Pollen Resource Repartition Between Managed Honey Bees (Apis mellifera L. 1758) and Unmanaged Bees in Three Italian National Parks
Abstract
1. Introduction
2. Materials and Methods
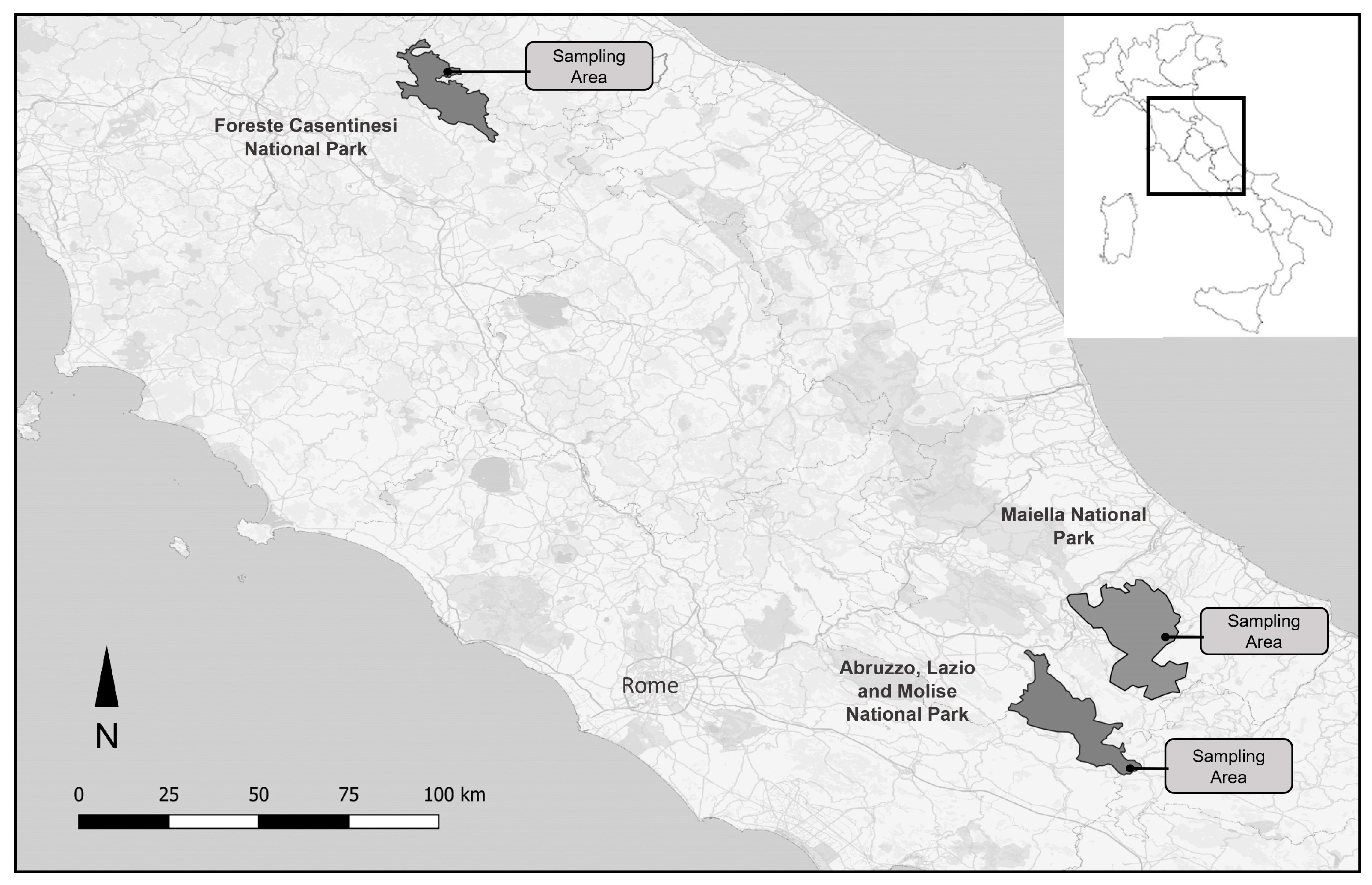
2.1. Study Area and Pollen Sampling
2.2. Palynological Analysis and Taxonomic Identification of Wild Bees
2.3. Data Analysis
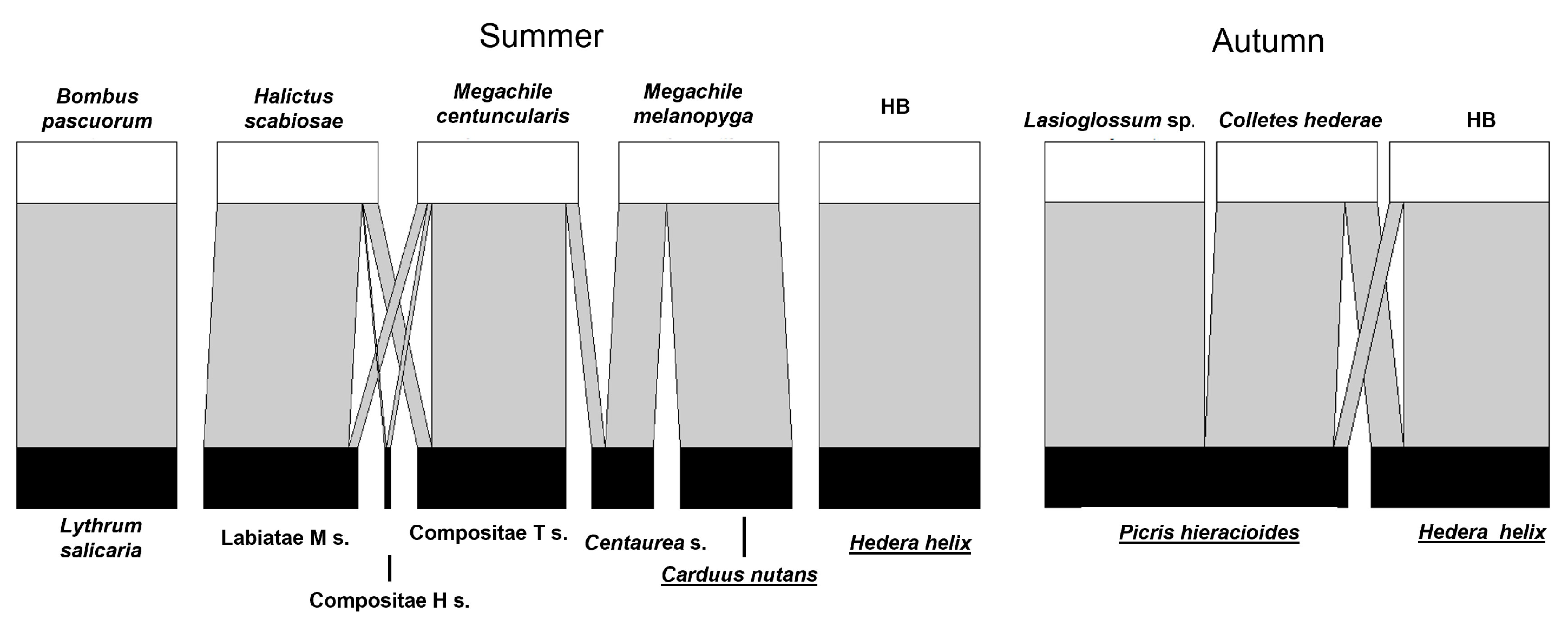
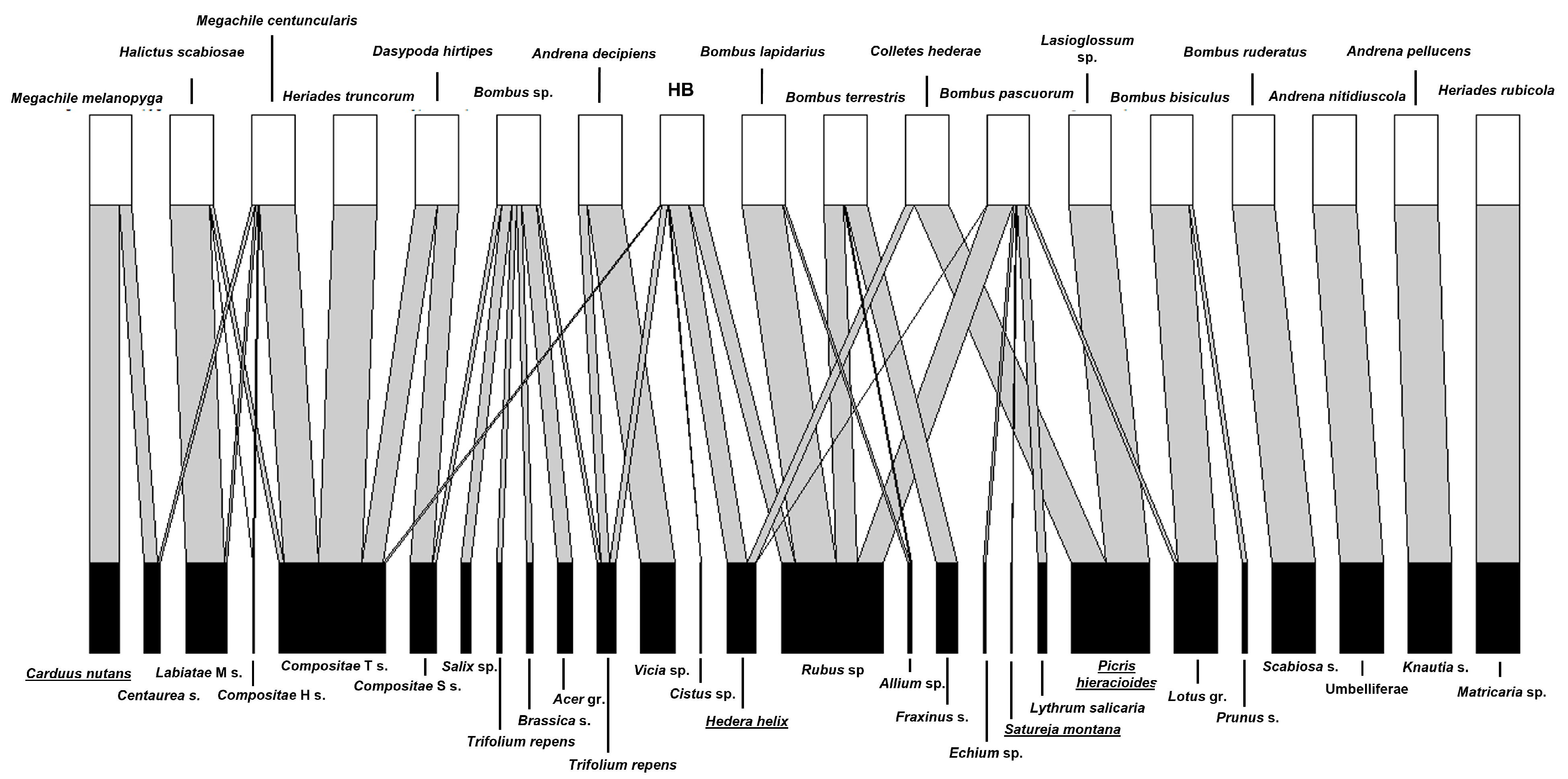
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, R.J.; Baldock, K.C.; Brown, M.J.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Garratt, M.P.; Gough, L.A.; Heard, M.S.; Holland, J.M. Protecting an ecosystem service: Approaches to understanding and mitigating threats to wild insect pollinators. In Advances in Ecological Research; Woodward, G., Bohan, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 135–206. [Google Scholar]
- Requier, F.; Pérez-Méndez, N.; Andersson, G.K.; Blareau, E.; Merle, I.; Garibaldi, L.A. Bee and non-bee pollinator importance for local food security. Trends Ecol. Evol. 2022, 38, 196–205. [Google Scholar] [CrossRef]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of Apoidea as Main Pollinators. Ecological and Economic Impact and Implications for Human Nutrition. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Biella, P.; Bogliani, G.; Cornalba, M.; Manino, A.; Neumayer, J.; Porporato, M.; Rasmont, P.; Milanesi, P. Distribution patterns of the cold adapted bumblebee Bombus alpinus in the Alps and hints of an uphill shift (Insecta: Hymenoptera: Apidae). J. Insect Conserv. 2017, 21, 357–366. [Google Scholar] [CrossRef]
- Sivakoff, F.; Gardiner, M.M. Soil lead contamination decreases bee visit duration at sunflowers. Urban Ecosys. 2017, 20, 1221–1228. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post (2013). Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Xun, E.; Zhang, Y.; Zhao, J.; Guo, J. Heavy metals in nectar modify behaviors of pollinators and nectar robbers: Consequences for plant fitness. Environ. Pollut. 2018, 242, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Paxton, R.J. The conservation of bees: A global perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Quaranta, M.; Cornalba, M.; Biella, P.; Comba, M.; Battistoni, A.; Rondinini, C.; Teofili, C. Lista Rossa IUCN delle Api Italiane Minacciate; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2018. [Google Scholar]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, A.; Bortolotti, L.; Cilia, G. Pathogens spillover from honey bees to other arthropods. Pathogens 2021, 10, 1044. [Google Scholar] [CrossRef]
