Implementation and Costs of an Agroforestry System in a Degraded Area of the Brazilian Semi-Arid Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Implantation Process and Costs
2.2. Characterization of the Study Area
2.3. Phytosociological Analysis
3. Results and Discussion
3.1. Implementation and Maintenance Cost
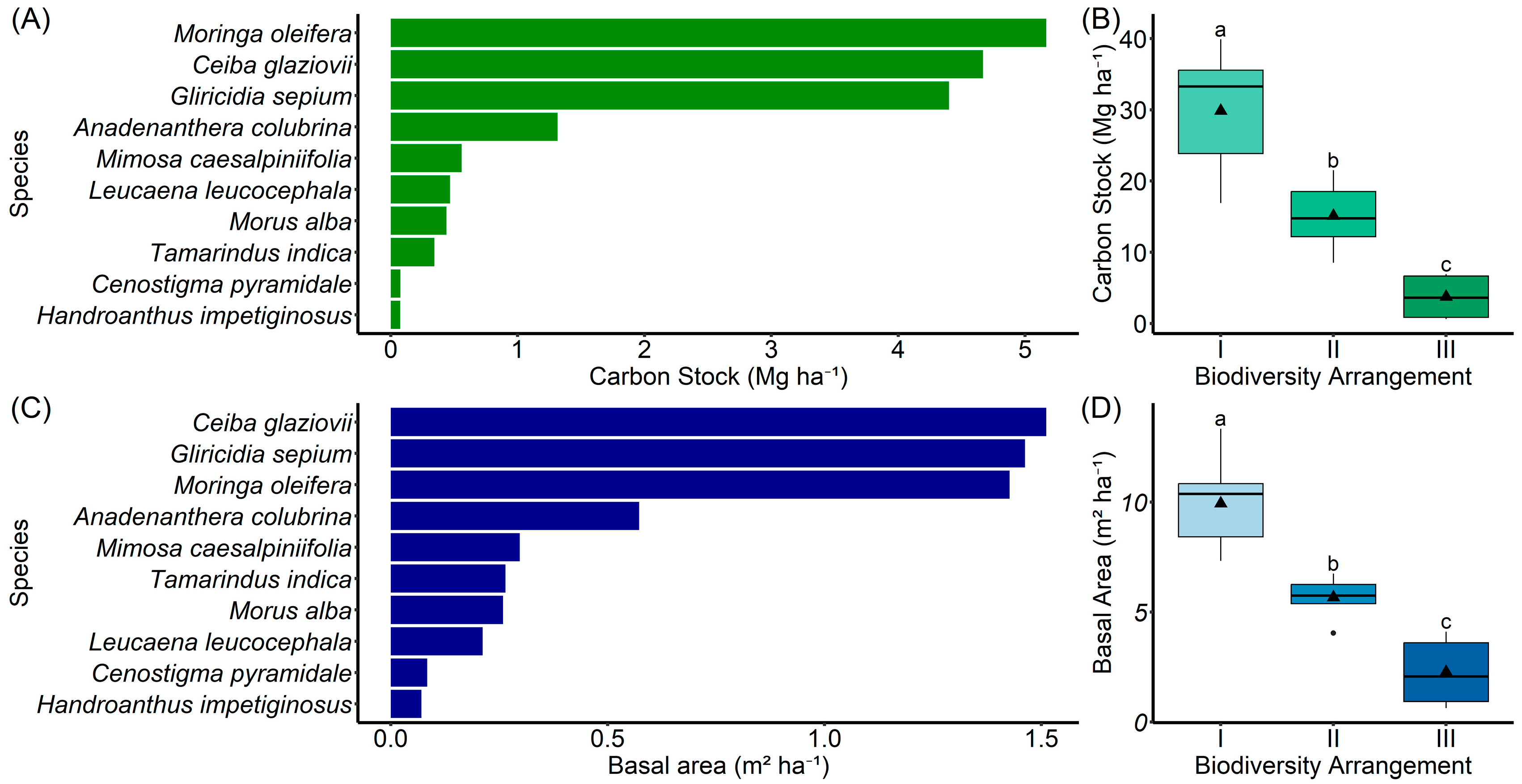
3.2. Species Composition, Diversity, and Structural Dominance in the Agroforestry System
3.3. Aboveground Carbon Stock, Basal Area, and Carbon Credit Potential in the Agroforestry System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, J.M.C.; Barbosa, L.C.F.; Leal, I.R.; Tabarelli, M. The caatinga: Urderstanding the challenges. In Caatinga: The Largest Tropical Dry Forest Region in South America; Silva, J.M.C., Leal, I.R., Tabarelli, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–19. [Google Scholar]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the Global Aridity Index and Potential Evapotranspiration Database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.M.; Aquino, D.N.; Chaves, L.C.G.; Lopes, F.B. Water as capital and its uses in the Caatinga. In Caatinga: The Largest Tropical Dry Forest Region in South America; Silva, J.M.C., Leal, I.R., Tabarelli, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 281–302. [Google Scholar]
- Teixeira, L.P.; Lughadha, E.N.; Silva MVCda Moro, M.F. How much of the Caatinga is legally protected? An analysis of temporal and geographical coverage of protected areas in the Brazilian semiarid region. Acta Bot. Bras. 2021, 35, 473–485. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Venticinque, E.M.; Lion, M.B.; Pinto, M.P. The Brazilian Caatinga protected areas: An extremely unbalanced conservation system. Environ. Conserv. 2021, 48, 287–294. [Google Scholar] [CrossRef]
- Antongiovanni, M.; Venticinque, E.M.; Fonseca, C.R. Fragmentation patterns of the Caatinga drylands. Landsc. Ecol. 2018, 33, 1353–1367. [Google Scholar] [CrossRef]
- Antongiovanni, M.; Venticinque, E.M.; Matsumoto, M.; Fonseca, C.R. Chronic anthropogenic disturbance on Caatinga dry forest fragments. J. Appl. Ecol. 2020, 57, 2064–2074. [Google Scholar] [CrossRef]
- Nelson, A.; Chomitz, K.M. Effectiveness of strict vs. multiple use protected areas in reducing Tropical Forest fires: A global analysis using matching methods. PLoS ONE 2011, 6, e22722. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A refuge for tropical biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lapola, D.M.; Martinelli, L.A.; Peres, C.A.; Ometto, J.P.; Ferreira, M.E.; Nobre, C.A.; Aguiar, A.P.D.; Bustamante, M.M.; Cardoso, M.F.; Costa, M.H.; et al. Pervasive transition of the Brazilian land-use system. Nat. Clim. Change 2014, 4, 27–35. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Agroecology and the reconstruction of a post-COVID-19 agriculture. J. Peasant. Stud. 2020, 47, 881–898. [Google Scholar] [CrossRef]
- Melo, F.P.L. The socio-ecology of the Caatinga: Understanding how natural resource use shapes an ecosystem. In Caatinga: The Largest Tropical Dry Forest Region in South America; Silva, J.M.C., Leal, I.R., Tabarelli, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 369–382. [Google Scholar]
- Schulz, C.; Koch, R.; Cierjacks, A.; Kleinschmit, B. Land change and loss of landscape diversity at the Caatinga phytogeographical domain—Analysis of pattern-process relationships with MODIS land cover products (2001–2012). J. Arid Environ. 2017, 136, 54–74. [Google Scholar] [CrossRef]
- Miller, P.M.; Kauffman, J.B. Effects of slash and burn agriculture on species abundance and composition of a tropical deciduous forest. Ecol. Manag. 1998, 103, 191–201. [Google Scholar] [CrossRef]
