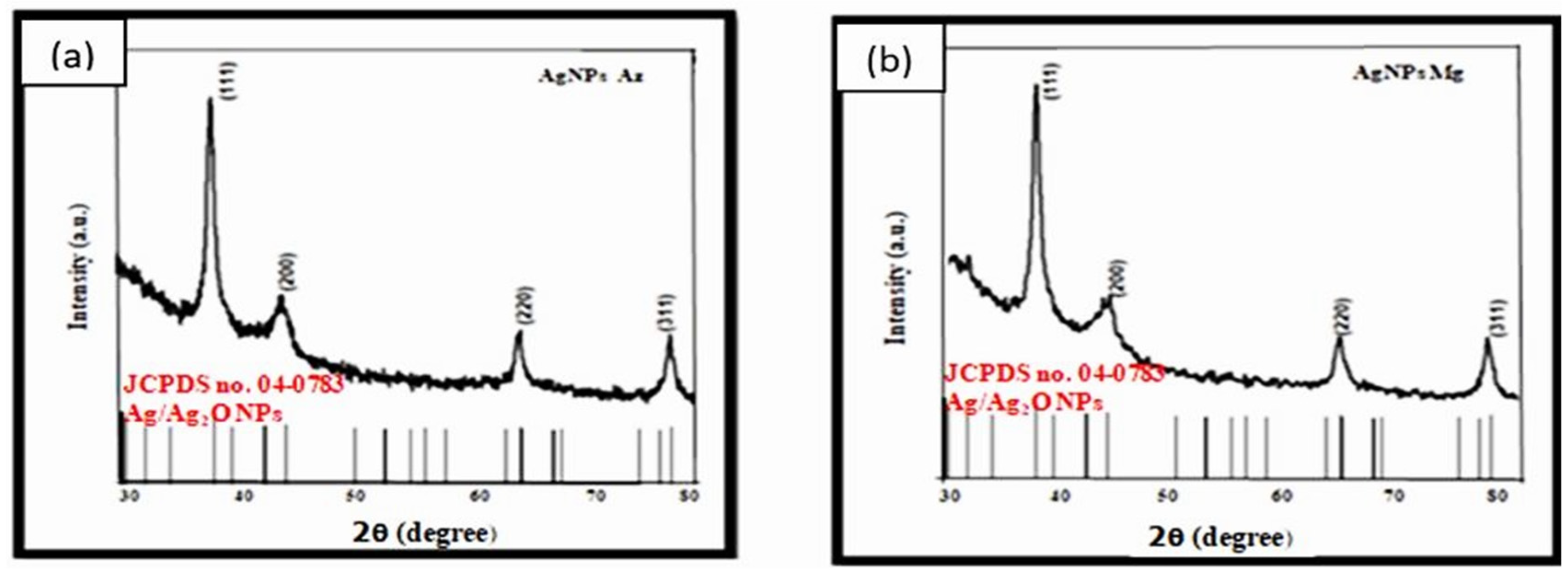
5.2. GIXRD (Grazing Incidence X-ray Diffraction)
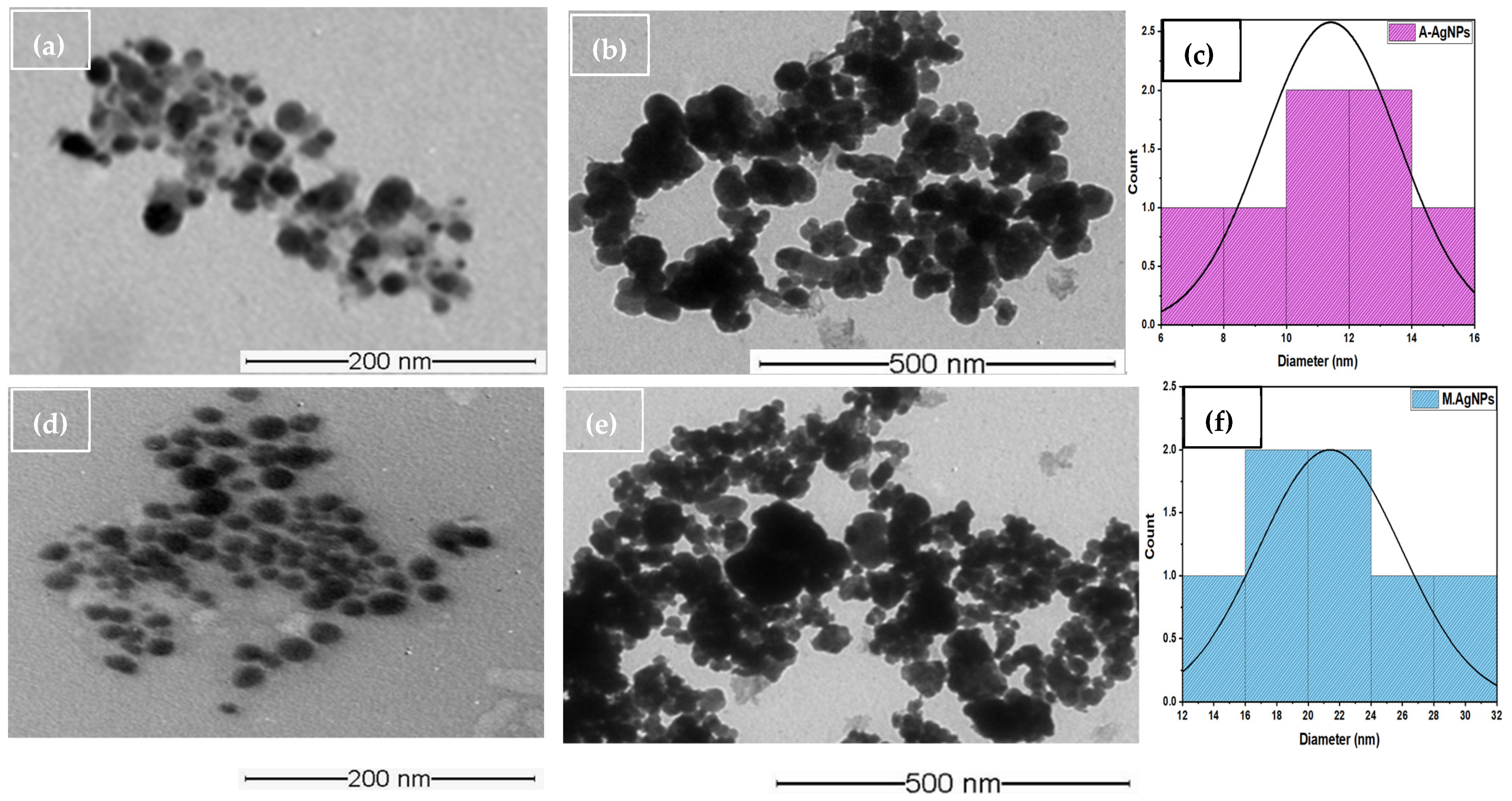
5.3. TEM (Size and Shape)
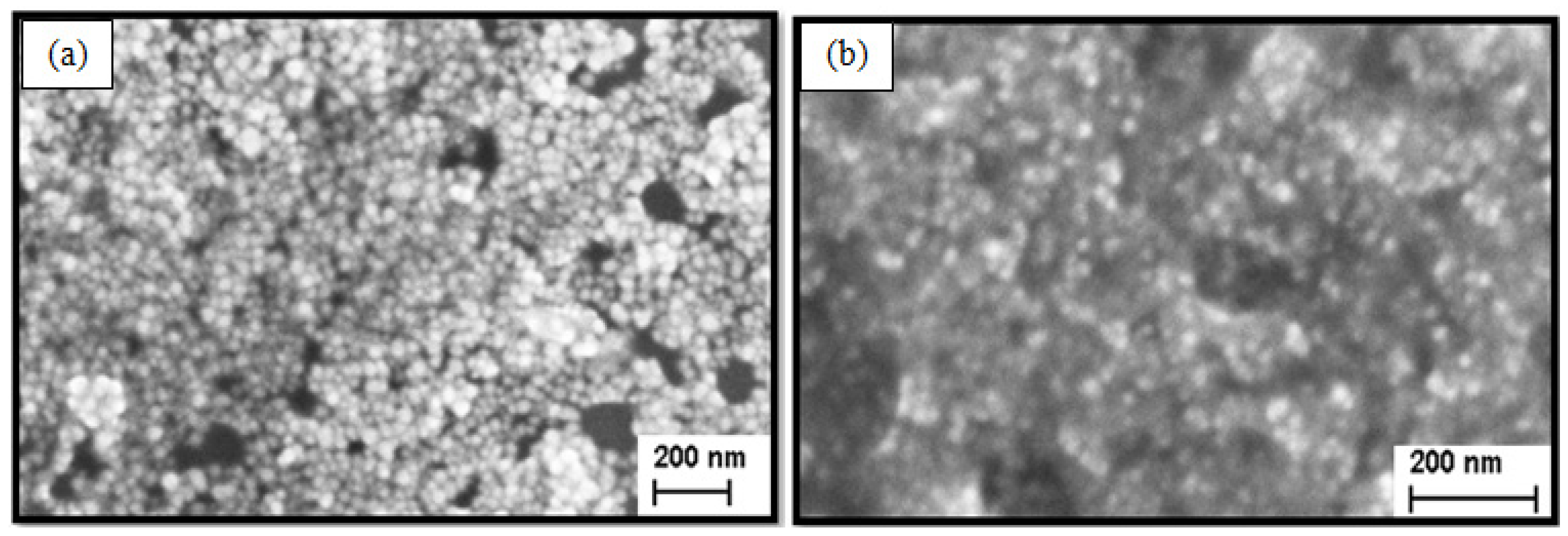
5.4. SEM (Morphology)
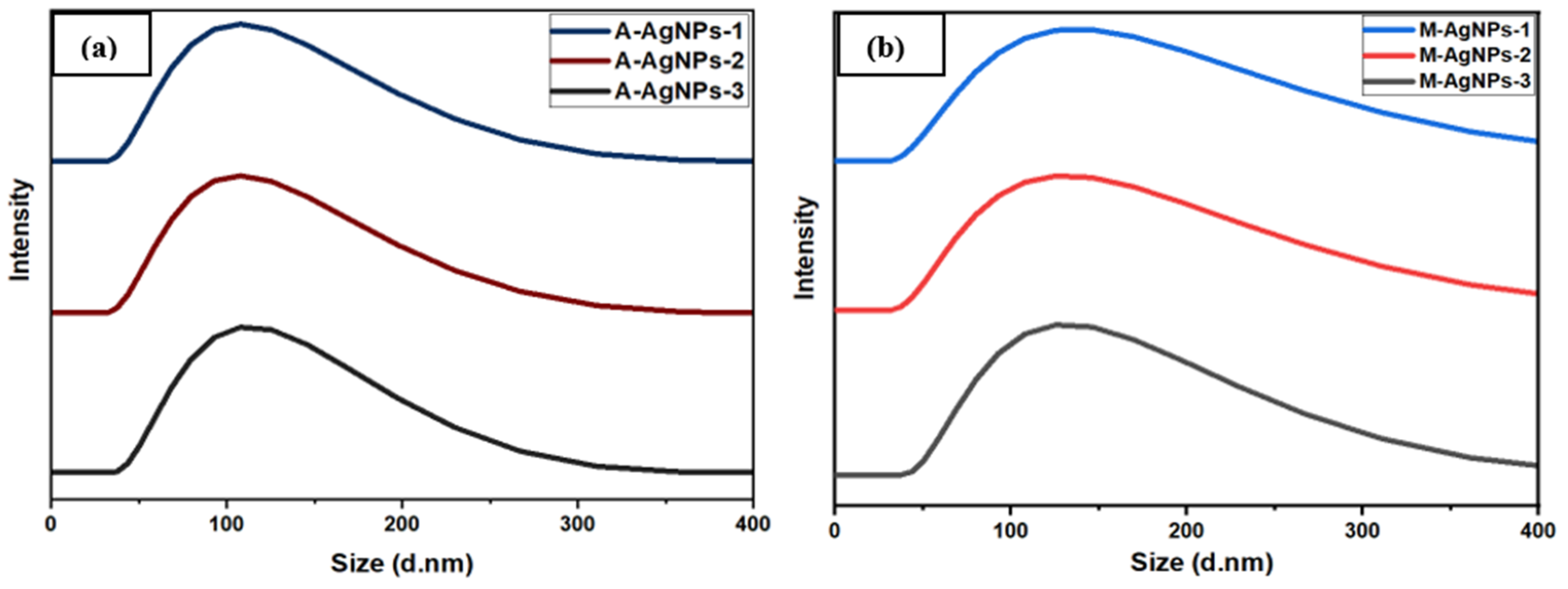
5.5. Particle Size Analysis
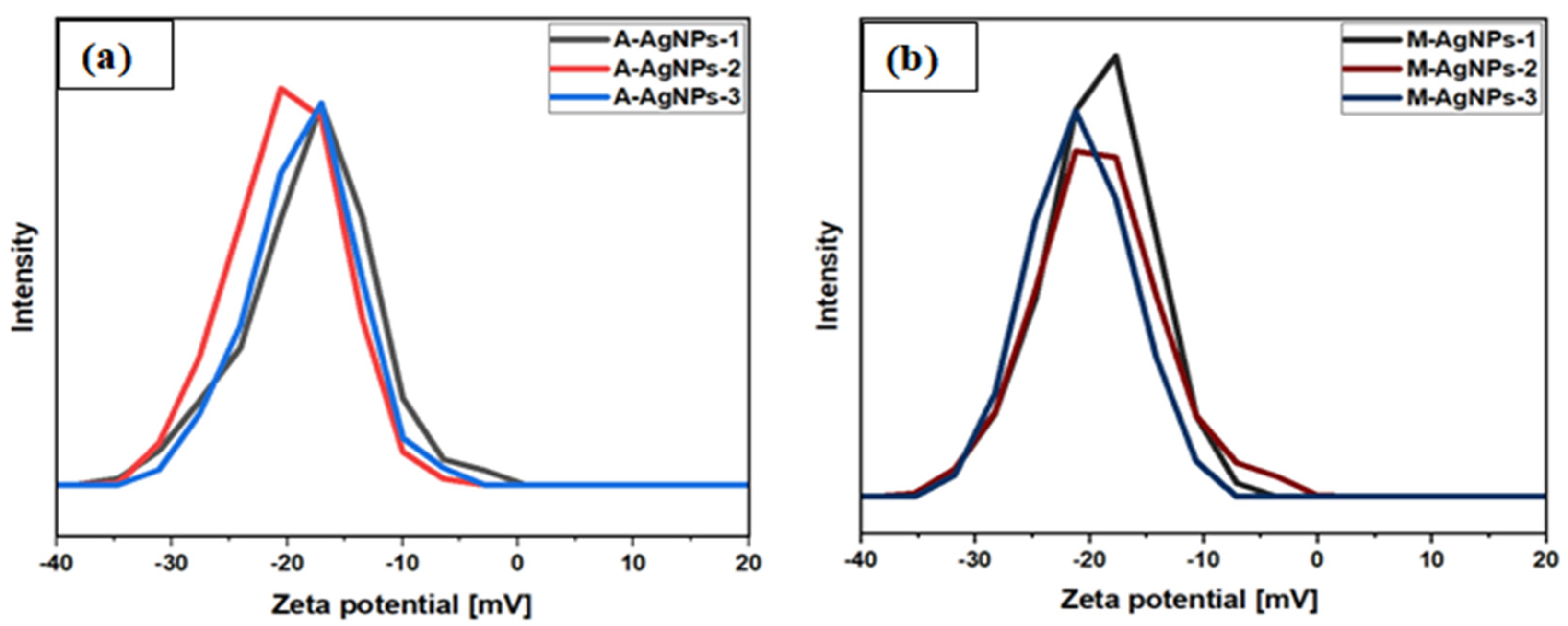
5.6. Stability
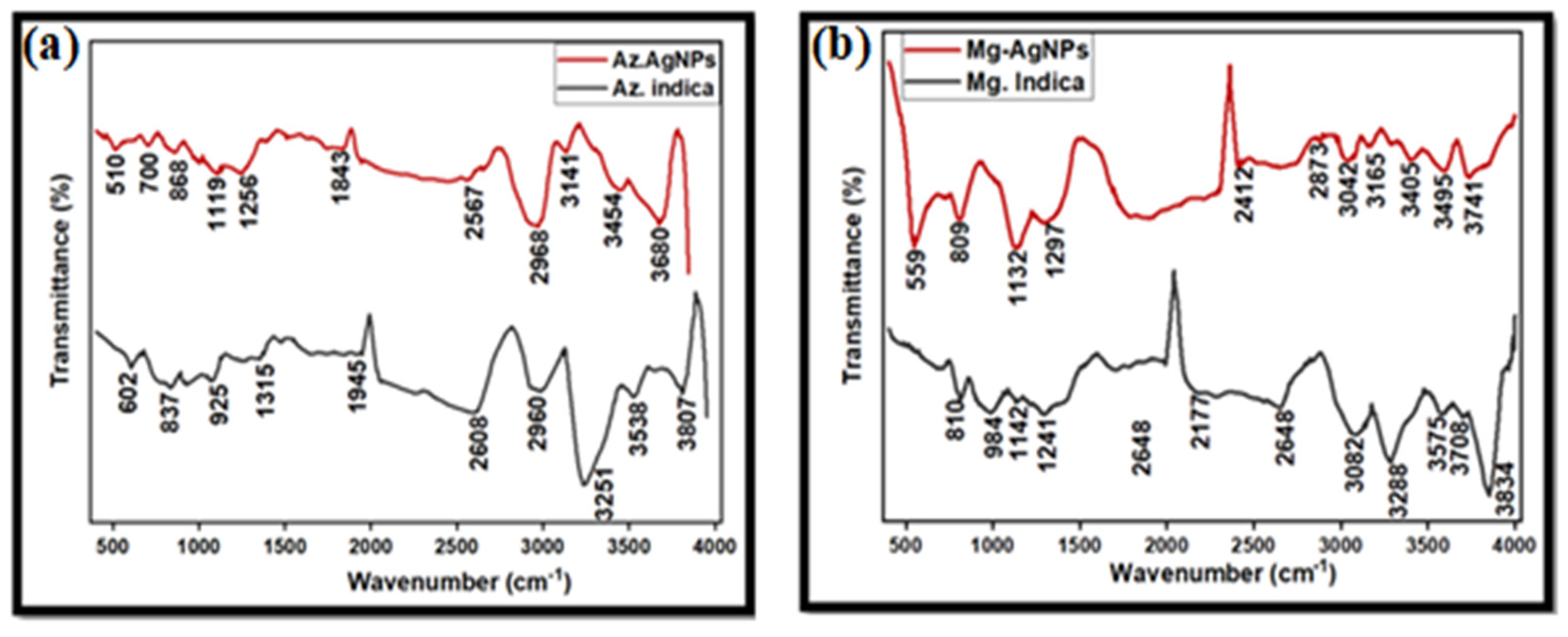
5.7. FTIR Spectroscopy Analysis
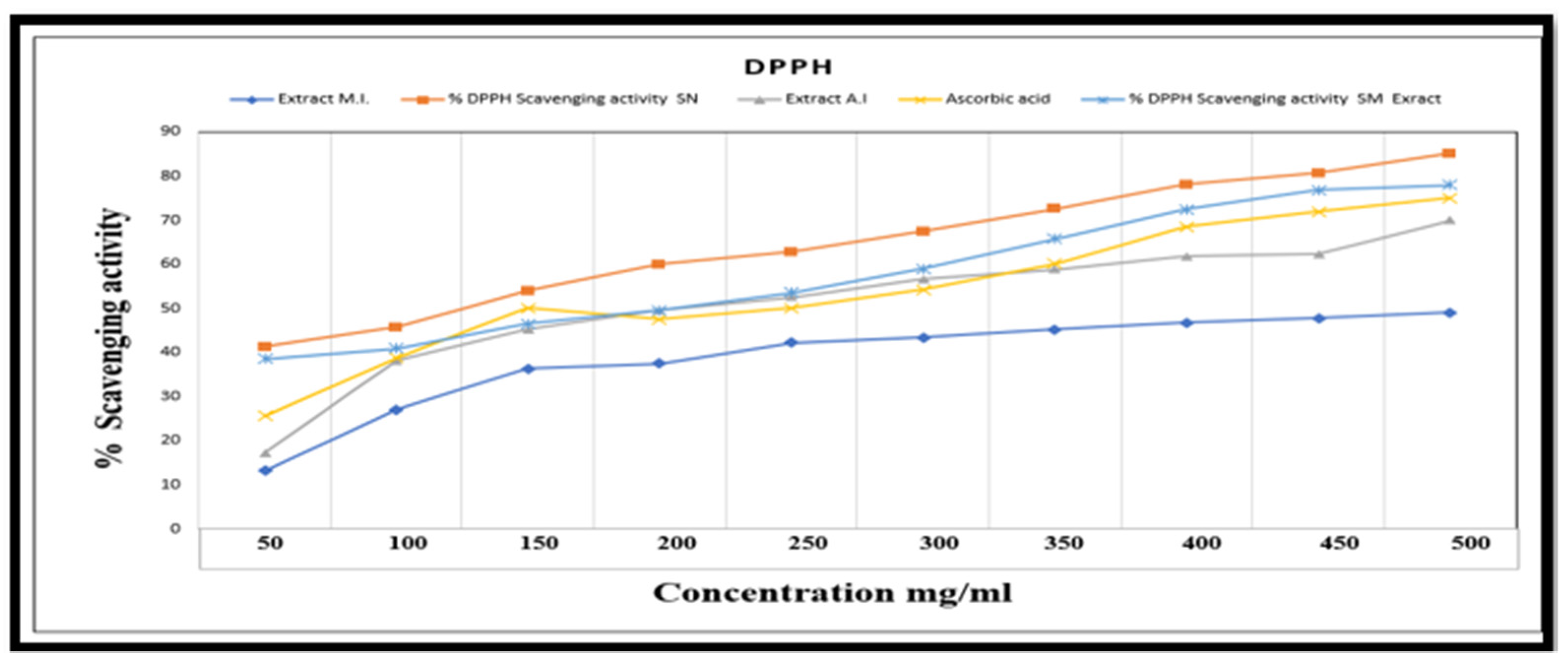
5.8. DPPH Scavenging
5.9. Antimicrobial Activity A-AgNPs and M-AgNPs
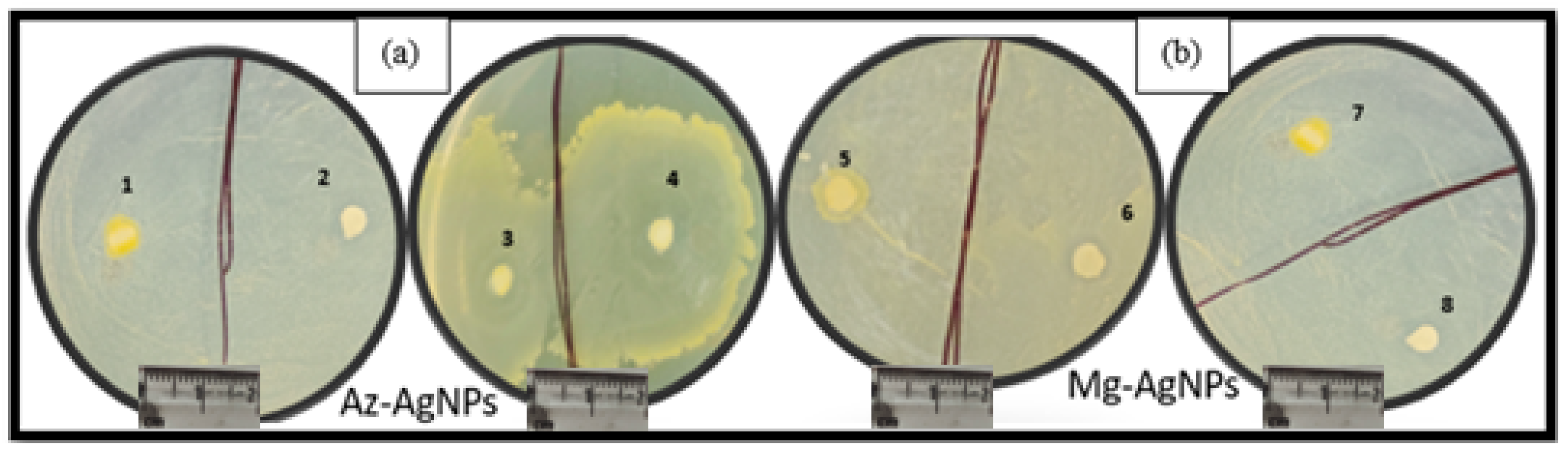
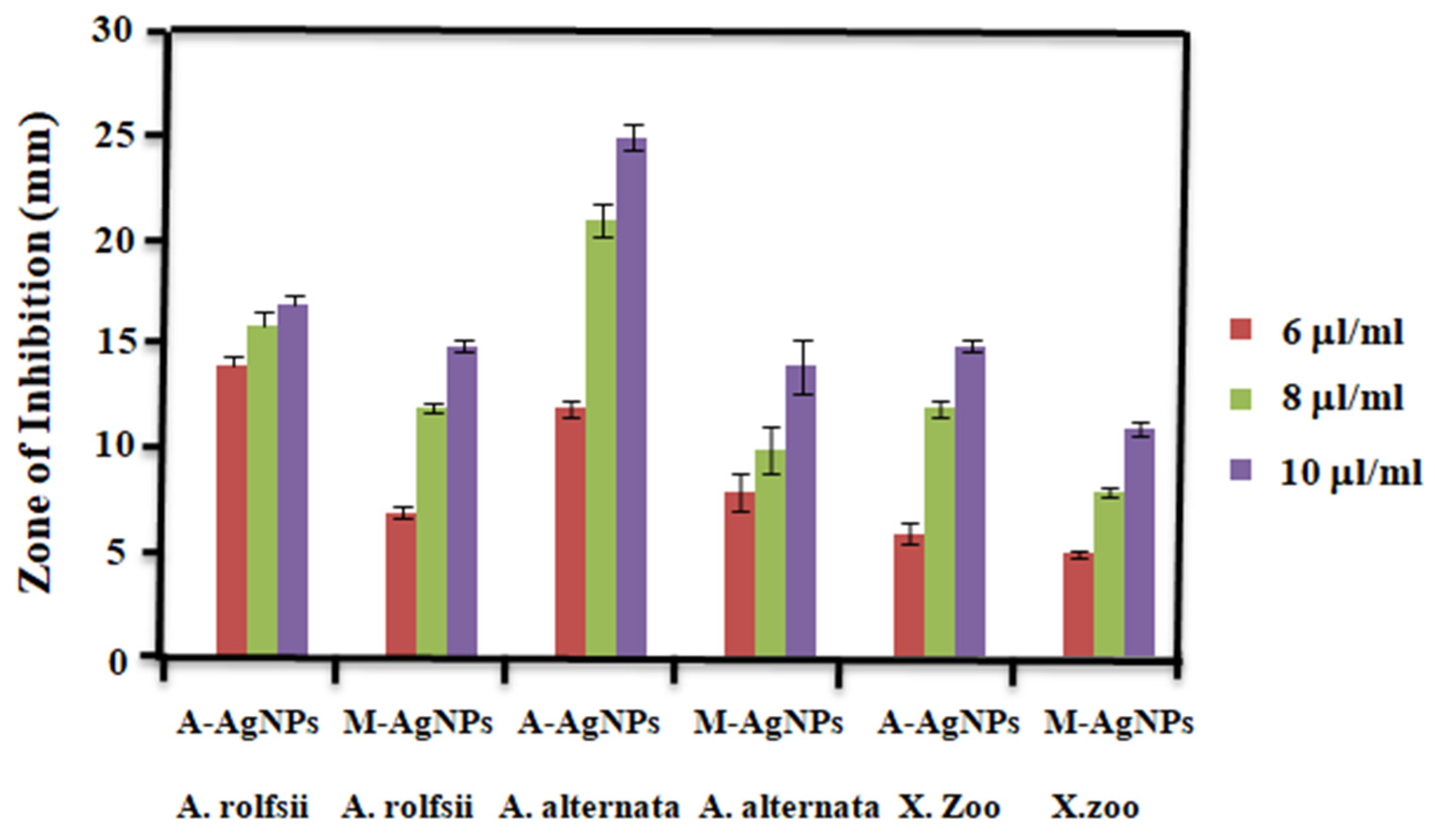
5.9.1. In Vitro Inhibitory Effect of A-AgNPs and M-AgNPs on A. alternata and A. rolfsii
5.9.2. Anti-Bacterial Activity
In Vitro Inhibitory Effect of A-AgNPs and M-AgNPs on the X. Oryzae Bacteria
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| A. indica | Azadirachta indica |
| M. indica | Mangifera indica |
| A. alternata | Alternaria alternata |
| A. rolfsii | Sclerotium rolfsii |
| X. oryzae | Xanthomonas oryzae |
| A-AgNPs | A. indicasilver nanoparticles |
| M-AgNPs | M. indica silver nanoparticles |
References
- Mohanta, Y.K.; Nayak, D.; Biswas, K.; Singdevsachan, S.K.; Abd Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Yadav, D.; Mohanta, T.K. Silver nanoparticles synthesized using wild mushroom show potential antimicrobial activities against food borne pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huo, Y.; Han, Y.X.; Li, J.F.; Ali, H.; Batjikh, I.; Hurh, J.; Pu, J.Y.; Yang, D.C. Biosynthesis of gold and silver nanoparticles from Scutellariabaicalensis roots and in vitro applications. Appl. Phys. A 2020, 126, 424. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017, 102, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kanawaria, S.K.; Sankhla, A.; Jatav, P.K.; Yadav, R.S.; Verma, K.S.; Velraj, P.; Kachhwah, S.; Kothari, S.L. (2018). Rapid biosynthesis and characterization of silver nanoparticles: An assessment of antibacterial and antimycotic activity. Appl. Phys. A 2018, 124, 320. [Google Scholar] [CrossRef]
- Ijaz, M.; Yousaf, M.; El Shafey, A.M. Arrhenius activation energy and Joule heating for Walter-B fluid with Cattaneo–Christov double-diffusion model. J. Therm. Anal. Calorim. 2021, 143, 3687–3698. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Rai, M.; Ribeiro, C.; Mattaso, L.; Duran, N. Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Chauhan, N.; Tyagi, A.; Kumar, P.; Malik, A. Antibacterial potential of Jatrophacurcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol. 2016, 7, 1748. [Google Scholar] [CrossRef]
