A Comparison of the Effect of Activator Cations (Sodium and Potassium) on the Fresh and Hardened Properties of Mine Tailing-Slag Binders
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.2. Mixture Proportions
2.3. Test Methods
3. Results and Discussion
3.1. Setting Times
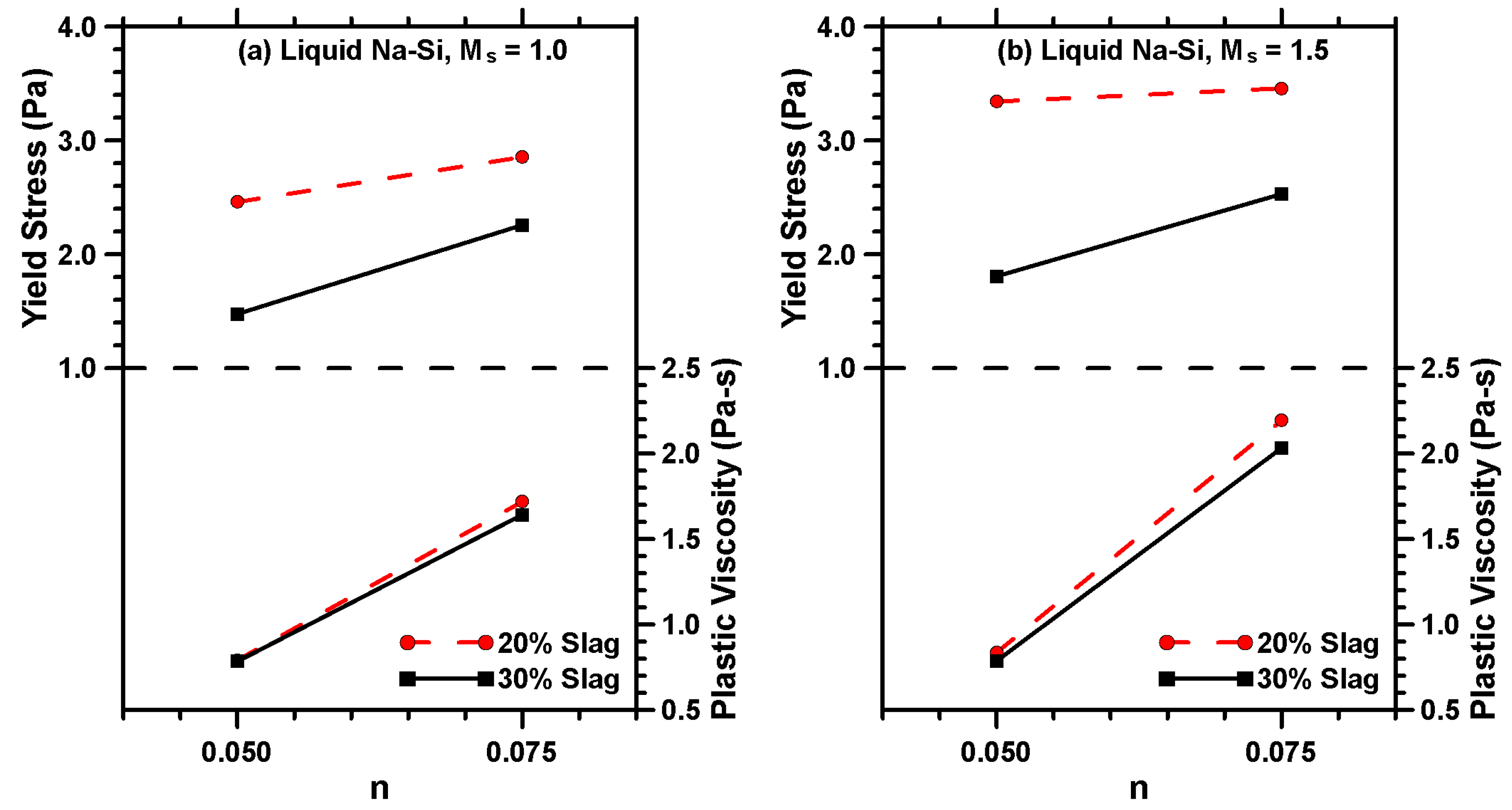
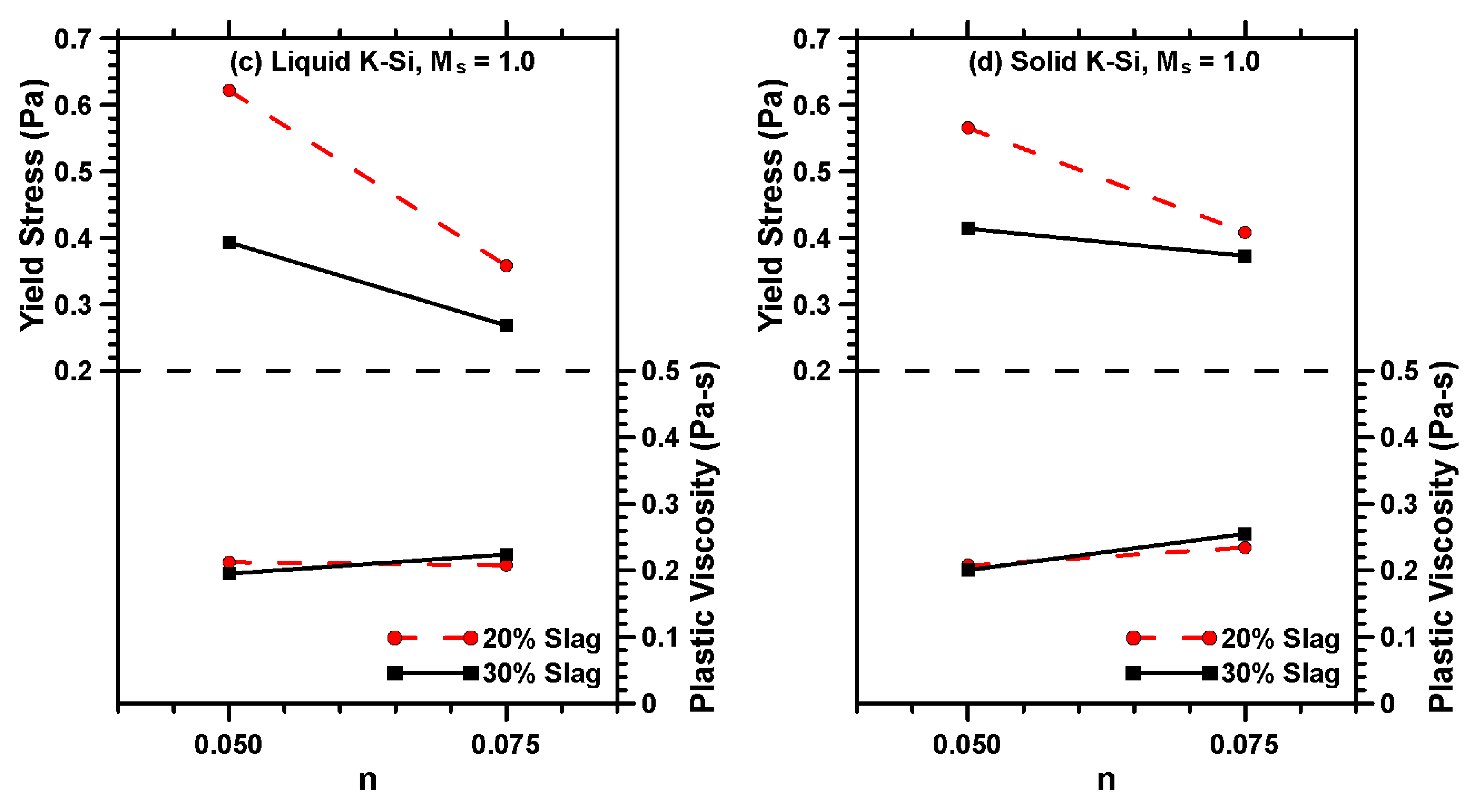
3.2. Rheological Characterization
3.3. Isothermal Calorimetry
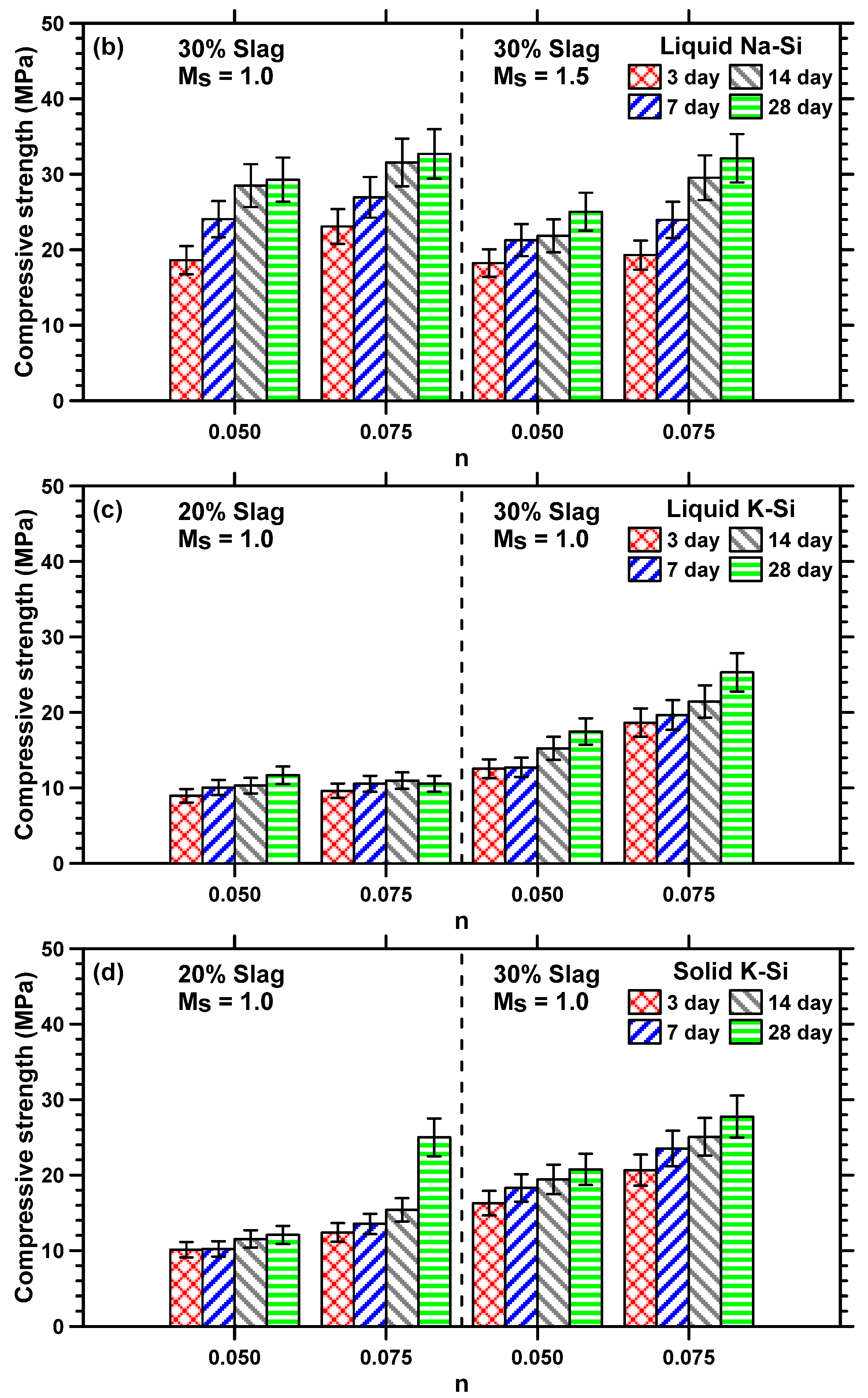
3.4. Compressive Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maruthupandian, S.; Chaliasou, A.; Kanellopoulos, A. Recycling mine tailings as precursors for cementitious binders—Methods, challenges and future outlook. Constr. Build. Mater. 2021, 312, 125333. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Zeyad, A.M.; de Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Ince, C.; Derogar, S.; Gurkaya, K.; Ball, R.J. Properties, durability and cost efficiency of cement and hydrated lime mortars reusing copper mine tailings of Lefke-Xeros in Cyprus. Constr. Build. Mater. 2021, 268, 121070. [Google Scholar] [CrossRef]
- Vilela, A.P.; Eugênio, T.M.C.; de Oliveira, F.F.; Mendes, J.F.; Ribeiro, A.G.C.; Brandão, L.E.V.D.S.; Mendes, R.F. Technological properties of soil-cement bricks produced with iron ore mining waste. Constr. Build. Mater. 2020, 262, 120883. [Google Scholar] [CrossRef]
- Dong, L.; Tong, X.; Li, X.; Zhou, J.; Wang, S.; Liu, B. Some developments and new insights of environmental problems and deep mining strategy for cleaner production in mines. J. Clean. Prod. 2019, 210, 1562–1578. [Google Scholar] [CrossRef]
- Nurcholis, M.; Yudiantoro, D.F.; Haryanto, D.; Mirzam, A. Heavy Metals Distribution in the Artisanal Gold Mining Area in Wonogiri. Indones. J. Geogr. 2017, 49, 133–144. [Google Scholar] [CrossRef]
- Macklin, M.G.; Brewer, P.A.; Balteanu, D.; Coulthard, T.J.; Driga, B.; Howard, A.J.; Zaharia, S. The long term fate and environmental significance of contaminant metals released by the January and March 2000 mining tailings dam failures in Maramureş County, upper Tisa Basin, Romania. Appl. Geochem. 2003, 18, 241–257. [Google Scholar] [CrossRef]
