Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions
Abstract
1. Introduction
2. Occurrence of Cyanobacteria in the Environment
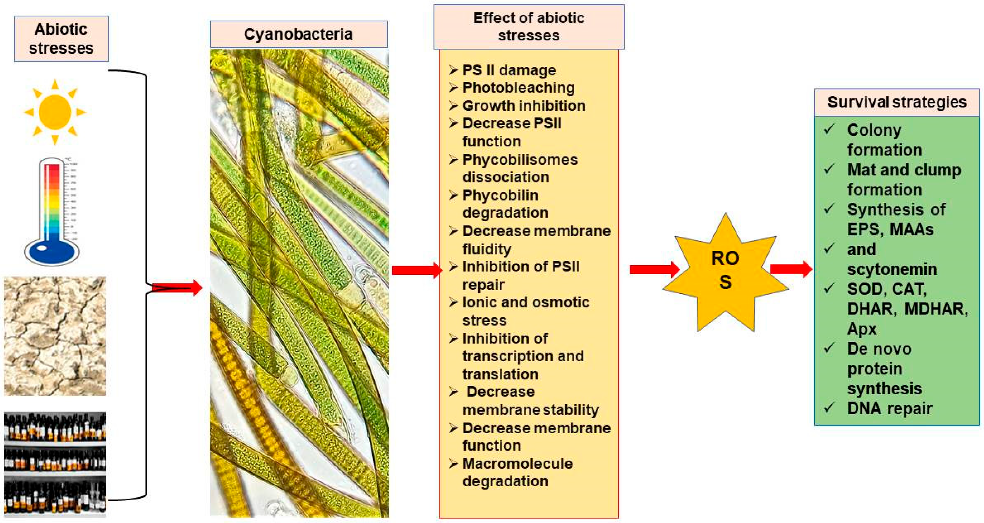
Cyanobacterial Stress Tolerance
3. General Mechanisms of Cyanobacteria to Survive under Extreme Habitat
3.1. Photosynthetic Active Radiation (P.A.R.)
3.2. Light Quality
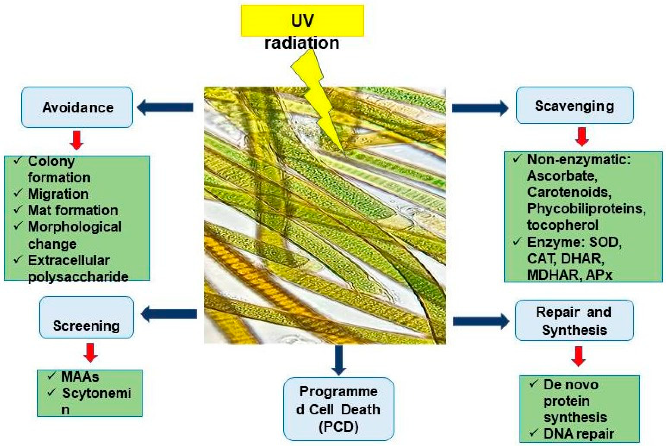
3.3. Ultraviolet Radiation (U.V.R.)
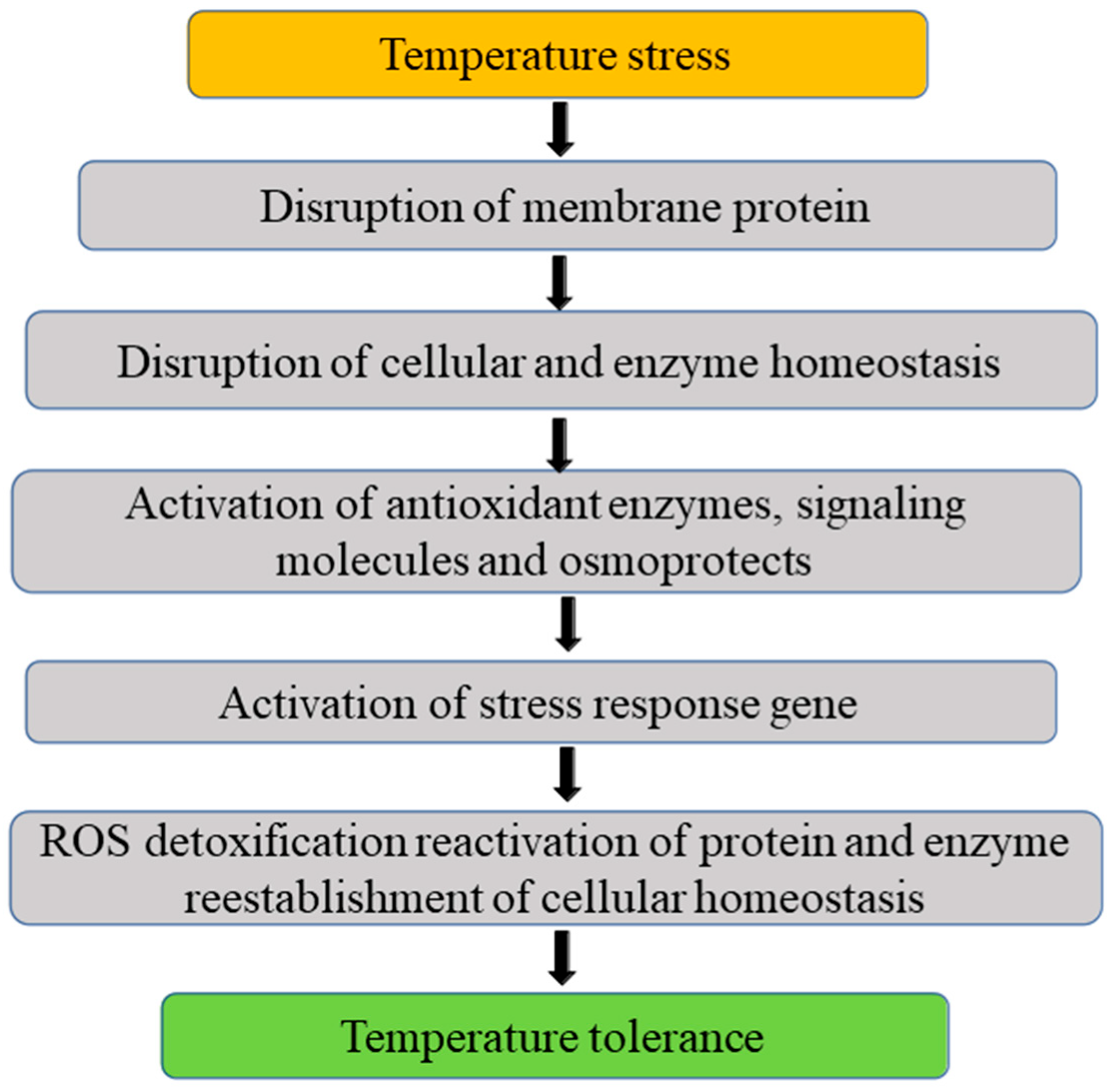
3.4. Temperature
3.4.1. Low Temperature
3.4.2. High Temperature
3.5. pH Stress
3.6. Salinity and Osmotic Stress
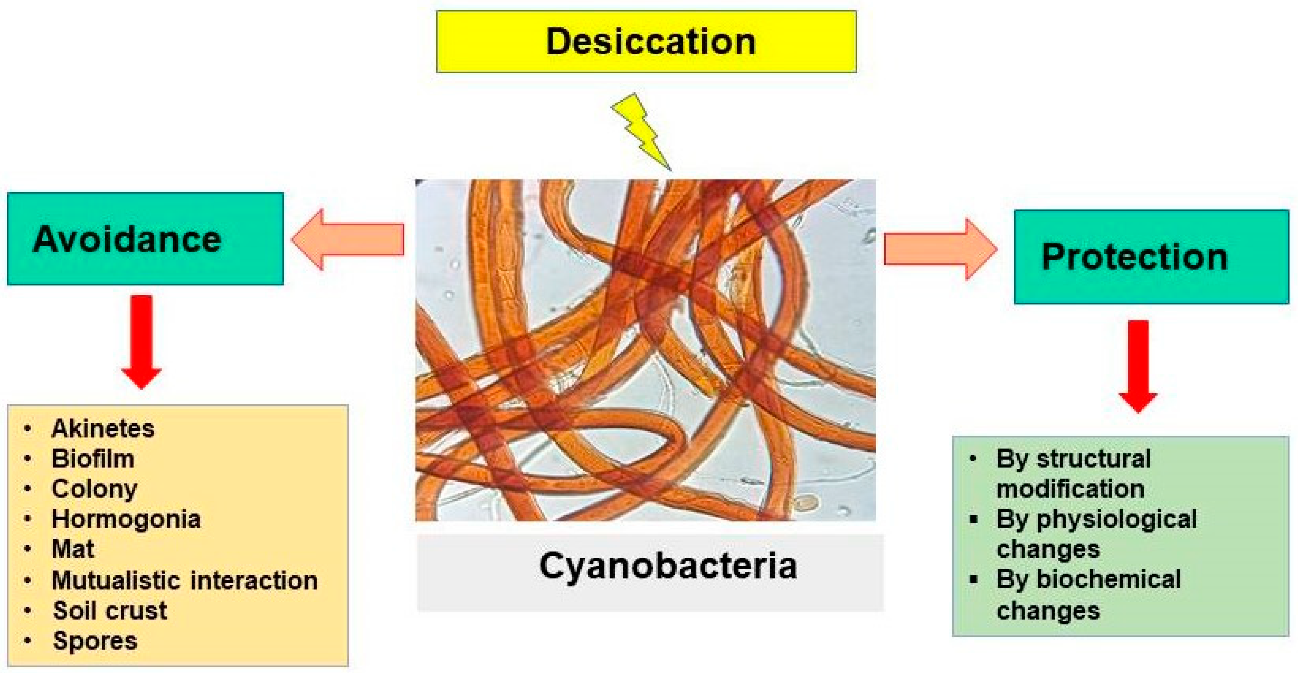
3.7. Desiccation
3.8. Freeze and Melting Cycles
3.9. Metals and Metalloid Stress
4. Proteomics and Cyanobacteria
5. Application of Cyanobacterial Secondary Metabolites in Imparting Abiotic Stress Tolerance
| Stress | Cyanobacteria | Plant | Mechanism | References |
|---|---|---|---|---|
| Cold | Anabaena sp. | Agrostissto lonifera L. | Growth modulation in plants via transformation in flavodoxin gene | [179] |
| Aphanothece halophytic | Populus alba | With the transformation in HSP70 gene | [180] | |
| Synechocystis sp. Synechococcus vulcanus | Nicotiana tabacum | Plant transformation with a Δ9 and Δ12 acyl–lipid desaturase genes | [181] | |
| Salinity | Nostoc flagelliforme | Arabidopsis thaliana | Transformation in plants using P-loop NTPase domain gene | [182] |
| Anabaena vaginicola ISB42, Nostoc calcicola ISB43, Trichormus ellipsosporus ISB44, and Cylindrospermum michailovskoense ISB45 | Mentha piperita | Growth promotion plant | [183] | |
| Nostoc carneum TUBT04 and N. carneum TUBT05 | Oryza sativa | Growth and yield enhancement in plants | [184] | |
| Lyngbya mucicola; Oscillatoria Princeps L.; Lyngbya phormidium Gloeocapsa sp.; Cryophilic sp. | Oryza sativa | Enhancement in soil organic matter and enhanced concentration of plant nutrients | [185] | |
| Anabaena oryzae, Anabaena doliolum, Phormidium fragile, Calothrix geitonos, Hapalosiphon intricatus, Aulosira fertilissima, Tolypothrix tenuis, Oscillatoria Acuta, and Plectonema boryanum | Oryza sativa | Growth promotion and modulation of phytohormone concentration | [186] | |
| Scytonema hofmanni | Oryza sativa | Production of gibberellin-like hormone | [187] | |
| Nostoc kihlmani and Anabaena cylindrica | Triticum aestivum | Enhanced growth and improved soil structure | [188] | |
| Anabaena sphaerica | Production of extracellular polysaccharides and proteins | [189] | ||
| Nostoc flagelliforme | Arabidopsis thaliana | Transformation in plants with P-loop NTPase domain gene | [182] | |
| Nostoc muscorum; Anabaena fertilissima, A. anomala | Oryza sativa | Increase in the rhizosphere biology or phytohormone | [190] | |
| Nostoc calcicola, Nostoc spongiaeformae, Nostoc linckia, and Nostoc muscorum | Wheat, maize, and rice | Increased seed vigor index and germination, vigor index | [191] | |
| Anabaena variabilis | Medicago truncatula | Transformation in plants with flavodoxin gene | [192] | |
| Anabaena sphaerica | Oryza sativa | Production of extracellular polysaccharides and proteins | [193] | |
| Drought | Nostoc flagelliforme | Arabidopsis thaliana | Enhance seed germination under salinity stress | [182] |
| Nostoc sp. and Microcoleus sp. | Senna notabilis and Acacia hilliana | Seeds priming and enhanced germination | [194] | |
| Anabaena sp. | Agrostis stolonifera L. | Transformation in plants with a flavodoxin gene | [179] | |
| Leptolyngbya sp., Microcoleus sp., Nostoc sp., and Scytonema sp. | Eucalyptus gamophylla and Grevillea wickhamii | Enhanced radicle initiation and improved shoot and root growth | [195] | |
| Anabaena sp. | Nicotiana tabacum | Plant transformation with a flavodoxin gene | [196,197] | |
| Osmotic stress | Anabaena sp. | Nicotiana tabacum | Transformation in plants with flavodoxin and a ferredoxin–NADP+ reductase gene | [198] |
| Heat | Anabaena sp. | Agrostis stolonifera L. | Plant transformation with a flavodoxin gene | [179] |
| Nostoc sp., Anabaena doliolum, Calothrix sp., Westiellopsis sp., and Phormidium papyraceum | Oryza sativa | Heavy metal stress tolerance and improved plant growth | [199] | |
| Heavy metal | Nostoc muscorum | Trigonella foenumgracum | Enhancement in the sugar, protein, and lipid content | [200] |
| Synechococcus sp. | A. thaliana | Transformation in plants with a metallothionein gene | [201] | |
| Nostoc sp., Anabaena doliolum, Calothrix sp., Westiellopsis sp., and Phormidium papyraceum | Oryza sativa | Tolerance of heavy metal stress and enhancement in plant growth | [202] | |
| Spirulina platensis | Zea mays L. | Activation of plant mechanisms | [203] |
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tashyreva, D.; Elster, J. Annual cycles of two cyanobacterial mat communities in hydro-terrestrial habitats of the high Arctic. Microb. Ecol. 2016, 71, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Zakhia, F.; Jungblut, A.D.; Taton, A.; Vincent, W.F.; Wilmotte, A. Cyanobacteria in cold ecosystems. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 121–135. [Google Scholar]
- Larsson, J.; Nylander, J.A.; Bergman, B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. B.M.C. Evol. Biol. 2011, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Burnap, R.L. Systems and photosystems: Cellular limits of autotrophic productivity in cyanobacteria. Front. Bioeng. Biotechnol. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiong, W.; Li, X.B.; Gao, C.F.; Zhang, Y.L.; Li, H.; Wu, Q.Y. Identification of the proteomic changes in Synechocystis sp. PCC 6803 following prolonged UV-B irradiation. J. Exp. Bot. 2009, 60, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, H.M.; Cui, S.X.; Suzuki, I.; Zhang, L.F.; Li, L.; Bo, T.T.; Wang, J.; Murata, N.; Huang, F. Proteomic study of the impact of Hik33 mutation in Synechocystis sp. PCC 6803 under normal and salt stress conditions. J. Proteome Res. 2012, 11, 502–514. [Google Scholar] [CrossRef]
