Genetic Variation and the Relationships Among Growth, Morphological, and Physiological Traits in Pterocarpus macrocarpus: Implications for Early Selection and Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.1.1. Seed Sources and Genetic Background
2.1.2. Seed Preparation and Germination Protocol
2.1.3. Nursery Management and Transplanting
2.1.4. Fertilisation and Environmental Controls
2.2. Trait Assessments
2.2.1. Growth and Morphology
2.2.2. Physiological Traits
2.3. Statistical and Genetic Analysis
2.3.1. Growth and Morphology
- µ—grand mean;
- Ri—ith replication effect, i = 1–16;
- Pj—jth population effect, j = 1–6;
- RPij—effect of replication-by-population interaction;
- Fk(Pj)—kth family effect within the jth population, k = 1, 2, …, n (n ranges from 13 to 27);
- εijk—residual error.
2.3.2. Physiological Traits
- µ—grand mean;
- Ri—ith replication effect, i = 1–5;
- Pj—jth population effect, j = 1–6;
- RPij—effect of replication-by-population interaction;
- Fk(Pj)—kth family effect within the jth population, k = 1, 2, …, n (n ranges from 13 to 27);
- Ll(FPjk)—lth leaf effect within the kth family within the jth population, l = 1–2;
- εijkl—residual error.
3. Results
3.1. Seedling Survival
3.2. Growth and Morphology
3.3. Physiological Traits
4. Discussion
4.1. Nursery Performance and Early Growth Dynamics
4.2. Growth and Morphological Traits
4.3. Physiological Traits
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitken, S.N.; Whitlock, M.C. Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 367–388. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Lv, Y.W.; He, Z.H.; Yeh, F.C.; Hu, X.S. A community-based framework integrates interspecific interactions into forest genetic conservation. Plants 2024, 13, 435. [Google Scholar] [CrossRef]
- Morgenstern, E.K. Geographic Variation in Forest Trees: Genetic Basis and Application of Knowledge in Silviculture; UBC Press: Vancouver, BC, Canada, 2011. [Google Scholar]
- He, Z.H.; Xiao, Y.; Lv, Y.W.; Yeh, F.C.; Wang, X.; Hu, X.S. Prediction of genetic gains from selection in tree breeding. Forests 2023, 14, 520. [Google Scholar] [CrossRef]
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Marcos, J.G.; Fluch, S.; Fraga, M.F.; Guevara, M.Á.; Abarca, D.; et al. Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol. Evol. 2013, 3, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, P.P.; Dean, C.A. Changes in the genetic control of growth of radiata pine to 16 years and efficiencies of early selection. Silvae Genet. 1988, 37, 138–146. [Google Scholar]
- Kramer, P.J. The role of physiology in forestry. Tree Physiol. 1986, 2, 297–308. [Google Scholar] [CrossRef]
- Bussotti, F.; Pallastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Major, J.E.; Johnsen, K.H. Family variation in photosynthesis of 22-year-old black spruce: A test of two models of physiological response to water stress. Can. J. For. Res. 1996, 26, 1922–1933. [Google Scholar] [CrossRef]
- Guerra, F.P.; Richards, J.H.; Fiehn, O.; Famula, R.; Stanton, B.J.; Shuren, R.; Sykes, R.; Davis, M.F.; Neale, D.B. Analysis of the genetic variation in growth, ecophysiology, and chemical metabolomic composition of wood of Populus trichocarpa provenances. Tree Genet. Genomes 2016, 12, 6. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Kučerová, J.; Konôpková, A.; Pšidová, E.; Kurjak, D.; Jamnická, G.; Slugenová, K.; Gömöry, K.; Ditmarová, L. Adaptive variation in physiological traits of beech provenances in Central Europe. iForest 2018, 11, 24–31. [Google Scholar] [CrossRef]
