Distribution Patterns, Nesting Ecology and Nest Characteristics of the Stingless Bees (Tetragonula pagdeni Schwarz) in West Bengal, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Stingless Bee Species
2.2. Survey on Nest Distribution Patterns
2.3. Nest Substrates
2.4. External Nest Characteristics
2.5. Internal Nest Characteristics
2.6. Statistical Analyses
3. Results
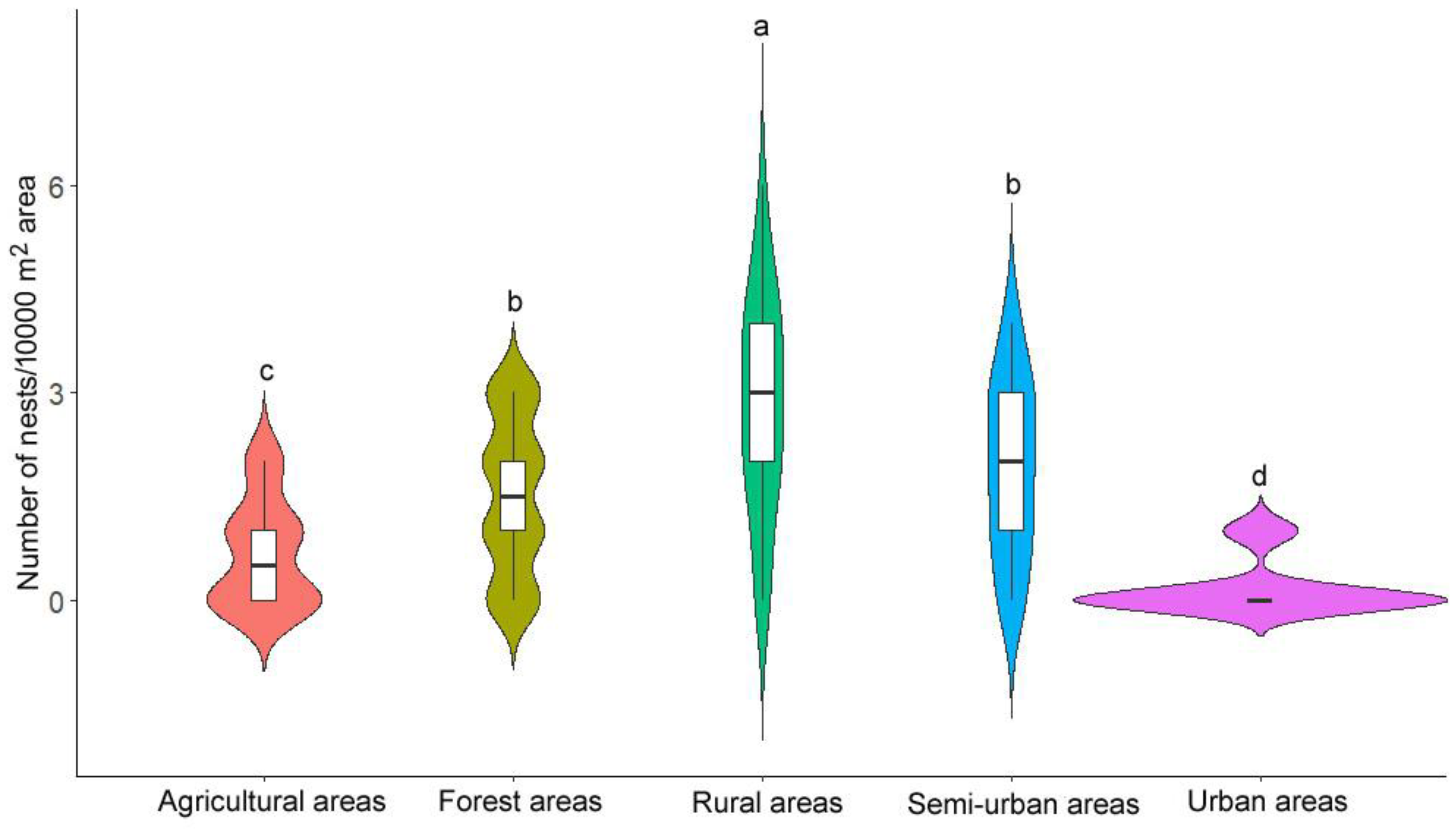
3.1. Nest Distribution Patterns
3.2. Nest Substrates
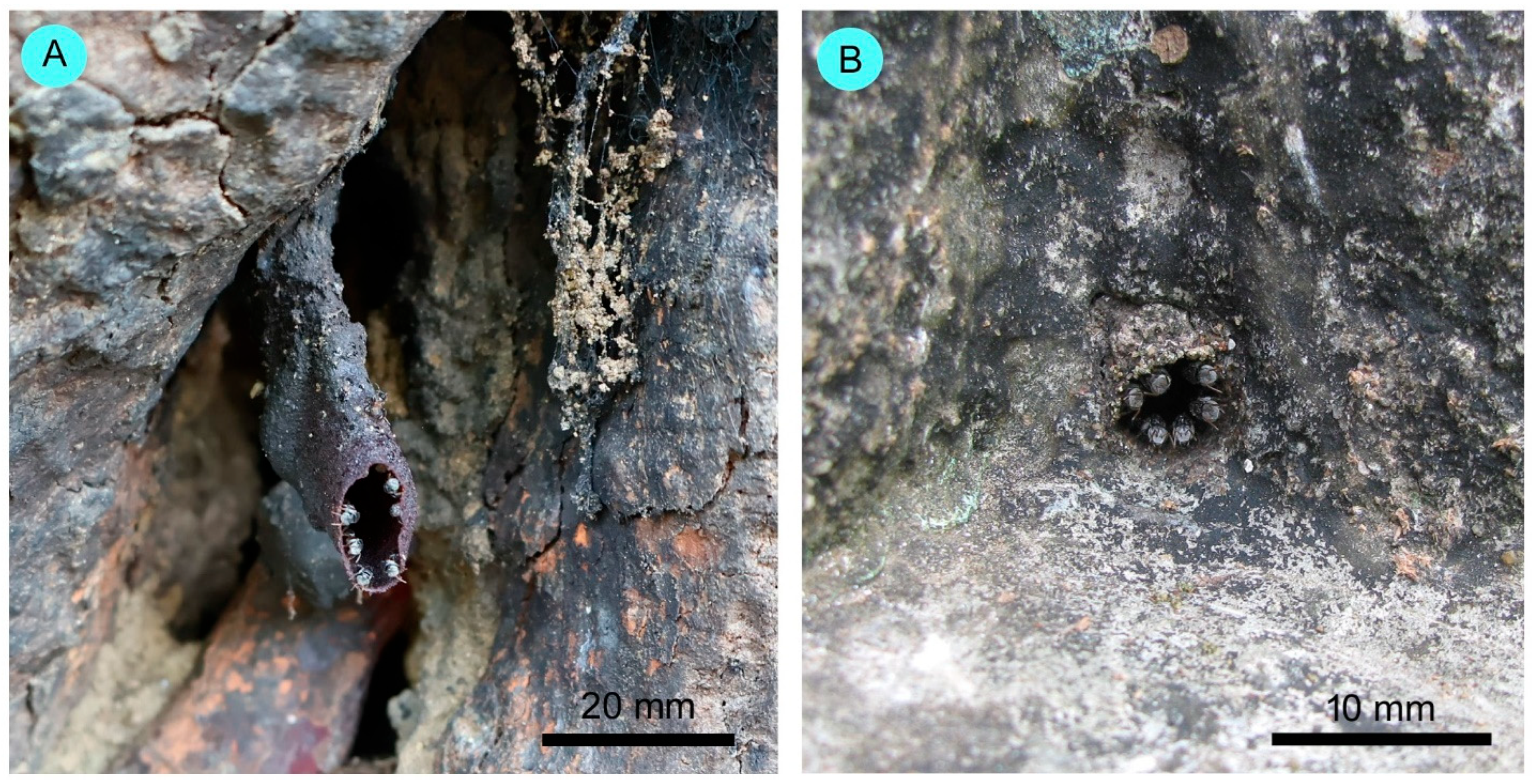
3.3. External Nest Characteristics
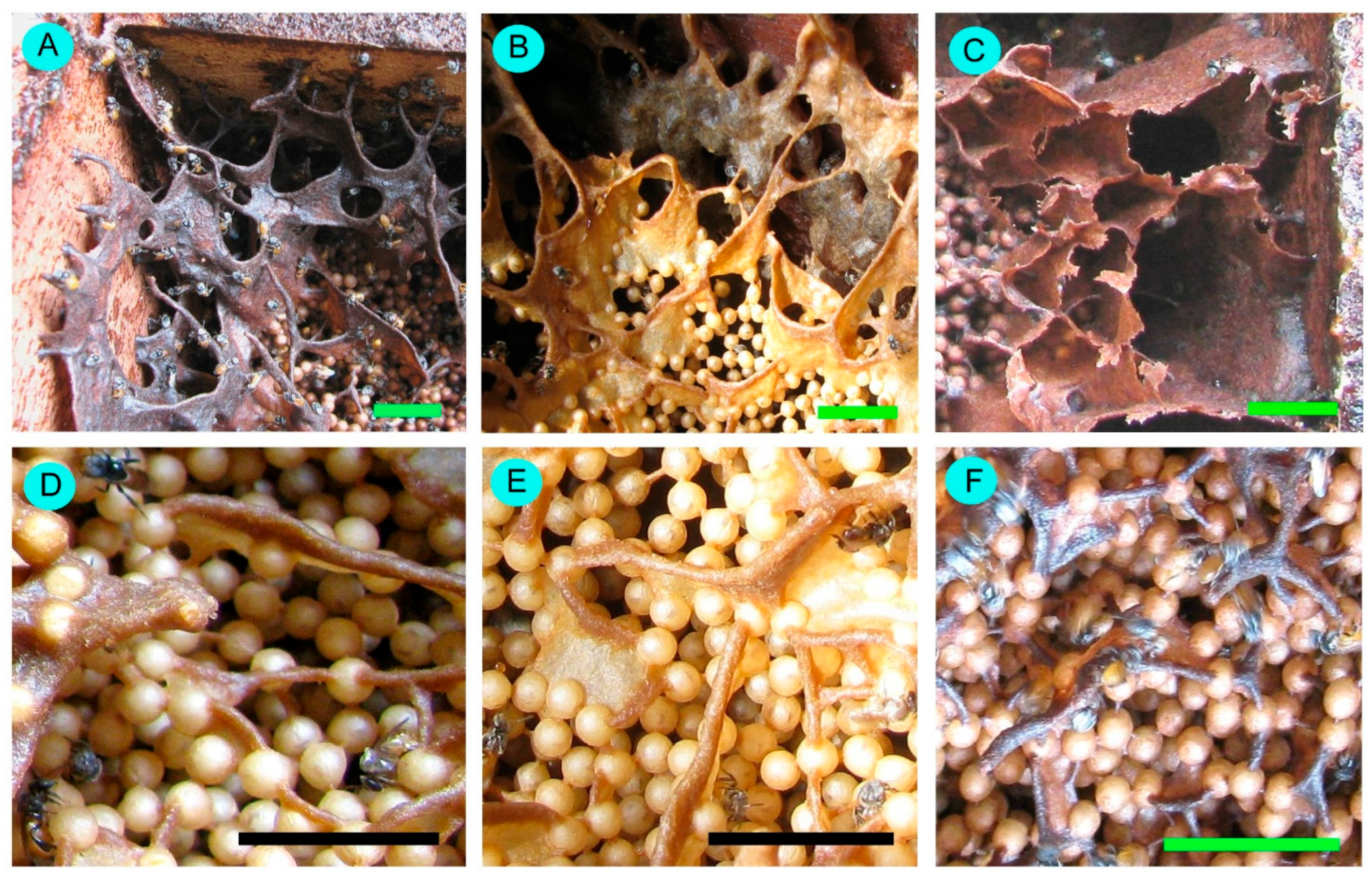
3.4. Internal Nest Characteristics
4. Discussion
4.1. Nest Distribution Patterns
4.2. Nest Substrates
4.3. External Nest Characteristics
4.4. Internal Nest Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerr, W.E.; Maule, V. Geographic distribution of stingless bees and its implications (Hymenoptera: Apidae). J. N. Y. Entomol. Soc. 1964, 72, 2–18. [Google Scholar]
- Camargo, J.M.F. Meliponinae (Hymenoptera, Apidae) da coleção do Instituto di entomologia agraria, Portici, Itália. Rev. Bras. Entomol. 1988, 32, 351–374. [Google Scholar]
- Rasmussen, C.; Cameron, S.A. Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol. J. Linn. Soc. 2010, 99, 206–232. [Google Scholar] [CrossRef]
- Ruano Iraheta, C.E.; Hernández Martínez, M.Á.; Alas Romero, L.A.; Claros Álvarez, M.E.; Arévalo, D.R.; Rodríguez González, V.A. Stingless bee distribution and richness in El Salvador (Apidae, Meliponinae). J. Apic. Res. 2015, 54, 1–10. [Google Scholar] [CrossRef]
- Roubik, D.W. Nest and colony characteristics of stingless bees from French Guiana (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 1979, 52, 443–470. [Google Scholar]
- Eardley, C.D. Taxonomic revision of the African stingless bees (Apoidea: Apidae: Apinae: Meliponini). Afr. Plant Prot. 2004, 10, 63–96. [Google Scholar]
- Cortopassi-Laurinoa, M.; Imperatriz-Fonsecab, V.L.; Roubik, D.W.; Dollind, A.; Hearde, T.; Aguilarf, I.; Venturieri, G.C.; Eardley, C.; Nogueira-Neto, P. Global meliponiculture: Challenge opportunities. Apidologie 2006, 37, 275–292. [Google Scholar] [CrossRef]