- Macklin, M.; Brewer, P.; Hudson-Edwards, K.; Bird, G.; Coulthard, T.; Dennis, I.; Lechler, P.; Miller, J.; Turner, J. A geomorphological approach to the management of rivers contaminated by metal mining. Geomorphology 2006, 79, 423–447. [Google Scholar] [CrossRef]
- Rico, M.; Benito, G.; Díez-Herrero, A. Floods from tailings dam failures. J. Hazard. Mater. 2008, 154, 79–87. [Google Scholar] [CrossRef]
- Liu, H.; Probst, A.; Liao, B. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci. Total Environ. 2005, 339, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Klimaszyk, P.; Marszelewski, W.; Borowiak, D.; Mleczek, M.; Nowiński, K.; Pius, B.; Niedzielski, P.; Poniedziałek, B. The chemistry and toxicity of discharge waters from copper mine tailing impoundment in the valley of the Apuseni Mountains in Romania. Environ. Sci. Pollut. Res. Int. 2017, 24, 21445–21458. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Alaba, P.A.; Rashedi, A. Selected performance of alkali-activated mine tailings as cementitious composites: A review. J. Build. Eng. 2022, 50, 104154. [Google Scholar] [CrossRef]
- Araujo, F.S.M.; Taborda-Llano, I.; Nunes, E.B.; Santos, R.M. Recycling and Reuse of Mine Tailings: A Review of Advancements and Their Implications. Geosciences 2022, 12, 319. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Liu, Q. Geopolymerization and Its Potential Application in Mine Tailings Consolidation: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 399–409. [Google Scholar] [CrossRef]
- Alonso, M.; Pasko, A.; Gascó, C.; Suarez, J.; Kovalchuk, O.; Krivenko, P.; Puertas, F. Radioactivity and Pb and Ni immobilization in SCM-bearing alkali-activated matrices. Constr. Build. Mater. 2018, 159, 745–754. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H. Practical Importance of Tailings for Cemented Paste Backfill. In Paste Tailings Management; Yilmaz, E., Fall, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 7–32. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- He, X.; Yuhua, Z.; Qaidi, S.; Isleem, H.F.; Zaid, O.; Althoey, F.; Ahmad, J. Mine tailings-based geopolymers: A comprehensive review. Ceram. Int. 2022, 48, 24192–24212. [Google Scholar] [CrossRef]
