Abstract
Ex situ conservation is a vital strategy of preserving plant species at risk, offering practical methods to obtain information regarding species-specific germination characteristics. Campanula pangea, a local endemic species of NE Greece, has been previously classified as vulnerable, partly due to the lack of knowledge about its biology. This study focused on the germination behaviour of C. pangea stored seeds by assessing their germination success under the effects of incubation temperature and gibberellic acid (GA3). To contextualize the experimental conditions, a bioclimatic profile of the species was developed using open-access temperature and precipitation data that characterize its natural habitat. The results showed that the optimal germination temperature range for C. pangea is 15–20 °C. Pre-treatment of seeds with GA3 solution (1000 mg L−1) widened the germination range of the seeds only at the low temperature of 10 °C. The experimentation results showed that the seeds of C. pangea exhibit dormancy. These findings contribute to the development of a species-specific germination protocol for ex situ propagation and conservation, enhance understanding of the species’ germination requirements, and thus support future conservation efforts and assessments of extinction risk, or other ornamental applications and/or targeted medicinal research.
1. Introduction
Over the past few decades, conservation strategies have evolved in response to growing global efforts to strengthen environmental policies and protect biodiversity. Despite notable progress, human activities continue to drive habitat loss, degradation, and fragmentation, leading to declines in species populations and genetic diversity [1]. The most widely recognised approach to preserving biodiversity is the management and monitoring of species populations and their habitats, commonly known as in situ conservation. However, its successful implementation often requires extensive coordination and investment, making it unfeasible in many cases [2,3]. Given these challenges, ex situ conservation has become an essential complementary strategy, providing practical means of preserving plant species at risk and serving as an effective back-up solution [4,5]. The conservation of plant species outside their natural habitat can be achieved in ex situ conservation facilities (seed banks, botanical gardens, and field gene banks), with seed collection and long-term storage being the most effective and widely used method [6].
Germination is critical for the survival of plant organisms and is the foundation for the recruitment of their populations [7]. Yet, seed germination is a highly susceptible stage of plant development [8], which relies on a sophisticated combination of endogenous and exogenous factors, with environmental conditions playing a major role [9]. The suitable conditions required for germination success vary from species to species, and for many threatened plants, such conditions are still unexplored [10]. Therefore, a key component in ex situ conservation of high-priority species is the investigation of their seed dormancy and germination characteristics [11]. This can be achieved through species-specific seed germination experiments, which can provide insight into their adaptive mechanisms, their resilience to pressures and threats, and their persistence to climate change, therefore enabling the development of appropriate and species-specific management measures [7].
Seed germination firstly encompasses seed hydration and concludes with the elongation of the radicle and its emergence from the surrounding anatomical structures [12]. The timing of seed germination is essential for the survival and growth of seedlings under favourable environmental conditions, specifically at the beginning of the growing season in their natural habitats, as it provides sufficient time for growth and establishment [13]. Seed dormancy is the main adaptation mechanism that delays the germination process until more suitable environmental conditions occur [14]. Hence, seed dormancy is an evolutionary strategy of plant organisms to avoid premature mortality of their individuals due to growth in unfavourable environmental conditions [15,16]. Dormancy is driven by blocks of germination imposed either by the embryo (endogenous), such as an underdeveloped embryo, chemical inhibitors, and physiological constraints, or by the embryo covering layers (exogenous), which may be interrupted with the removal, modification, or destruction of the covering layers [15,17]. In general, there are five main classes of dormancy, either simple (physiological, morphological, physical) or complex (morphophysiological and combinational dormancy) [18], with physiological dormancy (non-deep, intermediate, and deep) being the most common one [13,15].
The breaking of dormancy and the induction of germination are significantly influenced by environmental factors. Temperature has a critical role in breaking dormancy and controlling seed germination [19]. Specific temperature regimes are essential for breaking seed dormancy, after which a particular temperature range is required to facilitate germination of non-dormant seeds [13]. Within the temperature range where seed germination of a species occurs, there is an optimum point at which the highest germination rate is achieved, while lower or higher than optimal temperatures decrease the success of germination [20]. Temperature impacts several biochemical and physiological metabolic processes of the hydrated seed, as optimal temperatures can boost enzyme activity, facilitate the breakdown of stored nutrients into energy and key metabolic components, and accelerate germination rate [7,21]. The most essential hormones for the regulation of dormancy and germination processes are abscisic acid (ABA) and gibberellins (GAs), respectively [15,22]. The temporal shift in GA and ABA concentrations plays a vital role, inducing dormancy over germination due to high ABA/GA ratio, and triggering the inverse condition in case of low ratio [23,24]. Exogenous treatment of seeds with GA has the potential, under some circumstances, to interrupt dormancy and promote germination [25,26,27].
The Campanulaceae family is a large cosmopolitan angiosperm group with 84 accepted genera and ca. 2400 species, which is organised into five subfamilies [28,29]. Campanulaceae taxa (species and subspecies) are mostly herbaceous perennials, less often annuals or biennials, forming usually small and numerous seeds [30]. The genus Campanula, comprising approximately 450 species [31], is the most representative of this family and holds significant potential for sustainable use in the ornamental–horticultural sector, providing new and promising ornamental crops [32]. Campanula species are particularly abundant in the Mediterranean region and the Middle East [31] and exhibit significant diversity in both habitat preferences and morphological traits [33]. The seeds of the Campanulaceae family have a strong requirement for light for germination, especially the smaller seeds of Campanula spp., as their germination most often occurs close to the soil surface [34]. Campanula pangea Hartvig is a local endemic species of NE Greece, first described in 1998, and is known to occur exclusively on Mt Pangeo, Eastern Macedonia (single-mountain endemic) [35]. It grows within the beech belt around the mountain in an elevation range of 1000–1600 m above sea level [35]. The species C. pangea is a biennial hemicryptophyte, which flowers in the second year of its life cycle, usually in July. Given its endemic status, the species has been assessed in the national Red Data Book of Greece [36] where it was classified as vulnerable (VU) using the IUCN (International Union for the Conservation of Nature) criteria. The assessment of the species’ threat category was based on its narrow geographic distribution, the small size of its subpopulations, and the lack of knowledge regarding its biology, which still applies to date, and therefore, C. pangea has also recently been globally assessed as vulnerable [37].
