Optimization Using Central Composite Design of the Response Surface Methodology for the Compressive Strength of Alkali-Activated Material from Rice Husk Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Central Composite Design
2.3. Mixture Proportions and Specimen Preparation
2.4. Testing Methods
2.4.1. Compressive Strength
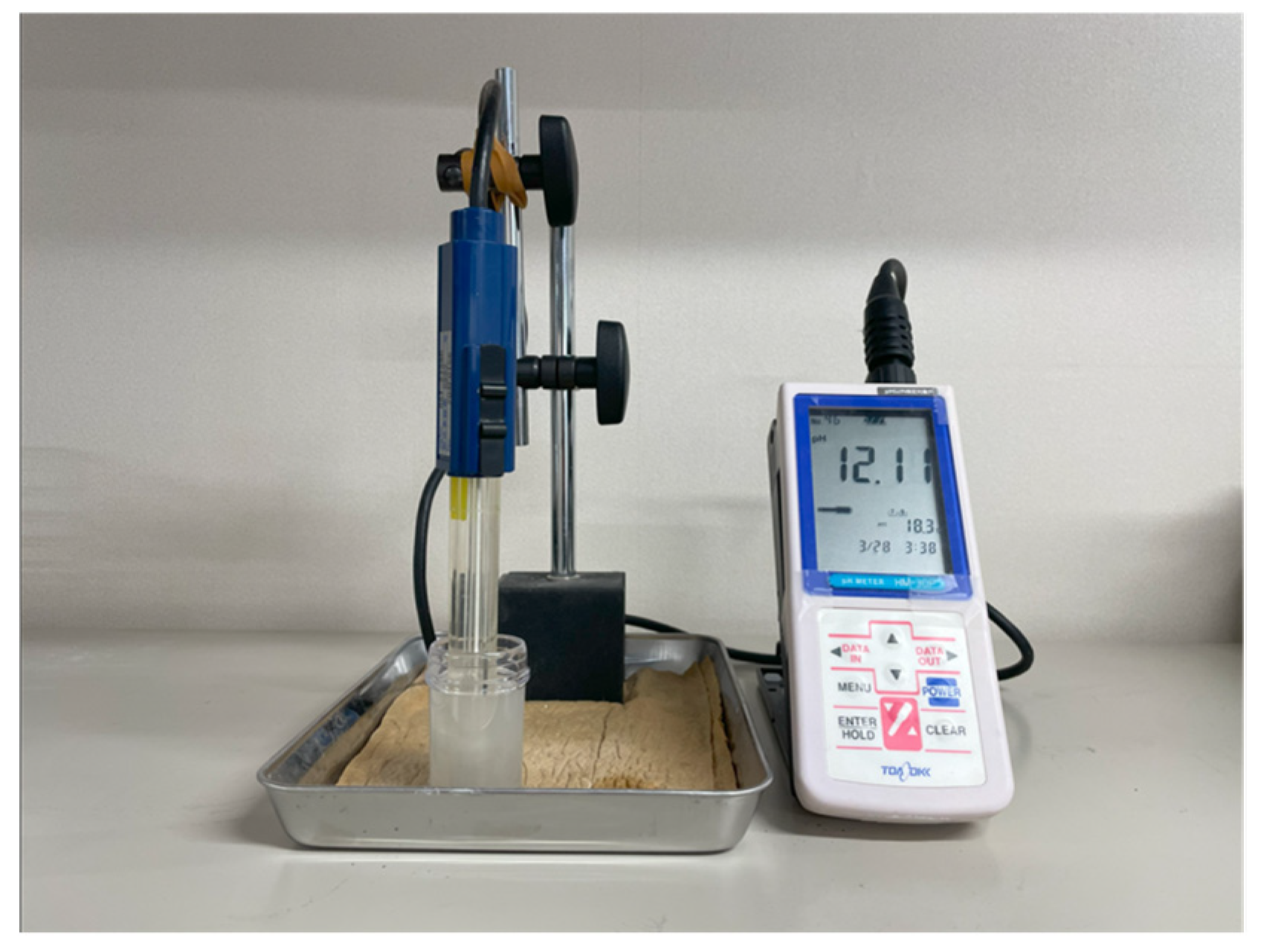
2.4.2. pH Value
