Birdfoot Violet (Viola pedata) in a Minnesota USA Dry Bluff Prairie: Population Assessment of a Preferred Host Plant of the Threatened Western Regal Fritillary Butterfly (Argynnis idalia occidentalis)
Abstract
1. Introduction
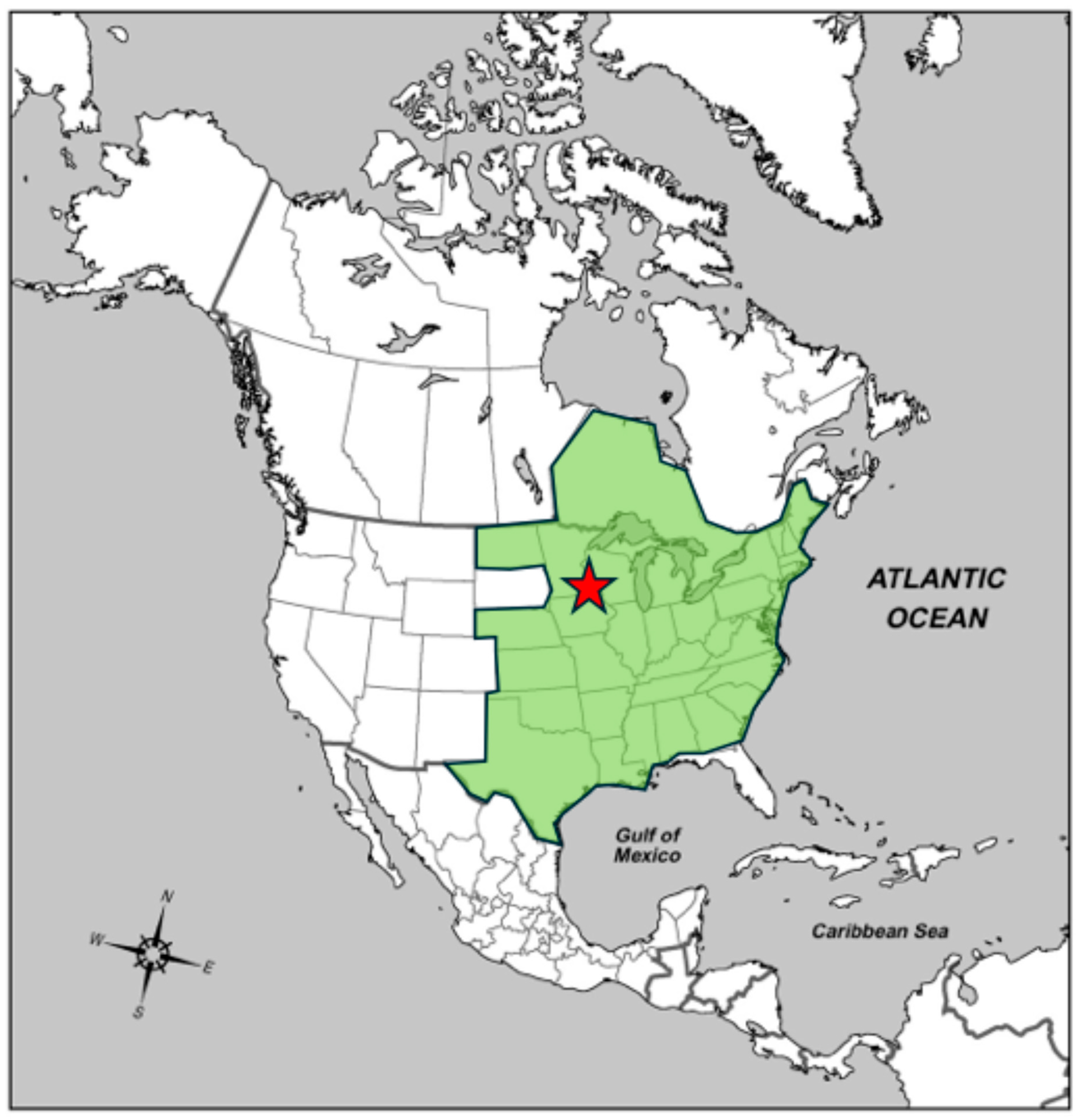
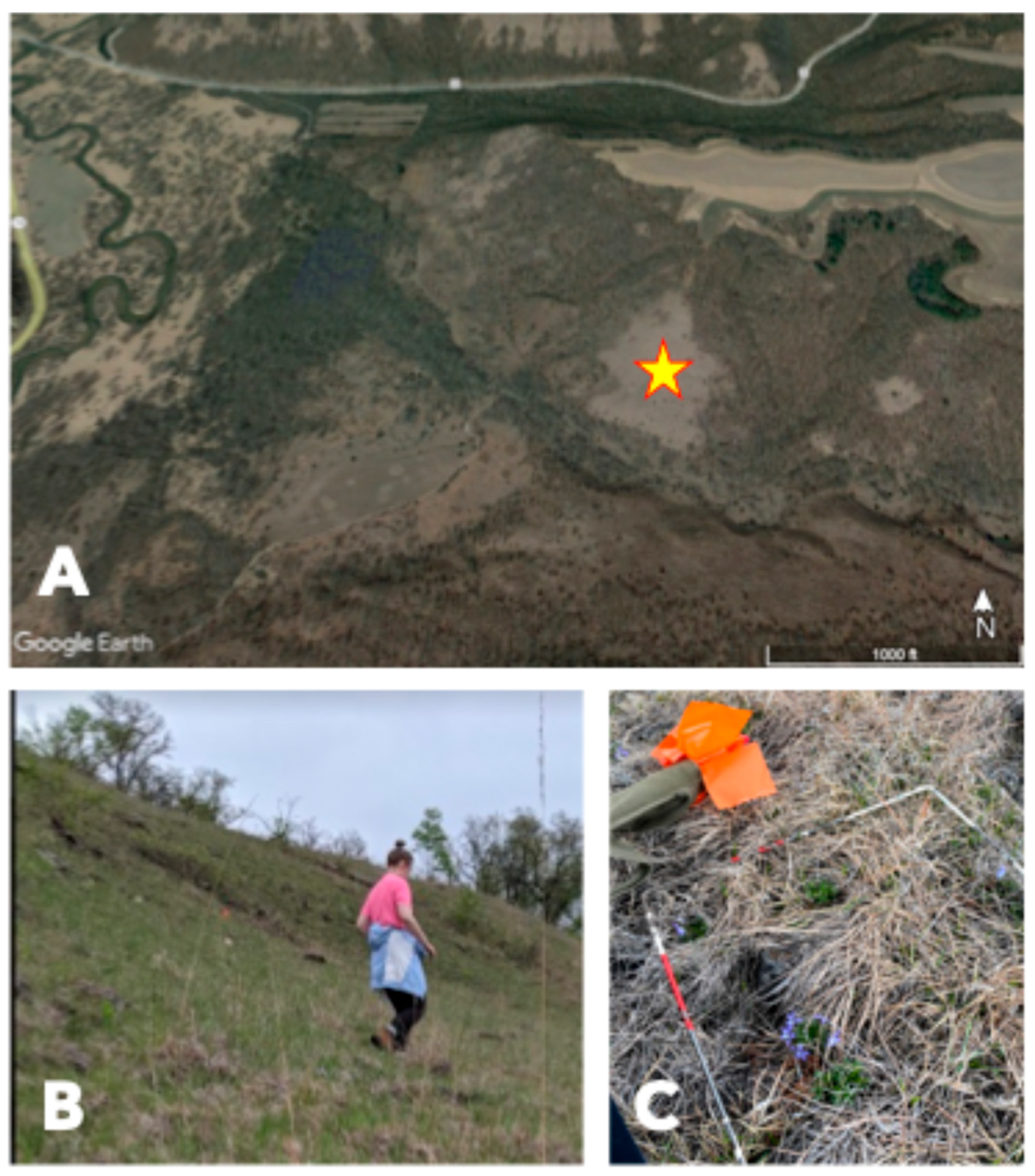
2. Study Site
3. Methods
