The Importance of Interphases in Energy Storage Devices: Methods and Strategies to Investigate and Control Interfacial Processes
Abstract
1. Introduction
2. Mechanisms of SEI Formation
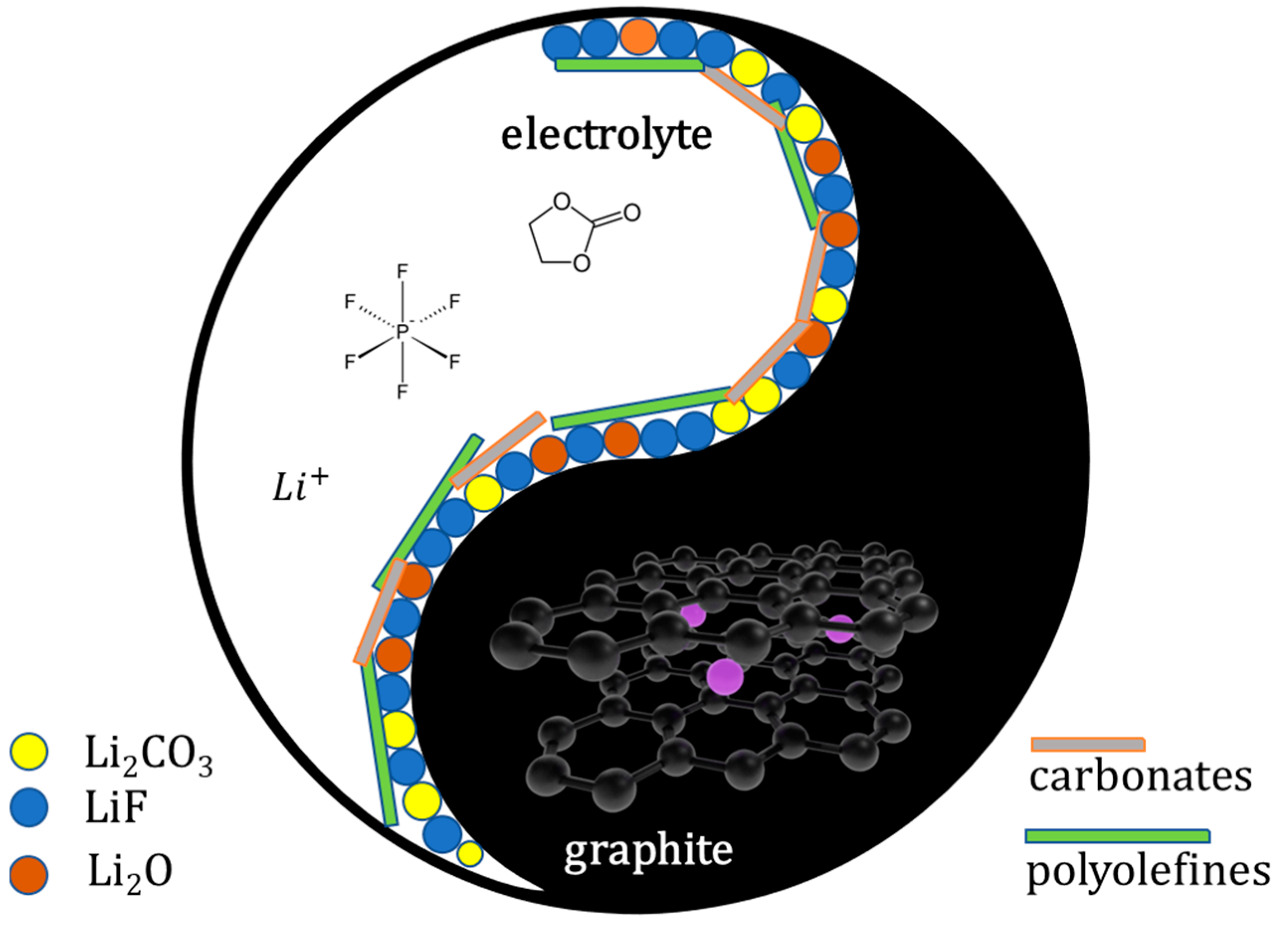
The Graphite/SEI Intephase
3. Interphase Investigation Methods
3.1. Diffraction-Based Techniques
3.2. X-rays/Electron Absorption Spectroscopies
3.3. Reflectometry and Scattering
3.4. Imaging and Microscopy
3.5. Other Spectroscopies and Electrochemistry-Based Approaches
| Technique | Ref. | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|---|
| RD | [27,28,29,30,31,32,33,34] | - | + | ++ | N.A. | + | = | - | ++ |
| Synch. Diffr. | [29,30] | - | ++ | ++ | N.A. | ++ | ++ | -- | |
| Neutron diffr. | [35,37] | -- | = | ++ | N.A. | = | - | + | -- |
| XAS | [28,40,41,42,43,44,45] | - | + | + | + | + | - | - | - |
| XPS | [48,49,50,51,52,53] | - | + | = | ++ | + | -- | -- | - |
| Reflectometry | [54,55,56,57,58] | + | ++ | N.A. | N.A. | - | = | + | = |
| Opt. Micr. | [80,81] | - | + | N.A. | N.A. | - | + | - | ++ |
| SEM | [82,83] | + | = | + | ++ | ++ | - | - | + |
| TEM | [86,87,88,89,90,91,92,93,94,95,96,97] | ++ | + | ++ | + | ++ | -- | -- | - |
| AFM | [98,99,100,101] | ++ | = | = | N.A. | = | + | - | ++ |
| IR | [103,104] | = | - | - | ++ | - | ++ | - | ++ |
| Raman | [105,106,107] | + | - | - | ++ | -- | + | - | + |
| NMR/MRI | [108,109,110,111] | = | -- | = | ++ | -- | - | = | = |
| XRT | [65,66,67,68,69,70,71] | ++ | ++ | ++ | N.A. | = | + | + | + |
| EIS | [125] | N.A. | -- | N.A. | N.A. | N.A. | ++ | ++ | ++ |
4. Future Developments
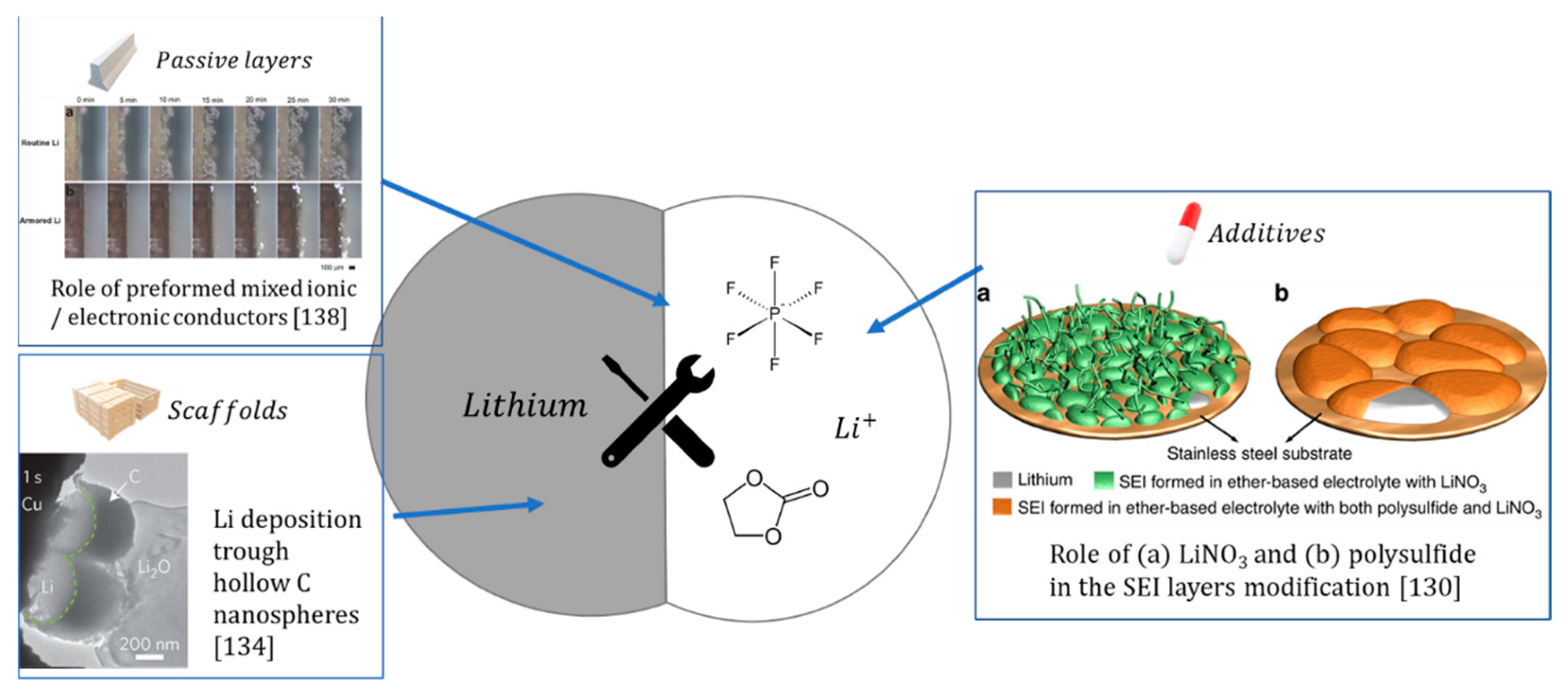
4.1. The Li/Electrolyte Interface
4.2. Beyond Graphite: Alloys for Negative Electrodes
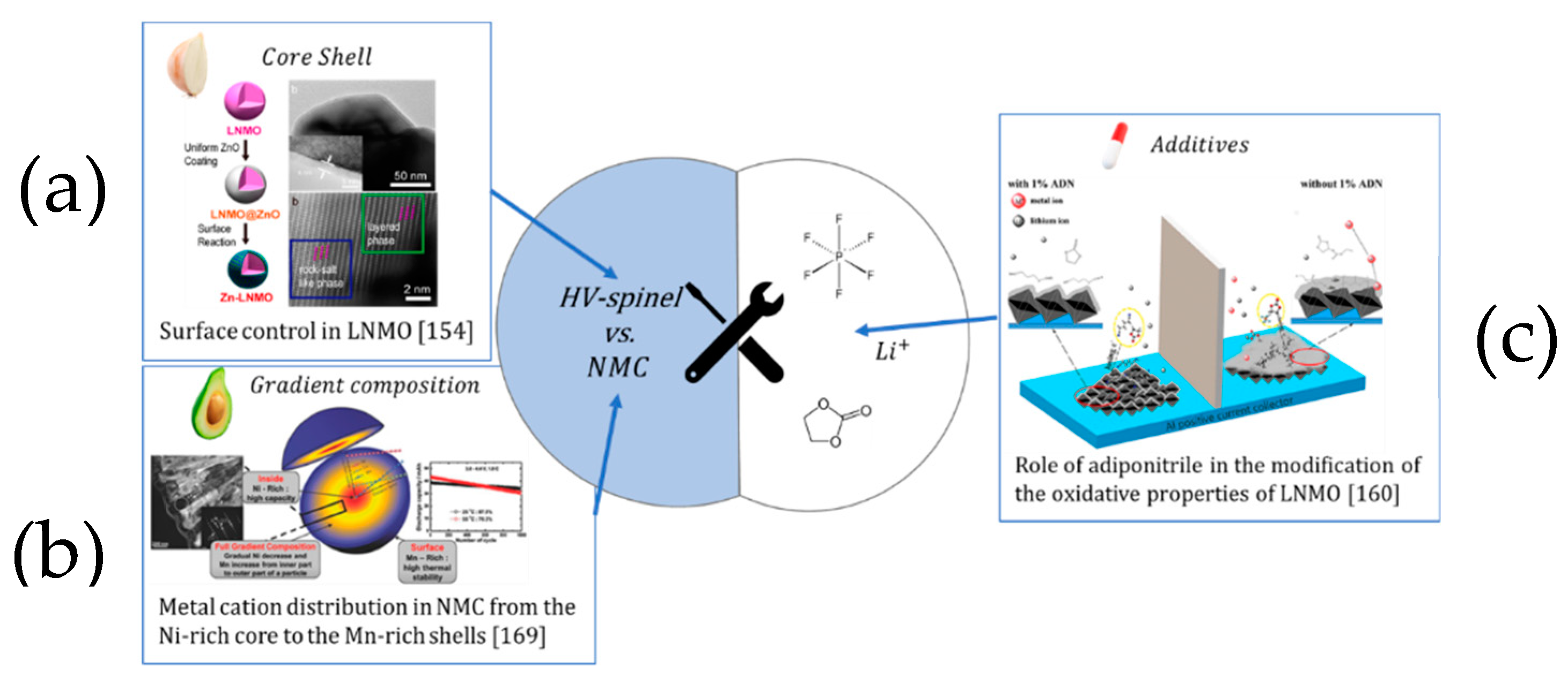
4.3. Towards High Voltage: The Emerging Role of the CEI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of the Acronyms Used in the Text
| AFM | Atomic Force Microscopy |
| ALD | Atomic Layer Deposition |
| CEI | Cathode Electrolyte Interphase |
| CEM | Cryogenic Electron Microscopy |
| DNP | Dynamic Nuclear Polarization |
