Abstract
The use of tunnel kiln firing in clay brick production offers a promising approach for disposing of Cl-containing solid waste, with lower chlorine (Cl) and heavy metal volatilization compared to cement kiln processes. However, the effects of Cl salts on brick properties and the volatilization mechanisms remain unclear. This study investigates the behaviors of NaCl, KCl, and CaCl2 during sintering. Adding 15 wt% Cl salts significantly alters pore structure, increasing water absorption by 80–100% and reducing compressive strength by 70–80%. At 1050 °C, 10.8–16.4% of Cl volatilizes mainly as HCl (g), 24.4–26.2% remains in original salt form, and over half is immobilized within the brick matrix. Thermodynamic and TG-MS analyses reveal Cl salts are stable below 800 °C but release HCl (g) at higher temperatures due to lower reaction energy barriers than Cl2 (g). Density functional theory (DFT) calculations show that H+ for HCl (g) formation primarily originates from water vapor (H2O), with organic decomposition having minimal effect. The presence of Cl salts promotes feldspar and silicate phase formation, enhancing densification but increasing porosity from HCl release. To reduce HCl emissions, a two-stage temperature control strategy is proposed: organic decomposition and moisture removal below 600 °C, followed by sintering at 800–1000 °C. This work clarifies the volatilization mechanisms of Cl salts and provides guidance for optimizing industrial brick production using Cl-containing waste.
1. Introduction
A substantial amount of wastewater containing Cl salts is generated across various industries, including chemical engineering, mining engineering [1,2], metallurgy [3], and electroplating [4,5] processes. Although Cl salts primarily exist as ions in aqueous solutions, a portion is still absorbed and co-precipitated in sludge, leading to a considerable accumulation of Cl salts in these waste residues. Fly ash and bottom slag, generated from the incineration of municipal solid waste, hazardous waste, medical waste, and industrial waste, also exhibit a high Cl salt content as a prominent characteristic [6,7,8,9]. The improper management of such solid waste can result in the release of Cl into the soil and groundwater, causing long-term environmental pollution [10,11]. Cl salts are known to inhibit microbial degradation activity, making high-Cl-content waste unsuitable for microbial biodegradation systems [12,13,14]. Additionally, solid waste containing Cl salts is generally undesirable in the building materials industry, as Cl salts interfere with cement setting time and strength development [15,16,17]. Secure landfilling, often combined with solidification or stabilization processes, remains the predominant method for disposing of Cl-containing solid waste. Other practical strategies include thermal treatment and vitrification, which are mainly applied in the metallurgy, cement, and ceramic industries. These sectors possess the necessary infrastructure and high-temperature processes to immobilize or transform Cl salts into more stable forms, thereby minimizing their environmental impact. However, given the increasing constraints on land resources and the growing emphasis on sustainable development, there is an urgent need to develop comprehensive strategies for the utilization of Cl-containing solid waste.
Cement kiln co-processing has become a preferred method for disposing of hazardous solid waste in China and the European Union due to its high operating temperatures (1400–1500 °C) and the ability to fully utilize solid waste. However, under high-temperature conditions, a significant portion of Cl is released into the exhaust gas, particularly in the presence of organochlorine compounds (e.g., oil/tar and char) and limestone. Previous studies [18,19] have reported that over 95% of Cl is emitted into flue gas and subsequently deposited in fly ash, while less than 5% remains as inorganic Cl in bottom ash during solid waste co-processing in cement kilns. The presence of Cl also enhances the volatilization of heavy metals in solid waste by forming volatile metal chlorides [18]. These volatilized metal chlorides tend to deposit on the surface of fly ash, where they act as catalysts and play a crucial role in the formation of toxic pollutants such as dioxins. Additionally, interactions between Cl and organic matter promote the generation of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and other organochlorine compounds [18,20]. Furthermore, Cl salts have been shown to negatively impact the quality of clinker and cement products [21,22]. Due to stringent regulations on Cl levels in cement products, cement factories in China generally accept solid waste with a Cl content of no more than 0.04%.
Tunnel kilns are widely used in the production of ceramics, bricks, tiles, and refractory materials, with typical firing temperatures varying depending on the product type. For example, low-fired ceramics and some traditional bricks may be fired at temperatures as low as 600–900 °C [23,24], whereas ceramic tiles and sanitaryware generally require higher firing temperatures around 900–1000 °C [25,26]. Although their operating temperatures are lower than those of cement kilns, tunnel kilns can effectively decompose harmful substances while ensuring consistent product quality through continuous preheating, firing, and cooling zones. The high silicon and aluminum oxide content in tunnel kilns creates an acidic environment, which effectively inhibits the volatilization of heavy metals and Cl [8]. Additionally, the denser structures of clay bricks, ceramics, and tiles further reduce Cl volatilization. Huang and Yang [27] investigated the interaction between heavy metals and Cl salts and found that while Cl significantly increased the volatilization rates of Cd and Cu by 30.8% and 14.2%, respectively, most heavy metals were still converted into stable mineral phases, substantially reducing their leaching risks. Although previous studies [27,28,29,30,31,32] have primarily focused on the transformation reactions and volatilization behaviors of heavy metals during the incorporation of solid waste in fired brick manufacturing, the volatilization and reaction mechanisms of Cl in tunnel kilns remain largely unexplored. Consequently, the distribution of Cl between exhaust gas and brick products, as well as its evaporation mechanism, is still unclear. Further investigation into the distribution, evaporation behavior, and effects of Cl on the physical and mechanical properties of products is therefore warranted. While the experimental work in this study was conducted at the laboratory scale, the sintering temperatures and heating curves were designed to simulate the operational conditions of industrial tunnel kilns. Therefore, references to tunnel kiln applications are made to evaluate the potential implications and possible corrosion risks associated with the incorporation of Cl-containing waste in real-world production lines.
The objectives of this study are to (1) investigate the influence of Cl salts on porosity evolution and analyze their role in the physical and mechanical properties of sintered bricks; (2) determine the distribution of Cl salts between the gas phase and brick matrix to elucidate their migration and evaporation characteristics; and (3) explore the interaction between Cl salts and other clay components to clarify their reaction behaviors during clay brick production. This study provides new insights into the evaporation and reaction mechanisms of Cl salts during the tunnel kiln co-processing of solid waste and offers valuable guidance for enhancing disposal efficiency and improving the quality of the final product.
2. Materials and Methods
2.1. Materials
The clay used in this study was sourced from a brick factory in Changzhou City, Jiangsu Province, China. The raw clay was first dried at 105 °C for 24 h, then ground in a ball mill at a rotational speed of 400 r/min for 30 min and subsequently passed through a 200-mesh sieve (particle size < 0.075 mm). The preparation procedure was based on commonly used methods in previous studies involving clay-based brick production [33,34], aiming to ensure uniform particle size distribution and adequate material homogeneity for subsequent testing. The particle size distribution and XRD of the clay are shown in Figure S1. The chemical composition of the clay was analyzed using X-ray fluorescence spectrometry (XRF, ZSX Primus III+, Tokyo, Japan). The instrument was calibrated using certified reference materials, and the analytical uncertainty for major oxides was within ±2 wt.%. The results, summarized in Table 1, indicate that SiO2, Al2O3, and Fe2O3 were the dominant components. To simulate Cl-containing solid waste, analytically pure chloride salts (AR grade, ≥99.0%), including NaCl, KCl, and CaCl2, all obtained from certified laboratory suppliers, were introduced into the clay matrix. The mixing ratios, detailed in Table 2, were selected to approximate the typical chloride content observed in municipal solid waste incineration fly ash, ensuring practical relevance to real-world applications.

Table 1.
Element kind and content in used clay.

Table 2.
Mixing ratios of raw materials in brick preparation.
2.2. Experimental Procedure
A mechanical mixing process was initially conducted to ensure uniform distribution of Cl salts within the clay matrix. Subsequently, approximately 40 g of the mixture was compacted into a mold (50 mm × 35 mm × 10 mm) with the addition of 3% water. The compaction process was carried out under a pressure of 45 MPa and maintained for 5 min using a hydraulic press. The compacted bricks were then dried in an oven at 105 °C for 24 h to remove residual moisture before being fired in a tube furnace (SK-G06143, Tianjin, China) at four different temperatures (750, 850, 950, and 1050 °C). The heating rate was set to 5 °C/min, and the firing duration was maintained at 3 h. The firing process was conducted in an air atmosphere, with a flow meter used to regulate the incoming air flow and deionized water employed to capture volatile gases. The concentration of Cl ions in the collected liquid was analyzed using ion chromatography, while metal ion concentrations were determined via atomic absorption spectrophotometry. After firing, the bricks were cooled to room temperature and subsequently subjected to a leaching test using deionized water at a liquid-to-solid ratio of 20:1 for 28 days. Both Cl and metal ion concentrations in the leachates were measured, and the distribution of Cl salts in the sintered bricks was calculated using Equations (1)–(3).
where ω0 is the total content of Cl and metal ions; ω1 and ω2 are the contents of Cl ions and metal ions in the collection solutions, respectively; ρ1 and ρ2 represent the volatilization and leaching rates of Cl and metal ions in the sintered bricks; and ρ3 denotes the solidification rate of Cl ions and metal ions.
To thoroughly investigate the role of potential hydrogen suppliers, glucose and sucrose esters (10 wt% of the brick) were introduced to simulate organic matter in solid waste. Additionally, water vapor was introduced during the firing process to replicate a high-humidity environment. The experimental setup for the hydration unit is illustrated in Figure S2. These conditions were designed to assess the effects of different hydrogen sources on the characteristics of the bricks.
A comprehensive evaluation of the interactions between Cl salts and clay at different temperatures was conducted using thermodynamic modeling. The interaction behavior of Cl with clay was examined over the temperature range of 100–1200 °C. In the thermodynamic calculations, 15 wt% of Cl salts was introduced, and the clay composition was defined as SiO2 (70%), Al2O3 (20%), and Fe2O3 (10%) by mass. Additionally, water accounted for 3% of the total mass.
2.3. Analysis Methods
The microstructure of the sintered bricks was examined using a scanning electron microscope (SEM, Hitachi Regulus 8100, Tokyo, Japan). The pore size distribution and pore volume were analyzed using a fully automated specific surface area and porosity analyzer (BET, ASAP 2020 Plus, Norcross, GA, USA). The concentration of Cl ions in solution was determined by ion chromatography (IC, Thermo Aquion, Waltham, MA, USA), while metal ion concentrations were measured using atomic absorption spectrometry (AAS, PinAAcle 900Z, Waltham, MA, USA). The mineral composition of the bricks was identified via X-ray powder diffraction (XRD, Smartlab9, Tokyo, Japan) at a scanning rate of 5°/min over a 2θ range of 5° to 90°. Thermogravimetric analysis coupled with mass spectrometry (TG-MS) was employed to obtain thermogravimetric curves and analyze the volatile forms of Cl salts under a heating rate of 10 °C/min up to 1100 °C.
Compressive strength tests were conducted following the ASTM C67/C67M-20 standard [33] using an electronic universal testing machine at a loading rate of 0.5 mm/min. The physical properties of the bricks were evaluated in accordance with the Test Methods for Wall Bricks (GB/T 2542-2012) [34]. Linear shrinkage was determined by measuring the length difference before and after firing, while mass loss was assessed by recording the weight change. The density was calculated based on the mass-to-volume ratio. Water absorption was measured by determining the mass difference between dry and water-saturated samples, with the latter being immersed in water for 24 h.
2.4. Computation Method
In the present study, all density functional theory (DFT) calculations were conducted using the Materials Studio 2023 software. This software has been widely used in studies involving Cl-containing solid waste [35,36]. The reactants and products were initially modeled employing the Materials Visualizer module, and subsequently, geometry optimization was carried out in the DMol3 module. The optimization employed the local density approximation (LDA) with the Perdew–Wang correlation (PWC) exchange-correlation functional. This included the use of plane wave pseudopotentials, a cutoff energy of 4.5 Å, and allowed up to 500 optimization iterations. Reaction pathways were investigated using the LST/QST method to determine transition states, and the transition state calculations were performed in the DMol3 module using the LDA/PWC functional, the DN basis set, and a medium-quality orbital cutoff. These calculations maintained an SCF tolerance of 1.0 × 10−5 over 500 SCF iterations. To confirm the transition states, the nudged elastic band (NEB) method was applied, ensuring path continuity and force projection to steer the system towards the minimum energy path (MEP).
