Heavy Metal Tolerance and Accumulation Potential of a Rare Coastal Species, Anthyllis vulneraria subsp. maritima
Abstract
1. Introduction
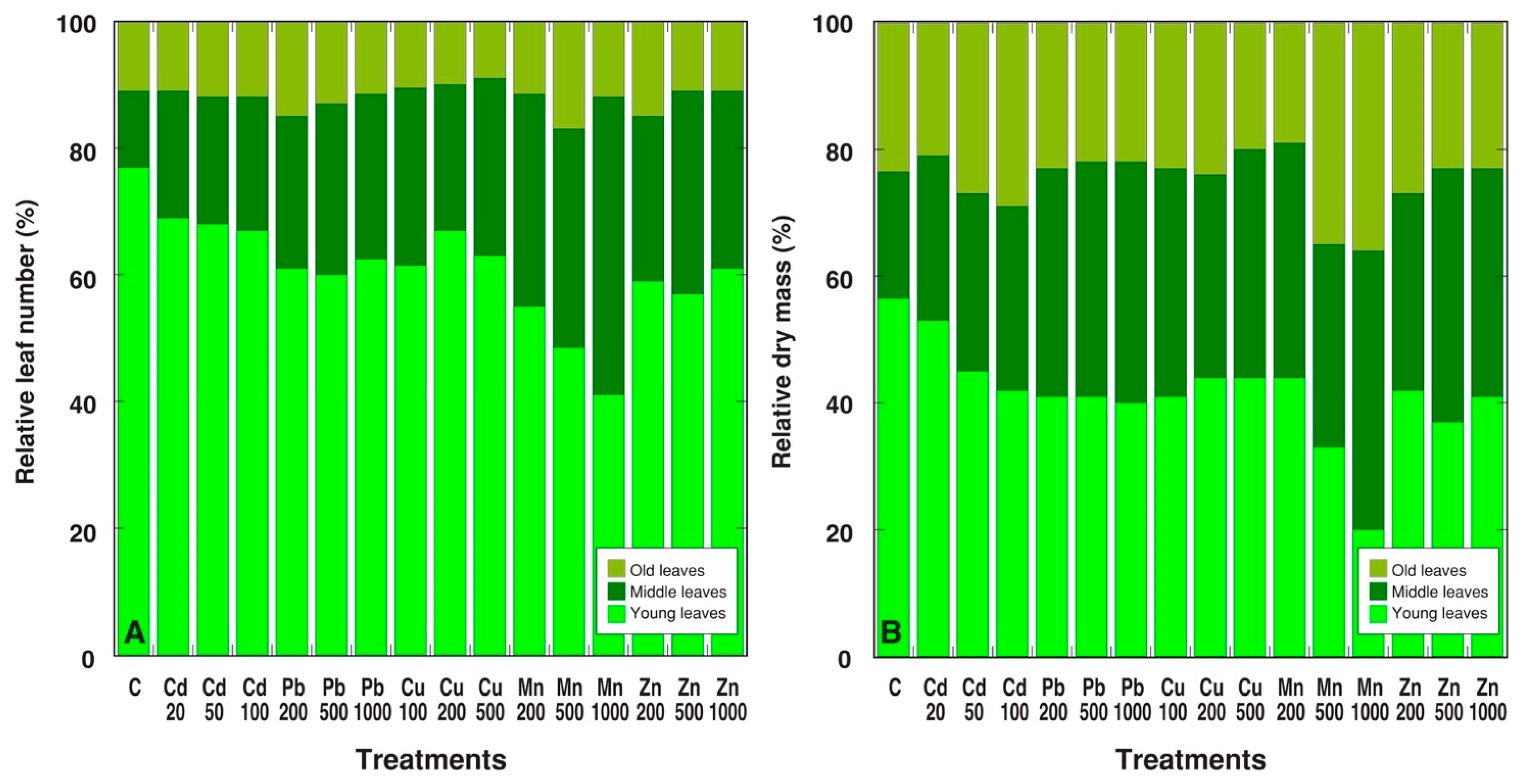
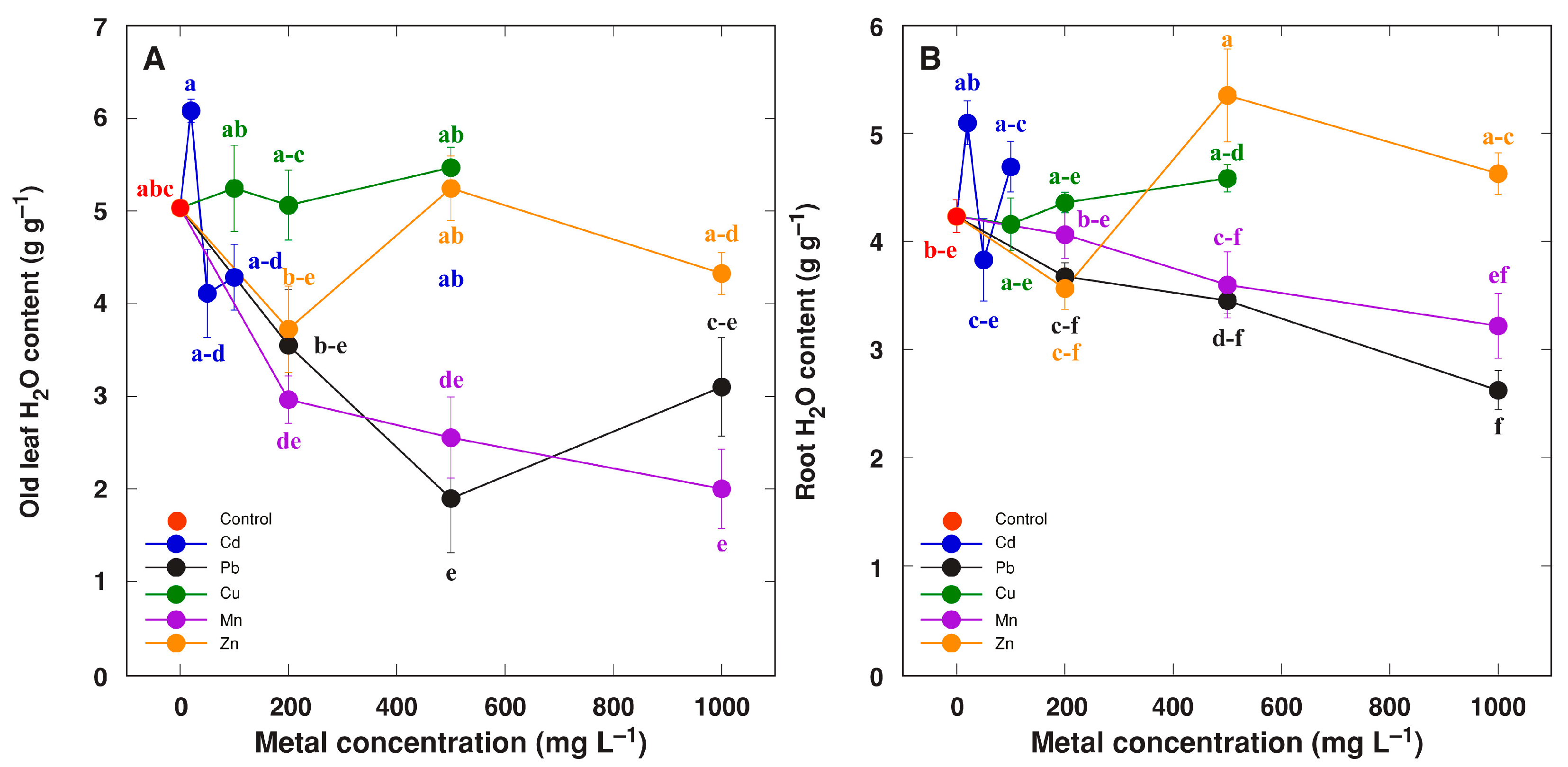
2. Results
3. Discussion
3.1. Tolerance to Heavy Metals
3.2. Metal Accumulation Potential
3.3. Changes in Oxidative Enzyme Activity
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprassanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [PubMed]
- Leguizamo, M.A.O.; Gómez, W.D.F.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Baltool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exp. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- Bothe, H. Plants in heavy metal soils. In Detoxification of Heavy Metals; Sherameti, I., Varma, A., Eds.; Soil Biology 30; Springer: Berlin/Heidelberg, Germany, 2011; pp. 35–57. [Google Scholar]
- Ernst, W.H.O. Evolution of metal tolerance in higher plants. Forest Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Skuza, L.; Szućko-Kociuba, I.; Filip, E.; Bożek, I. Natural molecular mechanisms of plant hyperaccumulation and hypertolerance towards heavy metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Rascio, N. Metal accumulation by some plants growing on zinc-mine deposits. Oikos 1977, 29, 250–253. [Google Scholar] [CrossRef]
- Punz, W. Metallophytes in the Eastern Alps with special emphasis on higher plants growing on calamine and copper localities. Phyton 1995, 35, 295–309. [Google Scholar]
- Bothe, H.; Słomka, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soils Sedim. 2017, 17, 1310–1320. [Google Scholar] [CrossRef]
- Hesami, R.; Salimi, A.; Ghaderian, S.M. Lead, zinc, and cadmium uptake, accumulation, and phytormediation by plants growing around Tang-e Douzan lead–zinc mine, Iran. Environ. Sci. Pollut. Res. 2018, 25, 8701–8714. [Google Scholar] [CrossRef] [PubMed]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laffont-Schwob, I. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a nondestructive and integrative approach. J. Environ. Manag. 2016, 183, 850–863. [Google Scholar] [CrossRef]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J.M. Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 2014, 217–218, 8–17. [Google Scholar] [CrossRef]
