Population Status and Ecological Features of the Endemic and Critically Endangered Ta Kou Bent-Toed Gecko (Cyrtodactylus takouensis) in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Surveys
2.2. Population Estimation
2.3. Microhabitat Characterization and Behaviors
2.4. Statistical Analysis
2.5. Threat Identification
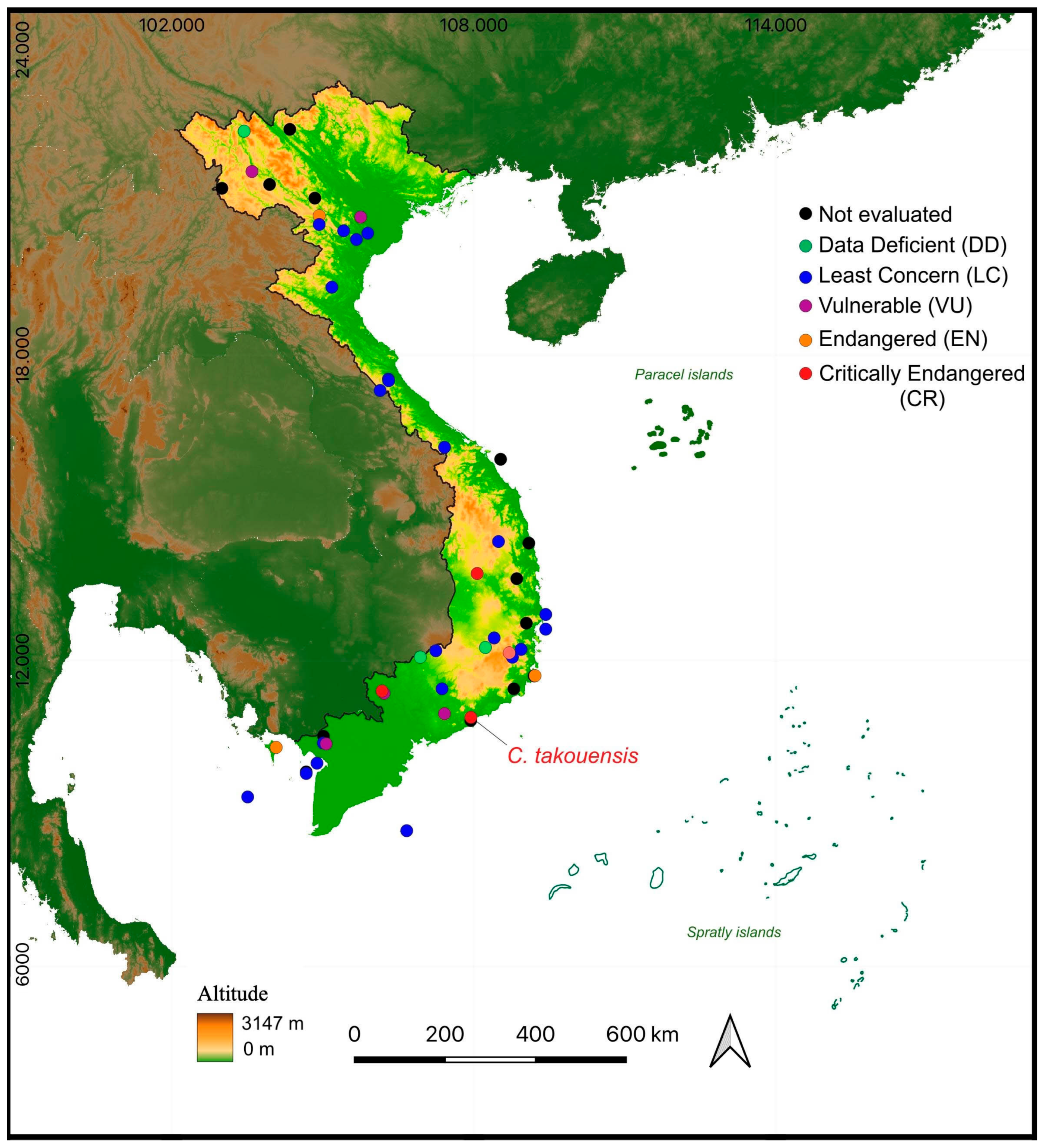
3. Results
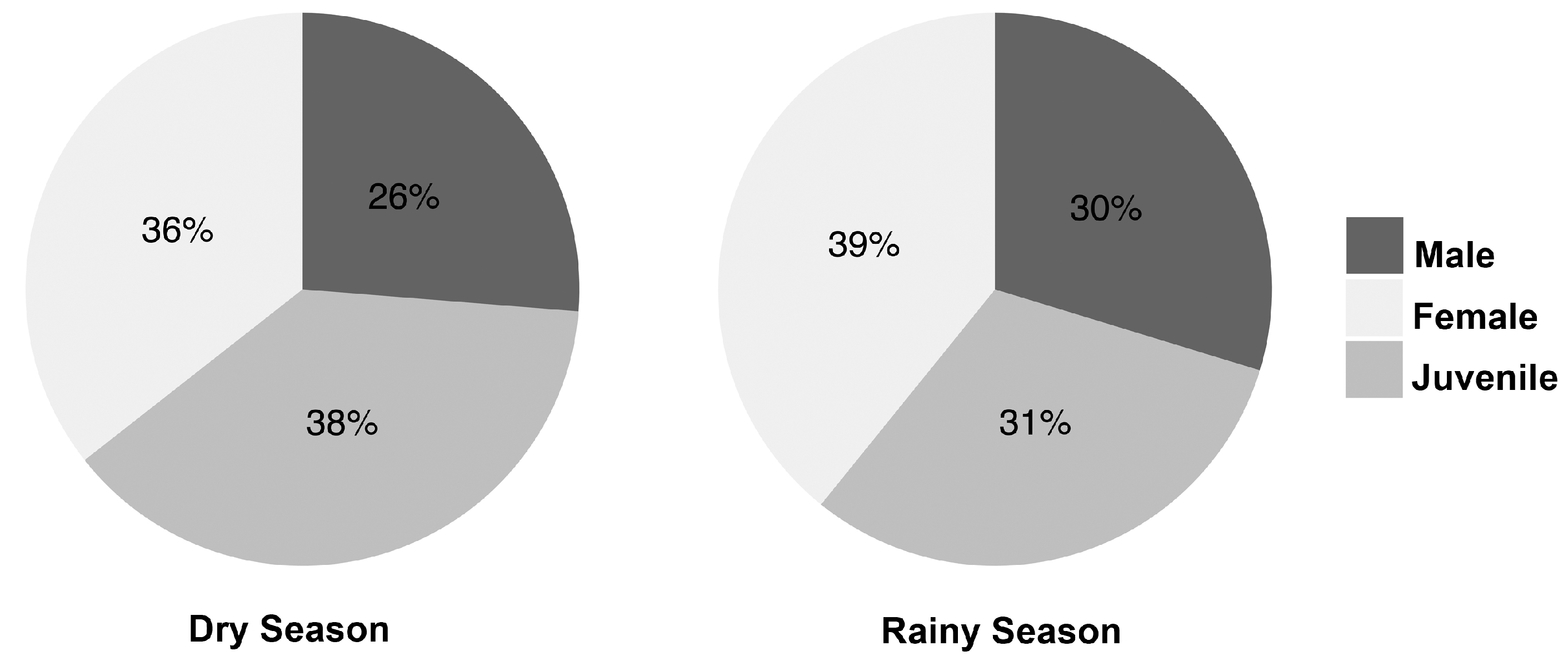
3.1. Population Status
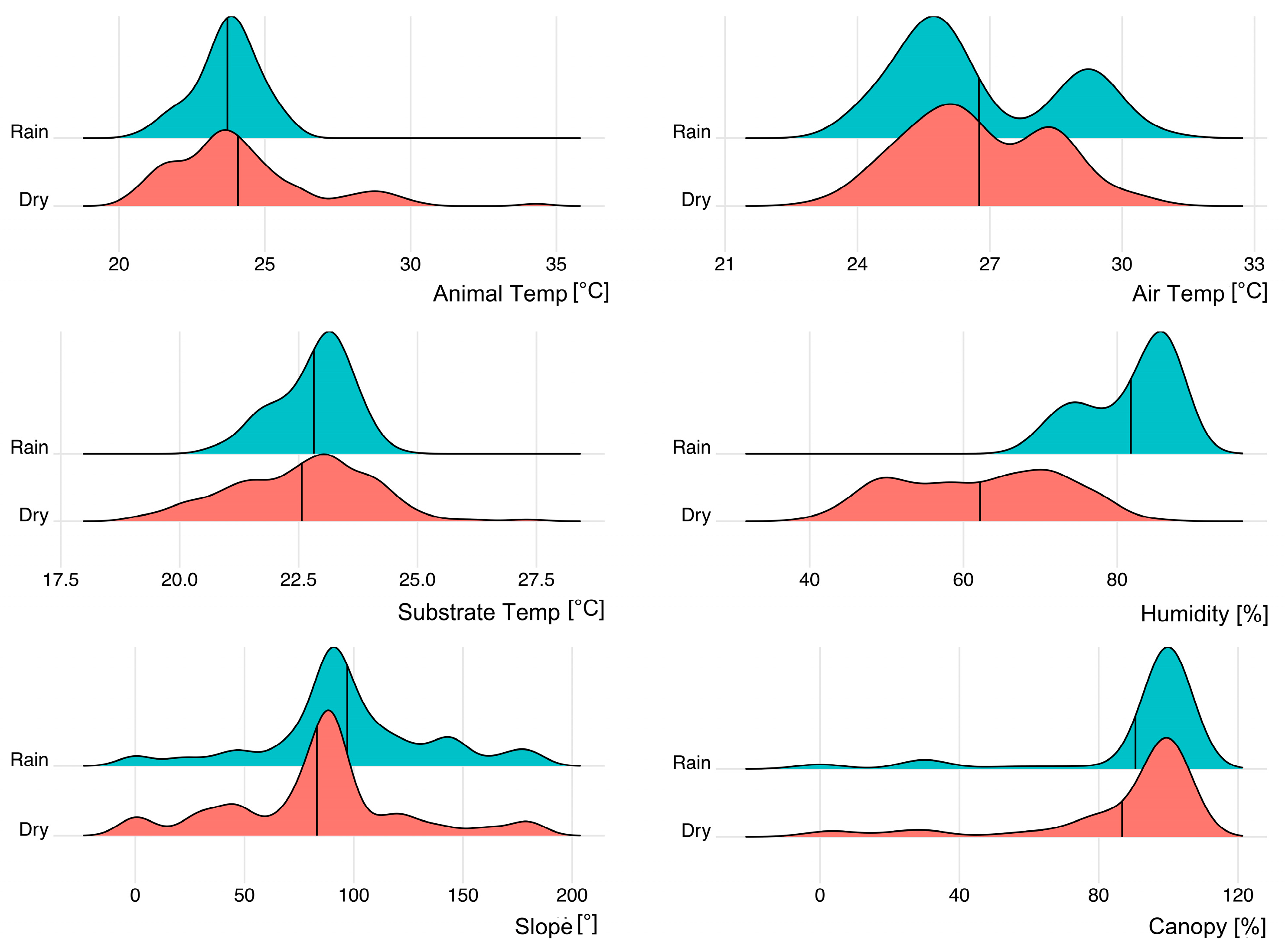
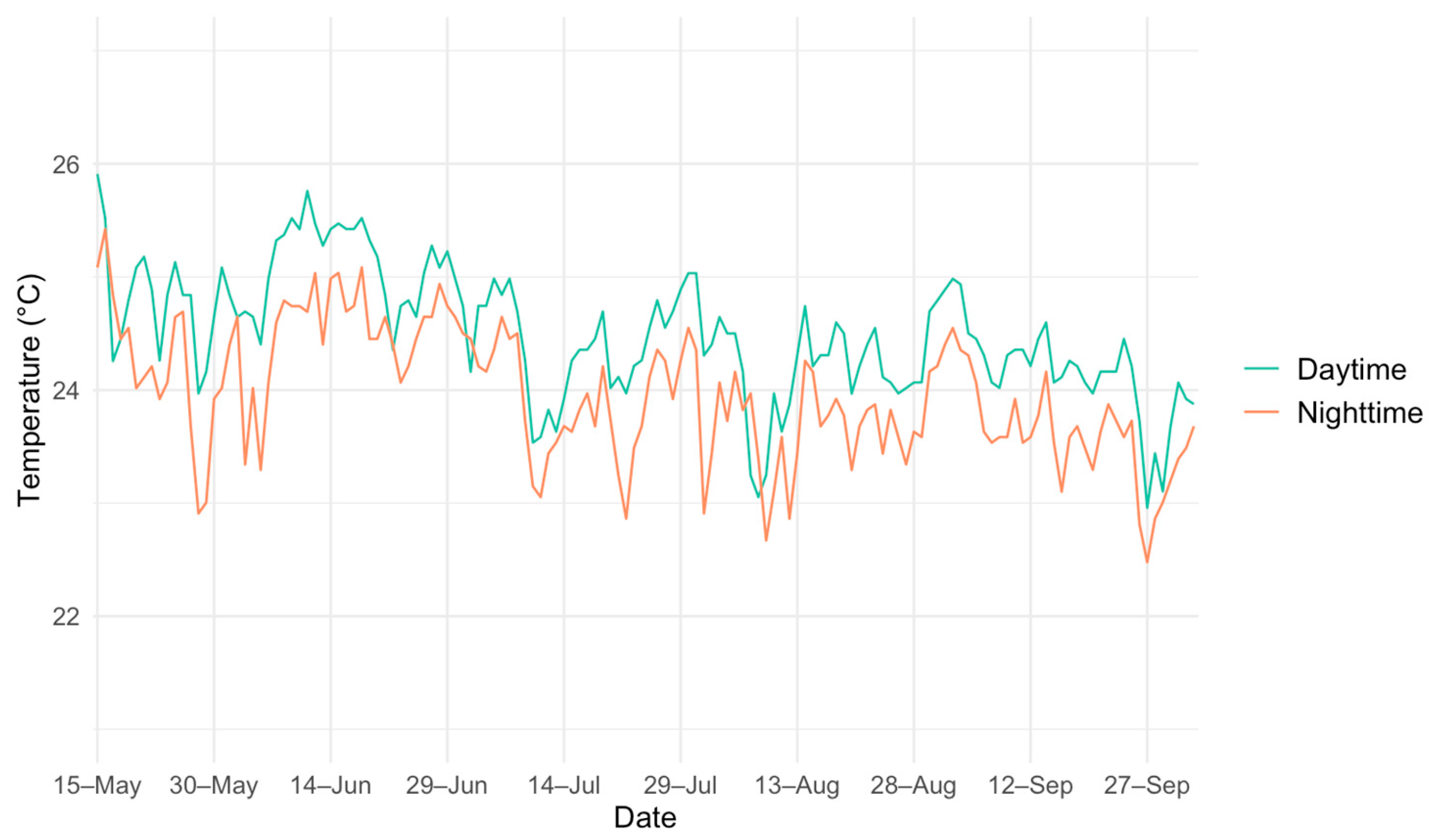
3.2. Microhabitat Selection and Behaviors
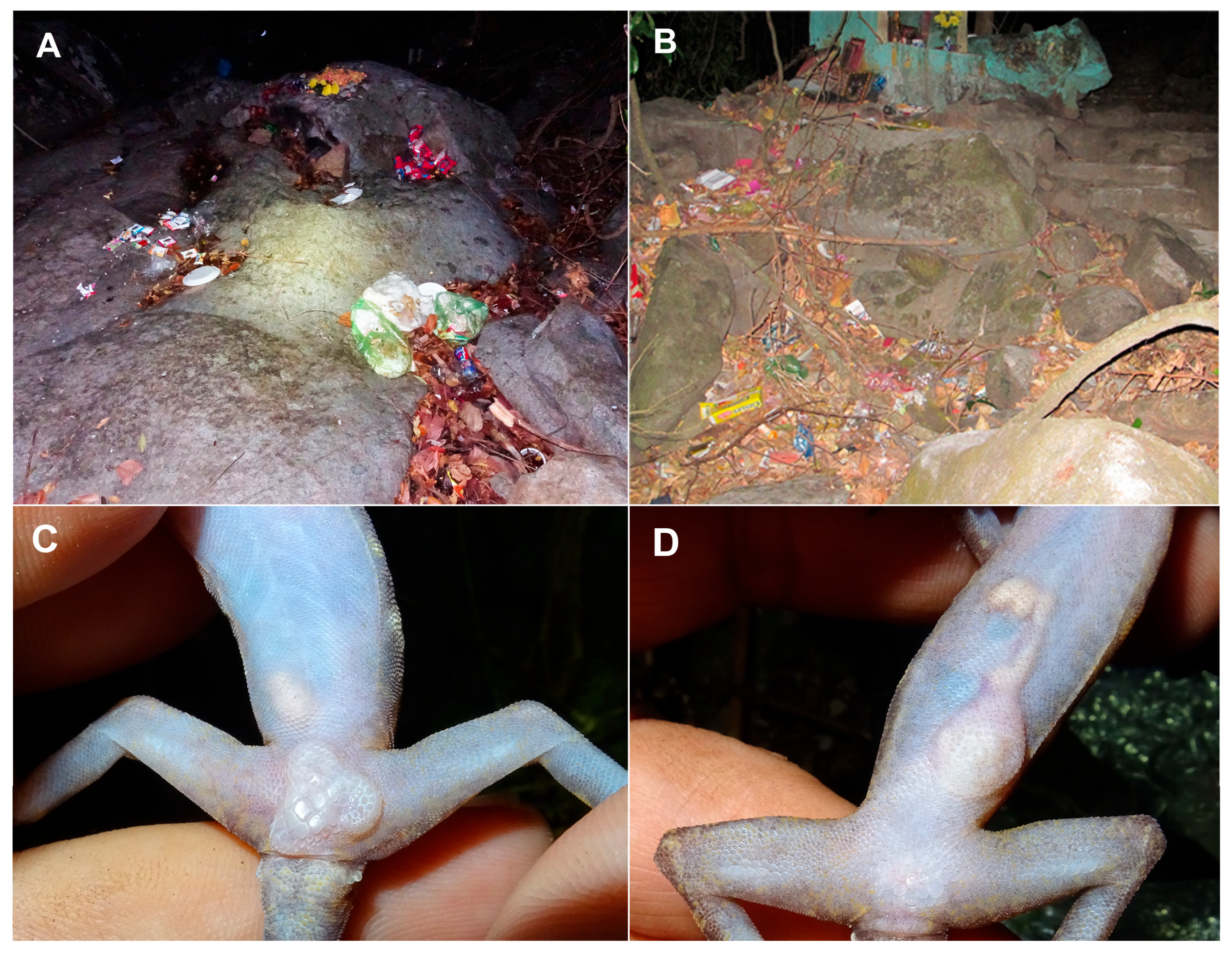
3.3. Main Threats
4. Discussion
4.1. Population Status
4.2. Microhabitat Selection and Behaviors
4.3. Main Threats
4.4. Conservation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Interview questions on the threat of the Ta Kou bent-toed gecko lizard Team member:................................................................................................................................. Date:............................................