Big and Fast: GPS Loggers Reveal Long-Range Movements in a Large, Riverine Turtle
Abstract
1. Introduction
2. Materials and Methods
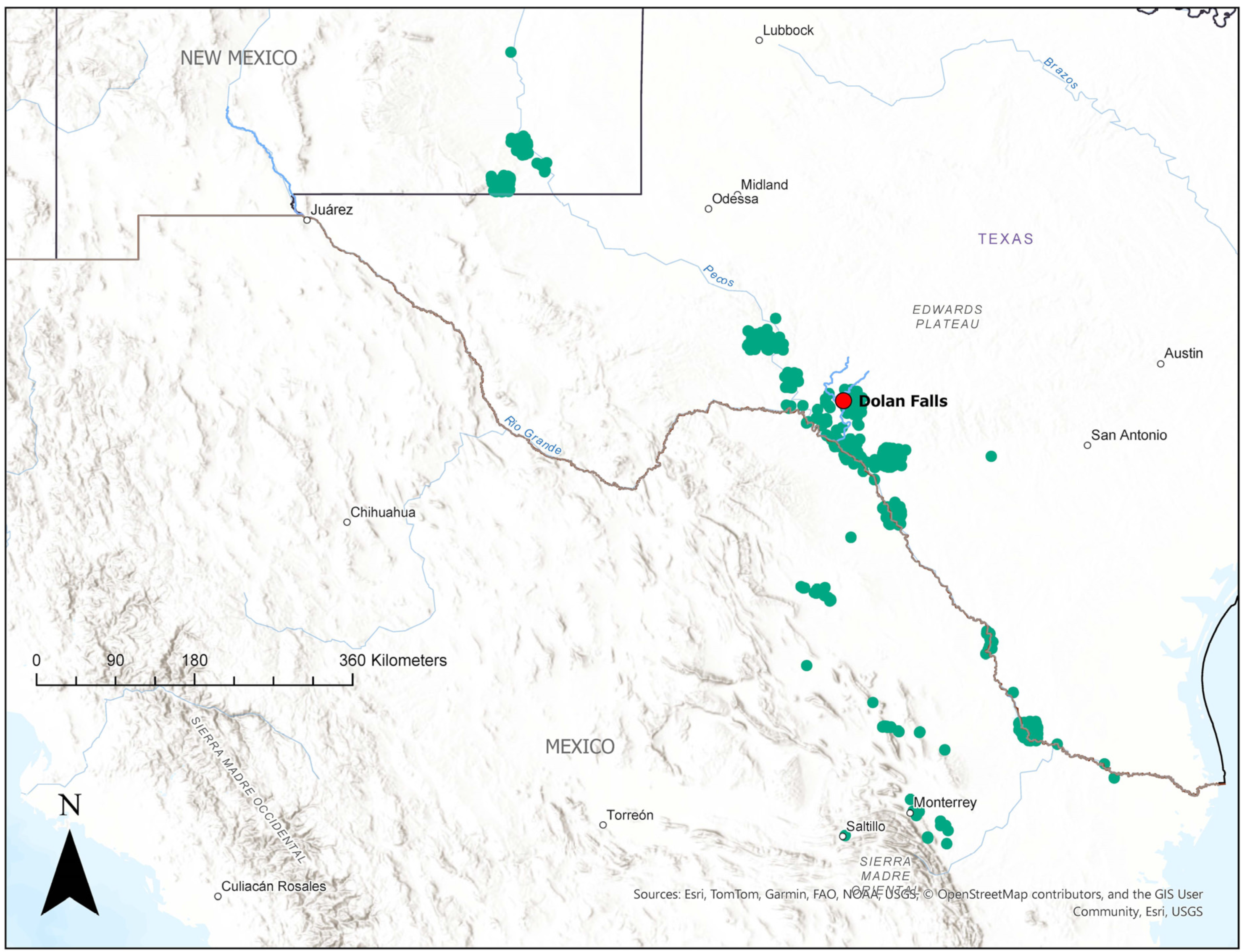
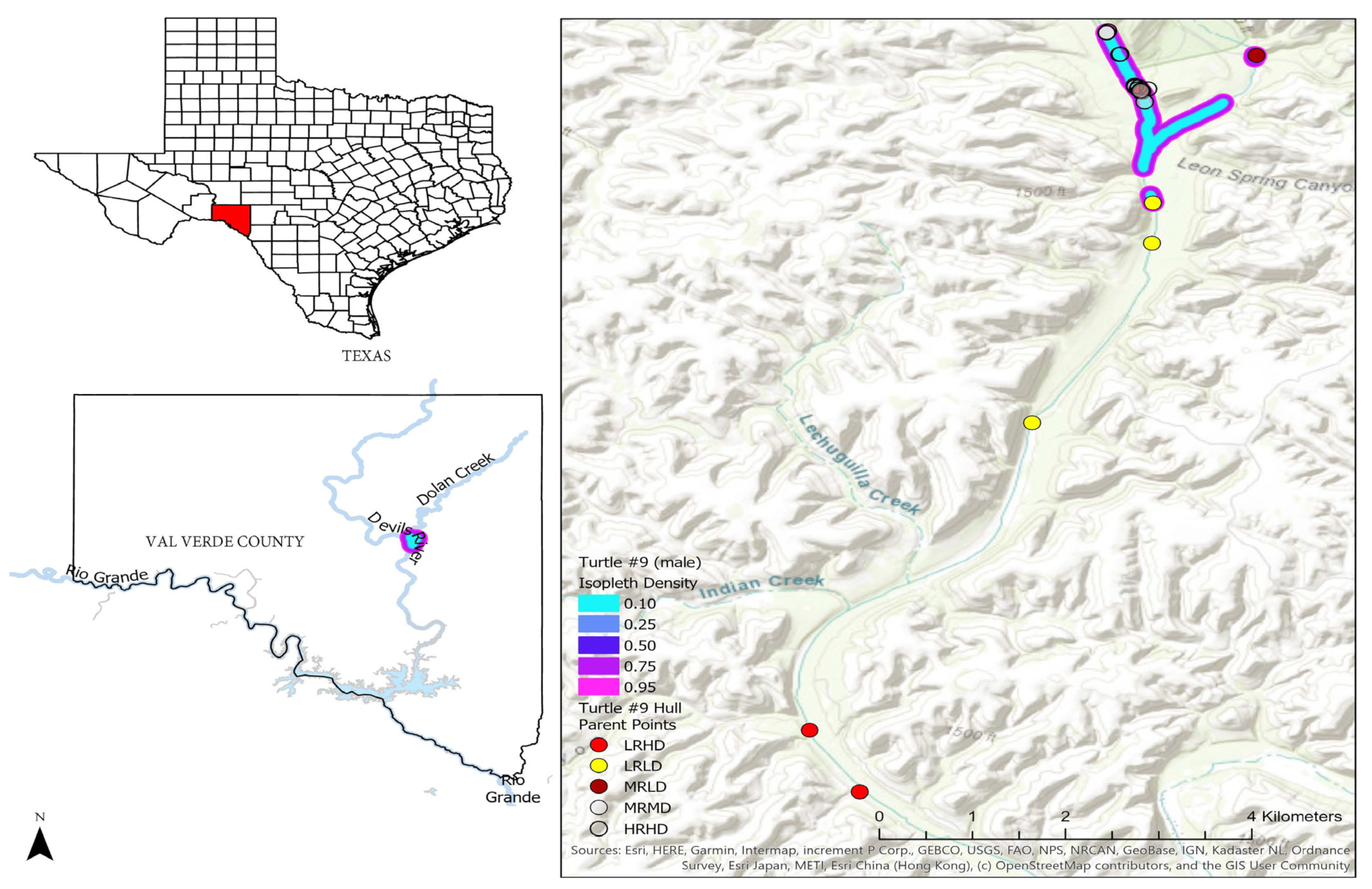
2.1. Study Site
2.2. Telemetry Study
2.2.1. Turtle Capture and Transmitter Attachment
2.2.2. Tracking
2.2.3. Movement Analysis
2.2.4. Home Range Analysis
2.2.5. CMR Study
3. Results
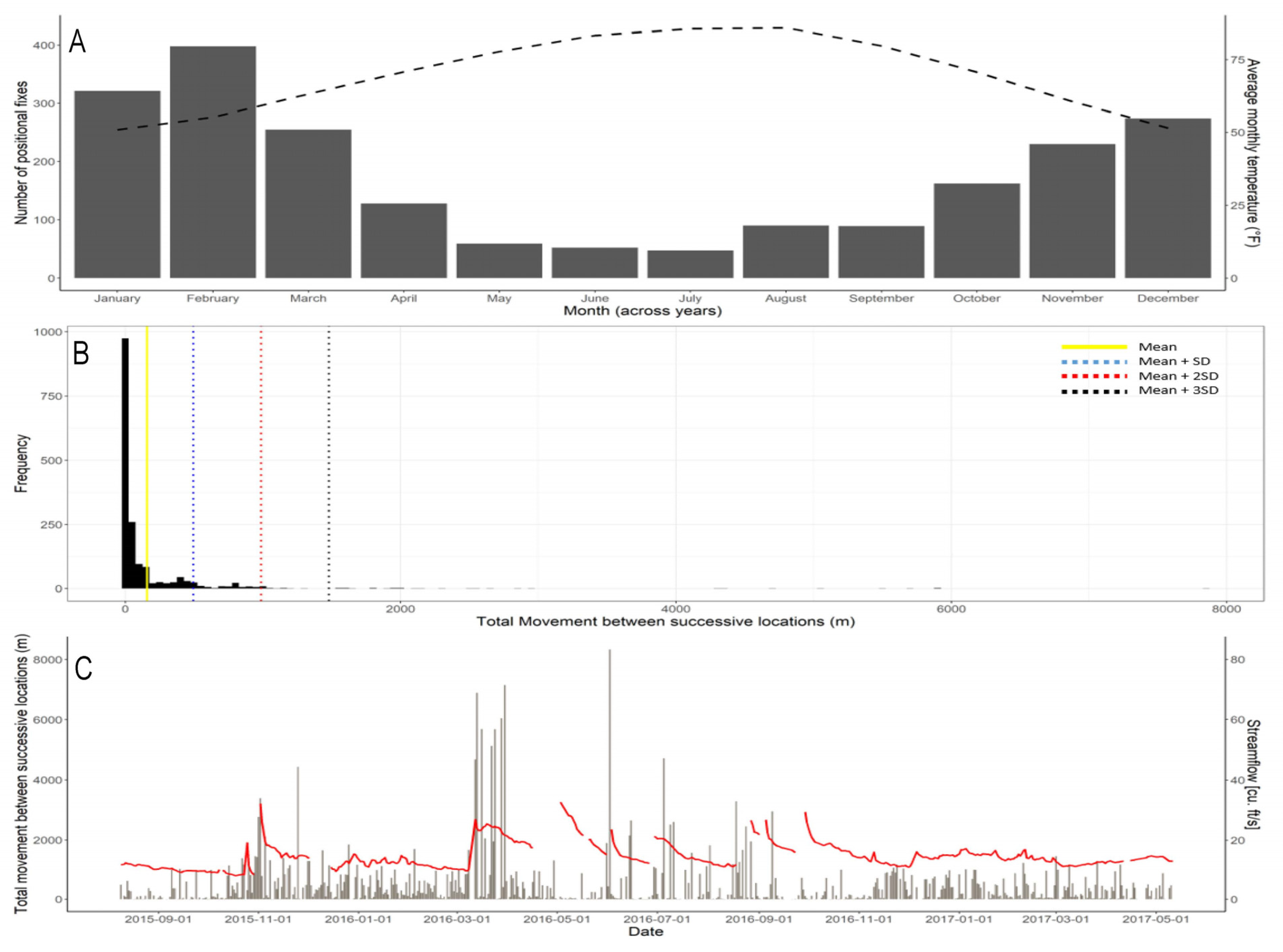
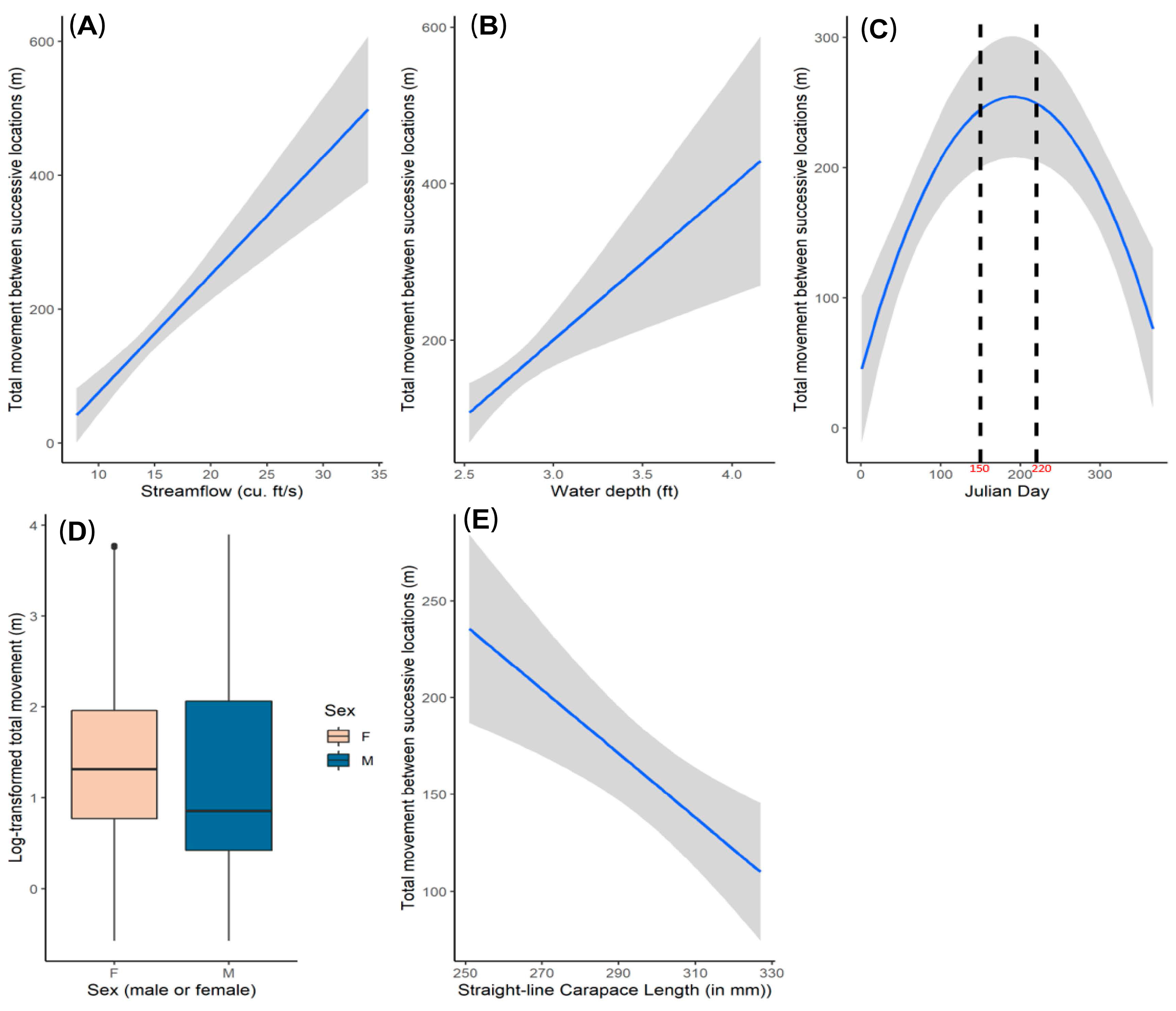
3.1. Movement Analysis
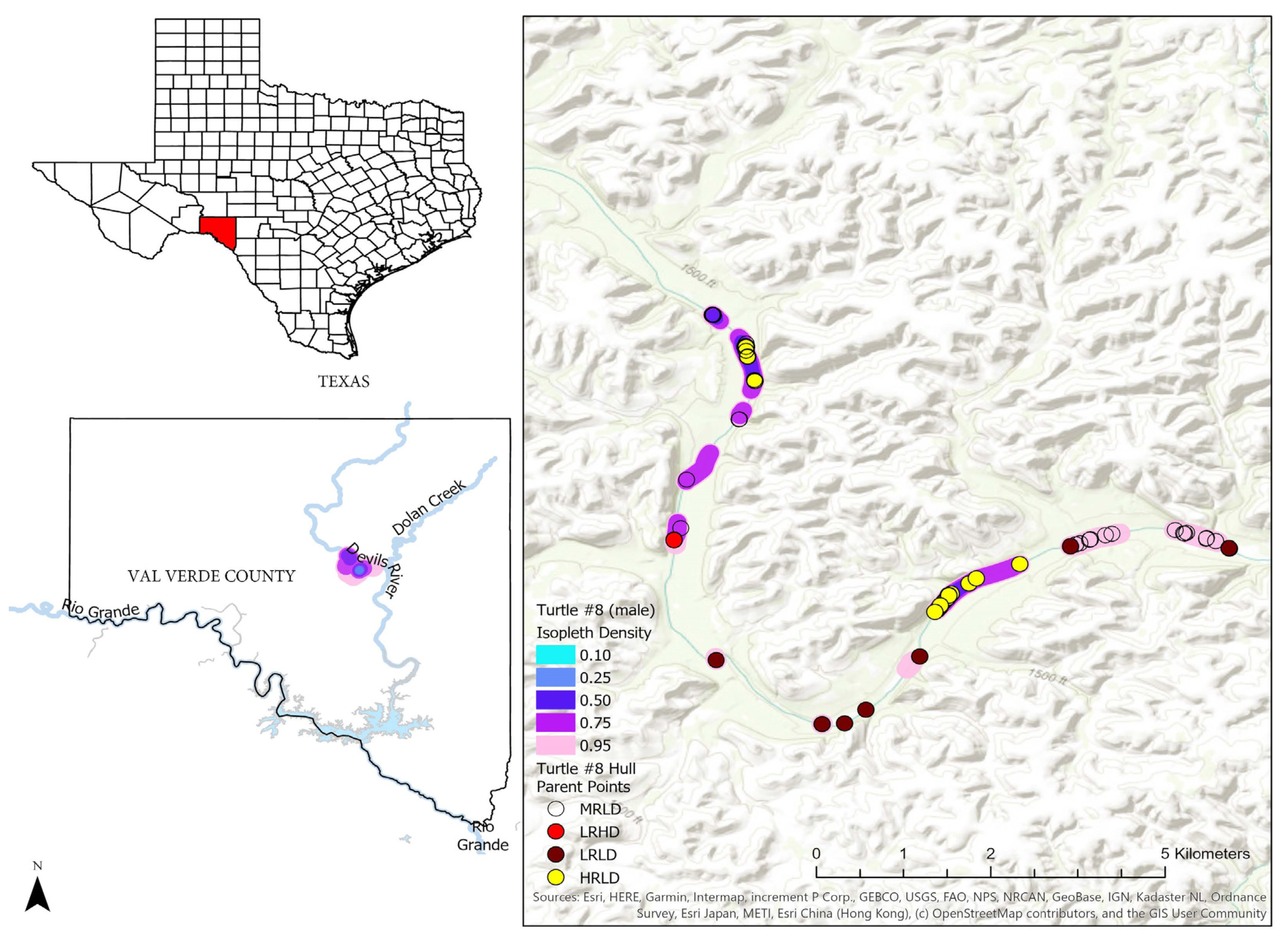
3.2. Home Range Analysis
3.3. CMR Study
4. Discussion
4.1. Movement Analysis
4.2. Home Range Analysis
4.3. Population Estimate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, R. An emerging movement ecology paradigm. Proc. Natl. Acad. Sci. USA 2008, 105, 19050–19051. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, R.Z.; Ayady, C.L.; Tatayah, V.; Jones, C.; Howey, P.W.; Froidevaux, J.S.; Racey, P.A.; Jones, G. The movement ecology of the Mauritian flying fox (Pteropus niger): A long-term study using solar-powered GSM/GPS tags. Mov. Ecol. 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef] [PubMed]
