Improving Concrete Infrastructure Project Conditions by Mitigating Alkali–Silica Reactivity of Fine Aggregates
Abstract
1. Introduction
2. Literature Review
- Local sources of fine aggregates were surveyed, and samples were obtained for ASR detection.
- Accelerated mortar bar tests for ASR detection in fine aggregates were conducted. The expansion of mortar bars was measured and compared with the permissible limits.
- Different percentages of SCMs, including microsilica and class C fly ash, were used to pour additional mortar bars for expansion measurements.
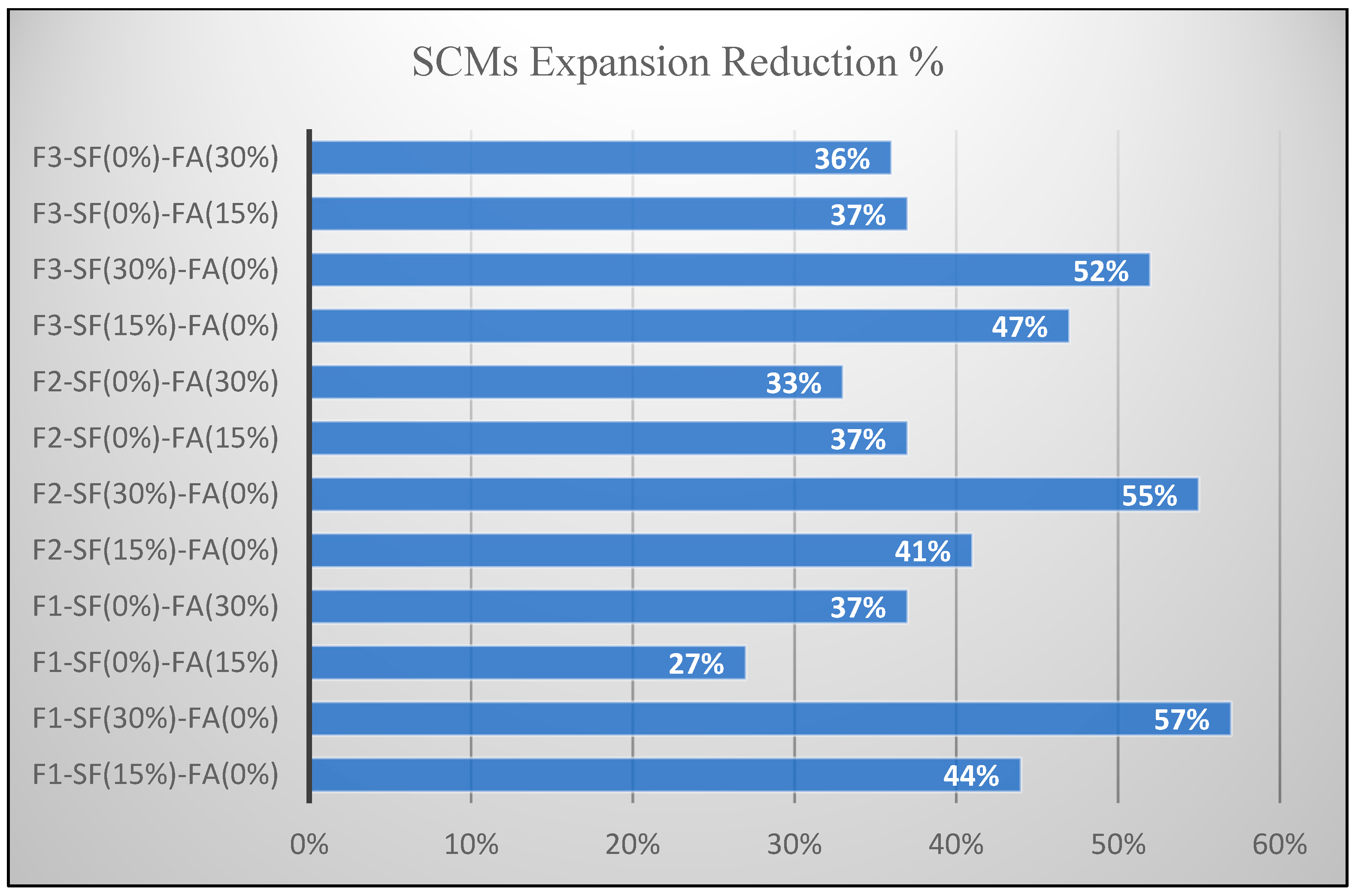
- The efficiency of SCMs in ASR mitigation was quantified through the decrease in bar expansion.