The conservation of threatened species demands a thorough comprehension of their biology and specifically their germination characteristics, which in many taxa remain poorly understood. Knowledge of the species’ seed dormancy type and the seed germination constraints is essential for effective in situ and ex situ conservation. Due to the limited data available on the germination behaviour of C. pangea, the present study aimed to (i) investigate how incubation temperature and gibberellic acid (GA3) affect seed germination success, (ii) determine the existence of seed dormancy and examine how GA3 affects its breaking, and (iii) identify the optimal conditions required for its successful germination, contributing to the development of a species-specific germination protocol. To link the results of the germination experiment with the species’ natural habitat conditions, a bioclimatic profile of the C. pangea was implemented based on environmental data (temperature and precipitation) of its total known distribution range on Mt Pangeo, NE Greece.
2. Materials and Methods
2.1. Seed Collection
The seeds of Campanula pangea were obtained from the seed bank of the Institute of Plant Breeding and Genetic Resources of the Hellenic Agricultural Organization-Demeter (ELGO Dimitra). The seeds were collected on 23 August 2020 from Mt. Pangeo (Figure 1) at geographical coordinates 40.913639 North and 24.103276 East, and altitude 1730 m above sea level, using a special collection permit issued by the competent Greek authorities (YPEN/DPD/64886/2959 of 6 July 2020). After taxonomic identification, the seedlot was designated with the International Plant Exchange Network (IPEN) reference accession number GR-1-BBGK-20,33 and was stored at 3–5 °C in a chamber with low humidity until the onset of germination experiments.

Figure 1.
Plant habit before (A) and after grazing by cattle (B), ascending flowering stems (C), and corolla (D) of the threatened (vulnerable) Campanula pangea, a local endemic plant of Mt. Pangeo, NE Greece (single-mountain endemic); all photos taken on 1 June 2020.
2.2. Seed Germination
Prior to the germination experiment, the seeds were cleaned manually from other tissue remnants and their mass was measured using a high precision analytical balance. The mass of 500 seeds weighed 0.028 g, with the average mass of a seed corresponding to 0.55 × 10−4 g. In addition, the seeds were photographed on 1 mm graph paper using a macro camera to capture their true size and dimensions (Figure 2A,B).
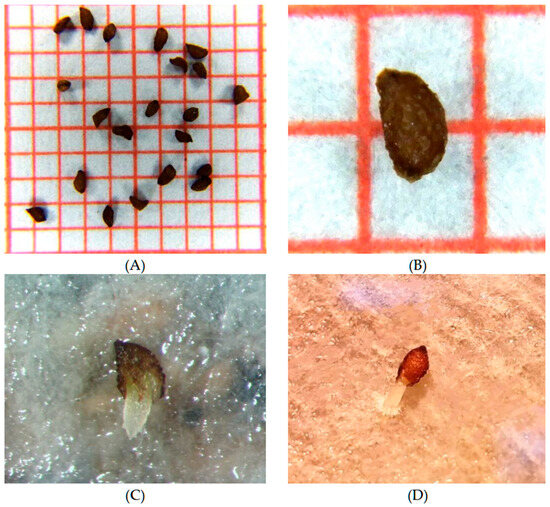
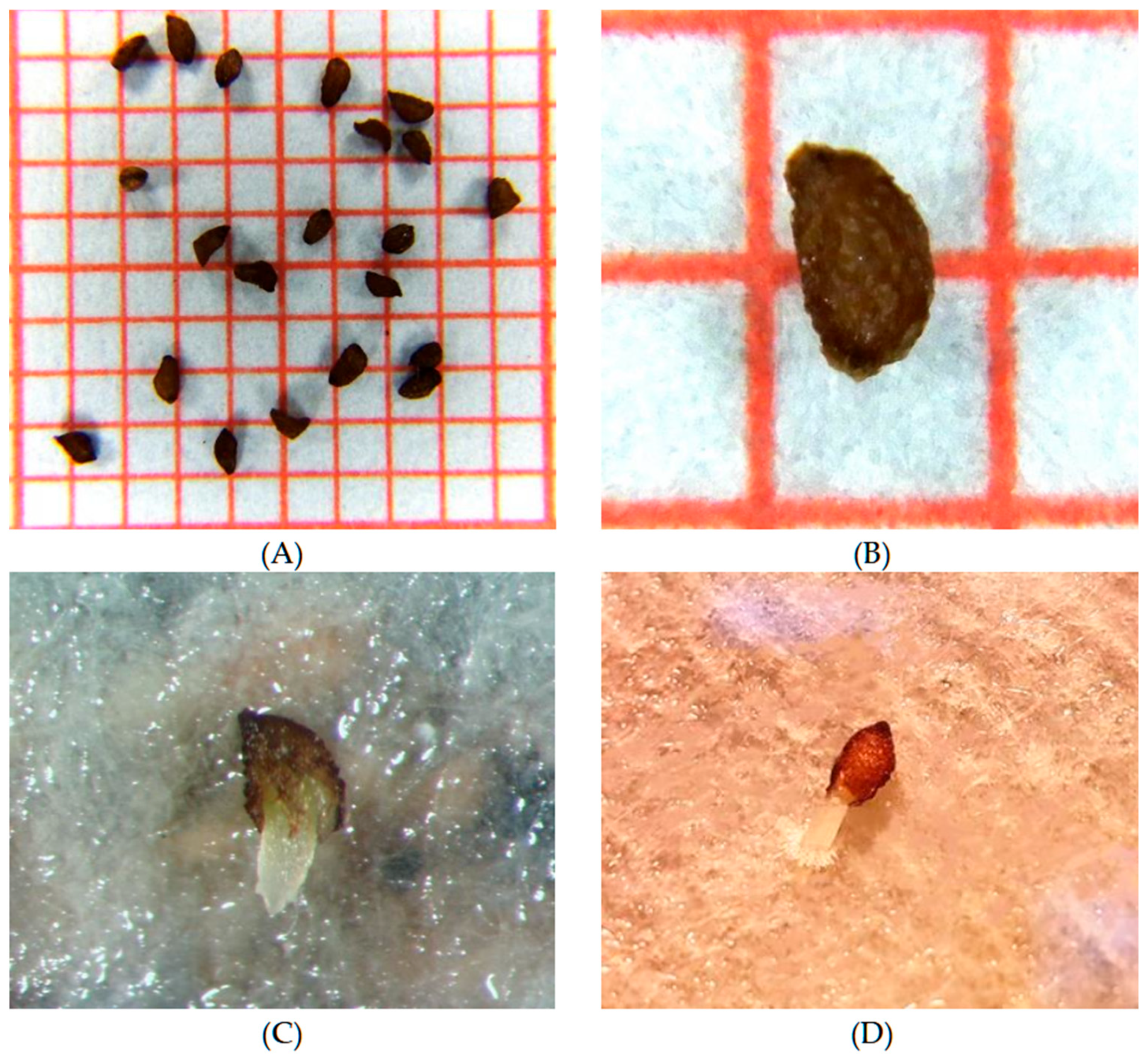
Figure 2.
