Evading the Ghost of Extinction: A Case Study for the Reintroduction of Ghost Bats (Macroderma gigas)
Abstract
1. Introduction
2. Materials and Methods
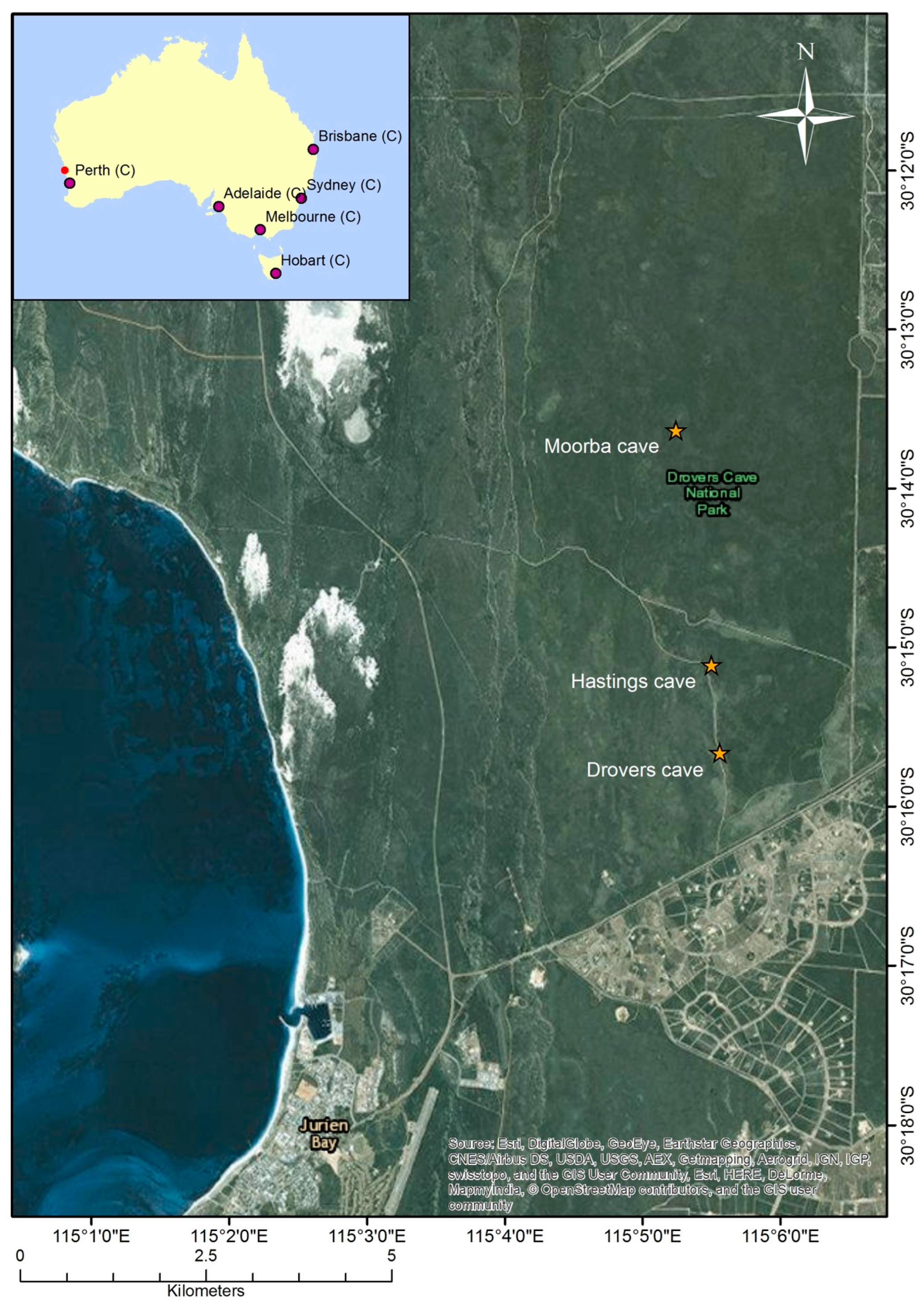
2.1. Microclimate Conditions at Drovers Cave
2.2. Food Supply at Drovers Cave National Park
2.3. Potential Prey Species at Drovers Cave National Park
3. Results
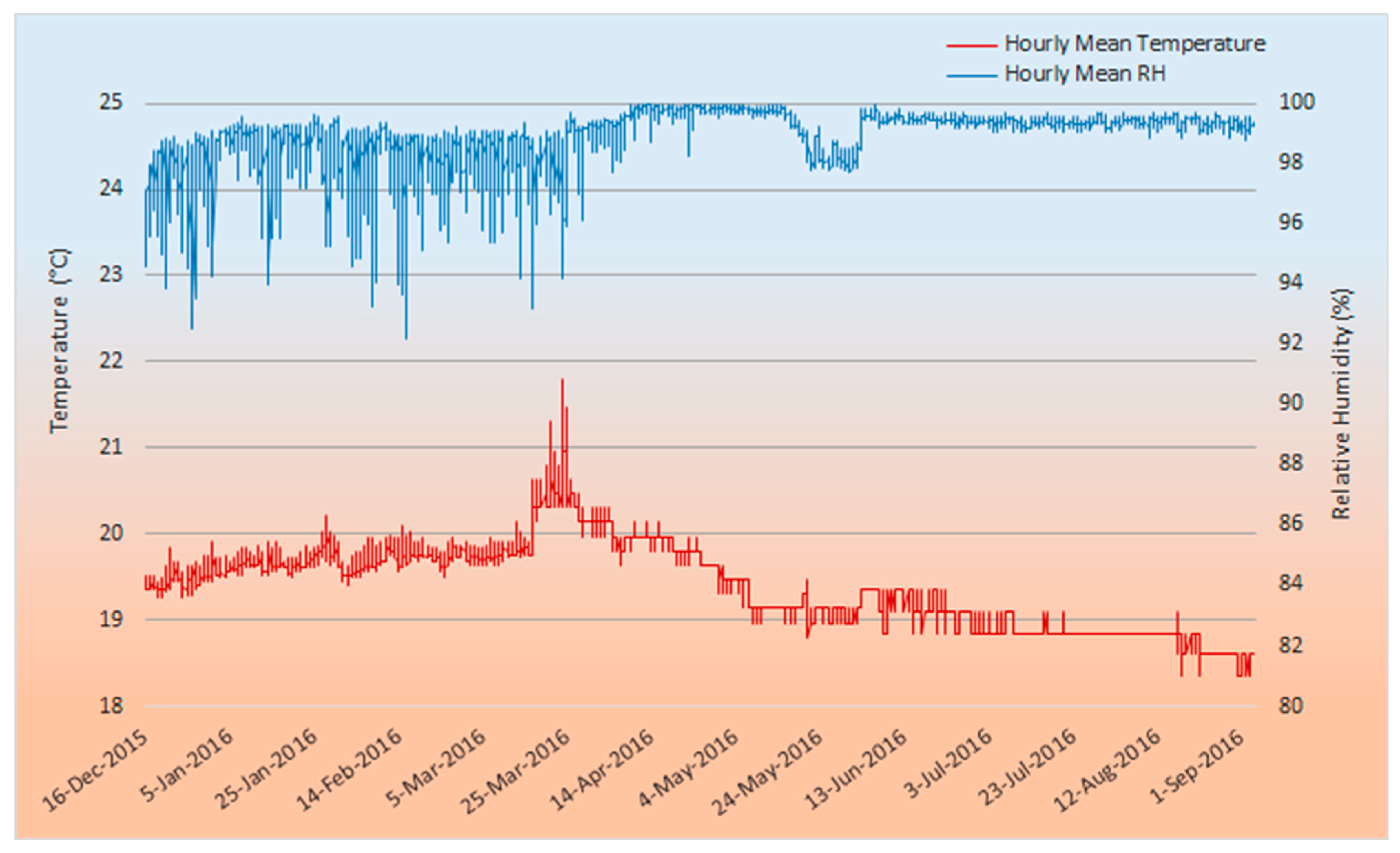
3.1. Microclimate Conditions at Drovers Cave
3.2. Food Supply at Drovers Cave National Park
Prey Species and Their Relative Abundance in the Pilbara Region
3.3. Potential Prey Species at Drovers Cave National Park
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards, G.C.; Hand, S.; Armstrong, K.A.; Hall, L.S. Ghost Bat Macroderma gigas. In The Mammals of Australia; Van Dyck, S., Strahan, R., Eds.; Reed New Holland: Sydney, Australia, 2008; pp. 449–450. [Google Scholar]
- Hudson, W.S.; Wilson, D.E. Macroderma gigas. Mamm. Species 1986, 260, 1–4. [Google Scholar] [CrossRef]
- McKenzie, N.; Hall, L. Macroderma gigas. The IUCN Red List of Threatened Species. Version 2015.4. 2008. Available online: http://www.iucnredlist.org (accessed on 6 August 2015).
- Woinarski, J.; Burbidge, A.; Harrison, P. Action Plan for Australian Mammals; CSIRO Publishing: Clayton, Australia, 2014. [Google Scholar]
- Churchill, S.K.; Helman, P.M. Distribution of the Ghost bat, Macroderma gigas, (Chiroptera: Megadermatidae) in central and South Australia. Aust. Mammal. 1990, 13, 149–156. [Google Scholar] [CrossRef]
- Baynes, A.; Baird, R.F. The original mammal fauna and some information on the original bird fauna of Uluru National Park, Northern Territory. Rangel. J. 1992, 14, 92–106. [Google Scholar] [CrossRef]
- Bat Call WA. A Review of Ghost Bat Ecology, Threats and Survey Requirements; Report prepared for the Department of Agriculture, Water and the Environment; CC BY-NC-ND 4.0; Department of Agriculture, Water and the Environment: Canberra, Australia, 2021. Available online: https://www.agriculture.gov.au/sites/default/files/documents/review-ghostbat-ecology-threats.pdf (accessed on 15 May 2024).