- Müller, C.; Hodecker, B.E.R.; De Barros, N.F.; Merchant, A. A physiological approach for pre-selection of Eucalyptus clones resistant to drought. iForest 2020, 13, 16–23. [Google Scholar] [CrossRef]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.M.; Barbaroux, C.; Le Thiec, D.; Bréchet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
- Elliott, S.; Kuaraksa, C. Producing Framework Tree Species for Restoring Forest Ecosystems in Northern Thailand. Small-Scale For. 2008, 7, 403–415. [Google Scholar] [CrossRef]
- Wangpakapattanawong, P.; Tiansawat, P.; Sharp, A. Forest restoration at the landscape level in Thailand. For. Landsc. Restor. Asia-Pac. For. 2016, 149. [Google Scholar]
- Liengsiri, C.; Yeh, F.C.; Boyle, T.J.B. Genetic structure of a tropical tree, Pterocarpus macrocarpus Kurz. from Thailand. For. Ecol. Manag. 1995, 74, 13–22. [Google Scholar] [CrossRef]
- White, T.L.; Wesley, T.A.; Neale, D.B. Forest Genetics; Cabi: Wallingford, UK, 2007. [Google Scholar]
- Greenwood, M.S.; Volkaert, H.A. Morphophysiological traits as markers for the early selection of conifer genetic families. Can. J. For. Res. 1992, 22, 1001–1008. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Xu, H.; Creed, I.F.; Blanco, J.A.; Wei, X.; Sun, G.; Asbjornsen, H.; Bishop, K. Forest water-use efficiency: Effects of climate change and management on the coupling of carbon and water processes. For. Ecol. Manag. 2023, 534, 120853. [Google Scholar] [CrossRef]
- Lambeth, C.C. Juvenile-mature correlations in Pinaceae and implications for early selection. For. Sci. 1980, 26, 571–580. [Google Scholar]
- Lu, P.X.; Yeh, F.C. Seedling growth response to nutrient and water treatments among Jack Pine open-pollinated families. Forests 2024, 15, 2062. [Google Scholar] [CrossRef]
- Liengsiri, C.; Boyle, T.J.B.; Yeh, F.C. Mating System in Pterocarpus macrocarpus Kurz in Thailand. J. Hered. 1998, 89, 216–221. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Tannor, C.B.; Bennett, J.M. Water-use efficiency in crop production. Bioscience 1984, 34, 36–40. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS; Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Satterthwaite, F.E. An approximate distribution of estimates of variance components. Biom. Bull. 1946, 2, 110–114. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Addison Wesley Longman: Harlow, UK, 1996. [Google Scholar]
- Nyquist, W.E. Estimation of heritability and prediction of selection response in plant populations. Crit. Rev. Plant Sci. 1991, 10, 235–322. [Google Scholar] [CrossRef]
- Robertson, A. The sampling variance of the genetic correlation coefficient. Biometrics 1959, 15, 469–485. [Google Scholar] [CrossRef]
- Bongarten, B.C.; Hanover, J.W. Accelerating seedling growth through photoperiod extension for genetic testing: A case study with blue spruce (Picea pungens). For. Sci. 1985, 31, 631–643. [Google Scholar]
- Diao, S.; Hou, Y.; Xie, Y.; Sun, X. Age trends of genetic parameters, early selection and family by site interactions for growth traits in Larix kaempferi open-pollinated families. BMC Genet. 2016, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Struve, D.K.; Joly, R.J. Transplanted red oak seedlings mediate transplant shock by reducing leaf surface area and altering carbon allocation. Can. J. For. Res. 1992, 22, 10. [Google Scholar] [CrossRef]
- Mainhart, D.E.; Christoffersen, B.O.; Thompson, R.A.; Reemts, C.M.; Fierro-Cabo, A. Preparing for the Worst: Enhancing Seedling Traits to Reduce Transplant Shock in Semi-Arid Regions. Forests 2024, 15, 1607. [Google Scholar] [CrossRef]
- Boltz, B.A.; Bongarten, B.C.; Teskey, R.O. Seasonal patterns of net photosynthesis of loblolly pine from diverse origins. Can. J. For. Res. 1986, 16, 1063–1068. [Google Scholar] [CrossRef]