- Burja, A.M.; Dhamwichukorn, S.; Wright, P.C. Cyanobacterial postgenomic research and systems biology. Trends Biotechnol. 2003, 21, 504–511. [Google Scholar] [CrossRef]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Yadav, P.; Gupta, R.K.; Singh, R.P.; Yadav, P.K.; Patel, A.K.; Pandey, K.D. Role of cyanobacteria in green remediation. In Sustainable Environmental Clean-Up; Elsevier: Amsterdam, The Netherlands, 2021; pp. 187–210. [Google Scholar]
- Gallon, J.R.; Jones, D.A.; Page, T.S. Trichodesmium, the paradoxical diazotroph. Arch. Hydrobiol. Suppl. Algol. Stud. 1996, 83, 215–243. [Google Scholar] [CrossRef]
- Reed, R.H.; Richardson, D.L.; Warr, S.R.; Stewart, W.D. Carbohydrate accumulation and osmotic stress in cyanobacteria. Microbiology 1984, 130, 1–4. [Google Scholar] [CrossRef]
- Dor, I.; Danin, A. Cyanobacterial desert crusts in the Dead Sea Valley, Israel. Arch. Hydrobiol. Algol. Stud. 1996, 83, 197–206. [Google Scholar] [CrossRef]
- Kann, E. Zur AutØkologie benthischer Cyanophyten in reinen europäischen Seen und Fliessgewässern. Algol. Stud. Arch. Hydrobiol. 1988, 50–53, 473–495. [Google Scholar]
- Castenholz, R.W. Species usage, concept, and evolution in the cyanobacteria (blue-green algae). J. Phycol. 1992, 28, 737–745. [Google Scholar] [CrossRef]
- Schulberg, O.M. Oscillatoialean cyanoprokaryotes and their application for algal culture technology. Arch. Hydrobiol. Algol. Stud. 1994, 75, 265–1278. [Google Scholar]
- Laamanen, M. Cyanoprokaryotes in the Baltic Sea ice and winter plankton. Arch. Hydrobiol. Suppl. Algol. Stud. 1996, 83, 423–433. [Google Scholar] [CrossRef]
- Van Landingham, S.L. Guide to the Identification, Environmental Requirements and Pollution Tolerance of Freshwater Blue-Green Algae (Cyanophyta); Environmental Monitoring and Support Laboratory, Office of Research and Development, U.S. Environmental Protection Agency: Las Vegas, NV, USA, 1982. [Google Scholar]
- Elster, J.; Lukesová, A.; Svoboda, J.; Kopecky, J.; Kanda, H. Diversity and abundance of soil algae in the polar desert, Sverdrup Pass, central Ellesmere Island. Polar Rec. 1999, 35, 231–254. [Google Scholar] [CrossRef]
- Marcozzi, C.; Camino, A.C.; Salerno, G.L. Role of NtcA, a cyanobacterial global nitrogen regulator, in the regulation of sucrose metabolism gene expression in Anabaena sp. PCC 7120. Arch. Microbiol. 2009, 191, 255–263. [Google Scholar] [CrossRef]
- Schwarz, R.; Forchhammer, K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: Emergence of a complex network of cellular responses. Microbiology 2005, 151, 2503–2514. [Google Scholar] [CrossRef]
- Hawco, N.J.; Fu, F.; Yang, N.; Hutchins, D.A.; John, S.G. Independent iron and light limitation in a low-light-adapted Prochlorococcus from the deep chlorophyll maximum. ISME J. 2021, 15, 359–362. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Konarzewska, Z.; Wiśniewska, K.; Konik, M. Photosynthetic pigments changes of three phenotypes of picocyanobacteria Synechococcus sp. under different light and temperature conditions. Cells 2020, 9, 2030. [Google Scholar] [CrossRef]
- Vincent, W.F. Cyanobacterial dominance in the polar regions. In The Ecology of Cyanobacteria; Whitton, B.A., Potts, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 321–340. [Google Scholar]
- El-Sheekh, M.M.; Alwaleed, E.A.; Ibrahim, A.; Saber, H. Detrimental effect of UV-B radiation on growth, photosynthetic pigments, metabolites and ultrastructure of some cyanobacteria and freshwater Chlorophyta. Int. J. Radiat. Biol. 2021, 97, 265–275. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.P. Resistance of blue-green algae to 60Co gamma radiation. Radiat. Bot. 1969, 9, 481–489. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.; Su, Z. Computational analysis of LexA regulons in Cyanobacteria. BMC Genom. 2010, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Apte, S.K. Effect of 60Co-Gamma Ionizing Radiation and Desiccation Stress on Protein Profile of Anabaena 7120. Protein J. 2018, 37, 608–621. [Google Scholar] [CrossRef]
- Harel, Y.; Ohad, I.; Kaplan, A. Activation of photosynthesis and resistance to photoinhibition in cyanobacteria within biological desert crust. Plant Physiol. 2004, 136, 3070–3079. [Google Scholar] [CrossRef]
- Ismaiel, M.M.; Piercey-Normore, M.D. Gene transcription and antioxidants production in Arthrospira (Spirulina) platensis grown under temperature variation. J. Appl. Microbiol. 2021, 130, 891–900. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Z.; Xu, H.; Liu, L.; An, J.; Ji, B.; Ye, S. Cold adaptation in drylands: Transcriptomic insights into cold-stressed Nostoc flagelliforme and characterization of a hypothetical gene with cold and nitrogen stress tolerance. Environ. Microbiol. 2021, 23, 713–727. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Responses to cold shock in cyanobacteria. J. Mol. Microbiol. Biotechnol. 1999, 1, 221–230. [Google Scholar]
- Martin, R.M.; Moniruzzaman, M.; Stark, G.F.; Gann, E.R.; Derminio, D.S.; Wei, B.; Hellweger, F.L.; Pinto, A.; Boyer, G.L.; Wilhelm, S.W. Episodic decrease in temperature increases mcy gene transcription and cellular microcystin in continuous cultures of Microcystis aeruginosa PCC 7806. Front. Microbiol. 2020, 11, 601864. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kujur, R.; Pandey, K.D.; Gupta, R.K. Cyanobacteria and salinity stress tolerance. In Cyanobacterial Lifestyle and its Applications in Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 253–280. [Google Scholar]
- Asthana, R.K.; Nigam, S.; Maurya, A.; Kayastha, A.M.; Singh, S.P. Trehalose-producing enzymes MTSase and MTHase in Anabaena 7120 under NaCl stress. Curr. Microbiol. 2008, 56, 429–435. [Google Scholar] [CrossRef]
- Kumar Srivastava, A.; Bhargava, P.; Mishra, Y.; Shukla, B.; Chand Rai, L. Effect of pretreatment of salt, copper and temperature on ultraviolet-B-induced antioxidants in diazotrophic cyanobacterium Anabaena doliolum. J. Basic Microbiol. 2006, 46, 135–144. [Google Scholar] [CrossRef]
- Apte, S.K.; Fernandes, T.A.; Iyer, V.; Alahari, A. Molecular basis of tolerance to salinity and drought stresses in photosynthetic nitrogen-fixing cyanobacteria. In Plant Molecular Biology and Biotechnology; Narosa Publications: New Delhi, India, 1997; pp. 258–268. [Google Scholar]