- Zhao, S.; Xia, M.; Yu, L.; Huang, X.; Jiao, B.; Li, D. Optimization for the preparation of composite geopolymer using response surface methodology and its application in lead-zinc tailings solidification. Constr. Build. Mater. 2021, 266, 120969. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Cárdenas, J.J.G.; Álvarez, G.E.S.; Rivera, V.B.A. Specimen size effects on the mechanical behaviors and failure patterns of the mine tailings-based geopolymer under uniaxial compression. Constr. Build. Mater. 2021, 281, 122525. [Google Scholar] [CrossRef]
- Ghazi, A.B.; Jamshidi-Zanjani, A.; Nejati, H. Utilization of copper mine tailings as a partial substitute for cement in concrete construction. Constr. Build. Mater. 2022, 317, 125921. [Google Scholar] [CrossRef]
- Xiaolong, Z.; Shiyu, Z.; Hui, L.; Yingliang, Z. Disposal of mine tailings via geopolymerization. J. Clean. Prod. 2021, 284, 124756. [Google Scholar] [CrossRef]
- Lyu, X.; Yao, G.; Wang, Z.; Wang, Q.; Li, L. Hydration kinetics and properties of cement blended with mechanically activated gold mine tailings. Thermochim. Acta 2020, 683, 178457. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Gao, P.; Li, Y. Effects of grinding media on grinding products and flotation performance of chalcopyrite. Miner. Eng. 2020, 145, 106070. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, ö; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Yao, X.; Zhu, Y. Effects of halloysite in kaolin on the formation and properties of geopolymers. Cem. Concr. Compos. 2012, 34, 709–715. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Parian, M.; Parapari, P.S.; Ghorbani, Y.; Rosenkranz, J. A comparative study on the effects of dry and wet grinding on mineral flotation separation–a review. J. Mater. Res. Technol. 2019, 8, 5004–5011. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-activated slag mortars: Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Zou, J.; Reid, A.; Wang, H. Toward an indexing approach to evaluate fly ashes for geopolymer manufacture. Cem. Concr. Res. 2016, 85, 163–173. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Reaction kinetics in sodium silicate powder and liquid activated slag binders evaluated using isothermal calorimetry. Thermochim. Acta 2012, 546, 32–43. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Dakhane, A.; Peng, Z.; Marzke, R.; Neithalath, N. Comparative Analysis of the Influence of Sodium and Potassium Silicate Solutions on the Kinetics and Products of Slag Activation. Adv. Civ. Eng. Matls. 2014, 3, 371–387. [Google Scholar] [CrossRef]
- Dakhane, A.; Neithalath, N. Reaction Kinetics and Characterization of Slag-Based, High Strength, ‘Just-Add-Water’ Type (One-Part) Alkali-Activated Binders. Recent Prog. Mater. 2022, 4, 6. [Google Scholar] [CrossRef]
- Vance, K.; Aguayo, M.; Dakhane, A.; Ravikumar, D.; Jain, J.; Neithalath, N. Microstructural, Mechanical, and Durability Related Similarities in Concretes Based on OPC and Alkali-Activated Slag Binders. Int. J. Concr. Struct. Mater. 2014, 8, 289–299. [Google Scholar] [CrossRef]
- Arora, A.; Aguayo, M.; Hansen, H.; Castro, C.; Federspiel, E.; Mobasher, B.; Neithalath, N. Microstructural packing- and rheology-based binder selection and characterization for Ultra-high Performance Concrete (UHPC). Cem. Concr. Res. 2018, 103, 179–190. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, C.; Zhang, Z. Recent progress in understanding setting and hardening of alkali-activated slag (AAS) materials. Cem. Concr. Compos. 2022, 134, 104795. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Koohestani, B.; Mokhtari, P.; Yilmaz, E.; Mahdipour, F.; Darban, A.K. Geopolymerization mechanism of binder-free mine tailings by sodium silicate. Constr. Build. Mater. 2021, 268, 121217. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F.P. Alkali sorption by C-S-H and C-A-S-H gels: Part II. Role of alumina. Cem. Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of Calcium Silicate Hydrates Formed in Alkaline-Activated Slag: Influence of the Type of Alkaline Activator. J. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Mueller, S.; Llewellin, E.W.; Mader, H.M. The rheology of suspensions of solid particles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 466, 1201–1228. [Google Scholar] [CrossRef]
