Effect of Headstarting Eggstrands of the Endangered Houston Toad (Bufo = [Anaxyrus] houstonensis) from a Captive Assurance Colony on Native Breeding Pond Microbiomes
Abstract
1. Introduction
2. Materials and Methods
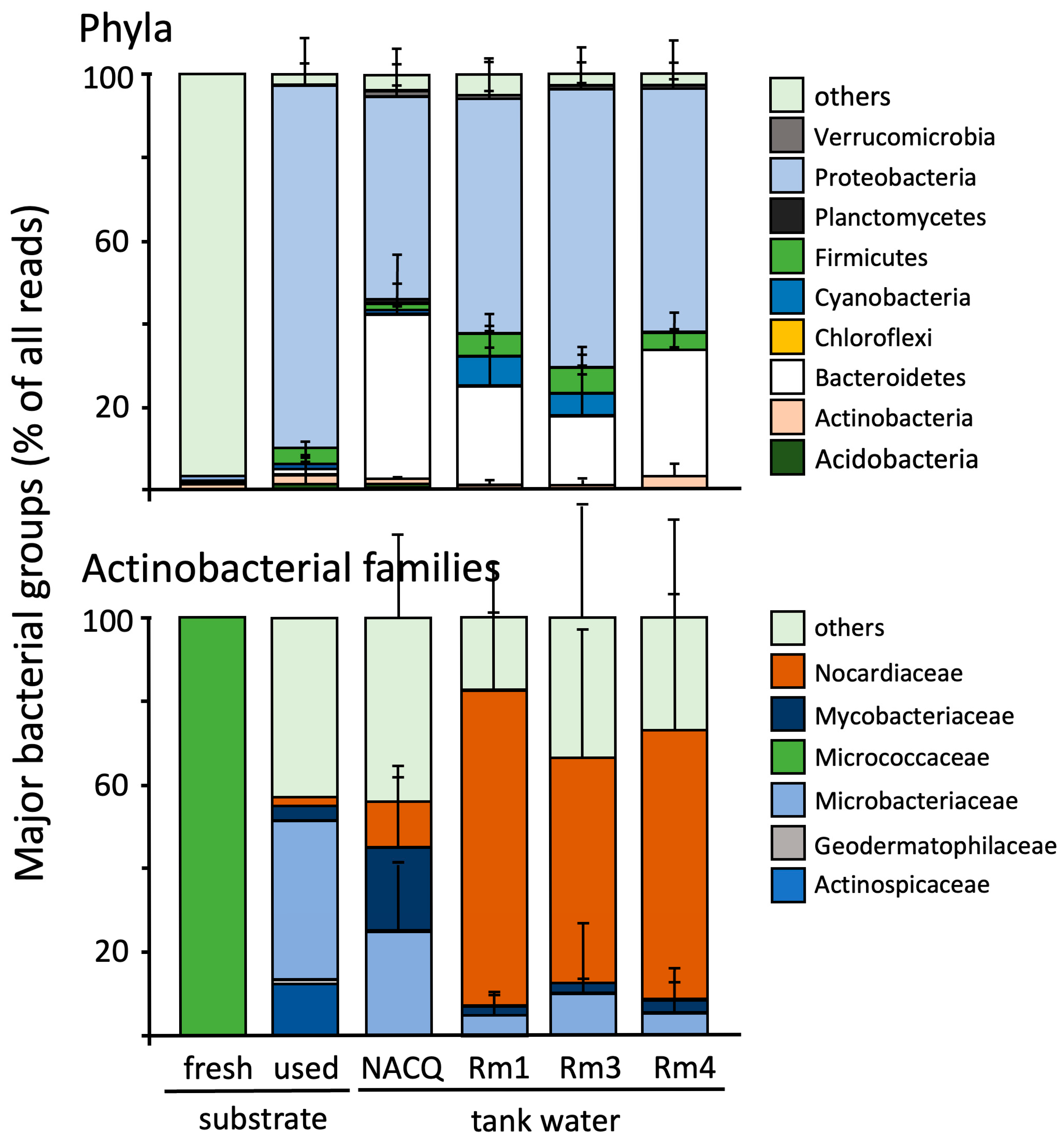
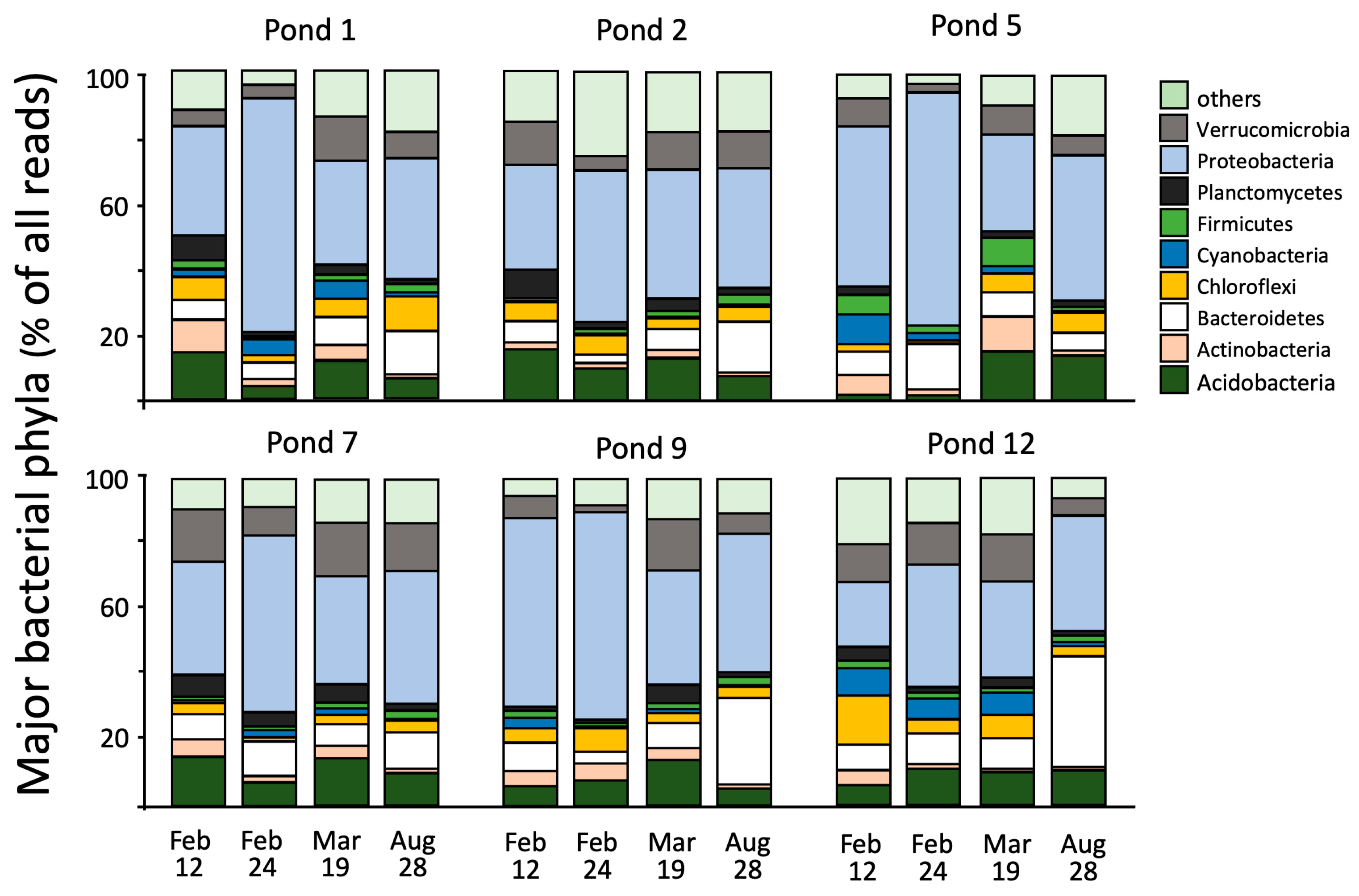
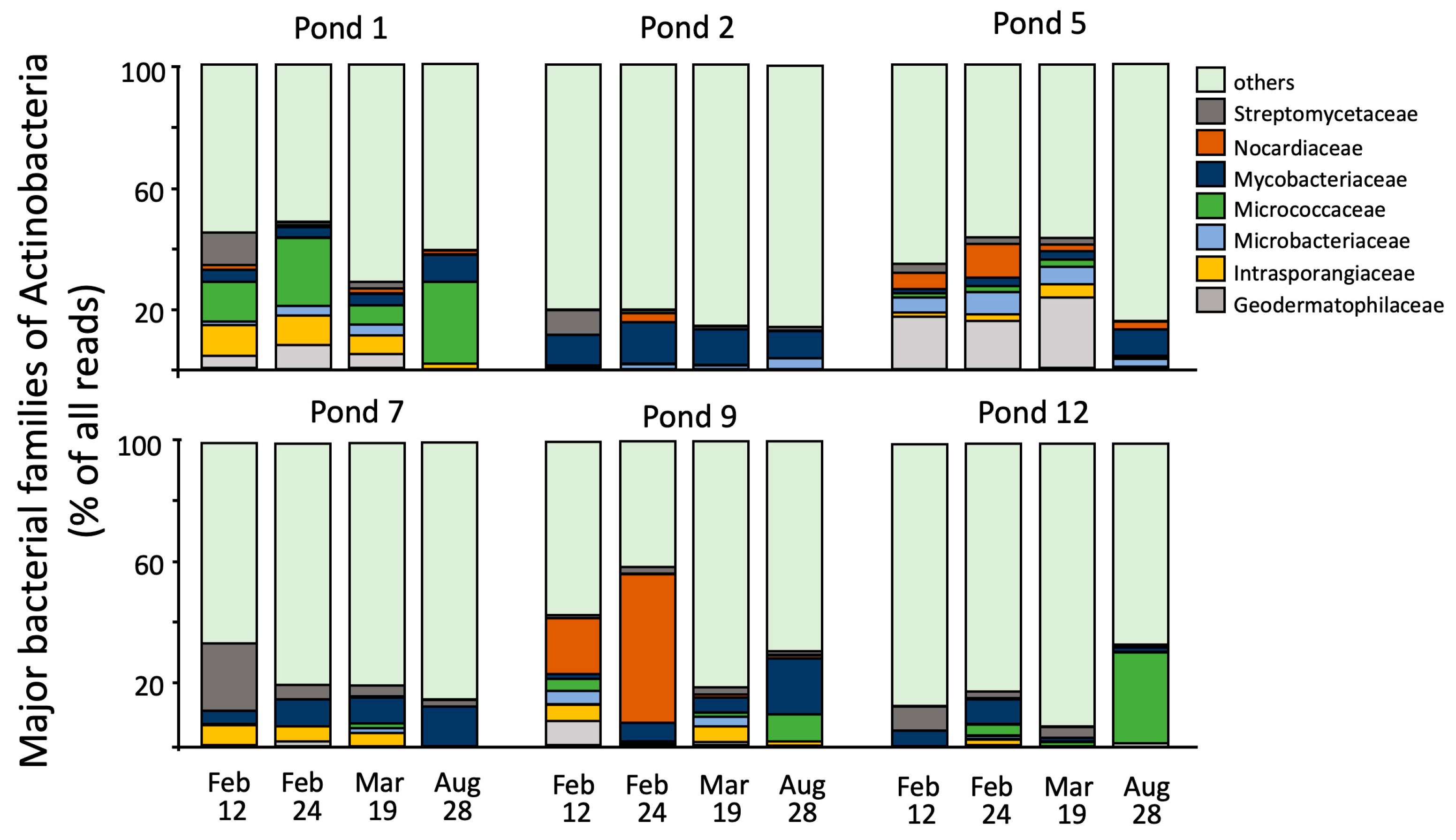
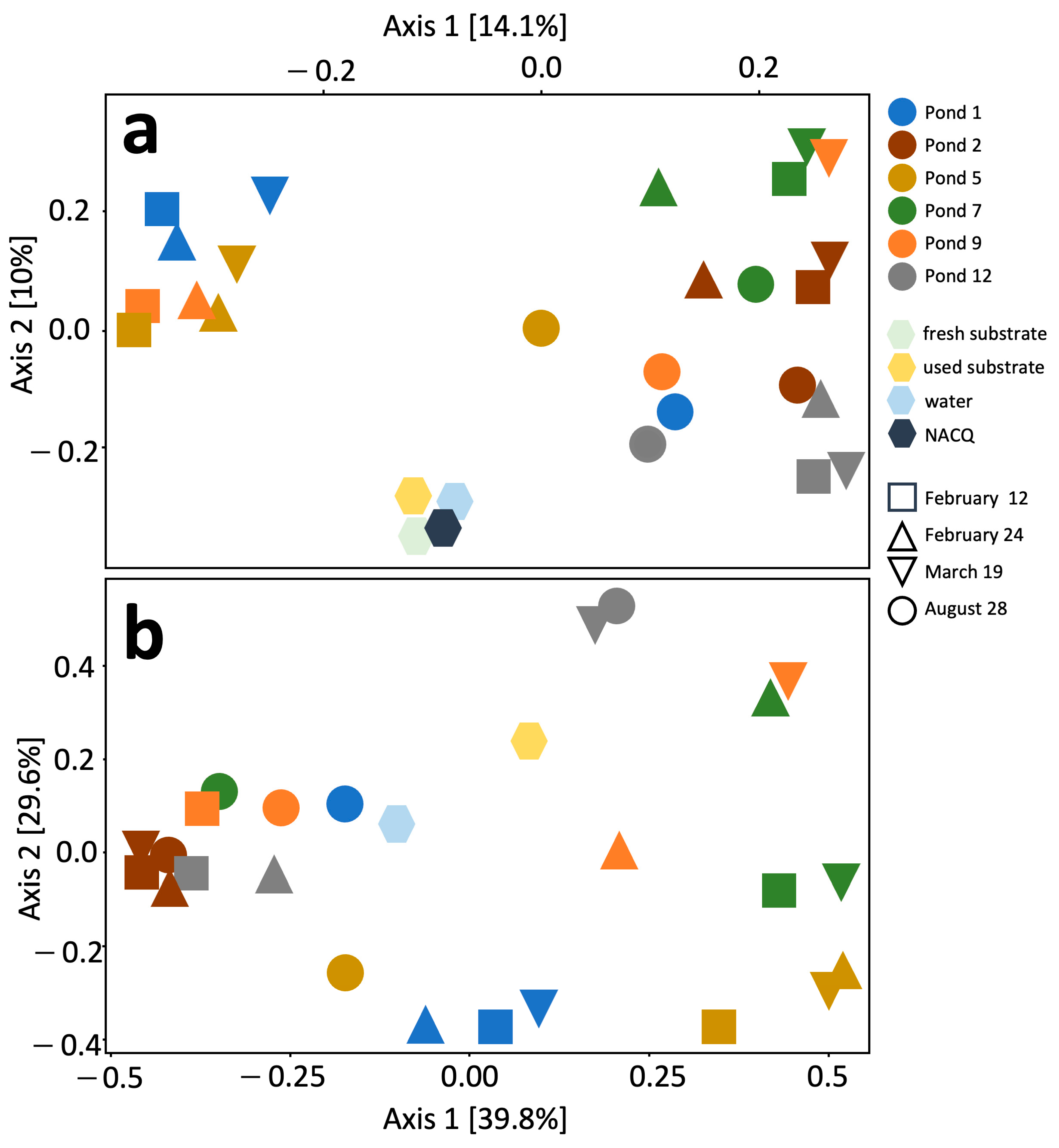
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaustein, A.R.; Urbina, J.; Snyder, P.W.; Reynolds, E.; Dang, T.; Hoverman, J.T.; Han, B.; Olson, D.H.; Searle, C.; Hambalek, N.M. Effects of emerging infectious diseases on amphibians: A review of experimental studies. Diversity 2018, 10, 81. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Blaustein, A.R.; Garcia, T.S.; Kats, L.B. Invasion complexities: The diverse impacts of nonnative species on amphibians. Copeia 2014, 4, 611–632. [Google Scholar] [CrossRef]
- Hermann, C.; Gilbreath, H. Revised recovery plan for the Houston toad (Anaxyrus [Bufo] houstonensis). In Species Profile for Houston Toad (Bufo houstonensis); U.S. Fish and Wildlife Service: Albuquerque, NM, USA, 2022; pp. 1–78. [Google Scholar]
- Tornabene, B.J.; Blaustein, A.R.; Briggs, C.J.; Calhoun, D.M.; Johnson, P.T.J.; McDevitt-Galles, T.; Rohr, J.R.; Hoverman, J.T. The influence of landscape and environmental factors on ranavirus epidemiology in a California amphibian assemblage. Freshw. Biol. 2018, 63, 639–651. [Google Scholar] [CrossRef]
- Klaus, J.M.; Noss, R.F. Specialist and generalist amphibians respond to wetland restoration treatments. J. Wildl. Manag. 2016, 80, 1106–1119. [Google Scholar] [CrossRef]