- Degtjareva, G.V.; Valiejo-Roman, C.M.; Samigullin, T.H.; Guara-Requena, M.; Sokoloff, D.D. Phylogenetics of Anthyllis (Leguminosae” Papilionoideae: Loteae): Partial incongruence between nuclear and plastid markers, a long brach problem and implications for morphological evolution. Mol. Phylogen. Evol. 2012, 67, 693–707. [Google Scholar] [CrossRef]
- Roze, I. Kidney vetch Anthyllis L. in the flora of Latvia. Proc. Latv. Acad. Sci. B 2004, 58, 61–69. [Google Scholar]
- Puidet, E.; Liira, J.; Paal, J.; Pärtel, M.; Pihu, S. Morphological variation in eight taxa of Anthyllis vulneraria s. lato (Fabaceae). Ann. Bot. Fennici 2005, 42, 293–304. [Google Scholar]
- Köster, E.; Bitocchi, E.; Papa, R.; Pihu, S. Genetic structure of the Anthyllis vulneraria L. s. l. species complex in Estonia based on AFLPs. Centr. Eur. J. Biol. 2008, 3, 442–450. [Google Scholar] [CrossRef]
- Daco, L.; Colling, G.; Matthies, D. Altitude and latitude have different effects on population characteristics of the widespread plant Anthyllis vulneraria. Oecologia 2021, 197, 537–549. [Google Scholar] [CrossRef]
- Rola, K. A morphometric study on Anthyllis vulneraria (Fabaceae) from Poland and its taxonomic implications. Biologia 2012, 67, 296–309. [Google Scholar] [CrossRef]
- Kesselring, H.; Hamann, E.; Armbruster, G.F.J.; Stöcklin, J.; Scheepens, J.F. Local adaptation is stronger between than within regions in alpine populations of Anthyllis vulneraria. Evol. Ecol. 2019, 33, 737–750. [Google Scholar] [CrossRef]
- Escarré, J.; Lefèbre, C.; Raboyeau, S.; Dossantos, A.; Gruber, W.; Marel, J.C.C.; Frérot, H.; Noret, N.; Mahieu, S.; Collin, C.; et al. Heavy metal concentration survey in soils and plants of the Les Malines mining district (Southern France): Implications for soil restoration. Water Air Soil Pollut. 2011, 216, 485–504. [Google Scholar] [CrossRef]
- Gaile, L.; Andersone-Ozola, U.; Samsone, I.; Elferts, D.; Ievisnh, G. Modification of growth and physiological response of coastal dune species Anthyllis maritima to sand burial by rhizobial symbiosis and salinity. Plants 2021, 10, 2584. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; El Moneim, D.A.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2008, 14, 43–50. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Mathé, C.; Barre, A.; Jourda, C.; Dunand, C. Evolution and expression of class III peroxidases. Arch. Biochem. Biophys. 2010, 500, 58–65. [Google Scholar] [CrossRef]
- Almagro, L.; Ros, L.V.G.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef]