................................................................................................... Time:............................................................................................................................................... Interview No..................................................................................................................................... Commune name:................................................................................................................................
| |
| If yes, What do you do in the forest? | If no, |
| |
| If yes, - How will I recognize the species? - Are there any local names for the Ta-Kou bent-toed gecko lizard? - What do you do if you see the Ta-kou bent-toed gecko lizard? - Do you know where I can find the Ta-kou bent-toed gecko lizard? - Do you know anyone else who are known about the Ta-kou bent-toed gecko lizard? (If yes, we ask some general information: name, where we can find him/her?) We show them the photo of the Ta-Kou bent-toed gecko lizards to confirm the species. | If no, show the photo of the Ta-Kou bent-toed gecko lizards. If they know this species, our conversation can continue. If they don’t know, the conversation stops. |
References
- Grismer, L.L.; Wood, P.L., Jr.; Poyarkov, N.A.; Le, M.D.; Kraus, F.; Agarwal, I.; Oliver, P.M.; Nguyen, S.N.; Nguyen, T.Q.; Karunarathna, S.; et al. Phylogenetic partitioning of the third-largest vertebrate genus in the world, Cyrtodactylus Gray, 1827 (Reptilia; Squa- mata; Gekkonidae) and its relevance to taxonomy and conservation. Vertebr. Zool. 2021, 71, 101–154. [Google Scholar] [CrossRef]
- Grismer, L.; Wood, P.L.; Poyarkov, N.A., Jr.; Le, M.D.; Karunarathna, S.; Chomdej, S.; Suwannapoom, C.; Qi, S.; Liu, S.; Che, J.; et al. Karstic landscapes are foci of species diversity in the world’s third-largest vertebrate genus Cyrtodactylus Gray, 1827 (Reptilia: Squamata: Gekkonidae). Diversity 2021, 13, e183. [Google Scholar] [CrossRef]
- Ngo, H.T.; Do, Q.H.; Pham, C.T.; Luu, V.Q.; Grismer, L.L.; Ziegler, T.; Nguyen, V.T.H.; Nguyen, T.Q.; Le, M.D. How many more species are out there? Current taxonomy substantially underestimates the diversity of bent-toed geckos (Gekkonidae, Cyrtodactylus) in Laos and Vietnam. Zookeys 2022, 1097, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P. The Reptile Database. Available online: https://reptile-database.reptarium.cz (accessed on 28 March 2025).
- IUCN (International Union for Conservation of Nature). Available online: https://www.iucnredlist.org (accessed on 28 February 2025).
- Luu, V.Q.; Van, O.L.; Hoang, T.T.; Van, T.P.; Duc, O.L.; Bordes, C.; Leprince, B.; Amori, G.; Luiselli, L. Ecological characteristics of a recently described, critically endangered gecko species, endemic to Central Highland, Vietnam. Trop. Zool. 2020, 33, 53–62. [Google Scholar] [CrossRef]
- Grismer, L.; Quah, E. Cyrtodactylus jelawangensis. The IUCN Red List of Threatened Species 2018: E.T101742396A101742480. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T101742396A101742480.en (accessed on 24 June 2025).