| EC | Ethylene Carbonate |
| EELS | Electron Energy Loss Spectroscopy |
| EIS | Electrochemical Impedance Spectroscopy |
| EPR | Electron Paramagnetic Resonance |
| EQCM | Electrochemical Quartz Crystal Microbalance |
| FEC | Fluoroethylene Carbonate |
| FTIR | Fourier Transform Infrared spectroscopy |
| HOMO | Highest Occupied Molecular Orbital |
| INS | Inelastic Neutron Scattering |
| LIB | Lithium-Ion Battery |
| LMB | Lithium Metal Battery |
| LNMO | LiNi0.5Mn1.5O4 |
| MRI | Magnetic Resonance Imaging |
| MS | Mass Spectroscopy |
| NDP | Neutron Depth Profiling |
| NMA | Nickel-Manganese-Aluminum oxide |
| NMCXYZ | LiNixMnyCozO2 |
| NMP | 1-methyl-2-pyrrolidone |
| NMR | Nuclear Magnetic Resonance |
| OM | Optical Microscopy |
| PC | Propylene Carbonate |
| Pair Distribution Function | |
| PEO | Poly(ethylene oxide) |
| SANS | Small Angle Neutron Scattering |
| SECM | Scanning ElectroChemical Microscopy |
| SEI | Solid Electrolyte Interphase |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| VC | Vinylene carbonate |
| XAS | X-rays Absorption Spectroscopy |
| XPS | X-rays Photoelectron Spectroscopy |
| XRD | X-rays Diffraction |
| XRR | X-rays Reflectometry |
| XRT | X-rays Tomography |
| XTM | X-rays Transmission Microscopy |
References
- Saubanère, M.; Filhol, J.-S.; Doublet, M.-L. Atomistic Modeling of Electrode Materials for Li-Ion Batteries: From Bulk to Interfaces. In Physical Multiscale Modeling and Numerical Simulation of Electrochemical Devices for Energy Conversion and Storage; Franco, A.A., Doublet, M.-L., Bessler, W.G., Eds.; Springer: London, UK, 2016; pp. 1–36. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Trasatti, S.; Parsons, R. Interphases in systems of conducting phases (IUPAC Recommendations 1985). Pure Appl. Chem. 1986, 58, 437–454. [Google Scholar] [CrossRef]
- Advanced Materials for Clean and Sustainable Energy and Mobility. Available online: https://emiri.eu/uploads/content_files/65/value__file/EMIRI%20Technology%20Roadmap%20-%20September%202019%20(cond).pdf (accessed on 23 February 2021).