- Van Pelt, R.; Sillett, S.C. Long-term diameter patterns and basal area growth of old-growth Douglas-fir trees in western Oregon. Can. J. For. Res. 2002, 32, 1232–1243. [Google Scholar]
- Xie, C.Y.; Johnstone, W.J.; Ying, C.C. Spacing and provenance effects on the performance of shore pine (Pinus contorta var. contorta): 20-year test results. Can. J. For. Res. 1995, 25, 567–576. [Google Scholar] [CrossRef]
- Hébert, F.; Krause, C.; Plourde, P.-Y.; Achim, A.; Prégent, G.; Ménétrier, J. Effect of Tree Spacing on Tree Level Volume Growth, Morphology, and Wood Properties in a 25-Year-Old Pinus banksiana Plantation in the Boreal Forest of Quebec. Forests 2016, 7, 276. [Google Scholar] [CrossRef]
- Keel, S.G.; Campbell, C.D.; Högberg, M.N.; Richter, A.; Wild, B.; Zhou, X.; Hurry, V.; Linder, S.; Näsholm, T.; Högberg, P. Allocation of carbon to fine root compounds and their residence times in a boreal forest depend on root size class and season. New Phytol. 2012, 194, 972–981. [Google Scholar] [CrossRef]
- Nikolova, P.S.; Bauerle, T.L.; Häberle, K.-H.; Blaschke, H.; Brunner, I.; Matyssek, R. Fine-root traits reveal contrasting ecological strategies in European beech and Norway spruce during extreme drought. Front. Plant Sci. 2020, 11, 1211. [Google Scholar] [CrossRef]
- Liengsiri, C. Effects of Temperature on Seed Germination in Pterocarpus macrocarpus. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 1987. [Google Scholar]
- Wu, H.X.; Yeh, F.C.; Pharis, R.P.; Dancik, B.P.; Jiang, I.B.; Dymock, I.; Dhir, N.K. Genetic parameters of greenhouse growth and performance of 2-year Pinus contorta subsp. latifolia. Scand. J. For. Res. 1995, 10, 12–21. [Google Scholar] [CrossRef]
- Popa, I.; Sidor, C.G.; Enescu, C.M. Genetic variation and early selection in Larix decidua Mill. from progeny test in Romania. Ann. For. Sci. 2019, 76, 81. [Google Scholar] [CrossRef]
- Parchman, T.L.; Benkman, C.W.; Jenkins, B.; Buerkle, C.A. Low levels of population genetic structure in Pinus contorta (Pinaceae) across a geographic mosaic of co-evolution†. Am. J. Bot. 2011, 98, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Y.; Kang, M.; Yi, G.; Huang, H. Spatial and temporal population genetic variation and structure of Nothotsuga longibracteata (Pinaceae), a relic conifer species endemic to subtropical China. Genet. Mol. Biol. 2013, 36, 598–607. [Google Scholar] [CrossRef]
- Breed, M.F.; Ottewell, K.M.; Gardner, M.G.; Marklund, M.H.K.; Dormontt, E.E.; Lowe, A.J. Mating patterns and pollinator mobility are critical traits in forest fragmentation genetics. Heredity 2015, 115, 108–114. [Google Scholar] [CrossRef]
- Wessinger, C.A. From pollen dispersal to plant diversification: Genetic consequences of pollination mode. New Phytol. 2020, 229, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Adams, W.T. Range-wide patterns of allozyme variation in Douglas-fir (Pseudotsuga menziesii). Can. J. For. Res. 1989, 19, 149–161. [Google Scholar] [CrossRef]
- Pretzsch, H. Genetic diversity reduces competition and increases tree growth on a Norway spruce (Picea abies [L.] Karst.) provenance mixing experiment. For. Ecol. Manag. 2021, 497, 119498. [Google Scholar] [CrossRef]
- Foster, G.S. Trends in genetic parameters with stand development and their influence on early selection for volume growth in loblolly pine. For. Sci. 1986, 32, 944–959. [Google Scholar] [CrossRef]
- St. Clair, J.B.; Adams, W.T. Relative family performance and variance structure of open-pollinated Douglas-fir seedlings grown in three competitive environments. Theor. Appl. Genet. 1991, 81, 541–550. [Google Scholar] [CrossRef]
- González-Rodríguez, V.; Villar, R.; Navarro-Cerrillo, R.M. Maternal influences on seed mass effect and initial seedling growth in four Quercus species. Acta Oecologica 2011, 37, 1–9. [Google Scholar] [CrossRef]