- Rasmussen, C. Stingless bees (Hymenoptera: Apidae: Meliponini) of the Indian subcontinent: Diversity, taxonomy and current status of knowledge. Zootaxa 2013, 3647, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, A.; Das, P.K.; Rajkumari, P.; Saikia, J.; Sharmah, D. Stingless bees (Hymenoptera: Apidae: Meliponini): Diversity and distribution in India. Int. J. Sci. Res. 2015, 4, 77–81. [Google Scholar]
- Viraktamath, S.; Roy, J. Description of five new species of Tetragonula (Hymenoptera: Apidae: Meliponini) from India. Biologia 2022, 77, 1769–1793. [Google Scholar] [CrossRef]
- Shaikh, A.K.; Wankhede, S.M.; Kale, S.N.; Ainarkar, A.A.; Mali, S.S. Nesting structure and biology of stingless bee, Tetragonula nr. pagdeni in Konkan region of Maharashtra. Pharma Innov. J. 2023, 12, 1000–1003. [Google Scholar]
- Layek, U.; Karmakar, P. Nesting characteristics, floral resources, and foraging activity of Trigona iridipennis Smith in Bankura district of West Bengal, India. Insectes Soc. 2018, 65, 117–132. [Google Scholar] [CrossRef]
- Gaona, F.P.; Guerrero, A.; Gusmán, E.; Espinosa, C.I. Pollen resources used by two species of stingless bees (Meliponini) in a tropical dry forest of southern Ecuador. J. Insect Sci. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Vossler, F.G. Main pollen characters in the diet of four stingless bees (Apidae: Meliponini) in the Chaco dry forest. Rodriguésia 2024, 75, e01892023. [Google Scholar] [CrossRef]
- Slaa, E.J.; Cevaal, A.; Sommeijer, M.J. Floral constancy in Trigona stingless bees foraging on artificial flower patches: A comparative study. J. Apic. Res. 1998, 37, 191–198. [Google Scholar] [CrossRef]
- Heard, T.A. The role of stingless bees in crop pollination. Annu. Rev. Entomol. 1999, 44, 183–206. [Google Scholar] [CrossRef]
- Slaa, E.J.; Chaves, L.A.S.; Malagodi-Braga, K.S.; Hofstede, F.E. Stingless bees in applied pollination: Practice and perspectives. Apidologie 2006, 37, 293–315. [Google Scholar] [CrossRef]
