Abstract
In most Sub-Saharan African countries, such as Togo, forest ecosystems provide ecosystem services to the local population. These ecosystem services are of vital importance to the local populations, who depend on the benefits derived from their use to meet their socio-economic needs. The permanent dependence of these populations on ecosystem services is a major factor accelerating the degradation of natural resources, which are already under pressure from climatic factors. The present study assesses the provisioning of ecosystem services provided by the relics forest in the southeast region of the Mono Biosphere Reserve in Togo. Individual interviews and group discussions were carried out with 420 households in fourteen villages around the reserve to identify the current uses of woody species. The results show that 100% of the respondents cited plant species, such as Mitragyna inermis, Lonchocarpus sericeus, and Diospyros mespiliformis, as used for wood. Species, such as Mimusops andogensis and Triplohiton scleroxylon, were cited as exclusively used for wood by 94% and 86%, respectively. Other species, such as Vitex doniana and Dialium guineense, in addition to their use for wood (93% and 70%), were cited, respectively, by 97% and 98% of respondents as used for fruit, and by 82% and 90% for their leaves. The heavy daily use of these species compromises their sustainability. An analysis of Sorensen’s similarity index, according to gender, age, ethnic group, and sector of activity, revealed a variation in this index ranging from 0.6 to 1, reflecting households’ knowledge of the use of these seven species. The local populations are already feeling the effects of the low availability of these commonly used species. According to them, the depletion of these resources is caused mainly by agricultural clearing, illegal logging, and bushfires.
1. Introduction
The concept of ecosystem service is used to highlight a set of goods and services that ecosystems provide directly or indirectly to humanity as a whole [1,2,3,4]. Each ecosystem has different functions and services. The functions and services provided by an ecosystem depend on the health of the ecosystem, the pressures on it, and the uses made of it by the communities within a biogeographical and geo-economic area [5].
Forest ecosystems provide a diversity of services, most of which are difficult to substitute [6,7]. In addition to conserving biological diversity, forests contribute to sustainable human development through their provisioning services, which often play a key role, accounting for up to 70% of all ecosystem services [8]. However, for decades, forest resources have been subject to disturbances due to both climatic hazards and, above all, pressures resulting from human activities. These disturbances have caused biodiversity loss at an alarming rate [9].
The forest ecosystems of southeast Togo (West Africa) have been the subject of several studies that led to their inclusion on UNESCO’s World Heritage List in 2017 as Togo’s Mono Biosphere Reserve (TMBR). These studies revealed the existence of several forest relics whose potential, despite their advanced state of degradation, still remains viable [10,11]. Since then, the Mono Biosphere Reserve (MBR) has been the subject of increased interest due to its revealed biological potential. This reserve has also been subject to a phenomenon of continuous degradation due to the combination of anthropogenic action and climate change. This has raised fears of the risk of residual forests being lost and/or converted to farmland in the long term if current cultivation practices continue [11]. Indeed, the ongoing degradation of forest resources in southeastern Togo is the result of a combination of numerous climatic and anthropogenic factors. Apart from agriculture, livestock farming, fishing, the collection of non-timber forest and timber products constitute an important source of income, food, and medicine for rural communities [12,13].
Studies have been carried out to identify and characterize the high-potential woody species used by the people living along the Benin side of the MBR [14]. To date, few studies have been carried out on the ecosystem services provided by the woody species in Togo’s MBR [15]. To this end, we need to assess local perceptions of the provisioning of ecosystem services provided by the woody species in the residual forest patches of the TMBR. This assessment requires an in-depth analysis of the diversity of woody species exploited in TMBR, which is a fundamental step towards biodiversity conservation and the sustainable management of forest resources [16]. This approach is in line with the recommendations of the Convention on Biological Diversity, aimed at preserving the variety of species against anthropogenic pressures [17]. As in Benin, precise knowledge of the floristic composition of TMBR will make it possible to identify and compare the woody species exploited, to categorize their ecological status, and to formulate appropriate conservation strategies [18]. Furthermore, an analysis of the local perceptions of the ecosystem services provided by the woody species, through the characterization of them, is part of an integrated approach to ecosystem management, and in line with the principles of the Millennium Ecosystem Assessment [4]. By detailing the specific contributions of these species to human needs, this characterization is consistent with the objectives of the Strategic Plan for Biological Diversity 2011–2020, by highlighting the links between biodiversity and human well-being [17]. Such an analysis will also contribute to the implementation of Sustainable Development Goals, particularly those related to ending poverty (SDG 1) and terrestrial life (SDG 15) [19]. Similarly, knowledge of the woody species whose harvesting has an impact on wildlife habitats [20] will make it possible to propose guidelines for the development and restoration of biodiversity.
The overall objective of this study is to assess local perceptions of the provisioning of the ecosystem services provided by woody species in the residual forest patches of TMBR. Specifically, the aim is to (i) analyze the diversity of woody species exploited in TMBR and (ii) characterize the ecosystem services provided by woody species in order to propose sustainable restoration and conservation strategies for the reserve’s residual forests.
2. Materials and Methods
2.1. Study Area
The study area (TMBR) is bounded between latitudes 6°11′3.59” and 7°3′47.30” north and longitudes 1°20′20.65″ and 1°48′41.10″ east (Figure 1), along the banks of the Mono River. It occupies an area of 203 224 ha [21]. It is characterized by a Guinean-type equatorial climate with a bimodal regime. Rainfall varies from 1000 to 1300 mm per year on average and is generally heaviest between March and July. Heavy rains are also recorded during the second rainy season between September and October. The average annual rainfall is low (900 to 1042.7 mm). The average annual temperature varies between 26.4 °C and 30.3 °C in the study area [22]. In addition to the Mono River, the reserve is drained by the Hondoulé, Afan, and Asrama tributaries, which are perennial rivers.
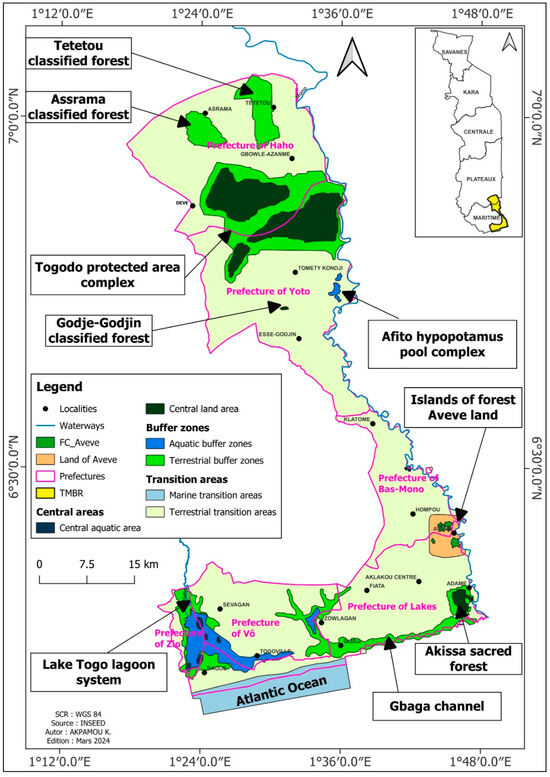
Figure 1.
Location of Togo’s Mono Biosphere Reserve.
TMBR is made up of a mosaic of landscapes and ecosystems, mainly comprising mangroves, savannahs, lagoons, flood plains, and patches of residual forest, including sacred forests, classified forests, and community forests. The flood zones correspond to the limits of the lower Mono valley). The hydromorphic soils, which are not very advanced, are made up of swelling clays, making them difficult to access during the rainy season [23].
The presence of watercourses allows for a wide diversification of economic activities, notably agriculture, vegetable gardening, fishing, and artisanal palm oil and wine production. Other activities include hunting, the collection of non-timber forest products (medicinal plants, harvested products, and honey), logging, and family farming.