2.4.3. Porosity
2.4.4. Ignition Loss
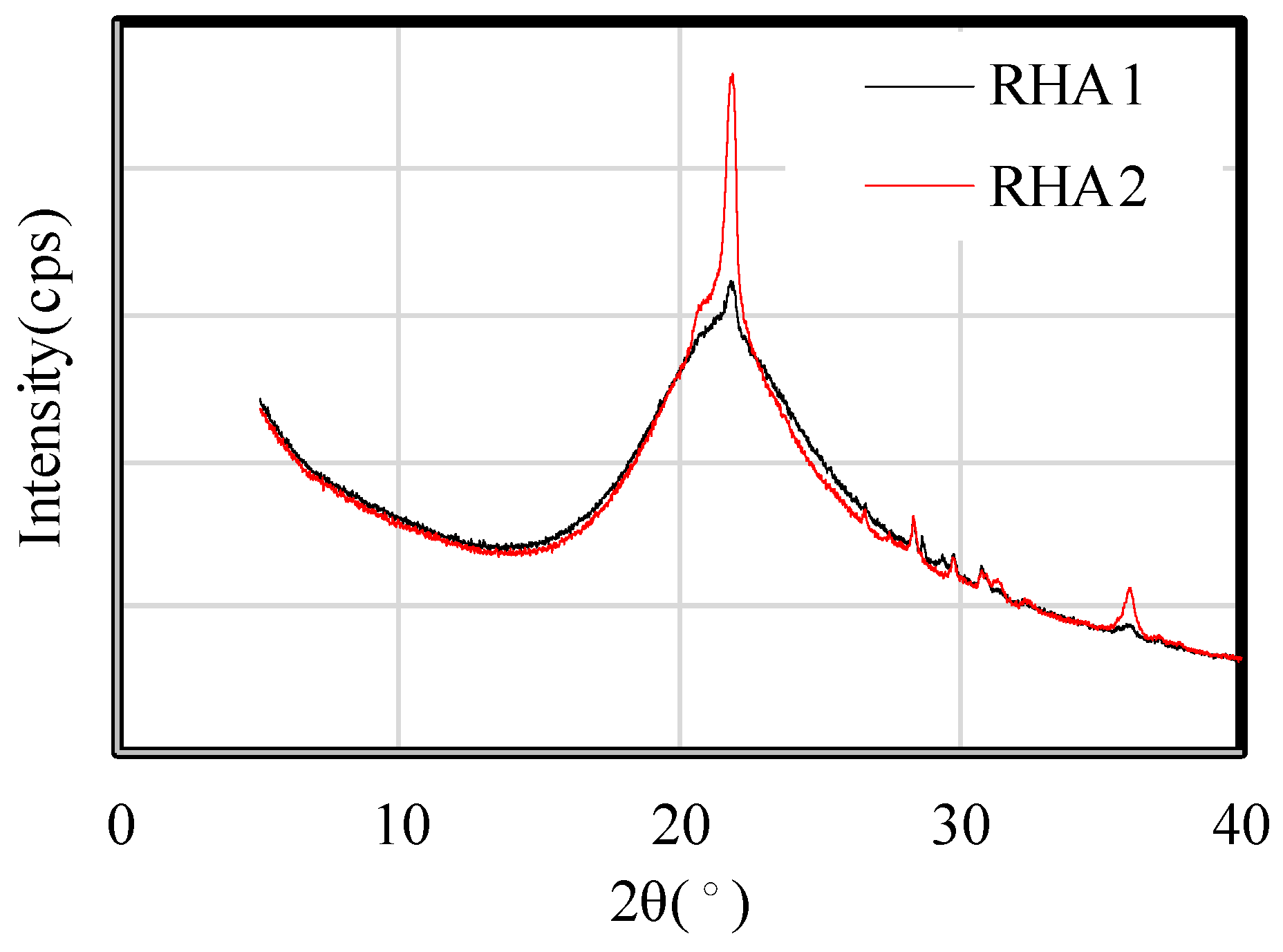
2.4.5. X-Ray Diffraction (XRD) Analysis
3. Results and Discussion
3.1. Compressive Strength Results
− 68.5X2 + 3.11X3 + 0.000118X12 + 0.370X22 + 0.0521X32
− 0.00225X1×X2 − 0.001167X1X3 − 0.0350X2X3
where X1 is the particle size (µm), X2 is the silica content (%), and X3 is the replacement ratio of RHA (%).
