Agroecological Weed Management and the Potential Role of Fungi-Based Bioherbicides in Conservation: Advantages, Applications and Future Prospects
Abstract
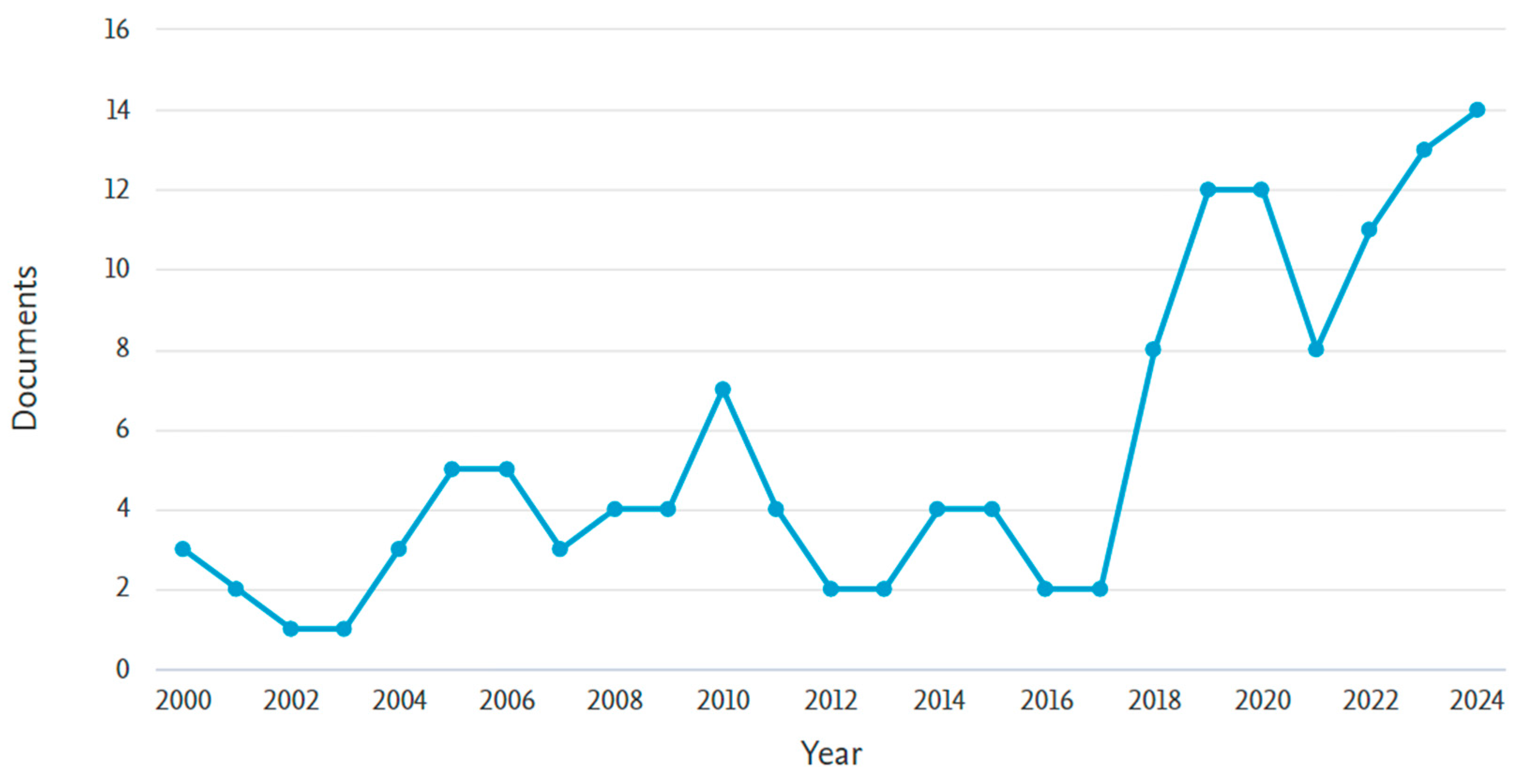
1. Introduction
2. Bioherbicides
2.1. Advantages of Bioherbicides Based on Fungi
2.2. Disadvantages of Bioherbicides Based on Fungi
2.3. Formulation of Bioherbicides
2.4. Selectivity
3. Effects of Fungi-Based Herbicides on Weeds
Effects of Fungal Bioherbicides in Combination with Herbicides
4. Current Status of Fungal Bioherbicides in Weed Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- van der Meulen, A.; Chauhan, B.S. A review of weed management in wheat using crop competition. Crop Prot. 2017, 95, 38–44. [Google Scholar] [CrossRef]
- Chauhan, B.S. Grand challenges in weed management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, V.; Godara, R.K.; Chauhan, B.S. Weed management using crop competition in the United States: A review. Crop Prot. 2017, 95, 31–37. [Google Scholar] [CrossRef]
- Peerzada, A.M.; Bukhari, S.A.H.; Dawood, M.; Nawaz, A.; Ahmad, S.; Adkins, S. Weed management for healthy crop production. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 225–256. ISBN 978-981-32-9783-8. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Neve, P.; Barney, J.N.; Buckley, Y.; Cousens, R.D.; Graham, S.; Jordan, N.R.; Lawton-Rauh, A.; Liebman, M.; Mesgaran, M.B.; Schut, M.; et al. Reviewing research priorities in weed ecology, evolution and management: A horizon scan. Weed Res. 2018, 58, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hamill, A.S.; Holt, J.S.; Mallory-Smith, C.A. Contributions of Weed Science to Weed Control and Management1. Weed Technol. 2004, 18 (Suppl. 1), 1563–1565. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Fernando, W.G.D.; Tennakoon, K.U. Achievements, Developments and Future Challenges in the Field of Bioherbicides for Weed Control: A Global Review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef]
- Triolet, M.; Guillemin, J.P.; Andre, O.; Steinberg, C. Fungal-based bioherbicides for weed control: A myth or a reality? Weed Res. 2020, 60, 60–77. [Google Scholar] [CrossRef]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef]
- Travlos, I.; Rapti, E.; Gazoulis, I.; Kanatas, P.; Tataridas, A.; Kakabouki, I.; Papastylianou, P. The herbicidal potential of different pelargonic acid products and essential oils against several important weed species. Agronomy 2020, 10, 1687. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 15 January 2024).
- Ziska, L.H. The role of climate change and increasing atmospheric carbon dioxide on weed management: Herbicide efficacy. Agric. Ecosyst. Environ. 2016, 231, 304–309. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, F.J.; Jensen, F.D.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest. Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Pacanoski, Z. Bioherbicides. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: London, UK, 2015; ISBN 978-953-51-2217-3. [Google Scholar]