- Conenna, I.; López-Baucells, A.; Rocha, R.; Ripperger, S.; Cabeza, M. Movement seasonality in a desert-dwelling bat revealed by miniature GPS loggers. Mov. Ecol. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Non-optimal animal movement in human-altered landscapes. Funct. Ecol. 2007, 21, 1003–1015. [Google Scholar] [CrossRef]
- Jeltsch, F.; Bonte, D.; Pe’er, G.; Reineking, B.; Leimgruber, P.; Balkenhol, N.; Schröder, B.; Buchmann, C.M.; Mueller, T.; Blaum, N. Integrating movement ecology with biodiversity research-exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 2013, 1, 1–13. [Google Scholar] [CrossRef]
- Parlin, A.F.; Nardone, J.A.; Kelly Dougherty, J.; Rebein, M.; Safi, K.; Schaeffer, P.J. Activity and movement of free-living box turtles are largely independent of ambient and thermal conditions. Mov. Ecol. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Rhodin, A.; Iverson, J.B.; Bour, R.; Fritz, U.; Georges, A.; Shaffer, H.B.; van Dijk, P.P. Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status; Rhodin, A.G.J., Iverson, J.B., van Dijk, P.P., Stanford, C.B., Goode, E.V., Buhlmann, K.A., Mittermeier, R.A., Eds.; Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group; IUCN/SSC Tortoise and Freshwater Turtle Specialist Group: Galápagos, Ecuador, 2021; pp. 1–472. [Google Scholar] [CrossRef]
- Congdon, J.D.; Dunham, A.E.; van Loben Sels, R. Delayed sexual maturity and demographics of Blanding’s turtles (Emydoidea blandingii): Implications for conservation and management of long-lived organisms. Conserv. Biol. 1993, 7, 826–833. [Google Scholar] [CrossRef]
- Heppell, S.S.; Crowder, L.B.; Crouse, D.T. Models to evaluate headstarting as a management tool for long-lived turtles. Ecol. Appl. 1996, 6, 556–565. [Google Scholar] [CrossRef]
- Georges, A. Setting conservation priorities for Australian freshwater turtles. In Herpetology in Australia: A. Diverse. Discipline; Lunney, D., Ayers, D., Eds.; Royal Zoological Society of New South Wales: Sydney, Australia, 1993; pp. 49–58. [Google Scholar]
- Reed, R.; Gibbons, J. Conservation Status of Live US Nonmarine Turtles in Domestic and International Trade; US Fish and Wildlife Service: Albuquerque, NM, USA, 2003; p. 92. [Google Scholar]