- Cilia, G.; Flaminio, S.; Ranalli, R.; Zavatta, L.; Nanetti, A.; Bortolotti, L.; Bogo, G. Presence of Apis mellifera pathogens in different developmental stages of wild Hymenoptera species. Bull. Insectology 2023, 76, 147–154. [Google Scholar]
- Fontana, P.; Costa, C.; Di Prisco, G.; Ruzzier, E.; Annoscia, D.; Battisti, A.; Caoduro, G.; Carpana, E.; Contessi, A.; Dal Lago, A.; et al. Appeal for biodiversity protection of native honey bee subspecies of Apis mellifera in Italy (San Michele all’Adige declaration). Bull. Insectology 2018, 2, 257–271. [Google Scholar]
- Tsuchida, K.; Yamaguchi, A.; Kanbe, Y.; Goka, K. Reproductive interference in an introduced bumblebee: Polyandry may mitigate negative reproductive impact. Insects 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.H. Not just cryptic, but a barcode bush: PTP reanalysis of global data for the bumblebee subgenus Bombus s. str. supports additional species (Apidae, genus Bombus). J. Nat. Hist. 2021, 55, 271–282. [Google Scholar] [CrossRef]
- Boni, C.B.; Coppola, F.; Quaranta, M.; Giannini, F.; Felicioli, A. Bombus terrestris terrestris (Linnaeus, 1758) and hybrids with the endemic Bombus xanthopus spotted on Capraia Island (Tuscan Archipelago, Italy): Some conservation management implications. Sci. Nat. 2023, 110, 14. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, V.A.; Morandin, L.A.; Davies Adams, L.; Rourke, K.E. Floral Resource Competition Between Honey Bees and Wild Bees: Is There Clear Evidence and Can We Guide Management and Conservation? Environ. Entomol. 2018, 47, 822–833. [Google Scholar] [CrossRef]
- Iwasaki, J.M.; Hogendoorn, K. Mounting evidence that managed and introduced bees have negative impacts on wild bees: An updated review. Curr. Res. Insect Sci. 2022, 2, 100043. [Google Scholar] [CrossRef] [PubMed]
- Paini, D.R.; Williams, M.R.; Roberts, J.D. No short-term impact of honey bees on the reproductive success of an Australian native bee. Apidologie 2005, 36, 613–621. [Google Scholar] [CrossRef]
- Goulson, D.; Sparrow, K.R. Evidence for competition between honeybees and bumblebees; effects on bumblebee worker size. J. Insect Conserv. 2009, 13, 177–181. [Google Scholar] [CrossRef]
- Hudewenz, A.; Klein, A.M. Red mason bees cannot compete with honey bees for floral resources in a cage experiment. Ecol. Evol. 2015, 5, 5049–5056. [Google Scholar] [CrossRef]
- Torné-Noguera, A.; Rodrigo, A.; Osorio, S.; Bosch, J. Collateral effects of beekeeping: Impacts on pollen-nectar resources and wild bee communities. Basic Appl. Ecol. 2016, 17, 199–209. [Google Scholar] [CrossRef]
