Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Methods and Sample Preparation
2.2.1. Preparation of Saturated Ca(OH)2 Solution
2.2.2. Interaction of TiNTs and Fe/TiNTs with Supersaturated Ca(OH)2 Solution
2.2.3. Effect of TiNTs and Fe/TiNTs on the Hydration of C3S
3. Results and Discussion
3.1. Characterization of Educts
3.2. Incorporation of Synthesized TiNTs and Fe/TiNTs in a Cementitious System
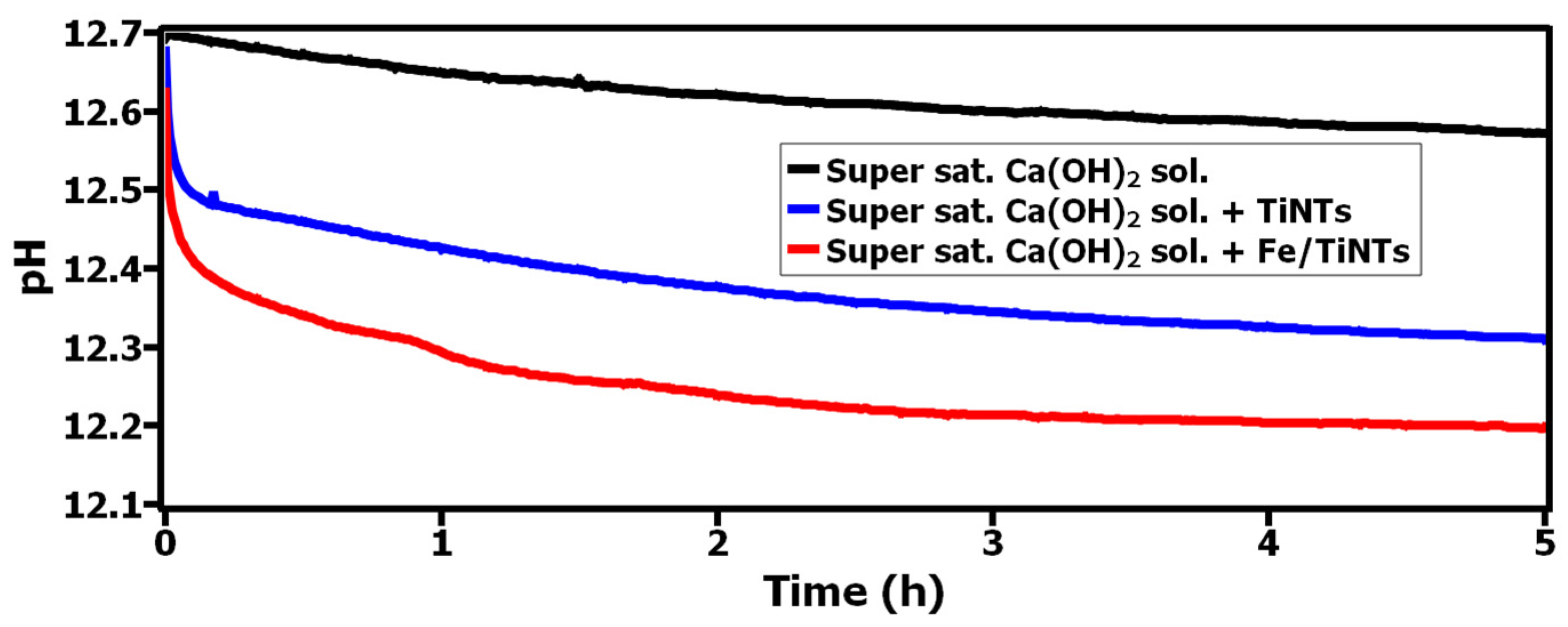
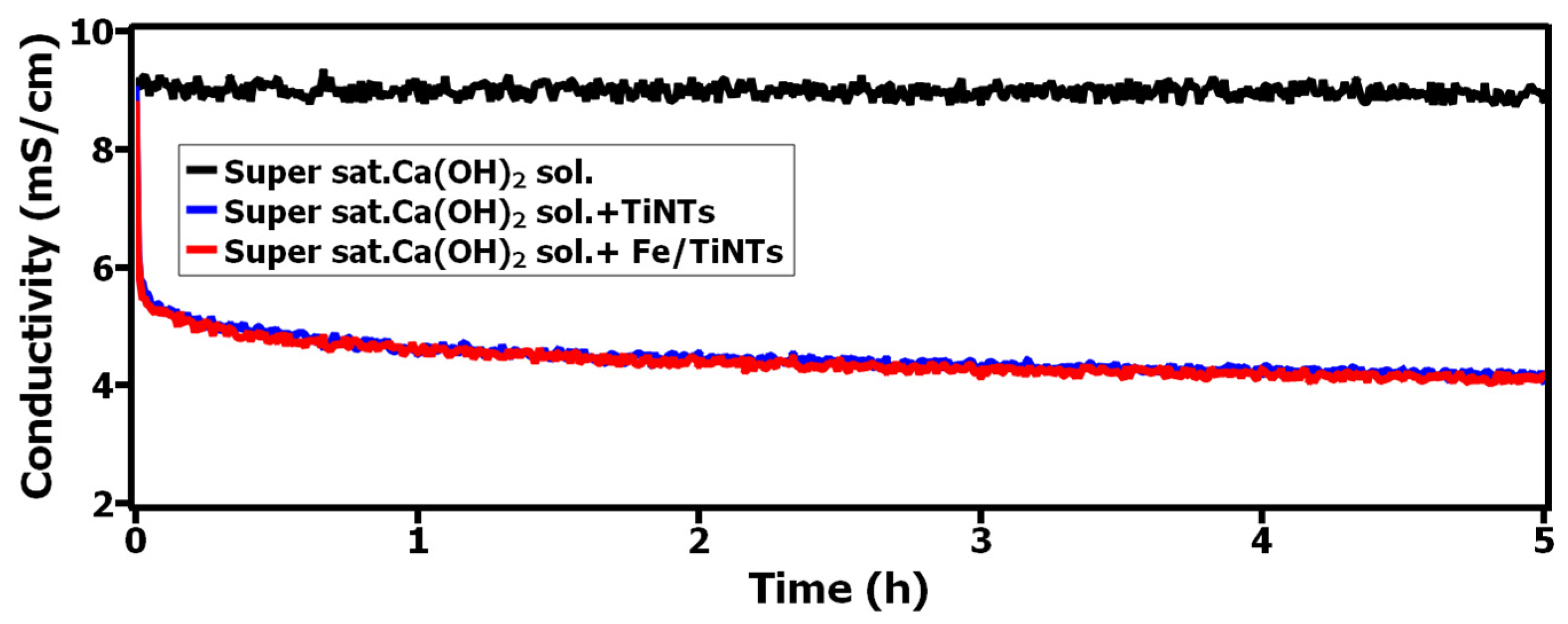