2.2. Data Collection
This assessment focused on timber supply services, as the exploitation of this category of product, while helping to improve the living conditions of the local populations, has a very significant impact on the degradation of the forest ecosystems in the area [24].
Surveys of TMBR households were carried out through individual interviews and focus groups [25] using the Kobocollect tool. These surveys were administered to a sample of 420 households in 14 villages (Figure 2), taking into account data from the general population census [26]. The majority of these households are indigenous (95.97%). They are 58.27% male and 41.23% female, with an average age of 44. Agriculture (68.96%) is the main activity of TMBR households, followed by trade (14.45%). Other activities include handicrafts (7.58%) and fishing (1.9%). The investigation focused on the parts of the woody species used, and their uses, previously identified through a preliminary survey.
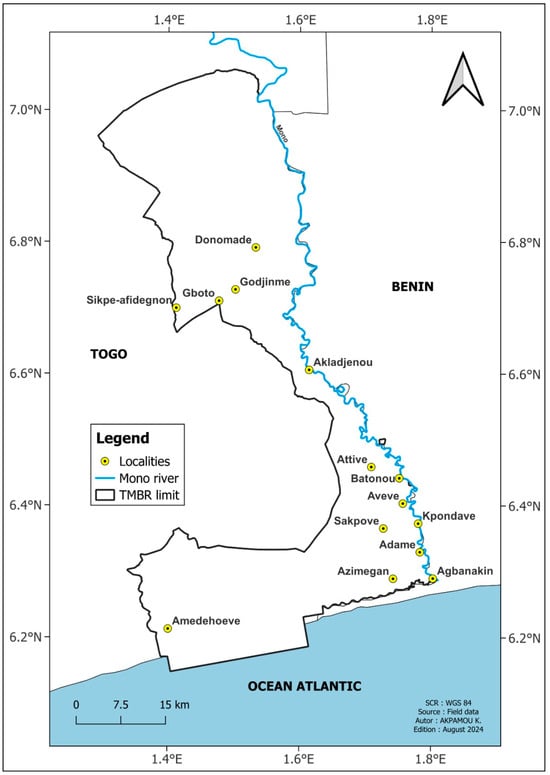
Figure 2.
Location of TMBR villages surveyed.
To ensure the representativeness of the sample and to take account of specific features, the number of households per village was chosen in accordance with the demographic weight of the village (Table 1). The representativeness of the sample is based on the Schwartz method, whose formula is as follows:
where n is the minimum sample size representative of the population, and z is the score for the desired risk of error. The risk of error is 5%, z =1.96 relative to a 95% confidence level, and p is the magnitude of the phenomenon in the population. This is a contingency study, i.e., a fictitious market. Conventionally, it is given a default value of 0.5; m is the margin of error, set here at 5%.

Table 1.
Distribution of parent population (POP) and sample (ECH) by target village in the study area.
These surveys aimed to gather information from the local populations of various, randomly selected localities around TMBR forest patches, concerning their knowledge of woody species, their uses, and any threats linked to the way these species are used. This information made it possible to understand the supply services provided by these woody species to the local populations who depend on them.
2.3. Data Processing and Analysis
The correlation matrix of the use categories and the seven species used was subjected to a principal component analysis (PCA) using R 3.1. software to determine the relationships between the most commonly used species and their uses.
The analysis focused on the types of ecosystem provisioning services provided by these woody species, an essential component of residual forest patches. To compare the importance and use of each ecosystem service and woody species, the Frequency of Citation (FC), use value (UV), and informant consensus factor (ICF) were calculated.
The Frequency of Citation (FC) is used to highlight not only the services provided by these species but also, and above all, to assess the pressure of the surrounding populations on these species. For each use or used part of each species, the formula [5,8] below is used
The use value (UV) is calculated for each type of supply service. The calculated UV is based on the formula expression of Ngom et al. [5] and Sambou et al. [8]:
where Ui = the number of citations for each type of use and n = the total number of citations for all types of use.
The informant consensus factor (ICF) of the respondents is calculated [5,8,27] according to the following formula:
where Ntu is the number of citations per type of use and Nt is the number of woody species used by the informants for that type of use.
The relationships between the socio-demographic factors and each index were verified using the Fisher analysis of variance with Minitab 16 software. Similarly, Sorensen’s similarity index was applied to analyze the level of similarity of knowledge about the seven species according to gender, sector of activity, ethnicity, and age, using Community Analysis Package (CAP) software.
The Sorensen index equals twice the number of elements common to both sets, divided by the sum of the number of elements in each set.
where |X| (|Y|, resp.) is the cardinality of the set X (Y, resp.), i.e., the number of elements in the set [28].
There is similarity between the elements when the Sorensen index is high. When the opposite occurs, there is no similarity between the elements.
3. Results
3.1. Diversity of Woody Species Exploited in TMBR
The local people living near TMBR reported a diversity of 54 woody species that they regularly use in their daily activities. These species belong to 47 genera and 26 families (Appendix A). Of this list of species, seven are the most widely used by the local communities (Lonchocarpus sericeus, Diospyros mespiliformis, Triplochiton scleroxylon, _Mimusops andongensis Vitex doniana, Dialium guineense and Mitragyna inermis), the other forty being little used or used as replacements in times of shortage. These seven most used species contribute to the provision of seven categories of ecosystem services: wood energy, timber, trade, food, fodder, ceremonies, and traditional medicine.
The principal component analysis (PCA) explains the variance in the use of the species in the study area, and the relationships between the distributions of the seven most commonly used species and their use categories. The overall variance expressed by the first two dimensions (75.1%) explains the variations observed (Table 2).

Table 2.
Description of PCA dimensions.
A principal component analysis (PCA) was used to discriminate the woody species according to their use (Figure 3). Three main groups were identified. They are as follows: (i) Woody species with predominantly timber and energy uses (G1), notably Lonchocarpus sericeus (Lombati), Diospyros mespiliformis (Ebony), Triplochiton scleroxylon (Wawa), and Mimusops andongensis (Djéhéga, Djéhéti). (ii) A group of woody species predominantly used for food (G2), namely Vitex doniana (fongni) and Dialium guineense (atitoeti). These species are also used for energy wood, timber, and traditional medicine. (iii) A group of woody species predominately used for energy wood and medicinal uses (G3), namely Mitragyna inermis (Nekpati or Linkpati).
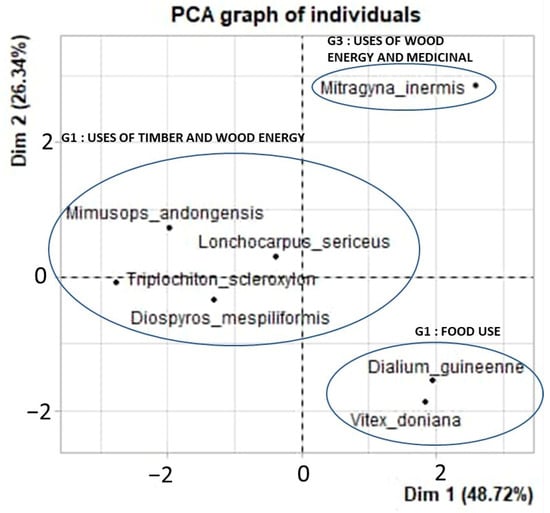
Figure 3.
Principal component analysis (PCA) of the matrix, for 7 woody species X 6 use categories.