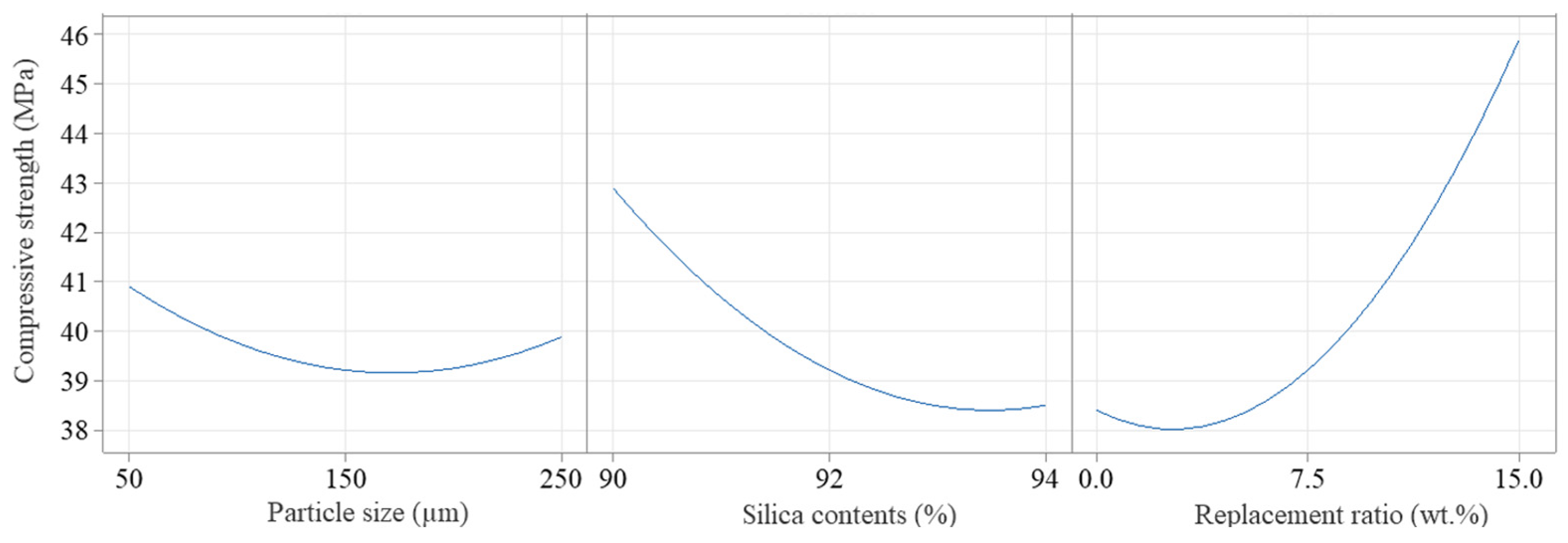
3.2. Main Effects
3.3. Interaction Effects
3.4. Optimization and Verification of Compressive Strength
3.5. Discussion on the Enhancement of the Compressive Strength of AAC with RHA
4. Conclusions
- It was found that the RHA replacement ratio in the mortar increased the compressive strength, indicating that the compressive strength is dependent on the ratio of RHA to BFS rather than the particle size and silica content. In addition, it was confirmed that the compressive strength decreased with an increase in the RHA silica content, which may be related to the presence of crystalline silica;
- Based on the analysis of variance results, the RHA replacement ratio resulted in a lower p-value in comparison with the particle size and silica content of RHA. The compressive strengths of the