- Lindström, S.A.; Herbertsson, L.; Rundlöf, M.; Bommarco, R.; Smith, H.G. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161641. [Google Scholar] [CrossRef] [PubMed]
- Ropars, L.; Affre, L.; Thébault, É.; Geslin, B. Seasonal dynamics of competition between honey bees and wild bees in a protected Mediterranean scrubland. Oikos 2022, 4, e08915. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 4711. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Rodet, G. Controlling the impact of the managed honeybee on wild bees in protected areas. Sci. Rep. 2018, 8, 9308. [Google Scholar] [CrossRef]
- Herrera, C.M. Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192657. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, A.; Müller, A.; Ebmer, A.W.; Dathe, H.H.; Scheuchl, E.; Schwarz, M.; Risch, S.; Pauly, A.; Devalez, J.; Tscheulin, T. Impacts of beekeeping on wild bee diversity and pollination networks in the Aegean Archipelago. Ecography 2021, 44, 1353–1365. [Google Scholar] [CrossRef]
- Huryn, V.M.B. Ecological impacts of introduced honey bees. Q. Rev. Biol. 1997, 72, 275–297. [Google Scholar]
- Horskins, K.; Turner, V.B. Resource use and foraging patterns of honeybees, Apis mellifera, and native insects on flowers of Eucalyptus costata. Austral Ecol. 1999, 24, 221–227. [Google Scholar] [CrossRef]
- Goras, G.; Tananaki, C.; Dimou, M.; Tscheulin, T.; Petanidou, T.; Thrasyvoulou, A. Impact of honeybee (Apis mellifera L.) density on wild bee foraging behaviour. J. Apic. Sci. 2016, 60, 49–62. [Google Scholar] [CrossRef]
- Paini, D.R. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: A review. Austral Ecol. 2004, 29, 399–407. [Google Scholar] [CrossRef]
- White, J.W. Honey. In Advances in Food Research; Chichester, C.O., Ed.; Academic Press: Cambridge, MA, USA, 1978; pp. 287–374. [Google Scholar] [CrossRef]
- Rodney, S.; Purdy, J. Dietary requirements of individual nectar foragers, and colony-level pollen and nectar consumption: A review to support pesticide exposure assessment for honey bees. Apidologie 2020, 51, 163–179. [Google Scholar] [CrossRef]
- Markwell, T.J.; Kell, D.; Duncan, K.W. Competition between honey bees (Apis mellifera) and wasps (Vespula spp.) in honeydew beech (Nothogafus solandri var. solandris) forest. N. Z. J. Ecol. 1993, 17, 85–93. [Google Scholar]