- Tran, T.T.; Do, Q.H.; Pham, C.T.; Phan, T.Q.; Ngo, H.T.; Le, M.D.; Ziegler, T.; Nguyen, T.Q. A new species of the Cyrtodactylus chauquangensis species group (Squamata, Gekkonidae) from Lao Cai Province, Vietnam. Zookeys 2024, 1192, 83–102. [Google Scholar] [CrossRef]
- Duong, T.V.; Vu, L.V.; Vu, H.T.T.; Mulcahy, D.; Bragin, A.M.; Poyarkov, N.A., Jr.; Grismer, L.L. Another new species of Cyrtodactylus Gray, 1927 (Squamata: Gekkonidae) of the angularis group from the karstic landscape of Phong Nha—Ke Bang National Park, central Vietnam. Zootaxa 2024, 5471, 555–571. [Google Scholar] [CrossRef]
- Nguyen, N.S.; Nguyen, T.Q.; Golynsky, E.; Milto, K. Cyrtodactylus nigriocularis. The IUCN Red List of Threatened Species 2018: E.T104695227A104718656. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T104695227A104718656.en (accessed on 29 March 2025).
- Nguyen, T.Q.; Nguyen, N.S.; Golynsky, E.; Milto, K. Cyrtodactylus phuquocensis. The IUCN Red List of Threatened Species 2018: E.T104698481A104718716. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T104698481A104718716.en (accessed on 24 June 2025).
- Nguyen, T.Q.; Nguyen, N.S. Cyrtodactylus otai. The IUCN Red List of Threatened Species 2018: E.T104696055A104718671. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T104696055A104718671.en (accessed on 24 June 2025).
- Nguyen, N.S.; Golynsky, E.; Milto, K. Cyrtodactylus caovansungi. The IUCN Red List of Threatened Species 2018: E.T104686599A104718436. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T104686599A104718436.en (accessed on 24 June 2025).
- Ngo, T.V.; Bauer, A.M. Descriptions of two new species of Cyrtodactylus Gray 1827 (Squamata: Gekkonidae) endemic to southern Vietnam. Zootaxa 2008, 1715, 27–42. [Google Scholar] [CrossRef]
- Nguyen, N.S.; Golynsky, E.; Milto, K. Cyrtodactylus takouensis. The IUCN Red List of Threatened Species 2018: E.T104699508A104718806. 2018. Available online: https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T104699508A104718806.en (accessed on 25 July 2025).
- Nguyen, T.Q.; Phung, T.M.; Schneider, N.; Botov, A.; Dao, T.A.T.; Ziegler, T. New records of amphibians and reptiles from southern Vietnam. Bonn Zool. Bull. 2014, 63, 148–156. [Google Scholar]
- Hoang, T.N.; Tran, T.T.; Le, Q.T.; Le, H.L.T.; Hoang, Q.X. New record of Cyrtodactylus cattienensis Geissler, Nazarov, Orlov, Bohme, Phung, Nguyen & Ziegler, 2009 (Squamata: Sauria: Gekkonidae) in Nui Ong Nature Reserve, Binh Thuan Province, Vietnam. Manag. For. Resour. Environ. 2022, 14, 40–45. [Google Scholar]
- Le, M.V.; Phan, K.D.; Phan, H.T.; Nguyen, S.N. The initial data on the herpetofauna of Thi Vai Mountain, Ba Ria—Vung Tau Province. J. For. Sci. Technol. 2022, 5, 101–108. (In Vietnamese) [Google Scholar]
- Le, T.D.T.; Tran, T.G. Species composition of reptiles in Da Huoai district, Di Linh Plateau, Lam Dong Province. Manag. For. Resour. Environ. 2022, 14, 14–20. [Google Scholar]
- van Schingen, M.; Pham, C.T.; An, H.T.; Bernardes, M.; Hecht, V.; Nguyen, T.Q.; Bonkowski, M.; Ziegler, T. Current status of the Crocodile Lizard Shinisaurus crocodilurus Ahl, 1930 in Vietnam with implications for conservation measures. Rev. Suisse Zoolgogie 2014, 121, 425–439. [Google Scholar]
- van Schingen, M.; Ha, Q.Q.; Pham, C.T.; Le, T.Q.; Nguyen, T.Q.; Bonkowski, M.; Ziegler, T. Discovery of a new crocodile lizard population in Vietnam: Population trends, future prognoses and identification of key habitats for conservation. Rev. Suisse Zoolgogie 2016, 123, 241–251. [Google Scholar]
- Ngo, H.N.; Nguyen, T.Q.; Nguyen, T.V.; Barsch, F.; Ziegler, T.; van Schingen, M. First population assessment of the endemic insular Psychedelic Rock Gecko (Cnemaspis psychedelica) in southern Vietnam with implications for conservation. Amphib. Reptile Conserv. 2016, 10, 18–26. [Google Scholar]
- Ngo, H.N.; Ziegler, T.; Nguyen, T.Q.; Pham, C.T.; Nguyen, T.T.; Le, M.D.; Van Schingen, M. First population assessment of two cryptic Tiger Geckos (Goniurosaurus) from northern Vietnam: Implications for conservation. Amphib. Reptile Conserv. 2016, 10, 34–45. [Google Scholar]
- Nguyen, T.Q.; Ngo, H.N.; Van, H.N.; Ngo, C.D.; van Schingen, M.; Ziegler, T. First population assessment of the Asian water dragon (Physignathus cocincinus Cuvier, 1829) in Thua thien Hue province, Vietnam. Nat. Conserv. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Park, I.-K.; Kim, D.-I.; Fong, J.J.; Park, D. Home Range size and overlap of the small nocturnal schlegel’s Japanese Gecko (Gekko japonicus), introduced into a City Park in Korea. Asian Herpetol. Res. 2019, 10, 261–269. [Google Scholar]
- Schnabel, Z.E. The estimation of the total fish population of a Lake. Am. Math. Mon. 1938, 45, 348–352. [Google Scholar] [CrossRef]
- Ngo, H.N.; Nguyen, T.Q.; Phan, T.Q.; van Schingen, M.; Ziegler, T. A case study on trade in threatened Tiger Geckos (Goniurosaurus) in Vietnam including updated information on the abundance of the Endangered G. catbaensis. Nat. Conserv. 2019, 33, 1–19. [Google Scholar] [CrossRef]
- Crow, E.L.; Gardner, R.S. Confidence intervals for the expectation of a Poissonvariable. Biometrika 1959, 46, 441–453. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 4th ed.; Addison-Wesley Educational Publishers, Inc.: Boston, MA, USA, 2015; Available online: https://www.zoology.ubc.ca/~krebs/books.html (accessed on 21 December 2022).