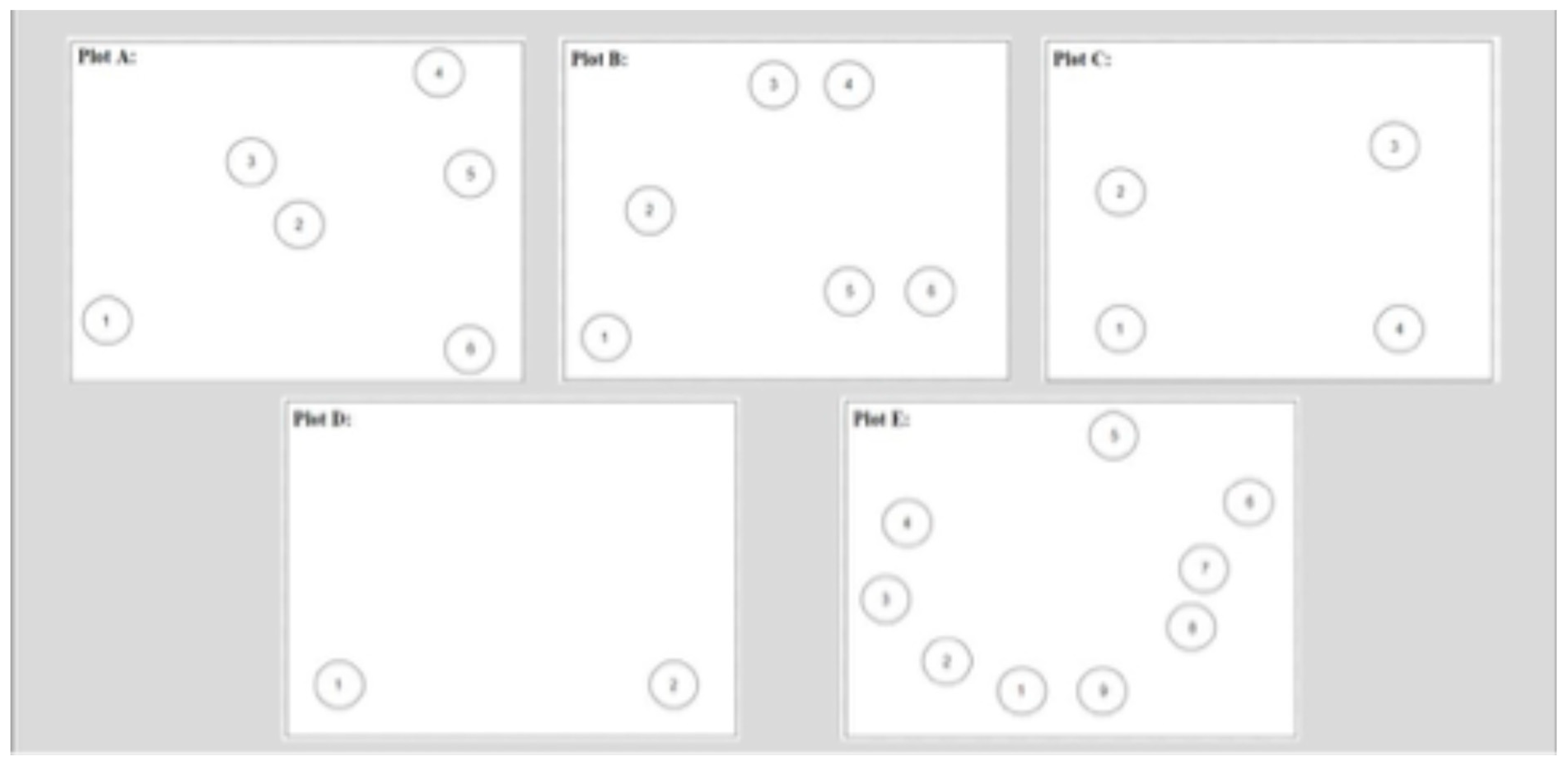
3.1. Field Studies
3.2. Data Analyses
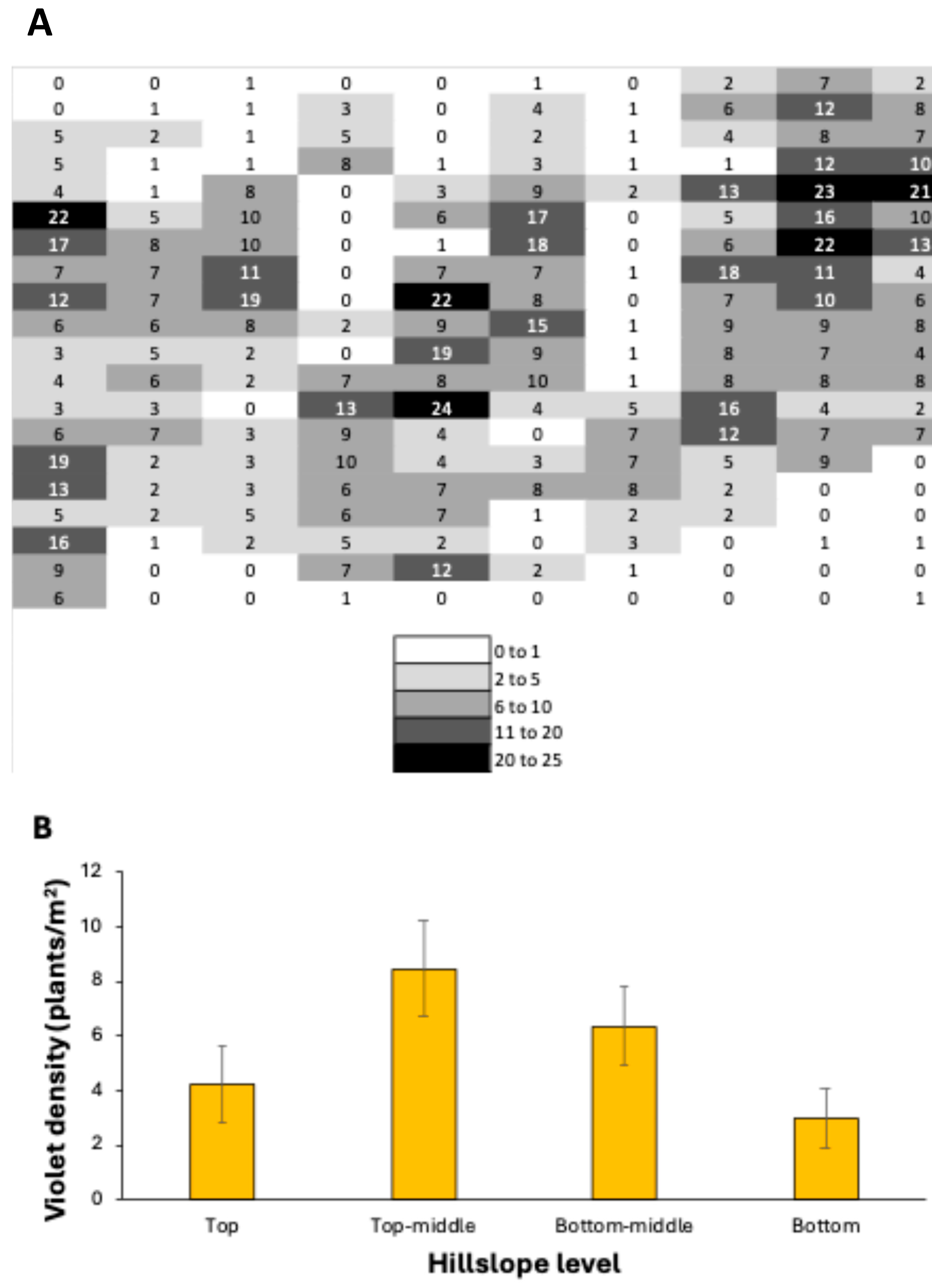
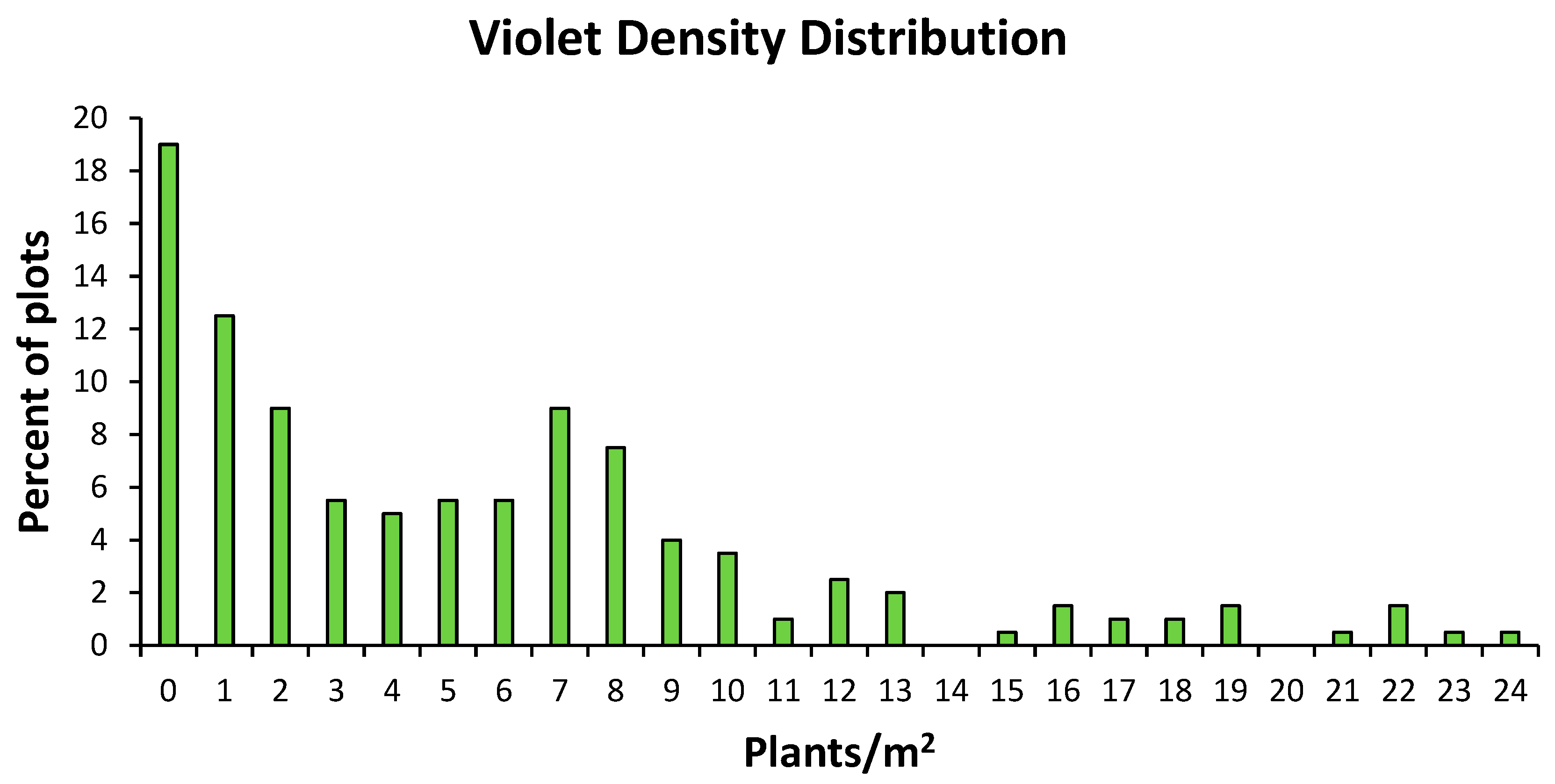