3.2. Local Knowledge of the Most Commonly Used Species and Availability of the Resources
According to the riparian populations surveyed around TMBR forest patches, the species best known for the provisioning services offered are Dialium guineense (atitoeti) (99.29%), Vitex doniana (99.53%), Mitragyna inermis (92.5%), and Lonchocarpus sericeus (91.94%). The least well-known are Triplochiton scleroxylon (49.76%), Mimusops andongensis (47.16%), and Diospyros mespiliformis (33.18%) (Figure 4). An analysis of Sorensen’s similarity index according to gender, age, ethnic group, and sector of activity revealed a variation in this index, ranging from 0.6 to 1. This shows that the households know the uses of all these species, regardless of gender, ethnic group, age category, or sector of activity. By ethnic group, the level of similarity of knowledge of the seven plant species is high among the Fons, Guins, Minas, and Watchis.
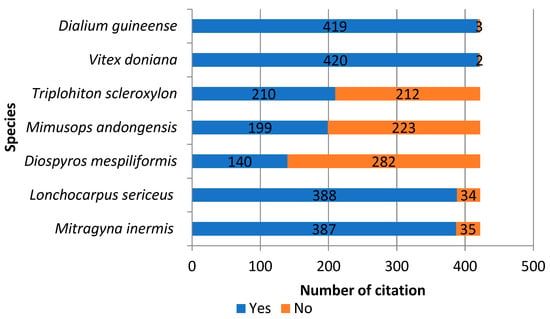
Figure 4.
Local people’s knowledge of woody species.
Concerning the perceived availability of the species mentioned, over 75% of the respondents stated that they had observed an increased current scarcity of all these species compared to a decade ago (Figure 5). The level of disappearance of the various species varied. Thus, 77% of the respondents said that Mitragyna inermis was becoming increasingly rare, 80% for Lonchocarpus sericeus, 94% for Diospyros mespiliformis, 85% for Mimusops andongensis, 83% for Triplochiton scleroxylon, 81% for Vitex doniana, and 82% for Dialium guineense.
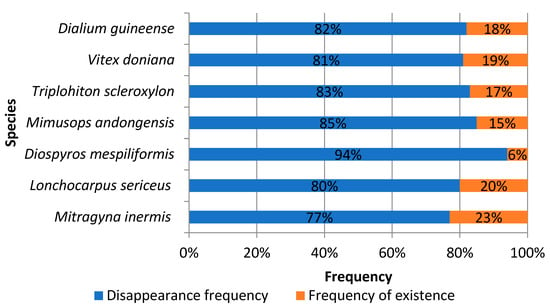
Figure 5.
Availability of woody species over the last ten (10) years.
3.3. Main Characteristics of the Usual Supply Services of the Species Surveyed
- Frequency of organ use by species
An analysis of the frequency of quotation indicates a divergence in the use of the organs or parts of a species (Table 3). The most frequently used are the wood, fruit, and leaves of the most prized species in TMBR. Wood comes first, with a score of over 60%, except for Triplochiton scleroxylon, with a score of 48.3%. Next come the fruits of two species, Dialium guineense (97.87%) and Vitex doniana (96.21%). Leaves come third, and are the most used part of two other species, Dialium guineense (90.05%) and Vitex doniana (81.52%). The most commonly used parts of the seven species are the wood (100%), fruit (100%), and leaves (99%), followed to a lesser extent by the bark (99%) (Figure 6). The frequency of use of the organs of these species varied according to ethnic group (P = 0.000). The Adja (92.18%) and Fon (86.09%) are the most frequent users of the organs of the seven species. The frequency of use was average among the Mina (42.25%), Guin (38.83%), and Watchi (12.56%). It was low among the Haoussa (1.08%) and Adangbé (0.74%). According to the sector of activity, the Fisher test is significant (P = 0.000). Farmers (100%) make greater use of the organs of the seven plants, followed by shopkeepers (44.01%) and craftsmen (21.83%). There was no variation in the use of plant organs by gender (P = 0.08). With respect to age groups, there was a significant difference (P = 0.000) in the use of the organs of these seven species. The 30–40 age group (82.63%) and the 40–50 age group (67.47%) make greater use of the organs of these species. Their use was average for the 20–30 (37.41%), 60–70 (31.35%), 50–60 (29.96%), and 70–80 (19.33%) age groups. The frequency of use of the organs of these seven species was low for the age range 80 to 90 (5.59%). Wood is the most commonly used organ, depending on the ethnicity, age group, sector of activity, and gender.

Table 3.
Frequency of organ use by species.
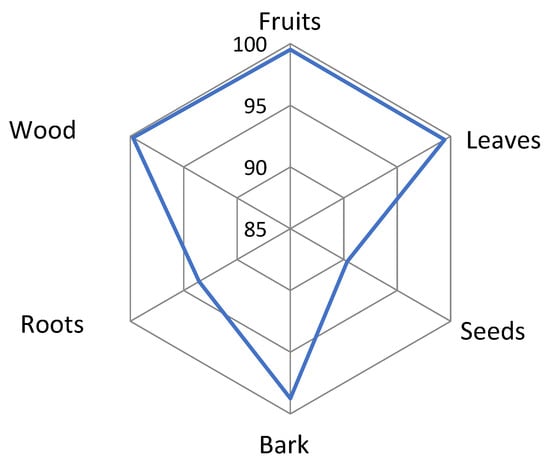
Figure 6.
Sought-after organ parts of seven most commonly used woody species.
- Use value of species
Seven use types or categories have been identified. These are wood energy, timber, food, fodder, trade, traditional ceremonies, and traditional medicine, with different use values. The use values were very high for Dialium guineense, Vitex doniana, and Mitragyna inermis (2.9, 2.9, and 2.7, respectively). They were average for Lonchocarpus sericeus, Diospyros mespiliformis, and Mimusops andongensis, at 1.9, 1.6, and 1.5, respectively. It was low for Triplochiton scleroxylon, with a use value of 1.2 (Table 4). An analysis of the variance in the use value of these species by ethnic group using Fisher’s test gives a p-value of less than 0.05 (P = 0.000). This reflects the variability in the species’ use by ethnic group. The use value was highest among the Adja (5.76) and Font (5.20), followed by the Mina (2.60) and Guin (2.41). It was low among the Watchi, Haoussa, and Adangbé, at 0.77, 0.6, and 0.04, respectively. According to gender, Fisher’s test gives a p-value of less than 0.05 (P = 0.01). This also reflects the variability in the use value of the species according to gender. The use value was higher for men (9.69) than for women (7.18). There was variability (P = 0.000) in the use of the seven species according to the sector of activity. It was highest among farmers (11.38), and lowest among shopkeepers (2.73) and craftsmen (1.36). The use value was very low for the other sectors. The use value of cash also varied according to age (P = 0.000). Its use value was high in the 30–40 age group (5.14) and in the 40–50 age group (4.20). It was low in the 20–30 (2.28), 60–70 (1.89), 50–60 (1.85), 70–80 (1.16), and 80–90 (0.33) age groups.

Table 4.
Use value of species studied.
The seven species studied have different uses (Table 5). Vitex doniana (96.45%) and Dialium guineense (96.21%) are the most widely used for food. Mitragyna inermis (90.05%), Diospyros mespiliformis (63.51%), Mimusops andogensis (61.14%), and Triplochiton scleroxylon (47.39%) are used more as timber. The same trend is observed for the use of Lonchocarpus sericeus and Mitragyna inermis for energy wood (88.39%). Dialium guineense (85.07%), Vitex doniana (77.49%), and Mitragyna inermis (52.61%) are the species most widely used in traditional medicine (19% to 21%).

Table 5.
Uses recorded for woody species listed in TMBR.
