Surface Coatings and Treatments for Controlled Hydrate Formation: A Mini Review
Abstract
:1. Introduction
2. Hydrate Adhesion and Deposition
3. Hydrate-Phobic Surface Treatments and Coatings
3.1. Functionalized Hydrate-Phobic Surfaces
3.2. Polymeric Coatings
3.3. Superhydrophobic and Omniphobic Coatings
3.4. Other Surface Treatments
4. Surface Active/Hydrate-Philic Coatings
5. Intellectual Property Generation/Commercialization
6. Conclusions and Future Perspectives
- Surface chemistry (hydrophobicity/hydrophilicity) and physical state (morphology) are both vital in defining a surface as hydrate-phobic or hydratephilic.
- Hydrate formation is a stochastic process, which depends on various parameters (several repetitions are required to obtain statistically significant results), which also make it impossible to compare the results across laboratories.
- Standard protocols indicating clearly the subcooling temperature, composition of THF/CP hydrate forming solutions, and parameters to assess the performance of a coating should be established for round robin measurements so that the results from different laboratories can be compared.
- The results obtained for THF/CP (type sII) model hydrates at atmospheric pressure should only be made with gaseous hydrates that form a similar hydrate structure at high pressure.
- In the future, screening of more robust and environmentally benign surface treatments/coatings, which are economic, can be prepared rapidly at large scale, and be multi-functional in nature, needs to be assessed.
- Systematic molecular modelling studies need to be pursued to unravel the mechanism by which a given surface alters the hydrate adhesion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 119, pp. 1–752. [Google Scholar]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Ripmeester, J.A.; Alavi, S. Some current challenges in clathrate hydrate science: Nucleation, decomposition and the memory effect. Curr. Opin. Solid State Mater. Sci. 2016, 20, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.D. Gas hydrates: Review of physical/chemical properties. Energy Fuels 1998, 12, 191–196. [Google Scholar] [CrossRef]
- Sum, A.K.; Koh, C.A.; Sloan, E.D. Developing a comprehensive understanding and model of hydrate in multiphase flow: From laboratory measurements to field applications. Energy Fuels 2012, 26, 4046–4052. [Google Scholar] [CrossRef]
- Creek, J.L. Efficient Hydrate Plug Prevention. Energy Fuels 2012, 26, 4112–4116. [Google Scholar] [CrossRef]
- Kinnari, K.; Hundseid, J.; Li, X.; Askvik, K.M. Hydrate management in practice. J. Chem. Eng. Data 2015, 60, 437–446. [Google Scholar] [CrossRef]
- Metaxas, P.J.; Lim, V.W.S.; McKay, S.F.; Morgan, J.E.P.; Johns, M.L.; Aman, Z.M.; May, E.F. High-fidelity evaluation of hybrid gas hydrate inhibition strategies. Energy Fuels 2012, 34, 15893–15989. [Google Scholar] [CrossRef]
- Song, S.; Shi, B.; Yu, W.; Ding, L.; Liu, Y.; Li, W.; Gong, J. Study on the optimization of hydrate management strategies in deepwater gas well testing operations. J. Energy Resour. Technol. 2020, 142, 033002. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Tohidi, B.; Anderson, R.; Mozaffar, H.; Tohidi, F. The return of kinetic hydrate inhibitors. Energy Fuels 2015, 29, 8254–8260. [Google Scholar] [CrossRef]
- Khurana, M.; Yin, Z.; Linga, P. A review of clathrate hydrate nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Yin, Z.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
- Zerpa, L.E.; Salager, J.-L.; Koh, C.A.; Sloan, E.D.; Sum, A.K. Surface chemistry and gas hydrates in flow assurance. Ind. Eng. Chem. Res. 2011, 50, 188–197. [Google Scholar] [CrossRef]
- Giavarini, C.; Hester, K. Gas Hydrate: Immense Energy Potential and Environmental Challenges, 1st ed.; Springer: London, UK, 2011; pp. 1–178. [Google Scholar]
- Eslamimanesh, A.; Mohammadi, A.H.; Richon, D.; Naidoo, P.; Ramjugernath, D. Application of gas hydrate formation in separation processes: A review of experimental studies. J. Chem. Thermodyn. 2012, 46, 62–71. [Google Scholar] [CrossRef]