The CASTEP tool in Materials Studio was used to simulate the electronic structure of O2 and H2O adsorbed by the NaCl surface. The adsorption model was constructed using Materials Visualizer and calculated using generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE) density functional theory. The plane wave pseudopotentials were the base group. The SCF tolerance was set to 2 × 10−6 eV/atom, the K-Point grid was 1 × 1 × 1, and the number of optimization iterations was 1000. The state density diagram was used to analyze the electron distribution, the differential charge density diagram showed the charge movement and polarization direction during bonding, and the Bader charge analysis was used to derive the valence of the atom.
3. Results and Discussion
3.1. Effect on Physical–Mechanical Properties of Brick
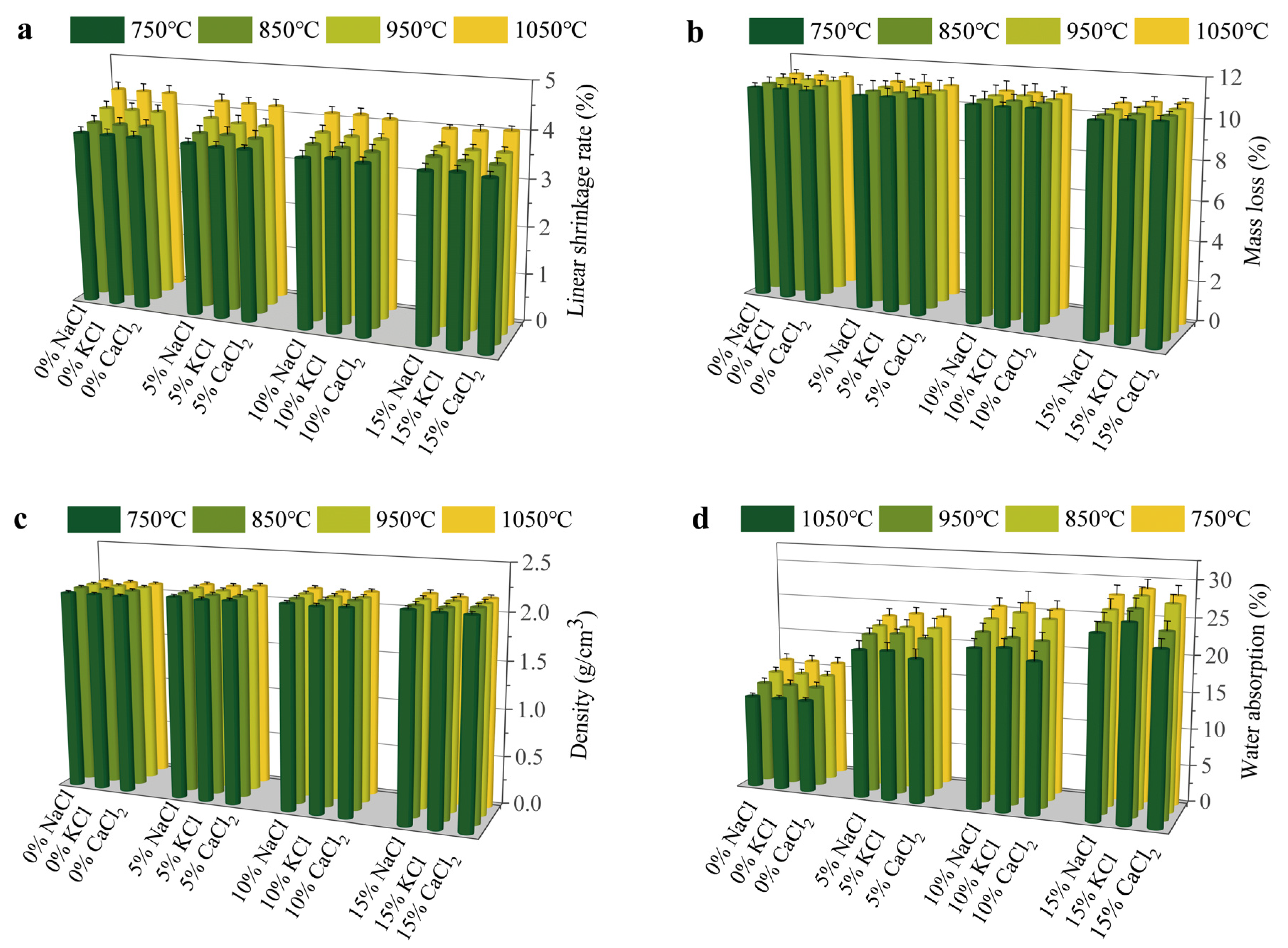
Figure 1 illustrates the effects of various types of Cl salts on the physical properties of sintered bricks. The addition of Cl salts had a minimal impact on the linear shrinkage of the sintered bricks. As the Cl salt content increased, a slight reduction in linear shrinkage was observed. The primary factors influencing the linear shrinkage of the bricks were the decomposition of organic matter and the evaporation of moisture. The linear shrinkage rates for bricks without Cl salts ranged from 3.7 to 4.3%, whereas the rates decreased to 3.52–4.03% with the inclusion of 15% Cl salts. The mass loss exhibited a similar trend to linear shrinkage, with higher Cl salt contents resulting in lower mass loss. The mass loss of sintered bricks was generally less than 1%. This mass loss primarily occurred due to the volatilization of water and the oxidative decomposition of organic matter in the clay. Since Cl salts do not contain organic matter and have relatively low moisture content, the reduction in brick mass after firing was minimal. Higher-density bricks typically exhibit greater compressive strength and durability. The density of the bricks increased with the sintering temperature. The density of bricks without Cl salts was approximately 2.1 g/cm3, while it increased to about 2.16 g/cm3 with the addition of 15% Cl salts. Water absorption of the bricks increased significantly with higher Cl salt content. This substantial increase was attributed to the volatilization or decomposition of Cl salts at elevated temperatures, which generated gases that formed bubbles and pores within the bricks. These bubbles and pores subsequently enhanced water absorption. Furthermore, some Cl salts remained in their initial state and dissolved upon soaking, creating additional internal pores in the bricks.
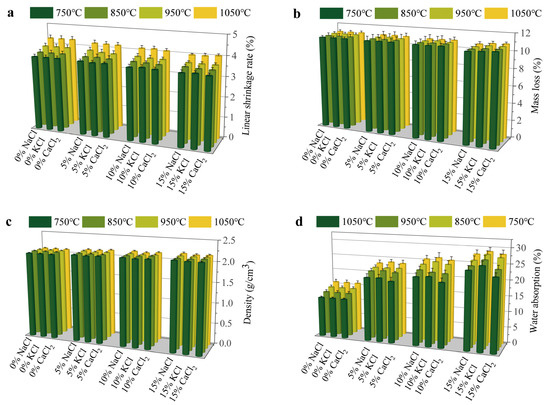
Figure 1.
Effect of Cl salts on physical properties of bricks: (a) linear shrinkage rate, (b) mass loss, (c) density, and (d) water absorption.
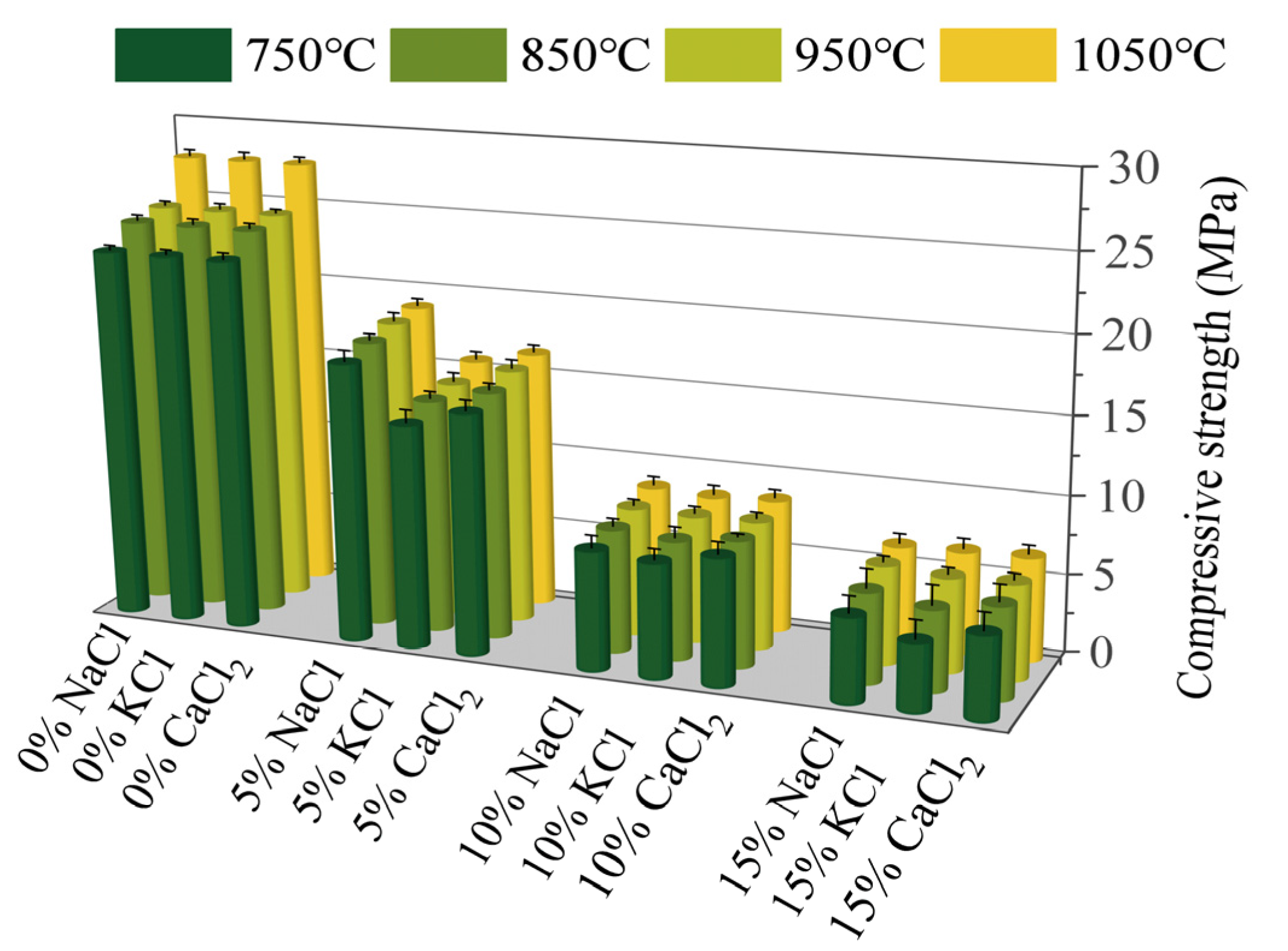
Figure 2 illustrates the effects of Cl salts on the mechanical strength of sintered bricks. An increase in the content of Cl salts led to a significant reduction in the compressive strength of the bricks. Specifically, the introduction of 15% Cl salts reduced the compressive strength by 70–80%, which was consistent with the observed increase in water absorption. Moreover, Cl salts are typically volatilized at high temperatures, producing bubbles and pores within the brick, which adversely affects the compressive strength [37]. Dai [38] investigated the use of electroplating sludge in the production of sintered ceramics and found that the mechanical properties were similarly negatively affected. The authors suggested that inorganic salts hindered the reaction between silicon and aluminum at high temperatures. Furthermore, previous studies have indicated that Cl salts lower the melting points of certain mineral phases, leading to the formation of liquid phases at lower temperatures. This impedes the normal growth of mineral crystals within the bricks. Additionally, the exchange of Na+, K+, and Ca2+ ions from Cl salts with other ions in the bricks at high temperatures disrupts the original mineral structure, weakening the crystal lattice and further diminishing the compressive strength [39]. During the compressive strength tests, samples with higher Cl salt contents exhibited brittle failure, often breaking suddenly into multiple fragments, whereas samples with lower Cl contents showed more compact and cohesive fracture surfaces. These findings suggest that Cl salts have a detrimental effect on the physical and mechanical properties of sintered bricks [1].