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Gauthier, M.A.M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.-H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C.; et al. Electrode–Electrolyte Interface in Li-Ion Batteries: Current Understanding and New Insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ji, Y.J.; Zhang, Z.R.; Yang, Y. Recent Progress in Research on High-Voltage Electrolytes for Lithium-Ion Batteries. ChemPhysChem 2014, 15, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Kazzazi, A.; Bresser, D.; Kuenzel, M.; Hekmatfar, M.; Schnaidt, J.; Jusys, Z.; Diemant, T.; Behm, R.; Copley, M.; Maranski, K.; et al. Synergistic electrolyte additives for enhancing the performance of high-voltage lithium-ion cathodes in half-cells and full-cells. J. Power Sources 2021, 482, 228975. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Guerard, D.; Herold, A. Intercalation of lithium into graphite and other carbons. Carbon 1975, 13, 337–345. [Google Scholar] [CrossRef]
- Dey, A.N.; Sullivan, B.P. The Electrochemical Decomposition of Propylene Carbonate on Graphite. J. Electrochem. Soc. 1970, 117, 222–224. [Google Scholar] [CrossRef]
- Yazami, R.; Touzain, P. A reversible graphite-lithium negative electrode for electrochemical generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Fong, R.; Von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009–2013. [Google Scholar] [CrossRef]
- Yoshino, A.; Sanechika, K.; Nakajima, T. Secondary Battery. U.S. Patent 4,668,595A, 26 May 1987. [Google Scholar]
- Yoshino, A. Lithium Ion Batteries; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Guyomard, D.; Tarascon, J.M. Rechargeable Li1+x Mn2O4/Carbon Cells with a New Electrolyte Composition: Potentiostatic Studies and Application to Practical Cells. J. Electrochem. Soc. 1993, 140, 3071–3081. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Zhu, Y.; Marin, T.W.; Abraham, D. Reduction of Carbonate Electrolytes and the Formation of Solid-Electrolyte Interface (SEI) in Lithium-Ion Batteries. 1. Spectroscopic Observations of Radical Intermediates Generated in One-Electron Reduction of Carbonates. J. Phys. Chem. C 2013, 117, 19255–19269. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Zhu, Y.; Marin, T.W.; Abraham, D. Reduction of Carbonate Electrolytes and the Formation of Solid-Electrolyte Interface (SEI) in Lithium-Ion Batteries. 2. Radiolytically Induced Polymerization of Ethylene Carbonate. J. Phys. Chem. C 2013, 117, 19270–19279. [Google Scholar] [CrossRef]
- Peled, E.; Bar Tow, D.; Merson, A.; Burnstein, L. Microphase structure of SEI on HOPG. J. New Mater. Electrochem. Syst. 2000, 3, 321–328. [Google Scholar]
- Jeong, S.-K.; Inaba, M.; Abe, T.; Ogumi, Z. Surface Film Formation on Graphite Negative Electrode in Lithium-Ion Batteries: AFM Study in an Ethylene Carbonate-Based Solution. J. Electrochem. Soc. 2001, 148, A989–A993. [Google Scholar] [CrossRef]
- Domi, Y.; Ochida, M.; Tsubouchi, S.; Nakagawa, H.; Yamanaka, T.; Doi, T.; Abe, T.; Ogumi, Z. In Situ AFM Study of Surface Film Formation on the Edge Plane of HOPG in Lithium-Ion Batteries. ECS Meet. Abstr. 2011, 115, 2548–25489. [Google Scholar] [CrossRef]
- Schmitz, R.W.; Murmann, P.; Schmitz, R.; Müller, R.; Krämer, L.; Kasnatscheew, J.; Isken, P.; Niehoff, P.; Nowak, S.; Röschenthaler, G.-V.; et al. Investigations on novel electrolytes, solvents and SEI additives for use in lithium-ion batteries: Systematic electrochemical characterization and detailed analysis by spectroscopic methods. Prog. Solid State Chem. 2014, 42, 65–84. [Google Scholar] [CrossRef]
- Santner, H.J.; Korepp, C.; Winter, M.; Besenhard, J.O. In-situ FTIR investigations on the reduction of vinylene electrolyte additives suitable for use in lithium-ion batteries. Anal. Bioanal. Chem. 2004, 379, 266–271. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Slegr, H.; Shu, Z.X.; Wang, W. Fluoroethylene carbonate electrolyte and its use in lithium ion batteries with graphite anodes. J. Power Sources 1999, 81–82, 20–26. [Google Scholar] [CrossRef]
- Shen, Y.; Pedersen, E.E.; Christensen, M.; Iversen, B.B. An electrochemical cell for in operando studies of lithium/sodium batteries using a conventional x-ray powder diffractometer. Rev. Sci. Instrum. 2014, 85, 104103. [Google Scholar] [CrossRef]
- Silberstein, K.E.; Lowe, M.A.; Richards, B.; Gao, J.; Hanrath, T.; Abruña, H.D. Operando X-ray Scattering and Spectroscopic Analysis of Germanium Nanowire Anodes in Lithium Ion Batteries. Langmuir 2015, 31, 2028–2035. [Google Scholar] [CrossRef]
- Arthur, Z.N.; Chiu, H.-C.; Lu, X.; Chen, N.; Emond, V.; Demopoulos, G.P.; Jiang, D.-T. In Operando XANES & XRD Investigation into the Rate-Dependent Transport Properties of Lithium Iron Silicate Cathodes. MRS Adv. 2017, 2, 419–424. [Google Scholar] [CrossRef]
- Alemu, T.; Wang, F.-M. In situ electrochemical synchrotron radiation for Li ion batteries. J. Synchrotron. Radiat. 2018, 25, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Van Hulzen, M.; Singh, D.P.; Brownrigg, A.; Wright, J.P.; Van Dijk, N.H.; Wagemaker, M. Direct view on the phase evolution in individual LiFePO4 nanoparticles during Li-ion battery cycling. Nat. Commun. 2015, 6, 8333. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Lipson, A.L.; Karmel, H.J.; Emery, J.D.; Fister, T.T.; Fenter, P.A.; Hersam, M.C.; Bedzyk, M.J. In Situ X-ray Study of the Solid Electrolyte Interphase (SEI) Formation on Graphene as a Model Li-ion Battery Anode. Chem. Mater. 2012, 24, 3038–3043. [Google Scholar] [CrossRef]
- Wagner, M.R.; Albering, J.H.; Moeller, K.-C.; Besenhard, J.O.; Winter, M. XRD evidence for the electrochemical formation of Li+(PC)yCn- in PC-based electrolytes. Electrochem. Commun. 2005, 7, 947–952. [Google Scholar] [CrossRef]
- Buchberger, I.; Seidlmayer, S.; Pokharel, A.; Piana, M.; Hattendorff, J.; Kudejova, P.; Gilles, R.; Gasteiger, H.A. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC impedance. J. Electrochem. Soc. 2015, 162, A2737. [Google Scholar] [CrossRef]
- Bianchini, M.; Leriche, J.B.; Laborier, J.-L.; Gendrin, L.; Suard, E.; Croguennec, L.; Masquelier, C. A New Null Matrix Electrochemical Cell for Rietveld Refinements of In-Situ or Operando Neutron Powder Diffraction Data. J. Electrochem. Soc. 2013, 160, A2176–A2183. [Google Scholar] [CrossRef]
- Boulet-Roblin, L.; Borel, P.; Sheptyakov, D.; Tessier, C.; Novák, P.; Villevieille, C. Operando Neutron Powder Diffraction Using Cylindrical Cell Design: The Case of LiNi0.5Mn1.5O4 vs Graphite. J. Phys. Chem. C 2016, 120, 17268–17273. [Google Scholar] [CrossRef]