3. Experimental Investigation
- Type I/II portland cement was used in pouring AMBT specimens. The same cement batch was used in the preparation of all specimens to ensure the consistency of test results.
- Fine aggregate specimens (F1, F2, and F3) were used to pour the mortar bars. Three bars were poured using the same aggregate sample.
- SCM-free AMBT was poured using high-energy paddle mixer using a cement-to-aggregate ratio of 1:2.25 by weight. Water-to-cement ratio of 0.5:1 was used to fabricate the AMBT.
- SCMs including microsilica and class C fly ash were used to pour additional mortar bars. Silica fume and class C fly ash were selected due to their availability in the local market, and due to their incorporation in standard DOT mixes. SCMs were used in stepwise replacement of portland cement using a 1:1 weight ratio. Mortar bar design combinations are shown in Table 2.
- SCMs have a fine particle size as compared with all granular mix constituents. The fine particle size results in an improved packing order of the mix constituents and a decreased void ratio. This lowers the rate of moisture ingress and reduces the rate of reactivity.
- SCMs result in a lower cement content, which reduces the alkaline content of the mix, and significantly reduces the alkali–silica reactivity within the mix.
- The incorporation of SCMs in concrete mix binds the alkaline content during the cement hydration process, which reduces the pH value of the mix and slows down deleterious ASR.
4. Summary and Conclusions
5. Recommendations for Future Research
Funding
Data Availability Statement
Conflicts of Interest
References
- Stark, D. Handbook for the Identification of Alkali-Silica Reactivity in Highway Structures. SHRP-C/FR-91-101; TRB National Research Council: Washington, DC, USA, 1993; 49p. [Google Scholar]
- FHWA. Guidelines for the Use of Lithium to Mitigate or Prevent Alkali-Silica Reaction (ASR); Federal Highway Administration Report No.: FHWA-RD-03-047; Federal Highway Administration: Washington, DC, USA, 2007.
- Stanton, T.E. Expansion of concrete through reaction between cement and aggregate. Proc. Am. Soc. Civ. Eng. 1940, 66, 1781–1811. [Google Scholar] [CrossRef]
- Xu, H. On the alkali content of cement in AAR. In Proceedings of the 7th International Conference in Concrete Alkali-Aggregate Reactions, Park Ridge, NJ, USA, 1987; pp. 451–455. [Google Scholar]
- Poole, A.B. Introduction to alkali-aggregate reaction in concrete. In The Alkali-Silica Reaction in Concrete; Swamy, R.N., Van Nostrand, R., Eds.; CRC Press: New York, NY, USA, 1992. [Google Scholar]
- Moreira, K.M.D.V.; Oliveira, P.V.G.; de Deus, E.P.; Cabral, A.E.B. Alkali-silica reaction: Understanding the phenomenon. J. Build. Pathol. Rehabil. 2021, 6, 5. [Google Scholar] [CrossRef]
- Akhnoukh, A.K.; Kamel, L.Z.; Barsoum, M.M. Alkali silica reaction mitigation and prevention measures for Arkansas local aggregates. Int. J. Civ. Environ. Eng. 2016, 10, 95–99. [Google Scholar] [CrossRef]