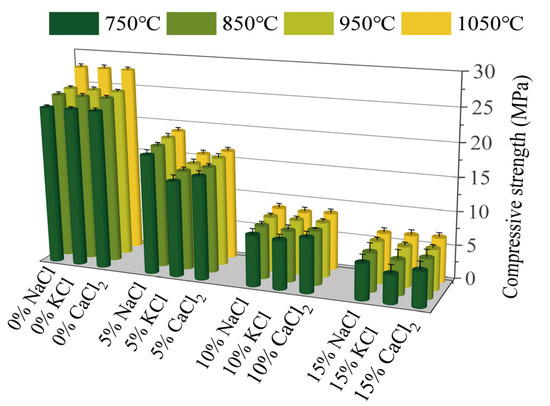
Figure 2.
The compressive strength of sintered bricks incorporated with different amounts of Cl salts.
3.2. Effect on Porosity Structure of Brick
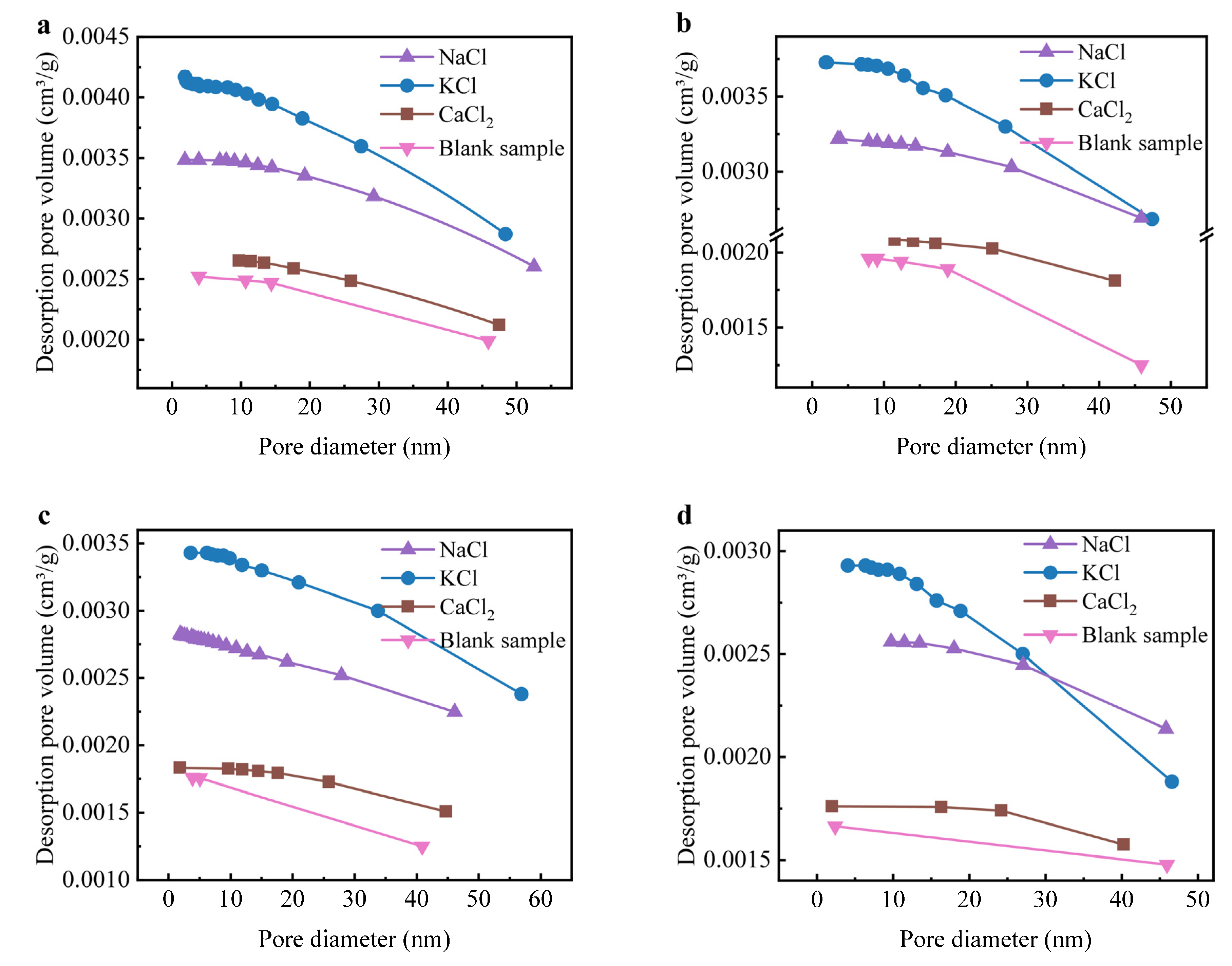
Figure S3 presents the XRD patterns of sintered bricks incorporating 10 wt% NaCl, KCl, and CaCl2. The introduction of Cl salts led to the formation of feldspar phases and CaSiO3, which became more prominent as the temperature exceeded 850 °C. NaCl and KCl were detected in sintered bricks at temperatures below 850 °C, but were not observed above 950 °C. CaCl2, however, was absent in all sintered bricks, suggesting that the interaction between silicon-aluminum and CaCl2 was more favorable compared to NaCl and KCl. The formation of feldspar phases and CaSiO3 was closely associated with the interaction of Na+, K+, and Ca2+ with the silicate-aluminate components. The amount of feldspar phases increased significantly at 950 °C, and the Cl salts were no longer detected, indicating that a temperature above 850 °C was required for the reaction between silicon-aluminum and Cl salts. The formation of feldspar phases and CaSiO3 generally enhances the compressive strength of sintered bricks by improving the compactness of the raw materials. However, the introduction of these Cl salts negatively impacted the compressive strength (Figure 2), suggesting that the Cl salts might disrupt the brick’s porosity structure. Figure 3 shows the accumulated pore volume of sintered bricks with Cl salts. The internal pores of the sintered bricks incorporating Cl salts were significantly more pronounced than those of the blank sample, with NaCl and KCl resulting in greater pore formation compared to CaCl2 [40]. This result aligns with the XRD patterns, indicating that Ca2+ tends to form a denser structure with silicon-aluminum. At temperatures above 850 °C, especially at 1050 °C, the porosity of the bricks containing NaCl and KCl increased significantly due to the enhanced volatilization of these salts. The evaporation of NaCl and KCl generates gas within the matrix, leading to the formation of internal pores. In contrast, Ca2+ in CaCl2 more readily reacts with aluminosilicate components in the clay to form stable silicate phases and Cl-containing mineral phases, thereby suppressing the volatilization of Cl and limiting pore development. As a result, the porosity in the CaCl2-containing samples remained relatively low, reflecting a more stable microstructure under high-temperature conditions.
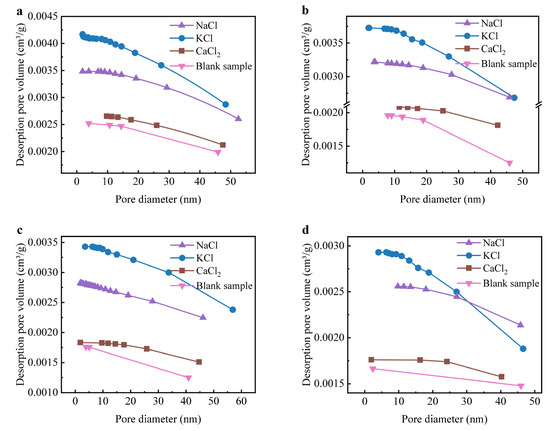
Figure 3.
Accumulated pore volume of sintered bricks incorporated with NaCl, KCl, and CaCl2: (a) 750 °C, (b) 850 °C, (c) 950 °C, and (d) 1050 °C.
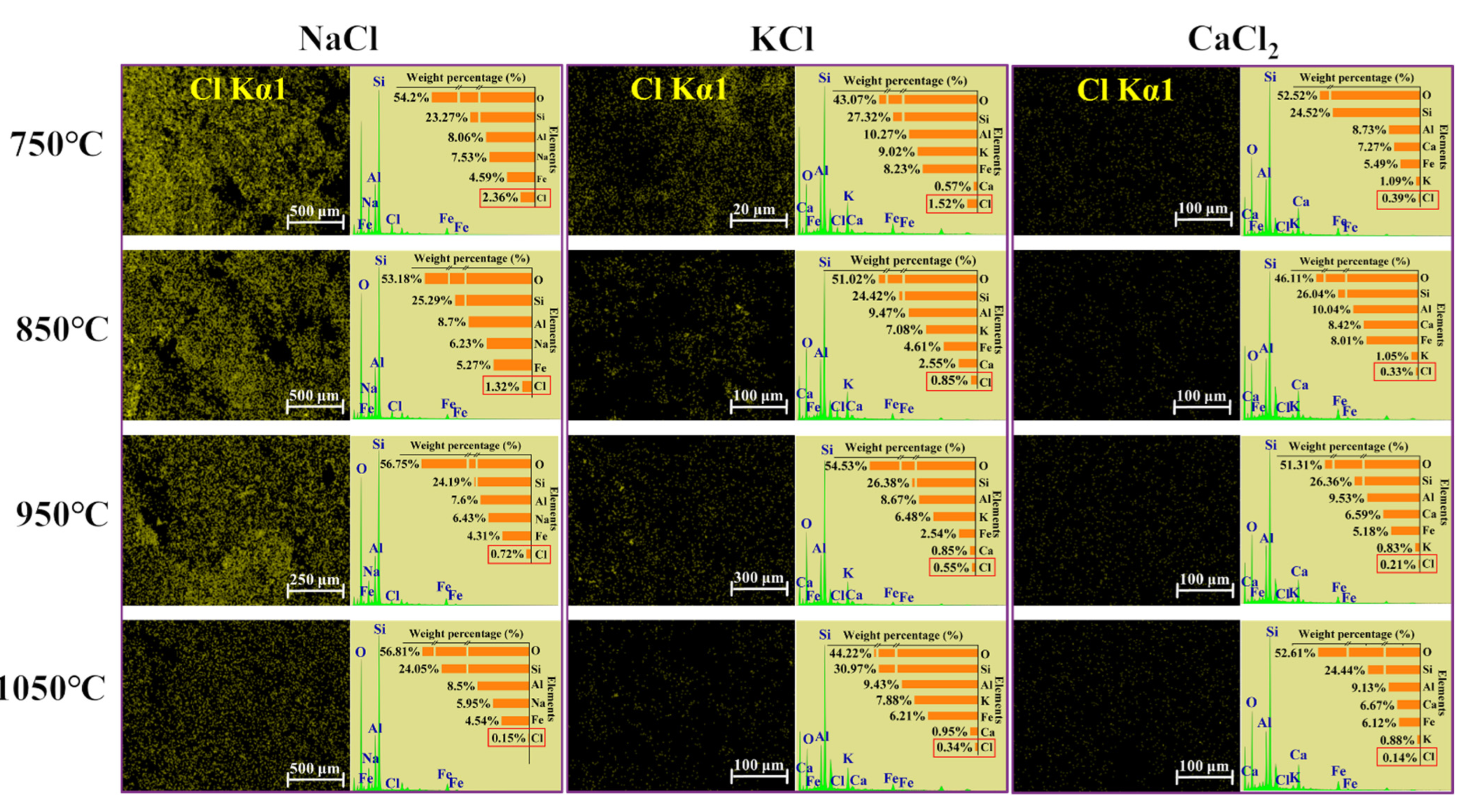
SEM images of sintered bricks with Cl salts are shown in Figure S4. As the temperature increased, the cross-sections of the bricks became denser, and the porosity decreased due to vitrification. The specific surface area of the bricks, shown in Figure S5, was measured to assess the internal pore structure. A smaller specific surface area typically correlates with higher brick density. The specific surface area gradually decreased with the increase in temperature. The specific surface area of bricks without Cl salts was less than 0.8 m2/g, which was significantly lower than that of bricks with Cl salts, suggesting that the presence of Cl salts increased the porosity of the bricks. Figure 4 shows the distribution and concentration of Cl in the sintered bricks. A decrease in Cl content was observed as the temperature increased. Additionally, the concentrations of Na+, K+, and Ca2+ in the sintered bricks were significantly higher than that of Cl, which can be attributed to the volatilization of Cl at high temperatures. The morphological and specific surface area data indicate that the evaporation of Cl salts led to the formation of internal pores within the bricks, which, in turn, affected the bricks’ strength.