- Shiotani, S.; Naka, T.; Morishima, M.; Yonemura, M.; Kamiyama, T.; Ishikawa, Y.; Ukyo, Y.; Uchimoto, Y.; Ogumi, Z. Degradation analysis of 18650-type lithium-ion cells by operando neutron diffraction. J. Power Sources 2016, 325, 404–409. [Google Scholar] [CrossRef]
- Key, B.; Morcrette, M.; Tarascon, J.-M.; Grey, C.P. Pair Distribution Function Analysis and Solid State NMR Studies of Silicon Electrodes for Lithium Ion Batteries: Understanding the (De)lithiation Mechanisms. J. Am. Chem. Soc. 2011, 133, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Allan, P.K.; Griffin, J.M.; Darwiche, A.; Borkiewicz, O.J.; Wiaderek, K.M.; Chapman, K.W.; Morris, A.J.; Chupas, P.J.; Monconduit, L.; Grey, C.P. Tracking Sodium-Antimonide Phase Transformations in Sodium-Ion Anodes: Insights from Operando Pair Distribution Function Analysis and Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2016, 138, 2352–2365. [Google Scholar] [CrossRef]
- Khalid, S.; Caliebe, W.; Siddons, P.; So, I.; Clay, B.; Lenhard, T.; Hanson, J.; Wang, Q.; Frenkel, A.; Marinkovic, N.; et al. Quick extended x-ray absorption fine structure instrument with millisecond time scale, optimized for in situ applications. Rev. Sci. Instrum. 2010, 81, 15105. [Google Scholar] [CrossRef]
- Li, C.; Sarapulova, A.; Pfeifer, K.; Luo, X.; Casati, N.P.M.; Welter, E.; Melinte, G.; Fu, Q.; Dsoke, S. Elucidating the Mechanism of Li Insertion into Fe1–xS/Carbon via In Operando Synchrotron Studies. ACS Appl. Mater. Interfaces 2020, 12, 52691–52700. [Google Scholar] [CrossRef]
- Dixon, D.; Ávila, M.; Ehrenberg, H.; Bhaskar, A. Difference in Electrochemical Mechanism of SnO2 Conversion in Lithium-Ion and Sodium-Ion Batteries: Combined in Operando and Ex Situ XAS Investigations. ACS Omega 2019, 4, 9731–9738. [Google Scholar] [CrossRef]
- Deng, S.; Sun, Q.; Li, M.; Adair, K.; Yu, C.; Li, J.; Li, W.; Fu, J.; Li, X.; Li, R.; et al. Insight into cathode surface to boost the performance of solid-state batteries. Energy Storage Mater. 2021, 35, 661–668. [Google Scholar] [CrossRef]
- Bleith, P.; van Beek, W.; Kaiser, H.; Novak, P.; Villevieille, C. Simultaneous in situ X-ray absorption spectroscopy and X ray diffraction studies on battery materials: The case of Fe0.5TiOPO4. J. Phys. Chem C 2015, 119, 3466–3471. [Google Scholar] [CrossRef]
- Arthur, Z.; Chiu, H.-C.; Lu, X.; Chen, N.; Emond, V.; Zaghib, K.; Jiang, D.-T.; Demopoulos, G.P. Spontaneous reaction between an uncharged lithium iron silicate cathode and a LiPF6-based electrolyte. Chem. Commun. 2015, 52, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Orikasa, Y.; Takamatsu, D.; Koyama, Y.; Mori, S.; Masese, T.; Mori, T.; Minato, T.; Tanida, H.; Uruga, T.; et al. Stabilization of the Electronic Structure at the Cathode/Electrolyte Interface via MgO Ultra-thin Layer during Lithium-ions Insertion/Extraction. Electrochemistry 2014, 82, 891–896. [Google Scholar] [CrossRef][Green Version]
- Wandt, J.; Freiberg, A.; Thomas, R.; Gorlin, Y.; Siebel, A.; Jung, R.; Gasteiger, H.A.; Tromp, M. Transition metal dissolution and deposition in Li-ion batteries investigated by operando X-ray absorption spectroscopy. J. Mater. Chem. A 2016, 4, 18300–18305. [Google Scholar] [CrossRef]
- Philippe, B.; Hahlin, M.; Edström, K.; Gustafsson, T.; Siegbahn, H.; Rensmo, H. Photoelectron Spectroscopy for Lithium Battery Interface Studies. J. Electrochem. Soc. 2015, 163, A178–A191. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novak, P. A review of the features and analysis of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Cherkashinin, G.; Motzko, M.; Schulz, N.; Späth, T.; Jaegermann, W. Electron Spectroscopy Study of Li[Ni,Co,Mn]O2/Electrolyte Interface: Electronic Structure, Interface Composition, and Device Implications. Chem. Mater. 2015, 27, 2875–2887. [Google Scholar] [CrossRef]
- Davis, A.L.; Garcia-Mendez, R.; Wood, K.N.; Kazyak, E.; Chen, K.-H.; Teeter, G.; Sakamoto, J.; Dasgupta, N.P. Electro-chemo-mechanical evolution of sulfide solid electrolyte/Li metal interfaces: Operando analysis and ALD interlayer effects. J. Mater. Chem. A 2020, 8, 6291–6302. [Google Scholar] [CrossRef]
- Schwöbel, A.; Hausbrand, R.; Jaegermann, W. Interface reactions between LiPON and lithium studied by in-situ X-ray photoemission. Solid State Ion. 2015, 273, 51–54. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Haasch, R.T.; Dillon, S.J. In situ X-ray photoelectron and Auger electron spectroscopic characterization of reaction mechanisms during Li-ion cycling. Chem. Commun. 2016, 52, 13257–13260. [Google Scholar] [CrossRef]
- Hirayama, M.; Sonoyama, N.; Abe, T.; Minoura, M.; Ito, M.; Mori, D.; Yamada, A.; Kanno, R.; Terashima, T.; Takano, M.; et al. Characterization of electrode/electrolyte interface for lithium batteries using in situ synchrotron X-ray reflectometry—A new experimental technique for LiCoO2 model electrode. J. Power Sources 2007, 168, 493–500. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Shimada, K.; Ichitsubo, T.; Yagi, S.; Matsubara, E. Surface-layer formation by reductive decomposition of LiPF6 at relatively high potentials on negative electrodes in lithium ion batteries and its suppression. J. Power Sources 2014, 271, 431–436. [Google Scholar] [CrossRef]
- Jerliu, B.; Hüger, E.; Horisberger, M.; Stahn, J.; Schmidt, H. Irreversible lithium storage during lithiation of amorphous silicon thin film electrodes studied by in-situ neutron reflectometry. J. Power Sources 2017, 359, 415–421. [Google Scholar] [CrossRef]
- Owejan, J.E.; Owejan, J.P.; Decaluwe, S.C.; Dura, J.A. Solid Electrolyte Interphase in Li-Ion Batteries: Evolving Structures Measured In situ by Neutron Reflectometry. Chem. Mater. 2012, 24, 2133–2140. [Google Scholar] [CrossRef]
- Hirayama, M.; Yonemura, M.; Suzuki, K.; Torikai, N.; Smith, H.; Watkinsand, E.; Majewski, J.; Kanno, R. Surface Characterization of LiFePO4 Epitaxial Thin Films by X-ray/Neutron Reflectometry. Electrochemistry 2010, 78, 413–415. [Google Scholar] [CrossRef]
- Metwalli, E.; Götz, K.; Lages, S.; Bär, C.; Zech, T.; Noll, D.M.; Schuldes, I.; Schindler, T.; Prihoda, A.; Lang, H.; et al. A novel experimental approach for nanostructure analysis: Simultaneous small-angle X-ray and neutron scattering. J. Appl. Crystallogr. 2020, 53, 722–733. [Google Scholar] [CrossRef]
- Hattendorff, J.; Seidlmayer, S.; Gasteiger, H.A.; Gilles, R. Li-ion half-cells studied operando during cycling by small-angle neutron scattering. J. Appl. Crystallogr. 2020, 53, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.; Wetjen, M.; Busch, S.; Gasteiger, H.; Gilles, R. Contrast Matched SANS for Observing SEI and Pore Clogging in Silicon-Graphite Anodes. J. Electrochem. Soc. 2019, 166, A1051–A1054. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, Z.-G.; Li, X.; He, L.; Yu, X.; Li, H.; Wang, F. Neutron-based characterization techniques for lithium-ion battery research. Chin. Phys. B 2020, 29, 018201. [Google Scholar] [CrossRef]