- Lee, Y.; Rubio, M.C.; Alassimone, J.; Geldner, N. A mechanism for localized lignin deposition in the endodermis. Cell 2013, 153, 402–412. [Google Scholar] [CrossRef]
- Mahieu, S.; Frérot, H.; Vidal, C.; Galiana, A.; Heulin, K.; Maure, L.; Brunel, B.; Lefèbvre, C.; Escarré, J.; Cleyer-Marel, J.-C. Anthyllis vulneraria/Mesorhizobium metallidurans, an efficient symbiotic nitrogen fixing association able to grow in mine tailings highly contaminated by Zn, Pb and Cd. Plant Soil 2011, 342, 405–417. [Google Scholar] [CrossRef]
- Soussou, S.; Mahieu, S.; Brunel, B.; Escarré, J.; Lebrun, M.; Banni, M.; Bousetta, H.; Cleyet-Marel, J.-C. Zinc accumulation patterns in four Anthyllis vulneraria subspecies supplemented with mineral nitrogen or grown in the presence of their symbiotic bacteria. Plant Soil 2013, 371, 423–434. [Google Scholar] [CrossRef]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative stress and heavy metals in plants. Rev. Environ. Contamin. Toxicol. 2017, 245, 129–156. [Google Scholar]
- Saraswat, S.; Rai, J.P.N. Complexation and detoxification of Zn and Cd in metal accumulating plants. Rev. Environ. Sci. Biotechnol. 2011, 10, 327–339. [Google Scholar] [CrossRef]
- Leitenmaier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef]
- Singer, C.E.; Havill, D.C. Manganese as an ecological factor in salt marshes. Vegetatio 1985, 62, 287–292. [Google Scholar] [CrossRef]
- Singer, C.E.; Havill, D.C. Resistance to divalent manganese of salt-marsh plants. J. Ecol. 1993, 81, 797–806. [Google Scholar] [CrossRef]
- Ievinsh, G.; Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A. Salinity and heavy metal tolerance, and phytoextraction potential of Ranunculus sceleratus plants from a sandy coastal beach. Life 2022, 12, 1959. [Google Scholar] [CrossRef]
- Ievinsh, G.; Dišlere, E.; Karlsons, A.; Osvalde, A.; Vikmane, M. Physiological responses of wetland species Rumex hydrolapathum to increased concentration of biogenous heavy metals Zn and Mn in substrate. Proc. Latv. Acad. Sci. B 2020, 74, 35–47. [Google Scholar] [CrossRef]