- Layek, U.; Kundu, A.; Bisui, S.; Karmakar, P. Impact of managed stingless bee and western honey bee colonies on native pollinators and yield of watermelon: A comparative study. Ann. Agric. Sci. 2021, 66, 38–45. [Google Scholar] [CrossRef]
- Wongsa, K.; Duangphakdee, O.; Rattanawannee, A. Pollination efficacy of stingless bees, Tetragonula pagdeni Schwarz (Apidae: Meliponini), on greenhouse tomatoes (Solanum lycopersicum Linnaeus). PeerJ 2023, 11, e15367. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Russell, F.D. Cerumen of Australian stingless bees (Tetragonula carbonaria): Gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Naturwissenschaften 2011, 98, 329–337. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-based medicinal properties of stingless bee products: Recent progress and future directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef]
- Chuttong, B.; Lim, K.; Praphawilai, P.; Danmek, K.; Maitip, J.; Vit, P.; Wu, M.C.; Ghosh, S.; Jung, C.; Burgett, M.; et al. Exploring the functional properties of propolis, geopropolis, and cerumen, with a special emphasis on their antimicrobial effects. Foods 2023, 12, 3909. [Google Scholar] [CrossRef]
- Suriawanto, N.; Atmowidi, T.; Kahono, S. Nesting sites characteristics of stingless bees (Hymenoptera: Apidae) in Central Sulawesi, Indonesia. J. Insect Biodivers. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Bisui, S.; Layek, U.; Karmakar, P. Comparing the pollen forage pattern of stingless bee (Trigona iridipennis Smith) between rural and semi-urban areas of West Bengal, India. J. Asia Pac. Entomol. 2019, 22, 714–722. [Google Scholar] [CrossRef]
- Mogho, N.M.T.; Akwanjoh, S.R.; Wittmann, D.; Kenneth, T. Nest architecture and colony characteristics of Meliponula bocandei (Hymenoptera, Apidae, Meliponini) in Cameroon. Int. J. Res. Agric. Sci. 2018, 5, 2348–3997. [Google Scholar]
- Eltz, T.; Brühl, C.A.; Van der Kaars, S.; Linsenmair, E.K. Determinants of stingless bee nest density in lowland dipterocarp forests of Sabah, Malaysia. Oecologia 2002, 131, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Njoya, M.T.M.; Akwanjoh, S.R.; Wittmann, D. Nest architecture and colony characteristics of Meliponula (Axestotrigona) ferruginea (Hymenoptera, Apidae, Meliponini) in Cameroon. J. Biosci. 2019, 44, 13. [Google Scholar] [CrossRef]