- Sacci, R.L.; Bañuelos, J.L.; Veith, G.M.; Littrell, K.C.; Cheng, Y.Q.; Wildgruber, C.U.; Jones, L.L.; Ramirez-Cuesta, A.J.; Rother, G.; Dudney, N.J.; et al. Structure of Spontaneously Formed Solid-Electrolyte Interphase on Lithiated Graphite Determined Using Small-Angle Neutron Scattering. J. Phys. Chem. C 2015, 119, 9816–9823. [Google Scholar] [CrossRef]
- Bridges, C.A.; Sun, X.-G.; Zhao, J.; Paranthaman, M.P.; Dai, S. In Situ Observation of Solid Electrolyte Interphase Formation in Ordered Mesoporous Hard Carbon by Small-Angle Neutron Scattering. J. Phys. Chem. C 2012, 116, 7701–7711. [Google Scholar] [CrossRef]
- Holler, M.; Guizar-Sicairos, M.; Tsai, E.H.; Dinapoli, R.; Müller, E.; Bunk, O.; Aeppli, G. High-resolution non-destructive three-dimensional imaging of integrated circuits. Nat. Cell Biol. 2017, 543, 402–406. [Google Scholar] [CrossRef]
- Heenan, T.M.; Tan, C.; Hack, J.; Brett, D.J.; Shearing, P.R. Developments in X-ray tomography characterization for electrochemical devices. Mater. Today 2019, 31, 69–85. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zuo, P. Probing Battery Electrochemistry with In Operando Synchrotron X-Ray Imaging Techniques. Small Methods 2018, 2, 1700293. [Google Scholar] [CrossRef]
- Wolf, M.; May, B.M.; Cabana, J. Visualization of Electrochemical Reactions in Battery Materials with X-ray Microscopy and Mapping. Chem. Mater. 2017, 29, 3347–3362. [Google Scholar] [CrossRef]
- Kimura, Y.; Tomura, A.; Fakkao, M.; Nakamura, T.; Ishiguro, N.; Sekizawa, O.; Nitta, K.; Uruga, T.; Okumura, T.; Tada, M.; et al. 3D Operando Imaging and Quantification of Inhomogeneous Electrochemical Reactions in Composite Battery Electrodes. J. Phys. Chem. Lett. 2020, 11, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Vanpeene, V.; Villanova, J.; Suuronen, J.-P.; King, A.; Bonnin, A.; Adrien, J.; Maire, E.; Roué, L. Monitoring the morphological changes of Si-based electrodes by X-ray computed tomography: A 4D-multiscale approach. Nano Energy 2020, 74, 104848. [Google Scholar] [CrossRef]
- Ziesche, R.F.; Arlt, T.; Finegan, D.P.; Heenan, T.M.M.; Tengattini, A.; Baum, D.; Kardjilov, N.; Markötter, H.; Manke, I.; Kockelmann, W.; et al. 4D imaging of lithium-batteries using correlative neutron and X-ray tomography with a virtual unrolling technique. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kardjilov, N.; Manke, I.; Woracek, R.; Hilger, A.; Banhart, J. Advances in neutron imaging. Mater. Today 2018, 21, 652–672. [Google Scholar] [CrossRef]
- Senyshyn, A.; Muhlbauer, M.J.; Nikolowski, K.; Pirling, T.; Ehrenberg, H. “In operando” neutron scattering studies on Li-ion batteries. J. Power Sources 2012, 203, 126–129. [Google Scholar] [CrossRef]
- Butler, L.G.; Schillinger, B.; Ham, K.; Dobbins, T.A.; Liu, P.; Vajo, J.J. Neutron imaging of a commercial Li-ion battery during discharge: Application of monochromatic imaging and polychromatic dynamic tomography. Nucl. Instrum. Methods Phys. Res. Sect. A 2011, 651, 320–328. [Google Scholar] [CrossRef]
- Goers, D.; Holzapfel, M.; Scheifele, W.; Lehmann, E.; Vontobel, P.; Novák, P. In situ neutron radiography of lithium-ion batteries: The gas evolution on graphite electrodes during the charging. J. Power Sources 2004, 130, 221–226. [Google Scholar] [CrossRef]
- Lanz, M.; Lehmann, E.; Imhof, R.; Exnar, I.; Novák, P. In situ neutron radiography of lithium-ion batteries during charge/discharge cycling. J. Power Sources 2001, 101, 177–181. [Google Scholar] [CrossRef]
- Goldner, R.B.; Haas, T.E.; Arntz, F.O.; Slaven, S.; Wong, K.K.; Wilkens, B.; Shepard, C.; Lanford, W. Nuclear reaction analysis profiling as direct evidence for lithium ion mass transport in thin film “rocking-chair” structures. Appl. Phys. Lett. 1993, 62, 1699–1701. [Google Scholar] [CrossRef]
- Lamaze, G.P.; Chen-Mayer, H.; Badding, M.; Laby, L. In situ measurement of lithium movement in thin film electrochromic coating using cold neutron depth profiling. Surf. Interface Anal. 1999, 27, 644–647. [Google Scholar] [CrossRef]
- Lamaze, G.; Chen-Mayer, H.; Becker, D.; Vereda, F.; Goldner, R.; Haas, T.; Zerigian, P. Cold neutron depth profiling of lithium-ion battery materials. J. Power Sources 2003, 119–121, 680–685. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.-N.; Baudry, P.; Lascaud, S. In situ study of dendritic growth inlithium/PEO-salt/lithium cells. Electrochim. Acta 1998, 43, 1569–1574. [Google Scholar] [CrossRef]
- Steiger, J.; Richter, G.; Wenk, M.; Kramer, D.; Mönig, R. Comparison of the growth of lithium filaments and dendrites under different conditions. Electrochem. Commun. 2015, 50, 11–14. [Google Scholar] [CrossRef]
- Orsini, F.; Du Pasquier, A.; Beaudouin, B.; Tarascon, J.; Trentin, M.; Langenhuizen, N.; De Beer, E.; Notten, P. In situ SEM study of the interfaces in plastic lithium cells. J. Power Sources 1999, 81–82, 918–921. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Lee, P.-C.; Tsao, C.-W.; Lee, L.-H.; Wang, D.-Y.; Wen, C.-Y. In situ Scanning Electron Microscopy Observation of MoS2 Nanosheets during Lithiation in Lithium Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 7066–7072. [Google Scholar] [CrossRef]
- Raimann, P.R.; Hochgatterer, N.S.; Korepp, C.; Moller, K.C.; Winter, M.; Schrottner, H.; Hofer, F.; Besenhard, J.O. Monitoring dynamics of electrode reactions in Li-ion batteryes by in situ ESEM. Ionics 2006, 12, 253–255. [Google Scholar] [CrossRef]
- Lee, S.-H.; You, H.-G.; Han, K.-S.; Kim, J.; Jung, I.-H.; Song, J.-H. A new approach to surface properties of solid electrolyte interphase on a graphite negative electrode. J. Power Sources 2014, 247, 307–313. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, L.; Baumann, D.; Mei, L.; Yao, Y.; Duan, X.; Shi, Y.; Huang, J.; Huang, Y.; Duan, X. In Situ Transmission Electron Microscopy for Energy Materials and Devices. Adv. Mater. 2019, 31, e1900608. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Johnson, G.; Zhang, S.; Su, D. In Situ Transmission Electron Microscopy for Energy Applications. Joule 2019, 3, 4–8. [Google Scholar] [CrossRef]
- Basak, S.; Ganapathy, S.; Malladi, S.K.; Vicarelli, L.; Schreuders, H.; Dam, B.; Kelder, E.M.; Wagemaker, M.; Zandbergen, H.W. Designing Reliable Operando TEM Experiments to Study (De)lithiation Mechanism of Battery Electrodes. J. Electrochem. Soc. 2019, 166, A3384–A3386. [Google Scholar] [CrossRef]