- Fernandes, T.A.; Iyer, V.; Apte, S.K. Differential responses of nitrogen-fixing cyanobacteria to salinity and osmotic stresses. Appl. Environ. Microbiol. 1993, 59, 899–904. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Black, T.A.; Jäger, K.; Panoff, J.M.; Wolk, C.P. Regulation of an osmoticum-responsive gene in Anabaena sp. strain PCC 7120. J. Bacteriol. 1998, 180, 6332–6337. [Google Scholar] [CrossRef]
- Kuritz, T.; Bocanera, L.V.; Rivera, N.S. Dechlorination of lindane by the cyanobacterium Anabaena sp. strain PCC7120 depends on the function of the nir operon. J. Bacteriol. 1997, 179, 3368–3370. [Google Scholar] [CrossRef]
- Srivastava, A.; Biswas, S.; Yadav, S.; Kumar, S.; Srivastava, V.; Mishra, Y. Acute cadmium toxicity and post-stress recovery: Insights into coordinated and integrated response/recovery strategies of Anabaena sp. PCC 7120. J. Hazard. Mater. 2021, 411, 124822. [Google Scholar] [CrossRef]
- Nagao, R.; Yokono, M.; Ueno, Y.; Suzuki, T.; Kato, K.; Kato, K.H.; Tsuboshita, N.; Jiang, T.Y.; Dohmae, N.; Shen, J.R.; et al. Molecular organizations and function of iron-stress-induced-A protein family in Anabaena sp. PCC 7120. Biochim. Biophys. Acta. 2021, 1862, 148327. [Google Scholar] [CrossRef]
- Stuart, R.K.; Dupont, C.L.; Johnson, D.A.; Paulsen, I.T.; Palenik, B. Coastal strains of marine Synechococcus species exhibit increased tolerance to copper shock and a distinctive transcriptional response relative to those of open-ocean strains. Appl. Environ. Microbiol. 2009, 75, 5047–5057. [Google Scholar] [CrossRef]
- Higo, A.; Ikeuchi, M.; Ohmori, M. cAMP regulates respiration and oxidative stress during rehydration in Anabaena sp. PCC 7120. FEBS Lett. 2008, 582, 1883–1888. [Google Scholar] [CrossRef]
- Potts, M. Desiccation tolerance of prokaryotes. Microbiol. storage. Soil Biol. Biochem. 1994, 23, 313–322. [Google Scholar]
- Singh, H.; Anurag, K.; Apte, S.K. High radiation and desiccation tolerance of nitrogen-fixing cultures of the cyanobacterium Anabaena sp. strain PCC 7120 emanates from genome/proteome repair capabilities. Photos. Res. 2013, 118, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Kiran, M.D.; Chintalapati, S. Perception and transduction of low temperature in bacteria. Phys. Biochem. Extrem. 2007, 194, 207. [Google Scholar]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Al-Ajani, A.F.; Abd Allah, E.F.; Gupta, R.K. Physiological and Biochemical Responses of Bicarbonate Supplementation on Biomass and Lipid Content of Green Algae Scenedesmus sp. BHU1 Isolated from Wastewater for Renewable Biofuel Feedstock. Front. Microbiol. 2022, 13, 839800. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Murakami, A.; Aizawa, K. Short-term and long-term adaptation of the photosynthetic apparatus: Homeostatic properties of thylakoids. In The Molecular Biology of Cyanobacteria; Bryant, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 667–692. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P. Arsenic metabolism in plants: An inside story. New Phytol. 2005, 168, 503–505. [Google Scholar] [CrossRef]
- Rai, A.N.; Mishra, A.K.; Tiwari, D.N. Cyanobacteria: From Basic Science to Applications; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Los, D.A.; Suzuki, I.; Zinchenko, V.V.; Murata, N. Stress responses in Synechocystis: Regulated genes and regulatory systems. In The Cyanobacteria: Molecular Biology, Genomics and Evolution; Academic Press: Cambridge, MA, USA, 2008; Volume 117, p. 157. [Google Scholar]
- Jose, F.; Jeanjean, R.; Hagemann, M. Dynamics of the response of cyanobacteria to salt stress: Deciphering the molecular events. Physiol. Plant 1996, 96, 738–744. [Google Scholar] [CrossRef]
- Tanghe, A.; Van Dijck, P.; Thevelein, J.M. Determinants of freeze tolerance in microorganisms, physiological importance, and biotechnological applications. Adv. Appl. Microbiol. 2003, 53, 129–176. [Google Scholar]
- Potts, M. Mechanisms of desiccation tolerance in cyanobacteria. Eur. J. Phycol. 1999, 34, 319–328. [Google Scholar] [CrossRef]
- Hihara, Y.; Kamei, A.; Kanehisa, M.; Kaplan, A.; Ikeuchi, M. D.N.A. microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 2001, 13, 793–806. [Google Scholar] [CrossRef]
- He, Y.Y.; Häder, D.P. Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem. Photobiol. Sci. 2002, 1, 729–736. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Marine Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- He, Y.Y.; Häder, D.P. UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: Protective effects of ascorbic acid and N-acetyl-L-cysteine. Photochem. Photobiol. B Biol. 2002, 66, 115–124. [Google Scholar] [CrossRef]
- Rothshield, L.J. Microbes and radiation. In Enigmatic Microorganisms and Life in Extreme Environments; Seckbach, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 551–562. [Google Scholar]
- Singh, S.C.; Sinha, R.P.; Hader, D.P. Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool. 2002, 41, 297–308. [Google Scholar]
- Řezanka, T.; Nedbalová, L.; Elster, J.; Cajthaml, T.; Sigler, K. Very-long-chain iso and anteiso branched fatty acids in N-acylphosphatidylethanolamines from a natural cyanobacterial mat of Calothrix sp. Phytochemistry 2009, 70, 655–663. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kinoshita, M.; Inaba, M.; Suzuki, I.; Murata, N. Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol. 2001, 125, 1842–1853. [Google Scholar] [CrossRef]
- Olie, J.J.; Potts, M. Purification and biochemical analysis of the cytoplasmic membrane from the desiccation-tolerant cyanobacterium Nostoc commune UTEX 584. Appl. Environ. 1986, 52, 706–710. [Google Scholar] [CrossRef]
- Sakurai, I.; Hagio, M.; Gombos, Z.; Tyystjarvi, T.; Paakkarinen, V.; Aro, E.M.; Wada, H. Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol. 2003, 133, 1376–1384. [Google Scholar] [CrossRef]