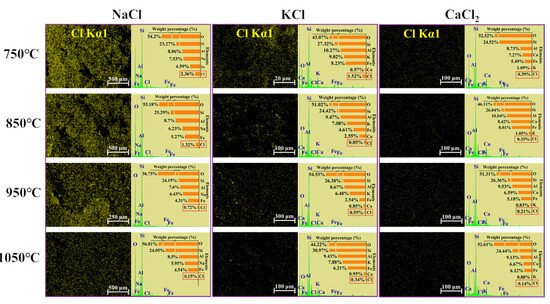
Figure 4.
The distribution and content of Cl in bricks incorporated with 10% of NaCl, KCl, and CaCl2 sintered at 750–1050 °C.
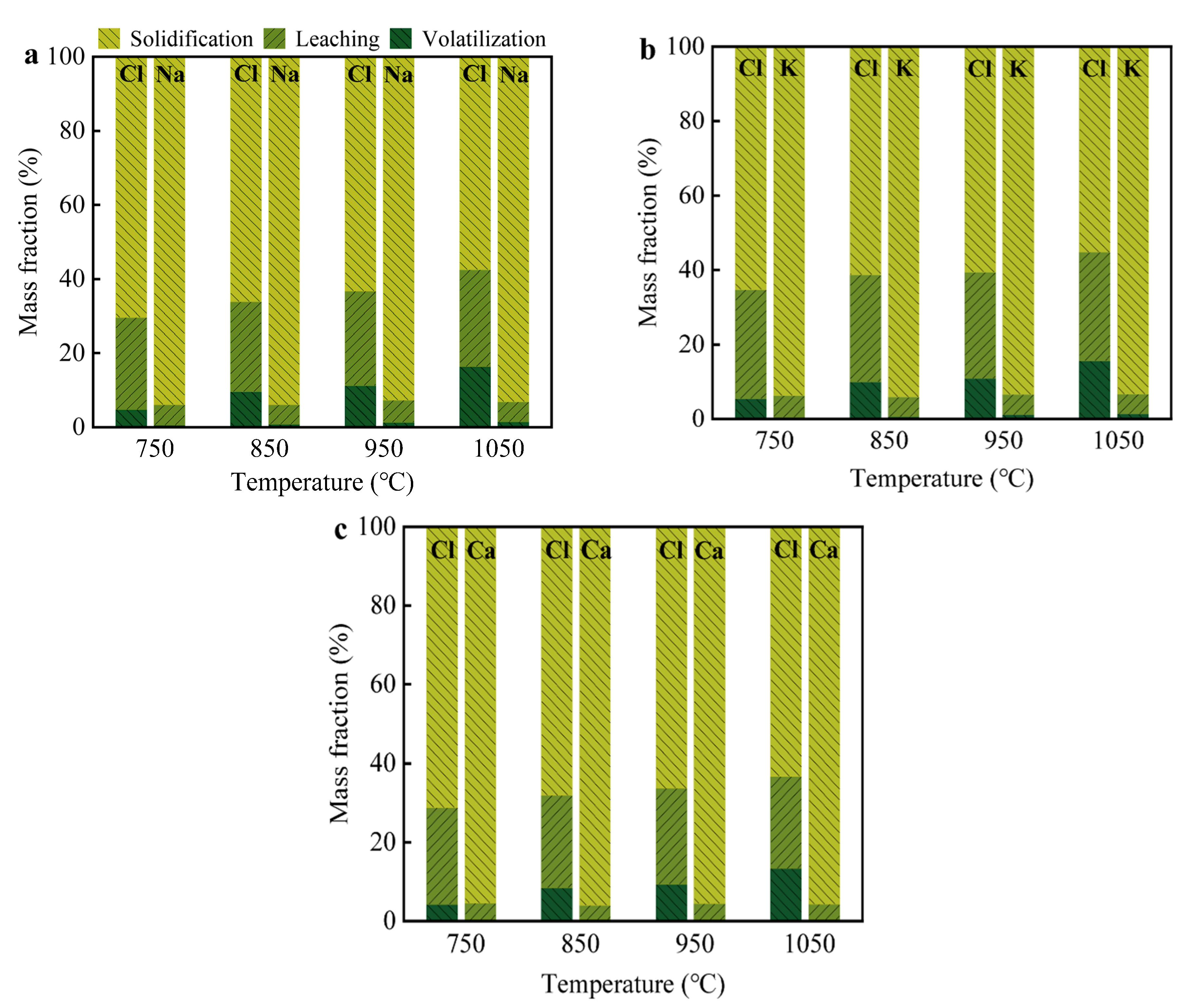
3.3. Migration and Distribution of Cl Salts
The gas volatilized from Cl salts at high temperatures created internal pores in the bricks, significantly affecting their physical properties. To investigate the volatilization and distribution of Cl salts, approximately 10 wt% NaCl, KCl, and CaCl2 were mixed with clay and used in the production of clay bricks. The distribution proportions of Na+, K+, Ca2+, and Cl− ions in the air and brick matrix after the firing process are presented in Figure 5. The proportions of these ions detected in absorption bottles represent the volatilized Cl that migrated into the air, while the leaching part by water indicates the free or original state of Cl salts. The remaining portion corresponds to Cl that solidified at high temperatures. It was found that the volatilization rate of Cl was significantly higher for Na+, K+, and Ca2+ and increased with the sintering temperature, which corresponds to the distribution of Cl shown in Figure 4. Approximately 10.78–16.38% of Cl evaporated and migrated into the exhaust gas at 1050 °C, while 24.35–26.16% of Cl remained in its original form. The proportions of Cl in both the evaporated and original states were similar for NaCl and KCl but higher for CaCl2. Nearly all Na+, K+, and Ca2+ remained stabilized during the firing process, with volatilization rates for these ions not exceeding 1.50%. The volatilization rates of Ca2+ were 0.25, 0.26, 0.29, and 0.39% at 750, 850, 950, and 1050 °C, respectively, which were much lower than those of Na+ (0.63, 0.84, 1.35, and 1.50% at the same temperatures).

Figure 5.
Distribution proportions of Cl−, Na+, K+, and Ca2+ ions during introducing (a) NaCl, (b) KCl, and (c) CaCl2 into the production of fired clay brick. The proportions detected in absorption bottles represented the volatilization amount into air, the leaching part by water represented the free or original state of Cl salts, and the rest could be assigned as the solidification part at high temperature.
Previous studies [41,42] reported that Na+ and K+ contributed to the formation of feldspar and pyroxene, while Ca2+ readily reacted with silica and aluminum in the clay to form stable compounds such as CaSiO3 and CaAl2Si2O8 [43]. The combination of these cations with silica and aluminum played a key role in immobilizing Cl salts. The substantial Cl evaporation rate (approximately 15%) suggests that the exhaust gas from the tunnel kiln contains significant amounts of HCl (g) or Cl2 (g), which could burden the waste gas treatment system and cause corrosion of the kiln walls and off-gas pipelines. Notably, this level of Cl volatilization may lead to HCl concentrations exceeding the recommended limits for flue gases in the ceramic industry, highlighting the need to control Cl salt content and optimize firing conditions to ensure compliance with environmental regulations [44].
Despite this, more than 20% of Cl remained in its original state within the brick matrix, which could be leached by aqueous solutions, potentially leading to efflorescence on the surface of the final product. Although efflorescence testing was not conducted in this study due to experimental limitations, this phenomenon may affect the aesthetic appearance and long-term durability of the bricks. Therefore, the systematic evaluation of efflorescence behavior will be addressed in future work. XRD results further indicated that some mineral phases containing Cl, such as Ca3Al2(SiO4)2Cl4 and Ca3SiO4Cl2, were detected in the sintered bricks, suggesting that Cl− ions were solidified through the formation of complex silicate minerals with the silica-aluminum substances in the clay. The possible reactions are shown in Equations (4)–(7). Furthermore, part of the Cl was encapsulated and incorporated by dense silica-aluminum components or the glass phase at high temperatures. In any case, the introduction of solid waste containing Cl salts in the production of fired bricks still suppressed the Cl evaporation rate when compared to cement production due to the lower temperature and less acidic environment, making this process more suitable for the disposal of Cl-containing solid waste.
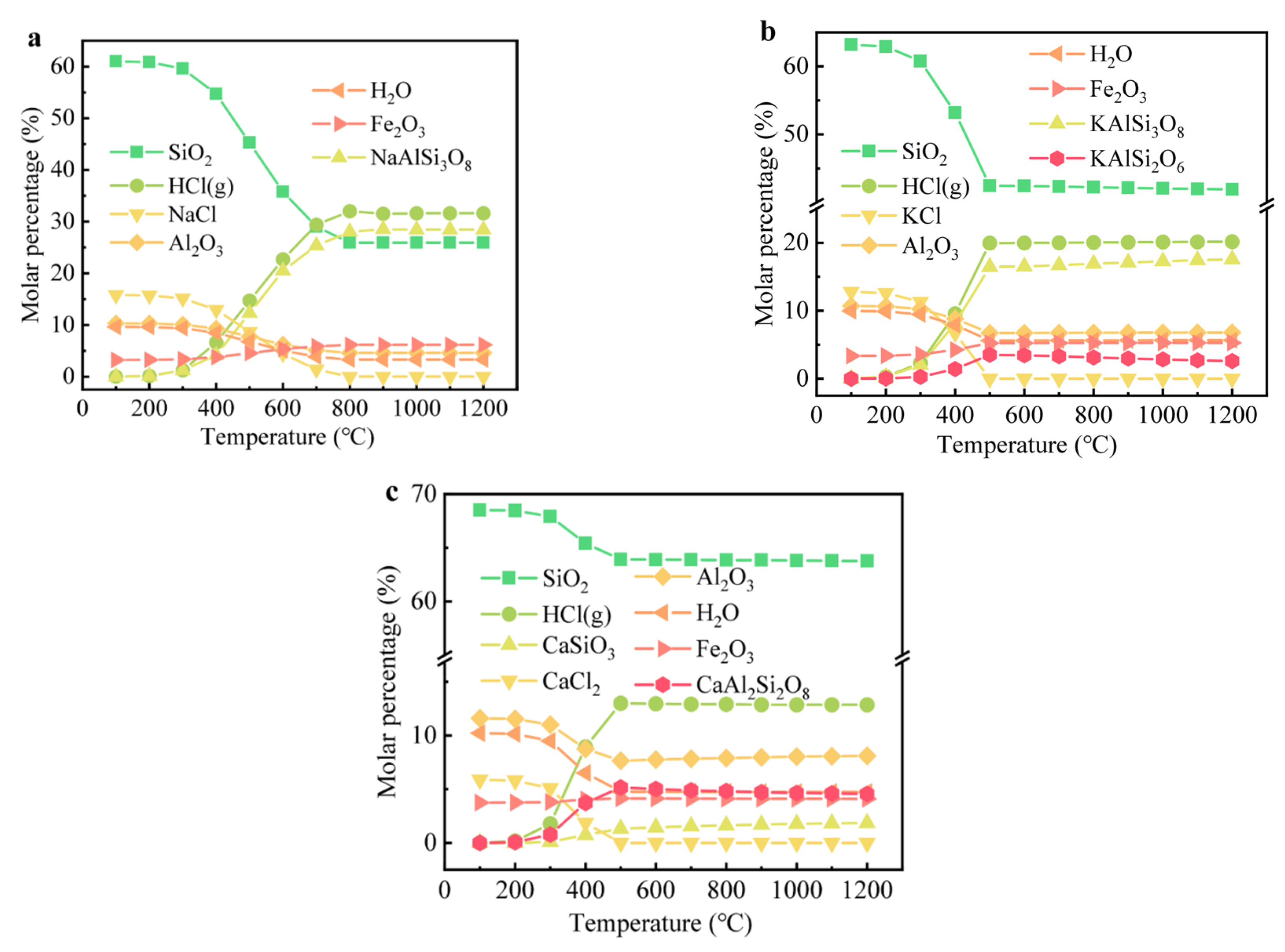
3.4. Volatilization Mechanism of Cl Salts
The species and changes in the binary system of Cl salts and clay at different temperatures were examined through thermodynamic calculations, with the results shown in Figure 6. To simulate the presence of both free and combined water in the soil, H2O (g) was incorporated into the simulation. Cl salts generally remain stable at temperatures below 400 °C but begin to transform into HCl (g) at temperatures exceeding 500 °C. HCl (g) was found to be the predominant Cl-hosting species rather than Cl2 (g) or FeCl3 (g). Above 700 °C, K+ and Na+ ions were observed to combine with SiO2 and Al2O3, forming KAlSi2O6, KAlSi3O8, and NaAlSi3O8, which coincided with a decrease in the amounts of SiO2 and Al2O3. In contrast to Na+ and K+, Ca2+ reacted with SiO2 to form CaSiO3, a finding that aligns with the XRD results. The presence of CaCl2 led to a decrease of only about 5% in the SiO2 content, which was significantly lower than the reductions observed with NaCl and KCl. The formation of low-melting substances, such as Na/K feldspar, may facilitate the combination reactions of Na+ or K+ with SiO2 during firing due to enhanced mass diffusion. Thermodynamic calculations indicated that Cl could transform into HCl (g) and evaporate at temperatures above 400 °C, consistent with previous studies [19,45,46].