- Duarte, A.; Brown, D.J.; Forstner, M.R.J. Documenting extinction in real time: Decline of the Houston toad on a primary recovery site. J. Fish. Wildl. Manag. 2014, 5, 363–371. [Google Scholar] [CrossRef]
- Brannelly, L.A.; Sharma, P.; Wallace, D.K. Captive breeding in the endangered alpine tree frog, Litoria verreauxii alpina. Peerj 2023, 11, e15179. [Google Scholar] [CrossRef]
- Clulow, J.; Trudeau, V.L.; Kouba, A.J. Amphibian Declines in the Twenty-First Century: Why We Need Assisted Reproductive Technologies. Adv. Exp. Med. Biol. 2014, 753, 275–316. [Google Scholar] [CrossRef]
- Silla, A.J.; Byrne, P.G. The role of reproductive technologies in amphibian conservation breeding programs. Annu. Rev. Anim. Biosci. 2019, 7, 499–519. [Google Scholar] [CrossRef]
- Forstner, M.R.J.; Crump, P. Houston toad population supplementation in Texas, USA. In Global Re-Introduction Perspectives; Soorae, P.S., Ed.; IUCN/SSC Re-introduction Specialist Group & Abu Dhabi Environmental Agency: Gland, Switzerland, 2011; pp. 71–76. [Google Scholar]
- Fratzke, A.; Howard, L.L.; Tocidlowski, M.E.; Armien, A.; Oliveira, F.; Ritchie, B.; Berlin, E.; Snook, E. Chlamydia pneumoniae Polioencephalomyelitis and Ganglionitis in Captive Houston Toads (Anaxyrus houstonensis). Vet. Pathol. 2019, 56, 789–793. [Google Scholar] [CrossRef]
- Browne, R.K.; Luo, Q.; Wang, P.; Mansour, N.; Kaurova, S.A.; Gakhova, E.N.; Shishova, N.V.; Uteshev, V.K.; Kramarova, L.I.; Venu, G.; et al. Ecological civilisation and amphibian sustainability through reproduction biotechnologies, biobanking, and conservation breeding programs (RBCs). Animals 2024, 14, 1455. [Google Scholar] [CrossRef]