- Worthington-Wilmer, J.; Hall, L.; Barratt, E.; Moritz, C. Genetic structure and male-mediated gene flow in the Ghost Bat (Macroderma gigas). Evolution 1999, 53, 1582–1591. [Google Scholar]
- Armstrong, K.N.; Anstee, S.D. The ghost bat in the Pilbara: 100 years on. Aust. Mammal. 2000, 22, 93–101. [Google Scholar] [CrossRef]
- Cramer, V.A.; Armstrong, K.N.; Bullen, R.D.; Cross, S.L.; Gibson, L.; Hanrahan, N.; Knuckey, C.G.; Ottewell, K.S.C.; Ruykus, L.; Shaw, R.E.; et al. Research priorities for the ghost bat (Macroderma gigas) in the Pilbara region of Western Australia. Aust. Mammal. 2022, 45, 1–12. [Google Scholar] [CrossRef]
- Hall, L.; McKenzie, N.; Dunlop, N. The importance of abandoned mines as habitat for bats. In Conservation Outside Nature Reserves; Hale, P., Lamb, D., Eds.; The University of Queensland: St. Lucia, QLD, Australia, 1997; pp. 326–334. [Google Scholar]
- CSIRO. Climate Change in Australia Technical Report; CSIRO and Bureau of Meteorology: Melbourne, Australia, 2015. [Google Scholar] [CrossRef]
- Weeks, A.R.; Moro, D.; Thavornkanlapachai, R.; Taylor, H.R.; White, N.E.; Weiser, E.L.; Heinze, D. Conserving and enhancing genetic diversity in translocation programs. In Advances in Reintroduction Biology of Australian and New Zealand Fauna; Armstrong, D.P., Hayward, M.W., Moro, D., Seddon, P.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2015; pp. 27–140. [Google Scholar]
- Armstrong, D.P.; Moro, D.; Hayward, M.W.; Seddon, P.J. Introduction: The development of reintroduction biology in New Zealand and Australia. In Advances in Reintroduction Biology of Australian and New Zealand Fauna; Armstrong, D.P., Hayward, M.W., Moro, D., Seddon, P.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2015; pp. 1–6. [Google Scholar]
- Short, J. The Characteristics and Success of Vertebrate Translocations within Australia; A Progress Report; Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2009. [Google Scholar]