- Adhikary, S.P. Heat shock proteins in the terrestrial epiphytic cyanobacterium Tolypothrix byssoidea. Biol. Plant. 2003, 47, 125–128. [Google Scholar] [CrossRef]
- Webb, R.; Sherman, L.A. The cyanobacterial heat-shock response and the molecular chaperones. In The Molecular Biology of Cyanobacteria; Springer: Dordrecht, The Netherlands, 1994; pp. 751–767. [Google Scholar]
- Billi, D.; Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef]
- Nienow, J.A.; McKay, C.P.; Friedmann, E.I. The cryptoendolithic microbial environment in the Ross Desert of Antarctica: Light in the photosynthetically active region. Microb. Ecol. 1988, 16, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Nienow, J.A.; McKay, C.P.; Friedmann, E.I. Cryptoendolithic microbial environment in the Ross Desert of Antarctica: Mathematical models of the thermal regime. Microb. Ecol. 1988, 16, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Aigner, S.; Herburger, K.; Holzinger, A.; Karsten, U. Epilithic Chamaesiphon (Synechococcales, Cyanobacteria) species in mountain streams of the Alps—Interspecific differences in photo-physiological traits. J. Appl. Psychol. 2018, 30, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Schwarz, R.; Grossman, A.R. Molecular responses to environmental stress. In The Ecology of Cyanobacteria; Springer: Dordrecht, The Netherlands, 2000; pp. 397–442. [Google Scholar]
- Berner, T.; Sukenik, A. Photoacclimation in photosynthetic microorganisms: An ultrastructural response. Isr. J. Plant Sci. 1998, 46, 141–146. [Google Scholar] [CrossRef]
- Kawamura, M.; Mimuro, M.; Fujita, Y. Quantitative relationship between two reaction centers in the photosynthetic system of blue-green algae. Plant Cell Physiol. 1976, 20, 697–705. [Google Scholar]
- Elster, J.; Benson, E.E. Life in the polar terrestrial environment with a focus on algae and cyanobacteria. In Life in the Frozen State; Fuller, B.J., Lane, N., Benson, E.E., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 111–150. [Google Scholar]
- Bonilla, S.; Villeneuve, V.; Vincent, W.F. Benthic and planktonic algal communities in a High Arctic Lake: Pigment structure and contrasting responses to nutrient enrichment. J. Phycol. 2005, 41, 1120–1130. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, A.N. Partitioning light spectra: Adaptive stratification of phytobenthic communities in Antarctic lakes. J. Theor. Biol. 2017, 424, 1–10. [Google Scholar] [CrossRef]
- Six, C.; Thomas, J.C.; Brahamsha, B.; Lemoine, Y.; Partensky, F. Photophysiology of the marine cyanobacterium Synechococcus sp. WH8102, a new model organism. Aquatic Micro. Eco. 2004, 35, 17–29. [Google Scholar] [CrossRef]
- Grossman, A.R.; Schaefer, M.R.; Chang, G.G.; Collier, J.L. The response of cyanobacteria to environmental conditions: Light and nutrients. In The Molecular Biology of Cyanobacteria; Bryant, A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 641–675. [Google Scholar]
- Toro, R.; Araya, C.; Labra, F.; Morales, L.; Morales, R.G. Trend and recovery of the total ozone column in South America and Antarctica. Clim. Dyn. 2017, 49, 3735–3752. [Google Scholar] [CrossRef]
- Häder, D.P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Effects of solar U.V. radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 267–285. [Google Scholar] [CrossRef]
- Donker, V.; Häder, D.P. Effects of solar and ultraviolet radiation on motility, photo movement and pigmentation in filamentous, gliding cyanobacteria. FEMS Microbiol. Lett. 1991, 86, 159–168. [Google Scholar] [CrossRef]
- Schulz, E.M.; Scherer, S. U.V. protection in cyanobacteria. Eur. J. Phycol. 1999, 34, 329. [Google Scholar] [CrossRef]
- Seckbach, J.; Oren, A. Oxygenic photosynthetic microorganisms in extreme environments: Possibilities and limitations. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 3–25. [Google Scholar]
- Pattanaik, B.; Schumann, R.; Karsten, U. Effects of ultraviolet radiation on cyanobacteria and their protective mechanisms. In Algae and Cyanobacteria in Extreme Environments; Springer: Dordrecht, The Netherlands, 2007; pp. 29–45. [Google Scholar]
- Quesada, A.; Vincent, W.F.; Lean, D.R. Community and pigment structure of Arctic cyanobacterial assemblages: The occurrence and distribution of UV-absorbing compounds. FEMS Microbiol. Ecol. 1999, 28, 315–323. [Google Scholar] [CrossRef][Green Version]
- Vincent, W.F.; Mueller, D.R.; Bonilla, S. Ecosystems on ice: The microbial ecology of Markham Ice Shelf in the high Arctic. Cryobiology 2004, 48, 103–112. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Donker, V.A.; Hader, D.P. Ultraviolet radiation effects on pigmentation in the cyanobacterium. Acta Protozool. 1997, 36, 49. [Google Scholar]
- Burnap, R.L.; Sherman, L.A. Deletion mutagenesis in Synechocystis sp. PCC6803 indicates that the manganese-stabilizing protein of photosystem II is not essential for oxygen evolution. Biochemistry 1991, 30, 440–446. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, R.P.; Häder, D.P. Effect of UV-B on enzymes of nitrogen metabolism in the cyanobacterium Nostoc calcicola. J. Plant Physiol. 1996, 148, 86–91. [Google Scholar] [CrossRef]
- Gao, X.; Ai, Y.F.; Qiu, B.S. Drought adaptation of a terrestrial macroscopic cyanobacterium, Nostoc flagelliforme, in arid areas: A review. Afr. J. Microbiol. Res. 2012, 6, 5728–5735. [Google Scholar]
- Schulz, M.E.; Schulz, S.; Wait, R.; Görg, A.; Scherer, S. The UV-B stimulon of the terrestrial cyanobacterium Nostoc commune comprises early shock proteins and late acclimation proteins. Mol. Microbiol. 2002, 46, 827–843. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, S.; Shrivastava, A.K.; Singh, P.K.; Agrawal, C.; Rai, L.C. Understanding the mechanisms of abiotic stress management in cyanobacteria with special reference to proteomics. In Stress Biology of Cyanobacteria: Molecular Mechanisms to Cellular Responses; Srivastava, A.K., Rai, A.N., Neilan, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 93, p. 112. [Google Scholar]
- Pachauri, R.K.; Reisinger, A. (Eds.) Forth Assessment Report: Climate Change 2007; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2007; Volume 104. [Google Scholar]