- Gong, C.; Pu, S.D.; Gao, X.; Yang, S.; Liu, J.; Ning, Z.; Rees, G.J.; Capone, I.; Pi, L.; Liu, B.; et al. Revealing the Role of Fluoride-Rich Battery Electrode Interphases by Operando Transmission Electron Microscopy. Adv. Energy Mater. 2021, 11, 2003118. [Google Scholar] [CrossRef]
- Fawey, M.H.; Chakravadhanula, V.S.K.; Munnangi, A.R.; Rongeat, C.; Hahn, H.; Fitchner, M.; Kuberl, C. First results from in situ transmission electron microscopy studies of all-solid-state fluoride ion batteries. J. Power Sources 2020, 466, 228283. [Google Scholar] [CrossRef]
- Mu, X.; Mazilkin, A.; Sprau, C.; Colsmann, A.; Kübel, C. Mapping structure and morphology of amorphous organic thin films by 4D-STEM pair distribution function analysis. Microscopy 2019, 68, 301–309. [Google Scholar] [CrossRef]
- Li, Z.; Tan, X.; Li, P.; Kalisvaart, P.; Janish, M.T.; Mook, W.M.; Luber, E.J.; Jungjohann, K.L.; Carter, C.B.; Mitlin, D. Coupling in Situ TEM and Ex Situ Analysis to Understand Heterogeneous Sodiation of Antimony. Nano Lett. 2015, 15, 6339–6348. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Huang, J.Y. In situ TEM electrochemistry of anode materials in lithium ion batteries. Energy Environ. Sci. 2011, 4, 3844–3860. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhong, L.; Wang, C.M.; Sullivan, J.P.; Xu, W.; Zhang, L.Q.; Mao, S.X.; Hudak, N.S.; Liu, X.H.; Subramanian, A.; et al. In Situ Observation of the Electrochemical Lithiation of a Single SnO2 Nanowire Electrode. Science 2010, 330, 1515–1520. [Google Scholar] [CrossRef]
- Sacci, R.L.; Dudney, N.J.; More, K.L.; Parent, L.R.; Arslan, I.; Browning, N.D.; Unocic, R.R. Direct visualization of initial SEI morphology and growth kinetics during lithium deposition by in situ electrochemical transmission electron microscopy. Chem. Commun. 2014, 50, 2104–2107. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, D.; Wang, S.; Liu, S.; Cai, X.; Zhang, L.; Zhao, R.; Li, B.; Kang, F. In Situ Observation of Interface Evolution on a Graphite Anode by Scanning Electrochemical Microscopy. ACS Appl. Mater. Interfaces 2020, 12, 37047–37053. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Huang, W.; Wang, H.; Zhou, W.; Li, Y.; Li, Y.; Xu, J.; Huang, W.; Chiu, W.; et al. Cathode-Electrolyte Interphase in Lithium Batteries Revealed by Cryogenic Electron Microscopy. Matter 2021, 4, 302–312. [Google Scholar] [CrossRef]
- Aurbach, D.; Cohen, Y. The Application of Atomic Force Microscopy for the Study of Li Deposition Processes. J. Electrochem. Soc. 1996, 143, 3525–3532. [Google Scholar] [CrossRef]
- Edstrom, K.; Herranen, M. Thermal Stability of the HOPG/Liquid Electrolyte Interphase Studied by In Situ Electrochemical Atomic Force Microscopy. J. Electrochem. Soc. 2000, 147, 3628–3632. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Yin, Y.; Guthrey, H.; Park, K.; Lee, S.-H.; Al-Jassim, M. Local electrical degradations of solid-state electrolyte by nm-scale operando imaging of ionic and electronic transports. J. Power Sources 2021, 481, 229138. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, K.; Jervis, R.; Shearing, P.R.; Miller, T.S.; Brett, D.J.L. Operando Electrochemical Atomic Force Microscopy of Solid–Electrolyte Interphase Formation on Graphite Anodes: The Evolution of SEI Morphology and Mechanical Properties. ACS Appl. Mater. Interfaces 2020, 12, 35132–35141. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Joho, F.; Lanz, M.; Rykart, B.; Panitz, J.-C.; Alliata, D.; Kötz, R.; Haas, O. The complex electrochemistry of graphite electrodes in lithium-ion batteries. J. Power Sources 2001, 97-98, 39–46. [Google Scholar] [CrossRef]
- Charles-Blin, Y.; Flahaut, D.; Guérin, K.; Dubois, M.; Monconduit, L.; Louvain, N.; Martinez, H. Surface atomic layer fluorination of Li4Ti5O12: Investigation of the surface electrode reactivity and the outgassing behavior in LiBs. Appl. Surf. Sci. 2020, 527, 146834. [Google Scholar] [CrossRef]
- Li, J.; Fang, J.; Su, H.; Sun, S. Interfacial processes of lithium ion batteries by FTIR spectroscopy. Prog. Chem. 2011, 23, 349–356. [Google Scholar]
- Bhattacharya, S.; Reza Riahi, A.; Alpas, A.T. Electrochemical cycling behavior of lithium carbonate (Li2CO3) pre-treated graphite anodes-SEI formation and graphite damage mechanisms. Carbon 2014, 77, 99–112. [Google Scholar] [CrossRef]
- Hy, S.; Felix; Chen, Y.-H.; Liu, J.-Y.; Rick, J.; Hwang, B.-J. In situ surface enhanced Raman spectroscopic studies of solid electrolyte interphase formation in lithium ion battery electrodes. J. Power Sources 2014, 256, 324–328. [Google Scholar] [CrossRef]
- Odziemkowski, M.; Krell, M.; Irish, D.E. A Raman Microprobe in Situ and Ex Situ Study of Film Formation at Lithium/Organic Electrolyte Interfaces. J. Electrochem. Soc. 1992, 139, 3052–3063. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Key, B.; Chen, H.; Best, A.S.; Hollenkamp, A.F.; Grey, C.P. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 2010, 9, 504–510. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Thienenkamp, J.H.; Huang, C.-J.; Tao, H.-C.; Rodehorst, U.; Hwang, B.J.; Winter, M.; Brunklaus, G. Revealing the Impact of Film-Forming Electrolyte Additives on Lithium Metal Batteries via Solid-State NMR/MRI Analysis. J. Phys. Chem. C 2021, 125, 252–265. [Google Scholar] [CrossRef]
- Märker, K.; Xu, C.; Grey, C.P. Operando NMR of NMC811/Graphite Lithium-Ion Batteries: Structure, Dynamics, and Lithium Metal Deposition. J. Am. Chem. Soc. 2020, 142, 17447–17456. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Jerschow, A. In situ and operando magnetic resonance imaging of electrochemical cells: A perspective. J. Magn. Reson. 2019, 308, 106600. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.A.; Rinkel, B.L.D.; Gunnarsdottir, A.B.; Marker, K.; Menkin, S.; Paul, S.; Sergeyev, I.V.; Grey, C.P. Selective NMR observation of the SEI-metal interface by dynamic nuclear polarization from lithium metal. Nat. Commun. 2020, 11, 2224. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zheng, G.; Liang, Z.; Jin, Y.; Liu, X.; Chen, S.; Zhou, K.; Zhu, J.; Lin, M.; He, H.; et al. Visualizing the growth process of sodium microstructures in sodium batteries by in-situ 23Na MRI and NMR spectroscopy. Nat. Nanotechnol. 2020, 15, 883–890. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, M.; Yu, X.; Zhang, Y.; Wang, J.-G.; Ren, X.; Cao, R.; Xu, W.; Baer, D.R.; Du, Y.; et al. Real-time mass spectrometric characterization of the solid–electrolyte interphase of a lithium-ion battery. Nat. Nanotechnol. 2020, 15, 224–230. [Google Scholar] [CrossRef]