- Adji, B.I.; Akaffou, D.S.; De Reffye, P.; Sabatier, S. Maternal environment and seed size are important for successful germination and seedling establishment of Pterocarpus erinaceus (Fabaceae). J. For. Res. 2022, 33, 977–990. [Google Scholar] [CrossRef]
- Lokmal, N. Genetic parameters of Gmelina arborea: Height and diameter growth. J. Trop. For. Sci. 1994, 7, 323–331. [Google Scholar]
- Lustri, P.A.; Siqueira, W.J.; Azevedo Filho, J.A.; Vianna, S.A.; Colombo, C.A. Estimates of genetic parameters for juvenile traits in macaw palm. Bragantia 2021, 80, e2821. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Yin, T.M.; DiFazio, S.P.; Tschaplinski, T.J.; Gunter, L.E.; Davis, M.F.; Tuskan, G.A. Phenotypic variation in growth and biomass distribution for two advanced-generation pedigrees of hybrid poplar. Can. J. For. Res. 2005, 35, 1779–1789. [Google Scholar] [CrossRef]
- Wu, D.; Shu, M.; Moran, E.V. Heritability of plastic trait changes in drought-exposed ponderosa pine seedlings. Ecosphere 2023, 14, e4454. [Google Scholar] [CrossRef]
- Pincebourde, S.; Woods, H.A. Climate uncertainty on leaf surfaces: The biophysics of leaf microclimates and their consequences for leaf-dwelling organisms. Funct. Ecol. 2012, 26, 844–853. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Xu, L.; Chen, Z.; He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef] [PubMed]
- Clair, J.B.S.; Adams, W.T. Family composition of Douglas-fir nursery stock as influenced by seed characters, mortality, and culling practices. New For. 1993, 7, 319–329. [Google Scholar] [CrossRef]
- Mullin, T.J.; Adams, G.W.; Simpson, J.D.; Tosh, K.J.; Greenwood, M.S. Genetic parameters and correlations in tests of open-pollinated black spruce families in field and retrospective nursery test environments. Can. J. For. Res. 1995, 25, 270–285. [Google Scholar] [CrossRef]
- Cregg, B.M. Seed-source variation in water relations, gas exchange, and needle morphology of mature ponderosa pine trees. Can. J. For. Res. 1993, 23, 749–755. [Google Scholar] [CrossRef]
- Vårhammar, A.; Wallin, G.; McLean, C.M.; Dusenge, M.E.; Medlyn, B.E.; Hasper, T.B.; Nsabimana, D.; Uddling, J. Photosynthetic temperature responses of tree species in Rwanda: Evidence of pronounced negative effects of high temperature in montane rainforest climax species. New Phytol. 2015, 206, 1000–1012. [Google Scholar] [CrossRef]
- Thakur, D.; Hadincová, V.; Schnablová, R.; Synková, H.; Haisel, D.; Wilhelmová, N.; Dostálek, T.; Münzbergová, Z. Differential effect of climate of origin and cultivation climate on structural and biochemical plant traits. Funct. Ecol. 2023, 37, 1436–1448. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Loik, M.E.; Smith, R.A.; Tissue, D.T. Effects of a Heat Wave on Nocturnal Stomatal Conductance in Eucalyptus camaldulensis. Forests 2018, 9, 319. [Google Scholar] [CrossRef]
- Dunlap, J.M.; Braatne, J.H.; Hinckley, T.M.; Stettler, R.F. Intraspecific variation in photosynthetic traits of Populus trichocarpa. Can. J. Bot. 1993, 71, 1304–1311. [Google Scholar] [CrossRef]
- Larsen, J.B.; Wellendorf, H. Early test in Picea abies full sibs by applying gas exchange, frost resistance and growth measurements. Scand. J. For. Res. 1990, 5, 369–380. [Google Scholar] [CrossRef]
- Dang, Q.L.; Xie, C.Y.; Ying, C.; Guy, R.D. Genetic variation of ecophysiological traits in red alder (Alnus rubra Bong.). Can. J. For. Res. 1994, 24, 2150–2156. [Google Scholar] [CrossRef]
- Zhang, J.; Marshall, J.D. Population differences in water-use efficiency of well-watered and water-stressed western larch seedlings. Can. J. For. Res. 1994, 24, 92–99. [Google Scholar] [CrossRef]