- Santamarı, I.; Mendoza, C.I. The rheology of concentrated suspensions of arbitrarily-shaped particles. J. Colloid Interface Sci. 2010, 346, 118–126. [Google Scholar] [CrossRef]
- Kamal, M.R.; Mutel, A. Rheological Properties of Suspensions in Newtonian and Non-Newtonian Fluids. J. Polym. Eng. 1985, 5, 293–382. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Brady, J.F. The rheological behavior of concentrated colloidal dispersions. J. Chem. Phys. 1993, 99, 567–581. [Google Scholar] [CrossRef]
- Mandal, R.; Panda, S.K.; Nayak, S. Rheology of Concrete: Critical Review, recent Advancements, and future prospectives. Constr. Build. Mater. 2023, 392, 132007. [Google Scholar] [CrossRef]
- Vance, K.; Arora, A.; Sant, G.; Neithalath, N. Rheological evaluations of interground and blended cement–limestone suspensions. Constr. Build. Mater. 2015, 79, 65–72. [Google Scholar] [CrossRef]
- Vance, K.; Dakhane, A.; Sant, G.; Neithalath, N. Observations on the rheological response of alkali activated fly ash suspensions: The role of activator type and concentration. Rheol. Acta 2014, 53, 843–855. [Google Scholar] [CrossRef]
- Zuo, Y.; Ye, G. Preliminary Interpretation of the Induction Period in Hydration of Sodium Hydroxide/Silicate Activated Slag. Materials 2020, 13, E4796. [Google Scholar] [CrossRef] [PubMed]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation kinetics. 1. In situ energy-dispersive X-ray diffractometry. Chem. Eng. Sci. 2007, 62, 2309–2317. [Google Scholar] [CrossRef]
- Ravikumar, D.; Neithalath, N. Effects of activator characteristics on the reaction product formation in slag binders activated using alkali silicate powder and NaOH. Cem. Concr. Compos. 2012, 34, 809–818. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Obenaus-Emler, R.; Falah, M.; Illikainen, M. Assessment of mine tailings as precursors for alkali-activated materials for on-site applications. Constr. Build. Mater. 2020, 246, 118470. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.R. “Kinetics of Alkaline Activation of Slag and Fly ash-Slag Systems” M.S., Arizona State University, United States—Arizona. Available online: https://www.proquest.com/docview/1269512488/abstract/3E68298DC62C47DBPQ/1 (accessed on 18 June 2023).