3.4. Informant Consensus Factors
The consensus factor for the uses made of the various woody species among the respondents is very high (ICF >90%) for all seven types of use, except for traditional ceremonies, for which the informant consensus factor is 67% (Table 6). This factor varies (P = 0.000) according to the ethnic group. It is high among the Fon (93.16%), Guin (87.01%), Adja (83.66%), Mina (80.71%), and Watchi (60.87%). It is low among the Adangbe and Haoussa groups. The Fisher test is not significant (P = 0.95) at the gender level for the informant consensus factor. This factor is 90.04% for men and 89.39% for women. The Fisher test is not as significant (P = 0.57) for the informant consensus factor at the age level. This factor is highest for the 60–70 age group (91.72%) and lowest for the 80–90 age group (28.23%).

Table 6.
Informant consensus factor for the uses of different woody species.
The respondents agree about the uses they make of the different species studied. Such agreement could be due to the sharing of information and best practices regarding their uses between the populations of the different villages bordering TMBR forest patches. Indeed, the wood from these species is used not only for energy purposes (100%), but also for the construction of buildings and habitats (100%). They are also traded (99%) between villages and supply the major urban centers of TMBR (Table 6; Figure 7).
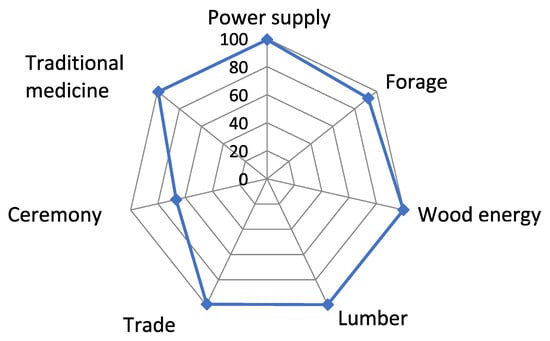
Figure 7.
Uses of seven woody species.
3.5. Pressure on Wood Resources
The local people consider land clearing, illegal logging, and bush fires to be the main reasons for biodiversity loss (Figure 8). They note that land clearing and illegal logging are the main factors in the destruction of biodiversity, for 96% and 92% of intentions, respectively. Bush fires account for only 16%, compared to 0% for climate change.
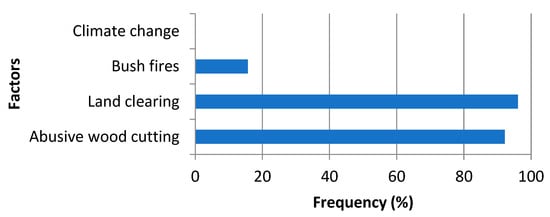
Figure 8.
Reasons for the disappearance of woody species.
4. Discussion
The ecosystem services provided by woody species in the residual forest patches of the Mono Biosphere Reserve in southeast Togo are perceived in different ways by the local populations. The assessment of the ecosystem supply services, based on the surveys, identified and characterized seven priority woody species (Lonchocarpus sericeus (Lombati), Diospyros mespiliformis (Ebène), Triplochiton scleroxylon (Wawa), Mimusops andongensis (Djéhéga, Djéhéti), Vitex doniana (fongni), Dialium guineense (atitoeti), and Mitragyna inermis (Nekpati ou Linkpati)) for the provision of seven categories of ecosystem supply services, including fuelwood, timber, trade, food, fodder, ceremonies, and traditional medicine. The results obtained are similar to those of other studies in Africa and beyond, carried out under more-or-less-similar conditions [8]. For instance, Sambou et al. [8] stated that the local people mentioned a diversity of ecosystem services provided by the forests and trees in their studies of the local people’s perceptions of ecosystem services in the Kalounayes classified and managed forest in Senegal. In other studies [29,30], populations have been shown to strongly perceive direct and indirect ecosystem services. However, the results obtained in this study differ from those obtained by Reyes-Arroyo et al. [31], Nyangoko et al. [32], and Gnansounou et al. [33]. This difference is linked to the difference in study environments. Indeed, they all conducted studies on mangrove ecosystem services in Mexico, Tanzania, and Togo–Benin, respectively. Although the present study is located in the Mono Transboundary Biosphere Reserve, the divergence of the results with those of Gnansounou et al. (2022) is linked to the survey sites. Indeed, Gnansounou et al. (2022) conducted their surveys in villages bordering mangroves in mangrove ecosystem services, whereas this type of habitat is not found at our study area.
The extent of the use of and the pressure exerted on plant species are obtained through the retrospective survey method. This method relies on the memory of the respondents and may be biased by the respondent’s assessment [34]. The importance attributed to the use of species is provided by individuals, who implicitly take into account a personal assessment, which often refers to their preference [35,36]. Despite these biases, this method is widely used in ethnobotany by many authors and has a history of producing fairly conclusive results [37,38,39].
For the people of the Reserve, various plant organs are used to satisfy their economic, dietary, and socio-cultural needs. These results are in line with the work of Ezebilo and Mattsson [12] and Dossou et al. [40]. The organs most often used are the wood, fruit, leaves, roots, bark, and sometimes even the flowers and bark exudates. This result is in line with the work of other authors [41,42,43]. The frequent use of wood across age, gender, sector, and ethnicity reflects the importance of plants to local communities. In addition, the assessment of ecosystem provisioning services carried out with the local communities in TMBR shows that Dialium guineense, Vitex doniana, and Mitragyna inermis have the highest use values. This is due to their contribution to the diet and therapeutic practices of local communities living in precarious conditions. Although these species are also used to supply service wood, the community preference is for Diospyros mespiliformis, Mimusops andogensis, and Lonchocarpus sericeus, which are more widely used for construction and energy wood. The pressure exerted on these woody species through land clearance, illegal logging, and vegetation fires has led to the degradation and fragmentation of flora and fauna habitats [24,44]. This is confirmed by the 12.94% loss of tree cover observed in Togo between 2001 and 2023 (www.globalforestwatch.org). According to regeneration inventory data, the high demand for these six species will be offset by the regeneration potential of Vitex doniana (78.57%), Diospyros mespiliformis (56.27%), Mimusops andogensis (85.23%), Lonchocarpus sericeus (37.53%), Dialium guineense (47.36%), and Mitragyna inermis (30.81%). Triplochiton scleroxylon receives little attention, as it is almost non-existent in the study area. The importance attributed to a species does not depend on its availability, but rather on its capacity to satisfy the needs of the populations in the various use categories [34]. An analysis of Sorensen’s similarity index according to gender, age, ethnic group, and sector of activity reveals a variation in this index, ranging from 0.6 to 1, reflecting the knowledge of the use of the seven species among households in the area. However, in Benin, the seven species with high use values identified by Hadonou-Yovo et al. [14] differ from the species identified in this study. Indeed, they found that the species with a high ethnobotanical use value (EUV) in the MBR in Benin are, respectively, Elaeis guineensis (EUV = 18.9), Ficus trichopoda (EUV = 18.5), Diospyros mespiliformis (EUV = 16), Azadirachta indica (EUV = 15.7), Vitex doniana (EUV = 15), and Mitragyna inermis (EUV = 14.8). By ethnic group, the level of similarity in the knowledge of the seven plants is high among the Fons, Guins, Minas, and Watchis. The slight differences in species knowledge among the ethnic groups is thought to be due to cultural heritage, with knowledge being passed down from generation to generation within the same ethnic group [45,46,47,48]. The analysis of the variance in the use value of species by ethnic group, using Fisher’s test, revealed variability in species use by ethnic group. This finding was also made by other authors in their studies [49,50,51]. The use value of species is higher among the Adja and Fons than among the Mina, Guin, Watchi, Haoussa, and Adangbé. This reflects the high importance of these seven species for the Adja and Fons [52]. The results of this study help to identify the useful and high-pressure species that should be prioritized in the management of residual forest patches to contribute to the sustainable economic and socio-cultural well-being of the populations who depend on them. It would, therefore, be judicious for the different managers of the sites of conservation in TMBR to privilege these seven species in silviculture operations, including reforestation and restoration, during the implementation of management plans for the different sites studied [14].