- Sabil, K.M.; Partoon, B. Recent advances on carbon dioxide capture through a hydrate-based gas separation process. Curr. Opin. Green Sustain. Chem. 2018, 11, 22–26. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Straume, E.O.; Morales, R.E.M.; Sum, A.K. Perspectives on gas hydrates cold flow technology. Energy Fuels 2019, 33, 1–15. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon dioxide sequestration via gas hydrates: A potential pathway toward decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of clathrate hydrate based desalination to strengthen energy–water nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of surfactants in promoting gas hydrate formation. Ind. Eng. Chem. Res. 2019, 54, 12217–12232. [Google Scholar] [CrossRef]
- Linga, P.; Clarke, M.A. A review of reactor design and materials employed for increasing the rate of gas hydrate formation. Energy Fuels 2017, 31, 1–12. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation. J. Nat. Gas Sci. Eng. 2015, 55, 452–465. [Google Scholar] [CrossRef]
- Song, Y.M.; Liang, R.-Q.; Wang, F.; Shi, J.-H.; Zhang, D.-B.; Yang, L. Enhancement of clathrate hydrate formation kinetics using carbon-based material promotion. Front. Chem. 2020, 8, 464. [Google Scholar] [CrossRef]
- Liu, N.; Chen, L.; Liu, C.; Yang, L.; Liu, D. Experimental study of carbon dioxide hydrate formation in the presence of graphene oxide. Energy 2020, 211, 118994. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, X.; Zhang, R.; Chao, K.; Wang, F. Promotion of activated carbon on the nucleation and growth kinetics of methane hydrates. Front. Chem. 2020, 8, 526101. [Google Scholar] [CrossRef]
- Hu, P.; Chen, D.; Zi, M.; Wu, G. Effects of carbon steel corrosion on the methane hydrate formation and dissociation. Fuel 2018, 230, 126–133. [Google Scholar] [CrossRef]
- Hu, P.; Wu, G.; Zi, M.; Li, L.; Chen, D. Effects of modified metal surface on the formation of methane hydrates. Fuel 2019, 255, 115720. [Google Scholar] [CrossRef]
- Chenwei, L.; Zhiyuan, W.; Jinlin, T.; Ci, Y.; Mingzhong, L. Fundamental investigation of the adhesion strength between cyclopentane hydrate deposition and solid surface materials. Chem. Eng. Sci. 2020, 217, 115524. [Google Scholar] [CrossRef]
- Esmail, S.; Beltran, J.G. Methane hydrate propagation on surfaces of varying wettability. J. Nat. Gas Sci. Eng. 2016, 35, 1535–1543. [Google Scholar] [CrossRef]
- Beltran, J.G.; Servio, P. Morphological investigations of methane-hydrate films formed on a glass surface. Cryst. Growth Des. 2010, 10, 4339–4347. [Google Scholar] [CrossRef]
- Li, H.; Wang, L. Hydrophobized particles can accelerate nucleation of clathrate hydrates. Fuel 2015, 140, 440–445. [Google Scholar] [CrossRef]
- Acharya, P.V.; Kar, A.; Shahriari, A.; Bhati, A.; Mhadeshwar, A.; Bahadur, V. Aluminium-base promotion of nucleation of carbon dioxide hydrates. J. Phys. Chem. Lett. 2020, 11, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Acharya, P.V.; Bhati, A.; Mhadeshwar, A.; Venkataraman, P.; Barckholtz, T.A.; Celio, H.; Mangolini, F.; Bahadur, V. Magnesium-promoted rapid nucleation of carbon dioxide hydrates. ACS Sustain. Chem. Eng. 2021, 9, 11137–11146. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Galib, M.; Nguyen, A.V. Critical review on gas hydrate formation at solid surfaces and in confined spaces—Why and how does interfacial regime matter? Energy Fuels 2020, 34, 6751–6760. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, S.; Lang, X. Reviews of gas hydrate inhibitors in gas-dominant pipelines and application of kinetic hydrate inhibitors in China. Chin. J. Chem. Eng. 2019, 27, 2118–2132. [Google Scholar] [CrossRef]
- Manakov, A.Y.; Penkov, N.V.; Rodionova, T.V.; Nesterov, A.N.; Fesenko, E.F., Jr. Kinetics of formation and dissociation of gas hydrates. Russ. Chem. Rev. 2017, 86, 845–869. [Google Scholar] [CrossRef]