- Morris, K.; Page, M.; Kay, R.; Renwick, J.; Desmond, A.; Comer, S.; Burbidge, A.; Kuchling, G.; Sims, C. Forty years of fauna translocations in Western Australia: Lessons learned. In Advances in Reintroduction Biology of Australian and New Zealand Fauna; Armstrong, P.D., Hayward, M.W., Moro, D., Seddon, P.J., Eds.; CSIRO Publishing: Melbourne, Australia, 2015; pp. 217–235. [Google Scholar]
- DSE. Relocating Flying-Foxes; revised ed.; Department of Sustainability and Environment, Victoria State Government: Melbourne, Australia, 2003. [Google Scholar]
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. A review of the global conservation status of bats. Oryx 2002, 36, 18–34. [Google Scholar] [CrossRef]
- Ruffell, J.; Parsons, S. Assessment of the short-term success of a translocation of lesser short-tailed bats Mystacina tuberculata. Endang. Species Res. 2009, 8, 33–39. [Google Scholar] [CrossRef]
- Ruffell, J.; Guilbert, J.; Parsons, S. Translocation of bats as a conservation strategy: Previous attempts and potential problems. Endang. Species Res. 2009, 8, 25–31. [Google Scholar] [CrossRef]
- Weinberger, I.C.; Bontadina, F.; Arlettaz, R. Translocation as a conservation tool to supplement relict bat colonies: A pioneer study with endangered horseshoe bats. Endanger. Species Res. 2009, 8, 41–48. [Google Scholar] [CrossRef][Green Version]
- Kelly, A. Further evidence for the post-release survival of hand-reared, orphaned bats based on radio-tracking and ring-return data. Anim. Welfare. 2012, 21, 27–31. [Google Scholar] [CrossRef]
- Douglas, A.M. The natural history of the ghost bat, Macroderma gigas (Microchiroptera, Megadermatidae), in Western Australia. West. Aust. Nat. 1967, 10, 125–137. [Google Scholar]
- Jones, F.W. The Mammals of South Australia; Govt. Printer: Adelaide, Australia, 1925; Volume 3, 458p. [Google Scholar]