- Souza, A.R.C.D.; Baldoni, D.B.; Lima, J.; Porto, V.; Marcuz, C.; Machado, C.; Ferraz, R.C.; Kuhn, R.C.; Jacques, R.J.C.; Guedes, J.V.C.; et al. Selection, isolation, and identification of fungi for bioherbicide production. Braz. J. Microbiol. 2017, 48, 101–108. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Khandelwal, A.; Phour, M.; Sehrawat, A. Bioherbicidal potential of rhizosphere microorganisms for ecofriendly weed management. In Role of Rhizospheric Microbes in Soil: Volume 1: Stress Management and Agricultural Sustainability; Meena, V.S., Ed.; Springer: Singapore, 2018; Volume 1, pp. 331–376. ISBN 978-981-10-8402-7. [Google Scholar]
- Gressel, J. Herbicides as Synergists for Mycoherbicides, and Vice Versa. Weed Sci. 2010, 58, 324–328. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Aneja, K.R.; Khan, S.A.; Aneja, A. Bioherbicides: Strategies, challenges and prospects. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S.K., Johri, B.N., Eds.; Springer: Singapore, 2017; pp. 449–470. ISBN 978-981-10-4768-8. [Google Scholar]
- Saitheja, V.; Thirukumaran, K.; Sendhilvel, V.; Karthikeyan, R.; Kalarani, M.K.; Vellaikumar, S.; Parasuraman, P.; Sangeetha, S.P.; Abhinaya, T. Scope and potential of herbicidal values of the fungal pathogens and its secondary metabolites for sustainable weed management. Plant Protect. Sci. 2024, 60, 109–126. [Google Scholar] [CrossRef]
- de Souza Barros, V.M.; Pedrosa, J.L.F.; Gonçalves, D.R.; Medeiros, F.C.L.D.; Carvalho, G.R.; Gonçalves, A.H.; Teixeira, P.V.V.Q. Herbicides of biological origin: A review. J. Hortic. Sci. Biotech. 2021, 96, 288–296. [Google Scholar] [CrossRef]
- Aneja, K.R. Non-chemical management of weeds through bioherbicides: Current status, market, development, constraints and future prospects. Braz. J. Dev. 2024, 10, e67432. [Google Scholar] [CrossRef]
- Bordin, E.R.; Frumi Camargo, A.; Stefanski, F.S.; Scapini, T.; Bonatto, C.; Zanivan, J.; Preczeski, K.; Modkovski, T.A.; Reichert, F., Jr.; Mossi, A.J.; et al. Current production of bioherbicides: Mechanisms of action and technical and scientific challenges to improve food and environmental security. Biocatal. Agric. Biotechnol. 2021, 39, 346–359. [Google Scholar] [CrossRef]
- Mathur, M.; Gehlot, P. Recruit the Plant Pathogen for Weed Management: Bioherbicide–A Sustainable Strategy. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 159–181. ISBN 978-981-13-0393-7. [Google Scholar]
- Bo, A.B.; Khaitov, B.; Umurzokov, M.; Cho, K.M.; Park, K.W.; Choi, J.S. Biological control using plant pathogens in weed management. Weed Turf. Sci. 2020, 9, 11–19. [Google Scholar] [CrossRef]
- Boyette, C.D.; Quimby, P.C.; Caesar, A.J.; Birdsall, J.L.; Connick, W.J.; Daigle, D.J.; Jackson, M.A.; Egley, G.H.; Abbas, H.K. Adjuvants, Formulations, and Spraying Systems for Improvement of Mycoherbicides. Weed Tech. 1996, 10, 637–644. [Google Scholar] [CrossRef]
- Auld, B.A.; Hetherington, S.D.; Smith, H.E. Advances in bioherbicide formulation. Weed Biol. Manag. 2003, 3, 61–67. [Google Scholar] [CrossRef]
- Morin, L. Progress in biological control of weeds with plant pathogens. Annu. Rev. Phytopathol. 2020, 58, 201–223. [Google Scholar] [CrossRef]