- Joshi, T.; Eom, K.; Yushin, G.; Fuller, T.F. Effects of Dissolved Transition Metals on the Electrochemical Performance and SEI Growth in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1915–A1921. [Google Scholar] [CrossRef]
- GirishKumar, G.; Bailey, W.H.; Peterson, B.K.; Casteel, W.J. Electrochemical and spectroscopic investigation of the overcharge behavior of stabile electrolyte salts in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A146–A153. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Mitlin, D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 2020, 10, 2002297. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Su, W.-N.; Hwang, B.J. In situ analytical techniques for battery interface analysis. Chem. Soc. Rev. 2018, 47, 736–851. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, F.; Liu, X.; Yang, S.; Ma, J. Formation and modification of cathode electrolyte interphase: A mini review. Electrochem. Commun. 2021, 122, 106870. [Google Scholar] [CrossRef]
- Bommier, C.; Chang, W.; Li, J.; Biswas, S.; Davies, G.; Nanda, J.; Steingart, D. Operando Acoustic Monitoring of SEI Formation and Long-Term Cycling in NMC/SiGr Composite Pouch Cells. J. Electrochem. Soc. 2020, 167, 020517. [Google Scholar] [CrossRef]
- Chang, W.; Bommier, C.; Fair, T.; Yeung, J.; Patil, S.; Steingart, D. Understanding Adverse Effects of Temperature Shifts on Li-Ion Batteries: An Operando Acoustic Study. J. Electrochem. Soc. 2020, 167, 090503. [Google Scholar] [CrossRef]
- Lemarié, Q.; Idrissi, H.; Maire, E.; Thivel, P.-X.; Alloin, F.; Roué, L. Impact of the binder nature on the morphological change of sulfur electrodes upon cycling investigated by in situ characterization methods. J. Power Sources 2020, 477, 228374. [Google Scholar] [CrossRef]
- Liu, T.; Lin, L.; Bi, X.; Tian, L.; Yang, K.; Liu, J.; Li, M.; Chen, Z.; Lu, J.; Amine, K.; et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat. Nanotechnol. 2019, 14, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kitz, P.G.; Lacey, M.J.; Novák, P.; Berg, E.J. Operando investigation of the solid electrolyte interphase mechanical and transport properties formed from vinylene carbonate and fluoroethylene carbonate. J. Power Sources 2020, 477, 228567. [Google Scholar] [CrossRef]
- Nangir, M.; Massoudi, A.; Teyebifard, S.A. Investigation of the lithium-ion depletion in the silicon-silicon carbode anode/electrolyte interface in lithium-ion battery via electrochemical impedance spectroscopy. J. Electroanal. Chem. 2020, 872, 114385. [Google Scholar] [CrossRef]
- Ebejer, N.; Schnippering, M.; Colburn, A.W.; Edwards, M.A.; Unwin, P.R. Localized High Resolution Electrochemistry and Multifunctional Imaging: Scanning Electrochemical Cell Microscopy. Anal. Chem. 2010, 82, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lochala, J.; Taverne, T.; Xiao, J. The interplay between solid electrolyte interface (SEI) and dendritic lithium growth. Nano Energy 2017, 40, 34–41. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Y.; Cui, Y. Lithium Metal Anode Materials Design: Interphase and Host. Electrochem. Energy Rev. 2019, 2, 509–517. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lin, D.C.; Li, Y.Z.; Chen, G.; Nix, O.; Li, Y.; Cui, Y. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 2018, 9, 3656. [Google Scholar] [CrossRef]
- Li, W.Y.; Yao, H.B.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.-M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Cheng, X.-B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Ma, L.; Kim, M.S.; Archer, L.A. Stable Artificial Solid Electrolyte Interphases for Lithium Batteries. Chem. Mater. 2017, 29, 4181–4189. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, G.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Lee, S.W.; Liang, Z.; Lee, H.W.; Yan, K.; Yao, H.; Wang, H.; Li, W.; Chu, S.; Cui, Y. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 2014, 9, 618–623. [Google Scholar] [CrossRef]
- Yan, K.; Lee, H.-W.; Gao, T.; Zheng, G.; Yao, H.; Wang, H.; Lu, Z.; Zhou, Y.; Liang, Z.; Liu, Z.; et al. Ultrathin Two-Dimensional Atomic Crystals as Stable Interfacial Layer for Improvement of Lithium Metal Anode. Nano Lett. 2014, 14, 6016–6022. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Chen, W.; Zhou, G.; Liu, K.; Dunn, B.; Cui, Y. Conformal Lithium Fluoride Protection Layer on Three-Dimensional Lithium by Nonhazardous Gaseous Reagent Freon. Nano Lett. 2017, 17, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liao, L.; Shi, F.; Lei, T.; Chen, G.; Pei, A.; Sun, J.; Yan, K.; Zhou, G.; Xie, J.; et al. Surface Fluorination of Reactive Battery Anode Materials for Enhanced Stability. J. Am. Chem. Soc. 2017, 139, 11550–11558. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cheng, X.-B.; Yao, Y.-X.; Shen, X.; Li, B.-Q.; Li, W.-J.; Zhang, R.; Huang, J.-Q.; Li, H.; Zhang, Q. An Armored Mixed Conductor Interphase on a Dendrite-Free Lithium-Metal Anode. Adv. Mater. 2018, 30, e1804461. [Google Scholar] [CrossRef] [PubMed]
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-Generation Lithium Metal Anode Engineering via Atomic Layer Deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, H.; Choo, M.-J.; Park, J.-K.; Kim, H.-T. Ionomer-Liquid Electrolyte Hybrid Ionic Conductor for High Cycling Stability of Lithium Metal Electrodes. Sci. Rep. 2015, 5, 14458. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-W.; Shi, Y.; Yin, Y.-X.; Zeng, X.-X.; Li, J.-Y.; Li, C.-J.; Wan, L.-J.; Wen, R.; Guo, Y.-G. Inside Cover: A Flexible Solid Electrolyte Interphase Layer for Long-Life Lithium Metal Anodes. Angew. Chem. Int. Ed. 2018, 57, 1422. [Google Scholar] [CrossRef]
- Belov, D.; Yarmolenko, O.; Peng, A.; Efimov, O. Lithium surface protection by polyacetylene in situ polymerization. Synth. Met. 2006, 156, 745–751. [Google Scholar] [CrossRef]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-amorphous core−shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef]
- Nie, M.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Silicon Solid Electrolyte Interphase (SEI) of Lithium Ion Battery Characterized by Microscopy and Spectroscopy. J. Phys. Chem. C 2013, 117, 13403–13412. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, N.; Lee, H.-W.; Zhao, J.; Li, W.; Li, Y.; Cui, Y. Nonfilling Carbon Coating of Porous Silicon Micrometer-Sized Particles for High-Performance Lithium Battery Anodes. ACS Nano 2015, 9, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Lee, H.-W.; Muralidharan, P.; Mari, C.M.; Ruffo, R.; Kim, D.K. Facile synthesis and electrochemical performance of ordered LiNi0.5Mn1.5O4 nanorods as a high power positive electrode for rechargeable Li-ion batteries. J. Power Sources 2011, 196, 10712–10716. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Liang, G.; Peterson, V.K.; See, K.W.; Guo, Z.; Pang, W.K. Developing high-voltage spinel LiNi0.5Mn1.5O4 cathodes for high-energy-density lithium-ion batteries: Current achievements and future prospects. J. Mater. Chem. A 2020, 8, 15373–15398. [Google Scholar] [CrossRef]