- Fitch, H.S. Sexual size differences in the genus Sceloporus. Univ. Kans. Sci. Bull. 1978, 51, 441–461. [Google Scholar] [CrossRef]
- McDiarmid, R.W.; Foster, M.S.; Guyer, C.; Gibbons, J.W.; Chernoff, N. Reptile Biodiversity: Standard Methods for Inventory and Monitoring, 1st ed.; University of California Press: Oakland, CA, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing v4.4.2; R Foundation for Statistical Computing; R Foundation: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 1 November 2024).
- Reed, D.H.; O’Grady, J.J.; Brook, B.W.; Ballou, J.D.; Frankham, R. Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biol. Conserv. 2003, 113, 23–34. [Google Scholar] [CrossRef]
- Traill, L.W.; Bradshaw, C.J.A.; Brook, B.W. Minimum viable population size: A meta-analysis of 30 years of published estimates. Biol. Conserv. 2007, 139, 159–166. [Google Scholar] [CrossRef]
- Khandekar, A.; Gaitonde, N.; Agarwal, I. Two new Cnemaspis Strauch, 1887 (Squamata: Gekkonidae) from the Shevaroy massif, Tamil Nadu, India, with a preliminary ND2 phylogeny of Indian Cnemaspis. Zootaxa 2019, 4609, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Bentz, E.J.; Rodriguez, M.J.R.; John, R.; Henderson, R.W.; Powell, R. Population densities, activity, microhabitats, and hermal biology of a unique crevice- and litter-dwelling assemblage of reptiles on Union Island, St. Vincent and the Grenadines. Herpetol. Conserv. Biol. 2011, 6, 40–50. [Google Scholar]
- Shepherd, C.; Janssen, J.; Noseworthy, J. A case for listing the Union Island Gecko Gonatodes daudini in the Appendices of CITES. Glob. Ecol. Conserv. 2019, 17, e00549. [Google Scholar] [CrossRef]
- Flecks, M.; Weinsheimer, F.; Böhme, W.; Chenga, J.; Lötters, S.; Rödder, D. Watching extinction happen: The dramatic population decline of the critically endangered Tanzanian Turquoise Dwarf Gecko Lygodactylus williamsi. Salamandra 2012, 48, 12–20. [Google Scholar]
- Vicente, L.A.; Santos, V.; Ribeiro, T.; Guimaraes, M.; Verrastro, L. Are Lizards sensitive to anomalous seasonal temperatures? Long-term thermobiological variability in subtropical species. PLoS ONE 2019, 14, e0226399. [Google Scholar] [CrossRef]
- Ngo, H.N.; Le, T.Q.; Pham, M.L.; Nguyen, T.Q.; Le, M.D.; van Schingen, M.; Ziegler, T. First record of the Cat Ba Tiger Gecko, Goniurosaurus catbaensis, from Ha Long Bay, Quang Ninh Province, Vietnam: Microhabitat selection, potential distribution, and evidence of threats. Amphib. Reptile Conserv. 2019, 13, 1–13. [Google Scholar]
- Shannon, G.; Larson, C.L.; Reed, S.E.; Crooks, K.R.; Angeloni, L.M. Ecological Consequences of Ecotourism for Wildlife Populations and Communities. In Ecotourism’s Promise and Peril; Blumstein, D., Geffroy, B., Samia, D., Bessa, E., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Dang, P.H.; Nguyen, D.D.; Nguyen, H.M.; Bui, H.T.; Hoang, H.Q.; Nguyen, S.T.; Vu, T.D.; Ly, T.N.; Le, H.M.; et al. Vietnam Red Data Book; Vol. 1 Animals; Natural Science and Technology Publishing House: Ha Noi, Vietnam, 2024; Volume 1, p. 1248.
- Red List Technical Working Group. Mapping Standards and Data quality for the IUCN Red List Categories and Criteria. Available online: https://nc.iucnredlist.org/redlist/resources/files/1539098236-Mapping_Standards_Version_1.16_2018.pdf (accessed on 12 May 2025).
- IUCN. IUCN Red List Categories and Criteria: Version 3.1. IUCN Species Survival Commission; IUCN: Gland, Switzerland; Cambridge, UK, 2001; p. 30. Available online: https://portals.iucn.org/library/sites/library/files/documents/RL-2001-001.pdf (accessed on 12 May 2025).