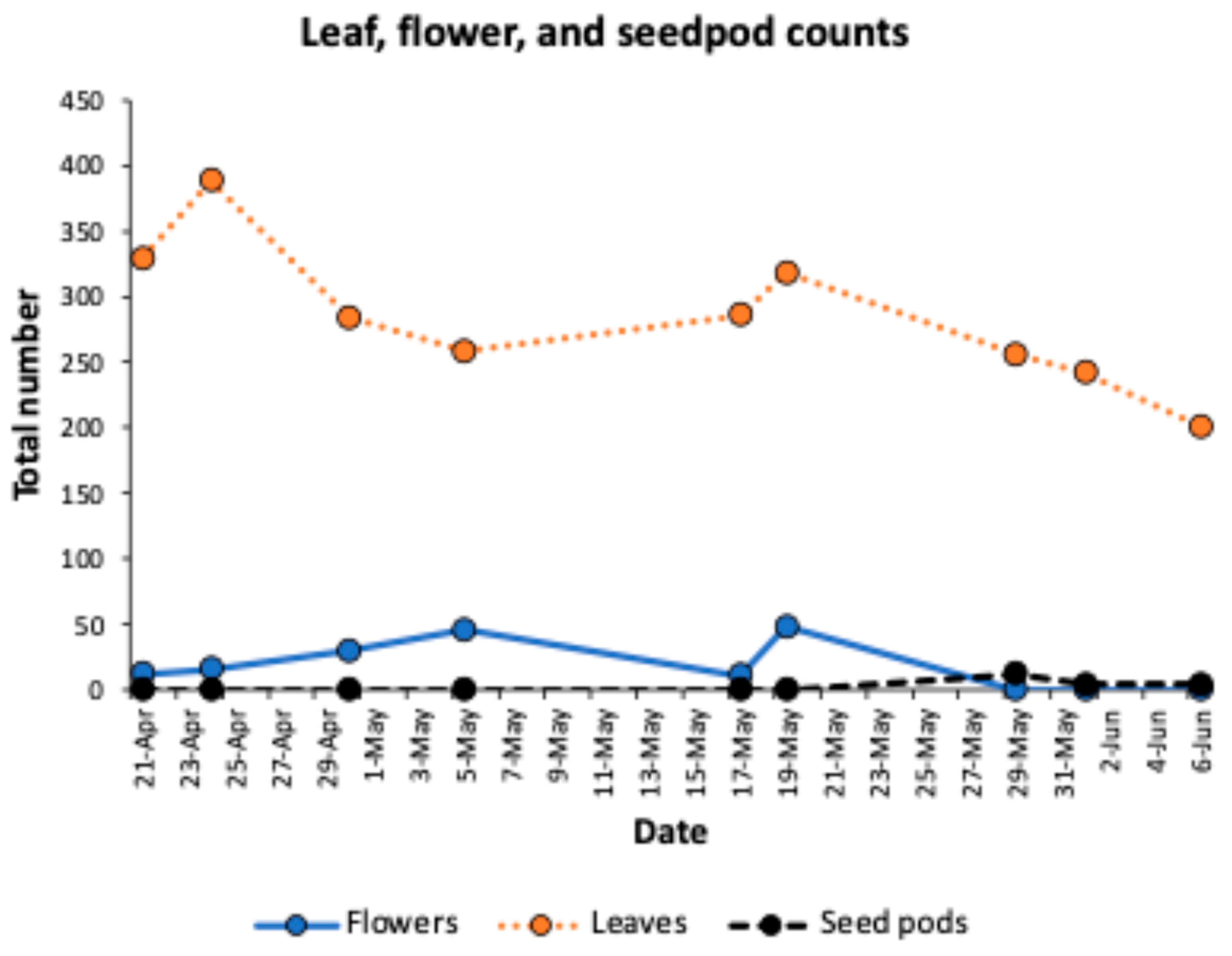
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture Natural Resources Conservation Service. Plant profile: Viola pedata L. birdfoot violet; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2025. Available online: https://plants.usda.gov/plant-profile/VIPE (accessed on 18 August 2025).