- Hubballi, M.; Nakkeeran, S.; Raguchander, T.; Anand, T.; Samiyappan, R. Effect of environmental conditions on growth of Alternaria alternata causing leaf blight of noni. World J. Agric. Sci. 2010, 6, 171–177. [Google Scholar]
- Gil, M.; Caniga, M.; Eckman, J.; McLeod, R.; Moy, L.; Wilhelm, A.; Woodhouse, J.; Hoover, J.Z.; Cicmil, M. Alternaria alternata induced inflammatory lung responses: A novel in vivo PK/PD model. J. Inflamm. 2013, 10, 10. [Google Scholar] [CrossRef]
- Chowdhary, A.; Agarwal, K.; Randhawa, H.S.; Kathuria, S.; Gaur, S.N.; Najafzadeh, M.J.; Roy, P.; Arora, N.; Khanna, G.; Meis, J.F. A rare case of allergic bronchopulmonary mycosis caused by Alternaria alternata. Med. Mycol. 2012, 50, 890–896. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.D.; Chong, X.Y.; Xin, Q.H.; Wang, D.X.; Bian, K. Seed-borne endophytic Bacillus velezensis LHSB1 mediate the biocontrol of peanut stem rot caused by Sclerotiumrolfsii. J. Appl. Microbiol. 2020, 128, 803–813. [Google Scholar] [CrossRef]
- Angeles-Shim, R.B.; Shim, J.; Vinarao, R.B.; Lapis, R.S.; Singleton, J.J. A novel locus from the wild allotetraploid rice species Oryza latifolia Desv. confers bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in rice (O. sativa). PLoS ONE 2020, 15, e0229155. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Trevino, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological synthesis of silver nanoparticles and prospects in plant disease management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, A.K.; Patade, V.Y.; Balakrishna, G.; Pandey, H.K.; Singh, A. Synthesis of silver nanoparticles using extract of Ocimum kilimandscharicum and its antimicrobial activity against plant pathogens. SN Appl. Sci. 2019, 1, 1652. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’(Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef]
- Castillo-Henriquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Aabed, K.F. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Wamunyokoli, F.; Magoma, G. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona muricata. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1231–1242. [Google Scholar] [CrossRef]
- Ismail, M.; Gul, S.; Khan, M.I.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange. Green Process. Synth. 2019, 8, 118–127. [Google Scholar] [CrossRef]
- Maji, S.; Modak, S. Neem: Treasure of natural phytochemicals. Chem. Sci. Rev. Lett. 2021, 10, 396–401. [Google Scholar] [CrossRef]