- Calatayud, N.E.; Jacobs, L.E.; Williams, C.L.; Steiner, C.C.; Shier, D.M. Recovering an endangered frog species through integrative reproductive technologies. Theriogenology 2022, 191, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Fonseca, L.D.; Afonso, A.M.; da Silva, M.G.; Saad, M.H.; Lilenbaum, W. A report of mycobacteriosis caused by Mycobacterium marinum in bullfrogs (Rana catesbeiana). Vet. J. 2006, 171, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Martinho, F.; Heatley, J.J. Amphibian mycobacteriosis. Vet. Clin. North. Am. Exot. Anim. Pr. 2012, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Morgado, J.M.; Gallagher, A.; Johnson, L.K. Mycobacterium gordonae infection in a colony of African clawed frogs (Xenopus tropicalis). Lab. Anim. 2009, 43, 300–303. [Google Scholar] [CrossRef]
- Pessier, A.P. An overview of amphibian skin disease. Semin. Avian Exot. Pet. 2002, 11, 162–174. [Google Scholar] [CrossRef]
- Vemulapally, S.; Villamizar, A.; Guerra, T.; Tocidlowski, M.E.; Spradley, M.; Mays, S.; Forstner, M.R.J.; Hahn, D. Mycobacteria in skin lesions and the habitat of the endangered Houston Toad (Anaxyrus houstonensis). J. Wildl. Dis. 2021, 57, 503–514. [Google Scholar] [CrossRef]
- Samant, S.; Sha, Q.; Iyer, A.; Dhabekar, P.; Hahn, D. Quantification of Frankia in soils using SYBR Green based qPCR. Syst. Appl. Microbiol. 2012, 35, 191–197. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Jansson, J.K.; Knight, R. The Earth Microbiome project: Successes and aspirations. BMC Biol. 2014, 12, 69. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; p. 571. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.R.; Lipman, D.J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 1988, 85, 2444–2448. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-joining Method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Am. Math. Soc. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Felsenstein, J. Confidence limits of phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Schulzerobbecke, R. Mycobacteria in the Environment. Immun. Infekt. 1993, 21, 126–131. [Google Scholar]
- Schulzerobbecke, R.; Janning, B.; Fischeder, R. Occurrence of Mycobacteria in biofilm samples. Tuber. Lung Dis. 1992, 73, 141–144. [Google Scholar] [CrossRef]
- Dovriki, E.; Gerogianni, I.; Petinaki, E.; Hadjichristodoulou, C.; Papaioannou, A.; Gourgoulianis, K. Isolation and identification of nontuberculous mycobacteria from hospitalized patients and drinking water samples-examination of their correlation by chemometrics. Environ. Monit. Assess. 2016, 188, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Modra, H.; Bartos, M.; Hribova, P.; Ulmann, V.; Hubelova, D.; Konecny, O.; Gersl, M.; Kudelka, J.; Voros, D.; Pavlik, I. Detection of mycobacteria in the environment of the Moravian Karst (Bull Rock Cave and the relevant water catchment area): The impact of water sediment, earthworm castings and bat guano. Vet. Med Czech 2017, 62, 153–168. [Google Scholar] [CrossRef]
- Peeters, C.; Depoorter, E.; Praet, J.; Vandamme, P. Extensive cultivation of soil and water samples yields various pathogens in patients with cystic fibrosis but not Burkholderia multivorans. J. Cyst. Fibros. 2016, 15, 769–775. [Google Scholar] [CrossRef][Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Fischeder, R.; Schulzerobbecke, R.; Weber, A. Occurrence of Mycobacteria in drinking-water samples. Zbl Hyg. Umweltmed. 1991, 192, 154–158. [Google Scholar]