- Butler, W.H. Occurrence of the Ghost Bat, Macroderma gigas, in the Great Victoria Desert, W.A. West. Aust. Nat. 1962, 8, 42–43. [Google Scholar]
- Hamilton-Smith, E. The geographical distribution of Australian cave-dwelling Chiroptera. Int. J. Speleol. 1966, 2, 91–104. [Google Scholar] [CrossRef][Green Version]
- Baynes, A. The Analysis of a Late Quaternary Mammal Fauna from Hastings Cave, Jurien, Western Australia. Ph.D. Thesis, Department of Zoology, The University of Western Australia, Perth, Australia, 1979. [Google Scholar]
- Lundelius, E. Post Pleistocene faunal succession in Western Australia and its climatic interpretation. In Proceedings of the 21st International Geological Congress, Copenhagen, Denmark, 15–25 August 1960; Part 4. pp. 42–53. [Google Scholar]
- Bridge, P.J.; Hodge, L.C.; Marsh, N.L.; Thomas, A.G. Chiropterite deposits in Moorba Cave, Jurien Bay, Western Australia. Helictite 1975, 13, 19–33. [Google Scholar]
- Woinarski, J.; Burbidge, A.; Harrison, P. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proc. Natl. Acad. Sci. USA 2015, 112, 4531–4540. [Google Scholar] [CrossRef]
- Saltré, F.; Rodríguez-Rey, M.; Brook, B.W.; Johnson, C.N.; Turney, C.S.M.; Alroy, J.; Cooper, A.; Beeton, N.; Bird, M.I.; Fordham, D.A.; et al. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 2016, 7, 10511. [Google Scholar] [CrossRef]
- King, D.; Smith, L.A. The distribution of the European Red Fox in Western Australia. Rec. West. Aust. Mus. 1985, 12, 197–202. [Google Scholar]
- Start, A.N. A review of the biology and conservation status of the Ngadji or Western Pebble-mound Mouse, Pseudomys chapmani Kitchener, 1980 (Rodentia: Muridae). CALMScience 2000, 3, 125–147. [Google Scholar]
- Hutson, A.M.; Mickleburgh, S.P.; Racey, P.A. Microchiropteran Bats: Global Status Survey and Conservation Action Plan; IUCN/SSC Chiroptera Specialist Group: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Doherty, T.S.; Davis, R.A.; Van Etten, E.J.B.; Algar, D.; Collier, N.; Dickman, C.R.; Edwards, G.; Masters, P.; Palmer, R.; Robinson, S. A continental-scale analysis of feral cat diet in Australia. J. Biogeo. 2015, 42, 964–975. [Google Scholar] [CrossRef]