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) Leaves: Nutritional composition phytochemical profile, and health- promoting bioactivities. Antioxidants 2021, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, M.; Kruszka, D.; Kachlicki, P.; Mondal, D.; Franklin, G. Uncovering the Phytochemical Basis and the Mechanism of Plant Extract-Mediated Eco-Friendly Synthesis of Silver Nanoparticles Using Ultra-Performance Liquid Chromatography Coupled with a Photodiode Array and High-Resolution Mass Spectrometry. ACS Sustain. Chem. Eng. 2022, 10, 562–571. [Google Scholar] [CrossRef]
- Omidi, S.; Sedaghat, S.; Tahvildar, K.; Derakhshi, P.; Motiee, F. Biosynthesis of silver nanoparticles with adiantum capillus-veneris L. leaf extract in the batch process and assessment of antibacterial activity. Green Chem. Lett. Rev. 2018, 11, 544–551. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, S.; Srivastava, M.; Ganesh, N.; Srivastava, S. Promising anti-inflammatory bio-efficiency of saponin loaded silver nanoparticles prepared from the plant Madhuca longifolia. Asian J. Nanosci. Mater. 2018, 1, 244–261. [Google Scholar] [CrossRef]
- Akintola, A.O.; Kehinde, B.D.; Ayoola, P.B.; Adewoyin, A.G.; Adewoyin, A.G.; Ajayi, J.F.; Ogunsona, S.B. Antioxidant properties of silver nanoparticles biosynthesized from methanolic leaf extract of Blighia sapida. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 012004. [Google Scholar] [CrossRef]
- Agrawal, S.; Popli, D.B.; Sircar, K.; Chowdhry, A. A review of the anticancer activity of Azadirachtaindica (Neem) in oral cancer. J. Oral Biol. Craniofacial Res. 2020, 10, 206–209. [Google Scholar] [CrossRef]
- Das, R.; Mukherjee, A.; Sinha, I.; Roy, K.; Dutta, B.K. Synthesis of potential bio-adsorbent from Indian Neem leaves (Azadirachtaindica) and its optimization for malachite green dye removal from industrial wastes using response surface methodology: Kinetics, isotherms and thermodynamic studies. Appl. Water Sci. 2020, 10, 117. [Google Scholar] [CrossRef]
- Bos, L. Beijerinck’s work on tobacco mosaic virus: Historical context and legacy. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1999, 354, 675–685. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Fu, P. Fungus-(Alternaria sp.) mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Saion, E.; Gharibshahi, E.; Naghavi, K. Size-Controlled and Optical Properties of Monodispersed Silver Nanoparticles Synthesized by the Radiolytic Reduction Method. Int. J. Mol. Sci. 2013, 14, 7880–7896. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa(Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Chaudhary, A.K.; Saini, S.; Srivastava, R.; Kumar, M.; Sharma, S.N. Ultrafast Transient Absorption Spectroscopic (UFTAS) and antibacterial Efficacy Studies of Phytofabricated Silver Nanoparticles using Ocimum Sanctum Leaf Extract. Inorg. Chem. Commun. 2023, 147, 110233. [Google Scholar] [CrossRef]
- Nourafkan, E.; Alamdari, A. Modelling of silver nanoparticles synthesis in ternary reverse microemulsion of Cyclohexane/Water/SDS. Part. Sci. Technol. 2014, 32, 215–223. [Google Scholar] [CrossRef]
- Mangini, V.; Dell’Aglio, M.; Stradis, A.D.; Giacomo, A.D.; Pascale, O.D.; Natile, G.; Arnesano, F. Amyloid Transition of Ubiquitin on Silver Nanoparticles Produced by Pulsed Laser Ablation in Liquid as a Function of Stabilizer and Single-Point Mutations. Chem. Eur. J. 2014, 20, 10745–10751. [Google Scholar] [CrossRef]
- Khannanov, A.A.; Rossova, A.A.; Ignatyeva, K.A.; Ulakhovich, N.A.; Gerasimov, A.V.; Boldyrev, A.E.; Evtugyn, V.G.; Rogov, A.M.; Cherosov, M.A.; Gilmutdinov, I.F.; et al. Superparamagentic cobalt nanoparticles in hyperbranched polyester polyol matric with anti-protease activity. J. Magn. Magn. Mater. 2021, 547, 168808. [Google Scholar] [CrossRef]
- Meena, R.K.; Meena, R.; Arya, D.K.; Jadoun, S.; Hada, R.; Kumari, R. Synthesis of silver nanoparticles by Phyllanthus emblica plant extract and their antibacterial activity. Mater. Sci. Res. India 2020, 17, 136–145. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): Synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef]
- Namburi, K.R.; Kora, A.J.; Chetukuri, A.; Kota, V.S.M.K. Biogenic silver nanoparticles as an antibacterial agent against bacterial leaf blight causing rice phytopathogens Xanthomonas oryzaepv. oryzae. Bioprocess Biosyst. Eng. 2021, 44, 1975–1988. [Google Scholar] [CrossRef]
- Pawar, O.; Deshpande, N.; Dagade, S.; Waghmode, S.; Nigam Joshi, P. Green synthesis of silver nanoparticles from purple acid phosphatase apoenzyme isolated from a new source Limoniaacidissima. J. Exp. Nanosci. 2015, 11, 28–37. [Google Scholar] [CrossRef]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3Biotech 2017, 7, 72. [Google Scholar] [CrossRef]
- Archana; Sharma, S.N.; Srivastava, R. Silver oxide nanoparticles synthesized by green method from Artocarpus Hetrophyllus for antibacterial and antimicrobial applications. Mater. Today Proc. 2020, 28, 332–336. [Google Scholar] [CrossRef]