- Fernández-Jiménez, A.; Zibouche, F.; Boudissa, N.; García-Lodeiro, I.; Abadlia, M.T.; Palomo, A. ‘Metakaolin-Slag-Clinker Blends.’ The Role of Na + or K + as Alkaline Activators of Theses Ternary Blends. J. Am. Ceram. Soc. 2013, 96, 1991–1998. [Google Scholar] [CrossRef]
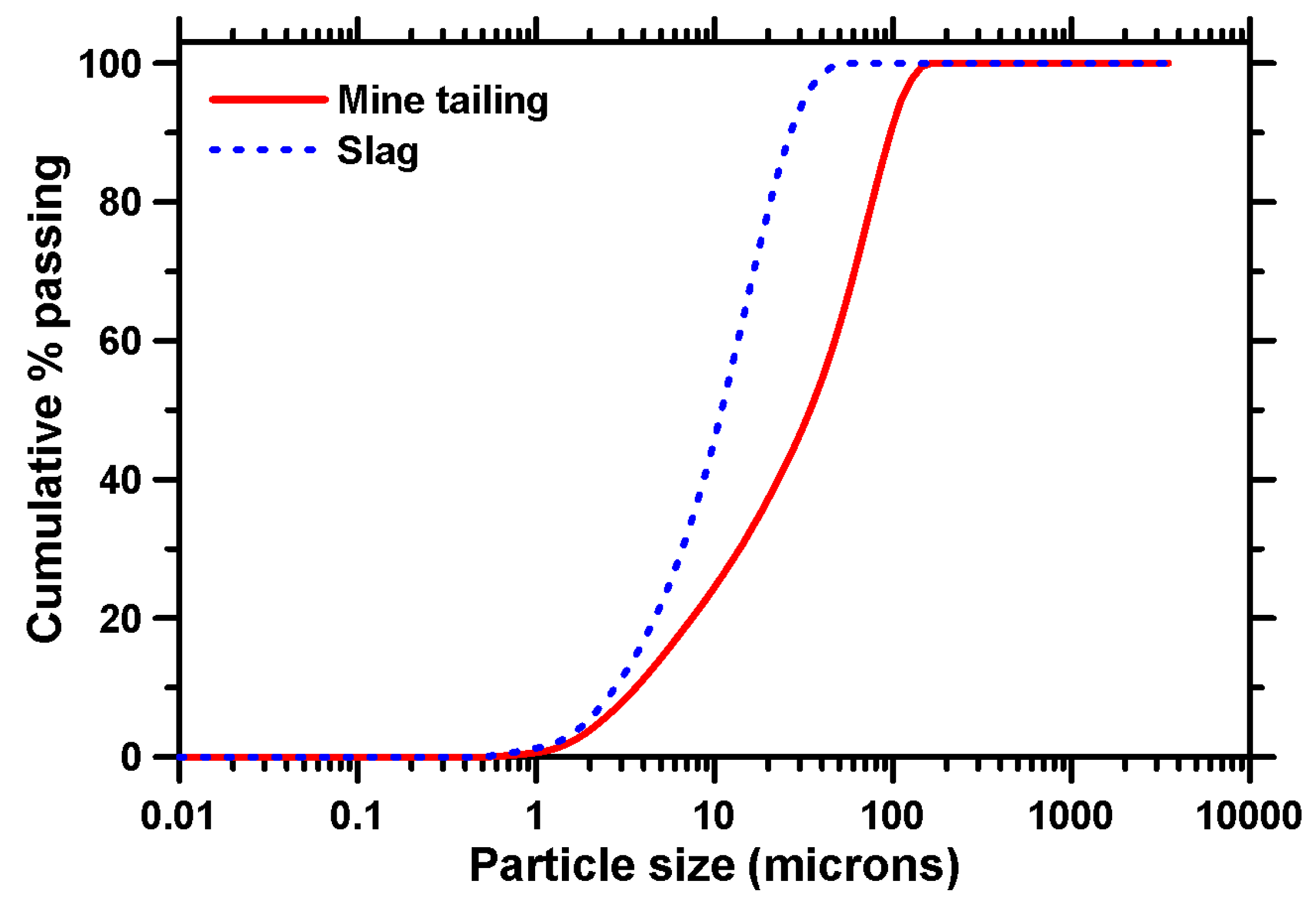



| Binder Ingredients | Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mine tailings # | SiO2 (%) | Al2O3 (%) | SO3 (%) | FexOy (%) | SnO (%) | MnO (%) | TiO2 (%) | Sb2O3 (%) | P2O5 (%) |
| 64.2 | 19.95 | 1.94 | 8.26 | 1.35 | 0.81 | 0.48 | 2.49 | 0.15 | |
| MgO (%) | Na2O (%) | K2O (%) | CaO (%) | LOI * (%) | |||||
| GGBFS | 39.4 | 8.49 | 2.83 | 0.37 | 12.05 | 0.27 | 0.80 | 35.53 | 1.31 |
| Activator Agent | Liquid Na–Si | Liquid K–Si | Solid K–Si | |||
|---|---|---|---|---|---|---|
| Ms | 1.0 | 1.5 | 1.0 | 1.5 | 1.0 | 1.5 |
| Na/K silicate (g) | 190.73 | 286.09 | 188.29 | 282.43 | 81.41 | 122.12 |
| NaOH/KOH (g) | 24.19 | 4.03 | 31.21 | 17.02 | 22.34 | 3.72 |
| Water (g) | 235.08 | 184.88 | 230.50 | 175.54 | 346.25 | 349.16 |
| Binder Composition | Activator Parameters | Initial Setting Time (min) | Final Setting Time (min) | ||
|---|---|---|---|---|---|
| Activator Type | Ms | n | |||
| 80 Mine tailing (MT)–20 slag (S) | Liquid sodium (Na)–silicate (Si) | 1.0 | 0.050 | 92 | 108 |
| 0.075 | 94 | 200 | |||
| 1.5 | 0.050 | 58 | 93 | ||
| 0.075 | 124 | 177 | |||
| 70 Mine tailing (MT)–30 slag (S) | 1.0 | 0.050 | 39 | 90 | |
| 0.075 | 58 | 101 | |||
| 1.5 | 0.050 | 42 | 63 | ||
| 0.075 | 74 | 106 | |||
| 80 Mine tailing (MT)–20 slag (S) | Liquid potassium (K)–silicate (Si) | 1.0 | 0.050 | 15 | 48 |
| 0.075 | 23 | 38 | |||
| 1.5 | 0.050 | 297 | 573 | ||
| 0.075 | >720 | - * | |||