Seeds of Campanula pangea: (A,B) Morphology and dimensions of dormant seeds on a millimetre graph paper, (C,D) germinated seeds showing clear radicle emergence.
The germination experiment of the seeds of C. pangea was carried out in February 2024 at the laboratory of Floriculture of the School of Agriculture of Aristotle University of Thessaloniki, Greece. To investigate the effect of the incubation temperature on seed germination, four constant temperatures (10, 15, 20, and 25 °C) were tested. To investigate the effect of gibberellic acid (GA3, Sigma-Aldrich, Saint Louis, MO, USA) on germination, two levels were tested: no treatment of seeds (control) and treatment with a solution of GA3. Informed by results of previous studies on seeds of Campanula species (C. cretica [38], C. pelviformis, and C. lyrata (unpublished data)) and due to the limited number of wild-sourced available seeds, the treatment of seeds with 1000 mg L−1 of GA3 was chosen for experimentation.
Based on the number of available seeds, for control and treatment with GA3, four replicates of 15 seeds were placed at each incubation temperature. The seeds of each replication were placed in 9 cm plastic Petri dishes on wet filter paper. For higher moisture retention in the Petri dishes, a layer of moist and sterile sand was placed under the filter papers. The seeds treated with GA3 were immersed in a GA3 solution at a concentration of 1000 mg L−1 for 24 h before being placed in the Petri dishes. Subsequently, the Petri dishes were placed in growth chambers (CRW-500SD, Chrysagis, Athens, Greece) with constant temperature at 10, 15, 20, and 25 °C, with a photoperiod of 12 h light/12 h dark and a light intensity of 82 μmol m−2 s−1 provided by cool white-fluorescent lamps (Philips, Hamburg, Germany). To avoid seed dehydration, water with fungicide (0.1% Captan 80 WG-Arysta) was added to the Petri dishes frequently or when deemed necessary. The recording of germinated seeds was carried out every four days for a period of two months. The criterion for determining seed germination was the evident emergence of the radicle through the tissues enclosing it (Figure 2C,D).
2.3. Statistical Analysis
The experimental setup encompassed a fully randomized factorial design with seed treatment (2 levels: control and 1000 mg L−1) and incubation temperature (4 levels: 10, 15, 20, and 25 °C) being the factors. Two-way ANOVA was employed to identify the significance of the effect of each factor on germination percentage, and the significance of their interaction. In advance of the analysis of variance, the normality and homogeneity of the data were examined. A test of multiple comparisons followed using the Least Significant Difference (LSD) criterion (significance level a = 0.05) for the comparison of means. All statistical analyses were performed using SPSS Statistics software (Version 25.0; IBM Corp., Chicago, IL, USA).
2.4. Bioclimatic Profile
The development of the bioclimatic profile of C. pangea followed the approach proposed by Krigas et al. [4]. In brief, eighty (n = 80) occurrence points of high accuracy, representing the entire known distribution of the species on Mt. Pangeo were exploited [37,39]. Climate data, regarding temperature, precipitation, and bioclimatic variables, were obtained from the Worldclim database (v.2.1) [40] for the period 1970–2000 at a spatial resolution of 30 arcseconds (~1 km2). Climate data extraction and graphic representation were conducted in R (R version 4.3.2; [41]) and RStudio software [42] with the packages geodata [43] and ggplot2 [44], respectively.
3. Results
3.1. Seed Germination
The two-way ANOVA analysis revealed that the effects of incubation temperature (p = 0.000) and GA3 (p = 0.003) on Campanula pangea seed germination, as well as the effects of their interaction (p = 0.001), were statistically significant (Table 1). Due to the significant effect of the examined factors’ interaction on germination, the interpretation of the main effects was considered less important and therefore only their interaction was further analysed.

Table 1.
Significance of factors (temperature, GA3) and their interaction on germination percentage of Campanula pangea seeds estimated by ANOVA.