Wood material supply services (firewood and charcoal) are the most important for all seven species. These results confirm the work of Lykke et al. [34], who showed that even in Sahelian countries, where famine is a regular occurrence, the supply of wood products is still the most important service provided by species such as Diospyros mespiliformis and Vitex doniana, whose fruits are highly prized in times of famine. According to the Greater Letaba (South Africa) study, among the ecosystem services, provisioning, timber, energy wood, and edible plants stood out as the most important [53]. The food and fuelwood services provided by woody plants are essential for local communities [54,55,56]. As a result, the vulnerability of these species remains high, threatening their availability. According to forest inventory data, the stand density of each of these species is 8 stems/ha for Dialium guineense, 2 stems/ha for Vitex doniana, 62 stems/ha for Mitragyna inermis, 11 stems/ha for Diospyros mespiliformis, 3 stems/ha for Mimusops andogensis, and 28 stems/ha for Lonchocarpus sericeus. Triplochiton scleroxylon was not observed in the field. This would indicate its probable disappearance from the study area.
There is a very high level of consensus among the respondents (ICF > 90%) on the uses made of the various woody species. These traditions are perpetuated from generation to generation, not only through bequests made by ascendants to descendants of the same siblings, or even of the same village, but also through the marriage links generally established between the populations of different riverside villages [27,57,58]. It can also be explained by the strong homogeneity of cultural and culinary practices linked to the predominantly Ewé ethnic origins observed in the area. Moreover, in the present study, this high ICF could also be explained by the fact that a preliminary survey was carried out that focused on the species frequently sought by local communities.
The results of this study show that, in terms of the determinants of forest resource degradation, human activities, such as uncontrolled clearing, illegal logging, and vegetation fires, are the main causes of biodiversity degradation [59,60,61]. For the local population, however, climate change is not directly responsible for the loss of resources in the area. This would appear to be linked to the low level of scientific knowledge that the local communities have about major climatic hazards and their inherent impacts on the zone’s various ecosystems. The most recurrent climatic hazard faced by the patches of residual forest in the southeast of TMBR is flooding, to which these ecosystems have been adapted for ages, as they are located in the hydromorphic zone of the lower Mono river basin. This phenomenon deserves to be studied in greater depth in the years to come, in order to establish a possible link between the effects of climate change on the availability of ecosystem services provided by the forest patches and the populations of the southeast of the Mono Biosphere Reserve in Togo.
5. Conclusions
This study reveals a diversity of 54 woody species that are a regular part of the supply chain of woody plants to riparian communities in the forest islets to the southeast of Togo’s Mono Biosphere Reserve. Among these species, the local populations indicated their preference for seven woody species, notably Dialium guineense, Vitex doniana, Mitragyna inermis, Diospyros mespiliformis, Mimusops andogensis, Lonchocarpus sericeus, and Triplochiton scleroxylon for the multiple uses they make of them in their daily activities. An analysis of the data shows that wood, bark, and fruit are the most widely used parts of the seven species studied. These multiple uses underline the importance of the existence of these species in people’s daily lives and, therefore, merit particular attention in the redesign of participatory systems for the sustainable management of these resources. Although these species have a considerable capacity for regeneration, pressure from the local populations seems to be getting the better of them. The local populations are already feeling the effects of the low availability of priority species. According to them, this scarcity of resources is mainly caused by land clearance, illegal logging, and vegetation fires. This study provides forest management, extension, and research institutions with benchmark data on the diversity and vulnerability of the forest species whose cultivation techniques need to be mastered to help the local communities living along the reserve’s forest patches improve their living conditions by restoring degraded forest land. Adopting good sustainable management practices and mastering silvicultural techniques for the native species studied will increase the resilience of the socio-ecological and economic services provided to the local populations. To this end, the promotion of reforestation based on these different species is recommended in future management programs. This study will provide sufficient qualitative and quantitative data to lay the foundations for a sustainable management strategy for forest relics in line with the needs of the local populations.
Author Contributions
Conceptualization, K.G.A. and K.K.; methodology, K.G.A. and H.E.; formal analysis, K.G.A., S.P., K.R.S., Y.W. and H.E.; investigation, G.H.S.; data curation, K.G.A., H.E. and K.K.; writing—original draft preparation, K.G.A. and D.D.; funding acquisition, K.G.A., L.L. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union Delegation’s support for the fight against climate change (PALCC/ENV/2017/385-911), as part of the development and management plan for the Avévé community forest.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of IDECC and the University of Lomé (protocol code 331, 17 November 2019) for the interviews with humans.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in this study.
Data Availability Statement
The data are not publicly available to protect the confidentiality of the participants, according to the obtained IRB approval.
Acknowledgments
Our sincere thanks to the coordinators of the “support for the fight against climate change (PALCC)” project of the Ministry of the Environment and Forest Resources, and to the European Union Delegation for funding this research work. We would like to express our gratitude to all the contributors for the quality of this scientific article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. List of Plant Species Inventoried in the Southeast of TMBR
| N° | Scientific Name of Species | Common Names | Common Names (Mina Language) | Level of Use | Family |
| 1 | Acacia auriculiformis A.Cunn. ex Benth. | Acacia in the shape of auricle | - | ++ | Fabaceae |
| 2 | Afzelia africana Sm. ex Pers. | Red Doussie | - | + | Fabaceae |
| 3 | Albizia glaberrima (Schum. & Thonn.) Benth. | - | - | + | Fabaceae |
| 4 | Albizia zygia (DC.) J.F.Macbr. | - | Ziwêti | + | Fabaceae |
| 5 | Alchornea cordifolia (Schum. & Thonn.) Müll.Arg. | - | - | + | Euphorbiaceae |
| 6 | Allophylus africanus P. Beauv. | - | Assiviato | + | Sapindaceae |