| 70 Mine tailing (MT)–30 slag (S) | 1.0 | 0.050 | 14 | 37 | |
| 0.075 | 15 | 27 | |||
| 1.5 | 0.050 | 146 | 196 | ||
| 0.075 | 287.75 | 766 | |||
| 80 Mine tailing (MT)–20 slag (S) | Solid potassium (K)–silicate (Si) | 1.0 | 0.050 | 26 | 61 |
| 0.075 | 27 | 47 | |||
| 1.5 | 0.050 | 718 | 825 | ||
| 0.075 | >720 | - | |||
| 70 Mine tailing (MT)–30 slag (S) | 1.0 | 0.050 | 16 | 29 | |
| 0.075 | 21 | 39 | |||
| 1.5 | 0.050 | 222 | 245 | ||
| 0.075 | 668 | 726 | |||
| Binder Composition | Activation Parameters | Magnitude of Peak (mW/g Binder) | Time to Peak (h) | Cumulative Heat (J/g Binder) | ||||
|---|---|---|---|---|---|---|---|---|
| Activator Type | Ms | n | First Peak | Second Peak | First Peak | Second Peak | ||
| 80 Mine tailing (MT)–20 slag (S) | Liquid sodium (Na)–silicate (Si) | 1.00 | 0.050 | 4.88 | 0.63 | 0.12 | 13.40 | 53.41 |
| 0.075 | 5.90 | - | 0.12 | - | 65.89 | |||
| 1.50 | 0.050 | 0.81 | 2.49 | 0.02 | 0.70 | 48.32 | ||
| 0.075 | 3.30 | 2.89 | 0.02 | 0.60 | 58.97 | |||
| 70 Mine tailing (MT)–30 slag (S) | 1.00 | 0.050 | 3.37 | 1.17 | 0.25 | 9.00 | 67.61 | |
| 0.075 | 4.26 | - | 0.28 | - | 78.81 | |||
| 1.50 | 0.050 | 0.91 | 3.28 | 0.02 | 0.58 | 57.84 | ||
| 0.075 | 3.94 | - | 0.65 | - | 61.65 | |||
| 80 Mine tailing (MT)–20 slag (S) | Liquid potassium (K)–silicate (Si) | 1.00 | 0.050 | 7.34 | - | 0.08 | - | 55.28 |
| 0.075 | 10.27 | - | 0.12 | - | 61.18 | |||
| 1.50 | 0.050 | 4.52 | - | 0.04 | - | 9.46 | ||
| 0.075 | 3.41 | - | 0.05 | - | 11.60 | |||
| 70 Mine tailing (MT)–30 slag (S) | 1.00 | 0.050 | 8.16 | - | 0.07 | - | 75.80 | |
| 0.075 | 12.00 | - | 0.21 | - | 80.19 | |||
| 1.50 | 0.050 | 1.65 | - | 0.02 | - | 6.25 | ||
| 0.075 | 3.76 | - | 0.03 | - | 6.41 | |||