3.2.1. Interaction of TiNTs and Fe/TiNTs with Supersaturated Ca(OH)2 Solution via pH, Conductivity and BET Specific Surface Area Measurement
3.2.2. Influence of TiNTs and Fe/TiNTs on the Hydration of C3S
- Course of hydration reaction
- 2.
- Study on the hydrated paste of C3S
- Characterization of C3S hydration products
- Phase composition and determination of the degree of hydration after 8 h, 1 d and 7 d
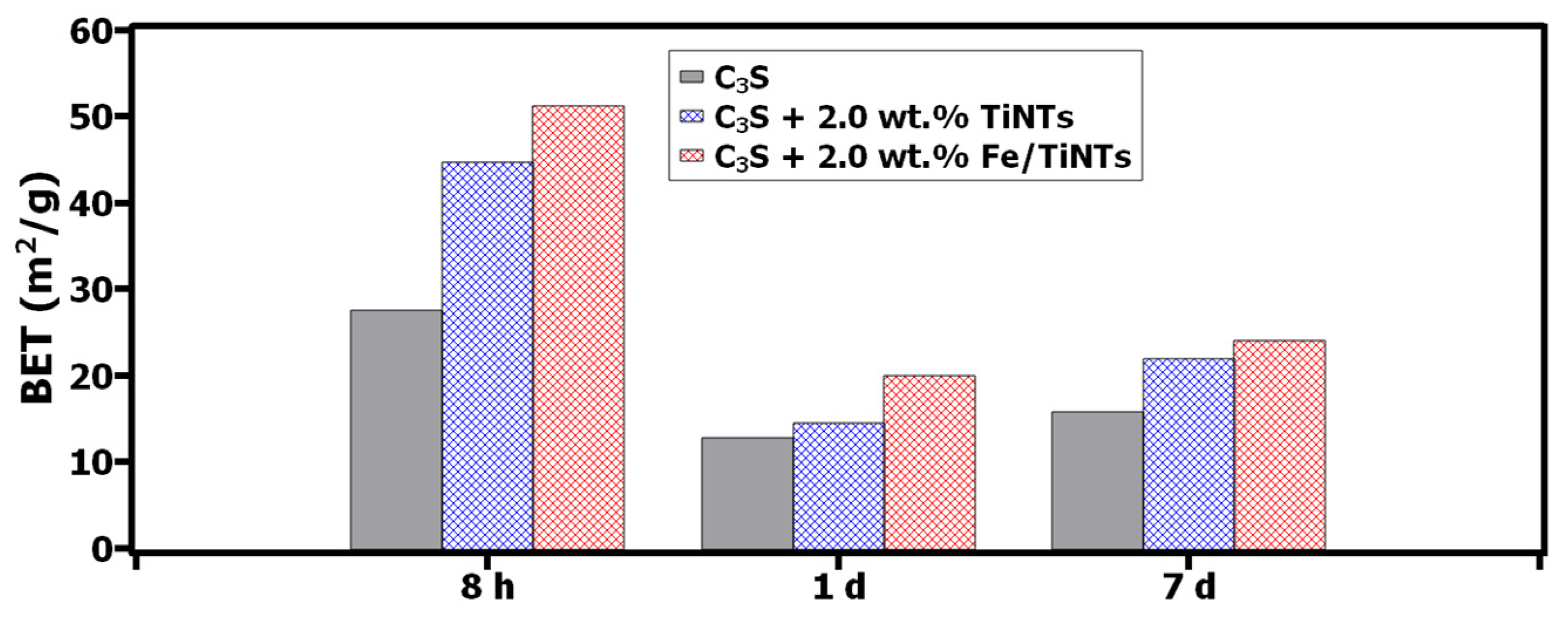
- Determination of BET specific surface area of hydration products