- Roubik, D. Foraging behavior of competing africanized honeybees and stingless bees. Ecology 1980, 61, 836–845. [Google Scholar] [CrossRef]
- Danaraddi, C.S.; Viraktamath, S.; Basavanagoud, K.; Bhat, A.R.S. Nesting habits and nest structure of stingless bee, Trigona iridipennis Smith at Dharwad, Karnataka. Karnataka J. Agric. Sci. 2009, 22, 310–313. [Google Scholar]
- Roubik, D.W. Stingless bee nesting biology. Apidologie 2006, 37, 124–143. [Google Scholar] [CrossRef]
- Chemurot, M.; Otim, A.S.; Namayanja, D.; Onen, H.; Angiro, C.; Mugume, R.; Kajobe, R.; Macharia, J.; Gikungu, M.; Abila, P.P.; et al. Stingless beekeeping in uganda: An industry in its infancy. Afr. Entomol. 2021, 29, 165–172. [Google Scholar] [CrossRef]
- Kajobe, R. Nesting biology of equatorial Afrotropical sting less bees (Apidae; Meliponini) in Bwindi Impenetrable National Park. Uganda J. Apic. Res. 2007, 46, 245–255. [Google Scholar] [CrossRef]
- Thakodee, T.; Deowanish, S.; Duangmal, K. Melissopalynological analysis of stingless bee (Tetragonula pagdeni) honey in Eastern Thailand. J. Asia Pac. Entomol. 2018, 21, 620–630. [Google Scholar] [CrossRef]
- Vu, T.T.; Tran, N.T.; Pham, T.H.; Nguyen, H.T.; Engel, M.S.; Nguyen, L.T.P. Confirmation of the occurrence of Tetragonula pagdeni (Schwarz, 1939) in Vietnam (Hymenoptera: Apidae). Orient. Insects, 2025; in Press. [Google Scholar] [CrossRef]
- Basanna, M.; Rajanand, M.C. Study of External Nest Appearances and Foraging Behavior of Tetragonula nr. pagdeni. SSR Inst. Int. J. Life Sci. 2021, 7, 2921–2930. [Google Scholar] [CrossRef]
- Wayo, K.; Haydon, D.T.; Piraonapicha, K.; Nelli, L. Habitat suitability for tropical Asian stingless bees across anthropogenic landscapes. J. Insect Conserv. 2025, 29, 21. [Google Scholar] [CrossRef]
- Pioker-Hara, F.C.; Drummond, M.S.; Kleinert, A.D.M.P. The influence of the loss of Brazilian savanna vegetation on the occurrence of nests of stingless bees (Apidae: Meliponini). Sociobiology 2014, 61, 393–400. [Google Scholar] [CrossRef]