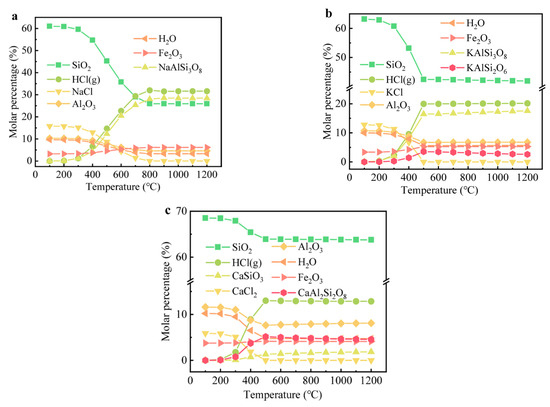
Figure 6.
The evolution of main components containing Cl in bricks at different temperatures: (a) NaCl, (b) KCl, and (c) CaCl2.
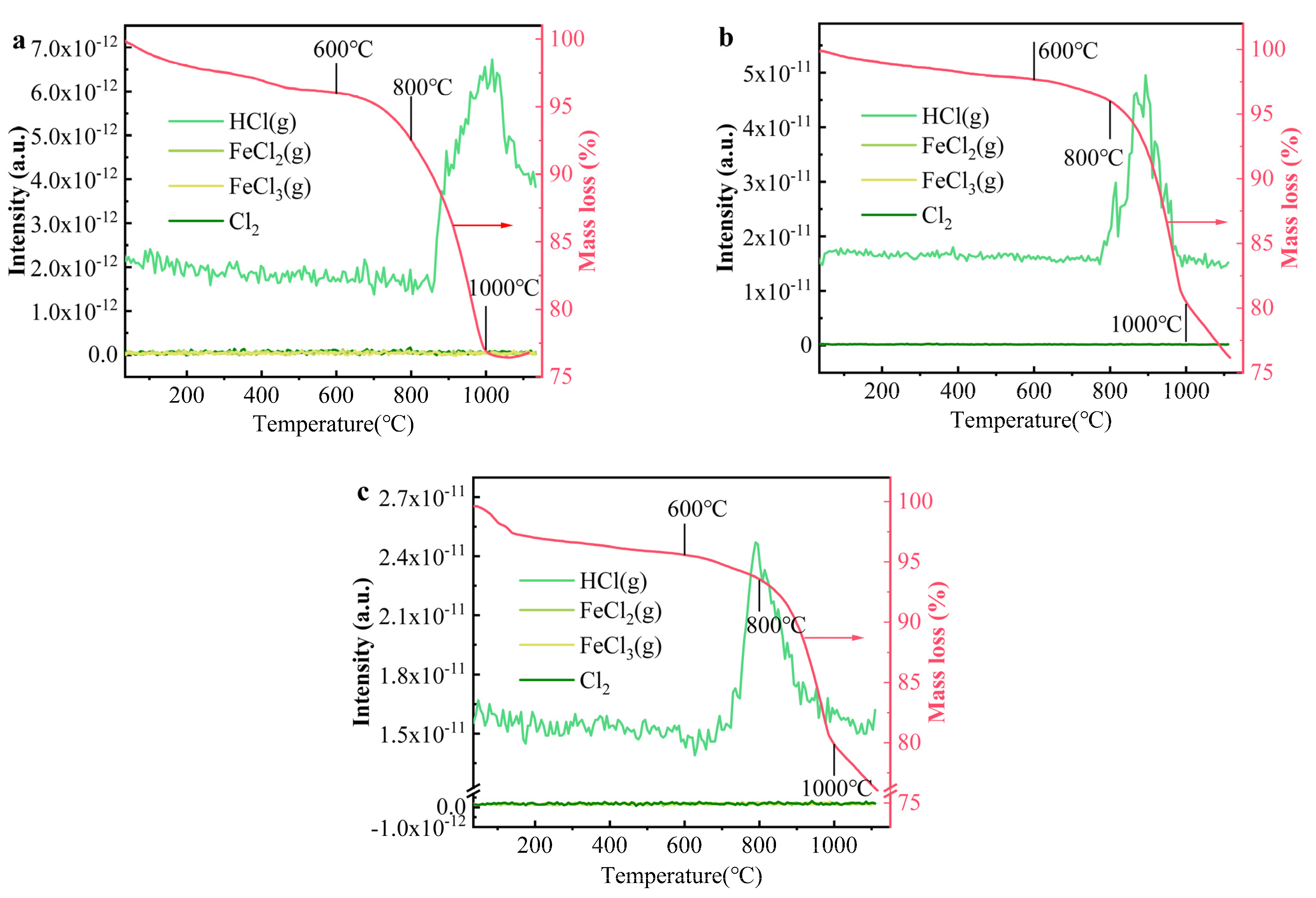
TG-MS was employed to measure the volatilization species of Cl, with the results presented in Figure 7. The thermogravimetric curves indicated that the mass loss of the clay from 0 to 600 °C was approximately 2.3–4.4%, which can be attributed to the decomposition of organic matter and the volatilization of both free and bound water. The mass loss between 600 and 1000 °C ranged from 15 to 19%, primarily due to the evaporation of Cl-containing gases. While potential Cl-containing gases include FeCl2 (g), FeCl3 (g), HCl (g), and Cl2 (g), HCl (g) was identified as the dominant species, with its concentration significantly higher than that of the other gases. Minimal quantities of Cl2 (g), NaCl (g), KCl (g), and CaCl2 (g) were detected in the gaseous phase. Notably, the substantial formation and release of HCl (g) above 800 °C corresponded well with the thermogravimetric curve. These findings suggest that Cl salts predominantly volatilized as HCl (g), consistent with both prior studies [45,46] and the thermodynamic simulation results shown in Figure 6. Additional gaseous species detected by TG-MS, aside from HCl (g), are presented in Figure S6. The O2 content in the system remained relatively stable, with no significant change, while CO2 levels increased markedly at temperatures above 600 °C. The rise in CO2 content between 200 and 600 °C is attributed to the decomposition and combustion of organic matter in the clay, while the increase at 800 °C is linked to the decomposition of carbonates [47].
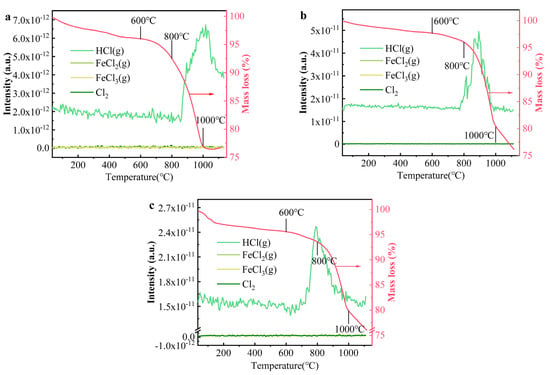
Figure 7.
The evolution of main components containing Cl in bricks at different temperatures: (a) NaCl, (b) KCl, and (c) CaCl2.
The above results demonstrate that Cl salts primarily volatilized as HCl (g) at high temperatures. However, the source of the hydrogen (H) required for HCl formation remained unclear. This study investigates two potential sources of H: organic matter within the clay and water vapor from the ambient air or bound water in the clay during firing. Figure S7 illustrates the effect of introducing glucose or sucrose ester as the hydrogen source on the volatilization of Cl salts. At 300 °C, no Cl was detected, and less than 2% was observed at 600 °C. Although organic substances such as glucose and sucrose ester fully decomposed at these temperatures, their presence did not significantly promote the volatilization of Cl salts. Even with a hydrogen source, the formation of HCl (g) from Cl salts was not observed. The Cl volatilization rates at 1000 °C were 18.39–20.69%, showing an increase of only 3–6% compared to the control sample without organic additives. Below 1000 °C, organic matter did not significantly enhance Cl volatilization or form chemical bonds with Cl, suggesting that organic matter in clay was not a source of H for HCl (g) formation.
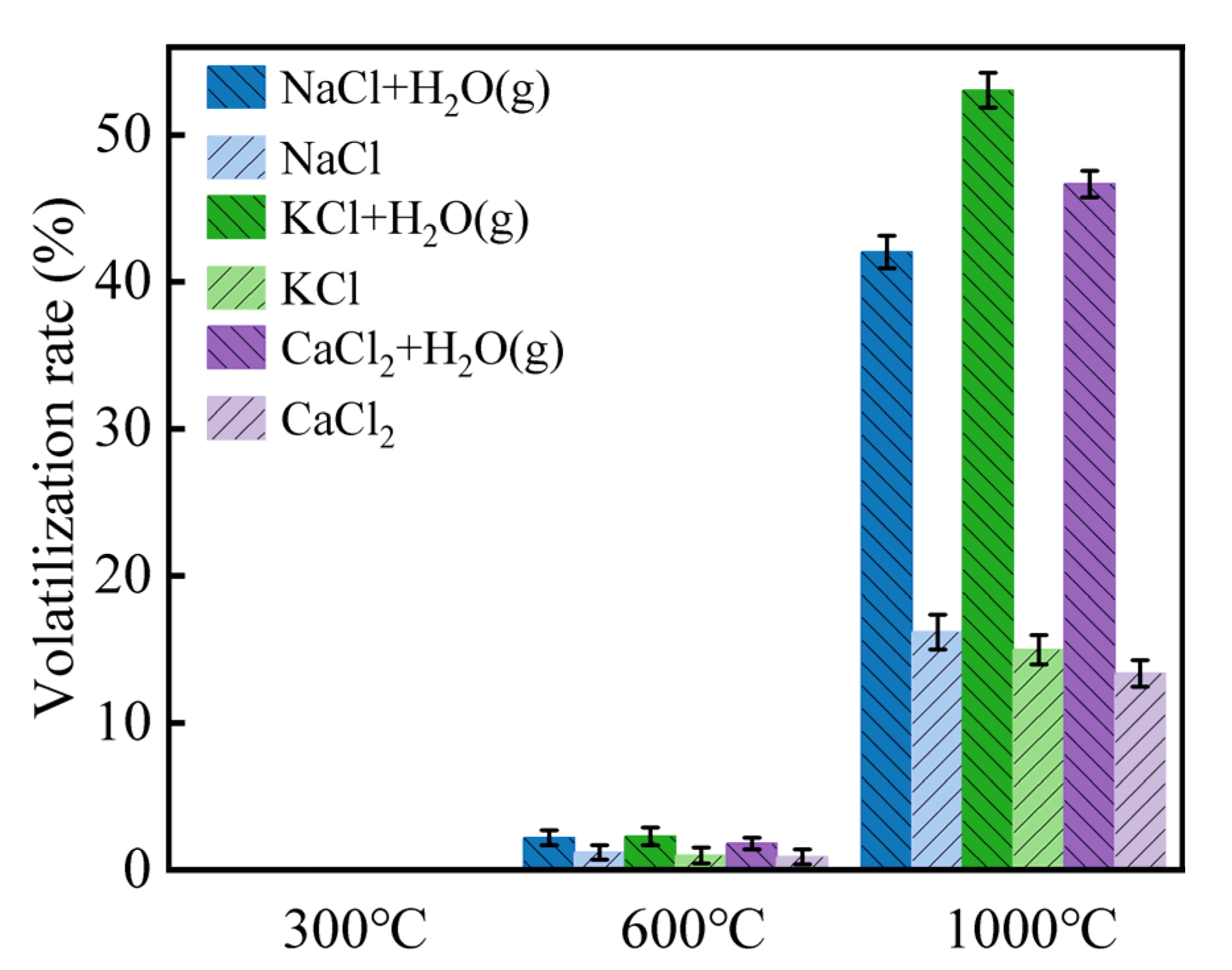
Figure 8 demonstrates the volatilization behavior of Cl salts when water vapor is introduced. At 600 °C, only 1.8–2.3% of Cl volatilized, but this increased to 42.03–53.04% at 1000 °C, indicating that a sufficient dissociation temperature was required for Cl salts to form HCl (g). The Cl volatilization rate in the presence of water vapor was higher than in the blank sample by 30%, supporting the idea that H2O (g) participates in HCl (g) formation and contributes the H source. It is proposed that H+ ions resulting from the dissociation of H2O (g) into OH− combine with Cl− to form HCl (g). This reaction is expressed in Equation (8). Moreover, the presence of water molecules as a solvent can alter the lattice energy of Cl salts, facilitating the migration of Cl and further promoting the formation of HCl (g).