- Pierce, L.; Stuart, J.; Ward, J.; Painter, C. Pseudemys gorzugi Ward 1984–Rio Grande Cooter, Western River Cooter, Tortuga de Oreja Amarilla, Jicotéa del Río Bravo. In Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group; Rhodin, A.G.J., Iverson, J.B., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Pritchard, P.C.H., Mittermeier, R., Eds.; Chelonian Research Monographs; IUCN/SSC Tortoise and Freshwater Turtle Specialist Group: Galápagos, Ecuador, 2016; Volume 5, pp. 100.1–100.12. [Google Scholar] [CrossRef]
- Ernst, C. Pseudemys gorzugi Ward. Rio Grande Cooter. In Catalogue of American Amphibians and Reptiles; The University of Texas Press: Austin, TX, USA, 1990; pp. 461.1–461.2. [Google Scholar]
- Ward, J. Relationship of Chrysemyd turtles of North America (Testudines:Emydidae); Special Publications—The Museum, Texas Tech University; Texas Tech Press: Lubbock, TX, USA, 1984; Volume 21, pp. 1–50. [Google Scholar]
- Mahan, L.B.; Bassett, L.G.; Duarte, A.; Forstner, M.R.; Mali, I. Effects of salinization on the occurrence of a long-lived vertebrate in a desert river. Sci. Rep. 2022, 12, 15907. [Google Scholar] [CrossRef]
- Bailey, L.; Dixon, J.; Hudson, R.; Forstner, M. Minimal Genetic Structure in the Rio Grande Cooter (Pseudemys gorzugi). Southwest. Nat. 2008, 53, 406–411. [Google Scholar] [CrossRef]
- Blackstun, D.; Woosley, L.; Flora, M. Water Resources-Issues in the Rio Grande–Rio Conchos to Amistad Reservoir Subarea; United States Department of the Interior: Washington, DC, USA, 1998; pp. 1–8. Available online: https://books.google.com/books?hl=en&lr=&id=175EAAAAYAAJ&oi=fnd&pg=PP3&dq=Water+Resources-Issues+in+the+Rio+Grande%E2%80%93Rio+Conchos+to+Amistad+Reservoir+Subarea&ots=ij7jD6SEuU&sig=Ych19UAxpSvFS5eUpoIm7HfeAZ4#v=onepage&q=Water%20Resources-Issues%20in%20the%20Rio%20Grande%E2%80%93Rio%20Conchos%20to%20Amistad%20Reservoir%20Subarea&f=false (accessed on 2 January 2021).
- Van Dijk, P. Rio Grande Cooter. In Pseudemys gorzugi. The IUCN Red List of Threatened Species 2011; 2011, e.T18459A974259282011. Available online: https://www.iucnredlist.org/species/18459/97425928 (accessed on 2 January 2021).