- Müller, A.; Diener, S.; Schnyder, S.; Stutz, K.; Sedivy, C.; Dorn, S. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee–flower relationships. Biol. Conserv. 2006, 130, 604–615. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Nicolson, S.W. Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Felicioli, A. The Rearing of Megachilids. In PP-ICON—Plant-Pollinator Integrated Conservation Approach: A Demonstrative Proposal Technical Handbook; 2015; Available online: https://www.fondazionevillaghigi.it/wp-content/uploads/2016/08/Dictamnus-report.pdf (accessed on 16 October 2024).
- Balfour, N.J.; Gandy, S.; Ratnieks, F.L. Exploitative competition alters bee foraging and flower choice. Behav. Ecol. Sociobiol. 2015, 69, 1731–1738. [Google Scholar] [CrossRef]
- Smith-Ramírez, C.; Ramos-Jiliberto, R.; Valdovinos, F.S.; Martínez, P.; Castillo, J.A.; Armesto, J.J. Decadal trends in the pollinator assemblage of Eucryphia cordifolia in Chilean rainforests. Oecologia 2014, 176, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hudewenz, A.; Klein, A.M. Competition between honey bees and wild bees and the role of nesting resources in a nature reserve. J. Insect Conserv. 2013, 17, 1275–1283. [Google Scholar] [CrossRef]
- Cini, A.; Benetello, F.; Bonifacino, M.; Salvati, V.; Monterastelli, E.; Pasquali, L.; Sistri, G.; Dani, F.R.; Dapporto, L. Simple and informative: Applying a basic Anthophila monitoring scheme in a simplified insular ecosystem. Bull. Insectology 2022, 75, 83–95. [Google Scholar]
- Hairston, N.G.; Smith, F.E.; Slobodkin, L.B. Community structure, population control, and competition. Am. Nat. 1960, 94, 421–425. [Google Scholar] [CrossRef]
- Tommasi, N.; Ferrari, A.; Labra, M.; Galimberti, A.; Biella, P. Harnessing the power of metabarcoding in the ecological interpretation of plant-pollinator DNA data: Strategies and consequences of filtering approaches. Diversity 2021, 13, 437. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jennions, M.D.; Zalucki, M.P.; Maron, M.; Watson, J.E.; Fuller, R.A. Protected areas and the future of insect conservation. Trends Ecol. Evol. 2022, 38, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Stolton, S.; Dudley, N.; Avcıoğlu Çokçalışkan, B.; Hunter, D.; Ivanić, K.Z.; Kanga, E.; Kettunen, M.; Kumagai, Y.; Maxted, N.; Senior, J.; et al. Values and benefits of protected areas. In Protected Area Governance and Management; Worboys, G.L., Lockwood, M., Kothari, A., Feary, S., Pulsford, I., Eds.; ANU Press: Canberra, Australia, 2015; pp. 145–168. [Google Scholar]
- Henry, M.; Rodet, G. The apiary influence range: A new paradigm for managing the cohabitation of honey bees and wild bee communities. Acta Oecol. 2020, 105, 103555. [Google Scholar] [CrossRef]