- Mamede Mde, A.; de Araújo, F.S. Effects of slash and burn practices on a soil seed bank of caatinga vegetation in Northeastern Brazil. J. Arid Environ. 2008, 72, 458–470. [Google Scholar] [CrossRef]
- Leal, L.C.; Andersen, A.N.; Leal, I.R. Anthropogenic disturbance reduces seed-dispersal services for myrmecochorous plants in the Brazilian Caatinga. Oecologia 2014, 174, 173–181. [Google Scholar] [CrossRef]
- Ribeiro, E.M.S.; Santos, B.A.; Arroyo-Rodríguez, V.; Tabarelli, M.; Souza, G.; Leal, I.R. Phylogenetic impoverishment of plant communities following chronic human disturbances in the Brazilian Caatinga. Ecology 2016, 97, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- FAO. HLPE Agroecological and Other Innovative Approaches for Sustainable Agriculture and Food Systems that Enhance Food Security and Nutrition; FAO: Rome, Italy, 2019. [Google Scholar]
- Mottet, A.; Bicksler, A.; Lucantoni, D.; De Rosa, F.; Scherf, B.; Scopel, E.; López-Ridaura, S.; Gemmil-Herren, B.; Bezner Kerr, R.; Sourisseau, J.M.; et al. Assessing transitions to sustainable agricultural and food systems: A tool for agroecology performance evaluation (TAPE). Front. Sustain. Food Syst. 2020, 4, 579154. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology: The science of natural resource management for poor farmers in marginal environments. Agric. Ecosyst. Environ. 2002, 93, 1–24. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for Ecosystem Services and Environmental Benefits: An Overview. Agroforest. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Cana Verde, B.S.; Brandão, H.C.R.S.; Souza, J.C.D.; Silva, D.M.L.D. Influence of agroforestry systems on phosphorus export from small tropical watersheds. Agrofor. Syst. 2023, 97, 785–798. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Sacramento, J.A.A.S.D.; Araújo, A.C.D.M.; Escobar, M.E.O.; Xavier, F.A.D.S.; Cavalcante, A.C.R.; Oliveira, T.S.D. Soil carbon and nitrogen stocks in traditional agricultural and agroforestry systems in the semiarid region of Brazil. Rev. Bras. Cienc. Solo 2013, 37, 784–795. [Google Scholar] [CrossRef]
- Gliessman, S.R. Agroecology: The Ecology of Sustainable Food Systems, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Menezes, R.S.C.; Salcedo, I.H.; Elliott, E.T. Microclimate and nutrient dynamics in a silvopastoral system of semiarid northeastern Brazil. Agrofor. Syst. 2002, 56, 27–38. [Google Scholar] [CrossRef]
- Bishaw, B.; Soolanayakanahally, R.; Karki, U.; Hagan, E. Agroforestry for sustainable production and resilient landscapes. Agrofor. Syst. 2022, 96, 447–451. [Google Scholar] [CrossRef]
- Sarto, M.V.; Borges, W.L.; Bassegio, D.; Nunes, M.R.; Rice, C.W.; Rosolem, C.A. Deep soil water content and forage production in a Tropical Agroforestry System. Agriculture 2022, 12, 359. [Google Scholar] [CrossRef]
- Fagundes, M.; Weisser, W.; Ganade, G. The role of nurse successional stages on species-specific facilitation in drylands: Nurse traits and facilitation skills. Ecol. Evol. 2018, 8, 5173–5184. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Navarro, A.; Verdú, M.; Querejeta, J.I.; Valiente-Banuet, A. Nurse plants transfer more nitrogen to distantly related species. Ecology 2017, 98, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Tonucci, R.G.; Vogado, R.F.; Silva, R.D.; Pompeu, R.C.F.F.; Oda-Souza, M.; Souza, H.A.D. Agroforestry system improves soil carbon and nitrogen stocks in depth after land-use changes in the Brazilian semi-arid region. Rev. Bras. Cienc. Solo 2023, 47, e0220124. [Google Scholar] [CrossRef]
- Vaarst, M.; Escudero, A.G.; Chappell, M.J.; Brinkley, C.; Nijbroek, R.; Arraes, N.A.; Andreasen, L.; Gattinger, A.; De Almeida, G.F.; Bossio, D.; et al. Exploring the concept of agroecological food systems in a city-region context. Agroecol. Sustain. Food Syst. 2018, 42, 686–711. [Google Scholar] [CrossRef]