- Harrison, S.A.; Boerma, H.R.; Ashley, D.A. Heritability of canopy apparent photosynthesis and its relationship to seed yield in soybeans. Crop Sci. 1981, 21, 222–226. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.; Quick, W.P.; Long, S.P. Evaluating natural variation, heritability, and genetic advance of photosynthetic traits in rice (Oryza sativa). Plant Breed. 2021, 140, 745–757. [Google Scholar] [CrossRef]
- Abidine, A.Z.E.; Stewart, J.D.; Bernier, P.Y.; Plamondon, A.P. Diurnal and seasonal variations in gas exchange and water relations of lowland and upland black spruce ecotypes. Can. J. Bot. 1995, 73, 716–722. [Google Scholar] [CrossRef]
- Fredericksen, T.S.; Steiner, K.C.; Skelly, J.M.; Joyce, B.J.; Kolb, T.E.; Kouterick, K.B.; Ferdinand, J.A. Diel and seasonal patterns of leaf gas exchange and xylem water potentials of different-sized Prunus serotina Ehrh. trees. For. Sci. 1996, 42, 359–365. [Google Scholar] [CrossRef]
- Mebrahtu, T.; Hanover, J.W. Family variation in gas exchange, growth and leaf traits of black locust half-sib families. Tree Physiol. 1991, 8, 185–193. [Google Scholar] [CrossRef]
- Zhang, J.; Marshall, J.D.; Jaquish, B.C. Genetic differentiation in carbon isotope discrimination and gas exchange in Pseudotsuga menziesii. Oecologia 1993, 93, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.; Oliveira, R.S.; Bittencourt, P.R.L.; Giles, A.L.; Coughlin, I.; de Britto Costa, P.; Domingues, T.; Ferreira, L.V.; Vasconcelos, S.S.; Junior, J.A.S.; et al. Plant traits controlling growth change in response to a drier climate. New Phytol. 2020, 229, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Chen, Q.; Lin, S.; Brueck, H.; Dittert, K.; Taube, F.; Schnyder, H. Tradeoffs between nitrogen and water-use efficiency in dominant species of the semiarid steppe of Inner Mongolia. Plant Soil 2011, 340, 227–238. [Google Scholar] [CrossRef]
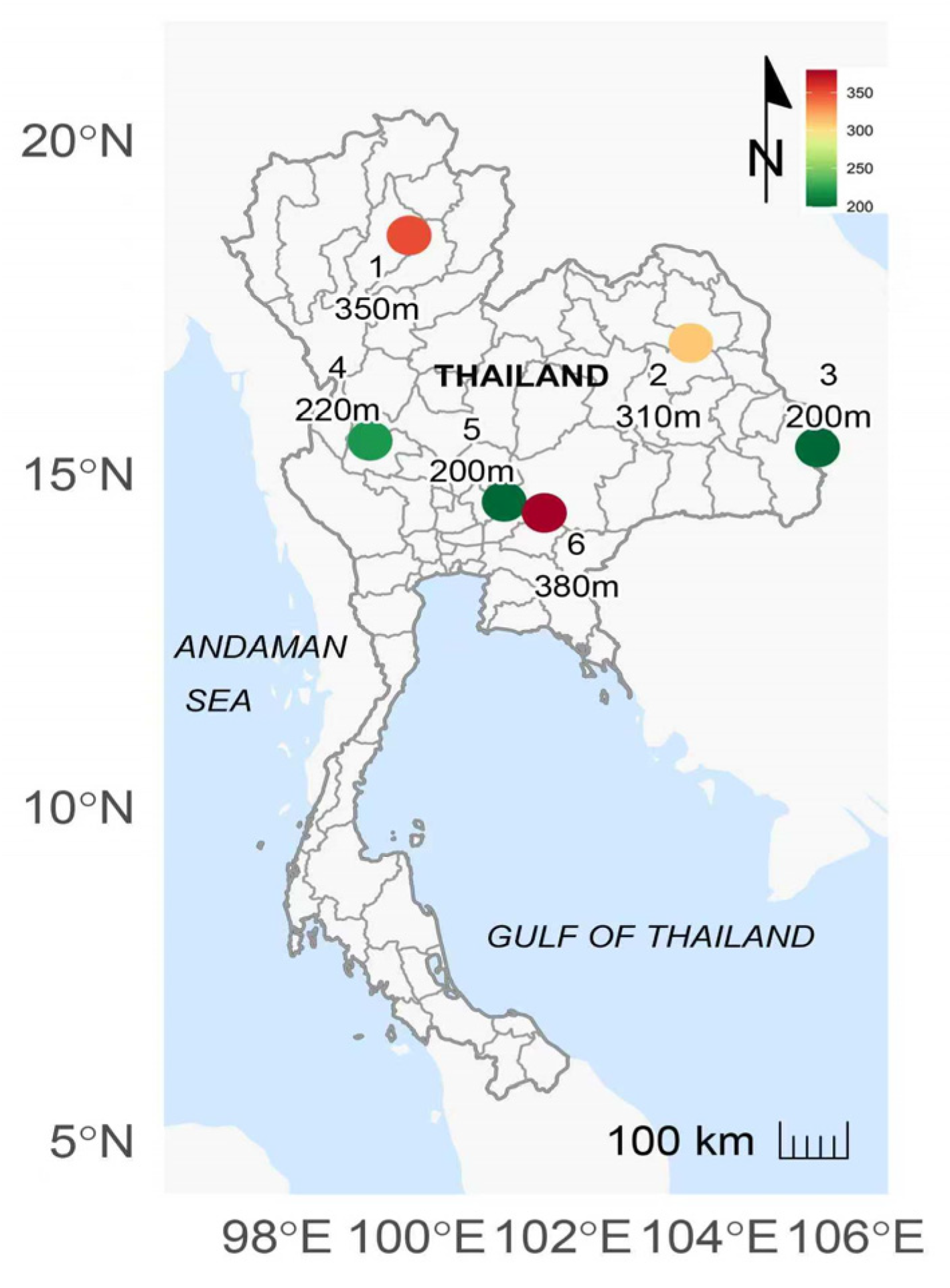




| Population | Latitude (°N) | Longitude (°E) | Elevation (m) | Mean Annual Temperature (°C) | Annual Rainfall (mm) | Forest Type a | F b | tm b | No. of Families | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Name | |||||||||
| 1 | Lampang | 18°35′ | 99°54 | 350 | 25.9 c | 1076 c | MDF | −0.010 | 0.945 | 15 |
| 2 | Phuphan-3 | 16°58′ | 103°45 | 310 | 26.1 c | 1587 c | DDF | 0.144 | 0.751 | 14 |
| 3 | Khong-chiam | 15°24′ | 105°29′ | 200 | 26.7 c | 1634 c | DDF | 0.219 | 0.895 | 13 |
| 4 | Uthaithani | 15°30′ | 99°22′ | 220 | N/A | N/A | DDF | 0.090 | 0.953 | 21 |
| 5 | Saraburi | 14°35′ | 101°12′ | 200 | 26.1 d | 1168 d | MDF | 0.108 | 0.898 | 22 |
| 6 | Sakaerat | 14°25′ | 101°45′ | 380 | 26.3 e | 1310 e | DDF | 0.048 | 0.947 | 27 |
| Population | No. of Families | Survival (%) | Range Among Families Survival (%) |
|---|---|---|---|
| 1 | 15 | 95.8 | 75.0–100 |
| 2 | 14 | 97.3 | 93.8–100 |
| 3 | 13 | 93.8 | 75.0–100 |
| 4 | 21 | 92.3 | 75.0–100 |
| 5 | 22 | 94.6 | 75.0–100 |
| 6 | 27 | 96.5 | 87.5–100 |
| Overall | 112 | 95.0 a | 75.0–100 |
| Source of Variation | df | H3 | H6 | H9 | H12 | H15 | H18 | H21 | H24 | H27 | H30 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Replication | 15 | 0.02 ns | 0.18 ns | 0.11 ns | 1.19 ** | 4.57 ** | 7.72 ** | 10.6 ** | 9.45 ** | 10.0 ** | 9.89 ** |
| Population | 5 | 11.8 ** | 9.40 ** | 15.2 ** | 17.6 ** | 11.6 ** | 7.74 ** | 8.9 ** | 9.67 ** | 10.6 ** | 11.4 ** |
| Replication × Population | 75 | 0.00 ns | 0.00 ns | 0.00 ns | 0.20 ns | 0.00 ns | 0.00 ns | 0.00 ns | 0.00 ns | 0.13 ns | 0.00 ns |
| Family (Population) | 106 | 24.1 ** | 25.4 ** | 30.7 ** | 28.1 ** | 21.6 ** | 15.1 ** | 13.4 ** | 13.6 ** | 13.5 ** | 12.8 ** |