| 80 Mine tailing (MT)–20 slag (S) | Powdered potassium (K)–silicate (Si) | 1.00 | 0.050 | 9.61 | - | 0.05 | - | 58.34 |
| 0.075 | 8.15 | - | 0.23 | - | 59.72 | |||
| 1.50 | 0.050 | 4.59 | - | 0.06 | - | 15.14 | ||
| 0.075 | 4.59 | - | 0.05 | - | 12.47 | |||
| 70 Mine tailing (MT)–30 slag (S) | 1.00 | 0.050 | 6.01 | 5.80 | 0.05 | 0.40 | 68.27 | |
| 0.075 | 12.79 | - | 0.22 | - | 78.89 | |||
| 1.50 | 0.050 | 2.32 | - | 0.04 | - | 8.36 | ||
| 0.075 | 2.52 | - | 0.03 | - | 8.95 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surehali, S.; Simon, A.; Ramasamy, R.K.; Neithalath, N. A Comparison of the Effect of Activator Cations (Sodium and Potassium) on the Fresh and Hardened Properties of Mine Tailing-Slag Binders. Constr. Mater. 2023, 3, 389-404. https://doi.org/10.3390/constrmater3040025
Surehali S, Simon A, Ramasamy RK, Neithalath N. A Comparison of the Effect of Activator Cations (Sodium and Potassium) on the Fresh and Hardened Properties of Mine Tailing-Slag Binders. Construction Materials. 2023; 3(4):389-404. https://doi.org/10.3390/constrmater3040025
Chicago/Turabian StyleSurehali, Sahil, Aswathy Simon, Rijul Kanth Ramasamy, and Narayanan Neithalath. 2023. "A Comparison of the Effect of Activator Cations (Sodium and Potassium) on the Fresh and Hardened Properties of Mine Tailing-Slag Binders" Construction Materials 3, no. 4: 389-404. https://doi.org/10.3390/constrmater3040025
APA StyleSurehali, S., Simon, A., Ramasamy, R. K., & Neithalath, N. (2023). A Comparison of the Effect of Activator Cations (Sodium and Potassium) on the Fresh and Hardened Properties of Mine Tailing-Slag Binders. Construction Materials, 3(4), 389-404. https://doi.org/10.3390/constrmater3040025