- Smith, J.D.; Meuler, A.J.; Bralower, H.; Venkatesan, R.; Subramanian, S.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Hydrate-phobic surfaces: Fundamental studies in clathrate hydrate adhesion reduction. Phys. Chem. Chem. Phys. 2012, 14, 6013–6020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aman, Z.M.; Sloan, E.D.; Sum, A.K.; Koh, C.A. Adhesion force interactions between cyclopentane hydrate and physically and chemically modified surfaces. Phys. Chem. Chem. Phys. 2014, 16, 25121–25128. [Google Scholar] [CrossRef] [PubMed]
- Sojoudi, H.; Walsh, M.R.; Gleason, K.K.; McKinley, G.H. Designing durable vapor-deposited surfaces for reduced hydrate adhesion. Adv. Mat. Interfaces 2015, 2, 1500003–1500015. [Google Scholar] [CrossRef] [Green Version]
- Sojoudi, H.; Walsh, M.R.; Gleason, K.K.; McKinley, G.H. Investigation into the formation and adhesion of cyclopentane hydrates on mechanically robust vapor-deposited polymeric coatings. Langmuir 2015, 31, 6186–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perfeldt, C.M.; Sharifi, H.; von Solms, N.; Englezos, P. Oil and gas pipelines with hydrophobic surfaces better equipped to deal with gas hydrate flow assurance issues. J. Nat. Gas Sci. Eng. 2015, 27, 852–861. [Google Scholar] [CrossRef]
- Hall, J.R.; Baures, P.W. Inhibition of tetrahydrofuran hydrate formation in the presence of polyol-modified glass surfaces. Energy Fuels 2017, 31, 7816–7823. [Google Scholar] [CrossRef]
- Das, A.; Farnham, T.A.; Subramanyam, S.B.; Varanasi, K.K. Designing ultra-low hydrate adhesion surfaces by interfacial spreading of water immiscible barrier films. ACS Appl. Mater. Interfaces 2017, 9, 21496–21502. [Google Scholar] [CrossRef]
- Brown, E.; Hu, S.; Wang, S.; Wells, J.; Nakatsuka, M.; Veedu, V.; Koh, C.A. Low-adhesion coatings as a novel gas hydrate mitigation strategy. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 1–4 May 2017. [Google Scholar]
- Di Lullo, A.; Zonta, P.P.; Pontarollo, A.; Correra, S. Measurment of adhesion strength of methane hydrates to wall. Recent Adv. Petrochem. Sci. 2018, 4, 59–61. [Google Scholar] [CrossRef]
- Sojoudi, H.; Arabnejad, H.; Raiyan, A.; Shirazi, S.A.; McKinley, G.H.; Gleason, K.K. Scalable and durable polymeric icephobic and hydrate-phobic coatings. Soft Matter 2018, 14, 3443. [Google Scholar] [CrossRef] [Green Version]
- Pickarts, M.A.; Brown, E.; Delgado-Linares, J.; Blanchard, G.; Veedu, V.; Koh, C.A. Deposition mitigation in flowing systems using coatings. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2019. [Google Scholar]
- Ragunathan, T.; Xu, X.; Shuhili, J.A.; Wood, C.D. Preventing hydrate adhesion with magnetic slippery surfaces. ACS Omega 2019, 4, 15789–15797. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Zhang, H.; Yang, G.; Wang, Y.; Li, G.; Lang, X. Reduction clathrate hydrates growth rates and adhesion forces on surfaces of inorganic or polymer coatings. Energy Fuels 2020, 34, 13566–13579. [Google Scholar] [CrossRef]
- Dong, S.; Li, M.; Liu, C.; Zhang, J.; Chen, G. Bio-inspired superhydrophobic coating with low hydrate adhesion for hydrate mitigation. J. Bionic Eng. 2020, 17, 1019–1028. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, S.; Wang, Y.; Lang, X.; Li, G. Preparation and performance of biomimetic superhydrophobic coating on X80 pipeline steel for inhibition of hydrate adhesion. Chem. Eng. J. 2021, 419, 129651. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a surface-active coating for promoted gas hydrate formation. Chem. Ing. Tech. 2019, 91, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.D.; Varanasi, K.K.; McKinley, G.H.; Cohen, R.E.; Meuler, A.J.; Bralower, H.L. Massachusetts Institute of Technology, Articles and Methods for Reducing Hydrate Adhesion. U.S. Patent US2012/0160362A1, 28 June 2012. [Google Scholar]
- Hatton, G.J.; Mehta, A.P.; Peters, D.J. Pipe Transportation System with Hydrophobic Wall. Chinese Patent CN102959301B, 19 August 2015. U.S. Patent US2013/0087207A1, 11 April 2013. [Google Scholar]
- Bhatnagar, G.; Crosby, D.L.; Hatton, G.J.; Huo, Z.; Shell Internationale Research Maatschappij B., V. Hydrate deposit inhibition with surface-chemical combination. World Patent WO2012/058144A3, 3 May 2012. [Google Scholar]
- DragX Flow Assurance & Efficiency Home Page. Available online: https://dragxsurfaces.com (accessed on 2 December 2021).