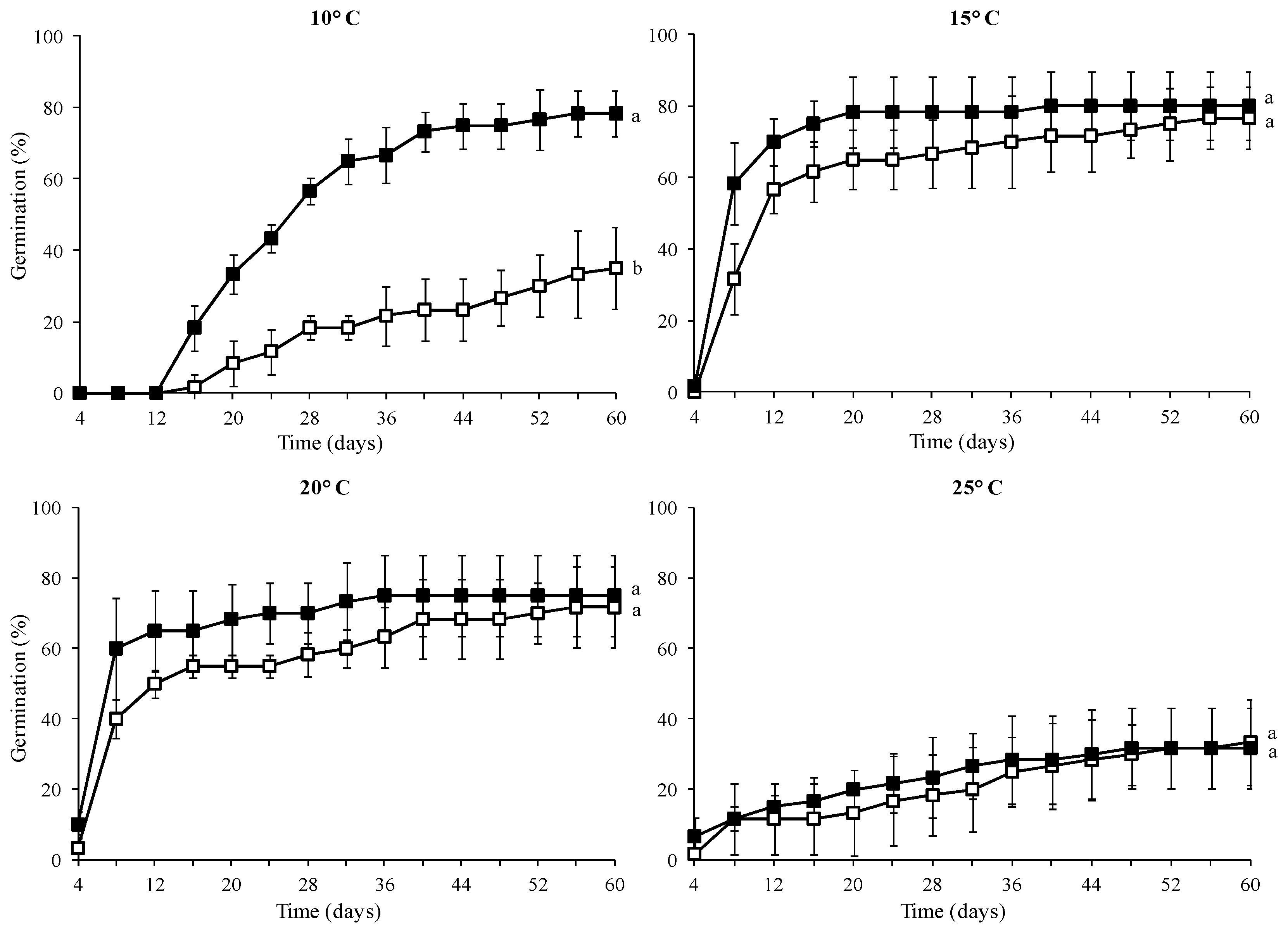
The control seeds (without GA3 treatment) showed a high germination percentage at 15 °C (77%) and 20 °C (72%), while at 10 °C and 25 °C, their germination was significantly lower (35% and 33%, respectively) (Table 2). The seeds treated with 1000 mg L−1 GA3 solution exhibited high germination percentage at 10, 15, and 20 °C (78, 80, and 75%, respectively) and low germination rate at 25 °C (31%).

Table 2.
Incubation temperature effect on germination of Campanula pangea seeds, including control (without treatment) or seeds treated with 1000 mg L−1 GA3. Means ± standard deviation values are given.
The seed treatment with GA3 promoted higher germination percentage and accelerated seed germination rate compared to the control at 10 °C (Figure 3). However, the incubation temperature of 25 °C seemed to notably reduce seed germination success in both the control and the 1000 mg L−1 GA3 treatment.
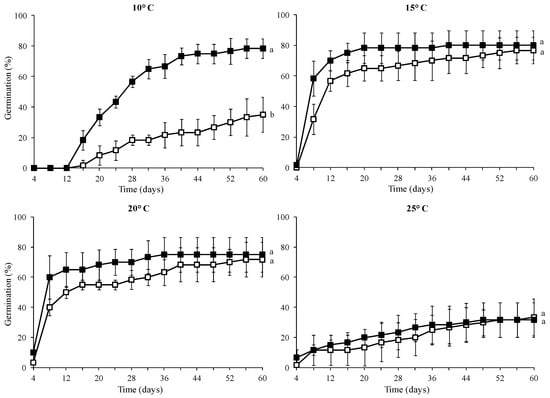
Figure 3.
Cumulative germination percentage diagrams at 10, 15, 20, and 25 °C of Campanula pangea seeds without pretreatment (□) or treated with 1000 mg L−1 GA3 (■). At each incubation temperature, means are statistically different at p < 0.05, when they do not share a common letter.
3.2. Bioclimatic Profiling
To better comprehend the environmental preferences of C. pangea, a bioclimatic profile was constructed using temperature and precipitation data of its natural habitat. The bioclimatic profile, illustrated in Figure 4, provides information about the species’ fundamental climatic requirements, which are further detailed below.