| 7 | Annona senegalensis Pers. | Apple cinnamon | Zogbegnigli | + | Anonaceae |
| 8 | Anthocleista vogelii Planch. | - | - | ++ | Gentianaceae |
| 9 | Antiaris toxicaria subsp. africana (Engl.) C.C.Berg | - | - | + | Moraceae |
| 10 | Azadirachta indica A.Juss. | Neem | Kiniti | ++ | Meliaceae |
| 11 | Blighia sapida K.D. Koenig | fricassee tree | Atchanti | + | Sapindaceae |
| 12 | Bombax costatum Pellegr. & Vuill. | - | ++ | Malvaceae | |
| 13 | Bridelia ferruginea Benth. | - | Akamatsi | + | Euphorbiaceae |
| 14 | Cassipounia congoensis R. Br. ex DC. | - | - | + | Rhizophoraceae |
| 15 | Ceiba pentandra (L.) Gaertn. | Kapok tree | Houti | ++ | Malvaceae |
| 16 | Celtis brownii Rendle | - | - | + | Ulmaveae |
| 17 | Celtis zenkeri Engl. | - | - | + | Ulmaveae |
| 18 | Chassalia kolly (Schumach.) Hepper | - | - | + | Rubiaceae |
| 19 | Citrus sinensis L. | Lemon tree | - | + | Rutaceae |
| 20 | Cola gigantea A.Chev. | - | - | ++ | Sterculiaceae |
| 21 | Cola laurifolia Mast. | - | - | + | Sterculiaceae |
| 22 | Dialium guineense Willd. | Black tamarind | Atitoeti | +++ | Fabaceae |
| 23 | Dichapetalum guineense (DC.) Keay | - | - | + | Dichapetalaceae |
| 24 | Diospyros mespiliformis Hochst. ex A.DC. | African ebony | Ebony, Dokotsou | +++ | Ebenaceae |
| 25 | Drypetes floribunda (Mull.Arg) Hutch | - | - | + | Euphorbiaceae |
| 26 | Eucalyptus camaldulensis Dehnh. | - | - | ++ | Myrtaceae |
| 27 | Ficus capensis Thunb. | - | - | + | Moraceae |
| 28 | Ficus exasperata Vahl | - | aklalê | + | Moraceae |
| 29 | Ficus sur Forssk. | - | - | + | Moraceae |
| 30 | Ficus vogelii (Miq.) Miq. | - | - | + | Moraceae |
| 31 | Flacourtia flavescens Wild. | - | - | + | Flacourtiaceae |
| 32 | Holarrhena floribunda (G. Don) T. Durand & Schinz | - | Séséwou/afeketsi | ++ | Apocynaceae |
| 33 | Khaya senegalensis (Desr.) A. Juss. | - | - | + | Meliaceae |
| 34 | Kigelia africana (Lam.) Benth. | - | - | ++ | Bignoniaceae |
| 35 | Lecaniodiscus cupanioides Planch. ex Benth. | - | - | + | Sapindaceae |
| 36 | Lonchocarpus sericeus (Poir.) Kunth Ex. DC. | River soap | Lombati | +++ | Fabaceae |
| 37 | Mallotus oppositifolius f. glabratus (Müll. Arg.) Pax | - | Nyati | + | Euphorbiaceae |
| 38 | Mangifera indica L. | - | Mangoti | ++ | Anacardiaceae |
| 39 | Mitragyna inermis (Willd.) Kuntze | Mitragyna | Linkpati | +++ | Rubiaceae |
| 40 | Morinda lucida Benth. | - | Dadaclan | + | Rubiaceae |
| 41 | Mimusops andongensis Hiern | Milk tree | Djéhéga, Djéhéti | +++ | Sapotaceae |
| 42 | Oncoba spinosa Forssk. | - | - | + | Salicaceae |
| 43 | Pouteria alnifolia (Baker) Roberty | - | - | + | Sapotaceae |
| 44 | Rhus natalensis Krauss | - | - | + | Anacardiaceae |
| 45 | Sarcocephalus latifolius (Sm.) E.A.Bruce | - | - | + | Rubiaceae |
| 46 | Senna siamea (Lam.) H.S. Irwin & Barneby | - | Atsragbé | ++ | Fabaceae |
| 47 | Sorindeia warnekei Engl. | - | aloti | ++ | Anacardiaceae |
| 48 | Spondias mombin L. | - | Aklikonksi | ++ | Anacardiaceae |
| 49 | Tectona grandis L.f. | - | - | ++ | Verbenaceae |
| 50 | Treculia africana Decne. | - | - | ++ | Moraceae |
| 51 | Triplochiton scleroxylon K. Schum. | Samba | Wawa | +++ | Malvaceae |
| 52 | Vitex chrysocarpa Planch. ex Benth. | - | - | ++ | Verbenaceae |
| 53 | Vitex doniana Sweet | Black plum | Fongni | +++ | Verbenaceae |
| 54 | Zanthoxylum zanthoxyloides (Lam.) Zepern. & Timler | - | Xéti | ++ | Rutaceae |
| +++: widely used; ++: moderately used; +: little used. | |||||
References
- Ehrlich, P.R.; Mooney, H.A. Extinction, substitution, and ecosystem services. BioScience 1983, 33, 248–254. Available online: https://people.wou.edu/~vanstem/391.W12/Ehrlich%20and%20Mooney.pdf (accessed on 20 March 2020). [CrossRef]
- Sabi Lolo Ilou, B.; Toko Imorou, I.; Vigninou, T.; Thoma, O. Caracterisation des Services Ecosystemiques dans la Reserve de Biosphere Transfrontaliere du W (RBTW) au Nord-Benin. Eur. Sci. J. 2019, 15, 278–295. [Google Scholar] [CrossRef]
- Sabi Lolo Ilou, B.; Houinato, I.M.; Sogbohossou, I.E.A. Impact des feux de végétation sur les services écosystémiques dans la réserve de biosphère de la Pendjari au Nord-Bénin. In Mémoire de Master en Géographie; Universite d’Abomey-Calavi: Cotonou, Benin, 2015; p. 67. [Google Scholar]
- MEA. Ecosystems and Human Well-Being; Island Press: Washington, DC, USA, 2005; 63p. [Google Scholar]
- Ngom, D.; Charahabil, M.M.; Sarr, O.; Bakhoum, A.; Akpo, L.E. Perceptions communautaires sur les services écosystémiques d’approvisionnement fournis par le peuplement ligneux de la Réserve de Biosphère du Ferlo (Sénégal). VertigO 2014, 14, 18. [Google Scholar] [CrossRef]
- Diouf, J.; Camara, A.A.; Mbaye, M.S.; Diouf, N.; Diop, D.; Ndour, S. Le Jardin Botanique du département de Biologie Végétale (FST/UCAD/SENEGAL): Structure de la flore d’un site de haute diversité floristique. Int. J. Dev. Res. 2020, 10, 37997–38004. [Google Scholar] [CrossRef]
- Daily, G.C. Nature’s Services: Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997; p. 10. [Google Scholar]
- Sambou, A.; Camara, B.; Goudiaby, A.O.K.; Coly, A.; Badji, A. Perception des populations locales sur les services écosystèmiques de la forêt classée et aménagée de Kalounayes (Sénégal). Rev. Francoph. Dév. Durable 2019, 69–86. Available online: https://rivieresdusud.uasz.sn/xmlui/handle/123456789/671 (accessed on 28 June 2022).
- Lévêque, C. Erosion de la Biodiversité: Enjeux et Débats; ISTE Group: London, UK, 2022; 263p. [Google Scholar]
- Kokou, K. Les Mosaïques Forestières au sud du Togo: Biodiversité, Dynamique et Activités Humaines. Ph.D. Thesis, Montpellier 2, Montpellier, France, 1998; 139p. Available online: https://www.theses.fr/1998MON20057 (accessed on 12 June 2022).
- Konko, Y.; Rudant, J.; Akpamou, G.; Noumonvi, K.; Kokou, K. Spatio-Temporal Distribution of Southeastern Community Forests in Togo (West Africa). J. Geosci. Environ. Prot. 2018, 6, 51–65. Available online: https://hal.science/hal-03239572/document (accessed on 20 March 2020). [CrossRef]
- Ezebilo, E.E.; Mattsson, L. Socio-economic benefits of protected areas as perceived by local people around Cross River National Park, Nigeria. For. Policy Econ. 2010, 12, 189–193. [Google Scholar] [CrossRef]
- Masengo, C.A.; Ngbolua, J.-P.K.-t.-N. Étude ethnobotanique et vulnérabilité de Vitex doniana Sweet (Lamiaceae) dans la forêt péri-urbaine de Gbado-Lite, République démocratique du Congo. Rev. Marocaine Sci. Agron. Vét. 2022, 10, 179–184. [Google Scholar]
- Hadonou-Yovo, A.G.; Houessou, L.G.; Lougbegnon, T.O.; Adebi, Y.; Sanni Sinasson, G.K.; Fifonsi Semevo, D.; Lange, U.; Boko, M. Diversité et formes d’utilisation des espèces ligneuses de la Réserve de biosphère du Mono (Bénin). VertigO 2019, 19. [Google Scholar] [CrossRef]
- Issifou, A.; Atakpama, W.; Segniagbeto, G.H.; Egbelou, H.; Ahuide, K.; Batawila, K.; Ketoh, G.K.; Akpagana, K. Use and vulnerability of fauna in the northern part of the Mono Basin in Togo, West Africa. Int. J. Avian Wildl. Biol. 2022, 6, 32–40. [Google Scholar] [CrossRef]
- CBD. Convention sur la Diversite Biologique; Convention on Biological Diversity: Montreal, QC, Canada, 1992; 32p, Available online: https://www.cbd.int/doc/legal/cbd-fr.pdf (accessed on 22 March 2021).