- Abbas, S.; Ahmed, A.; Nehdi, M.; Saeed, D.; Abbas, W.; Amin, F. Eco-friendly mitigation of alkali-silica reaction in concrete using waste marble powder. ASCE J. Mater. Civ. Eng. 2020, 31, 04020270. [Google Scholar] [CrossRef]
- Deschenes, R., Jr.; Hale, W. Alkali-silica reaction (ASR) in concrete with previously inert aggregates. ASCE J. Perform. Constr. Facil. 2017, 31, 04016084. [Google Scholar] [CrossRef]
- Deschenes, R., Jr.; Hale, W. Alkali-silica reaction mitigation and prevention measures-phase I. Ark. Dep. Transp. Mack Blackwell Rural. Transp. Cent. 2017, 1–86. [Google Scholar]
- Folliard, K.J.; Thomas, M.D.A.; Fournier, B.; Resendez, Y.; Drimalas, T.; Bentivegna, A. Evaluation of mitigation measures applied to ASR-affected concrete elements: Preliminary findings from Austin, TX Exposure Site. In Proceeding of the 14th International Conference on Alkali Aggregate Reaction (ICAAR), Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Akhnoukh, A.K.; Mallu, A.R. Detection of alkali-silica reactivity using field exposure site investigation. In Proceedings of the 58th Annual Associated Schools of Construction International Conference, Atlanta, GA, USA, 20–23 April 2022. [Google Scholar]
- Akhnoukh, A.K. The use of micro and nano-sized particles in increasing concrete durability. Part. Sci. Technol. J. 2020, 38, 529–534. [Google Scholar] [CrossRef]
- Akhnoukh, A.K. Overview of nanotechnology applications in construction industry in the United States. J. Micro Nano-Syst. 2013, 5, 147–153. [Google Scholar] [CrossRef]
- Wang, W.; Noguchi, T.; Maruyama, I. Mechanism understanding of alkali-silica reaction in alkali-activated materials system. Cem. Concr. Res. 2022, 156, 106768. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kolawole, J.T.; Almakrab, A. Alkali-silica reaction (ASR) in concrete structures: Mechanism, effects and evaluation test methods adopted in the United States. Case Stud. Constr. Mater. 2021, 15, e00563. [Google Scholar] [CrossRef]
- Abd-Elsammd, A.; Ma, Z.J.; Le Pape, Y. Influence of mineralogical and chemical composition of AAR carbonate aggregates on the expansion rate of concrete and AAR mitigation. Constr. Build. Mater. 2021, 261, 119916. [Google Scholar] [CrossRef]
- Abd-Elsammd, A.; Ma, Z.J.; LPape, Y.; Hayes, N.W.; Guimaraes, M. Effect of alkali-silica reaction expansion rate and confinement on concrete degradation. ACI Mater. J. 2020, 117, 265–277. [Google Scholar] [CrossRef]
- Kamel, L.Z. Alkali-Silica Reaction Mitigation for Arkansas Region. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2014. [Google Scholar]
- Fournier, B.; Berube, M.; Folliard, K.; Thomas, M. Report on the Diagnosis Prognosis and Mitigation of Alkali-Silica Reaction (ASR) in Transportation Structure; FHWA-HIF-09-004; Office of Pavement Technology: Urbana, IL, USA, 2010. [Google Scholar]
- AASHTO-T380; Standard Method of Test for Potential Alkali Reactivity of Aggregates and Effectiveness of ASR Mitigation Measures (Miniature Concrete Prism Test, MCPT). American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 2019.