- Webb, R. Drovers Cave, Western Australia: Cave destruction through management. In Proceedings of the 14th Biennial Conference, Adelaide, Australia, 3–7 January 1983; Pilkington, G., Ed.; Australian Speleological Federation: Sydney, Australia, 1984; pp. 41–48. [Google Scholar]
- Toop, G.J. Habitat requirements, survival strategies and ecology of the ghost bat, Macroderma gigas Dobson (Microchiroptera, Megadermatidae) in central coastal Queensland. Macroderma 1985, 1, 37–41. [Google Scholar]
- Kaita, A.A. Ecology and Flight Characteristics of Ghost Bats (Macroderma gigas): Pre-Requisites for In Situ Re-Introduction from Captivity. Master’s Thesis, Curtin University, Perth, Australia, 2012. [Google Scholar]
- Bridge, P.J. Paleo-distribution of Macroderma gigas in the southwest of Western Australia. Helictite 1975, 13, 34–36. [Google Scholar]
- Kern, A.M. Hydrogeology of the Coastal Plain, between Cervantes and Leeman, Perth Basin; Water and Rivers Commission Report Series; Report No. HG 3; Water and Rivers Commission: Perth, Australia, 1997. [Google Scholar]
- Beard, J.S. Biogeography of the Kwongan. In Kwongan: Plant Life of the Sand Plain: Biology of a South-West Australian Shrubland Ecosystem; Pate, J.S., Beard, J.S., Eds.; University of Western Australia Press: Nedlands, Australia, 1984. [Google Scholar]
- CALM. Management Plan for Lesueur National Park and Coomallo Nature Reserve 1995–2005. 1995. Available online: https://www.https://www.dbca.wa.gov.au/management/plans/lesueur-national-park-and-coomallo-nature-reserve (accessed on 20 November 2015).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.2.5; R Core Team: Vienna, Austria, 2016; Available online: https://www.r-project.org (accessed on 1 July 2016).
- DBCA. 2016. Available online: https://naturemap.dbca.wa.gov.au/default.aspx (accessed on 28 January 2016).