mortar samples were predicted using a regression equation. The optimum values for the replacement ratio, particle size, and silica content of RHA were suggested to be 15%, 50 µm, and 90%, respectively. Mortar samples were prepared based on the optimal particle size, silica content, and replacement ratio of RHA, and their compressive strengths were measured. Based on these results, the experimental and predicted values were confirmed to be approximately equal;
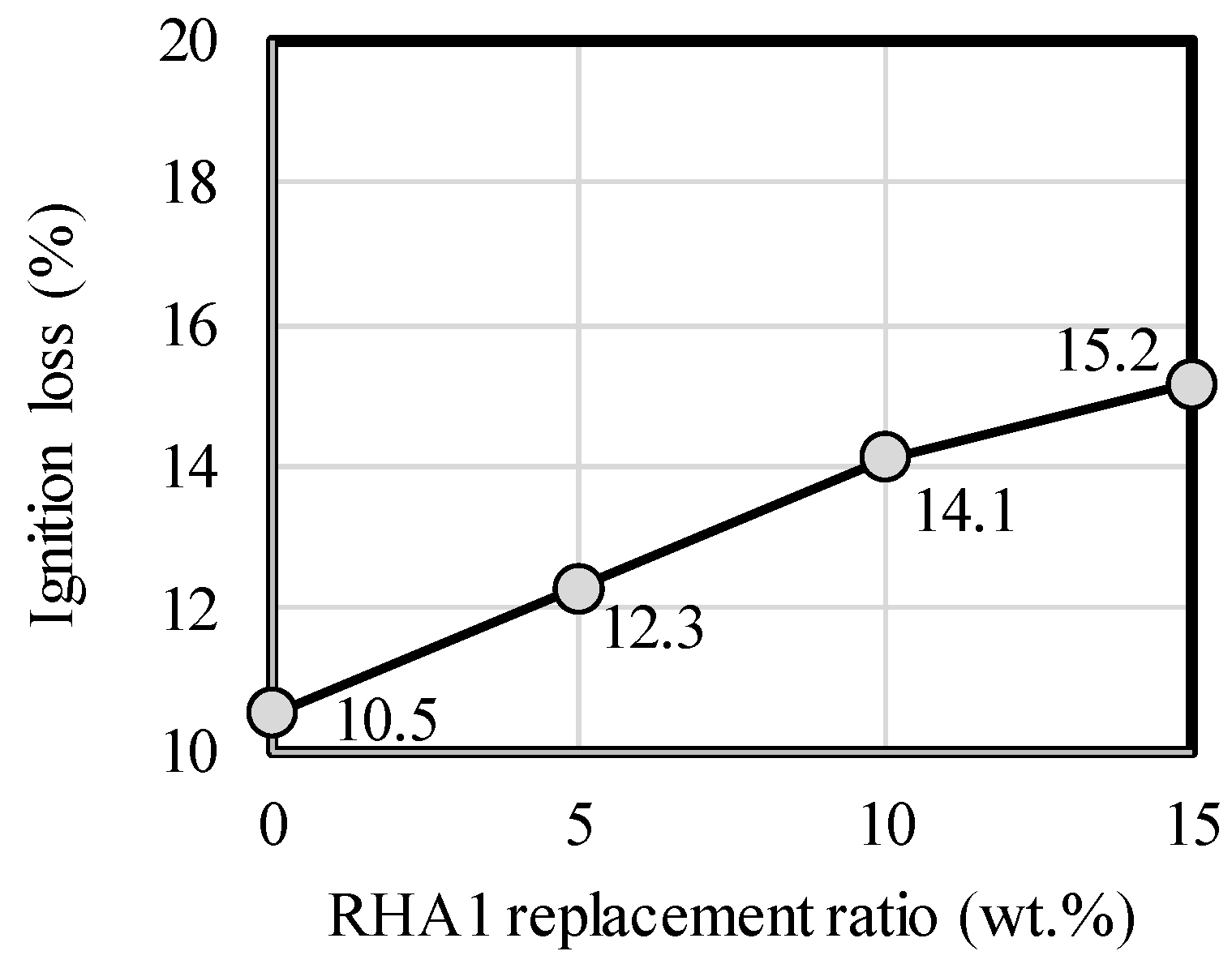
- From the results of the investigation of the microstructure and pore structure based on porosity, pH, ignition loss, and XRD analysis, it was confirmed that increasing the RHA replacement ratio in the paste sample decreased the porosity and pH and increased the ignition loss, which is related to the calcite and C-S-H in the paste.