- Elliot, B.; Wilson, R.; Shapcott, A.; Keller, A.; Newis, R.; Cannizzaro, C.; Burwell, C.; Smith, T.; Leonhardt, S.D.; Kamper, W.; et al. Pollen diets and niche overlap of honey bees and native bees in protected areas. Basic Appl. Ecol. 2021, 50, 169–180. [Google Scholar] [CrossRef]
- Mouillard-Lample, L.; Gonella, G.; Decourtye, A.; Henry, M.; Barnaud, C. Competition between wild and honey bees: Floral resources as a common good providing multiple ecosystem services. Ecosyst. Serv. 2023, 62, 101538. [Google Scholar] [CrossRef]
- Ricciardelli D’Albore, G.; Persano Oddo, L. Flora apistica italiana. In Edizione Istituto Sperimentale per la Zoologia Agrarian; GPR: Rome, Italy, 1978. [Google Scholar]
- Ricciardelli D’Albore, G. Textbook of Melissopalynology; Apimondia; Publishing House Bucharest: Bucharest, Romania, 1997. [Google Scholar]
- Ricciardelli D’Albore, G. Mediterranean Melissopalynology; Istituto di Entomologia Agraria, Università degli Studi, Perugia: Perugia, Italy, 1998. [Google Scholar]
- Palmieri, N.; Grillenzoni, F.V.; Corvucci, F.; Biondi, C.; Bedini, G.; Floris, I. Guida allo Studio della Melissopalinologia; CREA-Centro di Ricerca Agricoltura e Ambiente, Università di Sassari-Dipartimento di Agraria, Studio Naturalistico-Il Pianeta Naturale, Associazione Apicoltori della Sardegna Apiaresos de Abarèe; Tipografia Gallizzi: Sassari, Italy, 2017. [Google Scholar]
- Rasmont, P.; Ghisbain, G.; Terzo, M. Bumblebees of Europe and Neighbouring Regions; NAP Editions: Verrières-le-Buisson, France, 2021. [Google Scholar]
- Biella, P.; Tommasi, N.; Akter, A.; Guzzetti, L.; Klecka, J.; Sandionigi, A.; Labra, M.; Galimberti, A. Foraging strategies are maintained despite workforce reduction: A multidisciplinary survey on the pollen collected by a social pollinator. PLoS ONE 2019, 14, e0224037. [Google Scholar]
- Tommasi, N.; Biella, P.; Maggioni, D.; Fallati, L.; Agostinetto, G.; Labra, M.; Galimberti, A. DNA metabarcoding unveils the effects of habitat fragmentation on pollinator diversity, plant-pollinator interactions, and pollination efficiency in Maldive islands. Mol. Ecol. 2023, 32, 6394–6404. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Sponsler, D.B.; McMinn-Sauder, H.; Johnson, R.M. MetaCurator: A hidden Markov model-based toolkit for extracting and curating sequences from taxonomically-informative genetic markers. Methods Ecol. Evol. 2020, 11, 181–186. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Evol. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open J. Ecol. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Lecocq, T.; Biella, P.; Martinet, B.; Rasmont, P. Too strict or too loose? Integrative taxonomic assessment of Bombus lapidarius complex (Hymenoptera: Apidae). Zool. Scr. 2020, 49, 187–196. [Google Scholar] [CrossRef]