| Residual | 1479 | 64.0 | 65.1 | 54.1 | 52.9 | 62.3 | 69.4 | 67.1 | 67.3 | 65.7 | 65.9 |
| Source of Variation | df | D12 | D15 | D18 | D21 | D24 | D27 | D30 |
|---|---|---|---|---|---|---|---|---|
| Replication | 15 | 0.16 ns | 0.00 ns | 1.47 ** | 3.16 ** | 1.79 ** | 4.35** | 4.77 ** |
| Population | 5 | 2.72 * | 3.27 ** | 3.34 ** | 2.57 * | 2.61 * | 4.28** | 4.67 ** |
| Replication × Population | 75 | 0.78 ns | 0.55 ns | 1.43 * | 0.57 ns | 0.58 ns | 0.49 ns | 0.03 ns |
| Family (Population) | 106 | 18.1 ** | 19.4 ** | 18.6 ** | 21.0 ** | 18.0 ** | 16.5 ** | 17.0 ** |
| Residual | 1479 | 78.3 | 76.8 | 75.1 | 72.7 | 77.0 | 74.4 | 73.5 |
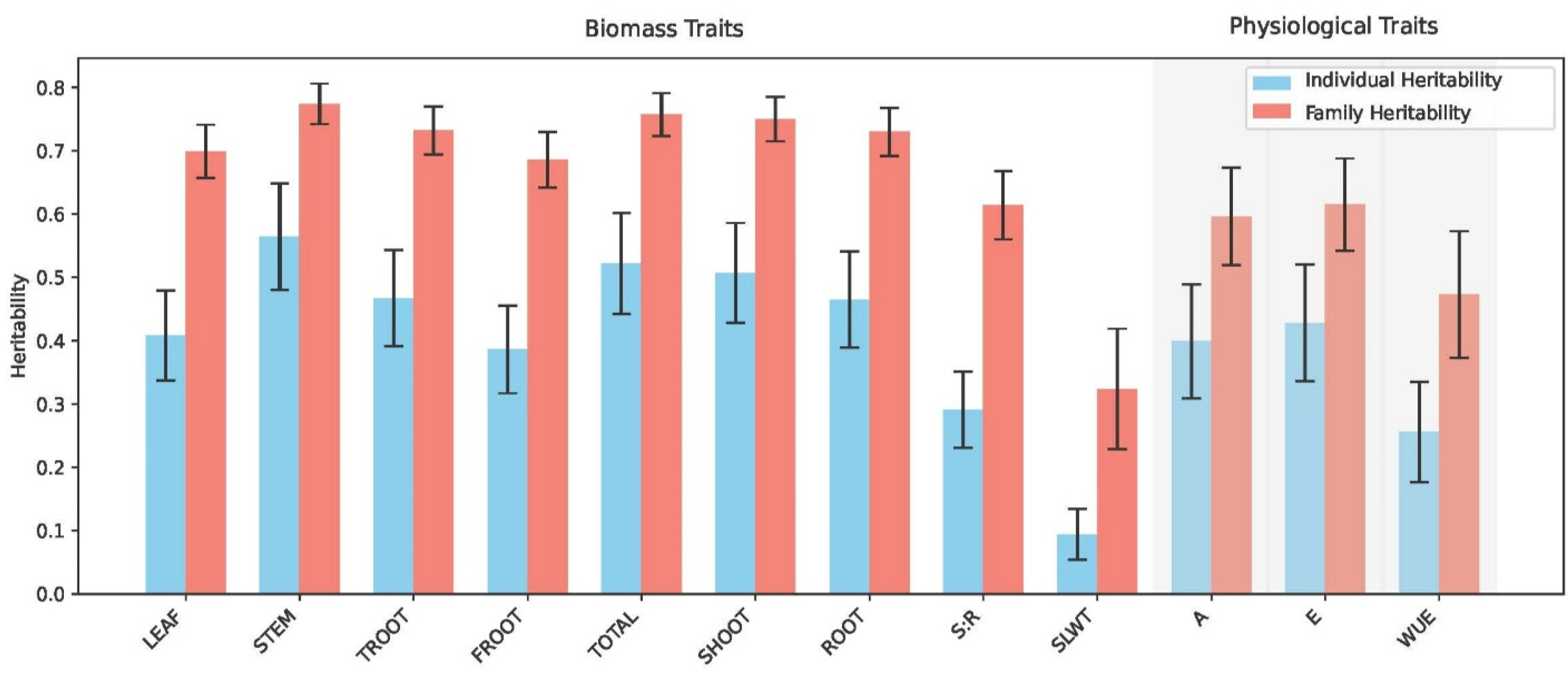
| Source of Variation | df | LEAF | STEM | TROOT | FROOT | TOTAL | SHOOT | ROOT | S:R | SLWT |
|---|---|---|---|---|---|---|---|---|---|---|
| Replication | 15 | 7.79 ** | 10.1 ** | 6.14 ** | 4.49 ** | 9.49 ** | 9.79 ** | 6.44 ** | 1.20 ** | 12.9 ** |
| Population | 5 | 15.2 ** | 10.2 ** | 7.59 ** | 4.46 ** | 11.0 ** | 13.2 ** | 7.09 ** | 8.04 ** | 2.28 ** |
| Replication × Population | 75 | 0.18 ns | 0.00 ns | 0.08 ns | 0.00 ns | 0.21 ns | 0.10 ns | 0.00 ns | 0.00 ns | 0.64 ns |
| Family (Population) | 106 | 10.3 ** | 14.8 ** | 13.3 ** | 11.6 ** | 13.7 ** | 12.9 ** | 3.26 ** | 8.72 ** | 2.62 ** |
| Residual | 1479 | 66.5 | 64.8 | 72.9 | 79.5 | 65.7 | 64.06 | 73.2 | 82.0 | 81.6 |
| H3 | H6 | H9 | H12 | H15 | H18 | H21 | H24 | H27 | H30 | |
|---|---|---|---|---|---|---|---|---|---|---|
| H3 | **** | 0.66 | 0.66 | 0.72 | 0.67 | 0.55 | 0.52 | 0.50 | 0.42 | 0.39 |
| H6 | 0.07 | **** | 0.88 | 0.81 | 0.79 | 0.70 | 0.70 | 0.69 | 0.64 | 0.60 |
| H9 | 0.07 | 0.03 | **** | 0.96 | 0.92 | 0.81 | 0.80 | 0.79 | 0.69 | 0.66 |
| H12 | 0.06 | 0.04 | 0.01 | **** | 0.96 | 0.86 | 0.85 | 0.83 | 0.73 | 0.70 |
| H15 | 0.07 | 0.05 | 0.03 | 0.01 | **** | 0.95 | 0.92 | 0.91 | 0.82 | 0.79 |
| H18 | 0.09 | 0.07 | 0.05 | 0.04 | 0.02 | **** | 0.98 | 0.96 | 0.92 | 0.89 |
| H21 | 0.09 | 0.07 | 0.05 | 0.04 | 0.03 | 0.01 | **** | 1.00 | 0.98 | 0.96 |
| H24 | 0.10 | 0.07 | 0.05 | 0.04 | 0.03 | 0.01 | 0.00 | **** | 0.99 | 0.98 |
| H27 | 0.10 | 0.08 | 0.07 | 0.06 | 0.05 | 0.03 | 0.01 | 0.01 | **** | 1.00 |
| H30 | 0.11 | 0.08 | 0.07 | 0.07 | 0.05 | 0.03 | 0.02 | 0.01 | 0.00 | **** |
| D12 | D15 | D18 | D21 | D24 | D27 | D30 | |
|---|---|---|---|---|---|---|---|
| D12 | **** | 0.97 | 0.91 | 0.88 | 0.89 | 0.87 | 0.86 |
| D15 | 0.02 | **** | 0.99 | 0.97 | 0.96 | 0.95 | 0.91 |
| D18 | 0.03 | 0.01 | **** | 0.98 | 0.98 | 0.98 | 0.95 |
| D21 | 0.04 | 0.02 | 0.01 | **** | 1.00 | 0.99 | 0.97 |
| D24 | 0.04 | 0.02 | 0.02 | 0.01 | **** | 0.99 | 0.97 |
| D27 | 0.05 | 0.03 | 0.02 | 0.01 | 0.01 | **** | 1.00 |
| D30 | 0.05 | 0.03 | 0.02 | 0.01 | 0.01 | 0.00 | **** |
| rg | H3 | H6 | H9 | H12 | H15 | H18 | H21 | H24 | H27 | H30 |
|---|---|---|---|---|---|---|---|---|---|---|
| D12 | 0.55 | 0.59 | 0.80 | 0.79 | 0.73 | 0.62 | 0.64 | 0.65 | 0.58 | 0.56 |
| s.e | 0.09 | 0.08 | 0.05 | 0.05 | 0.06 | 0.08 | 0.08 | 0.08 | 0.09 | 0.09 |
| D15 | 0.65 | 0.68 | 0.86 | 0.89 | 0.86 | 0.77 | 0.76 | 0.76 | 0.70 | 0.69 |
| s.e | 0.08 | 0.07 | 0.04 | 0.04 | 0.04 | 0.06 | 0.06 | 0.06 | 0.07 | 0.08 |
| D18 | 0.68 | 0.72 | 0.87 | 0.92 | 0.88 | 0.75 | 0.73 | 0.74 | 0.66 | 0.64 |
| s.e | 0.07 | 0.07 | 0.04 | 0.03 | 0.04 | 0.06 | 0.07 | 0.07 | 0.08 | 0.08 |
| D21 | 0.62 | 0.72 | 0.81 | 0.87 | 0.87 | 0.76 | 0.73 | 0.73 | 0.65 | 0.62 |
| s.e | 0.08 | 0.07 | 0.05 | 0.04 | 0.04 | 0.06 | 0.07 | 0.07 | 0.08 | 0.08 |
| D24 | 0.60 | 0.70 | 0.82 | 0.87 | 0.88 | 0.78 | 0.74 | 0.75 | 0.68 | 0.66 |
| s.e | 0.08 | 0.07 | 0.05 | 0.04 | 0.04 | 0.06 | 0.07 | 0.07 | 0.08 | 0.08 |
| D27 | 0.57 | 0.70 | 0.82 | 0.87 | 0.88 | 0.81 | 0.78 | 0.78 | 0.72 | 0.70 |