5. Future Study
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef]
- Al-Zu, M.; Fan, M.; Al Rjoub, Y.S.; Ashteyat, A.; Al-Kheetan, M.J.; Anguilano, L. The effect of length and inclination of carbon fiber reinforced polymer laminates on shear capacity of near-surface mounted retrofitted reinforced concrete beams. Struct. Concr. 2021, 22, 3677–3691. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.Q.; Wang, Y.Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Xu, Z.K.; Yue, J.C.; Pang, G.H.; Li, R.; Zhang, P.; Xu, S. Influence of the Activator Concentration and Solid/Liquid Ratio on the Strength and Shrinkage Characteristics of Alkali-Activated Slag Geopolymer Pastes. Adv. Civ. Eng. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Kheradmand, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Shrinkage Performance of Fly Ash Alkali-activated Cement Based Binder Mortars. KSCE J. Civ. Eng. 2018, 22, 1854–1864. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Dawood Abdul Khadar, S. Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Na, S.; Zhang, W.; Ichikawa, Y.; Komatsu, M.; Takemura, A. Fundamental Study on Alkali-Activated Slag System with Sodium Carbonate or Calcium Hydroxide. J. Mater. Sci. Chem. Eng. 2024, 12, 55–70. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of combined activator of Ca(OH)2 and Na2CO3 on workability and compressive strength of alkali-activated ferronickel slag system. Cem. Concr. Compos. 2021, 123, 104179. [Google Scholar] [CrossRef]
- Glushankova, I.; Ketov, A.; Krasnovskikh, M.; Rudakova, L.; Vaisman, I. Rice Hulls as a Renewable Complex Material Resource. Resources 2018, 7, 31. [Google Scholar] [CrossRef]
- De Sensale, G.R. Strength development of concrete with rice-husk ash. Cem. Concr. Compos. 2006, 28, 158–160. [Google Scholar] [CrossRef]
- Öztaş, A.; Pala, M.; Özbay, E.; Kanca, E.; Caglar, N.; Bhatti, M.A. Predicting the compressive strength and slump of high strength concrete using neural network. Constr. Build. Mater. 2006, 20, 769–775. [Google Scholar] [CrossRef]
- Saraswathy, V.; Song, H.-W. Corrosion performance of rice husk ash blended concrete. Constr. Build. Mater. 2007, 21, 1779–1784. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2018, 115, 389–400. [Google Scholar] [CrossRef]
- Alex, J.; Dhanalakshmi, J.; Ambedkar, B. Experimental investigation on rice husk ash as cement replacement on concrete production. Constr. Build. Mater. 2016, 127, 353–362. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Mishra, U.; Biswal, S.K. Influence of RHA on strength and durability properties of alkali activated concrete. Mater. Today Proc. 2023, 3–8. [Google Scholar] [CrossRef]
- Huo, Y.; Huang, J.; Lu, D.; Han, X.; Sun, H.; Liu, T.; Wang, J.; Wang, F.; Tan, P.; Wang, M.; et al. Durability of alkali-activated slag concrete incorporating silica fume and rice husk ash. J. Build. Eng. 2023, 78, 107637. [Google Scholar] [CrossRef]