- Kremer, R.J. Bioherbicide development and commercialization: Challenges and benefits. In Development and Commercialization of Biopesticides; Academic Press: Cambridge, MA, USA, 2023; Volume 7, pp. 119–148. [Google Scholar]
- Evidente, A. Specialized Metabolites Produced by Phytotopatogen Fungi to Control Weeds and Parasite Plants. Microorganisms 2023, 11, 843. [Google Scholar] [CrossRef]
- Klaic, R.; Kuhn, R.C.; Foletto, E.L.; Dal Prá, V.; Jacques, R.J.S.; Guedes, J.V.C.; Treichel, H.; Mossi, A.J.; Oliveira, D.; Oliveira, J.V.; et al. An overview regarding bioherbicide and their production methods by fermentation. In Fungal Biomolecules; Gupta, V.K., Mach, R.L., Sreenivasaprasad, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 183–199. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Deka, B.; Bharani, R.A.; Samrat, K.; Kavisri, M.; Moovendhan, M. Impact of formulation on the fungal biomass–based herbicidal activity and phytotoxic metabolite production. Biomass Conv. Bioref. 2023, 14, 24765–24786. [Google Scholar] [CrossRef]
- Gupta, S.; Dubey, S.; Singh, J. Plant Pathogenic Fungi and Their Phytotoxins as Bioherbicides. In Applied Mycology: Entrepreneurship with Fungi; Shukla, A.C., Ed.; Springer: Cham, Germany, 2022; pp. 365–377. ISBN 978-3-030-90649-8. [Google Scholar]
- Júnior, F.W.R.; Scariot, M.A.; Forte, C.T.; Pandolfi, L.; Dil, J.M.; Weirich, S.; Carezia, C.; Mulinari, J.; Mazutti, M.A.; Fongaro, G.; et al. New perspectives for weeds control using autochthonous fungi with selective bioherbicide potential. Heliyon 2019, 5, e01676. [Google Scholar] [CrossRef]
- Dubovik, V.; Dalinova, A.; Berestetskiy, A. Effect of adjuvants on herbicidal activity and selectivity of three phytotoxins produced by the fungus, Stagonospora cirsii. Plants 2020, 9, 1621. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Bello, O.M.; Pradeep, M.; Jolly, R.S. Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ. Tech. Innov. 2019, 13, 304–317. [Google Scholar] [CrossRef]
- Gressel, J.; Michaeli, D.; Kampel, V.; Amsellem, Z.; Warshawsky, A. Ultralow calcium requirements of fungi facilitate use of calcium regulating agents to suppress host calcium-dependent defenses, synergizing infection by a mycoherbicide. J. Agric. Food Chem. 2002, 50, 6353–6360. [Google Scholar] [CrossRef]
- Boyette, C.D.; Hoagland, R.E.; Stetina, K.C. Biological control of the weed hemp sesbania (Sesbania exaltata) in rice (Oryza sativa) by the fungus Myrothecium verrucaria. Agronomy 2014, 4, 74–89. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Prasad, G.S.; Adejumo, I.O. Effect of Lasiodiplodia pseudotheobromae isolates, a potential bioherbicide for Amaranthus hybridus L. in maize culture. Not. Sci. Biol. 2017, 9, 131–137. [Google Scholar] [CrossRef]
- Pes, M.P.; Mazutti, M.A.; Almeida, T.C.; Curioletti, L.E.; Melo, A.A.; Guedes, J.V.; Kuhn, R.C. Bioherbicide based on Diaporthe sp. secondary metabolites in the control of three tough weeds. Afr. J. Agric. Res. 2016, 11, 4242–4249. [Google Scholar] [CrossRef]