- Stritch, L. Plant of the week: Birdfoot violet (Viola pedata L.); United States Department of Agriculture US Forest Service: Washington, DC, USA, 2025. Available online: https://www.fs.usda.gov/wildflowers/plant-of-the-week/viola_pedata.shtml (accessed on 18 August 2025).
- Molano-Flores, B. Biological Assessment: Bird’s-Foot Violet; Illinois Natural History Survey, Center for Biodiversity Technical Report 1999 (8); Illinois Natural History Survey: Wilmington, IL, USA, 1999; Available online: https://core.ac.uk/download/4819592.pdf (accessed on 8 August 2025).
- Swengel, A.B. Habitat associations of sympatric violet-feeding fritillaries (Euptoieta, Speyeria, Boloria) (Lepidoptera: Nymphalidae) in tallgrass prairie. Great Lakes Entomol. 1997, 30, 1–18. [Google Scholar] [CrossRef]
- Shuey, J.A.; Metzler, E.H.; Iftner, D.C.; Calhoun, J.V.; Peacock, J.W.; Watkins, R.A.; Hooper, J.D.; Babcock, W.F. Status and habitats of potentially endangered Lepidoptera in Ohio. J. Lepid. Soc. 1987, 41, 1–12. [Google Scholar]
- Shuey, J.A.; Calhoun, J.V.; Iftner, D.C. Butterflies that are endangered, threatened, and of special concern in Ohio. Ohio J. Sci. 1987, 87, 98–106. [Google Scholar]
- Bernhardt, P.; Edens-Meier, R.; Jocson, D.; Zweck, J.; Ren, Z.-X.; Camilo, G.R.; Arduser, M. Comparative floral ecology of bicolour and concolour morphs of Viola pedata L. (Violaceae) following controlled burns. J. Pollinat. Ecol. 2016, 19, 57–70. [Google Scholar] [CrossRef]
- Beattie, A.J.; Lyons, N. Seed dispersal in Viola (Violaceae): Adaptations and strategies. Am. J. Bot. 1975, 62, 714–722. [Google Scholar] [CrossRef]
- Kelly, L.; Debinski, D.M. Relationship of host plant density to size and abundance of the regal fritillary Speyeria idalia Drury (Nymphalidae). J. Lepid. Soc. 1998, 52, 262–276. [Google Scholar]
- Kopper, B.J.; Charlton, R.E.; Margolies, D.C. Oviposition site selection by the regal fritillary, Speyeria idalia, as affected by proximity of violet host plants. J. Insect Behav. 2000, 13, 651–665. [Google Scholar] [CrossRef]
- Ross, G.N. Survey of the butterflies of the Wah’kon-tah Prairie, Missouri. Holarct. Lepid. 2005, 8, 1–30. [Google Scholar]
- Szuszwalak, J.; Riley, L. USFWS Proposes Endangered Species Act Protection for Both Subspecies of the Regal Fritillary Butterfly; United States Fish and Wildlife Service: Falls Creek, VA, USA, 2024. Available online: https://www.fws.gov/press-release/2024-08/usfws-proposes-esa-protections-both-subspecies-regal-fritillary-butterfly (accessed on 6 August 2025).
- United States Department of Agriculture Natural Resources Conservation Service. Regal Fritillary (Speyeria idalia); United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2020. Available online: https://www.fws.gov/sites/default/files/documents/Regal%20Fritillary%20Field%20Version-%20Final_0.pdf (accessed on 17 August 2025).