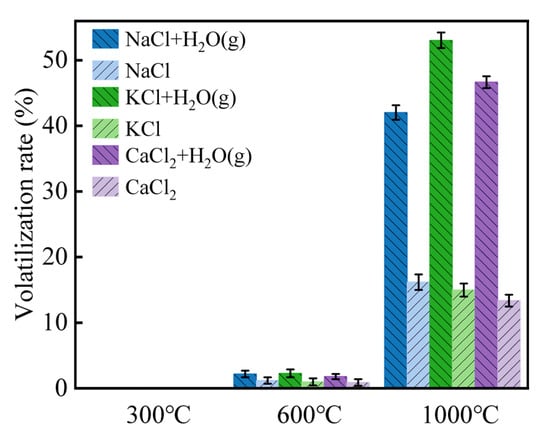
Figure 8.
Volatilization rates of Cl salts in the presence of water vapor at 300–1000 °C.
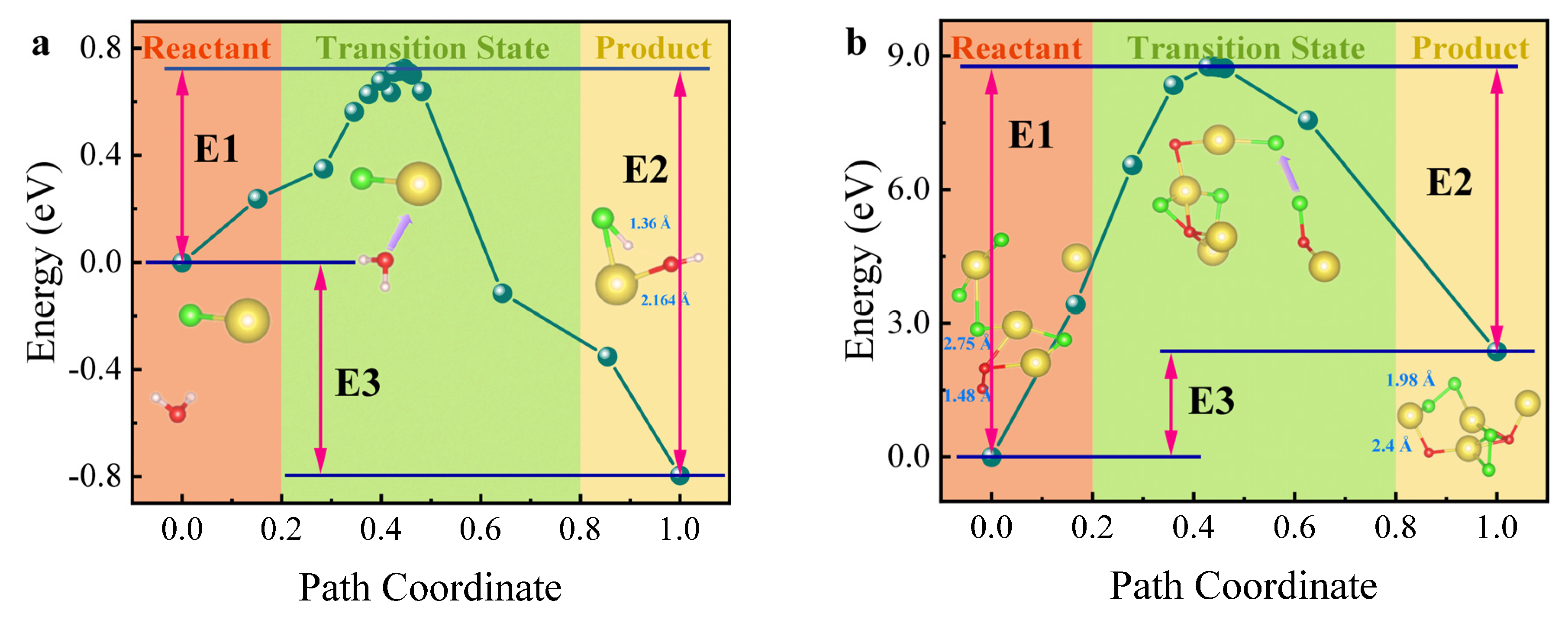
To confirm the formation pathway of HCl (g), the reaction barriers for HCl (g) (Equation (8)) and Cl2 (g) (Equation (9)) formation from the interaction of NaCl with H2O and O2 were calculated using DFT, and the results are shown in Figure 9. The energy difference between the transition products and reactants is referred to as activation energy (Ea), which represents the energy barrier for the reaction, denoted as E1. The reaction heat (ΔH) is key for assessing reaction spontaneity, determined from the values of E3 or the difference between E1 and E2. It is evident that the activation energy for the reaction between NaCl and O2 (Equation (9)) is significantly higher than for NaCl and H2O (Equation (8)). A higher activation energy typically results in a slower reaction rate, as a greater energy barrier must be overcome for the transformation of reactants into products. In Figure 9a, where E3 < 0 and E1−E2 < 0, indicating that ΔH is negative, the reaction is exothermic and spontaneous. Conversely, in Figure 9b, where E3 > 0 and E1−E2 > 0, resulting in a positive ΔH, the reaction is endothermic—requiring heat input—and is non-spontaneous. From both kinetic and thermodynamic perspectives, the presence of H2O (g) reduces the activation energy of the Cl salt reaction, thereby increasing the reaction rate and converting the process from endothermic to spontaneous and exothermic. This supports the formation pathway of HCl (g) as outlined in Equation (8).
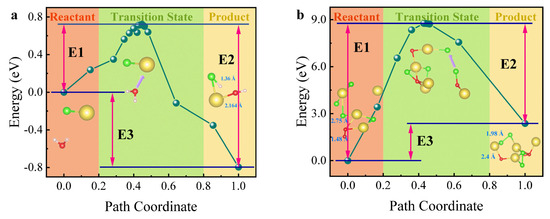
Figure 9.
DFT calculation results using NaCl as an example. (a) Reaction energy barrier diagram for NaCl + H2O; (b) reaction energy barrier diagram for NaCl + O2.
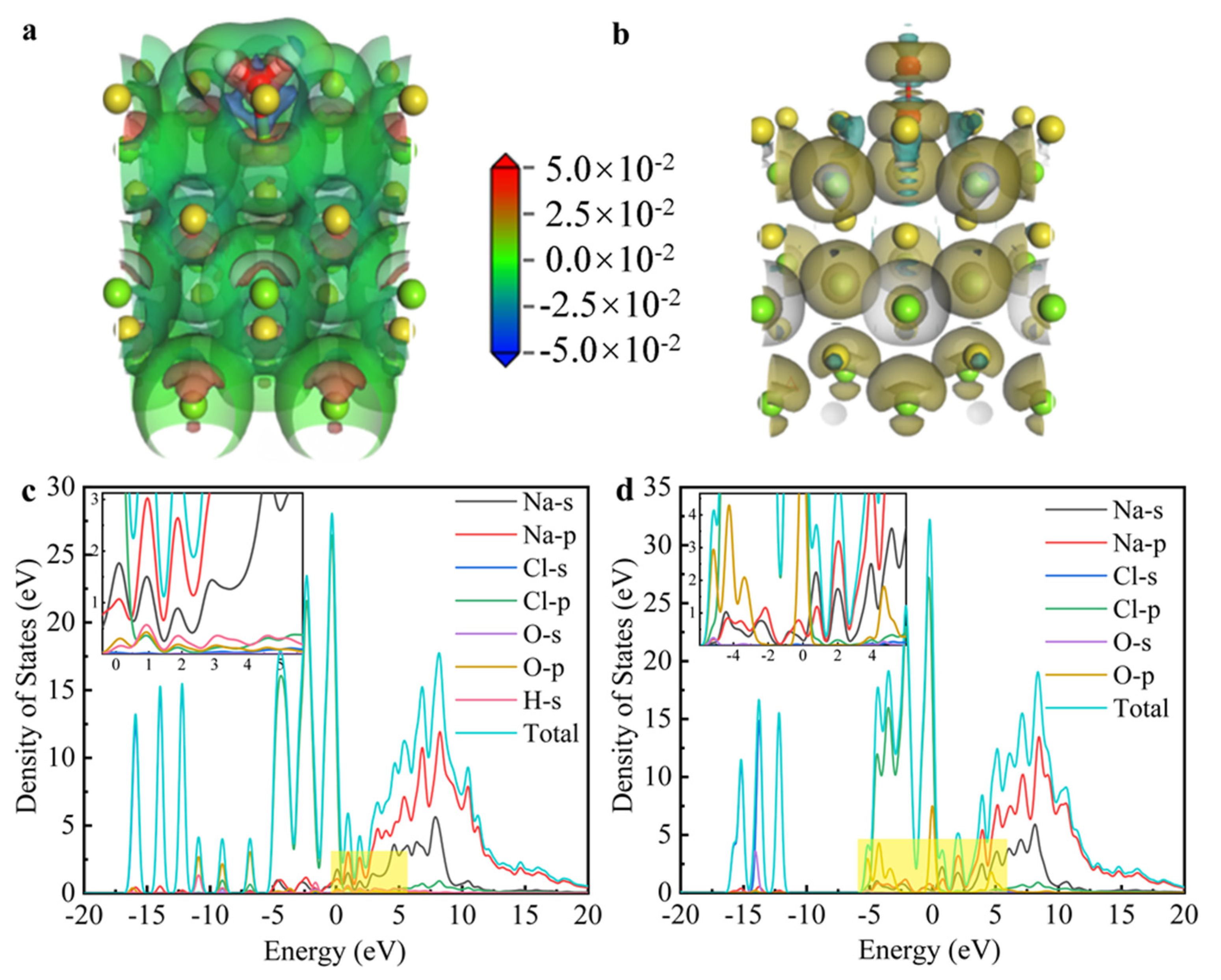
The charge density and density of states (DOS) differences between NaCl and the adsorption structures of H2O and O2 were calculated using DFT simulations, as shown in Figure 10. Figure 10a illustrates significant charge transfer between H and Cl in H2O following molecular dissociation. Specifically, the green-to-red regions in the charge density map indicate electron depletion near the H atom (blue/green) and accumulation near the adjacent Cl atom (red), confirming a directional charge transfer from H to Cl. This charge redistribution is characteristic of H–Cl bond formation, facilitated by the dissociation of H2O upon adsorption on the NaCl surface. In contrast, the charge transfer between NaCl crystals and O2 molecules, as shown in Figure 10b, was minimal, with almost symmetric and neutral charge distribution, suggesting a weak interaction. Figure 10c shows hybridization between Cl-p and H-s within the energy range of 0.5 to 1.5 eV, suggesting the formation of a chemical bond between H and Cl. On the other hand, in Figure 10d, Na-p and O-p hybridize between −3 and 1 eV, while Cl-s and Cl-p do not exhibit hybridization, indicating that the formation of Cl2 (g) is unfavorable. This observation is consistent with the reaction energy barrier presented in Figure 9. Overall, the differential charge and DOS results indicate that NaCl is unlikely to directly react with air to form Cl2 (g). In contrast, the presence of H from H2O facilitates the attraction of Cl, leading to the formation of HCl (g).