| s.e | 0.09 | 0.07 | 0.05 | 0.04 | 0.04 | 0.06 | 0.06 | 0.06 | 0.07 | 0.07 |
| D30 | 0.61 | 0.74 | 0.83 | 0.87 | 0.87 | 0.80 | 0.77 | 0.77 | 0.70 | 0.68 |
| s.e | 0.08 | 0.07 | 0.05 | 0.04 | 0.04 | 0.06 | 0.06 | 0.06 | 0.07 | 0.07 |
| LEAF | 0.30 | 0.58 | 0.53 | 0.55 | 0.66 | 0.75 | 0.78 | 0.79 | 0.82 | 0.81 |
| s.e | 0.12 | 0.09 | 0.09 | 0.09 | 0.08 | 0.07 | 0.06 | 0.06 | 0.05 | 0.05 |
| STEM | 0.54 | 0.74 | 0.79 | 0.82 | 0.88 | 0.89 | 0.88 | 0.88 | 0.86 | 0.84 |
| s.e | 0.09 | 0.06 | 0.05 | 0.05 | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.04 |
| TROOT | 0.50 | 0.50 | 0.53 | 0.57 | 0.67 | 0.73 | 0.63 | 0.59 | 0.58 | 0.56 |
| s.e | 0.10 | 0.10 | 0.09 | 0.09 | 0.07 | 0.07 | 0.08 | 0.09 | 0.09 | 0.09 |
| FROOT | 0.40 | 0.56 | 0.63 | 0.69 | 0.78 | 0.81 | 0.80 | 0.79 | 0.75 | 0.74 |
| s.e | 0.11 | 0.10 | 0.08 | 0.08 | 0.06 | 0.06 | 0.06 | 0.06 | 0.07 | 0.07 |
| TOTAL | 0.50 | 0.67 | 0.69 | 0.73 | 0.82 | 0.88 | 0.84 | 0.83 | 0.82 | 0.80 |
| s.e | 0.09 | 0.08 | 0.07 | 0.06 | 0.05 | 0.04 | 0.04 | 0.05 | 0.05 | 0.05 |
| SHOOT | 0.45 | 0.70 | 0.71 | 0.73 | 0.82 | 0.86 | 0.87 | 0.87 | 0.87 | 0.86 |
| s.e | 0.10 | 0.07 | 0.07 | 0.06 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.04 |
| ROOT | 0.51 | 0.55 | 0.59 | 0.64 | 0.75 | 0.80 | 0.72 | 0.68 | 0.67 | 0.65 |
| s.e | 0.10 | 0.09 | 0.08 | 0.08 | 0.06 | 0.05 | 0.07 | 0.07 | 0.08 | 0.08 |
| S:R | −0.12 | 0.21 | 0.16 | 0.12 | 0.08 | 0.07 | 0.22 | 0.26 | 0.31 | 0.32 |
| s.e | 0.13 | 0.13 | 0.14 | 0.13 | 0.13 | 0.14 | 0.14 | 0.14 | 0.13 | 0.13 |
| SLWT | −0.14 | 0.07 | 0.17 | 0.18 | 0.22 | 0.11 | 0.11 | 0.10 | 0.04 | 0.05 |
| s.e | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.19 | 0.19 | 0.19 | 0.20 | 0.20 |
| rg | D12 | D15 | D18 | D21 | D24 | D27 | D30 |
|---|---|---|---|---|---|---|---|
| LEAF | 0.49 | 0.62 | 0.57 | 0.58 | 0.56 | 0.60 | 0.59 |
| s.e. | 0.10 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 |
| STEM | 0.73 | 0.84 | 0.85 | 0.85 | 0.85 | 0.89 | 0.88 |
| s.e. | 0.07 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 |
| TROOT | 0.44 | 0.67 | 0.61 | 0.59 | 0.54 | 0.54 | 0.51 |
| s.e. | 0.11 | 0.08 | 0.09 | 0.09 | 0.09 | 0.09 | 0.10 |
| FROOT | 0.62 | 0.77 | 0.71 | 0.74 | 0.74 | 0.73 | 0.72 |
| s.e. | 0.09 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| TOTAL | 0.62 | 0.80 | 0.76 | 0.76 | 0.74 | 0.76 | 0.74 |
| s.e. | 0.08 | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| SHOOT | 0.64 | 0.78 | 0.76 | 0.76 | 0.76 | 0.80 | 0.78 |
| s.e. | 0.08 | 0.06 | 0.06 | 0.06 | 0.06 | 0.05 | 0.05 |
| ROOT | 0.52 | 0.74 | 0.68 | 0.67 | 0.63 | 0.63 | 0.60 |
| s.e. | 0.10 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 | 0.09 |
| S:R | 0.20 | 0.07 | 0.13 | 0.15 | 0.21 | 0.27 | 0.28 |
| s.e. | 0.14 | 0.14 | 0.14 | 0.13 | 0.14 | 0.13 | 0.13 |
| SLWT | 0.23 | 0.10 | 0.01 | 0.13 | 0.18 | 0.08 | 0.09 |
| s.e. | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 |
| LEAF | STEM | TROOT | FROOT | TOTAL | SHOOT | ROOT | S:R | SLWT | |
|---|---|---|---|---|---|---|---|---|---|
| LEAF | **** | 0.85 | 0.67 | 0.82 | 0.91 | 0.95 | 0.76 | 0.30 | −0.09 |
| STEM | 0.04 | **** | 0.71 | 0.87 | 0.94 | 0.97 | 0.80 | 0.24 | 0.02 |
| TROOT | 0.08 | 0.07 | **** | 0.66 | 0.87 | 0.72 | 0.98 | −0.40 | −0.31 |
| FROOT | 0.06 | 0.04 | 0.08 | **** | 0.89 | 0.88 | 0.80 | 0.03 | 0.04 |
| TOTAL | 0.03 | 0.02 | 0.03 | 0.03 | **** | 0.96 | 0.94 | 0.02 | −0.13 |
| SHOOT | 0.01 | 0.01 | 0.07 | 0.04 | 0.01 | **** | 0.81 | 0.27 | −0.03 |
| ROOT | 0.06 | 0.05 | 0.01 | 0.05 | 0.02 | 0.05 | **** | −0.31 | −0.24 |
| S:R | 0.14 | 0.13 | 0.12 | 0.15 | 0.14 | 0.13 | 0.13 | **** | 0.46 |
| SLWT | 0.21 | 0.19 | 0.20 | 0.21 | 0.20 | 0.20 | 0.20 | 0.22 | **** |