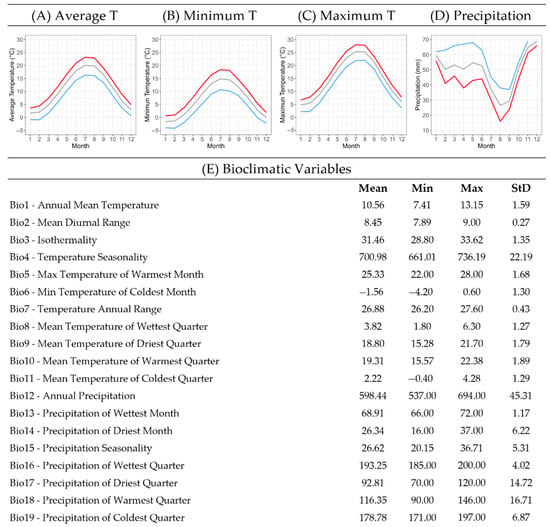
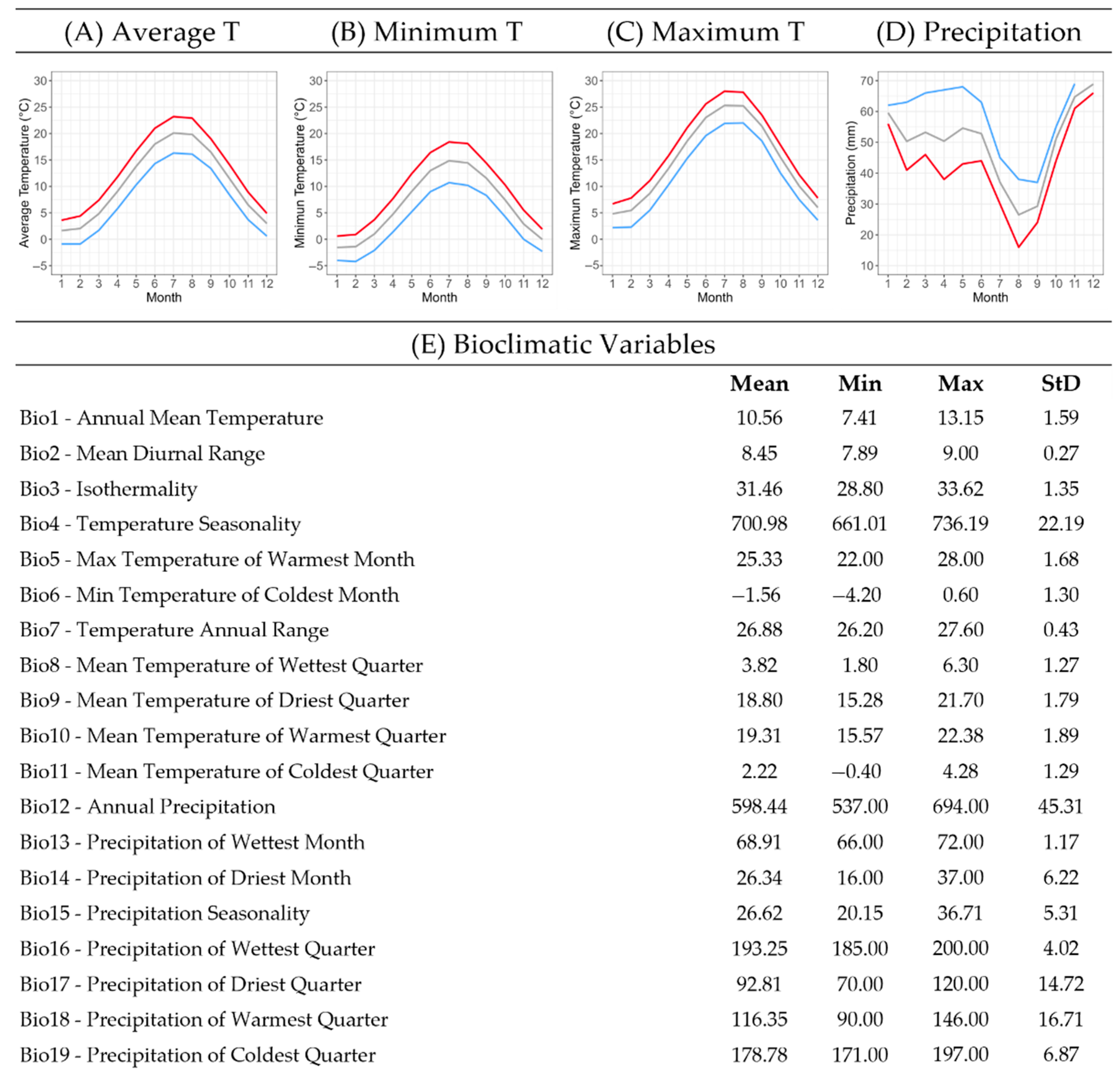
Figure 4.
Bioclimatic profile of the local endemic species Campanula pangea, which illustrates a summary of the climatic conditions at the 80 sites of its occurrence in Mt. Pangeo, NE Greece. Graphs of monthly data on (A) average, (B) minimum, and (C) maximum temperatures and (D) precipitation are included, as well as a table with mean, min, max, and std values of the 19 bioclimatic variables. Line graphs (A–C) show mean (grey), min (blue) and max (red) temperature values, and line graph (D) shows mean (grey), min (red), and max (blue) precipitation values. (E) Bioclimatic Variables.
The mean annual temperature (Bio1) across all recorded occurrence sites was 10.5 ± 1.6 °C. The species populations were found naturally adapted to a minimum temperature of −1.6 ± 1.3 °C in January and a maximum temperature of 25.3 ± 1.7 °C in July. The total annual precipitation at the occurrence sites averaged 598.4 ± 45.3 mm. The highest recorded monthly precipitation occurred in December, averaging 68.9 ± 1.2 mm, whereas the lowest (26.3 ± 6.2 mm) occurred in August (Figure 3). Notably, precipitation patterns exhibited significant variability among occurrence sites, with particularly high deviations observed in April and May.
4. Discussion
The scope of the current study was to determine the most favourable conditions for successful seed germination of the threatened local Greek endemic Campanula pangea. This knowledge provides a base for the development of a specific germination protocol for this species, facilitating its conservation efforts. The results of the germination experiment on seeds stored for four years at 3–5 °C of the threatened local endemic C. pangea indicated that temperature, GA3 treatment, and their interaction significantly influence their germination success. The seeds of C. pangea were shown to exhibit specific temperature requirements for germination, with an optimal range of 15–20 °C, where the stored seeds achieved maximum germination. Outside this temperature range (i.e., at 10 and 25 °C), the number of germinated seeds declined sharply.