- Boyette, C.D.; Hoagland, R.E.; Stetina, K.C. Efficacy improvement of a bioherbicidal fungus using a formulation-based approach. Americ. J. Plant Sci. 2016, 7, 2349–2358. [Google Scholar] [CrossRef]
- Kadir, J.; Charudattan, R. Dactylaria higginsii, a Fungal Bioherbicide Agent for Purple Nutsedge (Cyperus rotundus). Biol. Control 2000, 17, 113–124. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, J.C.; Hong, K.S.; Kim, C.J. Evaluation of a fungal strain, Myrothecium roridum F0252, as a bioherbicide agent. Plant Pathol. J. 2008, 24, 453–460. [Google Scholar] [CrossRef]
- Sotelo-Cerón, N.D.; Maldonado-Mendoza, I.E.; Leyva-Madrigal, K.Y.; Martínez-Álvarez, J.C. Isolation, selection, and identification of phytopathogenic fungi with bioherbicide potential for the control of field bindweed (Convolvulus arvensis L.). Weed Biol. Manag. 2023, 23, 99–109. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Watson, A.K.; Ditommaso, A.; Hurle, K. Effect of the pre-emergence bioherbicide Phomopsis convolvulus on seedling and established plant growth of Convolvulus arvensis. Weed Res. 1998, 38, 175–182. [Google Scholar] [CrossRef]
- Srisuksam, C.; Yodpanan, P.; Suntivich, R.; Tepboonrueng, P.; Wattananukit, W.; Jongsareejit, B.; Amnuaykanjanasin, A. The fungus Phoma multirostrata is a host-specific pathogen and a potential biocontrol agent for a broadleaf weed. Fungal Biol. 2022, 126, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Abdessemed, N.; Bahet, Y.A.; Zermane, N. Mycoherbicide potential of Alternaria alternata (Fries.) Kiessler and its formulations on the host weed Xanthium strumarium L. Biocontrol Sci. Tech. 2020, 30, 1300–1315. [Google Scholar] [CrossRef]
- Xiao, W.; Li, J.; Zhang, Y.; Guo, Y.; Fang, W.; Valverde, B.E.; Yin, J.; Qiang, S.; Chen, S. A fungal Bipolaris bicolor strain as a potential bioherbicide for goosegrass (Eleusine indica) control. Pest Manag. Sci. 2022, 78, 1251–1264. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, Y.; Guo, Q.; Xu, B. Biological weed control using Trichoderma polysporum strain HZ-31. Crop Prot. 2020, 134, 105161. [Google Scholar] [CrossRef]
- Hoagland, R.E. Chemical interactions with bioherbicides to improve efficacy. Weed Tech. 1996, 10, 651–674. [Google Scholar] [CrossRef]
- Boyette, C.D.; Hoagland, R.E.; Weaver, M.A.; Stetina, K.C. Interaction of the bioherbicide Myrothecium verrucaria and glyphosate for kudzu control. Am. J. Plant Sci. 2014, 5, 3943. [Google Scholar] [CrossRef]
- Hoagland, R.E.; Boyette, C.D.; Jordan, R.H.; Stetina, K.C. Interaction of the bioherbicide Myrothecium verrucaria with technical-grade glyphosate on glyphosate-susceptible and-resistant palmer amaranth. Am. J. Plant Sci. 2018, 9, 2306. [Google Scholar] [CrossRef]
- Weaver, M.A.; Jin, X.; Hoagland, R.E.; Boyette, C.D. Improved bioherbicidal efficacy by Myrothecium verrucaria via spray adjuvants or herbicide mixtures. Biol. Control 2009, 50, 150–156. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Yerkes, C.N.; Racine, S.R.; Lewis, E.H. The interaction of two potential fungal bioherbicides and a sub-lethal rate of glyphosate for the control of shattercane. Biol. Control 2008, 46, 391–399. [Google Scholar] [CrossRef]