- Hruska, K.; Kaevska, M. Mycobacteria in water, soil, plants and air: A review. Vet. Med. Czech 2012, 57, 623–679. [Google Scholar] [CrossRef]
- Roguet, A.; Therial, C.; Saad, M.; Boudahmane, L.; Moulin, L.; Lucas, F.S. High mycobacterial diversity in recreational lakes. Antonie Leeuwenhoek 2016, 109, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Villamizar, A.; Vemulapally, S.; Guerra, T.; Tocidlowski, M.E.; Forstner, M.R.J.; Hahn, D. Quantification of members of the Mycobacterium chelonae-abscessus complex in lesions of the endangered Houston toad (Anaxyrus houstonensis). Syst. Appl. Microbiol. 2022, 45, 126342. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection sources of a common non-tuberculous mycobacterial pathogen, Mycobacterium avium complex. Front Med. (Lausanne) 2017, 4, 27. [Google Scholar] [CrossRef]
- Feazel, L.M.; Baumgartner, L.K.; Peterson, K.L.; Frank, D.N.; Harris, J.K.; Pace, N.R. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. USA 2009, 106, 16393–16399. [Google Scholar] [CrossRef]
- Gomez-Smith, C.K.; LaPara, T.M.; Hozalski, R.M. Sulfate reducing bacteria and Mycobacteria dominate the biofilm communities in a chloraminated drinking water distribution system. Environ. Sci. Technol. 2015, 49, 8432–8440. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hu, G.; Qiu, L.; Meng, S.; Wu, W.; Zheng, Y.; Song, C.; Li, D.; Chen, J. Variations in bacterioplankton communities in aquaculture ponds and the influencing factors during the peak period of culture. Environ. Pollut. 2020, 258, 113656. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.Y.; Ham, J.H. Comparative analysis of the microbial community in the sediments of two constructed wetlands differentially influenced by the concentrated poultry feeding operations. J. Soils Sediments 2017, 17, 557–566. [Google Scholar] [CrossRef]