Pre-treatment with GA3 was shown to expand the temperature range that promotes high germination percentages, suggesting that C. pangea seeds exhibit a type of dormancy. The temperature range for successful germination after treatment of seeds with 1000 mg L−1 of GA3 extended from 10 to 20 °C, though germination remained low at 25 °C. It has been reported that some seeds with non-deep physiological dormancy may germinate over a narrow range of temperatures, and upon dormancy release, they may have a broader temperature range for germination [13]. Exogenous treatment with GA3 can release dormancy, promoting seed germination. The above results indicate that the seeds of C. pangea may have non-deep physiological dormancy; however, the possibility of morphological dormancy (underdeveloped embryo) cannot be excluded since the size of the embryo was not examined. Seeds of many herbaceous matorral species are characterized by non-deep physiological dormancy [25]. The seeds that possess this type of dormancy can easily escape dormancy by dry storage at temperatures of 20 °C or higher (after the ripening process), by cold moist stratification, or GA3 treatment [45]. However, the effect of incubation temperature and seed treatments (after ripening, cold stratification, and GA3 application) on germination remains unknown for freshly collected mature seeds of C. pangea, and therefore, further experimentation is suggested to provide a deeper insight into seed dormancy type. The significant reduction in germination percentage at the higher (25 °C) examined temperature (33%) raises some concerns about the future adaptability of this species to climate change impact, and therefore, it should be further investigated. Apart from temperature, another factor that affects seed germination is light, especially in plants with small seeds like those of Campanulaceae. Undoubtedly, it would be useful to conduct further experiments to draw conclusions about the effect of light on seed germination of C. pangea.
The bioclimatic profile of C. pangea that was developed using temperature and precipitation data at the species occurrence sites provided valuable insights into the macro-climatic conditions of its wild habitats. The bioclimatic profile of the species revealed that its wild-growing populations are naturally adapted to experience cold, relatively dry winters and mild summers with moderate temperature and precipitation levels. By integrating the bioclimatic profile with the germination experiment results, which showed that the favourable temperatures for seed germination were 15–20 °C, it can be inferred that, most probably, a part of the seeds in the natural habitats will germinate in early autumn, after their dispersal, namely towards late September when the average monthly air temperature is around 15 °C and the increased rainfall during this period is able to create the ideal soil moisture conditions. The plants derived from seeds that germinated in autumn may be able to survive the low temperatures and snow in winter. The non-germinated seeds will be exposed to the low temperatures of winter and will probably germinate in springtime under similar conditions. It is known that the exposure of seeds to low temperatures of winter (cold stratification), like the GA3 treatment, may extend the germination temperature range [25]. Therefore, germination of C. pangea will probably take place in early spring (March–April) when the average monthly air temperature is around 10 °C combined with favourable moisture levels in the wild habitats.
The germination requirements of the threatened and Greek endemic species C. pangea, which belongs to Sect. Involucratae [35], differ from those of other previously studied Greek endemic Campanula species of Sect. Quinqueloculares, reflecting both distinct taxonomic positioning and different ecological adaptations. These differences can mainly be attributed, as the bioclimatic profile also suggested, to the species’ geographic distribution in the northern part of Greece. The optimal temperatures for the species’ germination appear relatively high, compared to other perennial threatened Greek endemic Campanula species of Southern Greece, such as the C. cretica [38], C. pelviformis Lam. [46], and C. saxatilis L. subsp. saxatilis [47]. It is known that in Mediterranean-type ecosystems, which are characterized by high seasonality (hot dry summers and cold wet winters), the optimum temperature for seed germination of lowland species is often relatively low (≤15 °C) [38,46,47]. This outlines an ecological adaptation of several Mediterranean species which ensures that germination takes place in late autumn when rainfall is sufficient, thus increasing the growing season’s length before the onset of summer drought. However, mountain species in the Mediterranean are exposed to different environmental conditions and therefore have different seed germination behaviour than lowland species. In the present study, higher optimal germination temperatures probably resulted from the species’ natural adaptation to a higher latitude. This is further supported by comparing the conclusions of the present study with those of other Campanula species growing at similar latitudes and high altitudes, which show germination temperature preferences that correspond with the favourable range of C. pangea [48]. Furthermore, other studies have also observed that the seeds of many high-mountain Mediterranean species may promptly germinate without treatment, reaching an optimal level at relatively high temperatures [49]. Compared to low altitudes, the growing season at high altitudes occurs later in the year, as winter environmental conditions are harsher, and summer conditions are milder. Therefore, to ensure successful germination and establishment, plants of higher altitudes tend to germinate at higher temperatures. Moreover, the range of optimal germination temperatures and the seed dormancy type are likely linked to the species’ extensive altitudinal range. Despite its restricted geographical distribution exclusively in a single mountain (single-mountain endemic), C. pangea was found at elevations ranging from 350 to 1700 m. These germination characteristics of the species have likely contributed to its broad distribution in different altitudinal zones of Mt. Pangeo.
Previous studies, which have included C. pangea in comparative germination experiments, have indicated that the optimal germination temperature for C. pangea seeds is 25 °C [34]; however, such results do not align with the outcomes of the present study. The current investigation showed that even after treatment with GA3, the germination success at 25 °C remained low, which is a cause of concern. One way to deal with such inconsistency is to consider the effect of genetic variation that may arise between subpopulations of the species in Mt. Pangeo, depending on the altitudinal range over which they are naturally distributed. Unfortunately, this cannot be further evaluated as site-specific collection data are lacking in the previous work [34]. If the case of random or methodological error is excluded, this variation in germination behaviour highlights the necessity of exploring the germination requirements in different subpopulations of C. pangea from Mt Pangeo, investigating potential intraspecific differences in germination characteristics. Furthermore, previous studies have shown that, in addition to temperature, light has a significant effect on seed germination of the species, with C. pangea seeds placed in dark conditions showing zero germination percentages [34]. However, in artificial environments, this significant effect of light on germination of Campanulaceae species tends to be replaced by treatment with GA3, which promotes even higher germination success [34]. Similar findings on the effect of GA3 on germination were observed in the present study.