- Ulrich, A.; Müller, C.; Gasparetto, I.G.; Bonafin, F.; Diering, N.L.; Camargo, A.F.; Reichert, F.W., Jr.; Paudel, N.R.; Treichel, H.; Mossi, A.J. Bioherbicide effects of Trichoderma koningiopsis associated with commercial formulations of glyphosate in weeds and soybean plants. Crop Prot. 2023, 172, 106346. [Google Scholar] [CrossRef]
- Graham, G.L. Synergism of Colletotrichum truncatum with Herbicides for Control of Scentless Chamomile (Matricaria perforata). Ph.D. Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2004. [Google Scholar]
- Vurro, M.; Zonno, M.C.; Evidente, A.; Andolfi, A.; Montemurro, P. Enhancement of efficacy of Ascochyta caulina to control Chenopodium album by use of phytotoxins and reduced rates of herbicides. Biol. Control 2001, 21, 182–190. [Google Scholar] [CrossRef]
- Boyette, C.D.; Hoagland, R.E.; Stetina, K.C. Interaction of a bioherbicidal fungus with a phenoxy herbicide for controlling velvetleaf (Abutilon theophrasti). Biocontrol Sci. Tech. 2024, 34, 323–335. [Google Scholar] [CrossRef]
- Gera, V.K.; Kumar, P.R.; Babu, Y.R. Molecular identification and phylogenetic analysis of Alternaria perpunctulata GVKNSV7 causing leaf spot disease on Alternanthera philoxeroides (Mart.) Griseb: A first report in India. J. Appl. Nat. Sci. 2024, 16, 202–208. [Google Scholar] [CrossRef]
- Mohammed, Y.M.; Badawy, M.E. Potential of phytopathogenic fungal isolates as a biocontrol agent against some weeds. Egypt. J. Biol. Pest Control 2020, 30, 92. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Wang, H.; Luo, Z.; Chen, Y.; Jiang, M.; Lu, H.; Valverde, B.E.; Qiang, S.; Strasser, R.J.; et al. Action of the fungal compound citrinin, a bioherbicide candidate, on photosystem II. Pest Manag. Sci. 2024, 80, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Ding, Y.; Bourdôt, G.W.; Qiang, S. Evaluation of Bipolaris yamadae as a bioherbicidal agent against grass weeds in arable crops. Pest Manag. Sci. 2024, 80, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Rabuske, J.E.; Luft, L.; Confortin, T.C.; Todero, I.; Aita, B.C.; Zabot, G.L.; Mazutti, M.A. Powder containing biomolecules from Diaporthe schini for weed control. Environ. Technol. 2022, 43, 2135–2144. [Google Scholar] [CrossRef]
- Song, K.; Ai, Y.; Zhou, J.; Dun, B.; Yue, Q.; Zhang, L.; Xu, Y.; Wang, C. Isolation, Characterization, and Bioherbicidal Potential of the 16-Residue Peptaibols from Emericellopsis sp. XJ1056. J. Agric. Food Chem. 2024, 72, 6315–6326. [Google Scholar] [CrossRef]
- Mnciva, S.T.; Coombes, C.; Coetzee, J.A. Morphological and molecular characterisation of naturally occurring pathogenic fungi for Pontederia crassipes (water hyacinth) in South Africa. Biocontrol Sci. Tech. 2023, 33, 869–884. [Google Scholar] [CrossRef]