- Woodcock, J.; Edwards, P.; Tonne, C.; Armstrong, B.G.; Ashiru, O.; Banister, D.; Beevers, S.; Chalabi, Z.; Chowdhury, Z.; Cohen, A.; et al. Public health benefits of strategies to reduce greenhouse-gas emissions: Urban land transport. Lancet 2009, 374, 1930–1943. [Google Scholar] [CrossRef]
- Lucena, R.J.D.; Lima, J.R.D.; Bakke, I.A. Dynamic organization of two agroforestry systems in the semi-arid region of Paraíba and their contribution to improving the socio-economic conditions of farming families. Ciência Rural 2023, 53, e20200512. [Google Scholar] [CrossRef]
- Victório CP, Silva MGCF da. Urban and community agroforestry: An experience in Campo Grande, West Zone of Rio de Janeiro. InterEspaço 2020, 6, e202034. [Google Scholar] [CrossRef]
- Brancalion, P.H.; Meli, P.; Tymus, J.R.; Lenti, F.E.; Benini, R.M.; Silva, A.P.M.; Isernhagen, I.; Holl, K.D. What makes ecosystem restoration expensive? A systematic cost assessment of projects in Brazil. Biol. Conserv. 2019, 240, 108274. [Google Scholar] [CrossRef]
- Kimball, S.; Lulow, M.; Sorenson, Q.; Balazs, K.; Fang, Y.C.; Davis, S.J.; O’Connell, M.; Huxman, T.E. Cost-effective ecological restoration. Restor. Ecol. 2015, 23, 800–810. [Google Scholar] [CrossRef]
- Araújo, J.B.C.N.; Souza, A.N.; Joaquim, M.S.; Mattos, L.M.; Lustosa Junior, I.M. Use of the activity-based costing methodology (ABC) in the cost analysis of successional agroforestry systems. Agrofor. Syst. 2020, 94, 71–80. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Marques, M.C.M. How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic forest. Ecol. Manag. 2014, 329, 1–9. [Google Scholar] [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Levine, J.M.; Loreau, M.; Hector, A. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecol. Lett. 2013, 16, 116–127. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Isbell, F.; Arce-Plata, M.I.; Di Marco, M.; Harfoot, M.; Johnson, J.; Lerman, S.B.; Miller, B.W.; Morelli, T.L.; Mori, A.S.; et al. Biodiversity loss reduces global terrestrial carbon storage. Nat. Commun. 2024, 15, 4354. [Google Scholar] [CrossRef]
- Raupp, P.P.; Ferreira, M.C.; Alves, M.; Campos-Filho, E.M.; Sartorelli, P.A.R.; Consolaro, H.N.; Vieira, D.L.M. Direct seeding reduces the costs of tree planting for forest and savanna restoration. Ecol. Eng. 2020, 148, 105788. [Google Scholar] [CrossRef]
- Pérez, D.R.; González, F.; Ceballos, C.; Oneto, M.E.; Aronson, J. Direct seeding and outplantings in drylands of Argentinean Patagonia: Estimated costs, and prospects for large-scale restoration and rehabilitation. Restor. Ecol. 2019, 27, 1105–1116. [Google Scholar] [CrossRef]
- Nowak, D.J. Understanding i-Tree: 2021 Summary of Programs and Methods; USDA Forest Service: Madison, WI, USA, 2021. [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. Vol. 2, 4th ed.; Instituto Plantarum: Nova Odessa, Brazil, 2013. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. Vol. 1, 6th ed.; Instituto Plantarum de Estudos da Flora: São Paulo, Brazil, 2014. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. Vol. 3, 1st ed.; Instituto Plantarum: Nova Odessa, Brazil, 2009. [Google Scholar]
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras. Vol. 2; Embrapa Informação Tecnológica: Colombo, Brazil, 2006. [Google Scholar]
- Miccolis, A.; Peneireiro, F.M.; Vieira, D.L.M.; Marques, H.R.; Hoffmann, M.R.M. Restoration through agroforestry: Options for reconciling livelihoods with conservation in the Cerrado and Caatinga biomes in Brazil. Exp. Agric. 2019, 55, 208–225. [Google Scholar] [CrossRef]
- IBGE. Censo Brasileiro de 2022 2023. Available online: https://www.ibge.gov.br/estatisticas/downloads-estatisticas.html (accessed on 7 February 2024).