The above findings seem to imply another important issue: that seed germination requirements are both species-specific and habitat-specific. Information on the germination behaviour of a plant species and on its optimal environmental conditions for germination allows for deeper understanding of the plant’s germination ecology. This knowledge is crucial for ex situ conservation efforts, such as seed banking and plant propagation, to facilitate population reinforcement. It also supports in situ conservation by providing insights that help assess the potential impacts of climate change on population status and distribution, guiding the implementation of appropriate protection measures. A holistic approach that integrates both in situ and ex situ conservation efforts is essential for protecting high-priority species, including the threatened and local endemic species C. pangea studied herein. The outcomes of the study herein can contribute to the design of a species-specific germination protocol, which is fundamental for implementing targeted conservation actions tailored to the species’ bioclimatic requirements or may allow potential ornamental applications and/or targeted medicinal research, since Campanula members have recently raised medicinal interest [50].
5. Conclusions
The investigation of the germination behaviour of stored seeds of the threatened Greek local endemic species C. pangea highlighted the suitable temperature conditions for its successful germination. The bioclimatic profile of C. pangea, built on all known distribution sites of the species, provided insight into the climatic conditions of its wild habitats, shaping its natural adaptations. This information enables the development of a species-specific germination protocol. The establishment of a specialized protocol for the germination of the species’ seeds serves as a valuable tool for both in situ and ex situ conservation. In situ, it is aimed to aid in predicting the species’ extinction risk in changing environmental conditions, while ex situ, it may facilitate the cultivation of individuals to further study their adaptive mechanisms under controlled anthropogenic conditions or to ensure the potential for reintroduction (if necessary), or may allow potential ornamental applications and/or targeted medicinal research.
Author Contributions
Conceptualization, N.K., E.P. and I.T.; methodology, A.M., M.P., E.P., S.K., S.H., N.K. and G.T.; software, M.P.; validation, A.M., I.T., S.H., N.K., G.T., S.K. and E.P.; formal analysis, M.P. and E.P.; investigation, M.P. and N.K.; resources, S.H., S.K., N.K. and G.T.; data curation, M.P. and E.P.; writing—original draft preparation, M.P., E.P. and N.K.; writing—review and editing, A.M., I.T., S.K., S.H., G.T., E.P. and N.K.; visualization, M.P. and E.P.; supervision, I.T.; project administration, N.K. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the results of this study are included in the manuscript and the datasets are available on request.
Acknowledgments
The authors would like to thank the anonymous reviewers for their constructive comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heywood, V.H.; Iriondo, J.M. Plant conservation: Old problems, new perspectives. Biol. Conserv. 2003, 113, 321–335. [Google Scholar] [CrossRef]
- Heywood, V.H. Conserving plants within and beyond protected areas—Still problematic and future uncertain. Plant Divers. 2019, 41, 36–49. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.S.; Cogoni, D.; Fournaraki, C.; Gian Pietro, G.d.G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; et al. A common approach to the conservation of threatened island vascular plants: First results in the mediterranean basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef]
- Krigas, N.; Papadimitriou, K.; Mazaris, D. GIS and ex situ plant conservation. In Applied Geographical Information Systems; Alam, B.M., Ed.; Intech Open: Rijeka, Croatia, 2012; pp. 153–174. ISBN 978-953-51-0824-5. [Google Scholar]
- Radomir, A.-M.; Stan, R.; Florea, A.; Ciobotea, C.-M.; Bănuță, F.M.; Negru, M.; Neblea, M.A.; Sumedrea, D.I. Overview of the success of in vitro culture for ex situ conservation and sustainable utilization of endemic and subendemic native plants of Romania. Sustainability 2023, 15, 2581. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Hawkes, J.G. Complementary conservation strategies. In Plant Genetic Conservation; Maxted, N., Ford-Lloyd, B.V., Hawkes, J.G., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2000; pp. 15–39. ISBN 978-0-412-63730-8. [Google Scholar]
- Liu, X.; Xiao, Y.; Ling, Y.; Liao, N.; Wang, R.; Wang, Y.; Liang, H.; Li, J.; Chen, F. Effects of seed biological characteristics and environmental factors on seed germination of the critically endangered species Hopea chinensis (Merr.) Hand.-Mazz. in China. Forests 2023, 14, 1975. [Google Scholar] [CrossRef]
- Qaderi, M.M. Environmental regulation of weed seed dormancy and germination. Seeds 2023, 2, 259–277. [Google Scholar] [CrossRef]
- Chen, F.; Liu, L.; Chen, F.; Jia, G. The ecological characteristics of seed germination and seedling establishment of Manglietia patungensis: Implication for species conservation. Am. J. Plant Sci. 2012, 3, 1455–1461. [Google Scholar] [CrossRef]
- Margreiter, V.; Pagitz, K.; Berg, C.; Schwager, P.; Erschbamer, B. Pros and cons of using a standard protocol to test germination of alpine species. Plant Ecol. 2020, 221, 1045–1067. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, J.H.; Lee, J.H.; Kim, G.M.; Lee, M.H.; Park, C.Y.; Kim, D.H.; Lee, D.H.; Kim, K.M.; Na, C.S. Dormancy-release and germination improvement of korean bellflower (Campanula takesimana Nakai), a rare and endemic plant native to the Korean peninsula. PLoS ONE 2023, 18, e0292280. [Google Scholar] [CrossRef]
- Bewley, J. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Gonzalez-Andujar, J.L. An overview of environmental cues that affect germination of nondormant seeds. Seeds 2022, 1, 146–151. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef]
- Dadlani, M.; Yadava, D.K. Seed Science and Technology: Biology, Production, Quality; Springer Nature: Singapore, 2023; ISBN 978-981-19-5888-5F. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Mayer, A.M.; Poljakoff-Mayber, A. The Germination of Seeds, 3rd ed.; Mayer, A.M., Poljakoff-Mayber, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1982; ISBN 0-08-028854-5. [Google Scholar]
- Haj Sghaier, A.; Tarnawa, Á.; Khaeim, H.; Kovács, G.P.; Gyuricza, C.; Kende, Z. The effects of temperature and water on the seed germination and seedling development of rapeseed (Brassica napus L.). Plants 2022, 11, 2819. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; ISBN 978-0-12-416677-6. [Google Scholar]
- Ma, H.-Y.; Zhao, D.-D.; Ning, Q.-R.; Wei, J.-P.; Li, Y.; Wang, M.-M.; Liu, X.-L.; Jiang, C.-J.; Liang, Z.-W. A multi-year beneficial effect of seed priming with gibberellic acid-3 (GA3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 2018, 8, 13214. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhang, H.; Yang, Y.; Zhao, Y.; Tang, K.; Liu, F. Effects of gibberellin pre-treatment on seed germination and seedling physiology characteristics in industrial hemp under drought stress condition. Life 2022, 12, 1907. [Google Scholar] [CrossRef]
- Lammers, T.G. World Checklist and Bibliography of Campanulaceae; Kew Publishing, Royal Botanic Gardens, Kew: Richmond, UK, 2007. [Google Scholar]
- Bramley, G.; Trias-Blasi, A.; Wilford, R. The Kew Temperate Plant Families Identification Handbook; Kew Publishing, Royal Botanic Gardens, Kew: Richmond, UK, 2023. [Google Scholar]
- Lammers, T.G. Campanulaceae: Campanulaceae Jussieu, Gen. Pl. 163 (1789), Nom. Cons. In Flowering Plants Eudicots; Kadereit, J.W., Jeffrey, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 26–56. ISBN 978-3-540-31051-8. [Google Scholar]
- Campanula L. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 23 May 2025).