- Febriyanto, D.W.; Manan, A.; Sastyawan, M.W.R.; Soesanto, L. Application of Three Weed Pathogenic Fungi on Bok choy (Brassica rapa subsp. chinnensis). Biosaintifika J. Biol. Educ. 2022, 14, 408–416. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Phazang, P.; Sarin, N.B. Environmental Science and Pollution Research; Springer: Singapore, 2020; Volume 27, pp. 9919–9934. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Prasad, G. Environment, Development and Sustainability; Springer: Singapore, 2020; Volume 22, pp. 1977–1990. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Wang, X.B.; Li, Y.Q.; Ding, J.L.; Lan, M.X.; Gao, X.; Zhao, D.L.; Zhang, C.S.; Wu, G.X. Phytotoxic azaphilones from the mangrove-derived fungus Penicillium sclerotiorum HY5. Front. Microbiol. 2022, 13, 880874. [Google Scholar] [CrossRef]

| Source | Registered Name | Weeds | Country | Released |
|---|---|---|---|---|
| Fungus Colleotrichum gloeosporioides | Lubao | Cuscuta chinensis | China | 1963 |
| Fungus Phytophthora palmivora | DeVine | Morrenia odorata | USA | 1981 |
| Fungus Colleotrichum acutatum | Hakatak | Hakea gummosis and H. sericea | South Africa | 1990 |
| Fungus Cylindrobasidium leave | Stump out | Poa annua | South Africa | 1997 |
| Bacterium Xanthomonas campestris | Camperico | Poa annua | Japan | 1997 |
| Fungus Puccinia thalaspeos | Woad Warrior | Isatis tinctoria | USA | 2002 |
| Fungus Chondrostereu purpureum | MycoTech/Chontrol/Ecoclear | Woody plants of Rosaceae family | Canada | 2004 |
| Fungus Colleotrichum gloeosporioides | Lock Down | Aeschynomene virginica | USA | 2006 |
| Fungus Sclerotinia minor | Sarritor | Taraxacum officinale | Canada | 2007 |
| Bacterium Streptomyces acidiscabies | Opportune (MBI- 005) | Taraxacum officinale | USA, Japan | 2012 |
| Bacterium Gibbago trianthemae | Gibbatrianth | Trianthema portulacastrum | India | 2014 |
| Virus Tobamovirus cepa | Solvi Nix | Solanum viarum | USA | 2015 |
| Fungus Phoma macrostoma | Biophoma | broad spectrum of broad-leaved weeds | Canada | 2016 |
| Fungi Lasiodiplodia pseudotheobromae, Macrophomina phaseolina and Neoscytalidium novaehollandiae | Di-Bak Parkinsonia | Parkinsonia aculeata | Australia | 2021 |
| Pine oil + sugar | Bioweed | herbaceous and grass weeds | Australia | Unknown year |
| Fungi | Effect on Weeds | Reference |
|---|---|---|
| Myrothecium verrucaria | Reduction of Sesbania exaltata biomass by 95% | [39] |
| Lasiodiplodia pseudotheobromae | Reduction of annual weeds by 72% | [40] |
| Diaporthe sp. | Root sprouting prevention of Lolium multiflorum and Sorghum halepense | [41] |
| Reduction of Conyza sp. shoot dry biomass by 50% | ||
| Colletotrichum coccodes | 95% mortality of Solanum ptycanthum | [42] |
| Dactylaria higginsii | Reduction in shoot and tuber dry weights of Cyperus rotundus by 71% and 67%, respectively | [43] |
| Myrothecium roridum | 100% control of Xanthium strumarium, Abutilon avicennae, Digitaria sanguinalis and Echinochloa crus-galli | [44] |
| Macrophomina phaseolina and Alternaria alternata | Reduction of Convolvulus arvensis root dry weight by up to 80% | [45] |
| Phomopsis convolvulus | Biomass reduction of Convolvulus arvensis up to 98–100% | [46] |
| Phoma multirostrata | Control of Tridax procumbens | [47] |
| Alternaria alternata | Reduction of Xanthium strumarium dry weight by 58% | [48] |
| Bipolaris bicolor | Control of Eleusine indica and Setaria viridis | [49] |