- Puciariello, C.; Boscari, A.; Tagliani, A.; Brouquisse, R.; Perata, P. Exploring legume-rhizobia symbiotic models for waterlogging tolerance. Front. Plant Sci. 2019, 10, 578. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Habtewold, J.Z. Evaluation of legume–rhizobial symbiotic interactions beyond nitrogen fixation that help the host survival and diversification in hostile environments. Microorganisms 2023, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Chantreuil, C.; Berge, O.; Mauré, L.; Escarré, J.; Béna, G.; Brunel, B.; Cleyet-Marel, J.C. Mesorhizobium metallidurans sp. nov., a novel metal resistant symbiont of Anthyllis vulneria growing on metallicolous soil in Languedoc, France. Int. J. Syst. Evol. Microbiol. 2009, 59, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, S.; Soussou, S.; Cleyer-Marel, J.-C.; Brunel, B.; Mauré, L.; Lefèbvre, C.; Escarré, J. local adaptation of metallicolous and non0metallicolous Anthyllis vulneraria populations: Their utilization in soil restoration. Restor. Ecol. 2013, 21, 551–559. [Google Scholar] [CrossRef]
- Skujovska-Rybkowska, M.; Muszyńska, E.; Labudda, M. Structural adaptation and physiological mechanisms in the leaves of Anthyllis vulneraria L. from metallicolous and non-metallicolous populations. Plants 2020, 9, 662. [Google Scholar] [CrossRef]
- Grison, C.M.; Mazel, M.; Sellini, A.; Escande, V.; Biton, J.; Grison, C. The leguminous species Anthyllis vulneraria as a Zn-hyperaccumulator and eco-Zn catalyst resource. Environ. Sci. Pollut. Res. 2015, 22, 5667–5676. [Google Scholar] [CrossRef]
- van de Mortel, J.E.; Villanueva, L.A.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; van Themaat, E.V.L.; Koorneef, M.; Aarts, M.G.M. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Huguet, S.; Soussou, S.; Cleyet-Marel, J.C.; Trcera, N.; Isaure, M.P. Rhizostabilization of a mine tailing highly contaminated: Previous study of Cd localization and speciation in Anthyllis vulneraria. E3S Web Conf. 2013, 1, 19008. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Toakrz, K.; Muszyńska, E.; Makowski, W.; Jędrzejczyk, R.; Gajewski, Z.; Hanus-Fajerska, E. The acclimatization strategies of kidney vetch (Anthyllis vulneraria L.) to Pb toxicity. Environ. Sci. Pollut. Res. 2018, 25, 19739–19752. [Google Scholar] [CrossRef]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Comparison of in vitro and in planta heavy metal tolerance and accumulation potential of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G. Phytoremediation of lead: From fundamentals to application. In Lead Toxicity: Challenges and Solutions; Kumar, N., Jha, A.K., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2023; pp. 91–116. [Google Scholar]