- USFWS. Endangered and threatened wildlife and plants; three species not warranted for listing as endangered or threatened species. Fed Regist. 2022, 87, 14227–14232. [Google Scholar]
- Lovich, J.E.; Ennen, J.R. A quantitative analysis of the state of knowledge of turtles of the United States and Canada. Amphibia-Reptilia 2013, 34, 11–23. [Google Scholar] [CrossRef]
- Bogolin, A.P.; Davis, D.R.; Kline, R.J.; Rahman, A.F. A drone-based survey for large, basking freshwater turtle species. PLoS ONE 2021, 16, e0257720. [Google Scholar] [CrossRef]
- Mali, I.; Duarte, A.; Forstner, M.R. Comparison of hoop-net trapping and visual surveys to monitor abundance of the Rio Grande cooter (Pseudemys gorzugi). PeerJ 2018, 6, e4677. [Google Scholar] [CrossRef]
- Suriyamongkol, T.; Ortega-Berno, V.; Mahan, L.B.; Mali, I. Using stable isotopes to study resource partitioning between red-eared slider and Rio Grande cooter in the Pecos River watershed. Ichthyol. Herpetol. 2022, 110, 96–105. [Google Scholar] [CrossRef]
- Degenhardt, W.G.; Painter, C.W.; Price, A.H.; Conant, R. Amphibians and Reptiles of New Mexico; UNM Press: Albuquerque, NM, USA, 1996. [Google Scholar]
- Vandewege, M.W.; Gutierrez, J.; Davis, D.R.; Forstner, M.R.; Mali, I. Patterns of genetic divergence in the Rio Grande cooter (Pseudemys gorzugi), a riverine turtle inhabiting an arid and anthropogenically modified system. J. Hered. 2024, 115, 253–261. [Google Scholar] [CrossRef]
- Baguette, M. Long distance dispersal and landscape occupancy in a metapopulation of the cranberry fritillary butterfly. Ecography 2003, 26, 153–160. [Google Scholar] [CrossRef]
- Christensen, R.; Chow-Fraser, P. Use of GPS loggers to enhance radio-tracking studies of semi-aquatic freshwater turtles. Herpetol. Conserv. Biol. 2014, 9, 18–28. [Google Scholar]
- MacLaren, A.; Sirsi, S.; Foley, D., III; Forstner, M. Pseudemys gorzugi (Rio Grande Cooter). Long Distance Dispersal. Herpetol. Rev. 2017, 48, 180–181. [Google Scholar]
- Ross, J.P.; Bluett, R.D.; Dreslik, M.J. Movement and home range of the smooth softshell turtle (Apalone mutica): Spatial ecology of a river specialist. Diversity 2019, 11, 124. [Google Scholar] [CrossRef]
- Bohannon, A.M.; Bassett, L.G.; Sirsi, S.; MacLaren, A.R.; Foley, D.H.; Fritts, S.R.; Pharr, L.R.; Forstner, M.R. Reproductive Characteristics of Rio Grande Cooters (Pseudemys gorzugi) in Western Texas. Chelonian Conserv. Biol. 2022, 21, 246–255. [Google Scholar] [CrossRef]
- Suriyamongkol, T.; Mali, I. Aspects of the reproductive biology of the Rio Grande cooter (Pseudemys gorzugi) on the Black River, New Mexico. Chelonian Conserv. Biol. 2019, 18, 187–194. [Google Scholar] [CrossRef]
- Harrell, H.L. Response of the Devil’s River (Texas) fish community to flooding. Copeia 1978, 1978, 60–68. [Google Scholar] [CrossRef]
- Smith, K.N.; Cain, J.W., III; Morrison, M.L.; Wilkins, R.N. Nesting ecology of the black-capped vireo in southwest Texas. Wilson J. Ornithol. 2012, 124, 277–285. [Google Scholar] [CrossRef]
- Bailey, L. The Status of Pseudemys gorzugi (the Rio Grande River Cooter) in Texas Rivers; Texas State University: San Marcos, TX, USA, 2005. [Google Scholar]
- Boarman, W.; Goodlett, T.; Goodlett, G.; Hamilton, P. Review of radio transmitter attachment techniques for turtle research and recommendations for improvement. Herpetol. Rev. 1998, 29, 26–33. [Google Scholar]
- MacLaren, A.; Foley, D.; Sirsi, S.; Forstner, M. Updating methods of satellite transmitter attachment for long-term monitoring of the Rio Grande Cooter (Pseudemys gorzugi). Herpetol. Rev. 2017, 48, 48–52. [Google Scholar]
- Brown, R.; Cooke, S.; Anderson, W.; McKinley, R. Evidence to challenge the “2% rule” for biotelemetry. N. Am. J. Fish. Manag. 1999, 19, 867–871. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- D’Eon, R.G.; Delparte, D. Effects of radio-collar position and orientation on GPS radio-collar performance, and the implications of PDOP in data screening. J. Appl. Ecol. 2005, 42, 383–388. [Google Scholar] [CrossRef]
- Calenge, C. The package adehabitat for the R software: Tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Barrett, T.; Dowle, M.; Srinivasan, A.; Gorecki, J.; Chirico, M.; Hocking, T.; Schwendinger, B. Data. Table: Extension of ‘Data. Frame’. R Package Version 1.16.99. 2025. Available online: https://github.com/Rdatatable/data.table (accessed on 2 January 2021).
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Young, A.H.; Knapp, K.R.; Inamdar, A.; Hankins, W.; Rossow, W.B. The international satellite cloud climatology project H-Series climate data record product. Earth Syst. Sci. Data 2018, 10, 583–593. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.N.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–249. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.48.4. 2024. Available online: https://cran.r-project.org/package=MuMIn (accessed on 2 January 2021).