- Kajobe, R. Pollen foraging by Apis mellifera and stingless bees Meliponula bocandei and Meliponula nebulata in Bwindi Impenetrable National Park, Uganda. Afr. J. Ecol. 2007, 45, 265. [Google Scholar] [CrossRef]
- Roubik, D.W.; Villanueva-Gutierrez, R. Invasive Africanized honey bee impact on native solitary bees: A pollen resource and trap nest analysis. Biol. J. Linn. Soc. 2009, 98, 152–160. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Bluthgen, N. The same, but different: Pollen foraging in honeybee and bumblebee colonies. Apidologie 2012, 43, 449–464. [Google Scholar] [CrossRef]
- Nakamura, Y. Differences in Pollen Resource Usage and Foraging Periods between the Exotic Bumblebee Bombus terrestris and the Native B. pseudobaicalensis and B. hypocrita sapporoensis in Hokkaido, Japan. Eurasian J. For. Res. 2014, 17, 1–10. [Google Scholar]
- Ferreira, M.G.; Absy, M.L. Pollen analysis of honeys of Melipona (Michmelia) seminigra merrillae and Melipona (Melikerria) interrupta (Hymenoptera: Apidae) bred in Central Amazon, Brazil. Grana 2017, 56, 436–449. [Google Scholar] [CrossRef]
- Marín-Henao, D.; Quijano-Abril, M.; Sánchez, C.E.G.; Calvo-Cardona, S.J.; Zapata-Vahos, I.C. Limited foraging overlap between introduced Apis mellifera and native Melipona eburnea in a Colombian moist forest as revealed through pollen analysis. Palynology 2021, 46, 1–14. [Google Scholar] [CrossRef]
- Allen, G.; Davies, R.G. Canopy sampling reveals hidden potential value of woodland trees for wild bee assemblages. Insect Conserv. Divers. 2023, 16, 33–46. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Shimamura, T.; Kudo, G.; Yabe, K. Habitat use and floral resource partitioning of native and alien bumblebees in the coastal grassland—Rural landscape. J. Insect Conserv. 2019, 23, 677–687. [Google Scholar] [CrossRef]
- Novella-Fernandez, R.; Rodrigo, A.; Arnan, X.; Bosch, J. Interaction strength in plant-pollinator networks: Are we using the right measure? PLoS ONE 2019, 14, e0225930. [Google Scholar] [CrossRef] [PubMed]
- Librán-Embid, F.; Grass, I.; Emer, C.; Ganuza, C.; Tscharntke, T. A plant–pollinator metanetwork along a habitat fragmentation gradient. Ecol. Lett. 2021, 24, 2700–2712. [Google Scholar] [CrossRef]
- Eltz, T.; Brühl, C.A.; van der Kaars, S.; Chey, V.K.; Linsenmair, K.E. Pollen foraging and resource partitioning of stingless bees in relation to flowering dynamics in a Southeast Asian tropical rainforest. Insectes Soc. 2001, 48, 273–279. [Google Scholar] [CrossRef]