- Southwest Research Institute Press Releases Page. Available online: https://www.swri.org/press-release/superhydrophobic-coating-process-subsea-pipelines (accessed on 2 December 2021).
- DuBose, B. Materials Performance Coatings and Linings Section. Available online: https://www.materialsperformance.com/articles/coating-linings/2020/03/superhydrophobic-coating-developed-for-offshore-drilling-pipes (accessed on 2 December 2021).
- Evonik Products and Solutions Page. Available online: https://corporate.evonik.com/en/products/industry-teams/oil-gas/products-markets/flowlines-and-pipelines-123783.html (accessed on 2 December 2021).
- Lingelem, M.N.; Majeed, A.I.; Stange, E. Industrial experience in evaluation of hydrate formation, inhibition, and dissociation in pipeline design and operation. Ann. N. Y. Acad. Sci. 1994, 715, 75–93. [Google Scholar] [CrossRef]
- Austvik, T.; Li, X.; Gjertsen, L.H. Hydrate plug properties: Formation and removal of plugs. Ann. N. Y. Acad. Sci. 2000, 912, 294–303. [Google Scholar] [CrossRef]
- Nicholas, J.W.; Dieker, L.E.; Sloan, E.D.; Koh, C.A. Assessing the feasibility of hydrate deposition on pipeline walls–adhesion force measurements of clathrate hydrate particle on carbon steel. J. Colloid Interface Sci. 2009, 331, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Aspenes, G.; Dieker, L.E.; Aman, Z.M.; Hoiland, S.; Sum, A.K.; Koh, C.A.; Sloan, E.D. Adhesion force between cyclopentane hydrates and solid surface materials. J. Colloid Interface Sci. 2010, 343, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Zhang, G.; Koh, C.A. Direct measurements of the interactions between clathrate hydrate particles and water droplets. Phys. Chem. Chem. Phys. 2015, 17, 20021–20029. [Google Scholar] [CrossRef] [PubMed]
- Aman, Z.M.; Joshi, S.E.; Sloan, E.D.; Sum, A.K.; Koh, C.A. Micromechanical cohesion force measurements to determine cyclopentane hydrate interfacial properties. J. Colloid Interface Sci. 2012, 376, 283–288. [Google Scholar] [CrossRef]
- Aman, Z.M.; Koh, C.A. Interfacial phenomena in gas hydrate systems. Chem. Soc. Rev. 2016, 45, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.A.; Vijayamohan, P.; Sloan, E.D.; Koh, C.A.; Sum, A.K. Gas hydrate deposition in flowlines: A challenging problem in flow assurance. In Proceedings of the ASME, 32nd International Conference Ocean, Offshore and Arctic Engineering, Nantes, France, 9–14 June 2013. [Google Scholar]