- Ab’Sáber, A. Os Domínios de Natureza no Brasil: Potencialidades Paisagísticas; Ateliê Editorial: São Paulo, Brazil, 2003. [Google Scholar]
- De Oliveira, J.R.F. O Clima Urbano em Cidade de Pequeno Porte no Semiárido Cearense: O Caso de Crateús. Ph.D Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2020. [Google Scholar]
- de Sousa Monte, A.M. A planície fluvial do rio Poti nos municípios de Novo Oriente e Crateús-CE: Os barramentos e suas repercussões socioambientais. Rev. GeoUECE 2021, 3, 332–335. [Google Scholar]
- Costa, F.G. Geologia e Recursos Minerais da Folha Crateús—SB.24-V-C-III: Estados do Ceará e Piauí. Escala 1:100.000; CPRM: Fortaleza, Brazil, 2017. [Google Scholar]
- IPECE. Perfil Municipal 2017—Crateús; IPECE: Fortaleza, Brazil, 2018. [Google Scholar]
- Melo, E.T.; Sales, M.C.L.; de Oliveira, J.G.B. Aplicação do índice de vegetação por diferença normalizada (NDVI) para análise da degradação ambiental da microbacia hidrográfica do riacho dos Cavalos, Crateús-CE. Raega-O Espaço Geográfico Análise 2011, 23, 520–533. [Google Scholar] [CrossRef]
- Moro, M.F.; Martins, F.R. Métodos de levantamento do componente arbóreo-arbustivo. In Fitossociologia no Brasil: Métodos e Estudos de Caso; Felfili, J.M., Eisenlohr, P.V., Melo, M.M.d.R.F., Andrade, L.A., Meira Neto, J.A.A., Eds.; Editora UFV: Viçosa, Brazil, 2013; pp. 174–212. [Google Scholar]
- Scolforo, H.F.; Scolforo, J.R.S.; Mello, C.R.; Mello, J.M.; Ferraz Filho, A.C. Spatial distribution of aboveground carbon stock of the arboreal vegetation in Brazilian Biomes of Savanna, Atlantic Forest and Semi-Arid Woodland. PLoS ONE 2015, 10, e0128781. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Schuler, H.R.; Alarcon, G.G.; Joner, F.; dos Santos, K.L.; Siminski, A.; Siddique, I. Ecosystem Services from Ecological Agroforestry in Brazil: A Systematic Map of Scientific Evidence. Land 2022, 11, 83. [Google Scholar] [CrossRef]
- Tremblay, S.; Lucotte, M.; Revéret, J.P.; Davidson, R.; Mertens, F.; Passos, C.J.S.; Romana, C.A. Agroforestry systems as a profitable alternative to slash and burn practices in small-scale agriculture of the Brazilian Amazon. Agrofor. Syst. 2015, 89, 193–204. [Google Scholar] [CrossRef]
- Bresler, E.; Yasutomi, R. Drip irrigation technology in semi-arid regions and international cooperation. J. Irrig. Eng. Rural Plan. 1990, 1990, 48–62. [Google Scholar] [CrossRef]
- Cunha, F.N.; de Oliveira, R.C.; da Silva, N.F.; de Freitas Moura, L.M.; Teixeira, M.B.; Gomes Filho, R.R. Variabilidade temporal da uniformidade de distribuição em sistema de gotejamento. Rev. Bras. Agric. Irrig. 2013, 7, 248–257. [Google Scholar] [CrossRef]
- Abdul-Salam, Y.; Ovando, P.; Roberts, D. Understanding the economic barriers to the adoption of agroforestry: A Real Options analysis. J. Environ. Manag. 2022, 302, 113955. [Google Scholar] [CrossRef]
- Gori Maia, A.; Eusebio Gdos, S.; Fasiaben Mdo, C.R.; Moraes, A.S.; Assad, E.D.; Pugliero, V.S. The economic impacts of the diffusion of agroforestry in Brazil. Land Use Policy 2021, 108, 105489. [Google Scholar] [CrossRef]