- Sung, D.Y.; Kaplan, F.; Lee, K.J.; Guy, C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Brock, D.T.; Madigan, T.D. Biology of Microorganisms, 7th ed.; Chapters 18 and 19; Prentice Hall: Englewood Cliffs, NJ, USA, 1994. [Google Scholar]
- Chintalapati, S.; Prakash, J.S.S.; Gupta, P.; Ohtani, S.; Suzuki, I.; Sakamoto, T.; Murata, N.; Shivaji, S. A novel Δ9 acyl-lipid desaturase, DesC2, from cyanobacteria acts on fatty acids esterified to the sn−2 position of glycerolipids. Biochem. J. 2006, 398, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fort, D.G.; Herr, T.M.; Shaw, P.L.; Gutzman, K.E.; Starren, J.B. Mapping the evolving definitions of translational research. J. Clin. Transl. Sci. 2017, 1, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.; Bhargava, P.; Chaurasia, N.; Rai, L.C. Proteomic evaluation of the non-survival of Anabaena doliolum (Cyanophyta) at elevated temperatures. Eur. J. Phycol. 2009, 44, 551–565. [Google Scholar] [CrossRef][Green Version]
- Slabs, A.R.; Suzuki, I.; Murata, N.; Simon, W.J.; Hall, J.J. Proteomic analysis of the heat shock response in Synechocystis PCC6803 and a thermally tolerant knockout strain lacking the histidine kinase 34 gene. Proteomics 2006, 6, 845–864. [Google Scholar] [CrossRef]
- Hongsthong, A.; Sirijuntarut, M.; Yutthanasirikul, R.; Senachak, J.; Kurdrid, P.; Cheevadhanarak, S.; Tanticharoen, M. Subcellular proteomic characterization of the high-temperature stress response of the cyanobacterium Spirulina platensis. Proteome Sci. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Psenner, R.; Sattler, B. Life at the freezing point. Science 1998, 280, 2073–2074. [Google Scholar] [CrossRef]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Huner, N.P. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252. [Google Scholar] [CrossRef]
- Nishida, I.; Murata, N. Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. Annu. Rev. Plant. Biol. 1996, 47, 541–568. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Los, D.A. New insights in cyanobacterial cold stress responses: Genes, sensors, and molecular triggers. Biochim. Biophys. Acta 2016, 1860, 2391–2403. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Structure and expression of fatty acid desaturases. BBA-Lipids Lipid Metab. 1998, 1394, 3–15. [Google Scholar] [CrossRef]
- Inoue, N.; Taira, Y.; Emi, T.; Yamane, Y.; Kashino, Y.; Koike, H.; Satoh, K. Acclimation to the growth temperature and the high-temperature effects on photosystem II and plasma membranes in a mesophilic cyanobacterium, Synechocystis sp. PCC6803. Plant Cell Physiol. 2001, 42, 1140–1148. [Google Scholar] [CrossRef]
- Slabs, A.R.; Suzuki, I.; Simon, W.J. The heat shock response of Synechocystis sp. PCC 6803 analyzed by transcriptomics and proteomics. J. Exp. Bot. 2006, 57, 1573–1578. [Google Scholar]
- Giraldez-Ruiz, N.P.; Mateo, I.; Bonilla, I.; Fernandez-Pinas, F. The relationship between intracellular pH, growth characteristics and calcium in the cyanobacterium Anabaena sp. strain PCC7120 exposed to low pH. New Phytol. 1997, 137, 599–605. [Google Scholar] [CrossRef]
- Szabolcs, I. Soil and salinization. In Handbook of Plant and Crop Stress; Pessarakali, M., Ed.; Marcel Dekker: New York, NY, USA, 1994; p. 3. [Google Scholar]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef]
- Tijen, D.; Ismail, T. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar]
- Wei, Y.; Xu, X.; Tao, H.; Wang, P. Growth performance and physiological response in the halophyte Lycium barbarum grown at salt-affected soil. Ann. Appl. Biol. 2006, 149, 263–269. [Google Scholar] [CrossRef]
- Huang, F.; Fulda, S.; Hagemann, M.; Norling, B. Proteomic screening of salt-stress-induced changes in plasma membranes of Synechocystis sp. strain PCC 6803. Proteomics 2006, 6, 910–920. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhargava, P.; Rai, L.C. Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J. Microbiol. Biotechnol. 2005, 21, 1291–1298. [Google Scholar] [CrossRef]
- Singh, R.N. Reclamation of ‘Usar’ lands in India through blue-green algae. Nature 1950, 165, 325–326. [Google Scholar] [CrossRef]
- Kanesaki, Y.; Suzuki, I.; Allakhverdiev, S.I.; Mikami, K.; Murata, N. Salt stress and hyperosmotic stress regulate the expression of different sets of genes in Synechocystis sp. PCC 6803. Biochem. Biophys. 2002, 290, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Molitor, V.; Erber, W.; Peschek, G.A. Increased levels of cytochrome oxidase and sodium-proton antiporter in the plasma membrane of Anacystis nidulans after growth in sodium-enriched media. FEBS Lett. 1986, 204, 251–256. [Google Scholar] [CrossRef]
- Fulda, S.; Huckauf, J.; Schoor, A.; Hagemann, M. Analysis of stress responses in the cyanobacterial strains Synechococcus sp. PCC 7942, Synechocystis sp. PCC 6803, and Synechococcus sp. PCC 7418: Osmolyte accumulation and stress protein synthesis. J. Plant Physiol. 1999, 154, 240–249. [Google Scholar] [CrossRef]
- Fulda, S.; Huang, F.; Nilsson, F.; Hagemann, M.; Norling, B. Proteomics of Synechocystis sp. strain PCC 6803: Identification of periplasmic proteins in cells grown at low and high salt concentrations. Eur. J. Biochem. 2000, 267, 5900–5907. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Orthwein, T.; Alford, J.T.; Forchhammer, K. The Slr0058 protein from Synechocystis sp. PCC 6803 is a novel regulatory protein involved in PHB granule formation. Front. Microbiol. 2020, 11, 809. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Miyairi, S.; Yamamoto, H.; Inagaki, N.; Kanesaki, Y.; Murata, N. Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol. 2002, 130, 1443–1453. [Google Scholar] [CrossRef]
- Apte, S.K.; Fernandes, T.; Badran, H.; Ballal, A. Expression and possible role of stress-responsive proteins in Anabaena. J. Biosci. 1998, 23, 399–406. [Google Scholar] [CrossRef]
- Apte, S.K.; Reddy, B.R.; Thomas, J. Relationship between sodium influx and salt tolerance of nitrogen-fixing cyanobacteria. Appl. Environ. Microbiol. 1987, 53, 1934–1939. [Google Scholar] [CrossRef]