- ASTM C289-07 (Withdrawn 2016); Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method). Withdrawn 2016; ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM C1293-18 (Superseded); Standard Test Method for Determination of Length Change of Concrete Due to Alkali-Silica Reaction. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM C1260-14 (Superseded); Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Li, C.; Ideker, J.H.; Thomas, M.D.A. Evaluation of current ASTM standards for ASR prevention when fine lightweight aggregates are used. Adv. Civ. Eng. Mater. 2021, 10, 396–411. [Google Scholar] [CrossRef]
- Sanchez, L.F.M.; Fournier, B.; Jolin, M.; Bastien, J.; Mitchel, D. Tools for assessing damage in concrete affected by AAR coming from fine and coarse aggregates. Rev. IBRACON Estrut. Mater. 2017, 10, 84–91. [Google Scholar] [CrossRef]
- Johnson, R.; Shehata, M.H. The efficacy of accelerated test methods to evaluate alkali-silica reactivity of recycled concrete aggregates. Constr. Build. Mater. 2016, 112, 518–528. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Fournier, B.; Folliard, K.; Ideker, J.; Shehata, M. Test methods for evaluating preventive measures for controlling expansion due to alkali-silica reaction in concrete. Cem. Concr. Res. 2006, 36, 1842–1856. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Fournier, B.; Folliard, K.; Ideker, J.; Shehata, M. Test Methods for Evaluating Preventive Measures for Controlling Expansion Due to Alkali-Silica Reaction in Concrete; Report No. ICAR 302-1; International Center for Aggregate Research (ICAR): Austin, TX, USA, 2006; 62p. [Google Scholar]
- Davis, D.E.; Deschenes, R., Jr.; Hale, W. Development of a Field Exposure Site for predicting and Mitigating ASR; Final Report; TRC: Little Rock, AR, USA, 2018. [Google Scholar]
- Thomas, M.D.A.; Fournier, B.; Folliard, K.; Ideker, J.; Resendez, Y. Alkali-Silica Reactivity Field Identification Handbook (Report No. FHWA-HIF-12-022); Federal Highway Administration; U.S. Department of Transportation: Washington, DC, USA, 2011; 80p.
- Locati, F.; Zega, C.; Santos, G.; Marfil, S.; Falcone, D. Petrographic method to semi-quantify the content of particles with reactive components and residual mortar in ASR-affected fine recycled concrete aggregates. Cem. Concr. Res. 2021, 119, 104003. [Google Scholar] [CrossRef]
- Fernandez, I.; Ribeiro, M.; Martins, H.; Broekmans, M.; Sims, I.; Nixon, P.; Noronha, F. Assessment of concrete aggregates for ASR potential by petrography. Eng. Geol. Soc. Territ. 2015, 5, 37–40. [Google Scholar]
- Shi, Z.; Lothenbach, B. Role of aluminum and lithium in mitigating alkali-silica reaction—A review. Front. Mater. J. 2022, 8, 596. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. The effect of lithium ions on chemical sequence of alkali-silica reaction. Cem. Concr. Res. 2016, 79, 159–168. [Google Scholar] [CrossRef]
- Leemann, A.; Bernard, L.; Alahrache, S.; Winnefeld, F. ASR prevention—Effect of aluminum and lithium ions on the reaction products. Cem. Concr. Res. 2015, 76, 192–201. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Fournier, B.; Folliard, K.; Ideker, J.; Resendez, Y. The Use of Lithium to Prevent or Mitigate Alkali-Silica Reaction in Concrete Pavements and Structures; Report No. FHWA-HRT-06-133); Federal Highway Administration; U.S. Department of Transportation: Washington, DC, USA, 2007; 41p.