- Selby, G. Regal Fritillary (Speyeria idalia Drury): A Technical Conservation Assessment; United States Department of Agriculture US Forest Service, Rocky Mountain Region: Lakewood, CO, USA, 2007. [Google Scholar]
- Vaughan, D.M.; Shepherd, M.D. Species Profile: Speyeria idalia (Drury), 1773 Regal Fritillary (Nymphalidae: Argynninae). In Red List of Pollinator Insects of North America; Sheperd, M.D., Vaughan, D.M., Black, S.H., Eds.; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2005. [Google Scholar]
- Debinski, D.; Drobney, P. Regal fritillary and its host plant studied at Neal Smith National Wildlife Refuge (Iowa). Ecol. Restor. 2000, 18, 254–255. [Google Scholar]
- Shepherd, S.; Debinski, D.M. Reintroduction of regal fritillary (Speyeria idalia) to a restored prairie. Ecol. Restor. 2005, 23, 244–250. [Google Scholar] [CrossRef]
- Gehring, J.L.; Cusac, T.; Shaw, C.; Timian, A. Seed germination of Viola pedata, a key larval host of a rare butterfly. Native Plants 2013, 14, 205–2013. [Google Scholar] [CrossRef]
- Minnesota Wildflowers. Viola pedata (Birdfoot Violet). Minnesota Wildflowers: A Field Guide to the Flora of Minnesota: New Brighton, MN, USA. 2011. Available online: https://www.minnesotawildflowers.info/flower/birdfoot-violet (accessed on 18 August 2025).
- Minnesota Department of Natural Resources. Whitewater WMA; Minnesota Department of Natural Resources: Saint Paul, MN, USA, 2025; Available online: https://www.dnr.state.mn.us/areas/wildlife/whitewater_wma.html (accessed on 18 August 2025).
- Minnesota Department of Natural Resources. Whitewater Wildlife Management Area Master Plan, 2023–2033; Minnesota Department of Natural Resources: Saint Paul, MN, USA, 2023; Available online: https://files.dnr.state.mn.us/areas/wildlife/whitewater/master-plan.pdf?v=2025.04.09-12.03.00 (accessed on 18 August 2025).
- Red Wing Wildlife League. Karner Blue Butterfly Habitat Restoration at Whitewater Wildlife Management Area; Red Wing Wildlife League: Maiden Rock, WI, USA, 2006; Available online: https://files.dnr.state.mn.us/eco/nongame/projects/consgrant_reports/2006/swg_2006_lundquist.pdf (accessed on 18 August 2025).
- Minnesota Department of Natural Resources. Field Guide to the Native Plant Communities of Minnesota: The Eastern Broadleaf Forest Province; Minnesota Department of Natural Resources Ecological Land Classification Program, Minnesota County Biological Survey, and Natural Heritage and Nongame Research Program: Saint Paul, MN, USA, 2005. [Google Scholar]
- Evers, R.A.; Page, L.M. Some Unusual Natural Areas in Illinois; Biological Notes No. 100; Illinois Natural History Survey: Havana, IL, USA, 1977. [Google Scholar]
- Sarwacinski, M.I.; Berg-Binder, M.C. Relationships between plant community species richness, remnant area, and invasive leafy spurge cover in southeastern Minnesota bluff prairie habitats. Bios 2017, 88, 19–28. [Google Scholar] [CrossRef]
- Johanson, C.J.; Schmid, S.A.; Mundahl, N.D. Fire history and woody encroachment influence saxicolous microlichen community composition on dolomite outcrops in dry bluff prairies of the Minnesota Driftless Area. Prairie Nat. 2024, 56, 56–70. [Google Scholar]
- Dietz, T.E.; Marsh, M.K.; Mundahl, N.D. A rapidly expanding population of great Indian plantain (Arnoglossum reniforme) in southeastern Minnesota. Prairie Nat. 2025, 57. in press. [Google Scholar]
- Minnesota Department of Natural Resources. Types of Prairie; Minnesota Department of Natural Resources: Saint Paul, MN, USA, 2025; Available online: https://www.dnr.state.mn.us/prairie/native/types-prairies.html (accessed on 18 September 2025).
- Evans, C. Ecological Profile: Hill Prairies of Illinois; University of Illinois Urbana-Champaign, Illinois Extension: Urbana, IL, USA, 2020; Available online: https://extension.illinois.edu/blogs/naturalist-news/2020-10-16-ecological-profile-hill-prairies-illinois (accessed on 18 September 2025).