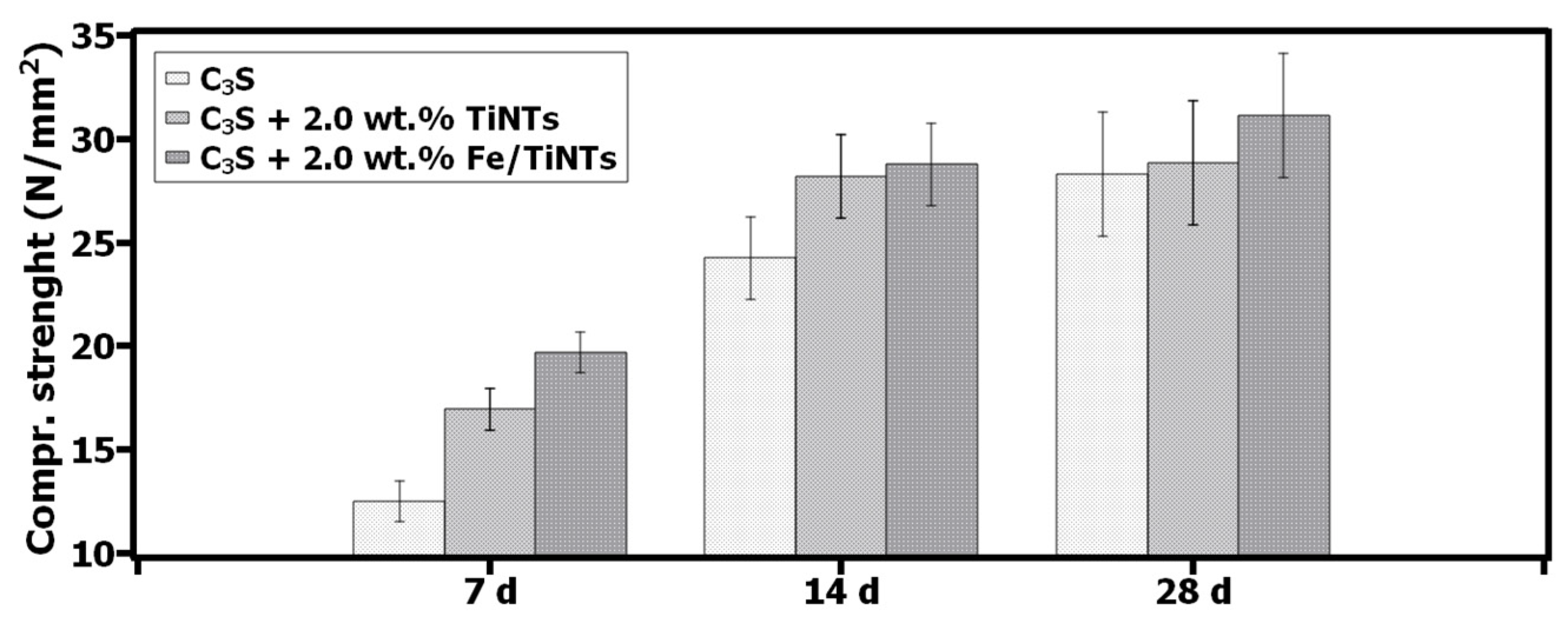
- Mechanical study of hydrated C3S prism
4. Conclusions
- Treatment with supersaturated Ca(OH)2 solution.
- A strong interaction between Ca2+-ions and both TiNTs and Fe/TiNTs was observed as a result of treatment with super saturated Ca(OH)2 solution. The results were proved by pH, conductivity and gas adsorption measurements.
- The pH and conductivity measurements showed a decrease as a result of Ca2+ ions being sorbed by the nanotubes.