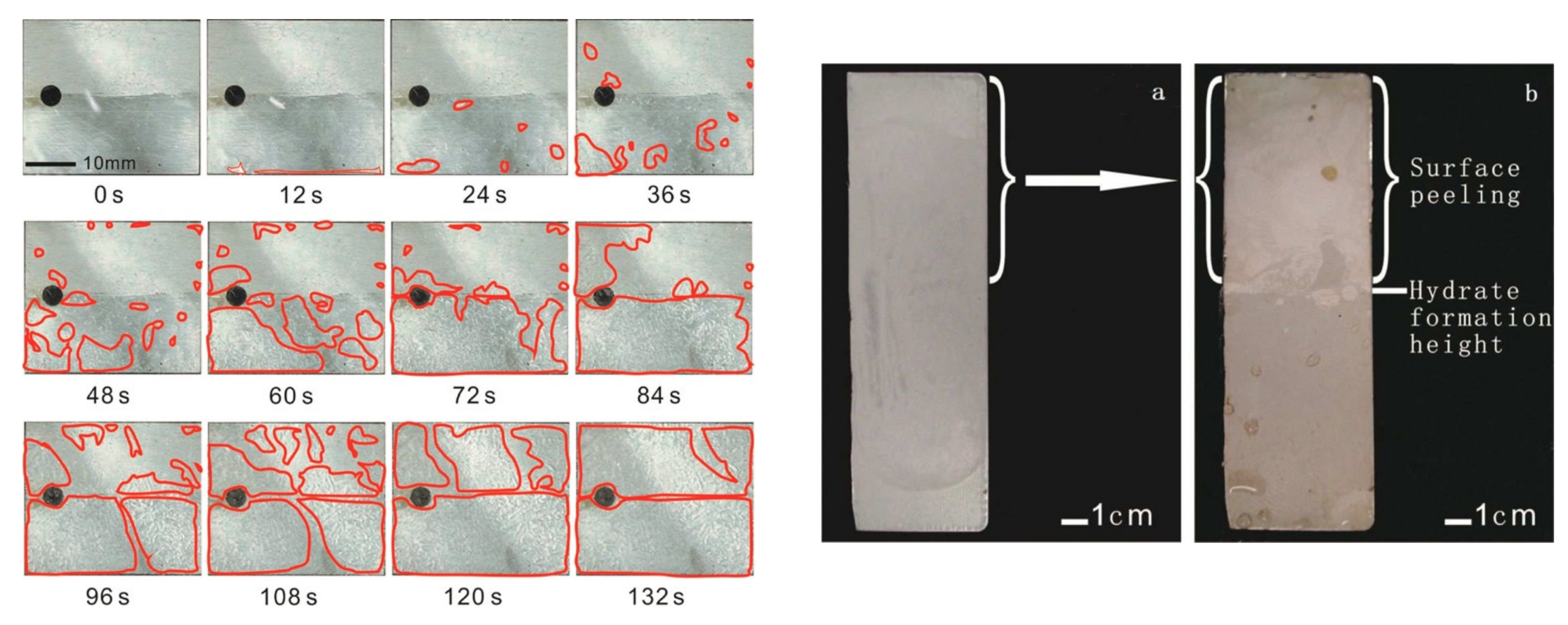
| Hydrate Formers | Substrates | Coatings | Experimental Conditions | References |
|---|---|---|---|---|
| Tetrahydrofuran (19.1 wt.% in water) | -Clean glass -Bare steel | -l-Butanethiol, -1H, 1H, 2H, 2H-Perfluorodecane-thiol, -Methyl 3-mercaptopropionate, -4-Mercapto-l-butanol, -50/50 Butanethiol/methyl 3-mercaptopropionate, -50/50 Butanethiol/4-Mercapto-1-butanol, -Trichloro (1H, 1H, 2H, 2H perfluorooctyl) silane, -Octadecyl trichlorosilane, -80 wt.%/20 wt.% PEMA/fluorodecyl POSS | Atmospheric pressure, < 4.4 °C | [40] |
| Cyclopentane (in water) | -Stainless steel (grade 309) | -Oleamide (CH3)(CH2)8(CH)2(CH2)7CONH2; -Citric acid ester, based on HOC(COOH)(CH2COOH)2, -Nonanedithiol HSCH2(CH2)7CH2SH, -Commercial Rain-Xs (anti-wetting agent), -Graphite (based on planar sheets of carbon) | Atmospheric Pressure, T–n.a. | [41] |
| Tetrahydrofuran (19.0 wt.% in water) | -Steel -Silicone | -poly-divinyl benzene/poly(perfl uorodecylacrylate) (pDVB/pPFDA) Bilayer (BL) -linker free grafted coatings (LFG) with pPFDA thickness of 10 and 40 nm | Atmospheric Pressure, −15 °C | [42] |
| Cyclopentane (in water) | -Rough Steel -Flat Silicone | -pDVB/pPFDA (BL-LFG) with pPFDA thickness of 10 and 40 nm | Atmospheric Pressure, −15 °C | [43] |
| Methane (in water and water + LDHIs) | -Stainless steel crystallizer | -perfluoroalkoxy alkane polymer from DuPont Teflon coating, Cantech Precision Coatings Inc. (PFA) | 41 bar, 6 °C | [44] |
| Tetrahydrofuran (1:15) | -Glass test tubes (Heavy wall borosilicate) | -(3-aminopropyl)triethoxysilane (APTES) -N-[3-(trimethoxysilyl)propyl]ethylenediamine (AEAPTMS) with succinic acid (SA) linker to a glycerol at 1- or 2-position. | Atmoshperic Pressure, 0 °C | [45] |
| Cyclopentane (in water) | -Silicon | -octadecyltricholorosilane (OTS) -tridecafluoro-1,1,2,2 tetrahydrooctyl trichlorosilane (FS) | Atmospheric Pressure, −5 °C | [46] |
| Cyclopentane (in water) & Methane (74.7 mol%) + Ethane (25.3 mol%) | -Carbon Steel (Pristine & corroded) | -Omniphobic -Superhydrophobic | Atmopsheric Pressure, 500 psig and 1 °C | [47] |
| Tetrahydrofuran & Methane | -Carbon Steel | -A green fluorinated ethylene propylene (FEP) -A blue polytetrafluoro ethylene (PTFE) -B black polytetrafluoro ethylene (PTFE) -B green fluorinated ethylene propylene (FEP) | Conditions n.a. | [48] |