- Sanjaya, V.; Astiani, D.; Sisillia, L. Studi habitat dan sumber pakan lebah kelulut di kawasan cagar alam Gunung Nyiut Desa Pisak Kabupaten Bengkayang. J. Hutan Lestari 2019, 7, 786–798. [Google Scholar] [CrossRef]
- Kiatoko, N.; Raina, S.K.; Van Langevelde, F. Impact of habitat degradation on species diversity and nest abundance of five African stingless bee species in a tropical rainforest of Kenya. Int. J. Trop. Insect Sci. 2017, 37, 189–197. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Johnson, L.K. Competition and nest spacing in a tropical stingless bee community. Ecology 1977, 58, 949–963. [Google Scholar] [CrossRef]
- Roubik, D.W. Nest and colony characteristics of stingless bees from Panama (Hymenoptera: Apidae). J. Kans. Entomol. Soc. 1983, 56, 327–355. [Google Scholar]
- Cameron, E.C.; Franck, P.; Oldroyd, B.P. Genetic structure of nest aggregations and drone congregations of the southeast Asian stingless bee Trigona collina. Mol. Ecol. 2004, 13, 2357–2364. [Google Scholar] [CrossRef]
- Vossler, F.G. Flower visits, nesting and nest defence behaviour of stingless bees (Apidae: Meliponini): Suitability of the bee species for meliponiculture in the Argentinean Chaco region. Apidologie 2012, 43, 139–161. [Google Scholar] [CrossRef]
- Silva, M.D.E.; Ramalho, M.; Monteiro, D. Diversity and habitat use by stingless bees (Apidae) in the Brazilian Atlantic Forest. Apidologie 2013, 44, 699–707. [Google Scholar] [CrossRef]
- Gajanan, S.; Mohite, S.; Kuberappa, G.C.; Kencharaddi, R.N. The nest architecture of stingless bee Trigona iridipennis. Indian Bee J. 2005, 67, 36–40. [Google Scholar]
- Macedo, C.R.D.C.; Aquino, I.D.S.; Borges, P.D.F.; Barbosa, A.D.S.; Medeiros, G.R.D. Nesting behavior of stingless bees. Ciênc. Anim. Bras. 2020, 21, e58736. [Google Scholar] [CrossRef]
- Nayak, P.P.; Reddy, S.M.; Jayaprakash. Nesting pattern preferences of Stingless Bee, Trigona iridipennis Smith (Hymenoptera: Apidae) in Jnanabharathi Campus, Karnataka, India. Int. Res. J. Biol. Sci. 2013, 2, 44–50. [Google Scholar]