- Chen, L.Z.; Li, D.H.; Song, L.R.; Hu, C.X.; Wang, G.H.; Liu, Y.D. Effects of salt stress on carbohydrate metabolism in desert soil alga Microcoleus vaginatus Gom. J. Integr. Plant Biol. 2006, 48, 914–919. [Google Scholar] [CrossRef]
- Eisenhut, M.; Kahlon, S.; Hasse, D.; Ewald, R.; Lieman-Hurwitz, J.; Ogawa, T.; Ruth, W.; Bauwe, H.; Kaplan, A.; Hagemann, M. The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiol. 2006, 142, 333–342. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhargava, P.; Thapar, R.; Rai, L.C. Salinity-induced physiological and proteomic changes in Anabaena doliolum. Environ. Exp. Bot. 2008, 64, 49–57. [Google Scholar] [CrossRef]
- Hejduková, E.; Elster, J.; Nedbalová, L. Annual cycle of freshwater diatoms in the High Arctic revealed by multiparameter fluorescent staining. Microb. Ecol. 2020, 80, 559–572. [Google Scholar] [CrossRef]
- Alpert, P. The limits and frontiers of desiccation-tolerant life. Integer. Comp. Biol. 2005, 45, 685–695. [Google Scholar] [CrossRef]
- Shirkey, B.; McMaster, N.J.; Smith, S.C.; Wright, D.J.; Rodriguez, H.; Jaruga, P.; Birincioglu, M.; Helm, R.F.; Potts, M. Genomic D.N.A. of Nostoc commune (Cyanobacteria) becomes covalently modified during long-term (decades) desiccation but is protected from oxidative damage and degradation. Nucleic Acids Res. 2003, 31, 2995–3005. [Google Scholar] [CrossRef][Green Version]
- Lipman, C.B. The successful revival of Nostoc commune from a herbarium specimen eighty-seven years old. Bull. Torrey Bot. Club 1941, 68, 664–666. [Google Scholar] [CrossRef]
- Cameron, R.E. Species of Nostoc vaucher occurring in the Sonoran Desert in Arizona. Trans. Am. Microsc. Soc. 1962, 81, 379–384. [Google Scholar] [CrossRef]
- Scheibe, R.; Beck, E. Drought, desiccation, and oxidative stress. In Plant Desiccation Tolerance; Springer: Berlin/Heidelberg, Germany, 2011; pp. 209–231. [Google Scholar]
- Palmer, R.J.; Friedmann, E.I. Water relations and photosynthesis in the cryptoendolithic microbial habitat of hot and cold deserts. Microb. Ecol. 1990, 19, 111–118. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. R.O.S. defense systems and terminal oxidases in bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef]
- Smith, M.G. Survival of E. coli and Salmonella after chilling and freezing in liquid media. J. Food Sci. 1995, 60, 509–512. [Google Scholar] [CrossRef]
- Prát, S. Ustoychivosttermalnykhsinezelenykhvodorosley k nizkimtemperaturam [Stability of thermal blue-green algae to low temperatures]. Bull. Int. Acad. Tcheque Sci. 1950, 20, 1–6. [Google Scholar]
- Ninagawa, T.; Eguchi, A.; Kawamura, Y.; Konishi, T.; Narumi, A. A study on ice crystal formation behavior at intracellular freezing of plant cells using a high-speed camera. Cryobiology 2016, 73, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Stokes, M.D.; Korsmeyer, K.E. Overwintering strategies of Antarctic organisms. Environ. Rev. 2000, 8, 1–19. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Hantke, K. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol 2005, 8, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Y.S.; Peng, R.H.; Xiong, A.S.; Zhu, B.; Hou, X.L.; Yao, Q.H. Cyanobacteria MT gene SmtA enhance zinc tolerance in Arabidopsis. Mol. Biol. Rep. 2010, 37, 1105–1110. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Gupta, R.K. Role of cyanobacteria in germination and growth of paddy seedlings. Int. J. Phytol. Res. 2022, 2, 11–18. [Google Scholar]
- Wang, Z.; Li, D.; Li, G.; Liu, Y. Mechanism of photosynthetic response in Microcystis aeruginosa PCC7806 to low inorganic phosphorus. Harmful Algae. 2010, 9, 613–619. [Google Scholar] [CrossRef]
- Blindauer, C.A. Bacterial metallothioneins: Past, present, and questions for the future. J. Biol. Inorg. Chem. 2011, 16, 1011–1024. [Google Scholar] [CrossRef]
- Murata, N.; Suzuki, I. Exploitation of genomic sequences in a systematic analysis to access how cyanobacteria sense environmental stress. J. Exp. Bot. 2006, 57, 235–247. [Google Scholar] [CrossRef]
- Bhargava, P.; Mishra, Y.; Srivastava, A.K.; Narayan, O.P.; Rai, L.C. Excess copper induces anoxygenic photosynthesis in Anabaena doliolum: A homology-based proteomic assessment of its survival strategy. Photos. Res. 2008, 96, 61–74. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, R.; Rai, L.C. Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J. Proteom. 2012, 75, 921–937. [Google Scholar] [CrossRef]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J.; et al. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crops Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Chardonnet, S.; Sakr, S.; Cassier-Chauvat, C.; Le Maréchal, P.; Chauvat, F.; Lemaire, S.D.; Decottignies, P. First proteomic study of S-glutathionylation in cyanobacteria. J. Proteome Res. 2015, 14, 59–71. [Google Scholar] [CrossRef]
- Babele, P.K.; Kumar, J.; Chaturvedi, V. Proteomic deregulation in cyanobacteria in response to abiotic stresses. Front. Microbiol. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Graves, P.R.; Haystead, T.A. Molecular biologist’s guide to proteomics. Microbiol. Mol. Biol. Rev. 2002, 66, 39–63. [Google Scholar] [CrossRef]
- Monteoliva, L.; Albar, J.P. Differential proteomics: An overview of gel and non-gel based approaches. Brief. Funct. Genom. 2004, 3, 220–239. [Google Scholar] [CrossRef]
- Kashino, Y.; Lauber, W.M.; Carroll, J.A.; Wang, Q.; Whitmarsh, J.; Satoh, K.; Pakrasi, H.B. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 2002, 41, 8004–8012. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Chitnis, P.R. Proteomic study of the peripheral proteins from thylakoid membranes of the cyanobacterium Synechocystis sp. PCC 6803. Electrophoresis 2000, 21, 1746–1754. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Chen, W.; Ding, L.; Li, P.; Zhao, X.; Wang, X.; Li, A.; Bao, Q. Identification of differentially expressed proteins of Arthrospira (Spirulina) plantensis-YZ under salt-stress conditions by proteomics and qRT-PCR analysis. Proteome Sci. 2013, 11, 6. [Google Scholar] [CrossRef]