| Cyclopentane | -Silicon -Steel | -poly-divinylbenzene (pDVB)/poly-perfluorodecylacrylate (pPFDA) bilayer covalently bonded and grafted to substrates using iCVD process. | Atmospheric Pressure, −15 and 5 °C | [49] |
| Methane (74.7 mol%) + Ethane (25.3 mol%) | -Stainless Steel | -Omniphobic -Superomniphobic | 1000 psig and 2.5 °C | [50] |
| Methane | -Glass -Steel 316 L | -ethyltriethoxysilane (ES) -n-dodecyltriethoxysilane (DS) -n-octyltriethoxysilane (OE) | 4 °C, 100 bar (stationary experiments) and 150 bar (transient experiments) | [55] |
| Tetrahydrofuran (19.1 wt.% in water) | -Neodymium Magnet | -Ferrofluid (Iron oxide/magnetite 8% v/v, oil-soluble dispersant 14% v/v, light hydrocarbon oil 78% v/v) | Atmospheric Pressure, −5 °C; Mechanical tests for adhesion were performed at −15 °C | [51] |
| Tetrahydrofuran (19.0 wt.% in water) & Methane | -X70 pipeline steel -X80 pipeline steel -Zirconia (ZrO2) -Tin plate | -Fluoro-coating (F-coating) -Polydimethylsiloxane (PDMS) -Hexagonal boron nitride (HBN) -Hydrophobic fuming SiO2 (HB-630, diameter ≤ 300 nm) | Atmospheric Pressure, −3 °C; −10 °C, 8.2 MPa | [52] |
| Cyclopentane | -X90 pipeline steel | -Copper oxide (CuO) layer modified with Stearic acid | Atmospheric pressure, 1 °C | [53] |
| Cyclopentane | -X80 pipeline steel | -Cerium oxide (CeO2)/polydopamine (pDA) | Atmospheric pressure, n.a. (0 °C > T > 7.7 °C) | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamash, T.; Esperança, J.M.S.S.; Tariq, M. Surface Coatings and Treatments for Controlled Hydrate Formation: A Mini Review. Physchem 2021, 1, 272-287. https://doi.org/10.3390/physchem1030021
Altamash T, Esperança JMSS, Tariq M. Surface Coatings and Treatments for Controlled Hydrate Formation: A Mini Review. Physchem. 2021; 1(3):272-287. https://doi.org/10.3390/physchem1030021
Chicago/Turabian StyleAltamash, Tausif, José M. S. S. Esperança, and Mohammad Tariq. 2021. "Surface Coatings and Treatments for Controlled Hydrate Formation: A Mini Review" Physchem 1, no. 3: 272-287. https://doi.org/10.3390/physchem1030021
APA StyleAltamash, T., Esperança, J. M. S. S., & Tariq, M. (2021). Surface Coatings and Treatments for Controlled Hydrate Formation: A Mini Review. Physchem, 1(3), 272-287. https://doi.org/10.3390/physchem1030021