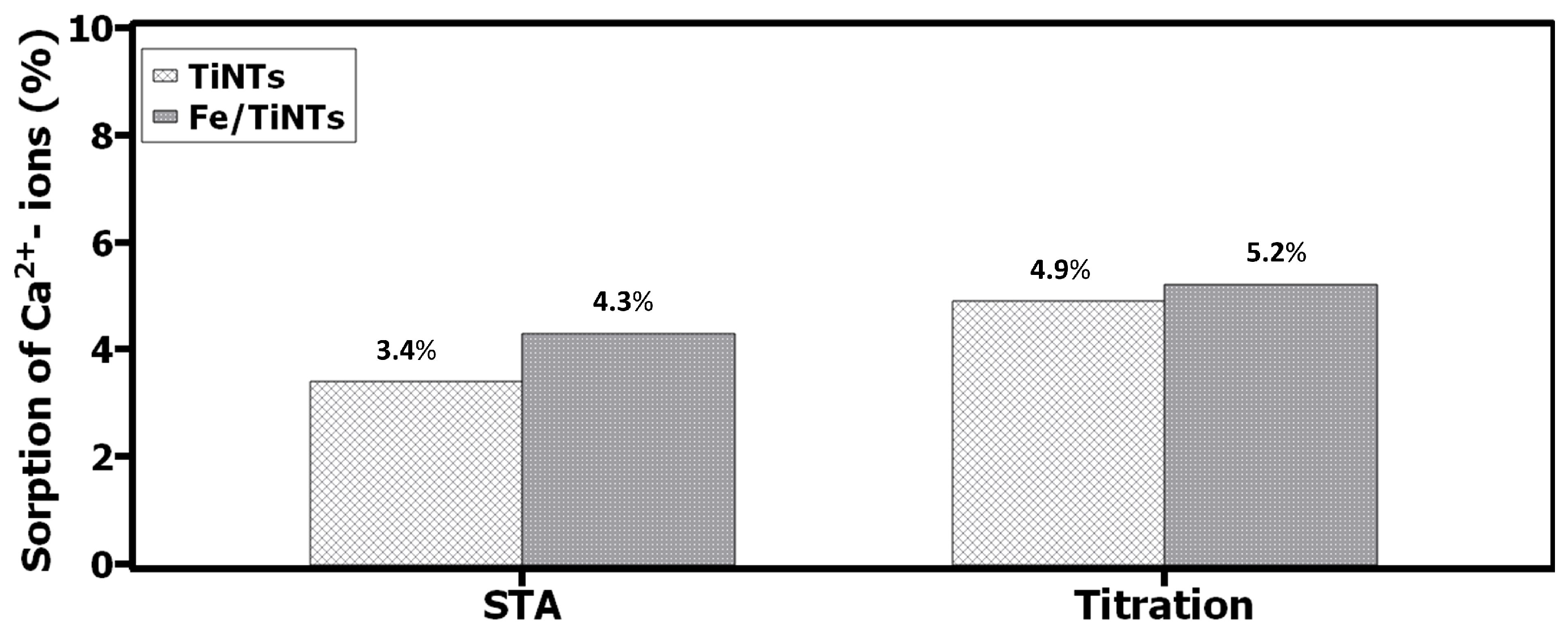
- The amount of sorbed Ca2+-ions by nanotubes were quantitatively determined via EDTA titration and thermal analysis using STA.
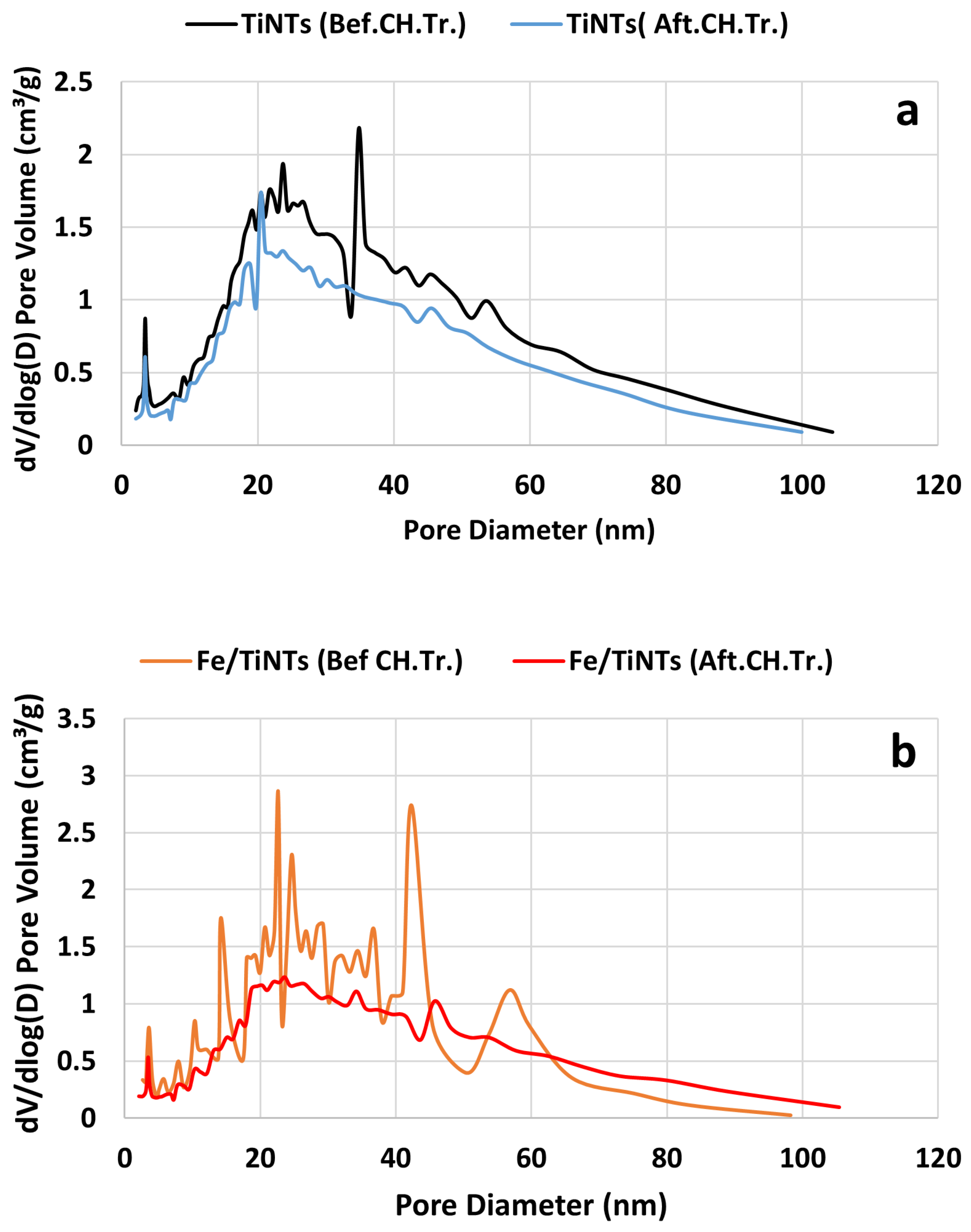
- The gas adsorption measurement showed lower BET specific surface area and porosity for both type of nanotubes after treatment with super saturated Ca(OH)2 solution, indicating sorption of Ca2+-ions by nanotubes.