- Arteaga Claramunt, A.M.; White, N.E.; Bunce, M.; O’Connell, M.; Bullen, R.D.; Mawson, P.R. Determination of the diet of the Ghost bat (Macroderma gigas) in the Pilbara region of Western Australia from dried prey remains and DNA metabarcoding. Aust. J. Zool. 2019, 66, 195–200. [Google Scholar] [CrossRef]
- Start, A.N.; McKenzie, N.L.; Bullen, R.D. Notes on bats in the diets of ghost bats (Macroderma gigas: Megadermatidae) in the Pilbara region of Western Australia. Rec. West. Aust. Mus. 2019, 34, 51–53. [Google Scholar] [CrossRef]
- Storr, G.; Smith, L.A.; Johnstone, R.E. Lizards of Western Australia. I. Skinks; Western Australian Museum: Perth, Australia, 1999. [Google Scholar]
- Johnstone, R.E.; Storr, G.M. Volume 2—Passerines. In Handbook of Western Australian Birds; Western Australian Museum: Perth, Australia, 2004. [Google Scholar]
- Van Dyck, S.; Strahan, R. The Mammals of Australia; Reed New Holland: Sydney, Australia, 2008. [Google Scholar]
- BOM. Summary Statistics for Jurien Bay; Commonwealth of Australia Bureau of Meteorology: Melbourne, Australia, 2016. Available online: http://www.bom.gov.au (accessed on 16 February 2017).
- Baudinette, R.V.; Churchill, S.K.; Christian, K.A.; Nelson, J.E.; Hudson, P.J. Energy, water balance and the roost microenvironment in three Australian cave-dwelling bats (Microchiroptera). J. Comp. Physiol. B. 2000, 170, 439–446. [Google Scholar] [CrossRef]
- Churchill, S. Australian Bats; Reed New Holland: Sydney, Australia, 1998. [Google Scholar]
- Leitner, P.; Nelson, J.E. Body temperature, oxygen consumption and heart rate in the Australian false vampire, Macroderma gigas. Comp. Biochem. Physiol. 1967, 21, 65–74. [Google Scholar] [CrossRef]
- Bullen, R.D.; Reiffer, S.; Trainer, J. Satellite tracking Ghost bats (Macroderma gigas) in the Pilbara, Western Australia. Rec. West. Aust. Mus. 2023, 38, 1–10. [Google Scholar] [CrossRef]
- Hoyle, S.D.; Pople, A.R.; Toop, G.J. Mark–recapture may reveal more about ecology than about population trends: Demography of a threatened ghost bat (Macroderma gigas) population. Austral Ecol. 2001, 26, 80–92. [Google Scholar]
- White, A.W.; Morris, I.; Madani, G.; Archer, M. Are Cane Toads Rhinella marina impacting Ghost Bats Macroderma gigas in Northern Australia? Aust. Zool. 2016, 38, 183–191. [Google Scholar] [CrossRef]
- Kearney, M.; Phillips, B.L.; Tracy, C.R.; Christian, K.A.; Betts, G.; Porter, W.P. Modelling species distributions without using species distributions: The cane toad in Australia under current and future climates. Oikos 2008, 31, 423–434. [Google Scholar] [CrossRef]
- Seebacher, F.; Franklin, C.E. Physiology of invasion: Cane toads are constrained by thermal effects on physiological mechanisms that support locomotor performance. J. Exp. Biol. 2011, 214, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Tidemann, C.R.; Priddel, D.M.; Nelson, J.E.; Pettigrew, J.D. Foraging behaviour of the Australian ghost bat, Macroderma gigas (Microchiroptera: Megadermatidae). Aust. J. Zool. 1985, 33, 705–713. [Google Scholar] [CrossRef]
- Boles, W.E. Avian prey of the Australian ghost bat Macroderma gigas (Microchiroptera: Megadermatidae): Prey characteristics and damage from predation. Aust. Zool. 1999, 31, 82–91. [Google Scholar] [CrossRef]
- Johnston, M.; Herrod, A.; Little, N.; Bould, L.; Gigliotti, F. An opportunistic observation of Ghost Bat, (Macroderma gigas) predation on six bird species within Karijini National Park. J. R. Soc. West. Aust. 2015, 98, 89–91. [Google Scholar]
- Schultz, M. Vertebrate prey of the Ghost Bat, Macroderma gigas, at Pine Creek, Northern Territory. Macroderma 1986, 2, 59–62. [Google Scholar]
- Kulzer, E.; Nelson, J.E.; McKean, J.L.; Möhres, F.P. Prey-catching behaviour and echolocation in the Australian ghost bat, Macroderma gigas (Microchiroptera: Megadermatidae). Aust. Mammal. 1984, 7, 37–50. [Google Scholar] [CrossRef]
- Biodiversity Conservation Act 2016, Biodiversity Conservation (Listing of Native Species) (Fauna) Order 2023. Available online: https://www.dbca.wa.gov.au/sites/default/files/2023-07/Biodiversity%20Conservation%20Listing%20of%20Native%20Species%20Flora%20Order%202022.pdf (accessed on 4 July 2024).