- Gori Maia, A.; Burney, J.A.; Morales Martínez, J.D.; Cesano, D. Improving production and quality of life for smallholder farmers through a climate resilience program: An experience in the Brazilian Sertão. PLoS ONE 2021, 16, e0251531. [Google Scholar] [CrossRef] [PubMed]
- Dutra, I.; Martins, M.; Parré, J. A produção da agricultura familiar e os efeitos dos programas de incentivo. Rev. Política Agríc. 2021, 30, 94–106. [Google Scholar]
- Oliveira, G.C.; Torezan-Silingardi, H.M.; Anjos, D.V.; Abrahão, M.; Alves-de-Lima, L. Financial Analysis and Cost Implications of Implementing an Agroforestry System in Brazil. Agric. Rural Stud. 2024, 2, 0023. [Google Scholar] [CrossRef]
- Gonçalves, A.L.R. Sistemas Agroflorestais No Semiárido Brasileiro: Estratégias para Combate à Desertificação e Enfrentamento Às Mudanças Climáticas; Centro Sábia/Caatinga: Recife, Brazil, 2016. [Google Scholar]
- Tranchina, M.; Reubens, B.; Frey, M.; Mele, M.; Mantino, A. What challenges impede the adoption of agroforestry practices? A global perspective through a systematic literature review. Agrofor. Syst. 2024, 98, 1817–1837. [Google Scholar] [CrossRef]
- Martinelli Gdo, C.; Schlindwein, M.M.; Padovan, M.P.; Gimenes, R.M.T. Decreasing uncertainties and reversing paradigms on the economic performance of agroforestry systems in Brazil. Land Use Policy 2019, 80, 274–286. [Google Scholar] [CrossRef]
- Altieri, M.A.; Funes-Monzote, F.R.; Petersen, P. Agroecologically efficient agricultural systems for smallholder farmers: Contributions to food sovereignty. Agron. Sustain. Dev. 2012, 32, 1–13. [Google Scholar] [CrossRef]
- Manrique-Ascencio, A.; Prieto-Torres, D.A.; Villalobos, F.; Mercado Gómez, J.; Guevara, R. Limited drought tolerance in the neotropical seasonally dry forest plants impairs future species richness. Plant Biol. 2024. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Cardoso, D.; Schrire, B.D.; Lewis, G.P.; Pennington, R.T.; Brummer, T.J.; Rotella, J.; Lavin, M. Stability structures tropical woody plant diversity more than seasonality: Insights into the ecology of high legume-succulent-plant biodiversity. S. Afr. J. Bot. 2013, 89, 42–57. [Google Scholar] [CrossRef]
- Miccolis, A.; Peneireiro, F.M.; Marques, H.R.; Vieira, D.L.; Arco-Verde, M.F.; Hoffmann, M.R.; Rehder, T.; Pereira, A.V. Agroforestry Systems for Ecological Restoration: How to Concile Conservation and Production—Options for Brazil’s Cerrado and Caatinga Biomes; Instituto Sociedade, População e Natureza—ISPN/World Agroforestry Centre—ICRAF: Brasília, Brazil, 2016. [Google Scholar]
- Herrera, A.M.; de Mello, A.C.L.; de Oliveira Apolinário, V.X.; Dubeux Júnior, J.C.B.; da Cunha, M.V.; dos Santos, M.V.F. Potential of Gliricidia sepium (jacq.) Kunth ex Walp. and Mimosa caesalpiniifolia Benth. in silvopastoral systems intercropped with signalgrass [Urochloa decumbens (Stapf) R.D. Webster]. Agrofor. Syst. 2021, 95, 1061–1072. [Google Scholar] [CrossRef]
- Pereira, A.C.H.; Endringer, D.C. Composição química, atividades biológicas e aplicabilidade da gliricídia em sistemas agroflorestais. In Café Sombreado: Uma Abordagem Multidisciplinar; Araújo, J.B.S., de Siqueira, H.M., da P. Padovan, M., Sales, E.F., Eds.; Incaper: Vitória, Brazil, 2024; pp. 173–205. [Google Scholar]