- The effect of Fe/TiNTs on the sorption of Ca2+-ions in supersaturated were superior compared to TiNTs.
- Incorporation of Fe/TiNTs and TiNTs with C3S.
- In terms of hydration course, a shortening of the induction period and earlier acceleration period was observed for the samples containing nanotubes. However, the effect of Fe/TiNTs was found to be superior compared to the control sample (pure C3S) and the sample with TiNTs.
- Concerning the studies on hydration products, more hydration products were formed in the presence of Fe/TiNTs and TiNTs, particularly for the samples with Fe/TiNTs content.
- The degree of hydration increased, especially in the presence of Fe/TiNTs. The BET specific surface area of hydration products was quantitatively determined via combing XRD and gas adsorption data.
- A 36%, 16% and 9% increase in compressive strength of Fe/TiNTs samples for 7 d, 14 d and 28 d hydrated C3S paste was obtained.
- On the basis of above obtained results, the following conclusions were achieved:
- Iron-doped TiNTs (Fe/TiNTs) can serve as a promising reinforcement nanomaterial for cement-based construction materials.
- Lowering the values of pH and conductivity, decreasing the concentration of Ca2+-ions in supersaturated Ca(OH)2 solution and decreasing the specific surface area of nanotubes after the treatment with supersaturated Ca(OH)2 are indications of improvement in hydration reactions.
- The main hydration products of cement, such as C-S-H and CH phases, are formed in the media of saturated Ca(OH)2 solution. Finding a strong interaction between Fe/TiNTs and Ca2+-ions from the Ca(OH)2 solution treatment is an indication of improvement of C3S hydration, resulting in a higher amount of hydration products.
- One of the remarkable features of Fe/TiNTs is their enhanced photocatalytic activity at higher wavelengths. This makes them a viable option for the production of self-cleaning concrete in indoor environments where the intensity of UV radiation is low.
- The fabricated nanotube can directly incorporate into concrete when there is a need for this material in a particular case, such as the surface of concrete, but not within the bulk of concrete. The fabricated Fe/TiNTs have high specific surface area of Fe/TiNTs; even fewer percentages (1–2% or even less) of the nanotube is sufficient to use in the construction industry compared to other additives, which can stabilize the cost-benefit. If we take this into consideration, we only need less material and only the top layer of concrete should be modified; then, the overall cost will be not that high.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pustovgar, E.; Sangodkar, R.P.; Andreev, A.S.; Palacios, M.; Chmelka, B.F. Understanding Silicate Hydration from Quantitative Analyses of Hydrating Tricalcium Silicates. Nat. Commun. 2016, 10952, 10952. [Google Scholar] [CrossRef]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Alizadeh, R.; Raki, L.; Makar, J.M.; Beaudoin, J.J.; Moudrakovskid, I. Hydration of Tricalcium Silicate in the Presence of Synthetic Calcium–Silicate–Hydrate. J. Mater. Chem. 2009, 19, 7937–7946. [Google Scholar] [CrossRef]
- Jong, B.J.G.M.d.; Stein, H.N.; Stevels, J.M. Hydration of Tricalcium Silicate. J. Mater. Chem. 1967, 17. [Google Scholar] [CrossRef]
- Sakalli, Y.; Trettin, R. Investigation of C3S Hydration by Environmental Scanning Electron Microscope. J. Microsc. 2015, 259, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Trettin, R. Reactivity and Mechanism of Hydration of Cement Phases. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997. [Google Scholar]
- Trettin, R.; Wieker, W. Zur Hydratation von Tricalciumsilicat I. Ursachen Der Induktionsperiod, Silikattechnik. Silikattechnik 1986, 37, 75–78. [Google Scholar]