- Sánchez, D.; Solórzano-Gordillo, E.; Vandame, R. A study on intraspecific resource partitioning in the stingless bee Scaptotrigona mexicana Guérin (Apidae, Meliponini) using behavioral and molecular techniques. Neotrop. Entomol. 2016, 45, 518–523. [Google Scholar] [CrossRef]
- Pleasants, J.M. Bumblebee response to variation in nectar availability. Ecology 1981, 62, 1648–1661. [Google Scholar] [CrossRef]
- Thomson, D.M. Detecting the effects of introduced species: A case study of competition between Apis and Bombus. Oikos 2006, 114, 407–418. [Google Scholar] [CrossRef]
- Franco, E.L.; Aguiar, M.; Ferreira, V.S.; de Oliveira-Reboucas, P.L. Plant use and niche overlap between the introduced honey bee (Apis mellifera) and the native bumblebee (Bombus atratus) (Hymenoptera: Apidae) in an area of tropical mountain vegetation in northeastern Brazil. Sociobiology 2009, 53, 141–150. [Google Scholar]
- Thomson, D.M. Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecol. Lett. 2016, 19, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, A.; Bonaldi, G.; Mei, M.; Paniccia, D.; Cerretti, P.; Marini, L. Functional traits of plants and pollinators explain resource overlap between honeybees and wild pollinators. Oecologia 2022, 198, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A. Intraspecific Resource Partitioning in the Bumble Bees Bombus Ternarius and B. Pennsylvanicus. Ecology 1986, 67, 133–138. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Gratton, C. Flexible foraging shapes the topology of plant–pollinator interaction networks. Ecology 2016, 97, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Corbet, S.A. A typology of pollination systems: Implications for crop management and the conservation of wild plants. In Plant Pollinator Interactions from Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 315–340. [Google Scholar]
- Hawkins, J.; de Vere, N.; Griffith, A.; Ford, C.R. Using DNA metabarcoding to Identify the floral composition of honey: A new tool for investigating honey bee foraging preferences. PLoS ONE 2015, 10, e0134735. [Google Scholar] [CrossRef]
- Felicioli, A.; Sabatini, A.G.; Pinzauti, M. Risorsa trofica e competizione tra Osmia rufa L. e Osmia cornuta Latr. (Hymenoptera, megachilidae) in tre ecosistemi. In Proceedings of the Api Lombardia 98. Giornate di Studio Sull’apicoltura, Minoprio (Como), Italy, 25–27 September 1998; Sabatini, A.G., Colombo, M., Spreafico, M., Eds.; Litografia Polaris: Sondrio, Italy, 1999. [Google Scholar]