- CBD. Perspective Mondiale de la Diversité Biologique; Convention on Biological Diversity: Montreal, QC, Canada, 2010; 94p, Available online: https://www.cbd.int/doc/gbo/gbo2/cbd-gbo2-fr.pdf (accessed on 22 March 2021).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. Available online: https://www.nature.com/articles/35002501 (accessed on 15 July 2022). [CrossRef] [PubMed]
- ONU. Transformer Notre Monde: Le Programme de Développement Durable à L’horizon 2030. Résolution Adoptée par l’Assemblée Générale le 25 Septembre 2015. 2015, pp. 1–13. Available online: https://press.un.org/fr/2015/ag11688.doc.htm (accessed on 25 April 2022).
- Segniagbeto, G.H.; Akpamou, K.G.; Konko, Y.; Gaglo, J.K.T.; Ketoh, G.K.; Dendi, D.; Fa, J.E.; Luiselli, L. Diversity and relative abundance of ungulates and other medium and large mammals in flooded forests in the Dahomey gap (Togo). Animals 2022, 12, 3041. [Google Scholar] [CrossRef]
- GIZ. Evaluation de la Situation Socio-Économique, du Cadre de Gouvernance, du Genre et de l’accès aux Ressources dans les Aires Cibles de la RBT-DM; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ): Togo, 2016; p. 144. Available online: https://crepsad.tg/publication/evaluation-de-la-situation-socio-economique-du-cadre-de-gouvernance-du-genre-et-de (accessed on 26 August 2024).
- ANAMET. Agence Nationale de la Méteorologie. 2023. Available online: https://www.anamet-togo.com (accessed on 26 August 2024).
- Lamouroux, M. Note Explicative N 34: Carte Pédologique du Togo au 1/1.000.000; ORSTOM: Paris, France, 1969; 91p. [Google Scholar]
- Akpamou, G.K.; Konko, Y.; Kokou, K. Monitoring of Residual Forest Ecosystems Dynamics in the Mono Biosphere Reserve (Southeast Togo). Nat. Resour. 2021, 12, 271–289. [Google Scholar] [CrossRef]
- Atakpama, W.; Batawila, K.; Dourma, M.; Pereki, H.; Wala, K.; Dimobe, K.; Akpagana, K.; Gbeassor, M. Ethnobotanical knowledge of Sterculia setigera Del. in the Sudanian zone of Togo (West Africa). ISRN Bot. 2012, 2012, 8. [Google Scholar] [CrossRef]
- RGPH. Quatrieme Recensement General de la Population et de L’habitat–Novembre 2010; Editions l’Héritage: Lomé, Togo, 2010. [Google Scholar]
- Mesfin, F.; Demissew, S.; Teklehaymanot, T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J. Ethnobiol. Ethnomed. 2009, 5, 1–18. Available online: https://ethnobiomed.biomedcentral.com/articles/10.1186/1746-4269-5-28 (accessed on 23 September 2023). [CrossRef] [PubMed]
- Li, X.; Wang, C.; Zhang, X.; Sun, W. Generic sao similarity measure via extended sørensen-dice index. IEEE Access 2020, 8, 66538–66552. Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=9050516 (accessed on 23 September 2023). [CrossRef]
- Martín-López, B.; Iniesta-Arandia, I.; García-Llorente, M.; Palomo, I.; Casado-Arzuaga, I.; Amo, D.G.D.; Gómez-Baggethun, E.; Oteros-Rozas, E.; Palacios-Agundez, I.; Willaarts, B. Uncovering ecosystem service bundles through social preferences. PLoS ONE 2012, 7, e38970. [Google Scholar] [CrossRef]
- Muhamad, D.; Okubo, S.; Harashina, K.; Gunawan, B.; Takeuchi, K. Living close to forests enhances people׳ s perception of ecosystem services in a forest–agricultural landscape of West Java, Indonesia. Ecosyst. Serv. 2014, 8, 197–206. [Google Scholar] [CrossRef]
- Reyes-Arroyo, N.; Camacho-Valdez, V.; Saenz-Arroyo, A.; Infante-Mata, D. Socio-cultural analysis of ecosystem services provided by mangroves in La Encrucijada Biosphere Reserve, southeastern Mexico. Local Environ. 2021, 26, 86–109. Available online: https://sii.ecosur.mx/Content/ProductosActividades/archivos/38400/textocompleto-12-01-2021-15-27.pdf (accessed on 10 September 2022). [CrossRef]
- Nyangoko, B.P.; Berg, H.; Mangora, M.M.; Gullström, M.; Shalli, M.S. Community perceptions of mangrove ecosystem services and their determinants in the Rufiji Delta, Tanzania. Sustainability 2020, 13, 63. [Google Scholar] [CrossRef]
- Gnansounou, S.C.; Salako, K.V.; Sagoe, A.A.; Mattah, P.A.; Aheto, D.W.; Glèlè Kakaï, R. Mangrove Ecosystem Services, Associated Threats and Implications for Wellbeing in the Mono Transboundary Biosphere Reserve (Togo-Benin), West-Africa. Sustainability 2022, 14, 2438. [Google Scholar] [CrossRef]
- Lykke, A.; Kristensen, M.; Ganaba, S. Valuation of local use and dynamics of 56 woody species in the Sahel. Biodivers. Conserv. 2004, 13, 1961–1990. [Google Scholar] [CrossRef]
- Mokoso, J.d.D.M.; Kavatsurwa, S.M.; Birhashirwa, R.N.; Habimana, H.N. Utilisation des ressources forestieres ligneuses par la population habitant la zone submontagnarde du parc national de kahuzi-biega (RD Congo) [use of woody forestry resources by the population living in the submountain area of kahuzi-biega national park (DR Congo)]. Int. J. Innov. Appl. Stud. 2015, 11, 508. [Google Scholar]
- Hessou, H.K. Diversité, ethnobotanique et état de conservation des espèces de sous-bois des plantations de Tectona grandis Lf au sud du Bénin. Sci. Agron. 2019, 7, 9. Available online: http://publication.lecames.org/index.php/svt/article/viewFile/1458/907 (accessed on 3 March 2022).
- Camou-Guerrero, A.; Reyes-García, V.; Martínez-Ramos, M.; Casas, A. Knowledge and use value of plant species in a Rarámuri community: A gender perspective for conservation. Hum. Ecol. 2008, 36, 259–272. [Google Scholar] [CrossRef]
- Nguenang, G.; Fedoung, E.F.; Nkongmeneck, B. Importance des forêts secondaires pour la collecte des plantes utiles chez les Badjoué de l’Est Cameroun. Tropicultura 2010, 28, 238–245. Available online: http://www.tropicultura.org/text/v28n4.pdf#page=48 (accessed on 24 March 2023).
- Gouwakinnou, G.N.; Lykke, A.M.; Assogbadjo, A.E.; Sinsin, B. Local knowledge, pattern and diversity of use of Sclerocarya birrea. J. Ethnobiol. Ethnomed. 2011, 7, 1–9. Available online: https://ethnobiomed.biomedcentral.com/articles/10.1186/1746-4269-7-8 (accessed on 24 March 2023). [CrossRef]
- Dossou, M.E.; Houessou, G.L.; Lougbégnon, O.T.; Tenté, A.H.B.; Codjia, J.T.C. Etude ethnobotanique des ressources forestières ligneuses de la forêt marécageuse d’Agonvè et terroirs connexes au Bénin. Tropicultura 2012, 30, 41–48. [Google Scholar]
- Codjia, J.T.C.; Ekue, M.; Condé, S.K. L’habitat du phacochère (Phacochoerus africanus) dans la forêt classée des Trois Rivières au Bénin. Quelles Aires Protégées Pour L’afrique L’ouest 2007, 238–246. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers15-04/010044696.pdf (accessed on 26 March 2022).