- Korpa, A.; Trettin, R. The Influence of Different Drying Methods on Cement Paste Microstructures as Reflected by Gas Adsorption-Comparison between Freeze-Drying (F-Drying), D-Drying, P-Drying and Oven-Drying Methods. Cem. Concr. Res. 2006, 36, 634–649. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Hydration Behaviour, Structure and Morphology of Hydration Phases in Advanced Cement-Based Systems Containing Micro and Nanoscale Pozzolanic Additives. Cem. Concr. Res. 2008, 38, 955–962. [Google Scholar] [CrossRef]
- Korpa, A.; Trettin, R.; Böttger, K.-G.; Thieme, J.; Schmidt, C. Pozzolanic Reactivity of Nanoscale Pyrogene Oxides and Their Strength Contribution in Cement-Based Systems. Adv. Cem. Res. 2008, 20, 35–46. [Google Scholar] [CrossRef]
- Stötzel, T.; Ali, M.; Trettin, R. Neue Nanopartikel Zur Verbesserung Der Eigenschaften von Zementären Bindemitteln, Posterpräsentation. In GDCh Tagungsband: Tagung Bauchemie, Hamburg; GDCh Tagungsband: Hamburg, Germany, 2011; pp. 344–349. [Google Scholar]
- Trettin, R.; Satava, V.; Wieker, W. Studies on Physico-Chemical Changes in Time of Initial Setting of C3S. In First International Workshop on Hydration and Setting, Dijon 1991; RILEM, Ed.; E&FN Spon: London, UK, 1992; pp. 289–297. [Google Scholar]
- Korpa, A.; Trettin, R. Nanoscale Pozzolans for Improving Ultra High Performance Cementitious Binders. Cem. Int. 2007, 1, 74–83. [Google Scholar]
- Wang, J. Steady-State Chloride Diffusion Coefficient and Chloride Migration Coefficient of Cracks in Concrete. J. Mater. Civ. Eng. 2017, 29, 4017117. [Google Scholar] [CrossRef]
- Qinfei, L.; Wang, Y.; Heng, C.; Pengkun, H.; Xin, C. Effect of Calcium Chloride on Hydration Kinetics and Pore Structure of Hydrated Tricalcium Silicate. In International Conference on Optoelectronic Science and Materials 2019; Materials Science and Engineering; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2020; p. 12055. [Google Scholar]
- Dorn, T.; Blask, O.; Stephan, D. Acceleration of Cement Hydration–A Review of the Working Mechanisms, Effects on Setting Time, and Compressive Strength Development of Accelerating Admixtures. Constr. Build. Mater. 2022, 323, 126554. [Google Scholar] [CrossRef]
- Mingli, C.; Xing, M.; Kaiyu; Li, L.; Shirley, S. Effect of Macro-, Micro- and Nano-Calcium Carbonate on Properties of Cementitious Composites—A Review. Materials 2019, 12, 781. [Google Scholar]
- Birgisson, B.; Mukhopadhyay, A.K.; Geary, G.; Khan, M. Nanotechnology in Concrete Materials; Transportation Research Board: Washington, DC, USA, 2012. [Google Scholar]
- Dorn, T.; Hirsch, T.; Stephan, D. Working Mechanism of Calcium Nitrate as an Accelerator for Portland Cement Hydration. J. Am. Ceram. Soc. 2022, 106, 752–766. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E.; Li, L. Characterization on the Recycling of Waste Seashells with Portland Cement towards Sustainable Cementitious Materials. J. Clean. Prod. 2019, 220, 235–252. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Wang, J. Effects of Aggregate Micro Fines (AMF), Aluminum Sulfate and Polypropylene Fiber (PPF) on Properties of Machine-Made Sand Concrete. Appl. Sci. 2019, 9, 2250. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and Concrete Nanoscience and Nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef]
- Sahin, R.; Oltulu, M. New Materials For Concrete Technology: Nano Powders. In Proceedings of the 33rd Conference on OUR World in Concrete & Structures, Singapore, 25–27 August 2008; CI-Premier PTE LTD: Singapore, 2008. [Google Scholar]
- Hunashyal, A.M.; Tippa, S.V.; Quadri, S.S.; Banapurmath, N.R. Experimental Investigation on Effect of Carbon Nanotubes and Carbon Fibres on the Behavior of Plain Cement Mortar Composite Round Bars under Direct Tension. Int. Sch. Res. Netw. 2011, 2011, 856849. [Google Scholar] [CrossRef]
- Chena, J.; Akono, A.-T. Influence of Multi-Walled Carbon Nanotubes on the Hydration Products of Ordinary Portland Cement Paste. Cem. Concr. Res. 2020, 137, 106197. [Google Scholar] [CrossRef]
- Ge, Z.; Gao, Z. Applications of Nanotechnology and Nanomaterials in Construction. In Proceedings of the First International Conference on Construction in Developing Countries (ICCIDC–I)“Advancing and Integrating Construction Education, Research & Practice”, Karachi, Pakistan, 4–5 August 2008. [Google Scholar]