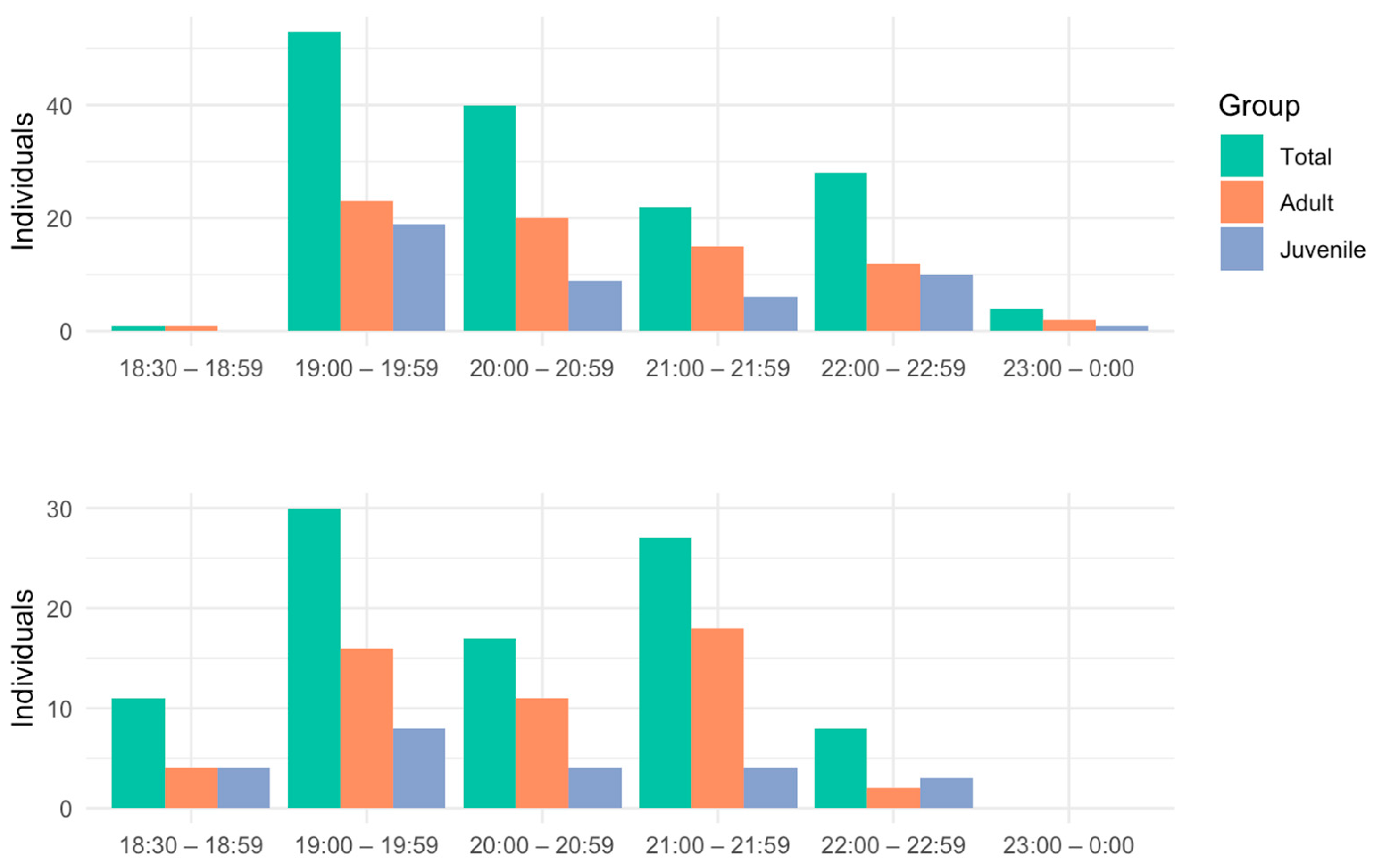
| Transects | Transect 1 (T1) | Transect 2 (T2) | Transect 3 (T3) | Total |
|---|---|---|---|---|
| Dry Season—April 2022 | ||||
| Total Adults | 14 | 21 | 38 | 73 |
| Total Obs | 19 | 42 | 87 | 148 (NA = 30) |
| Area (km2) | 2.36 | 2.97 | 6.09 | 11.42 |
| DA (Adults/km2) | 5.93 | 7.07 | 6.24 | 6.39 |
| DAD (Adults/km2/day) | 1.48 | 1.77 | 1.56 | 1.60 |
| D (ind./km2) | 8.05 | 14.14 | 14.29 | 12.96 |
| DD (ind./km2/day) | 2.01 | 3.54 | 3.57 | 3.24 |
| N–Total Schnabel | 27 | 109 | 179 | 315 |
| Variance | 1.48 × 10−6 | 1.19 × 10−5 | 0.0156 | 0.015638 |
| Standard error | 0.00122 | 0.00345 | 0.125 | 0.1297 |
| Lower 95% confidence limits | 119 | 56 | 14 | 189 |
| Upper 95% confidence limits | 294 | 233 | 54 | 581 |
| Rainy season—September 2022 | ||||
| Total Adults | 8 | 7 | 36 | 51 |
| Total Obs | 11 | 12 | 72 | 95 (NA = 19) |
| Area (km2) | 1.23 | 0.52 | 6.09 | 7.84 |
| DA (Adults/km2) | 6.50 | 13.46 | 5.91 | 6.51 |
| DAD (Adults/km2/day) | 2.17 | 4.49 | 1.97 | 2.17 |
| D (ind./km2) | 8.94 | 23.08 | 11.82 | 12.12 |
| DD (ind./km2/day) | 2.98 | 7.69 | 2.96 | 3.03 |
| N–Total Schnabel | 14 | 24 * | 142 | 180 |
| Variance | 0.00108 | - | 2.93 × 10−6 | - |
| Standard error | 0.00171 | - | 0.0329 | - |
| Lower 95% confidence limits | 6 | 10 | 92 | 108 |
| Upper 95% confidence limits | 35 | 75 | 251 | 361 |
| Human impacts | Severely disturbed | Intact | Disturbed | |
| Parameters | Dry Season | Rainy Season | Wilcoxon Test |
|---|---|---|---|
| Canopy cover [%] | 0–100 (86.71 ± 2.3) (n = 123) | 0–100 (90.54 ± 2.57) (n = 93) | W = 4757.5, r = 0.18 < 0.5, CI lower = 0.05, CI upper = 0.32, p-value = 0.007 < 0.05 -> no significant seasonal differences |
| Elevation [m] | 267–700 (434.25 ± 14.15) (n = 146) | 265–678 (358.14 ± 0.05) (n = 93) | |
| Animal Temp. [°C] | 20.3–34.2 (24.08 ± 0.22) (n = 118) | 20.8–25.8 (23.72 ± 0.12) (n = 76) | W = 4450, r = 0.006 < 0.5, CI lower = 0.002, CI upper = 0.16, p-value = 0.93 > 0.05 -> no significant seasonal differences |
| Substrate Temp. [°C] | 19.1–27.3 (22.57 ± 0.12) (n = 144) | 20.8–24.4 (22.82 ± 0.08) (n = 93) | W = 6034.5, r = 0.08 < 0.5, CI lower = 0.01, CI upper = 0.20, p-value = 0.20 > 0.05 -> no significant seasonal differences |
| Air Temp. [°C] | 23.3–30.5 (26.76 ± 0.13) (n = 145) | 23.8–30.9 (26.75 ± 0.19) (n = 93) | W = 6958, r = 0.03 < 0.5, CI lower = 0.002, CI upper = 0.16, p-value = 0.68 > 0.05 -> no significant seasonal differences |
| Air Humidity [%] | 40–85 (62.16 ± 0.87) (n = 145) | 69–88 (81.77 ± 0.58) (n = 93) | W = 644, r = 0.76 > 0.5, CI lower = 0.72, CI upper = 0.80, p-value < 2.2 × 10−16 < 0.05 -> significant seasonal differences |