- Zhao, W.; Ji, C.; Sun, Q.; Gu, Q. Preparation and microstructure of alkali-activated rice husk ash-granulated blast furnace slag tailing composite cemented paste backfill. Materials 2022, 15, 4397. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Huyn, T.-P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Xu, W.; Lo, T.Y.; Wang, W.; Ouyang, D.; Wang, P.; Xing, F. Pozzolanic Reactivity of Silica Fume and Ground Rice Husk Ash as Reactive Silica in a Cementitious System: A Comparative Study. Materials 2016, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kumar, A.; Yvaz, A.; Sala, B. Central composite design application in the optimization of the effect of pumice stone on lightweight concrete properties using RSM. Case Stud. Constr. Mater. 2023, 18, e01958. [Google Scholar] [CrossRef]
- Sinkhonde, D.; Onchiri, R.O.; Oyawa, W.O.; Mwero, J.N. Response surface methodology-based optimisation of cost and compressive strength of rubberised concrete incorporating burnt clay brick powder. Heliyon 2021, 7, e08565. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Atarashi, D.; Itoh, T.; Osaki, M. Sodium carbonate and calcium nitrite in combination hydration reaction analysis of blast furnace slag. Cem. Sci. Concr. Technol. 2022, 76, 108–114. [Google Scholar] [CrossRef]
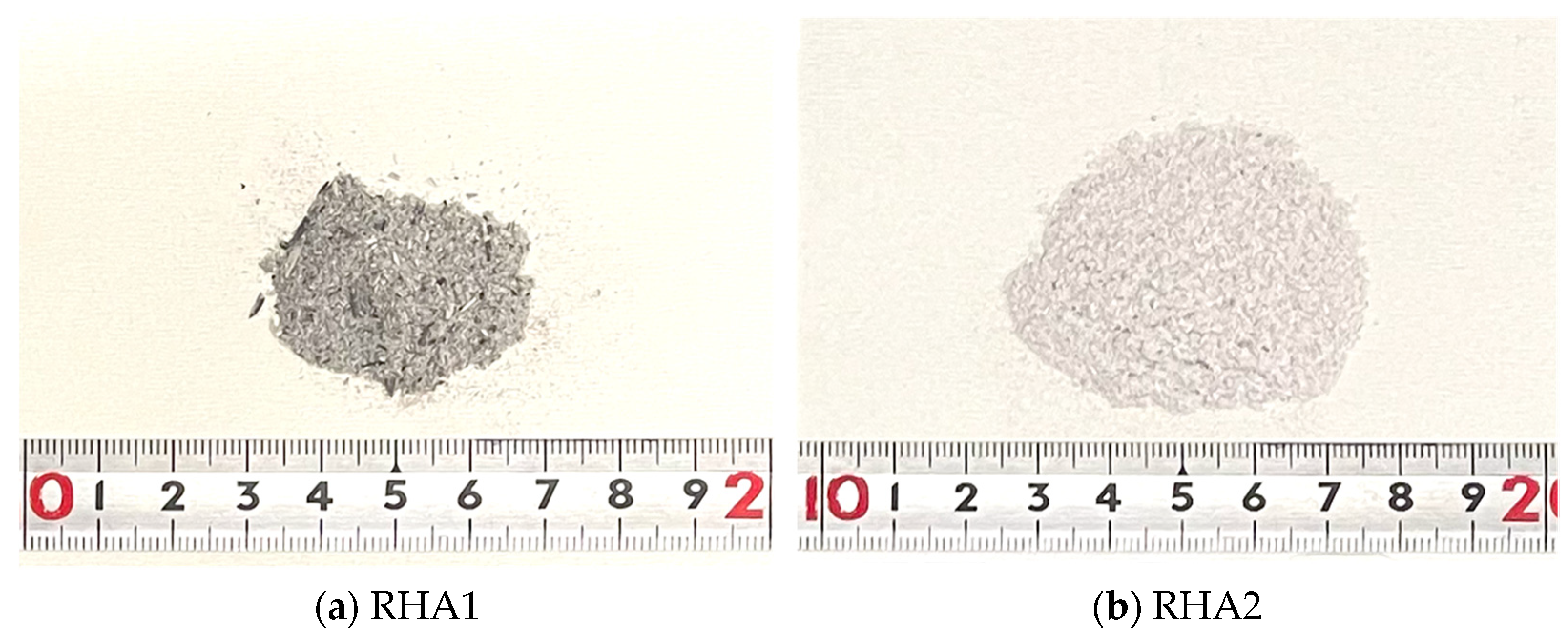









| Materials | SiO2 | Al2O3 | CaO | P2O3 | K2O | Fe2O3 | CO2 |
|---|---|---|---|---|---|---|---|
| RHA1 | 89.9 | 0.6 | 1.9 | 0.8 | 0.7 | 0.6 | 4.5 |
| RHA2 | 93.5 | 0.2 | 0.4 | 0.3 | 0.3 | 0.2 | 4.2 |
| Level of Factor | Particle Size (µm) | Silica Contents (%) | Replacement Ratio (%) |
|---|---|---|---|
| −1 | 50 | 90 (RHA1) | 0 |
| 0 | 150 | 92 (RHA1:RHA2 mixed) | 7.5 |
| 1 | 250 | 94 (RHA2) | 15.0 |
| Particle Size (µm) | Silica Contents (%) | Replacement Ratio (wt.%) |
|---|---|---|
| 50 | 90 | 0 |
| 250 | 94 | 0 |
| 250 | 90 | 15 |
| 50 | 94 | 15 |
| 150 | 92 | 7.5 |
| 250 | 90 | 0 |
| 50 | 94 | 0 |
| 50 | 90 | 15 |
| 250 | 94 | 15 |
| 50 | 92 | 7.5 |
| 250 | 92 | 7.5 |
| 150 | 90 | 7.5 |
| 150 | 94 | 7.5 |
| 150 | 92 | 0 |
| 150 | 92 | 15 |
| 150 | 92 | 7.5 |
| 150 | 92 | 7.5 |
| Factors | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|
| Particle Size (µm) | Silica Contents (%) | Replacement Ratio (%) | Measured Value | Predicted Value | Difference (Meas. − Pred.) |
| 50 | 90 | 0 | 42.5 | 46.9 | −4.4 |
| 250 | 94 | 0 | 38.9 | 44.6 | −5.7 |
| 250 | 90 | 15 | 49.4 | 55.2 | −5.8 |
| 50 | 94 | 15 | 47.3 | 52.9 | −5.6 |
| 150 | 92 | 7.5 | 37.7 | 44.3 | −6.6 |
| 250 | 90 | 0 | 42.9 | 48.5 | −5.6 |
| 50 | 94 | 0 | 39.4 | 44.8 | −5.4 |
| 50 | 90 | 15 | 51.6 | 57.2 | −5.6 |
| 250 | 94 | 15 | 42.4 | 49.2 | −6.8 |