- Panda, B.; Basu, B.; Rajaram, H.; Apte, S.K. Comparative proteomics of oxidative stress response in three cyanobacterial strains native to Indian paddy fields. J. Proteomics 2015, 127, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Basu, B.; Rajaram, H.; Kumar Apte, S. Methyl viologen responsive proteome dynamics of Anabaena sp. strain PCC7120. Proteomics 2014, 14, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Hikari, J.; Österholm, J.; Kopf, M.; Battchikova, N.; Wahlsten, M.; Aro, E.M.; Hess, W.R.; Sivonen, K. Transcriptomic and proteomic profiling of Anabaena sp. strain 90 under inorganic phosphorus stress. Appl. Environ. Microbiol. 2015, 81, 5212–5222. [Google Scholar]
- Shrivastava, A.K.; Chatterjee, A.; Yadav, S.; Singh, P.K.; Singh, S.; Rai, L.C. UV-B stress-induced metabolic rearrangements explored with comparative proteomics in three Anabaena species. J. Proteom. 2015, 121, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Singh, G.; Kumar, A.; Tyagi, M.B. Induction and differential expression of certain novel proteins in Anabaena L31 under UV-B radiation stress. Front. Microbiol. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Zhang, W. Proteomic and metabolomic analyses reveal metabolic responses to 3-hydroxy propionic acid synthesized internally in cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9, 209. [Google Scholar] [CrossRef]
- Mehta, A.; López-Maury, L.; Florencio, F.J. Proteomic pattern alterations of the cyanobacterium Synechocystis sp. PCC 6803 in response to Cadmium, nickel and cobalt. J. Proteomics 2014, 102, 98–112. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, J.; Chen, L.; Tian, X.; Huang, S.; Ren, X.; Zhang, W. Quantitative iTRAQ LC-MS/MS proteomics reveals metabolic responses to biofuel ethanol in cyanobacterial Synechocystis sp. PCC 6803. J. Proteome Res. 2012, 11, 5286–5300. [Google Scholar] [CrossRef]
- Tian, X.; Chen, L.; Wang, J.; Qiao, J.; Zhang, W. Quantitative proteomics reveals dynamic responses of Synechocystis sp. PCC 6803 to next generation biofuel butanol. J. Proteomics 2013, 78, 326–345. [Google Scholar]
- Singh, A.K.; Singh, P.P.; Tripathi, V.; Verma, H.; Singh, S.K.; Srivastava, A.K.; Kumar, A. Distribution of cyanobacteria and their interactions with pesticides in paddy field: A comprehensive review. J. Environ. Manag. 2018, 224, 361–375. [Google Scholar] [CrossRef]
- Hashtroudi, M.S.; Ghassempour, A.; Riahi, H.; Shariatmadari, Z.; Khanjir, M. Endogenous auxins in plant growth-promoting Cyanobacteria—Anabaena vaginicola and Nostoc calcicola. J. Appl. Phycol. 2013, 25, 379–386. [Google Scholar] [CrossRef]
- Haroun, S.A.; Hussein, M.H. The Promotive Effect of Algal Biofertilizers on Growth, Protein Pattern and Some Metabolic Activities of Lupinus termis Plants Grown in Siliceous Soil. Asian J. Plant Sci. 2003, 2, 944–951. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Verma, S.; Meena, K.K.; Yandigeri, M. Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech. 2017, 7, 134. [Google Scholar] [CrossRef]
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef]
- Panda, D.; Pramanik, K.; Nayak, B.R. Use of seaweed extracts as plant growth regulators for sustainable agriculture. Int. J. Bio-resour. Stress Manag. 2012, 3, 404–411. [Google Scholar]
- Errani, A.; Nardi, S.; Francioso, O.; Sanchez-Cortes, S.; Foggia, M.D.; Schiavon, M. Effects of two protein hydrolysates obtained from Chickpea (Cicer arietinum L.) and Spirulina platensis on Zea mays (L.) plants. Front. Plant Sci. 2019, 10, 954. [Google Scholar]
- Singh, D.P.; Prabha, R.; Yandigeri, M.S.; Arora, D.K. Cyanobacteria-mediated phenylpropanoids and phytohormones in rice (Oryza sativa) enhance plant growth and stress tolerance. Antonie Van Leeuwenhoek 2011, 100, 557–568. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, S.; Jia, H.; Gao, F.; Zhou, M.; Yuan, N.; Wu, P.; Hu, Q.; Sun, D.; Luo, H. Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnol. 2017, 15, 433–446. [Google Scholar] [CrossRef]
- Takabe, T.; Uchida, A.; Shinagawa, F.; Terada, Y.; Kajita, H.; Tanaka, Y.; Takabe, T.; Hayashi, T.; Kawai, T.; Takabe, T. Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytic enhances growth rate as well as abiotic stress tolerance of poplar plants. Plant Growth Regul. 2008, 56, 265–273. [Google Scholar] [CrossRef]
- Gerasymenko, I.M.; Sakhno, L.A.; Kyrpa, T.N.; Ostapchuk, A.M.; Hadjiev, T.A.; Goldenkova-Pavlova, I.V.; Sheludko, Y.V. Characterization of Nicotiana tabacum plants expressing hybrid genes of cyanobacterial Δ9 or Δ12 acyl-lipid desaturases and thermostable lichenase. Russ. J. Plant Physiol. 2015, 62, 283–291. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Y.; Yang, Y.; Ye, S.; Luo, H.; Qiu, B.; Gao, X. The drnf1 gene from the drought-adapted cyanobacterium Nostoc flagelliforme improved salt tolerance in transgenic Synechocystis and Arabidopsis plant. Genes 2018, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Shariatmadari, Z.; Riahi, H.; Abdi, M.; Hashtroudi, M.S.; Ghassempour, A.R. Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L. J. Appl. Phycol. 2015, 27, 2279–2287. [Google Scholar] [CrossRef]
- Chittapun, S.; Limbipichai, S.; Amnuaysin, N.; Boonkerd, R.; Charoensook, M. Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: A pot experiment. J. Appl. Soc. Psychol. 2018, 30, 79–85. [Google Scholar] [CrossRef]
- Jan, Z.; Ali, S.; Sultan, T.; Ahmad, W. The role of cyanobacteria in the availability of major plant nutrients and soil organic matter to rice crop under saline soil conditions. Sarhad J. Agric. 2017, 33, 566–572. [Google Scholar] [CrossRef]
- Singh, N.K.; Dhar, D.W. Cyanobacterial reclamation of salt-affected soil. In Genetic Engineering, Biofertilization, Soil Quality and Organic Farming; Springer: Dordrecht, The Netherlands, 2010; pp. 243–275. [Google Scholar]
- Rodríguez, A.A.; Stella, A.M.; Storni, M.M.; Zulpa, G.; Zaccaro, M.C. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef]