- Lyons, A.J.; Turner, W.C.; Getz, W.M. Home range plus: A space-time characterization of movement over real landscapes. Mov. Ecol. 2013, 1, 2. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Pro 2.4.1; ESRI: Redlands, CA, USA, 2019. [Google Scholar]
- Buhlmann, K.; Tuberville, T.; Gibbons, J.W. Turtles of the Southeast; University of Georgia Press: Athens, GA, USA, 2008. [Google Scholar]
- Schwarz, C.; Arnason, A. Jolly-Seber models in MARK. In MARK: A Gentle Introduction; Cooch, E., White, G., Eds.; Colorado State University: Fort Collins, CO, USA, 2009; Available online: http://www.phidot.org/software/mark/docs/book/pdf/chap12.pdf (accessed on 2 January 2021).
- White, G. Program MARK. 2001. Available online: http://www.phidot.org/software/mark/docs/book/pdf/chap1.pdf (accessed on 2 January 2021).
- Kéry, M.; Schaub, M. Bayesian Population Analysis Using WinBUGS: A Hierarchical Perspective; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Mazerolle, M.J. Estimating detectability and biological parameters of interest with the use of the R environment. J. Herpetol. 2015, 49, 541–559. [Google Scholar] [CrossRef]
- von Hildebrand, P. La Tortuga Charapa (Podocnemis expansa) en el Bajo río Caquetá, Amazonas, Colombia: Aspectos de la Biología Reproductiva y Técnicas para su Manejo; Disloque Editores: Santafé de Bogotá, Colombia, 1997; p. 152. [Google Scholar]
- Moll, D.; Moll, E. The Ecology, Exploitation and Conservation of River Turtles; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Quaglietta, L.; Martins, B.H.; de Jongh, A.; Mira, A.; Boitani, L. A low-cost GPS GSM/GPRS telemetry system: Performance in stationary field tests and preliminary data on wild otters (Lutra lutra). PLoS ONE 2012, 7, e29235. [Google Scholar] [CrossRef]
- Cochrane, M.M.; Brown, D.J.; Moen, R.A. GPS technology for semi-aquatic turtle research. Diversity 2019, 11, 34. [Google Scholar] [CrossRef]
- Guilhon, A.; Vogt, R.C.; Schneider, L.; Ferrara, C.R. Assessment of turtle tracking technoligies in the Brazilian Amazon. Herpetol. Rev. 2011, 42, 525–530. [Google Scholar]
- Thompson, D.G.; Swystun, T.; Cross, J.; Cross, R.L.; Chartrand, D.; Edge, C.B. Fine-and Coarse-Scale Movements and Habitat Use by Wood Turtles (Glyptemys insculpta) Based on Probabilistic Modeling of Radio-and GPS-Telemetry Data. Can. J. Zool. 2018, 96, 1153–1164. [Google Scholar] [CrossRef]
- Ennen, J.R.; Scott, A.F. Home range characteristics and overwintering ecology of the stripe-necked musk turtle (Sternotherus minor peltifer) in middle Tennessee. Chelonian Conserv. Biol. 2013, 12, 199–203. [Google Scholar] [CrossRef]
- Ernst, C. Environmental temperatures and activities in wild spotted turtles, Clemmys guttata. J. Herpetol. 1982, 16, 112–120. [Google Scholar] [CrossRef]
- Johnston, G.R.; Mitchell, J.C.; Suarez, E.; Thomas, T.M. Pseudemys suwanniensis (Suwannee Cooter). Long distance movement and homing. Herpetol. Rev. 2017, 48, 623–624. [Google Scholar]
- Jones, M.T.; Sievert, P.R. Effects of stochastic flood disturbance on adult wood turtles, Glyptemys insculpta, in Massachusetts. Can. Field-Nat. 2009, 123, 313–322. [Google Scholar] [CrossRef]
- Moll, E.; Legler, J. The Life History of a Neotropical Slider Turtle Pseudemys scripta (Schoepff) in Panama; Scientific Bulletin—Natural History Museum of Los Angeles County; Los Angeles Natural History Museum: Los Angeles, CA, USA, 1971; Volume 11, pp. 1–102. [Google Scholar]
- Pluto, T.G.; Bellis, E.D. Seasonal and annual movements of riverine map turtles, Graptemys geographica. J. Herpetol. 1988, 22, 152–158. [Google Scholar] [CrossRef]
- Bury, R. Habits and Home Range of the Pacific Pond Turtle, Clemmys marmorata, in a Stream Community; UCB Press: Berkeley, CA, USA, 1972. [Google Scholar]
- Buhlmann, K.; Vaughan, M. Ecology of the Turtle Pseudemys concinna in the New River, West Virginia. J. Herpetol. 1991, 25, 72–78. [Google Scholar] [CrossRef]
- Jones, R. Home range and seasonal movements of the turtle Graptemys flavimaculata. J. Herpetol. 1996, 30, 376–385. [Google Scholar] [CrossRef]