- Pourrut, B.; Shahid, M.; Douay, F.; Dumat, C.; Pinelli, E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In Heavy Metal Stress in Plants; Gupta, D.K., Corpas, F.J., Palma, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 121–147. [Google Scholar]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Irtelli, B.; Petrucci, W.A.; Navari-Izzo, F. Nicotianamine and histidine/proline are, respectively, the most important copper chelators in xylem sap of Brassica carinata under conditions of copper deficiency and excess. J. Exp. Bot. 2009, 60, 269–277. [Google Scholar] [CrossRef]
- Ievinsh, G.; Osvalde, A.; Karlsons, A.; Andersone-Ozola, U. Hylotelephium maximum from coastal drift lines is a promising Mn and Zn accumulator with a high tolerance to biogenous heavy metals. Stresses 2022, 2, 450–466. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef]
- Bihanic, C.; Petit, E.; Perrot, R.; Cases, L.; Garcia, A.; Pelissier, F.; Poullain, C.; Rivard, C.; Hossaert-McKey, M.; McKey, D.; et al. Manganese distribution in the Mn-hyperaccumulator Grevillea meisneri from New Caledonia. Sci. Rep. 2021, 11, 23780. [Google Scholar] [CrossRef]
- Liu, P.; Tang, X.; Gong, C.; Xu, G.D. Manganese tolerance and accumulation in six Mn hyperaccumulators or accumulators. Plant Soil 2011, 335, 385–395. [Google Scholar] [CrossRef]
- Fagorzi, C.; Checcucci, A.; diCenzo, G.C.; Debiec-Andrzejewska, K.; Dziewit, L.; Pini, F.; Mengoni, A. Harnessing rhizobia to improve heavy-metal phytoremediation by legumes. Genes 2018, 9, 542. [Google Scholar] [CrossRef]
- Jach, M.E.; Sajnaga, E.; Ziaja, M. Utilization of legume-nodule bacterial symbiosis in phytoremediation of heavy metal-contaminated soils. Biology 2022, 11, 676. [Google Scholar] [CrossRef]
- Ayub, M.A.; ur Rehman, M.Z.; Umar, W.; Zuhra, N.; Shabaan, M. Role of legumes in phytoremediation of heavy metals. In Advances in Legumes for Sustainable Intensification; Meena, R.S., Kumar, S., Eds.; Academic Press: London, UK, 2022; pp. 345–360. [Google Scholar]
- Senani, N.; Bedouhene, S.; Houali, K. Peroxidase activity as a biochemical marker of insecticide use in vegetables. Acta Agric. Slov. 2023, 119, 1–9. [Google Scholar] [CrossRef]