- Mania, I.; Gorra, R.; Colombo, N.; Freppaz, M.; Martin, M.; Anesio, A.M. Prokaryotic diversity and distribution in different habitats of an alpine rock glacier-pond system. Microb. Ecol. 2019, 78, 70–84. [Google Scholar] [CrossRef]
- Barrows, M.; Koeppel, K.; Michel, A.; Mitchell, E. Mycobacterial arthritis and synovitis in Painted reed frogs (Hyperolius marmoratus). J. Comp. Pathol. 2017, 156, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Green, S.L.; Lifland, B.D.; Bouley, D.M.; Brown, B.A.; Wallace, R.J.; Ferrell, J.E. Disease attributed to Mycobacterium chelonae in South African clawed frogs (Xenopus laevis). Comp. Med. 2000, 50, 675–679. [Google Scholar]
- Haridy, M.; Tachikawal, Y.; Yoshida, S.; Tsuyuguchi, K.; Tomita, M.; Maeda, S.; Wada, T.; Ibi, K.; Sakai, H.; Yanai, T. Mycobacterium marinum infection in Japanese forest green tree frogs (Rhacophorus arboreus). J. Comp. Pathol. 2014, 151, 277–289. [Google Scholar] [CrossRef]
- Gcebe, N.; Michel, A.L.; Hlokwe, T.M. Non-tuberculous Mycobacterium species causing mycobacteriosis in farmed aquatic animals of South Africa. Bmc Microbiol. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.; Yang, C.; An, J.; Qin, J.; Song, F.; Zeng, W. Iridovirus Infection in Chinese Giant Salamanders, China, 2010. Emerg. Infect. Dis. 2011, 17, 2388–2389. [Google Scholar] [CrossRef]
- Chen, Z.; Gui, J.; Gao, X.; Pei, C.; Hong, Y.; Zhang, Q. Genome architecture changes and major gene variations of Andrias davidianus ranavirus (ADRV). Vet Res. 2013, 44, 101. [Google Scholar] [CrossRef]