- Seglie, L.; Scariot, V.; Larcher, F.; Devecchi, M.; Chiavazza, P.M. In vitro seed germination and seedling propagation in Campanula spp. Plant Biosyst. 2012, 146, 15–23. [Google Scholar] [CrossRef]
- Liveri, E.; Crowl, A.A.; Cellinese, N. Past, Present and future of Campanula (Campanulaceae) systematics—A review. Bot. Chron. 2019, 22, 209–222. [Google Scholar]
- Koutsovoulou, K.; Daws, M.I.; Thanos, C.A. Campanulaceae: A family with small seeds that require light for germination. Ann. Bot. 2014, 113, 135–143. [Google Scholar] [CrossRef]
- Hartvig, P. Campanula pangea, a new species of C. Sect. Involucratae from Mt Pangeon, NE Greece. Willdenowia 1998, 28, 65–68. [Google Scholar] [CrossRef][Green Version]
- Krigas, N.; Constantinidis, T. Campanula pangea Hartvig, Vulnerable (VU). In The Red Data Book of Rare and Threatened Plants of Greece; Hellenic Botanical Society: Patras, Greece, 2009; Volume 1 (A–D). [Google Scholar]
- Liveri, E.; Paradisioti, M.; Tsiripidis, I. Campanula pangea. Available online: https://www.iucnredlist.org/species/224625769/225911626 (accessed on 21 May 2025).
- Panagiotidou, T.-N.; Anestis, I.; Pipinis, E.; Kostas, S.; Tsoktouridis, G.; Hatzilazarou, S.; Krigas, N. GIS Bioclimatic profile and seed germination of the endangered and protected Cretan endemic plant Campanula cretica (A. DC.) D. Dietr. for conservation and sustainable utilization. Agriculture 2025, 15, 1161. [Google Scholar] [CrossRef]
- Paradisiotis, M. Conservation Status Assessment and Establishment of Monitoring Scheme for the Local Endemic Species Campanula pangea by Employing Individual-Based Functional Trait Measurements. Master’s Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2024. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.r-project.org/ (accessed on 10 May 2025).
- Posit Team RStudio: Integrated Development Environment for R. 2023. Available online: https://posit.co/downloads/ (accessed on 10 May 2025).
- Hijmans, R.J.; Barbosa, M.; Ghosh, A.; Mandel, A. Geodata: Download Geographic Data. 2024. Available online: https://CRAN.R-project.org/package=geodata (accessed on 10 May 2025).
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant graphics for data analysis (2nd Ed.). Meas-Interdiscip. Res. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of non-deep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Anestis, I.; Pipinis, E.; Kostas, S.; Papaioannou, E.; Karapatzak, E.; Dariotis, E.; Tsoulpha, P.; Koundourakis, E.; Chatzileontari, E.; Tsoktouridis, G.; et al. GIS-facilitated germination of stored seeds from five wild-growing populations of Campanula pelviformis Lam. and fertilization effects on growth, nutrients, phenol content and antioxidant potential. Horticulturae 2023, 9, 877. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Anestis, I.; Pipinis, E.; Kostas, S.; Avramakis, M.; Greveniotis, V.; Dariotis, E.; Tsoktrouridis, G.; Krigas, N. GIS-facilitated seed germination of six local endemic plants of Crete (Greece) and multifaceted evaluation in three economic sectors. J. Biol. Res. Thessalon. 2023, 30, 5. [Google Scholar] [CrossRef]
- Yücel, G.; Erken, K. Optimal germination methods, ornamental plant features, and ex situ conservation of endemic Campanula grandis Fisch & C.A. Mey. J. Environ. Eng. Landsc. Manag. 2023, 31, 132–141. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; Pérez-García, F. Seed germination of high mountain mediterranean species: Altitudinal, interpopulation and interannual variability. Ecol. Res. 2005, 20, 433–444. [Google Scholar] [CrossRef]
- Anestis, I.; Pipinis, E.; Karapatzak, E.; Kostas, S.; Menexes, G.; Dariotis, E.; Tsoktouridis, G.; Hatzilazarou, S.; Krigas, N. Effect of genotype and altitude on the germination of freshly collected seeds from wild-growing populations of Campanula pelviformis Lam. and Petromarula pinnata (L.) A. DC. (Campanulaceae). Horticulturae 2024, 10, 1149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).