- Bekheta, M.A.G.A.; Sahbaz, R.; Lieberei, R. Uniconazole-induced changes of stress responses of Vicia faba: Polyphenol oxidase activation pattern serves as an indicator of membrane stability. J. Appl. Bot. Food Qual. 2006, 80, 129–134. [Google Scholar]
- Zoz, T.; Steiner, F.; Guimarȃes, V.F.; Castagnara, D.D.; Meinerz, C.C.; Fey, R. Peroxidase activity as an indicator of water deficit tolerance in soybean cultivars. Biosci. J. 2013, 29, 1664–1671. [Google Scholar]
- Sleimi, N.; Guerfall, S.; Bankaji, I. Biochemical indicators of salt stress in Plantago maritima: Implications for environmental stress assessment. Ecol. Indic. 2015, 48, 570–577. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, J.P.; Bansal, R. Biochemical responses as stress indicator to water logging in pigeon pea (Cajanus cajan L.). Indian J. Biochem. Biophys. 2017, 54, 300–305. [Google Scholar]
- Dorleku, W.-P.; Gbewonyo, W.S.K. levels of polyphenol oxidase activity in leaves of Milicia seedlings is indicative of the resistance to Phyolyma lata-induced gall disease. Agric. Forest Entom. 2021, 23, 518–526. [Google Scholar] [CrossRef]
- Bavi, K.; Kholdebarin, B.; Moraadshahi, A. Effect of cadmium on growth, protein content and peroxidase activity in pea plants. Pak. J. Bot. 2011, 43, 1467–1470. [Google Scholar]
- Liu, J.; Lv, Y.; Li, M.; Wu, Y.; Li, B.; Wang, C.; Tao, Q. Peroxidase in plant defense: Novel insights for cadmium accumulation in rice (Oryza sativa L.). J. Hazard. Mater. 2024, 474, 134826. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Fecht-Christoffers, M.M.; Führs, H.; Braun, H.-P.; Horst, W.J. The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiol. 2006, 140, 1451–1463. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Burchett, M.D. Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar. Pollut. Bull. 2001, 42, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Samsone, I.; Ievinsh, G. Comparison of the effects of gradual and acute treatment with Mn on physiological responses of Rumex hydrolapathum plants. Stresses 2024, 4, 225–237. [Google Scholar] [CrossRef]
- Richter, H.; Lieberei, R.; von Schwatzenberger, K. Identification and characterisation of a bryophyte polyphenol oxidase encoding gene from Physcomitrella patens. Plant Biol. 2005, 7, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Ieviņa, S.; Karlsons, A.; Osvalde, A.; Andersone-Ozola, U.; Ievinsh, G. Coastal wetland species Rumex hydrolapathum: Tolerance against flooding, salinity and heavy metals for its potential use in phytoremediation and environmental restoration technologies. Life 2023, 13, 1604. [Google Scholar] [CrossRef] [PubMed]