- Marin, A.M.P.; Menezes, R.S.C.; Silva, E.D.; Sampaio, E.V.D.S.B. Efeito da Gliricidia sepium sobre nutrientes do solo, microclima e produtividade do milho em sistema agroflorestal no Agreste Paraibano. Rev. Bras. Cienc. Solo 2006, 30, 555–564. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Walter, D.; Jose, S. Carbon sequestration by forests and agroforests: A reality check for the United States. Carbon Footpr. 2022, 2, 2. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Nair, V.D.; Mohan Kumar, B.; Showalter, J.M. Chapter Five—Carbon Sequestration in Agroforestry Systems. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; Volume 108, pp. 237–307. [Google Scholar] [CrossRef]
- Lehmann, J.; Peter, I.; Steglich, C.; Gebauer, G.; Huwe, B.; Zech, W. Below-ground interactions in dryland agroforestry. Ecol. Manag. 1998, 111, 157–169. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Xavier, F.A.S.; Oliveira, T.S.; Mendonça, E.S.; Araújo Filho, J.A. Organic carbon pools in a Luvisol under agroforestry and conventional farming systems in the semi-arid region of Ceará, Brazil. Agrofor. Syst. 2007, 71, 127–138. [Google Scholar] [CrossRef]
- Ramachandran Nair, P.K.; Mohan Kumar, B.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- Shi, L.; Feng, W.; Xu, J.; Kuzyakov, Y. Agroforestry systems: Meta-analysis of soil carbon stocks, sequestration processes, and future potentials. Land. Degrad. Dev. 2018, 29, 3886–3897. [Google Scholar] [CrossRef]
- Ramos, H.M.N.; Vasconcelos, S.S.; Kato, O.R.; Castellani, D.C. Above- and belowground carbon stocks of two organic, agroforestry-based oil palm production systems in eastern Amazonia. Agrofor. Syst. 2018, 92, 221–237. [Google Scholar] [CrossRef]
- Cardinael, R.; Mao, Z.; Chenu, C.; Hinsinger, P. Belowground functioning of agroforestry systems: Recent advances and perspectives. Plant Soil 2020, 453, 1–13. [Google Scholar] [CrossRef]
- Henry, M.; Tittonell, P.; Manlay, R.J.; Bernoux, M.; Albrecht, A.; Vanlauwe, B. Biodiversity, carbon stocks and sequestration potential in aboveground biomass in smallholder farming systems of western Kenya. Agric. Ecosyst. Environ. 2009, 129, 238–252. [Google Scholar] [CrossRef]
- Feliciano, D.; Ledo, A.; Hillier, J.; Nayak, D.R. Which agroforestry options give the greatest soil and above ground carbon benefits in different world regions? Agric. Ecosyst. Environ. 2018, 254, 117–129. [Google Scholar] [CrossRef]
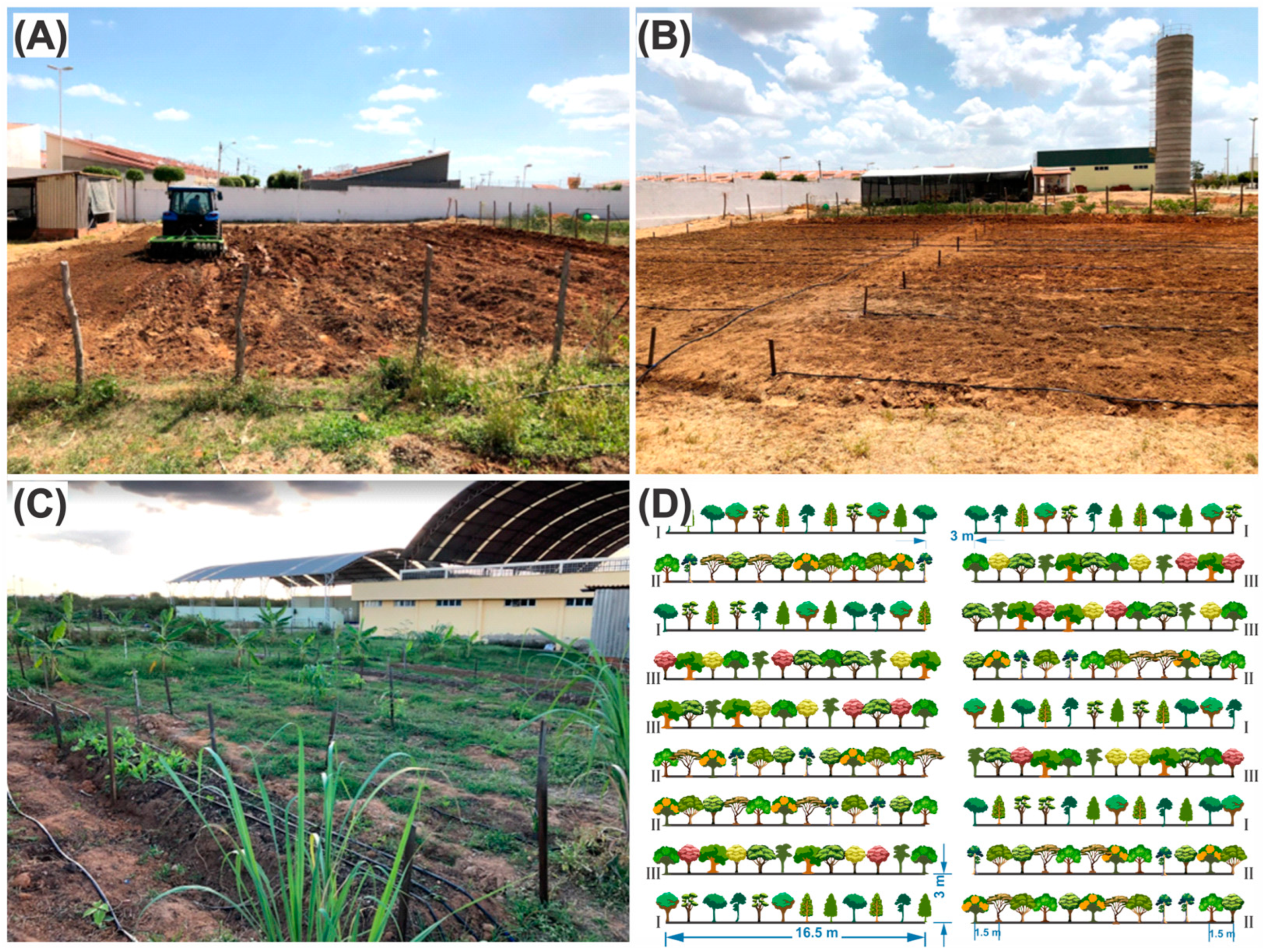
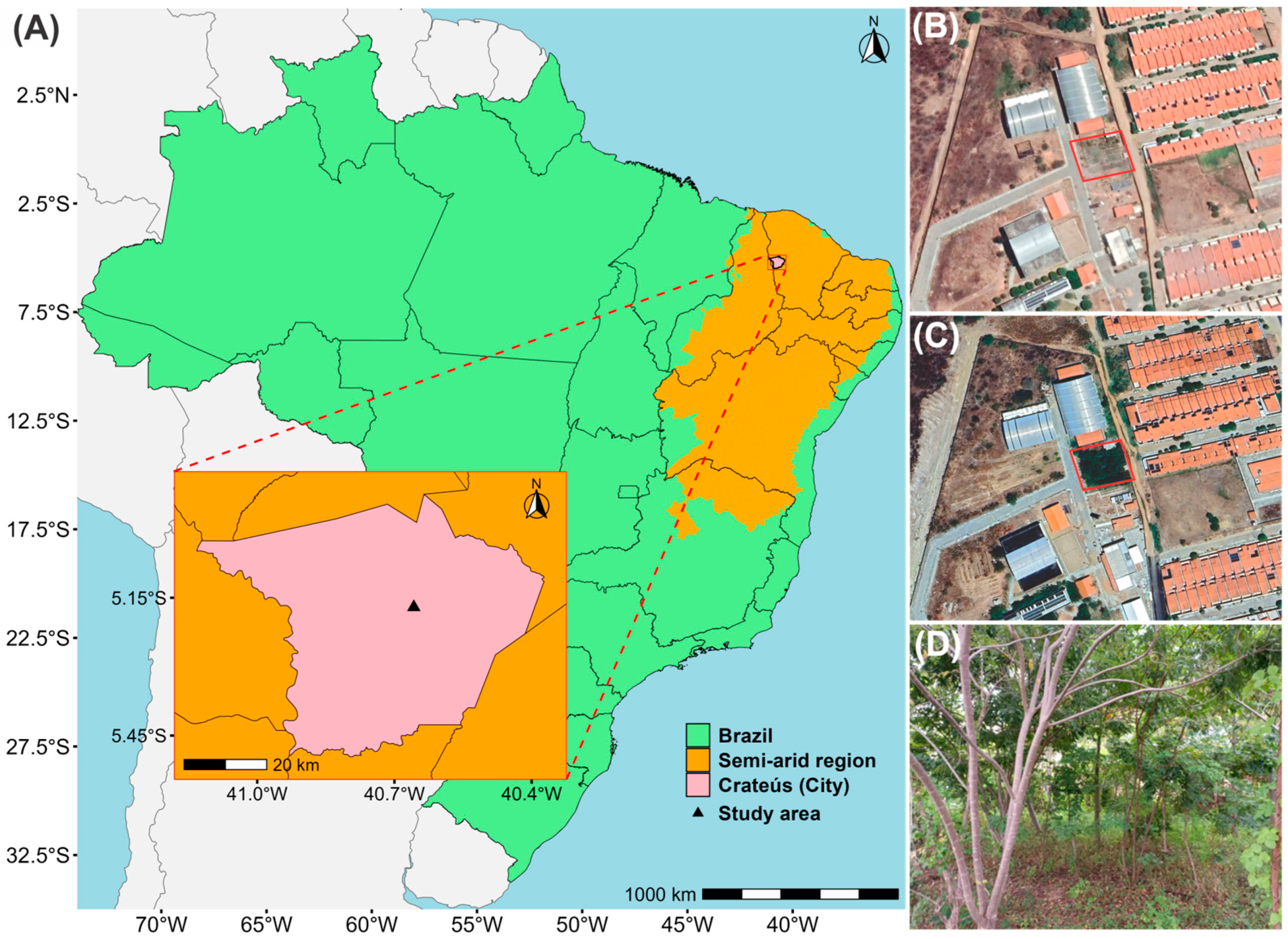
| Species | Original Biodiversity Arrangements | Post-Replacement Biodiversity Arrangements | Use | Wood Density (g cm−3) | Reference |
|---|---|---|---|---|---|
| Tamarindus indica | I (12) | I (15) | Fruit | 0.98 | [47] |
| Anadenanthera colubrina | I (12) | I (13) | Timber/Forage | 0.93 | [48] |
| Moringa oleifera | I (12) | I (11) | Forage | 0.26 | [47] |
| Astronium urundeuva | I (12) | - | Timber | 1.19 | [49] |
| Malpighia emarginata | I (12) | I (9), II (1) | Fruit | 0.70 | [47] |
| Ceiba glaziovii | I (12) | I (15) | Timber | 0.59 | [48] |
| Hymenaea sp. | II (12) | - | Timber | 0.95 | [50] |