- Kowald, T.; Trettin, R. Improvement of Cementitious Binders by Multi-Walled Carbon Nanotubes. Nanotechnol. Constr. 2009, 3, 261–266. [Google Scholar]
- Gammampila, R.; Mendis, P.; Ngo, T.; Aye, L.; Jayalath, A.S.; Rupasinghe, R.A.M. Application Of Nanomaterials In The Sustainable Built Environment. In Proceedings of the International Conference on Sustainable Built Environment (ICSBE-2010), Kandy, Sri Lanka, 13–14 December 2010. [Google Scholar]
- Abd Elrahman, M.; Sikora, P.; Chung, S.; Stephan, D. The Performance of Ultra-Lightweight Foamed Concrete Incorporating Nanosilica. Arch. Civ. Mech. Eng. 2021, 21, 79. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kurtis, K.E. Influence of TiO2 Nanoparticles on Early C3S Hydration. J. Am. Ceram. Soc. 2010, 93, 3399–3405. [Google Scholar] [CrossRef]
- Katya, N.K.; Ahluwalia, S.C.; Parkash, R. Effect of TiO2 on the Hydration of Tricalcium Silicate. Cem. Concr. Res. 1999, 29, 1851–1855. [Google Scholar] [CrossRef]
- Cook, R.; Ma, H.; Kumar, A. Influence of size-classified and slightly soluble mineral additives on hydration of tricalcium silicate. J. Am. Ceram. Soc. 2019, 103, 2764–2779. [Google Scholar] [CrossRef]
- Wu, L.; Mei, M.; Li, Z.; Liu, S.; Wang, X. Study on Photocatalytic and Mechanical Properties of TiO2 Modified Pervious Concrete. Case Stud. Constr. Mater. 2022, 17, e01606. [Google Scholar] [CrossRef]
- Sadeghi Nik, A.; Bahari, A.; Amiri, B. Nanostructural Properties of Cement–Matrix Composite. J. Basic Appl. Sci. Res. 2011, 1, 2167–2173. [Google Scholar]
- Yu, X.; Kang, S.; Long, X. Compressive Strength of Concrete Reinforced by TiO2 Nanoparticles. In 3rd International Conference on Materials Science, Resource and Environmental Engineering (MSREE 2018); AIP Publishing: Melville, NY, USA, 2018; p. 030006. [Google Scholar]
- Li, Z.; Wang, J.; Li, Y.; Yu, X.; Baoguo, H. Investigating Size Effect of Anatase Phase Nano TiO2 on the Property of Cement-Based Composites. Mater. Res. Express 2018, 5, 85034. [Google Scholar] [CrossRef]
- Jee, H.; Park, J.; Zalnezhad, E.; Jeong, K.; Woo, S.M.; Seok, S.; Sungchul, B. Characterization of Titanium Nanotube Reinforced Cementitious Composites: Mechanical Properties, Microstructure, and Hydration. Materials 2019, 12, 1617. [Google Scholar] [CrossRef]
- Siang, N.D.; Chandra, P.S.; Anggraini, V.; Kong, S.Y.; Shams, Q.T.; Romero, R.C.; Liu, Q.; Šavija, B. Influence of SiO2, TiO2 and Fe2O3 Nanoparticles on the Properties of Fly Ash Blended Cement Mortars. Constr. Build. Mater. 2020, 258, 119627. [Google Scholar] [CrossRef]
- Stötzel, T. Influence of Titanate Nanotubes on the Hydration of Tricalciumsilicate; Siegen University: Siegen, Germany, 2014. [Google Scholar]
- Liu, J.; Suh, H.; Jee, H.; Xu, J.; Zal Nezhad, E.; Choi, C.-S.; Bae, S. Synergistic Effect of Carbon Nanotube/TiO2 Nanotube Multi-Scale Reinforcement on Mechanical Properties and Hydration Process of Portland Cement Paste. Constr. Build. Mater. 2021, 293, 123447. [Google Scholar] [CrossRef]
- Mohd, S.; Qattali, Y. Modification of TiO2 Nanotubes via Doping with Fe3+-Ion Dopant and Its Application in Cementitious Systems. Ph.D. Thesis, University of Siegen, Siegen, Germany, 2020. [Google Scholar]
- Moni, S.M.; Oatalli SM, Y.; Pritzel, C.; Trettin, R. Interaction of Titania Nanotubes with Ca(OH)2 and C3S via Hydrothermal Approach. ACI Mater. J. 2020, 117, 233–239. [Google Scholar]
- Pérez-Nicolás, M.; Navarro-Blasco, Í.; Fernández, J.M.; Alvarez, J.I. The Effect of TiO2 Doped Photocatalytic Nano-Additives on the Hydration and Microstructure of Portland and High Alumina Cements. Nanomaterials 2017, 7, 329. [Google Scholar] [CrossRef]
- Trettin, R.; Ali, M.; Stötzel, T. The Effect of Titanate Nanotubes on Cementitious Systems for UHPC Application. In NICOM5; Springer International Publishing: Chicago, IL, USA, 2015. [Google Scholar]