| Population | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| Trait | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Grand Mean | C.V.a (%) |
| A b | 7.98 (3.54) | 7.93 (3.09) | 7.88 (4.18) | 8.54 (3.17) | 9.08 (3.67) | 8.40 (3.32) | 8.39 | 41.7 |
| Range | 5.66–10.0 | 5.99–11.6 | 5.31–10.7 | 6.29–11.7 | 6.12–11.0 | 5.15–11.1 | ||
| E c | 1.49 (0.76) | 1.39 (0.65) | 1.31 (0.73) | 1.41 (0.62) | 1.35 (0.63) | 1.44 (0.74) | 1.40 | 49.2 |
| Range | 1.13–2.18 | 1.09–1.96 | 0.89–1.86 | 0.99–2.03 | 0.98–1.90 | 0.91–2.48 | ||
| WUE d | 5.82 (2.35) | 6.26 (2.23) | 6.45 (3.01) | 6.58 (2.46) | 7.28 (2.51) | 6.54 (2.58) | 6.56 | 39.1 |
| Range | 4.32–7.78 | 5.34–7.20 | 4.95–8.40 | 5.28–9.31 | 6.03–8.86 | 4.86–8.20 | ||
| Source of Variation | df | A | E | WUE |
|---|---|---|---|---|
| Replication | 4 | 0.27 ns | 3.90 ** | 14.6 ** |
| Population | 5 | 0.00 ns | 0.00 ns | 2.34 ** |
| Replication × Population | 20 | 3.58 ** | 1.44 * | 0.98 ns |
| Family (Population) | 106 | 12.7 ** | 13.4 ** | 6.94 ** |
| Leaf (Family Population) | 112 | 0.00 ns | 0.00 ns | 0.00 ns |
| Residual | 842 | 83.5 | 81.3 | 75.1 |
| rg | A | E | WUE |
|---|---|---|---|
| H3 | 0.07 (0.14) | −0.02 (0.14) | 0.16 (0.16) |
| H6 | 0.05 (0.14) | −0.05 (0.14) | 0.15 (0.16) |
| H9 | −0.02 (0.14) | −0.11 (0.13) | 0.19 (0.15) |
| H12 | 0.04 (0.14) | −0.05 (0.13) | 0.17 (0.15) |
| H15 | 0.06 (0.14) | −0.07 (0.14) | 0.19 (0.16) |
| H18 | 0.06 (0.15) | −0.02 (0.14) | 0.09 (0.17) |
| H21 | 0.07 (0.15) | −0.08 (0.14) | 0.24 (0.16) |
| H24 | 0.10 (0.15) | −0.06 (0.14) | 0.26 (0.16) |
| H27 | 0.09 (0.15) | −0.12 (0.14) | 0.31 (0.16) |
| H30 | 0.07 (0.15) | −0.15 (0.15) | 0.32 (0.16) |
| D12 | −0.05 (0.14) | −0.13 (0.14) | 0.13 (0.16) |
| D15 | −0.08 (0.15) | −0.16 (0.14) | 0.14 (0.16) |
| D18 | −0.03 (0.15) | −0.09 (0.14) | 0.09 (0.16) |
| D21 | −0.01 (0.14) | −0.100 (0.14) | 0.04 (0.16) |
| D24 | −0.02 (0.14) | −0.09 (0.14) | 0.07 (0.16) |
| D27 | −0.04 (0.14) | −0.14 (0.14) | 0.11 (0.16) |
| D30 | −0.02 (0.14) | −0.14 (0.14) | 0.14 (0.16) |
| LEAF | −0.17 (0.15) | −0.32 (0.14) | 0.27 (0.17) |
| STEM | 0.01 (0.15) | −0.14 (0.14) | 0.22 (0.16) |
| TROOT | 0.01 (0.15) | −0.13 (0.14) | 0.20 (0.16) |
| FROOT | −0.08 (0.15) | −0.14 (0.15) | 0.03 (0.17) |
| TOTAL | −0.06 (0.15) | −0.22 (0.14) | 0.24 (0.16) |
| SHOOT | −0.07 (0.15) | −0.23 (0.14) | 0.26 (0.16) |
| ROOT | −0.02 (0.15) | −0.15 (0.14) | 0.17 (0.17) |
| S:R | −0.13 (0.15) | −0.19 (0.14) | 0.12 (0.16) |
| SLWT | 0.14 (0.15) | 0.06 (0.15) | 0.06 (0.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyasit, L.; Yeh, F.C. Genetic Variation and the Relationships Among Growth, Morphological, and Physiological Traits in Pterocarpus macrocarpus: Implications for Early Selection and Conservation. Conservation 2025, 5, 50. https://doi.org/10.3390/conservation5030050
Chaiyasit L, Yeh FC. Genetic Variation and the Relationships Among Growth, Morphological, and Physiological Traits in Pterocarpus macrocarpus: Implications for Early Selection and Conservation. Conservation. 2025; 5(3):50. https://doi.org/10.3390/conservation5030050
Chicago/Turabian StyleChaiyasit, Liengsiri, and Francis C. Yeh. 2025. "Genetic Variation and the Relationships Among Growth, Morphological, and Physiological Traits in Pterocarpus macrocarpus: Implications for Early Selection and Conservation" Conservation 5, no. 3: 50. https://doi.org/10.3390/conservation5030050
APA StyleChaiyasit, L., & Yeh, F. C. (2025). Genetic Variation and the Relationships Among Growth, Morphological, and Physiological Traits in Pterocarpus macrocarpus: Implications for Early Selection and Conservation. Conservation, 5(3), 50. https://doi.org/10.3390/conservation5030050