| Mimosa caesalpiniaefolia | - | II (8), III (1) | Timber/Forage | 1.10 | [51] |
| Amburana cearensis | II (12) | II (1) | Timber/Forage | 0.60 | [49] |
| Gliricidia sepium | II (12) | I (1), II (12) | Forage | 0.58 | [47] |
| Citrus sp. | II (12) | II (5) | Fruit | 0.74 | [47] |
| Annona squamosa | II (12) | II (8) | Fruit/Forage | 0.49 | [47] |
| Cenostigma pyramidale | II (12) | II (13) | Timber/Forage | 0.95 | [49] |
| Talisia esculenta | III (12) | III (2) | Fruit | 1.10 | [49] |
| Libidibia ferrea | III (12) | III (9) | Forage/Timber | 1.22 | [49] |
| Aspidosperma pyrifolium | III (12) | III (5) | Timber | 0.79 | [48] |
| Morus alba | III (12) | III (4) | Fruit | 0.55 | [47] |
| Anacardium occidentale | III (12) | III (2) | Fruit | 0.42 | [49] |
| Handroanthus impetiginosus | III (12) | III (6) | Timber | 0.96 | [49] |
| Spondias purpurea | - | III (1) | Fruit/Forage | 0.33 | [47] |
| Leucaena leucocephala | - | I (1), III (5) | Forage | 0.69 | [47] |
| Material | Cost Type | Unit | Quantity | Unit Cost | Total Cost |
|---|---|---|---|---|---|
| Irrigation water | Maintenance | m3 | 64.8 m3 month−1 | BRL 4.92 m−3 (USD 0.95 m−3) | BRL 1912.8 * (USD 369.33) |
| 3/4″ Hose | Preparation | m | 100 | BRL 136.27 (USD 26.32) | BRL 136.27 (USD 26.31) |
| Drip Tape | Preparation | m | 270 | BRL 0.40 (USD 0.08) | BRL 110.16 (USD 21.27) |
| 90° Double Internal Elbow 3/4″ | Preparation | unit | 4 | BRL 0.76 (USD 0.15) | BRL 3.04 (USD 0.59) |
| 3/4″ × 1/2″ Triple Reduction Tee | Preparation | unit | 18 | BRL 3.50 (USD 0.68) | BRL 63.00 (USD 12.16) |
| Drip Tape Plug | Preparation | unit | 18 | BRL 2.50 (USD 0.48) | BRL 45.00 (USD 8.69) |
| 3/4″ Ball Valve | Preparation | unit | 2 | BRL 38.90 (USD 7.51) | BRL 77.80 (USD 15.02) |
| 3/4″ Triple Tee | Preparation | unit | 4 | BRL 5.60 (USD 1.08) | BRL 22.40 (USD 4.33) |
| Fencing service (tractor-assisted) | Preparation | hour | 1 | BRL 360.00 (USD 69.51) | BRL 360.00 (USD 69.51) |
| Seedlings (nursery production) | Planting | unit | 246 | BRL 5.00 (USD 0.97) | BRL 1230.00 (USD 237.49) |
| Labor for planting and preparation | Preparation/Planting | day | 10 | BRL 80.00 (USD 15.45) | BRL 800.00 (USD 154.47) |
| Brush clearing | Maintenance | day | 3 | BRL 120.00 (USD 23.17) | BRL 360.00 (USD 69.51) |
| Total | BRL 5120.47 (USD 988.68) | ||||
| Family | Species | Common Name | n | AD (ind ha−1) | RD (%) | DoA (m2) | DoR (%) | AF (%) | RF (%) | IVI (%) | Carbon (g ind−1) | Carbon Stock (Mg ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malvaceae | Ceiba glaziovii (Kuntze) K.Schum. | Paineira-branca | 15 | 168.35 | 10.14 | 1.51 | 23.67 | 38.89 | 7.87 | 13.89 | 27,734.05 ± 15,568.88 | 4.6690 |
| Fabaceae | Gliricidia sepium (Jacq.) Kunth ex Walp. | Gliricidia | 13 | 145.90 | 8.78 | 1.46 | 22.90 | 38.89 | 7.87 | 13.18 | 30,162.24 ± 16,014.7 | 4.4008 |
| Moringaceae | Moringa oleifera Lam. | Moringa | 11 | 123.46 | 7.43 | 1.43 | 22.34 | 38.89 | 7.87 | 12.55 | 41,852.69 ± 36,211.27 | 5.1670 |
| Fabaceae | Anadenanthera colubrina (Vell.) Brenan | Angico | 13 | 145.90 | 8.78 | 0.57 | 8.97 | 38.89 | 7.87 | 8.54 | 9013.17 ± 8437.41 | 1.3151 |
| Fabaceae | Tamarindus indica L. | Tamarindo | 15 | 168.35 | 10.14 | 0.26 | 4.14 | 38.89 | 7.87 | 7.38 | 2048.93 ± 1502.98 | 0.3449 |