- Rowe, J.; Dalgarn, S. Home range size and daily movements of midland painted turtles (Chrysemys picta marginata) in relation to body size, sex, and weather patterns. Herpetol. Conserv. Biol. 2010, 5, 461–473. [Google Scholar]
- Palacios, D.M.; Bailey, H.; Becker, E.A.; Bograd, S.J.; DeAngelis, M.L.; Forney, K.A.; Hazen, E.L.; Irvine, L.M.; Mate, B.R. Ecological correlates of blue whale movement behavior and its predictability in the California Current Ecosystem during the summer-fall feeding season. Mov. Ecol. 2019, 7, 1–21. [Google Scholar] [CrossRef]
- Plummer, M.; Shirer, H. Movement Patterns in a River Population of the Softshell Turtle, Trionyx muticus; Occasional papers of the Museum of Natural History; The University of Kansas: Lawrence, KS, USA, 1975; Volume 43, pp. 1–26. [Google Scholar]
- Sexton, O.J. Spatial and temporal movements of a population of the painted turtle, Chrysemys picta marginata (Agassiz). Ecol. Monogr. 1959, 29, 113–140. [Google Scholar] [CrossRef]
- Webb, R. North American Recent Soft-Shelled Turtles (Family Trionychidae); University of Kansas Publications, Museum of Natural History, The University of Kansas: Lawrence, KS, USA, 1962; Volume 13, pp. 429–611. [Google Scholar]
- Bovee, K.; Scott, M. Implications of flood pulse restoration for Populus regeneration on the upper Missouri River. River Res. Appl. 2002, 18, 287–298. [Google Scholar] [CrossRef]
- Flessa, K.W.; Glenn, E.P.; Hinojosa-Huerta, O.; de la Parra-Rentería, C.A.; Ramírez-Hernández, J.; Schmidt, J.C.; Zamora-Arroyo, F.A. Flooding the Colorado River delta: A landscape-scale experiment. EOS Trans. Am. Geophys. Union 2013, 94, 485–486. [Google Scholar] [CrossRef]

| Turtle ID | Transmitter Frequency (MHz) | SCL (mm) | Weight (in g) | Sex | Number of Positional Fixes | Duration (in Days) | Maximum Net Displacement (km) |
|---|---|---|---|---|---|---|---|
| One | 150.400 | 319 | 4210 | F | 215 | 330 | 3.13 |
| Two | 150.420 | 328 | 4430 | F | 382 | 606 | 10.28 |
| Three | 150.440 | 283 | 3290 | F | NA | NA | NA |
| Four | 150.460 | 279 | 2370 | M | 220 | 594 | 2.67 |
| Five | 150.480 | 291 | 3130 | F | 188 | 245 | 35.5 |
| Six | 150.500 | 320 | 3900 | F | 336 | 601 | 1.18 |
| Seven | 150.520 | 291 | 3240 | F | 386 | 640 | 5.31 |
| Eight | 150.540 | 251 | 2170 | M | 207 | 517 | 16.15 |
| Nine | 150.560 | 264 | 2310 | M | 171 | 565 | 10.97 |
| Ten | 150.580 | 267 | 2040 | M | NA | NA | NA |
| Variable | Estimate | SE | Z | p Value |
|---|---|---|---|---|
| Intercept | 5.664 | 0.176 | 32.23 | <0.001 |
| SCL | −0.663 | 0.190 | −3.48 | <0.001 |
| SexM | −0.900 | 0.404 | −2.23 | 0.026 |
| (Day of Year)2 | −0.533 | 0.071 | −7.49 | <0.001 |
| Day of Year | 0.218 | 0.116 | 1.89 | 0.059 |
| Gage Height | 0.127 | 0.045 | 2.84 | 0.005 |
| Streamflow | 0.304 | 0.050 | 6.39 | <0.001 |
| Model | K | QAICc | ΔQAICc | w | ∑ w |
|---|---|---|---|---|---|
| φ(.)p(t)b(t)N(.) | 46 | 2022.06 | 0.00 | 0.979 | 0.979 |
| φ(t)p(t) b(t)N(.) | 66 | 2029.77 | 7.71 | 0.021 | 1.00 |
| φ(.)p(.)b(t)N(.) | 25 | 2067.05 | 44.99 | 0.00 | 1.00 |
| φ(t)p(.) b(t)N(.) | 45 | 2081.51 | 59.45 | 0.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirsi, S.; MacLaren, A.R.; Foley, D.H.; Bohannon, A.M.A.; Rose, J.P.; Halstead, B.J.; Forstner, M.R.J. Big and Fast: GPS Loggers Reveal Long-Range Movements in a Large, Riverine Turtle. Conservation 2025, 5, 6. https://doi.org/10.3390/conservation5010006
Sirsi S, MacLaren AR, Foley DH, Bohannon AMA, Rose JP, Halstead BJ, Forstner MRJ. Big and Fast: GPS Loggers Reveal Long-Range Movements in a Large, Riverine Turtle. Conservation. 2025; 5(1):6. https://doi.org/10.3390/conservation5010006
Chicago/Turabian StyleSirsi, Shashwat, Andrew R. MacLaren, Daniel H. Foley, Austin M. A. Bohannon, Jonathan P. Rose, Brian J. Halstead, and Michael R. J. Forstner. 2025. "Big and Fast: GPS Loggers Reveal Long-Range Movements in a Large, Riverine Turtle" Conservation 5, no. 1: 6. https://doi.org/10.3390/conservation5010006
APA StyleSirsi, S., MacLaren, A. R., Foley, D. H., Bohannon, A. M. A., Rose, J. P., Halstead, B. J., & Forstner, M. R. J. (2025). Big and Fast: GPS Loggers Reveal Long-Range Movements in a Large, Riverine Turtle. Conservation, 5(1), 6. https://doi.org/10.3390/conservation5010006