- Singh, V.; Shrivastava, A.; Wahi, N. Biosynthesis of silver nanoparticles by plants crude extracts and their characterization using UV, XRD, TEM and EDX. Afr. J. Biotechnol. 2015, 14, 2554–2567. [Google Scholar] [CrossRef]
- Rodenbough, P.P. Crystallite Size Dependency of the Pressure and Temperature Response in Nanoparticles of Ceria and Other Oxides; Columbia University: New York, NY, USA, 2016. [Google Scholar]
- Sur, U.K.; Ankamwar, B.; Karmakar, S.; Halder, A.; Das, P. Green synthesis of Silver nanoparticles using the plant extract of Shikakai and Reetha. Mater. Today Proc. 2018, 5, 2321–2329. [Google Scholar] [CrossRef]
- Sajanlal, P.R.; Sreeprasad, T.S.; Samal, A.K.; Pradeep, T. SAnisotropic nanomaterials: Structure, growth, assembly, and functions. Nano Rev. 2011, 2, 5883. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzaepv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichodermaharzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Khatami, M.; Mehnipor, R.; Poor, M.H.S. Facile biosynthesis of silver nanoparticles using Descurainiasophia and evaluation of their antibacterial and antifungal properties. J. Clust. Sci. 2016, 27, 1601–1612. [Google Scholar] [CrossRef]
- Torres-Martínez, Y.; Arredondo-Espinoza, E.; Puente, C.; González-Santiago, O.; Pineda-Aguilar, N.; Balderas-Rentería, I.; López, I.; Ramírez-Cabrera, M.A. Synthesis of silver nanoparticles using a Mentha spicata extract and evaluation of its anticancer and cytotoxic activity. PeerJ 2019, 7, e8142. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show the highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef]
- Kiran, M.S.; Betageri, V.S.; Ranjuth kumar, C.R.; Vinay, S.P.; Latha, M.S. In-Vitro Antibacterial, Antioxidant and Cytotoxic Potential of Silver Nanoparticles Synthesized Using Novel Eucalyptus tereticornis Leaves Extract. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2916–2925. [Google Scholar] [CrossRef]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthusamarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 40. [Google Scholar] [CrossRef]
- Javed, B.; Akhtar, N. Optimization, characterization and antimicrobial activity of silver nanoparticles against plant bacterial pathogens phyto-synthesized by Menthalongifolia. Mater. Res. Express 2020, 7, 085406. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Saha, P.; Ochiai, B. Green synthesis and catalytic activity of silver nanoparticles based on Piper chaba stem extracts. Nanomaterials 2020, 10, 1777. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Pandian, S.R.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Karmakar, S. Particle Size Distribution and Zeta Potential Based on Dynamic Light Scattering: Techniques to Characterise Stability and Surface distribution of Charged Colloids. In Recent Trends in Materials Physics and Chemistry; Studium Press (India) Pvt Ltd.: New Delhi, India, 2019; pp. 117–159. [Google Scholar]
- Meva, F.E.; Ntoumba, A.A.; Kedi, P.B.E. Silver and palladium nanopaarticles produced using a plant extract as reducing agent, stabilized with an ionic liquid: Sizing by X-ray powder diffraction and dynamic light scattering. J. Mater. Res. Technol. 2019, 8, 1991–2000. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. A kinetic study on the degradation and biodegradability of silver nanoparticles catalyzed Methyl Orange and textile effluents. Heliyon 2019, 5, e01356. [Google Scholar] [CrossRef]
- Phanjom, P.; Ahmed, G. Biosynthesized of Silver Nanoparticles by Aspergillus oryzae (MTCC No. 1846) and Its Characterizations. Nanosci. Nanotechnol. 2015, 5, 14–21. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V.J.A.N. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Tomah, A.A.; Alamer, I.S.A.; Li, B.; Zhang, J.Z. Mycosynthesis of Silver Nanoparticles Using Screened Trichoderma Isolates and Their Antifungal Activity against Sclerotiniasclerotiorum. Nanomaterials 2020, 10, 1955. [Google Scholar] [CrossRef]
- Menon, S.; Agarwal, H.; Kumar, S.R.; Kumar, S.V. Green synthesis of silver nanoparticles using medicinal plant Acalypha indica leaf extracts and its application as an antioxidant and antimicrobial agent against foodborne pathogens. Int. J. Appl. Pharm. 2017, 9, 42–50. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Radhakrishnan, M.; Gopikrishnan, V.; Pazhanimurugan, R.; Balagurunathan, R. A study of the bactericidal, anti-biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 680–687. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Farag, R.S.; Badei, A.Z.M.A.; Hewedi, F.M.; El-Baroty, G.S.A. Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J. Am. Oil Chem. Soc. 1989, 66, 792–799. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Kenari, R.E.; Asnaashari, M. Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci. Nutr. 2019, 7, 465–475. [Google Scholar] [CrossRef]
- Rizwana, H.; Alwhibi, M.S.; Aldarsone, H.A. Green synthesis, characterization, and antimicrobial activity of silver nanoparticles prepared using Trigonelllafoenum-graecum L. leaves grown in Saudi Arabia. Green Process. Synth. 2021, 10, 421–429. [Google Scholar] [CrossRef]