- Roopa, A.N.; Eswarappa, G.; Sajjanar, S.M.; Gowda, G. Study on nesting characteristics and biology of Stingless Bee (Trigona iridipennis Smith.). IOSR J. Agric. Vet. Sci. 2015, 8, 34–36. [Google Scholar]
- Miharja, J.; Atmowidi, T.; Priawandiputra, W.; Perwitasari, D.; Kahono, S. Species richness and nest entrance characteristics of stingless bees (Hymenoptera: Apidae: Meliponini) in Ujung Kulon National Park, Banten, Indonesia. Biodiversitas 2024, 25, 4961–4970. [Google Scholar] [CrossRef]
- Pooley, A.C.; Michener, C.D. Observations on nests of stingless bees in Natal (Hymenoptera: Apidae). J. Entomol. Soc. S. Afr. 1969, 32, 423–430. [Google Scholar]
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]


| Substrates | Occurrence of Nests (%) | |||||
|---|---|---|---|---|---|---|
| Agricultural Areas | Forest Areas | Rural Areas | Semi-Urban Areas | Urban Areas | Overall | |
| Tree trunks | 85 | 86.67 | 40.47 | 31.58 | 40 | 52.13 |
| Walls | 0 | 0 | 58.33 | 64.91 | 60 | 42.18 |
| Rock crevices | 0 | 13.33 | 0 | 0 | 0 | 2.84 |
| Agricultural ridges | 15 | 0 | 0 | 0 | 0 | 1.42 |
| Poles | 0 | 0 | 1.19 | 3.51 | 0 | 1.42 |
| Parameters | Available Information |
|---|---|
| Elevation from ground level | |
| Range: 0–13.46; 2.81 ± 3.17 (mean ± SD) |
| ≤2 m (59.24%), >2–4 m (24.17%), >4–6 m (7.11%), >6–8 m (5.21%), >8–10 m (3.32%), >10 m (0.95%) |
| Orientation of nest entrance | East (13.74%), west (13.27%), north (18.48%), south (4.27%), northeast (17.54%), northwest (11.37%), southeast (10.90%), southwest (10.43%) |
| External tunnel | |
| Tree trunks (58.18%), walls (8.99%), rock crevices (16.67%), agricultural ridges (33.33%), pole (0%); overall (35.07%) |
| Range: 13–84; 64.21 ± 14.61 (mean ± SD) |
| Nest entrance | |
| Circular (54.98%), oval (39.34%), slit-like (4.27%), irregular (1.42%) |
| Range: 6–17; 10.50 ± 2.94 (mean ± SD) |
| Range: 6–10; 8.12 ± 1.02 (mean ± SD) |
| Parameters | Available Information |
|---|---|
| Internal tunnel length (in mm, n = 10) | Range: 93–287; 177.20 ± 57.62 (mean ± SD) |
| Cavity dimensions | |