- Eckerter, P.W.; Albrecht, M.; Herzog, F.; Entling, M.H. Floral resource distribution and fitness consequences for two solitary bee species in agricultural landscapes. Basic Appl. Ecol. 2022, 65, 1–15. [Google Scholar] [CrossRef]
- Roubik, D.W.; Moreno, J.E.; Vergara, C.; Wittmann, D. Sporadic Food Competition with the African Honey Bee: Projected Impact on Neotropical Social Bees. J. Trop. Ecol. 1986, 2, 97–111. [Google Scholar] [CrossRef]
- Bischoff, I.; Eckelt, E.; Kuhlmann, M. On the biology of the ivy-bee Colletes hederae Schmidt and Westrich, 1993 (Hymenoptera, Apidae). Bonn. Zool. Beitr. 2005, 53, 27–36. [Google Scholar]
- Teppner, H.; Brosch, U. Pseudo-oligolecty in Colletes hederae (Apidae-Colletinae, Hymenoptera). Linz. Biol. Beitr. 2015, 47, 301–306. [Google Scholar]
- Samways, M.J.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Solutions for humanity on how to conserve insects. Biol. Conserv. 2020, 242, 108427. [Google Scholar] [CrossRef]
- Iwasaki, J.M.; Hogendoorn, K. How protection of honey bees can help and hinder bee conservation. Curr. Opin. Insect Sci. 2021, 46, 112–118. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, C.B.; Coppola, F.; Sagona, S.; Quaranta, M.; Flaminio, S.; Biella, P.; Tempesti, S.; Lazzeri, A.M.; Di Santo, M.; Felicioli, A. Pollen Resource Repartition Between Managed Honey Bees (Apis mellifera L. 1758) and Unmanaged Bees in Three Italian National Parks. Conservation 2025, 5, 5. https://doi.org/10.3390/conservation5010005
Boni CB, Coppola F, Sagona S, Quaranta M, Flaminio S, Biella P, Tempesti S, Lazzeri AM, Di Santo M, Felicioli A. Pollen Resource Repartition Between Managed Honey Bees (Apis mellifera L. 1758) and Unmanaged Bees in Three Italian National Parks. Conservation. 2025; 5(1):5. https://doi.org/10.3390/conservation5010005
Chicago/Turabian StyleBoni, Chiara Benedetta, Francesca Coppola, Simona Sagona, Marino Quaranta, Simone Flaminio, Paolo Biella, Stefano Tempesti, Anna Marta Lazzeri, Marco Di Santo, and Antonio Felicioli. 2025. "Pollen Resource Repartition Between Managed Honey Bees (Apis mellifera L. 1758) and Unmanaged Bees in Three Italian National Parks" Conservation 5, no. 1: 5. https://doi.org/10.3390/conservation5010005
APA StyleBoni, C. B., Coppola, F., Sagona, S., Quaranta, M., Flaminio, S., Biella, P., Tempesti, S., Lazzeri, A. M., Di Santo, M., & Felicioli, A. (2025). Pollen Resource Repartition Between Managed Honey Bees (Apis mellifera L. 1758) and Unmanaged Bees in Three Italian National Parks. Conservation, 5(1), 5. https://doi.org/10.3390/conservation5010005