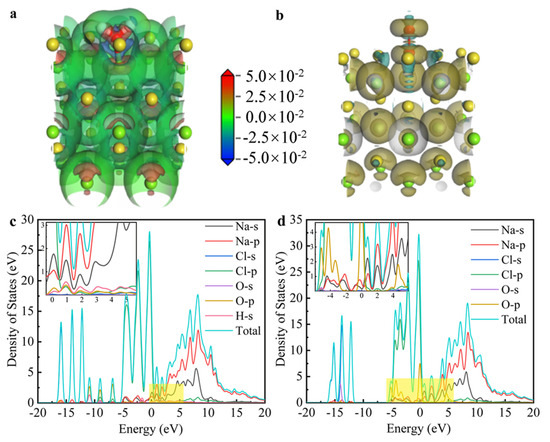
Figure 10.
The calculated charge density difference in (a) NaCl + H2O system and (b) NaCl + O2 system; the calculated state density in (c) NaCl + H2O system and (d) NaCl + O2 system.
The DFT calculations reveal that the reaction between NaCl and H2O proceeds with a substantially lower activation energy and is both exothermic and thermodynamically spontaneous, whereas the reaction between NaCl and O2 requires a higher activation energy and is endothermic and non-spontaneous. Complementary charge density and density of states analyses indicate that water vapor significantly facilitates H–Cl bond formation on the NaCl surface, thereby promoting HCl generation. In contrast, the interaction between NaCl and O2 is weak, disfavoring Cl2 formation. Therefore, the hydrogen in HCl originates from water vapor, which is consistent with and supported by the aforementioned results.
3.5. Reaction Behavior of Cl Salts
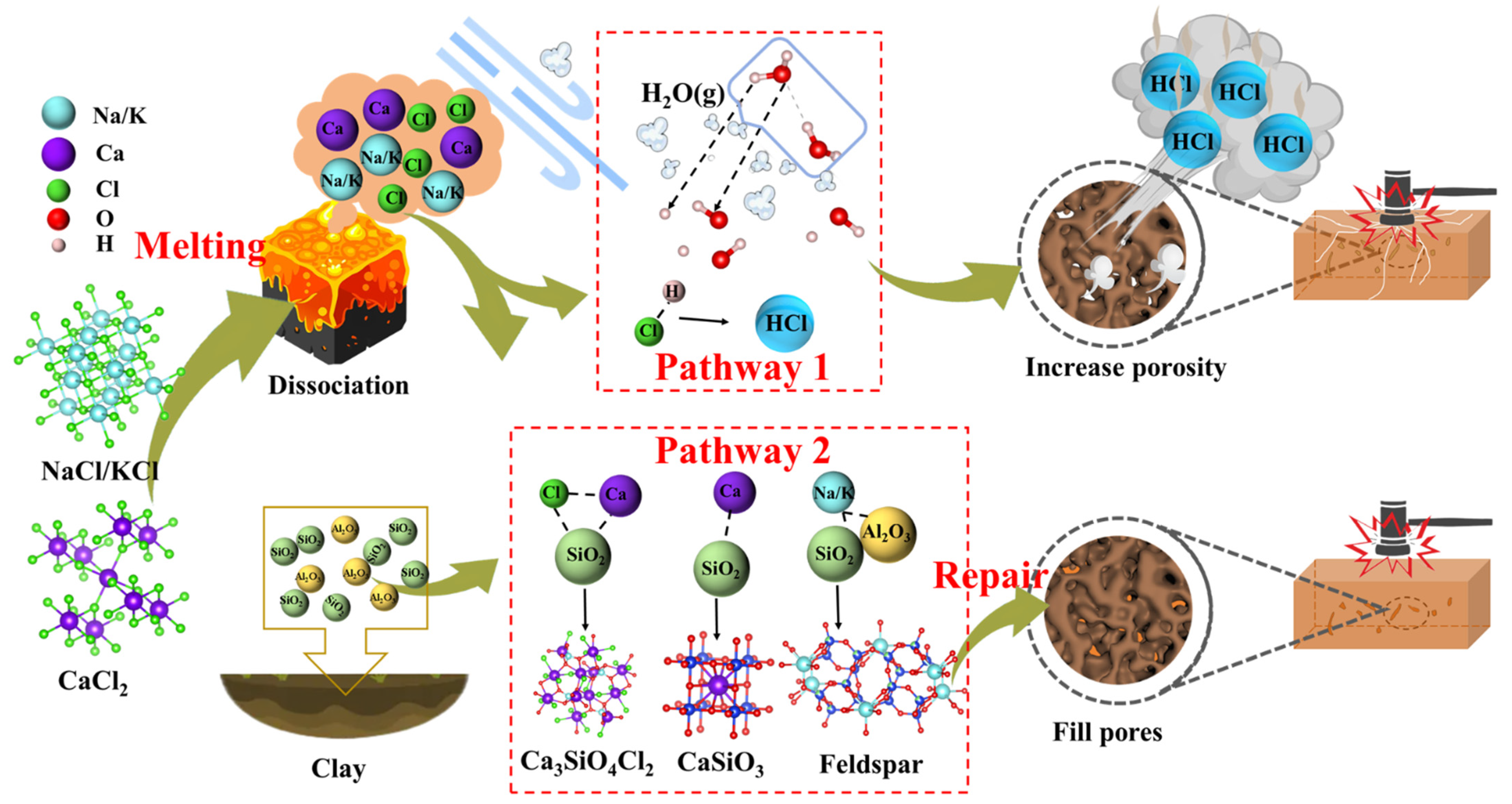
Based on the results above, the reaction behaviors of Cl salts (Figure 11) involve two main pathways: the formation and evaporation of HCl (g) (Path 1) and the combination of cations with aluminosilicates (Path 2). Below 800 °C, Cl salts remain stable, and their interaction and evaporation behaviors are minimal. However, at temperatures above 800 °C, significant reactions occur between cations (Na+, K+, Ca2+) and aluminosilicates, leading to the formation of Na/K feldspars, CaSiO3, and CaAl2Si2O8. These reactions are promoted as the temperatures exceed the melting points of NaCl (801 °C), KCl (770 °C), and CaCl2 (772 °C), causing the release of free cations. The formation of Na/K feldspars, CaSiO3, and CaAl2Si2O8 generally helps reduce porosity and improve the physical and mechanical properties of the bricks. However, Cl− ions combine with H+ from water vapor to form HCl (g) rather than Cl2 (g) due to the lower reaction energy barrier. Additionally, some Cl salts react to form Cl-containing minerals, such as Ca3Al2(SiO4)2Cl4 and Ca3SiO4Cl2, with silica-aluminum minerals. The overall reaction formulas for Cl salts interacting with clay components are represented by Equations (10)–(13). The Gibbs free energies for these reactions, shown in Figure S8, become negative above 400 °C, indicating that higher temperatures favor the occurrence of these reactions. The formation of HCl (g) generates pores in the brick matrix, increases the pore volume, and disrupts the homogeneous structure, thereby reducing the properties of the final product.
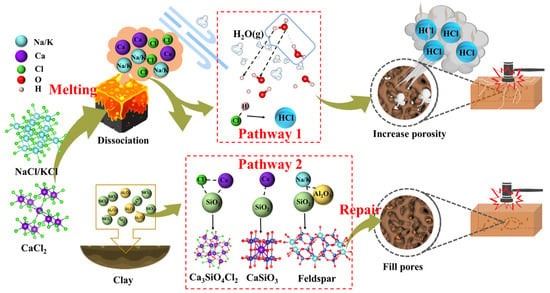
Figure 11.
Reaction mechanism diagram of Cl salts while introducing solid waste in the production of sintered brick.
During the combustion of medical waste [48] or dyeing sludge [49], HCl (g) typically forms between 400 and 700 °C, which is lower than the temperatures observed in this study. The formation of HCl (g) is thermodynamically more favorable than that of Cl2 (g) at temperatures below 1000 °C. In organic wastes containing Cl, the formation of HCl (g) is primarily driven by the collapse of component structures and the release of Cl− ions. These released Cl− ions can then react with H from water or organic substances to form HCl (g). In contrast, this study highlights that the liquefaction and dissociation of Cl salts are critical to HCl (g) formation, which explains why HCl (g) forms predominantly at temperatures above 800 °C. Furthermore, the presence of an H donor is essential for HCl (g) formation. Organic substances typically decompose completely below 800 °C, thus not contributing H to the formation of HCl (g). However, water vapor in the atmosphere plays a crucial role by supplying the necessary H element for HCl (g) formation. Therefore, during tunnel kiln co-processing of Cl-containing solid waste, a possible approach to suppress HCl (g) formation is to regulate the kiln temperature below 600 °C for an extended period to fully decompose organic substances and evaporate water, followed by a gradual increase to 800–1000 °C to complete the sintering process. This two-stage temperature control strategy shows potential for reducing HCl emissions without compromising product quality. From a practical standpoint, this approach is compatible with the multi-zone heating structure of tunnel kilns, which already feature preheating and sintering zones. As such, the proposed strategy may be implemented with minimal modifications to existing infrastructure. In terms of cost and scalability, this method avoids the need for additional additives or equipment and leverages existing process controls, making it a relatively low-cost and scalable solution. Nonetheless, further techno-economic assessment and pilot-scale validation are necessary to confirm its feasibility and industrial applicability.
4. Conclusions
In this study, the chemical form distribution and reaction mechanisms of Cl salts during the production of sintered bricks were systematically investigated. The key findings are summarized as follows:
(1) The incorporation of Cl salts significantly alters the pore structure of the bricks, leading to a deterioration in their physical and mechanical properties. Specifically, water absorption increases by 80–100%, while compressive strength decreases by 70–80%.
(2) The volatilization rates of Cl salts during sintering remain below 20%, with HCl (g) identified as the predominant Cl-containing gaseous species. The volatilization rates of Na+, K+, and Ca2+ do not exceed 1.5%, with Ca2+ exhibiting the lowest volatilization tendency. This is attributed to its strong affinity for silica-aluminum components in the clay, facilitating the formation of feldspar and silicate minerals.
(3) The melting and dissociation of Cl salts at temperatures exceeding 800 °C are essential for the formation of HCl (g). Additionally, the presence of H2O (g) significantly enhances the formation and volatilization of HCl (g), increasing Cl volatilization by more than 30%. The complete reaction pathways of Cl salts in the brick production process are described in Equations (10)–(13).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/constrmater5020034/s1, Figure S1: Properties of clay. (a) Particle size distribution and (b) XRD; Figure S2: Schematic diagram for the evaporation of Cl salts with the introduction of water vapor; Figure S3: XRD patterns of sintered bricks incorporated 10 wt% of Cl salts: (a) NaCl, (b) KCl, and (c) CaCl2; Figure S4: SEM photos of sintered bricks with different types of chloride salts incorporated; Figure S5: Specific surface area of sintered bricks doped with different types of Cl salts; Figure S6: Evolution profiles of O2, H2O (g), and CO2 released during the heating of clay mixed with (a) NaCl, (b) KCl, and (c) CaCl2; Figure S7: Volatilization rates of Cl salts at 300, 600, and 1000 °C with the introduction of glucose and sucrose ester; Figure S8: Calculated Gibbs free energies for Equations (10)–(13) at temperatures of 0–1200 °C.