- Gandham, R.G.; Rohan, B. Green synthesis and antibacterial activity of silver nanoparticles from the aqueous extracts of Cassia alata. Lett. Appl. NnaoBioSci. 2020, 9, 1037–1041. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, H.; Alzahrani, T.; Alwahibi, M.S.; Aljowaie, R.M.; Aldehaish, H.A.; Alsaggabi, N.S.; Ramadan, R. Phytofabrication of Silver Nanoparticles and Their Potent Antifungal Activity against Phytopathogenic Fungi. Processes 2022, 10, 2558. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Choi, O.; Yu, C.P.; Fernández, G.E.; Hu, Z. Esteban Fernández, and Zhiqiang Hu Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res. 2010, 44, 6095–6103. [Google Scholar] [CrossRef]
- Arya, G.; Sharma, N.; Mankamna, R.; Nimesh, S. Antimicrobial silver nanoparticles: Future of nanomaterials. In Microbial Nanobionics; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2019; pp. 89–119. [Google Scholar] [CrossRef]
- Singh, R.; Cheng, S.; Singh, S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcusradiodurans. 3Biotech 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Bawazir Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatorialimnetica. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Marwah, M.B. Evaluation of silver nanoparticles toxicity against toxic black mold Stachybotrys chartarum. J. Plant Pathol. Microbiol. 2017, 8, 1000408. [Google Scholar] [CrossRef]
- Devi, M.; Devi, S.; Sharma, V.; Rana, N.; Bhatia, R.K.; Bhatt, A.K. Green synthesis of silver nanoparticles using methanolic fruit extract of Aeglemarmelos and their antimicrobial potential against human bacterial pathogens. J. Tradit. Complement. Med. 2019, 10, 158–165. [Google Scholar] [CrossRef]
- Amna; Mahmood, T.; Khan, U.N.; Amin, B.; Javed, M.T.; Mehmood, S.; Farooq, M.A.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Characterization of bio-fabricated silver nanoparticles for distinct anti-fungal activity against sugarcane phytopathogens. Microsc. Res. Tech. 2021, 84, 1522–1530. [Google Scholar] [CrossRef]
- Vanti, G.L.; Kurjogi, M.; Basavesha, K.N.; Teradal, N.L.; Masaphy, S.; Nargund, V.B. Synthesis and antibacterial activity of solanumtorvum mediated silver nanoparticle against Xxanthomonasaxonopodispv. punicae and Ralstoniasolanacearum. J. Biotechnol. 2020, 309, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Hong, X.; Ibrahim, E.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeyadadantii. Molecules 2019, 24, 2303. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef]