- Korpa, A.; Kowald, T.; Treein, R. Principles of development, phase composition and nanostructural features of multiscale Ultra High Performance Concrete modified with pyrogenic nanoparticles–A review article. Am. J. Mater. Sci. Appl 2014, 2, 17–30. [Google Scholar]
- Feng, D.; Xie, N.; Gong, C.; Leng, Z.; Xiao, H.; Li, H.; Shi, X. Portland Cement Paste Modified by TiO2 Nanoparticles: A Microstructure Perspective. Ind. Eng. Chem. Res. 2013, 52, 11575–11582. [Google Scholar] [CrossRef]
- Liu, J.; Jee, H.; Lim, M.; Kim, J.H.; Kwon, S.J.; Lee, K.M.; Nezhad, E.Z.; Bae, S. Photocatalytic Performance Evaluation of Titanium Dioxide Nanotube-Reinforced Cement Paste. Materials 2020, 13, 5423. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Gao, Y. Effect of TiO2 Nanoparticles on Physical and Mechanical Properties of Cement at Low Temperatures. Adv. Mater. Sci. Eng. 2018, 2018, 8934689. [Google Scholar] [CrossRef]





| Free Lime Franke Method (wt.%) | Mineralogical Composition, XRD (wt.%) | Mean Particle Size. LG (µm) | ||
|---|---|---|---|---|
| C | C | C3S | C2S | d50 |
| 0.2 | 0.0 | 100 | 0 | 10.5 |
| Sample | End of Induction Period (h) | Duration of Acceleration Period (h) | Duration of Main Peak (h) |
|---|---|---|---|
| C3S | 4.5 | 6.8 | 15.3 |
| C3S + 1.0% TiNTs | 3.5 | 6.7 | 15.1 |
| C3S + 1.0% Fe/TiNTs | 3.0 | 6.8 | 15.4 |
| C3S + 2.0% TiNTs | 2.3 | 6.8 | 15.2 |
| C3S + 2.0% Fe/TiNTs | 2.1 | 7.0 | 15.9 |
| Age | Sample | C3S 4331-ICSD (%) | CH 61185-ICSD (%) | Cc 40107-ICSD (%) | Amorphous (tot. (%)) | Tot. Phy. Water (%) |
|---|---|---|---|---|---|---|
| 8 h | C3S | 63.47 | 0.75 | 0.50 | 11.30 | 23.97 |
| C3S + 2.0 wt.% TiNTs | 51.38 | 1.94 | 0.00 | 23.49 | 23.18 | |
| C3S + 2.0 wt.% Fe/TiNTs | 57.93 | 2.37 | 0.42 | 16.15 | 23.12 | |
| 1 d | C3S | 34.81 | 3.74 | 0.52 | 39.94 | 20.98 |
| C3S + 2.0 wt.% TiNTs | 32.87 | 6.80 | 0.48 | 39.75 | 20.10 | |
| C3S + 2.0 wt.% Fe/TiNTs | 31.85 | 6.82 | 0.61 | 40.25 | 20.46 | |
| 7 d | C3S | 22.90 | 12.67 | 1.38 | 44.18 | 18.86 |
| C3S + 2.0 wt.% TiNTs | 19.18 | 11.39 | 1.97 | 49.33 | 18.13 | |
| C3S + 2.0 wt.% Fe/TiNTs | 20.24 | 13.74 | 1.22 | 46.08 | 18.72 |
| Age | Sample | Weight Loss <105 °C | Chemically Bound Water (105–550 °C) | Tot. C3S | Excess Water | Ca(OH)2 (400–550 °C) | CaCO3 (550–700 °C) |
|---|---|---|---|---|---|---|---|
| 8 h | C3S | 0.44 | 2.27 | 66.67 | 31.53 | 3.37 | 0.84 |
| C3S + 2.0 wt.% TiNTs | 0.86 | 3.89 | 66.67 | 30.17 | 6.45 | 0.86 | |
| C3S + 2.0 wt.% Fe/TiNTs | 0.69 | 4.19 | 66.67 | 30.07 | 7.40 | 0.00 | |
| 1 d | C3S | 1.30 | 8.88 | 66.67 | 26.55 | 16.20 | 1.95 |
| C3S + 2.0 wt.% TiNTs | 1.21 | 10.25 | 66.67 | 25.69 | 16.86 | 0.95 | |
| C3S + 2.0 wt.% Fe/TiNTs | 1.34 | 10.07 | 66.67 | 25.73 | 17.51 | 0.00 | |
| 7 d | C3S | 1.08 | 14.03 | 66.67 | 23.26 | 24.87 | 3.45 |
| C3S + 2.0 wt.% TiNTs | 2.69 | 14.12 | 66.67 | 22.13 | 21.87 | 4.68 | |
| C3S + 2.0 wt.% Fe/TiNTs | 1.13 | 14.31 | 66.67 | 23.03 | 24.58 | 3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qattali, S.M.Y.; Pritzel, C.; Kowald, T.; Moni, S.M.F.K.; Killian, M.S.; Trettin, R. Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Constr. Mater. 2023, 3, 259-275. https://doi.org/10.3390/constrmater3020017
Qattali SMY, Pritzel C, Kowald T, Moni SMFK, Killian MS, Trettin R. Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Construction Materials. 2023; 3(2):259-275. https://doi.org/10.3390/constrmater3020017
Chicago/Turabian StyleQattali, S. Mohd. Yonos, Christian Pritzel, Torsten Kowald, S. M. Fuad Kabir Moni, Manuela S. Killian, and Reinhard Trettin. 2023. "Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate" Construction Materials 3, no. 2: 259-275. https://doi.org/10.3390/constrmater3020017
APA StyleQattali, S. M. Y., Pritzel, C., Kowald, T., Moni, S. M. F. K., Killian, M. S., & Trettin, R. (2023). Effect of Iron-Doped TiO2 Nanotubes on the Hydration of Tricalcium Silicate. Construction Materials, 3(2), 259-275. https://doi.org/10.3390/constrmater3020017