- Hai, B.; Shukla, A.K.; Duncan, H.; Chen, G. The effect of particle surface facets on the kinetic properties of LiNi0.5Mn1.5O4 cathode materials. J. Mater. Chem A 2013, 1, 759–769. [Google Scholar] [CrossRef]
- Ma, J.; Cui, G.; Chen, L. Surface and Interface Issues in Spinel LiNi0.5Mn1.5O4: Insights into a Potential Cathode Material for High Energy Density Lithium Ion Batteries. Chem. Mater. 2016, 28, 3578–3606. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Zeng, W.; Cao, F.; Na, J.; Tina, W.; Mu, S. Synthesis, Modification, and Lithium-Storage Properties of Spinel LiNi0.5Mn1.5O4. ChemElectroChem 2021, 8, 608–624. [Google Scholar] [CrossRef]
- Piao, J.-Y.; Gu, L.; Wei, Z.; Ma, J.; Wu, J.; Yang, W.; Gong, Y.; Sun, Y.-G.; Duan, S.-Y.; Tao, X.-S.; et al. Phase Control on Surface for the Stabilization of High Energy Cathode Materials of Lithium Ion Batteries. J. Am. Chem. Soc. 2019, 141, 4900–4907. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, H.; Liu, J.; Sun, Q.; Wang, B.; Kaliyappan, K.; Zhao, Y.; Banis, M.N.; Liu, Y.; Xueliang, S.; et al. Nanoscale Manipulation of Spinel Lithium Nickel Manganese Oxide Surface by Multisite Ti Occupation as High-Performance Cathode. Adv. Mater. 2017, 29, 1703764. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, D.H.; Oh, D.Y.; Lee, H.; Kim, J.H.; Lee, J.H.; Jung, Y.S. Surface chemistry of LiNi0.5Mn1.5O4 particles coated by Al2O3 using atomic layer deposition for lithium-ion batteries. J. Power Sources 2015, 274, 1254–1262. [Google Scholar] [CrossRef]
- Zou, Z.; Xu, H.; Zhang, H.; Tang, Y.; Cui, G. Electrolyte Therapy for Improving the Performance of LiNi0.5Mn1.5O4 Cathodes Assembled Lithium–Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 21368–21385. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Xing, L.; Li, W. Enhanced cyclability of LiNi0.5Mn1.5O4 cathode in carbonate based electrolyte with incorporation of tris(trimethylsilyl)phosphate (TMSP). J. Power Sources 2014, 261, 148–155. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Choi, I.; Kim, J.J. Quercetin as electrolyte additive for LiNi0.5Mn1.5O4 cathode for lithium-ion secondary battery at elevated temperature. J. Power Sources 2016, 336, 316–324. [Google Scholar] [CrossRef]
- Wang, X.; Xue, W.-D.; Hu, K.; Li, Y.; Li, Y.; Huang, R.-Y. Adiponitrile as Lithium-Ion Battery Electrolyte Additive: A Positive and Peculiar Effect on High-Voltage Systems. ACS Appl. Energy Mater. 2018, 1, 5347–5354. [Google Scholar] [CrossRef]
- Wu, B.; Ren, Y.; Mu, D.; Liu, X.; Wu, F. Electrochemical performance of 5 V LiNi0.5Mn1.5O4 cathode modified with lithium carbonate addition in electrolyte. J. Power Sources 2014, 272, 183–189. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Zhang, H.; Liu, J.; Jing, J.; Cui, X.; Li, S. Compatibility between LiNi0.5Mn1.5O4 and electrolyte based upon lithium bis(oxalate)borate and sulfolane for high voltage lithium-ion batteries. Electrochim. Acta 2013, 104, 134–139. [Google Scholar] [CrossRef]
- Ren, X.; Chen, S.; Lee, H.; Mei, D.; Engelhard, M.H.; Burton, S.D.; Zhao, W.; Zheng, J.; Li, Q.; Ding, M.S.; et al. Localized High-Concentration Sulfone Electrolytes for High-Efficiency Lithium-Metal Batteries. Chem 2018, 4, 1877–1892. [Google Scholar] [CrossRef]
- Matsumoto, K.; Martinez, M.; Gutel, T.; Mailley, S.; De Vito, E.; Patoux, S.; Inoue, K.; Utsugi, K. Stability of trimethyl phosphate non-flammable based electrolyte on the high voltage cathode (LiNi0.5Mn1.5O4). J. Power Sources 2015, 273, 1084–1088. [Google Scholar] [CrossRef]
- Zheng, X.; Liao, Y.; Zhang, Z.; Zhu, J.; Ren, F.; He, H.; Xiang, Y.; Zheng, Y.; Yang, Y. Exploring high-voltage fluorinated carbonate electrolytes for LiNi0.5Mn1.5O4 cathode in Li-ion batteries. J. Energy Chem. 2020, 42, 62–70. [Google Scholar] [CrossRef]
- Cao, X.; He, X.; Wang, J.; Liu, H.; Röser, S.; Rad, B.R.; Evertz, M.; Streipert, B.; Li, J.; Wagner, R.; et al. High Voltage LiNi0.5Mn1.5O4/Li4Ti5O12 Lithium Ion Cells at Elevated Temperatures: Carbonate- versus Ionic Liquid-Based Electrolytes. ACS Appl. Mater. Interfaces 2016, 8, 25971–25978. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Dong, N.; Xia, Y.; Chen, S.; Luo, H.; Liu, Y.; Liu, Z. Localized concentrated high-concentration electrolyte enhanced stability and safety for high voltage Li-ion batteries. Electrochim. Acta 2019, 320, 134633. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li [NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.-T.; Oh, S.-M.; Sun, Y.-K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2016, 6, 1501010. [Google Scholar] [CrossRef]
- Lim, B.-B.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Comparative Study of Ni-Rich Layered Cathodes for Rechargeable Lithium Batteries: Li[Ni0.85Co0.11Al0.04]O2 and Li[Ni0.84Co0.06Mn0.09Al0.01]O2 with Two-Step Full Concentration Gradients. ACS Energy Lett. 2016, 1, 283–289. [Google Scholar] [CrossRef]
- Li, W.; Dolocan, A.; Oh, P.; Celio, H.; Park, S.; Cho, J.; Manthiram, A. Dynamic behaviour of interphases and its implication on high-energy-density cathode materials in lithium-ion batteries. Nat. Commun. 2017, 8, 14589. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, C.; Ruffo, R.; Mustarelli, P. The Importance of Interphases in Energy Storage Devices: Methods and Strategies to Investigate and Control Interfacial Processes. Physchem 2021, 1, 26-44. https://doi.org/10.3390/physchem1010003
Ferrara C, Ruffo R, Mustarelli P. The Importance of Interphases in Energy Storage Devices: Methods and Strategies to Investigate and Control Interfacial Processes. Physchem. 2021; 1(1):26-44. https://doi.org/10.3390/physchem1010003
Chicago/Turabian StyleFerrara, Chiara, Riccardo Ruffo, and Piercarlo Mustarelli. 2021. "The Importance of Interphases in Energy Storage Devices: Methods and Strategies to Investigate and Control Interfacial Processes" Physchem 1, no. 1: 26-44. https://doi.org/10.3390/physchem1010003
APA StyleFerrara, C., Ruffo, R., & Mustarelli, P. (2021). The Importance of Interphases in Energy Storage Devices: Methods and Strategies to Investigate and Control Interfacial Processes. Physchem, 1(1), 26-44. https://doi.org/10.3390/physchem1010003