| Treatment (mg L−1 of Metal) | Salt | Total Amount of Salt (g per L of Soil) | Amount of Salt (g per L of Soil) | ||
|---|---|---|---|---|---|
| 1st Treatment | 2nd Treatment | 3rd Treatment | |||
| Control | – | – | – | – | – |
| Cd 20 | CdCl2 2.5H2O | 0.041 | 0.041 | – | – |
| Cd 50 | CdCl2 2.5H2O | 0.103 | 0.041 | 0.062 | – |
| Cd 100 | CdCl2 2.5H2O | 0.205 | 0.041 | 0.062 | 0.102 |
| Pb 200 | PbOAc 3H2O | 0.366 | 0.366 | – | – |
| Pb 500 | PbOAc 3H2O | 0.916 | 0.366 | 0.550 | – |
| Pb 1000 | PbOAc 3H2O | 1.832 | 0.366 | 0.550 | 0.916 |
| Cu 100 | CuSO4 5H2O | 0.390 | 0.390 | – | – |
| Cu 200 | CuSO4 5H2O | 0.780 | 0.390 | 0.390 | – |
| Cu 500 | CuSO4 5H2O | 1.950 | 0.390 | 0.390 | 1.170 |
| Mn 200 | MnSO4 H2O | 0.600 | 0.600 | – | – |
| Mn 500 | MnSO4 H2O | 1.500 | 0.600 | 0.900 | – |
| Mn 1000 | MnSO4 H2O | 3.000 | 0.600 | 0.900 | 1.500 |
| Zn 200 | ZnSO4 7H2O | 0.880 | 0.880 | – | – |
| Zn 500 | ZnSO4 7H2O | 2.200 | 0.880 | 1.320 | – |
| Zn 1000 | ZnSO4 7H2O | 4.400 | 0.880 | 1.320 | 2.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersone-Ozola, U.; Jēkabsone, A.; Karlsons, A.; Osvalde, A.; Banaszczyk, L.; Samsone, I.; Ievinsh, G. Heavy Metal Tolerance and Accumulation Potential of a Rare Coastal Species, Anthyllis vulneraria subsp. maritima. Stresses 2025, 5, 6. https://doi.org/10.3390/stresses5010006
Andersone-Ozola U, Jēkabsone A, Karlsons A, Osvalde A, Banaszczyk L, Samsone I, Ievinsh G. Heavy Metal Tolerance and Accumulation Potential of a Rare Coastal Species, Anthyllis vulneraria subsp. maritima. Stresses. 2025; 5(1):6. https://doi.org/10.3390/stresses5010006
Chicago/Turabian StyleAndersone-Ozola, Una, Astra Jēkabsone, Andis Karlsons, Anita Osvalde, Lidia Banaszczyk, Ineta Samsone, and Gederts Ievinsh. 2025. "Heavy Metal Tolerance and Accumulation Potential of a Rare Coastal Species, Anthyllis vulneraria subsp. maritima" Stresses 5, no. 1: 6. https://doi.org/10.3390/stresses5010006
APA StyleAndersone-Ozola, U., Jēkabsone, A., Karlsons, A., Osvalde, A., Banaszczyk, L., Samsone, I., & Ievinsh, G. (2025). Heavy Metal Tolerance and Accumulation Potential of a Rare Coastal Species, Anthyllis vulneraria subsp. maritima. Stresses, 5(1), 6. https://doi.org/10.3390/stresses5010006