- Lisachova, L.S.; Lisachov, A.P.; Ermakov, O.A.; Svinin, A.O.; Chernigova, P.I.; Lyapkov, S.M.; Zamaletdinov, R.I.; Pavlov, A.V.; Zaks, S.S.; Fayzulin, A.I.; et al. Continent-Wide Distribution of CMTV-Like Ranavirus, from the Urals to the Atlantic Ocean. Ecohealth, 2025; 1–12, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamizar, A.; Vemulapally, S.; Guerra, T.; Tocidlowski, M.E.; Forstner, M.R.J.; Hahn, D. Effect of Headstarting Eggstrands of the Endangered Houston Toad (Bufo = [Anaxyrus] houstonensis) from a Captive Assurance Colony on Native Breeding Pond Microbiomes. Conservation 2025, 5, 25. https://doi.org/10.3390/conservation5020025
Villamizar A, Vemulapally S, Guerra T, Tocidlowski ME, Forstner MRJ, Hahn D. Effect of Headstarting Eggstrands of the Endangered Houston Toad (Bufo = [Anaxyrus] houstonensis) from a Captive Assurance Colony on Native Breeding Pond Microbiomes. Conservation. 2025; 5(2):25. https://doi.org/10.3390/conservation5020025
Chicago/Turabian StyleVillamizar, Andrea, Spandana Vemulapally, Trina Guerra, Maryanne E. Tocidlowski, Michael R. J. Forstner, and Dittmar Hahn. 2025. "Effect of Headstarting Eggstrands of the Endangered Houston Toad (Bufo = [Anaxyrus] houstonensis) from a Captive Assurance Colony on Native Breeding Pond Microbiomes" Conservation 5, no. 2: 25. https://doi.org/10.3390/conservation5020025
APA StyleVillamizar, A., Vemulapally, S., Guerra, T., Tocidlowski, M. E., Forstner, M. R. J., & Hahn, D. (2025). Effect of Headstarting Eggstrands of the Endangered Houston Toad (Bufo = [Anaxyrus] houstonensis) from a Captive Assurance Colony on Native Breeding Pond Microbiomes. Conservation, 5(2), 25. https://doi.org/10.3390/conservation5020025