- Ghada, S.F.; Ahmed, D.A. Improved soil characteristics and wheat germination as influenced by inoculation of Nostoc kihlmani and Anabaena cylindrica. Rend. Lincei. 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Manchanda, H. Influence of cyanobacterial filtrate on growth of rice seedlings under saline conditions. IJRAR 2018, 5, 19–22. [Google Scholar]
- Abbas, H.H.; Ali, M.E.; Ghazal, F.M.; El-Gaml, N.M. Impact of Cyanobacteria inoculation on rice (Oryza sativa) yield cultivated in saline soil. Am. J. Sci. 2015, 11, 13–19. [Google Scholar]
- Arora, M.; Kaushik, A.; Rani, N.; Kaushik, C.P. Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination. J. Environ. Biol. 2010, 31, 701–704. [Google Scholar]
- Coba de la Pena, T.; Redondo, F.J.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Nitrogen fixation persists under conditions of salt stress in transgenic Medicago truncatula plants expressing a cyanobacterial flavodoxin. Plant Biotechnol. J. 2010, 8, 954–965. [Google Scholar] [CrossRef]
- Manchanda, R.K. Significance of prognostic factor research in clinical verification. Indian J. Res. Homoeopathy 2018, 12, 1. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Chilton, A.; Liyanage, G.S.; Erickson, T.E.; Merritt, D.J.; Neilan, B.A.; Ooi, M.K.J. Effects of indigenous soil cyanobacteria on seed germination and seedling growth of arid species used in restoration. ISO4 2018, 429, 91–100. [Google Scholar] [CrossRef]
- Chua, M.; Erickson, T.E.; Merritt, D.J.; Chilton, A.M.; Ooi, M.K.; Muñoz-Rojas, M. Bio-priming seeds with cyanobacteria: Effects on native plant growth and soil properties. Restore Ecol. 2020, 28, S168–S176. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Palatnik, J.F.; Fillat, M.F.; Melzer, M.; Hajirezaei, M.R.; Valle, E.M.; Carrillo, N. Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell 2006, 18, 2035–2050. [Google Scholar] [CrossRef]
- Gharechahi, J.; Hajirezaei, M.R.; Salekdeh, G.H. Comparative proteomic analysis of tobacco expressing cyanobacterial flavodoxin and its wild type under drought stress. J. Plant Physiol. 2015, 175, 48–58. [Google Scholar] [CrossRef]
- Giró, M.; Ceccoli, R.D.; Poli, H.O.; Carrillo, N.; Lodeiro, A.F. An in vivo system involving co-expression of cyanobacterial flavodoxin and ferredoxin–NADP+ reductase confers increased tolerance to oxidative stress in plants. FEBS Open Bio 2011, 1, 7–13. [Google Scholar] [CrossRef]
- Mohsen, A.; Dowidar, S.; Abo-Hamad, S.; Khalaf, B. Role of cyanobacteria in amelioration of toxic effects of copper in ‘Trigonella foenumgracum’. Aust. J. Crop Sci. 2013, 7, 1488–1493. [Google Scholar]
- Tripathi, R.D.; Dwivedi, S.; Shukla, M.K.; Mishra, S.; Srivastava, S.; Singh, R.; Rai, U.N.; Gupta, D.K. Role of blue-green algae biofertilizer in ameliorating the nitrogen demand and fly-ash stress to the growth and yield of rice (Oryza sativa L.) plants. Chemosphere 2008, 70, 1919–1929. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Xie, S.Q. A comprehensive review on recent developments in quality function deployment. Int. J. Product. Qual. Manag. 2010, 6, 457–494. [Google Scholar] [CrossRef]
- Bhagwat, A.A.; Apte, S.K. Comparative analysis of proteins induced by heat shock, salinity, and osmotic stress in the nitrogen-fixing cyanobacterium Anabaena sp. strain L-31. J. Bacteriol. 1989, 171, 5187–5189. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Hassani, S.B.; Aliniaeifard, S. Seed priming by cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against cadmium toxicity. J. Plant Growth Regul. 2020, 39, 1009–1021. [Google Scholar] [CrossRef]
| S. No. | Types of Stresses | References | |
|---|---|---|---|
| 1 | Nutrient deficiency | [19,20] | |
| 2 | Light intensity | [21,22,23] | |
| 3 | Radiation | U.V. | [24,25,26] |
| Gamma | [27,28,29] | ||
| 4 | Temperature | High | [30,31] |
| Low | [32,33,34] | ||
| 5 | Salt | Salinity | [35,36,37] |
| Osmoticum | [38,39,40] | ||
| 6 | Chemicals | Pesticides | [41] |
| Heavy metals | [42,43,44] | ||
| 7 | Desiccation | [45,46,47] | |
| Cyanobacterial Strain | Stress | Methodologies | Proteins Identified | References |
|---|---|---|---|---|
| Anabaena sp. PCC 7120 | Arsenic | 2-DE, MALDI-TOF/ MS, RT-PCR | 45 | [153] |
| Arthrospira plantensis-YZ | Salt | 2DE, MALDI-TOF/ TOF, qRT-PCR | 141 | [161] |
| Anabaena Doliolum, Anabaena PCC7120, and Anabaena L-31 | Methyl viologen | 2DE, MALDI-TOF MS/MS | 103, 92, and 41, respectively | [162,163] |
| Anabaena sp. strain 90 | Inorganic phosphorus (Pi) | 2D-DIGE and LC-MS/MS | 43 | [164] |
| Anabaena L31, Anabaena doliolum, and Anabaena sp. PCC 7120 | UV-B stress | 2DE, MALDI-TOF MS/MS | 90, 91, and 98, respectively | [165] |
| Anabaena sp. strain L31 | UV-B | 2DE, MALDI TOF MS/MS, Bioinformatics | 21 | [166] |
| Synechocystis sp. PCC 6803 | 3-hydroxypro pionic acid (3-HP) | iTRAQ-LC -MS/MS L.C.–MS-based targeted metabolomics | 2264 | [167] |
| Synechocystis sp. PCC 6803 | Cobalt, cadmium, and nickel | 2-DE MALDI TOF/MS RT-PCR | 20 (Nickel) 26 (Cobalt) 13 (Cadmium) | [168] |
| Synechocystis sp. PCC 6803 | Butanol | iTRAQ and LC-MS/MS | 303 | [169,170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; Singh, R.P.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Gupta, R.K.; Kumar, A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses 2022, 2, 531-549. https://doi.org/10.3390/stresses2040036
Yadav P, Singh RP, Rana S, Joshi D, Kumar D, Bhardwaj N, Gupta RK, Kumar A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses. 2022; 2(4):531-549. https://doi.org/10.3390/stresses2040036
Chicago/Turabian StyleYadav, Priya, Rahul Prasad Singh, Shashank Rana, Diksha Joshi, Dharmendra Kumar, Nikunj Bhardwaj, Rajan Kumar Gupta, and Ajay Kumar. 2022. "Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions" Stresses 2, no. 4: 531-549. https://doi.org/10.3390/stresses2040036
APA StyleYadav, P., Singh, R. P., Rana, S., Joshi, D., Kumar, D., Bhardwaj, N., Gupta, R. K., & Kumar, A. (2022). Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses, 2(4), 531-549. https://doi.org/10.3390/stresses2040036