| Slope [°] | 0–180 (83.12 ± 3.51) (n = 136) | 0–180 (97.03 ± 4.04) (n = 91) | W = 4682.5, r = 0.21 < 0.5, CI lower = 0.09, CI upper = 0.34, p-value = 0.002 < 0.05 -> no significant seasonal differences |
| Species | Locality | Density Estimation | Method Notes | References |
|---|---|---|---|---|
| Cnemaspis thackerayi | Salam District, India | 1,000,000–2,000,000 individual/km2 (10–15 individuals/7.5 m2) | Herpetofauna surveys | [35] |
| Gonatodes daudini | Grenadines and Saint Vincent | 8700–21,900 individuals/km2 (87–218 individuals/ha) | These figures resulted from surveys targeting four cryptic reptile species. The process involved sifting through litter and lifting various types of cover, such as rocks, logs, and deadfall, by two researchers along the transects, while a third person monitored the edges to observe any escaping animals | [36,37] |
| Lygodactylus williamsi | Tanzania | 35,300 individuals/km2 (353 individuals/ha) | Observations by three researchers in the mornings and afternoons | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, H.T.; Do, Q.H.; Ngo, H.N.; Nguyen, H.Q.; Pham, A.V.; Pham, C.T.; Nguyen, L.T.; Trinh, H.L.T.; Nguyen, T.Q.; Ziegler, T.; et al. Population Status and Ecological Features of the Endemic and Critically Endangered Ta Kou Bent-Toed Gecko (Cyrtodactylus takouensis) in Vietnam. Conservation 2025, 5, 52. https://doi.org/10.3390/conservation5030052
Ngo HT, Do QH, Ngo HN, Nguyen HQ, Pham AV, Pham CT, Nguyen LT, Trinh HLT, Nguyen TQ, Ziegler T, et al. Population Status and Ecological Features of the Endemic and Critically Endangered Ta Kou Bent-Toed Gecko (Cyrtodactylus takouensis) in Vietnam. Conservation. 2025; 5(3):52. https://doi.org/10.3390/conservation5030052
Chicago/Turabian StyleNgo, Hanh Thi, Quyen Hanh Do, Hai Ngoc Ngo, Huy Quoc Nguyen, Anh Van Pham, Cuong The Pham, Luan Thanh Nguyen, Ha Le Thi Trinh, Truong Quang Nguyen, Thomas Ziegler, and et al. 2025. "Population Status and Ecological Features of the Endemic and Critically Endangered Ta Kou Bent-Toed Gecko (Cyrtodactylus takouensis) in Vietnam" Conservation 5, no. 3: 52. https://doi.org/10.3390/conservation5030052
APA StyleNgo, H. T., Do, Q. H., Ngo, H. N., Nguyen, H. Q., Pham, A. V., Pham, C. T., Nguyen, L. T., Trinh, H. L. T., Nguyen, T. Q., Ziegler, T., & Le, M. D. (2025). Population Status and Ecological Features of the Endemic and Critically Endangered Ta Kou Bent-Toed Gecko (Cyrtodactylus takouensis) in Vietnam. Conservation, 5(3), 52. https://doi.org/10.3390/conservation5030052