- Fontaine, J.; Kennedy, P. Meta-analysis of avian and small-mammal response to fire severity and fire surrogate treatments in U.S. fire-prone forests. Ecol. Appl. 2012, 22, 1547–1561. [Google Scholar] [PubMed]
- Doherty, T.; Davis, R.; Van Etten, E. A game of cat-and-mouse: Microhabitat influences rodent foraging in recently burnt but not long unburnt shrublands. J. Mammal. 2015, 96, 324–331. [Google Scholar] [CrossRef]
- Lamont, B.; Groom, P.K.; Richards, M.; Witkowski, E.T.F. Recovery of Banksia and Hakea communities after fire in mediterranean Australia—The role of species identity and functional attributes. Divers. Distrib. 1999, 5, 15–26. [Google Scholar] [CrossRef]
- Johnston, T.R.; Stock, W.; Mawson, P.R. Foraging by Carnaby’s cockatoo on the Swan coastal plain, Western Australia. Emu 2016, 116, 284–293. [Google Scholar] [CrossRef]
- Bullen, R.D.; McKenzie, N.L. Recent developments in studies of the community structure, foraging ecology and conservation of Western Australian bats. In The Biology and Conservation of Australasian Bats; Law, B., Eby, P., Lunney, D., Lumsden, L., Eds.; Royal Zoological Society of NSW: Mosman, NSW, Australia, 2011; pp. 31–43. [Google Scholar]



| December | January | February | March | April | May | June | July | August | September | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Mean ± S.E. | 19.47 ± 0.006 | 19.69 ± 0.004 | 19.73 ± 0.004 | 20.08 ± 0.01 | 19.89 ± 0.01 | 19.18 ± 0.01 | 19.16 ± 0.01 | 18.88 ± 0.01 | 18.76 ± 0.01 | 18.51 ± 0.04 |
| Max–min | 19.83–19.25 | 20.22–19.45 | 20.10–19.40 | 21.80–19.64 | 20.30–19.30 | 19.63–18.80 | 19.35–18.85 | 19.10–18.85 | 19.10–18.35 | 18.60–18.35 | |
| Sample size | 4836 | 9672 | 9048 | 6192 | 2160 | 2232 | 1490 | 1488 | 1488 | 150 | |
| Ambient mean (Max–min) | 23.9 (28.9–16.0) | 24.8 (30.0–19.4) | 25.8 (30.8–17.8) | 24.5 (29.4–17.9) | 20.1 (25.5–15.9) | 16.0 (22.8–10.4) | 13.3 (19.9–9.7) | 13.0 (19.3–8.9) | 13.3 (19.4–8.6) | 14.9 (19.9–7.6) | |
| RH (%) | Mean ± S.E. | 97.35 ± 0.068 | 98.48 ± 0.036 | 97.86 ± 0.048 | 98.19 ± 0.06 | 99.64 ± 0.02 | 99.19 ± 0.03 | 99.41 ± 0.03 | 99.39 ± 0.02 | 99.40 ± 0.02 | 99.26 ± 0.08 |
| Max–min | 99.02–92.52 | 99.62–93.95 | 99.36–92.18 | 99.73–93.16 | 100–97.73 | 100–97.69 | 99.95–97.83 | 99.70–99,05 | 99.70–98.83 | 99.60–98.80 | |
| Sample size | 4836 | 9672 | 9048 | 7584 | 5040 | 5208 | 3030 | 2976 | 2976 | 300 | |