| Fabaceae | Mimosa caesalpiniifolia Benth. | Sabiá | 9 | 101.01 | 6.08 | 0.30 | 4.66 | 38.89 | 7.87 | 6.20 | 5541.54 ± 6211.17 | 0.5598 |
| Fabaceae | Cenostigma pyramidale (Tul.) Gagnon & G.P.Lewis | Catingueira | 13 | 145.90 | 8.78 | 0.08 | 1.32 | 33.33 | 6.74 | 5.62 | 527.55 ± 420.97 | 0.0770 |
| Malpighiaceae | Malpighia emarginata DC. | Acerola | 10 | 112.23 | 6.76 | 0.06 | 1.01 | 33.33 | 6.74 | 4.84 | 315.04 ± 292.34 | 0.0353 |
| Annonaceae | Annona squamosa L. | Ata | 8 | 89.79 | 5.41 | 0.03 | 0.48 | 33.33 | 6.74 | 4.21 | 177.75 ± 89.39 | 0.0160 |
| Fabaceae | Libidibia ferrea (Mart. ex Tul.) L.P.Queiroz | Jucá | 9 | 101.01 | 6.08 | 0.05 | 0.78 | 27.78 | 5.62 | 4.16 | 422.88 ± 631.47 | 0.0427 |
| Fabaceae | Leucaena leucocephala (Lam.) de Wit | Leuceana | 6 | 67.34 | 4.05 | 0.21 | 3.32 | 22.22 | 4.49 | 3.96 | 6945.99 ± 5846.56 | 0.4677 |
| Bignoniaceae | Handroanthus impetiginosus (Mart. ex DC.) Mattos | Ipê | 6 | 67.34 | 4.05 | 0.07 | 1.11 | 22.22 | 4.49 | 3.22 | 1098.68 ± 738.3 | 0.074 |
| Moraceae | Morus alba L. | Amora | 4 | 44.89 | 2.70 | 0.26 | 4.05 | 11.11 | 2.25 | 3.00 | 9789.3 ± 7258.18 | 0.4395 |
| Rutaceae | Citrus sp. | Limão | 5 | 56.12 | 3.38 | 0.03 | 0.43 | 22.22 | 4.49 | 2.77 | 354.05 ± 382.48 | 0.0199 |
| Apocynaceaea | Aspidosperma pyrifolium Mart. & Zucc. | Pereiro | 5 | 56.12 | 3.38 | 0.01 | 0.13 | 22.22 | 4.49 | 2.67 | 46.29 ± 59.85 | 0.0026 |
| Anacardiaceae | Anacardium occidentale L. | Caju | 2 | 22.45 | 1.35 | 0.03 | 0.53 | 11.11 | 2.25 | 1.37 | 2386.44 ± 2906.56 | 0.0536 |
| Sapindacea | Talisia esculenta (Cambess.) Radlk. | Pitomba | 2 | 22.45 | 1.35 | 0.00 | 0.03 | 11.11 | 2.25 | 1.21 | 27.94 ± 31.49 | 0.0006 |
| Anacardiaceae | Spondias purpurea L. | Seriguela | 1 | 11.22 | 0.68 | 0.01 | 0.11 | 5.56 | 1.12 | 0.64 | 186.87 | 0.0021 |
| Fabaceae | Amburana cearensis (Allemão) A.C.Sm. | Amburana | 1 | 11.22 | 0.68 | 0.00 | 0.02 | 5.56 | 1.12 | 0.61 | 19.54 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quadro, I.P.d.; Souza, A.G.d.C.d.; Nogueira, D.B.; Bomfim, I.G.A.; Giroldo, A.B. Implementation and Costs of an Agroforestry System in a Degraded Area of the Brazilian Semi-Arid Region. Conservation 2025, 5, 20. https://doi.org/10.3390/conservation5020020
Quadro IPd, Souza AGdCd, Nogueira DB, Bomfim IGA, Giroldo AB. Implementation and Costs of an Agroforestry System in a Degraded Area of the Brazilian Semi-Arid Region. Conservation. 2025; 5(2):20. https://doi.org/10.3390/conservation5020020
Chicago/Turabian StyleQuadro, Israel Pereira de, Antônio Gilvan da Cruz de Souza, Danilo Batista Nogueira, Isac Gabriel Abrahão Bomfim, and Aelton Biasi Giroldo. 2025. "Implementation and Costs of an Agroforestry System in a Degraded Area of the Brazilian Semi-Arid Region" Conservation 5, no. 2: 20. https://doi.org/10.3390/conservation5020020
APA StyleQuadro, I. P. d., Souza, A. G. d. C. d., Nogueira, D. B., Bomfim, I. G. A., & Giroldo, A. B. (2025). Implementation and Costs of an Agroforestry System in a Degraded Area of the Brazilian Semi-Arid Region. Conservation, 5(2), 20. https://doi.org/10.3390/conservation5020020