- Achigan-Dako, E.G.; Pasquini, M.W.; Assogba Komlan, F.; N’danikou, S.; Yédomonhan, H.; Dansi, A.; Ambrose-Oji, B. Traditional Vegetables in Benin; Institut National des Recherches Agricoles du Bénin, Imprimeries du CENAP: Cotonou, Benin, 2010; 282p, Available online: https://www.researchgate.net/profile/Bianca-Ambrose-Oji/publication/271074574_Traditional_Vegetables_in_Benin/links/5a65e14e0f7e9b6b8fdcb763/Traditional-Vegetables-in-Benin.pdf (accessed on 11 April 2022).
- Kouakou, K.A. Disponibilité et Vulnérabilité des Espèces Sources de Produits Forestiers Non Ligneux D’origine Végétale de la Forêt Classée du Haut-Sassandra et sa Périphérie Après la Décennie de Crise au Centre-Ouest de la Côte d’Ivoire. Ph.D. Thesis, Univerisité Jean Lorougnon Guédé, Daloa, Côte d’Ivoire, 2019; 189p. Available online: https://theses.hal.science/tel-03033353/document (accessed on 21 March 2022).
- Yuan, R.; Zhang, N.; Zhang, Q. The impact of habitat loss and fragmentation on biodiversity in global protected areas. Sci. Total Environ. 2024, 931, 173004. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0048969724031516 (accessed on 11 January 2024). [CrossRef]
- Issa, I.; Wala, K.; Dourma, M.; Atakpama, W.; Kanda, M.; Akpagana, K. Valeur ethnobotanique de l’espèce, Khaya senegalensis (Desr.) A. Juss (meliaceae) auprès des populations riveraines de la chaîne de l’Atacora au Togo. Rev. Marocaine Sci. Agron. Vét. 2018, 6, 64–72. Available online: https://agrimaroc.org/index.php/Actes_IAVH2/article/view/512/570 (accessed on 2 February 2022).
- Chikou, S.L.; Bissiriou, M.A.; Marcy, H.O.N. Fondements socioculturels de la participation des enfants à la peche dans la vallée de l’Oueme. Hall 2024, 23. Available online: https://revues.acaref.net/wp-content/uploads/sites/3/2024/05/12-Sandrine-Liliose-Chikou.pdf (accessed on 12 August 2024).
- Trent, L. L’alchimie de L’esprit: Sagesse et Mystères Cachés; Ahzuria Publishing, 2024; 276p, Available online: https://books.google.tg/books/about/L_alchimie_de_L_esprit.html?id=fesIEQAAQBAJ&redir_esc=y (accessed on 23 July 2024).
- Houètchégnon, T.; Gbèmavo, D.; Ouinsavi, C.; Sokpon, N. Ethnobotanical knowledge and traditional management of african mesquite (Prosopis africana Guill., Perrot. et Rich.) populations in Benin, West Africa. J. Ethnobiol. Trad. Med. 2015, 125, 1124–1135. [Google Scholar]
- Fandohan, B.; Assogbadjo, A.E.; Kakaï, R.G.; Kyndt, T.; Caluwé, E.D.; Codjia, J.T.C.; Sinsin, B. Women’s traditional knowledge, use value, and the contribution of tamarind (Tamarindus indica L.) to rural households’ cash income in Benin. Econ. Bot. 2010, 64, 248–259. [Google Scholar] [CrossRef]
- Assogbadjo, A.E.; Glèlè Kakaï, R.; Adjallala, F.H.; Azihou, A.F.; Vodouhê, G.F.; Kyndt, T.; Codjia, J.T.C. Ethnic differences in use value and use patterns of the threatened multipurpose scrambling shrub (Caesalpinia bonduc L.) in Benin. J. Med. Plants Res. 2011, 5, 1549–1557. Available online: https://academicjournals.org/journal/JMPR/article-full-text-pdf/817627F17728 (accessed on 12 March 2023).
- Wédjangnon, A.; Houètchégnon, T.; Ouinsavi, C. Caractéristiques ethnobotaniques et importance socio-culturelle de Mansonia altissima A. Chev. au Bénin, Afrique de l’Ouest. J. Anim. Plant Sci. 2016, 29, 4678–4690. [Google Scholar]
- Badjaré, B.; Kokou, K.; Bigou-laré, N.; Koumantiga, D.; Akpakouma, A.; Adjayi, M.B.; Abbey, G.A. Étude ethnobotanique d’espèces ligneuses des savanes sèches au Nord-Togo: Diversité, usages, importance et vulnérabilité. BASE 2018, 22, 152–171. [Google Scholar] [CrossRef]
- Mensah, S. Selected Key Ecosystem Services, Functions, and the Relationship with Biodiversity in Natural Forest Ecosystems. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016; 133p. Available online: https://scholar.sun.ac.za/server/api/core/bitstreams/1ffba654-4312-41b7-b13e-72257338a7d2/content (accessed on 26 August 2024).
- Fagerholm, N.; Käyhkö, N.; Ndumbaro, F.; Khamis, M. Community stakeholders’ knowledge in landscape assessments–Mapping indicators for landscape services. Ecol. Indic. 2012, 18, 421–433. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1470160X11004067 (accessed on 12 June 2023). [CrossRef]
- Badiane, M.; Camara, B.; Ngom, D.; Diédhiou, M.A.A. Perception communautaire des parcs agroforestiers traditionnels à Faidherbia albida (Del.) Chev. en Basse Casamance, Sénégal. Afr. Sci. 2019, 15, 214–226. Available online: https://rivieresdusud.uasz.sn/bitstream/handle/123456789/658/badiane_article%202019.pdf?sequence=1&isAllowed=y (accessed on 24 July 2023).
- Camara, B.; Sagna, B.; Ngom, D.; Niokane, M.; Gomis, Z.D. Importance socio-économique de Elaeis guineensis Jacq. (Palmier à huile) en Basse Casamance (SENEGAL). 2017, 13. [Google Scholar] [CrossRef]
- Atakpama, W. Étude des Formations à Sterculia setigera Del. dans la Zone Écofloristique I du Togo: Aspects Structural et Socio-économique; Université de Lomé: Lomé, Togo, 2010. [Google Scholar]
- Yetein, M.H.; Houessou, L.G.; Lougbégnon, T.O.; Teka, O.; Tente, B. Ethnobotanical study of medicinal plants used for the treatment of malaria in plateau of Allada, Benin (West Africa). J. Ethnopharmacol. 2013, 146, 154–163. Available online: https://www.sciencedirect.com/journal/journal-of-ethnopharmacology (accessed on 12 February 2022). [CrossRef] [PubMed]
- Ouattara, B.; Sanou, L.; Koala, J.; Mipro, H. Perceptions locales de la dégradation des ressources naturelles du corridor forestier de la Boucle du Mouhoun au Burkina Faso. Bois For. Trop. 2022, 352, 43–60. [Google Scholar] [CrossRef]
- Solly, B.; Diéye, E.H.B.; Mballo, I.; Sy, O.; Sane, T.; Thior, M. Dynamique spatio-temporelle des paysages forestiers dans le Sud du Sénégal: Cas du département de Vélingara. Physio-Géo. Géographie Phys. Environ. 2020, 15, 41–67. [Google Scholar] [CrossRef]
- Kombate, B.; Atakpama, W.; Klevor, K.J.A.; Egbelou, H.; Kanda, M.; Dourma, M.; Batawila, K.; Akpagana, K. Le feu de végétation entraîne la dégradation et la déforestation du Parc National Fazao-Malfakassa (PNFM) au Togo. Afr. J. Land Policy Geospat. Sci. 2024, 7, 218–229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).