Author Contributions
Writing—original draft preparation Z.L. (Zhu Liu); resources, supervision, project administration, and funding acquisition, J.W. and Z.L. (Zhongquan Liu); writing—review and editing, validation, and visualization, Y.L. and L.M.; conceptualization and methodology, S.W. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guizhou Provincial Energy Bureau science and technology project (Project number: 2023-68; Project name: Technical Research and Development of Coal Gangue Underground Backfill Mining Technology and Its Pilot Application by Guizhou Zhaping Coal Industry Co., Ltd.).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Caneda-Martínez, L.; Frías, M.; Medina, C.; de Rojas, M.I.S.; Rebolledo, N.; Sánchez, J. Evaluation of chloride transport in blended cement mortars containing coal mining waste. Constr. Build. Mater. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Prasad, B.; Maiti, D.; Kumar, A. Ground water quality evaluation in the lean period of a mining township. Appl. Water Sci. 2017, 7, 3553–3560. [Google Scholar] [CrossRef]
- Duan, L.; Yun, Q.; Jiang, G.; Teng, D.; Zhou, G.; Cao, Y. A review of chloride ions removal from high chloride industrial wastewater: Sources, hazards, and mechanisms. J. Environ. Manag. 2024, 353, 120184. [Google Scholar] [CrossRef] [PubMed]
- Kamar, M.T.; Hoda, E.; Mahmoud, A.S.; Peters, R.W.; Mostafa, M.K. A critical review of state-of-the-art technologies for electroplating wastewater treatment. Int. J. Environ. Anal. Chem. 2024, 104, 4143–4176. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Shu, Y.; Song, H.; Shu, X.; Ma, Y.; Hao, L.; Zhang, X.; Ren, X.; Wang, Z.; et al. Composition and Leaching Toxicity of Hydrochloric Acid Pickling Sludge Generated from the Hot-Dip Galvanized Steel Industry. ACS Omega 2022, 7, 13826–13840. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, Y.; Tian, C.; Zhang, W.; Zhang, T.; Shao, Y.; Ma, J. Preparation of municipal solid waste incineration fly ash/granite sawing mud ceramsite and the morphological transformation and migration properties of chlorine. Waste Manag. 2024, 173, 1–9. [Google Scholar] [CrossRef]
- Li, J.-S.; Xue, Q.; Wang, P.; Wang, H.-Q.; Zhang, T.-T. Leaching characteristics of chlorine from municipal solid waste incineration fly ash by up-flow percolation column tests. Environ. Earth Sci. 2015, 75, 31. [Google Scholar] [CrossRef]
- Huang, J.; Jin, Y.; Chu, X.; Shu, Z.; Ma, X.; Liu, J. Recovery of lead and chlorine via thermal co-treatment of municipal solid waste incineration fly ash and lead-rich waste cathode-ray tubes: Analysis of chlorination volatilization mechanism. J. Hazard. Mater. 2024, 462, 132752. [Google Scholar] [CrossRef]
- Long, Y.; Hu, Y.; Liu, D.; Shen, D.; Gu, F. Source analysis of chlorine in municipal solid waste under waste classification: A case study of Hangzhou, China. Environ. Sci. Pollut. Res. 2024, 31, 31054–31063. [Google Scholar] [CrossRef]
- Song, J.; Liu, M.; Sun, X. Model analysis and experimental study on scaling and corrosion tendencies of aerated geothermal water. Geothermics 2020, 85, 101766. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, V. A review of the management and treatment of brine solutions. Environ. Sci. Water Res. Technol. 2017, 3, 625–658. [Google Scholar] [CrossRef]
- Figler, A.; B-Béres, V.; Dobronoki, D.; Márton, K.; Nagy, S.A.; Bácsi, I. Salt Tolerance and Desalination Abilities of Nine Common Green Microalgae Isolates. Water 2019, 11, 2527. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World J. Microbiol. Biotechnol. 2016, 32, 135. [Google Scholar] [CrossRef]
- Zhou, Y.; Xingbin, F.; Ruzhuang, Z.; Songbo, Q.; Jicheng, W.; Lu, J. Salt-tolerant microorganisms treating hypersaline organic wastewater and the microbial population dynamics. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2854–2859. [Google Scholar] [CrossRef]
- Shu, Y.-H.; Zhang, J.-S.; Fang, A.-N.; Wu, Y.-Q.; Qin, R.-H.; Miao, P. Effect of coupled chloride salt erosion and Freeze-thaw on the dynamic mechanical properties of EPS cement soils. Constr. Build. Mater. 2023, 375, 130951. [Google Scholar] [CrossRef]
- Yang, G.; Na, B.; Jin, W.; Zhi, F.; Zhang, J.; Zhang, L.; Jiang, L. Effect of chloride salt types on corrosion resistance of reinforcing steel in cement mortar mixed with DNA primer inhibitor. Cem. Concr. Compos. 2024, 148, 105454. [Google Scholar] [CrossRef]
- Zuo, Y. Effect of chloride salt on the phase evolution in alkali-activated slag cements through thermodynamic modelling. Appl. Geochem. 2022, 136, 105169. [Google Scholar] [CrossRef]
- Long, H.; Liao, Y.; Cui, C.; Liu, M.; Liu, Z.; Li, L.; Hu, W.; Yan, D. Assessment of popular techniques for co-processing municipal solid waste in Chinese cement kilns. Front. Environ. Sci. Eng. 2021, 16, 51. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Bourtsalas, A.C.; Themelis, N.J.; Chi, Y.; Yan, J. Review on fate of chlorine during thermal processing of solid wastes. J. Environ. Sci. 2019, 78, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhan, J.; Zheng, M.; Li, L.; Li, C.; Jiang, X.; Wang, M.; Zhao, Y.; Jin, R. Field pilot study on emissions, formations and distributions of PCDD/Fs from cement kiln co-processing fly ash from municipal solid waste incinerations. J. Hazard. Mater. 2015, 299, 471–478. [Google Scholar] [CrossRef]
- Chang, H.; Wang, X.; Wang, Y.; Li, C.; Guo, Z.; Fan, S.; Zhang, H.; Feng, P. Chloride binding behavior of cement paste influenced by metakaolin dosage and chloride concentration. Cem. Concr. Compos. 2023, 135, 104821. [Google Scholar] [CrossRef]
- Long, W.; Yu, Y.; He, C.; Li, X.; Xiong, C.; Feng, G. Review on Chloride Ion Ingress Resistance and Chloride Binding Performance of Nano-reinforced Cement-based Composites. Mater. Rep. 2024, 38, 22090138-10. [Google Scholar] [CrossRef]
- Bilaç, O.; Topateş, G.; Duran, C. Production and characterization of glass/cordierite/hBN composites for low temperature co-fired ceramic applications. Ceram. Int. 2025, 51, 12381–12386. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, S.; Wang, Z.; Li, Y.; Rao, Z. Crystal structure, Raman spectra and microwave dielectric properties of a novel low-fired garnet-type ceramic NaCa2Zn2V3O12. Ceram. Int. 2025; advance online publication. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, C.; Lu, Y.; Li, J.; Zhu, D. Preparation and properties of low-temperature self-expanding foamed ceramics utilizing lepidolite mica ore and ceramic tile polishing waste. Case Stud. Therm. Eng. 2024, 58, 104455. [Google Scholar] [CrossRef]
- Allaoui, D.; Nadi, M.; Hattani, F.; Majdoubi, H.; Haddaji, Y.; Mansouri, S.; Oumam, M.; Hannache, H.; Manoun, B. Eco-friendly geopolymer concrete based on metakaolin and ceramics sanitaryware wastes. Ceram. Int. 2022, 48, 34793–34802. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Cui, C.; Long, H.; Huang, X.; Liu, M.; Li, L.; Xu, S.; Wang, M.; Yan, D. Heavy metal and metalloid emissions during co-processing of waste in a sintering kiln: Migration characteristics in the kiln and long-term leaching from bricks. J. Environ. Manag. 2022, 322, 116145. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, B.; Wei, J.; Zhang, T.; Yu, Q.; Zhang, W. Effects of chlorine on the volatilization of heavy metals during the co-combustion of sewage sludge. Waste Manag. 2017, 62, 204–210. [Google Scholar] [CrossRef]
- Xu, S.; Liu, T.; Yang, Y.; Yang, Z.; Huang, Q. Influence of chlorine on co-processing of hazardous wastes in brick kilns. J. Environ. Manag. 2024, 354, 120464. [Google Scholar] [CrossRef]
- Zhang, B.; Bogush, A.; Wei, J.; Xu, W.; Zeng, Z.; Zhang, T.; Yu, Q.; Stegemann, J. Influence of Chlorine on the Fate of Pb and Cu during Clinkerization. Energy Fuels 2018, 32, 7718–7726. [Google Scholar] [CrossRef]
- Jing, M.; Zhao, P.; Chen, T.; Li, J. Synergistic effect of polyvinyl chloride and coal ash on thermal separation of heavy metals from MSWI fly ash through molten salt process. Waste Manag. Res. 2022, 40, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zha, F.; Xu, L.; Yang, C.; Chu, C.; Tan, X. Effect of chloride attack on strength and leaching properties of solidified/stabilized heavy metal contaminated soils. Eng. Geol. 2018, 246, 28–35. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Cui, H.; Zhang, W.; Hu, L.; Mao, L. Effects of electric arc furnace slag on promoting quality and environmental safety of fired bricks incorporating municipal solid waste incineration fly ash. Constr. Build. Mater. 2022, 345, 128327. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, H.; Zhang, W.; Peng, M.; Liu, Y.; Hu, L.; Gao, B.; Mao, L. The introduction of wet dyeing sludge pellets in the production of clay brick: A novel approach to promote the disposal efficiency. J. Clean. Prod. 2023, 385, 135675. [Google Scholar] [CrossRef]
- Pašti, I.A.; Gavrilov, N.M.; Mentus, S.V. DFT study of chlorine adsorption on bimetallic surfaces—Case study of Pd3M and Pt3M alloy surfaces. Electrochim. Acta 2014, 130, 453–463. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Gao, Y.; Liu, R.; Wang, D.; Wang, B.; You, Y. Simultaneous realization of heavy metal Cd solidification and chloride separation in municipal solid waste incineration fly ash: Mechanism and DFT analysis. Chemosphere 2024, 348, 140741. [Google Scholar] [CrossRef]
- Todorović, J.; Janssen, H. The impact of salt pore clogging on the hygric properties of bricks. Constr. Build. Mater. 2018, 164, 850–863. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Y.; Hu, L.; Zhang, W.; Mao, L. Evaluating physical-mechanical properties and long periods environmental risk of fired clay bricks incorporated with electroplating sludge. Constr. Build. Mater. 2019, 227, 116716. [Google Scholar] [CrossRef]
- Umama, M.A.; Zahid, C.Z.B.; Sarder, N.; Joy, J.A.; Ifty, I.I. Temperature and humidity effects on salt crystallization in burnt clay bricks. Results Mater. 2024, 21, 100541. [Google Scholar] [CrossRef]
- Niu, L.; Qian, J.; Zheng, S.; Guan, J.; Gao, S. Experimental investigation on the seismic resistance of brick walls with constructional columns after chloride salt treatment: A further research. Structures 2025, 72, 108248. [Google Scholar] [CrossRef]
- Breiter, K.; Tvrdy, J.; Jedlička, P. Petrological diversity of leucocratic rocks at the sodium-potassium feldspar deposit Krásno—Vysoký kámen. Geosci. Res. Rep. 2023, 56, 14–20. [Google Scholar] [CrossRef]
- Duan, G.; Ram, R.; Xing, Y.; Etschmann, B.; Brugger, J. Kinetically driven successive sodic and potassic alteration of feldspar. Nat. Commun. 2021, 12, 4435. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Kuang, J.; Yang, Y.; Huang, Z.; Yang, D.; Yu, M. Synergistic strengthening mechanism of Ca2+-sodium silicate to selective separation of feldspar and quartz. Int. J. Min. Met. Mater. 2024, 31, 1985–1995. [Google Scholar] [CrossRef]
- Vidak Vasić, M. Techno-economic analysis in the service of traditional ceramic industry. The first review. Struct. Integr. Life 2024, 24, 199–205. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, Y.; Xie, M.; Xiao, Y.; Chen, B.; Wang, Y.; Zhou, T. Novel insight of the thermal degradation kinetics, environmental impact and transformation behavior of heavy metals and chlorine of municipal solid waste incineration fly ash in nitrogen. Fuel 2023, 353, 129275. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Hu, H.; Guo, G.; Gong, L.; Dong, L.; Xu, S.; Yao, H. Fate of sulfur and chlorine during co-incineration of municipal solid waste and industrial organic solid waste. Sci. Total Environ. 2024, 920, 171040. [Google Scholar] [CrossRef]
- Martinez, J.; Wardini, J.L.; Zheng, X.; Moghimi, L.; Rakowsky, J.; Means, J.; Guo, H.; Kuzmenko, I.; Ilavsky, J.; Zhang, F.; et al. Precision Calcination Mechanism of CaCO3 to High-Porosity Nanoscale CaO CO2 Sorbent Revealed by Direct In Situ Observations. Adv. Mater. Interfaces 2024, 11, 2300811. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, W.; Jiang, X.; Yan, J.; Chi, Y. Study on HCl removal for medical waste pyrolysis and combustion using a TG-FTIR analyzer. Front. Environ. Sci. Eng. 2015, 9, 230–239. [Google Scholar] [CrossRef]
- Shi, X.; Li, J.; Shang, L.; Wang, S.; Chen, S.; Liu, J.; Mei, M.; Xue, Y.; Wang, T. Microplastics in dyeing sludge: Whether do they affect sludge incineration? J. Hazard. Mater. 2022, 437, 129394. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).