| 50 | 92 | 7.5 | 40.1 | 46.0 | −5.9 |
| 250 | 92 | 7.5 | 42.2 | 45.0 | −2.8 |
| 150 | 90 | 7.5 | 43.2 | 47.8 | −4.6 |
| 150 | 94 | 7.5 | 39.7 | 43.8 | −4.1 |
| 150 | 92 | 0 | 37.7 | 43.5 | −5.8 |
| 150 | 92 | 15 | 48.1 | 51.0 | −2.9 |
| 150 | 92 | 7.5 | 42.4 | 44.3 | −1.9 |
| 150 | 92 | 7.5 | 41.5 | 44.3 | −2.8 |
| Source | Sum of | Degree of | Mean | F | p-Value |
|---|---|---|---|---|---|
| Squares | Freedom | Square | Value | Prob > F | |
| Model | 11 | 248.236 | 22.567 | 5.82 | 0.032 |
| Blocks | 2 | 5.352 | 2.676 | 0.69 | 0.544 |
| Linear | 3 | 190.438 | 63.479 | 16.36 | 0.005 |
| Particle size | 1 | 2.601 | 2.601 | 0.67 | 0.450 |
| Silica contents | 1 | 47.961 | 47.961 | 12.36 | 0.017 |
| Replacement ratio | 1 | 139.876 | 139.876 | 36.05 | 0.002 |
| Square | 3 | 33.687 | 11.229 | 2.89 | 0.141 |
| size × size | 1 | 3.102 | 3.102 | 0.8 | 0.412 |
| contents × contents | 1 | 4.882 | 4.882 | 1.26 | 0.313 |
| ratio × ratio | 1 | 19.142 | 19.142 | 4.93 | 0.077 |
| 2-Way Interaction | 3 | 9.95 | 3.317 | 0.85 | 0.521 |
| size × contents | 1 | 1.62 | 1.62 | 0.42 | 0.547 |
| size × ratio | 1 | 6.125 | 6.125 | 1.58 | 0.264 |
| Contents × ratio | 1 | 2.205 | 2.205 | 0.57 | 0.485 |
| Error | 5 | 19.403 | 3.881 | ||
| Lack-of-Fit | 4 | 18.998 | 4.749 | 11.73 | 0.215 |
| Pure Error | 1 | 0.405 | 0.405 | ||
| Total | 16 | 267.639 |
| Variable | Setting |
|---|---|
| Particle size (µm) | 50 |
| Silica contents (%) | 90 |
| Replacement ratio of RHA (wt.%) | 15 |
| Suggested value (MPa) | 51.7 |
| Measured value (MPa) | 49.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, S.; Zhang, W.; Kitagawa, M.; Hirooka, A.; Komatsu, M. Optimization Using Central Composite Design of the Response Surface Methodology for the Compressive Strength of Alkali-Activated Material from Rice Husk Ash. Constr. Mater. 2025, 5, 5. https://doi.org/10.3390/constrmater5010005
Na S, Zhang W, Kitagawa M, Hirooka A, Komatsu M. Optimization Using Central Composite Design of the Response Surface Methodology for the Compressive Strength of Alkali-Activated Material from Rice Husk Ash. Construction Materials. 2025; 5(1):5. https://doi.org/10.3390/constrmater5010005
Chicago/Turabian StyleNa, Seunghyun, Wenyang Zhang, Mai Kitagawa, Atsushi Hirooka, and Masaya Komatsu. 2025. "Optimization Using Central Composite Design of the Response Surface Methodology for the Compressive Strength of Alkali-Activated Material from Rice Husk Ash" Construction Materials 5, no. 1: 5. https://doi.org/10.3390/constrmater5010005
APA StyleNa, S., Zhang, W., Kitagawa, M., Hirooka, A., & Komatsu, M. (2025). Optimization Using Central Composite Design of the Response Surface Methodology for the Compressive Strength of Alkali-Activated Material from Rice Husk Ash. Construction Materials, 5(1), 5. https://doi.org/10.3390/constrmater5010005