- Prairie Moon Nursery. Viola pedata Bird’s Foot Violet; Prairie Moon Nursery: Winona, MN, USA, 2022; Available online: https://www.prairiemoon.com/viola-pedata-birds-foot-violet (accessed on 18 August 2025).
- Bray, T. Nectar-seeking visits by butterflies in a tallgrass prairie remnant in eastern Nebraska. Trans. Nebraska Acad. Sci. 1994, 21, 63–72. [Google Scholar]
- Debinski, D.M.; Kelly, L. Decline of Iowa populations of the regal fritillary (Speyeria idalia) Drury. J. Iowa Acad. Sci. 1998, 105, 16–22. [Google Scholar]
- Shirley, S. Restoring the Tallgrass Prairie; University of Iowa Press: Iowa City, IA, USA, 1994. [Google Scholar]
- Becker, W.A.; Ewart, L.C. Pollination, seed set and pollen tube growth investigations in Viola pedata L. Acta Hortic. 1990, 272, 33–36. [Google Scholar] [CrossRef]
- Leuenberger, W.; Doser, J.W.; Belitz, M.W.; Ries, L.; Haddad, N.M.; Thogmartin, W.E.; Zipkin, E.F. Three decades of declines restructure butterfly communities in the Midwestern United States. Proc. Natl. Acad. Sci. USA 2025, 122, e2501340122. [Google Scholar] [CrossRef]
- Janousek, W.M.; Douglas, M.R.; Cannings, S.; Clément, M.A.; Delphia, C.M.; Everett, J.G.; Hatfield, R.G.; Keinath, D.A.; Koch, J.B.U.; McCabe, L.M.; et al. Recent and future declines of a historically widespread pollinator linked to climate, land cover, and pesticides. Proc. Natl. Acad. Sci. USA 2023, 120, e2211223120. [Google Scholar] [CrossRef]
- Cornelisse, T.; Inouye, D.W.; Irwin, R.E.; Jepsen, S.; Mawdsley, J.R.; Ormes, M.; Daniels, J.; Debinski, D.M.; Griswold, T.; Klymko, J.; et al. Elevated extinction risk in over one-fifth of native North American pollinators. Proc. Natl. Acad. Sci. USA 2025, 122, e2418742122. [Google Scholar] [CrossRef]
- Edwards, C.B.; Zipkin, E.F.; Henry, E.H.; Haddad, N.M.; Forister, M.L.; Burls, K.J.; Campbell, S.P.; Crone, E.E.; Diffendorfer, J.; Douglas, M.R.; et al. Rapid butterfly declines across the United States during the 21st century. Science 2025, 387, 1090–1094. [Google Scholar] [CrossRef] [PubMed]

| Statistics | Leaves | Flowers | Seed Pods |
|---|---|---|---|
| Mean ± 95% CI | 10.58 ± 2.21 | 0.66 ± 0.57 | 0.08 ± 0.15 |
| Median | 9 | 0 | 0 |
| Range | 3 to 35 | 0 to 9 | 0 to 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, C.; Duffrin, J.; Mundahl, N.D. Birdfoot Violet (Viola pedata) in a Minnesota USA Dry Bluff Prairie: Population Assessment of a Preferred Host Plant of the Threatened Western Regal Fritillary Butterfly (Argynnis idalia occidentalis). Conservation 2025, 5, 58. https://doi.org/10.3390/conservation5040058
Peterson C, Duffrin J, Mundahl ND. Birdfoot Violet (Viola pedata) in a Minnesota USA Dry Bluff Prairie: Population Assessment of a Preferred Host Plant of the Threatened Western Regal Fritillary Butterfly (Argynnis idalia occidentalis). Conservation. 2025; 5(4):58. https://doi.org/10.3390/conservation5040058
Chicago/Turabian StylePeterson, Chloe, James Duffrin, and Neal D. Mundahl. 2025. "Birdfoot Violet (Viola pedata) in a Minnesota USA Dry Bluff Prairie: Population Assessment of a Preferred Host Plant of the Threatened Western Regal Fritillary Butterfly (Argynnis idalia occidentalis)" Conservation 5, no. 4: 58. https://doi.org/10.3390/conservation5040058
APA StylePeterson, C., Duffrin, J., & Mundahl, N. D. (2025). Birdfoot Violet (Viola pedata) in a Minnesota USA Dry Bluff Prairie: Population Assessment of a Preferred Host Plant of the Threatened Western Regal Fritillary Butterfly (Argynnis idalia occidentalis). Conservation, 5(4), 58. https://doi.org/10.3390/conservation5040058