| Trichoderma polysporum | Reduction of Elsholtzia densa, Polygonum lapathifolium and Chenopodium album weight by 91%, 89% and 88%, respectively | [50] |
| Fungi | Herbicide | Weed | Reference |
|---|---|---|---|
| Myrothecium verrucaria | glyphosate | Pueraria lobata, Amaranthus palmeri | [52,53] |
| Colletotrichum graminicola | glyphosate | Sorghum bicolor | [55] |
| Gloeocercospora sorghi | glyphosate | Sorghum bicolor | [55] |
| Trichoderma koningiopsis | glyphosate | Euphorbia heterophylla | [56] |
| Colletotrichum truncatum | clopyralid plus MCPA ester and metribuzin | Matricaria perforata | [57] |
| Ascochyta caulina | metribuzin | Chenopodium album | [58] |
| Fusarium lateritium | 2,4-DB | Abutilon theophrast | [59] |
| Fungi | Weed | Reference |
|---|---|---|
| Alternaria sp. | Alternathera philoxeroides | [60] |
| Alternaria sp. | Rumex dentatus, Sonchus oleraceus, Avena fatua, Polypogon monspeliensis, Setaria viridis, Echinochloa crus-galli, E. colona and Plantago major | [61] |
| Aspergillus sp. | Ageratina adenophora | [62] |
| Bipolaris sp. | Eleusine indica | [49] |
| Bipolaris sp. | Microstegium vimineum | [63] |
| Diaporthe sp. | Bidens pilosa, Amaranthus viridis, Echinochloa crus-galli. and Lolium multiflorum | [64] |
| Emericellopsis sp. | Amaranthus retroflexus | [65] |
| Fusarium sp. | Pontederia crassipes | [66] |
| Fusarium sp. | Brassica rapa | [67] |
| Lasiodiplodia sp. | Amaranthus hybridus and Echinochloa crus-galli | [68] |
| Lasiodiplodia sp. | Chromolaena odorata and Echinocholoa crus-galli | [69] |
| Penicillium sp. | Ageratina adenophora | [62] |
| Penicillium sp. | Amaranthus retroflexus | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petraki, D.; Kanatas, P.; Zannopoulos, S.; Kokkini, M.; Antonopoulos, N.; Gazoulis, I.; Travlos, I. Agroecological Weed Management and the Potential Role of Fungi-Based Bioherbicides in Conservation: Advantages, Applications and Future Prospects. Conservation 2024, 4, 847-859. https://doi.org/10.3390/conservation4040050
Petraki D, Kanatas P, Zannopoulos S, Kokkini M, Antonopoulos N, Gazoulis I, Travlos I. Agroecological Weed Management and the Potential Role of Fungi-Based Bioherbicides in Conservation: Advantages, Applications and Future Prospects. Conservation. 2024; 4(4):847-859. https://doi.org/10.3390/conservation4040050
Chicago/Turabian StylePetraki, Dimitra, Panagiotis Kanatas, Stavros Zannopoulos, Metaxia Kokkini, Nikolaos Antonopoulos, Ioannis Gazoulis, and Ilias Travlos. 2024. "Agroecological Weed Management and the Potential Role of Fungi-Based Bioherbicides in Conservation: Advantages, Applications and Future Prospects" Conservation 4, no. 4: 847-859. https://doi.org/10.3390/conservation4040050
APA StylePetraki, D., Kanatas, P., Zannopoulos, S., Kokkini, M., Antonopoulos, N., Gazoulis, I., & Travlos, I. (2024). Agroecological Weed Management and the Potential Role of Fungi-Based Bioherbicides in Conservation: Advantages, Applications and Future Prospects. Conservation, 4(4), 847-859. https://doi.org/10.3390/conservation4040050