| Ambient mean | 53 | 69 | 54 | 61 | 76 | 78 | 84 | 75 | 75 | 68 |
| Class | Insecta | Amphibia | Reptilia | Aves | Mammalia | Total Species No. | ||
|---|---|---|---|---|---|---|---|---|
| Order | 1 | 1 | 1 | 4 | 3 | |||
| Dasyuridae | Rodentia | Chiroptera | ||||||
| Family | 1 | 2 | 4 | 9 | 1 | 1 | 3 | |
| Genus | 4 | 2 | 4 | 10 | 4 | 4 | 3 | |
| Species | 4 | 2 | 4 | 11 | 6 | 5 | 4 | 36 |
| Percentage (%) | 11 | 5.5 | 11 | 30.5 | 17 | 14 | 11 | 100 |
| Taxa | Location | Ranked Mass/Length | No. Species | No. Records | Percentage of Total Individuals |
|---|---|---|---|---|---|
| Insects | Pilbara | S | 4 | 8 | 100 |
| M | 0 | 0 | 0 | ||
| L | 0 | 0 | 0 | ||
| Drovers Cave NP | S | 12 | 18 | 100 | |
| M | 0 | 0 | 0 | ||
| L | 0 | 0 | 0 | ||
| Amphibians | Pilbara | S | 2 | 2 | 100 |
| M | 0 | 0 | 0 | ||
| L | 0 | 0 | 0 | ||
| Drovers Cave NP | S | 7 | 48 | 100 | |
| M | 0 | 0 | 0 | ||
| L | 0 | 0 | 0 | ||
| Reptiles | Pilbara | S | 0 | 0 | 0 |
| M | 3 | 3 | 75 | ||
| L | 1 | 1 | 25 | ||
| Drovers Cave NP | S | 9 | 160 | 32 | |
| M | 11 | 279 | 55 | ||
| L | 2 | 65 | 13 | ||
| Birds | Pilbara | S | 6 | 24 | 58 |
| M | 3 | 15 | 37 | ||
| L | 2 | 2 | 5 | ||
| Drovers Cave NP | S | 25 | 220 | 92 | |
| M | 3 | 5 | 2 | ||
| L | 1 | 14 | 6 | ||
| Mammals | Pilbara | S | 11 | 87 | 70 |
| M | 4 | 38 | 30 | ||
| L | 0 | 0 | 0 | ||
| Drovers Cave NP | S | 6 | 317 | 100 | |
| M | 0 | 0 | 0 | ||
| L | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claramunt, A.M.A.; Bencini, R.; Mawson, P.R. Evading the Ghost of Extinction: A Case Study for the Reintroduction of Ghost Bats (Macroderma gigas). Conservation 2024, 4, 378-394. https://doi.org/10.3390/conservation4030025
Claramunt AMA, Bencini R, Mawson PR. Evading the Ghost of Extinction: A Case Study for the Reintroduction of Ghost Bats (Macroderma gigas). Conservation. 2024; 4(3):378-394. https://doi.org/10.3390/conservation4030025
Chicago/Turabian StyleClaramunt, Alba M. Arteaga, Roberta Bencini, and Peter R. Mawson. 2024. "Evading the Ghost of Extinction: A Case Study for the Reintroduction of Ghost Bats (Macroderma gigas)" Conservation 4, no. 3: 378-394. https://doi.org/10.3390/conservation4030025
APA StyleClaramunt, A. M. A., Bencini, R., & Mawson, P. R. (2024). Evading the Ghost of Extinction: A Case Study for the Reintroduction of Ghost Bats (Macroderma gigas). Conservation, 4(3), 378-394. https://doi.org/10.3390/conservation4030025