| Range: 136–432; 198.31 ± 86.36 (mean ± SD) |
| Range: 124–181; 142.73 ± 17.28 (mean ± SD) |
| Brood cells for workers & drones | |
| Light brown |
| Range: 2.50–3.00; 2.86 ± 0.23 (mean ± SD) |
| Range: 1.50–2.00; 1.95 ± 0.15 (mean ± SD) |
| Range: 48–71; 59.20 ± 7.43 (mean ± SD) |
| Brood cells for queens | |
| Light brown |
| Range: 3.50–4.50; 4.10 ± 0.32 (mean ± SD) |
| Range: 2.50–3.50; 2.90 ± 0.39 (mean ± SD) |
| Honey pots | |
| Light brown, dark brown |
| Range: 6.00–7.50; 6.87 ± 0.52 (mean ± SD) |
| Range: 5.00–6.50; 5.70 ± 0.44 (mean ± SD) |
| Range: 6–12; 9.00 ± 1.83 (mean ± SD) |
| Pollen pots | |
| Light brown, dark brown |
| Range: 5.00–6.50; 5.94 ± 0.63 (mean ± SD) |
| Range: 4.00–5.50; 4.92 ± 0.48 (mean ± SD) |
| Range: 12–20; 16.20 ± 2.66 (mean ± SD) |
| Supporting elements | |
| Light brown and dark brown, flat, broad, attachment portion with substrate mostly narrow and cylindrical, sometimes flat |
| Light brown and dark brown, flat, broad, attachment portion with substrate mostly narrow and cylindrical, sometimes flat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layek, U.; Karmakar, P. Distribution Patterns, Nesting Ecology and Nest Characteristics of the Stingless Bees (Tetragonula pagdeni Schwarz) in West Bengal, India. Conservation 2025, 5, 63. https://doi.org/10.3390/conservation5040063
Layek U, Karmakar P. Distribution Patterns, Nesting Ecology and Nest Characteristics of the Stingless Bees (Tetragonula pagdeni Schwarz) in West Bengal, India. Conservation. 2025; 5(4):63. https://doi.org/10.3390/conservation5040063
Chicago/Turabian StyleLayek, Ujjwal, and Prakash Karmakar. 2025. "Distribution Patterns, Nesting Ecology and Nest Characteristics of the Stingless Bees (Tetragonula pagdeni Schwarz) in West Bengal, India" Conservation 5, no. 4: 63. https://doi.org/10.3390/conservation5040063
APA StyleLayek, U., & Karmakar, P. (2025). Distribution Patterns, Nesting Ecology and Nest Characteristics of the Stingless Bees (Tetragonula pagdeni Schwarz) in West Bengal, India. Conservation, 5(4), 63. https://doi.org/10.3390/conservation5040063