- Roy, A.; Singh, V.; Sharma, S.; Ali, D.; Azad, A.K.; Kumar, G.; Emran, T.B. Antibacterial and Dye Degradation Activity of Green Synthesized Iron Nanoparticles. J. Nanomater. 2022, 2022, 3636481. [Google Scholar] [CrossRef]
- Salve, P.; Vinchurkar, A.; Raut, R.; Chondekar, R.; Lakkakula, J.; Roy, A.; Hossain, M.J.; Alghamdi, S.; Almehmadi, M.; Abdulaziz, O.; et al. An Evaluation of Antimicrobial, Anticancer, Anti-Inflammatory and Antioxidant Activities of Silver Nanoparticles Synthesized from Leaf Extract of Madhuca longifolia Utilizing Quantitative and Qualitative Methods. Molecules 2022, 27, 6404. [Google Scholar] [CrossRef] [PubMed]

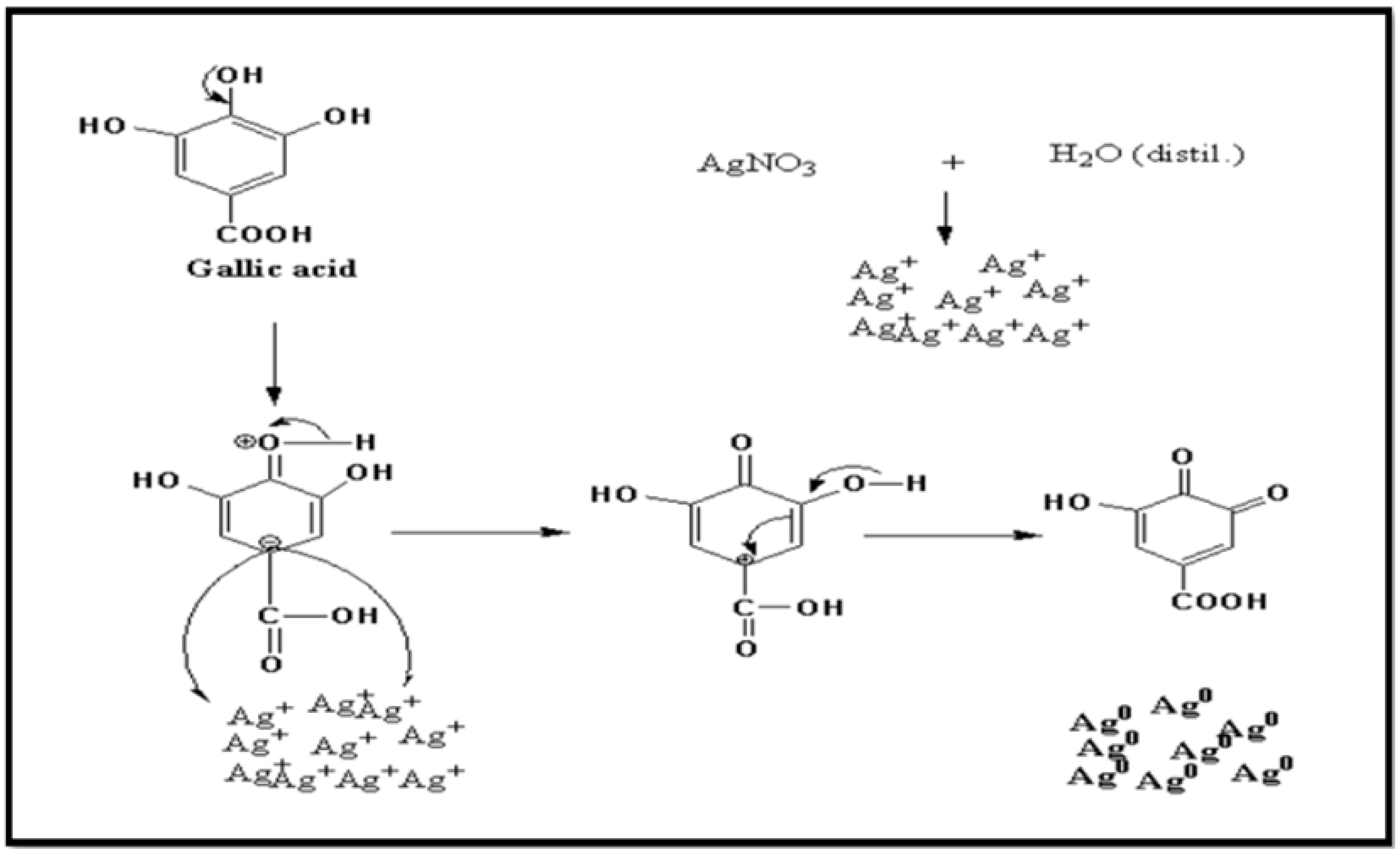
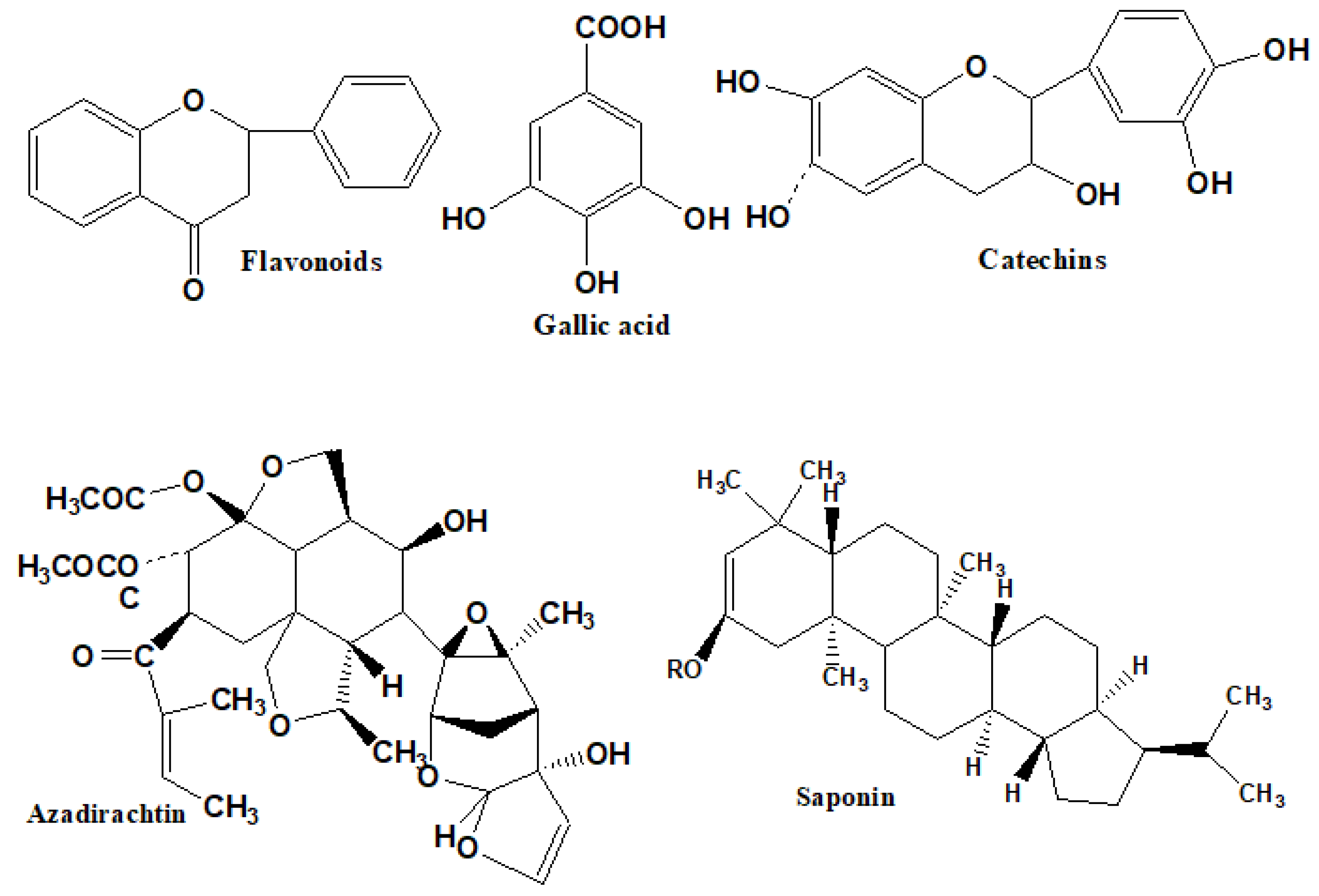


| Natural Chemical | Presence | Natural Chemical | Presence |
|---|---|---|---|
| Azadirachtin | + | Gallic acid | + |
| Nimbins | + | Saponins | + |
| Flavonoids | + | Limonoids | + |
| Alkaloids | + | Nimbosterol | + |
| Phenols | + | Tannins | + |
| Steroids | + | Polyphenols | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, A.; Kumari, A.; Chaudhary, A.K.; Srivastava, R.; Kamil, D.; Vashishtha, P.; Sharma, S.N. An Investigation of Antimicrobial Activity for Plant Pathogens by Green-Synthesized Silver Nanoparticles Using Azadirachta indica and Mangifera indica. Physchem 2023, 3, 125-146. https://doi.org/10.3390/physchem3010010
Rana A, Kumari A, Chaudhary AK, Srivastava R, Kamil D, Vashishtha P, Sharma SN. An Investigation of Antimicrobial Activity for Plant Pathogens by Green-Synthesized Silver Nanoparticles Using Azadirachta indica and Mangifera indica. Physchem. 2023; 3(1):125-146. https://doi.org/10.3390/physchem3010010
Chicago/Turabian StyleRana, Archana, Anjali Kumari, Amit Kumar Chaudhary, Ritu Srivastava, Deeba Kamil, Parth Vashishtha, and Shailesh Narain Sharma. 2023. "An Investigation of Antimicrobial Activity for Plant Pathogens by Green-Synthesized Silver Nanoparticles Using Azadirachta indica and Mangifera indica" Physchem 3, no. 1: 125-146. https://doi.org/10.3390/physchem3010010
APA StyleRana, A., Kumari, A., Chaudhary, A. K., Srivastava, R., Kamil, D., Vashishtha, P., & Sharma, S. N. (2023). An Investigation of Antimicrobial Activity for Plant Pathogens by Green-Synthesized Silver Nanoparticles Using Azadirachta indica and Mangifera indica. Physchem, 3(1), 125-146. https://doi.org/10.3390/physchem3010010