- Tuan, C.Y.; Kelly, M.T.; Sun, H.; Buss, M.E. Evaluation of use of lithium nitrate in controlling alkali-silica reactivity in existing concrete pavement. Transp. Res. Rec. J. Transp. Res. Board 2005, 1914, 34–44. [Google Scholar] [CrossRef]
- Feng, X.; Thomas, M.D.A.; Bremmer, T.W.; Balcom, B.J.; Folliard, K.J. Studies on lithium salts to mitigate ASR-induced expansion in new concrete: A critical review. Cem. Concr. Res. 2005, 35, 1789–1796. [Google Scholar] [CrossRef]
- Kawamura, M.; Fuwa, H. Effects of lithium salts on ASR gel composition and expansion of mortars. Cem. Concr. Res. 2003, 33, 913–919. [Google Scholar] [CrossRef]
- Lumley, J.S. ASR suppression by lithium compounds. Cem. Concr. Res. 1997, 27, 235–244. [Google Scholar] [CrossRef]
- Akhnoukh, A.K.; Buckhalter, C.R. Ultra-high-performance concrete: Constituents, mechanical properties, applications, and current challenges. J. Case Stud. Constr. Mater. 2021, 15, e00511. [Google Scholar] [CrossRef]
- Akhnoukh, A.K.; Elia, H. Developing high performance concrete for precast/prestressed concrete industry. J. Case Stud. Constr. Mater. 2019, 11, e00290. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Coelho, L.; Azenha, M.; De Almeida, J.M.; Jorge, P.A.S.; Silva, C.J.R. Alkali-silica reaction in concrete: Mechanism, mitigation, and test methods. Constr. Build. Mater. 2019, 222, 903–931. [Google Scholar] [CrossRef]
- Mahyar, M.; Erdogan, S.T.; Tokyay, M. Extension of the chemical index model for estimating alkali-silica reaction mitigation efficiency to slags and natural pozzolans. Constr. Build. Mater. 2018, 179, 587–597. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K. The mechanism of limited inhibition by fly ash on expansion due to alkali-silica reaction at the pessimum proportion. Cem. Concr. Res. 2017, 92, 1–15. [Google Scholar] [CrossRef]
- Deschenes, R.; Murray, C.D.; Hale, W. Mitigation of alkali-silica reaction and freezing and thawing through surface treatment. ACI Mater. J. 2017, 114, 307–314. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Folliard, K.J.; Fournier, B.; Drimalas, T.; Rivard, P. Study of remedial actions on highway structures affected by ASR. In Proceedings of the 14th International Conference on Alkali-Aggregate Reaction (ICAR), Austin, TX, USA, 20–25 May 2012. [Google Scholar]
- Ideker, J.H.; Drimalas, T.; Bentivegna, A.F.; Folliard, K.J.; Fournier, B.; Thomas, M.D.A.; Hooten, R.D.; Rogers, C.A. The importance of outdoor exposure site testing. In Proceedings of the 14th International Conference on Alkali Aggregate Reaction (ICAAR), Austin, TX, USA, 20–25 May 2012. [Google Scholar]





| ASR Damage Rating | Nature and Extent of Damage Features |
|---|---|
| Low |
|
| Moderate |
|
| High |
|
| Specimen | Aggregate | Silica Fume | Class C Fly Ash |
|---|---|---|---|
| F1-SF(0%)-FA(0%) | Fine Aggregate (F1) | 0% | |
| F1-SF(15%)-FA(0%) | 15% | 0% | |
| F1-SF(30%)-FA(0%) | 30% | 0% | |
| F1-SF(0%)-FA(15%) | 0% | 15% | |
| F1-SF(0%)-FA(30%) | 0% | 30% | |
| F2-SF(0%)-FA(0%) | Fine Aggregate (F2) | 0% | |
| F2-SF(15%)-FA(0%) | 15% | 0% | |
| F3-SF(30%)-FA(0%) | 30% | 0% | |
| F4-SF(0%)-FA(15%) | 0% | 15% | |
| F5-SF(0%)-FA(30%) | 0% | 30% | |
| F3-SF(0%)-FA(0%) | Fine Aggregate (F3) | 0% | |
| F3-SF(15%)-FA(0%) | 15% | 0% | |
| F3-SF(30%)-FA(0%) | 30% | 0% | |
| F3-SF(0%)-FA(15%) | 0% | 15% | |
| F3-SF(0%)-FA(30%) | 0% | 30% | |
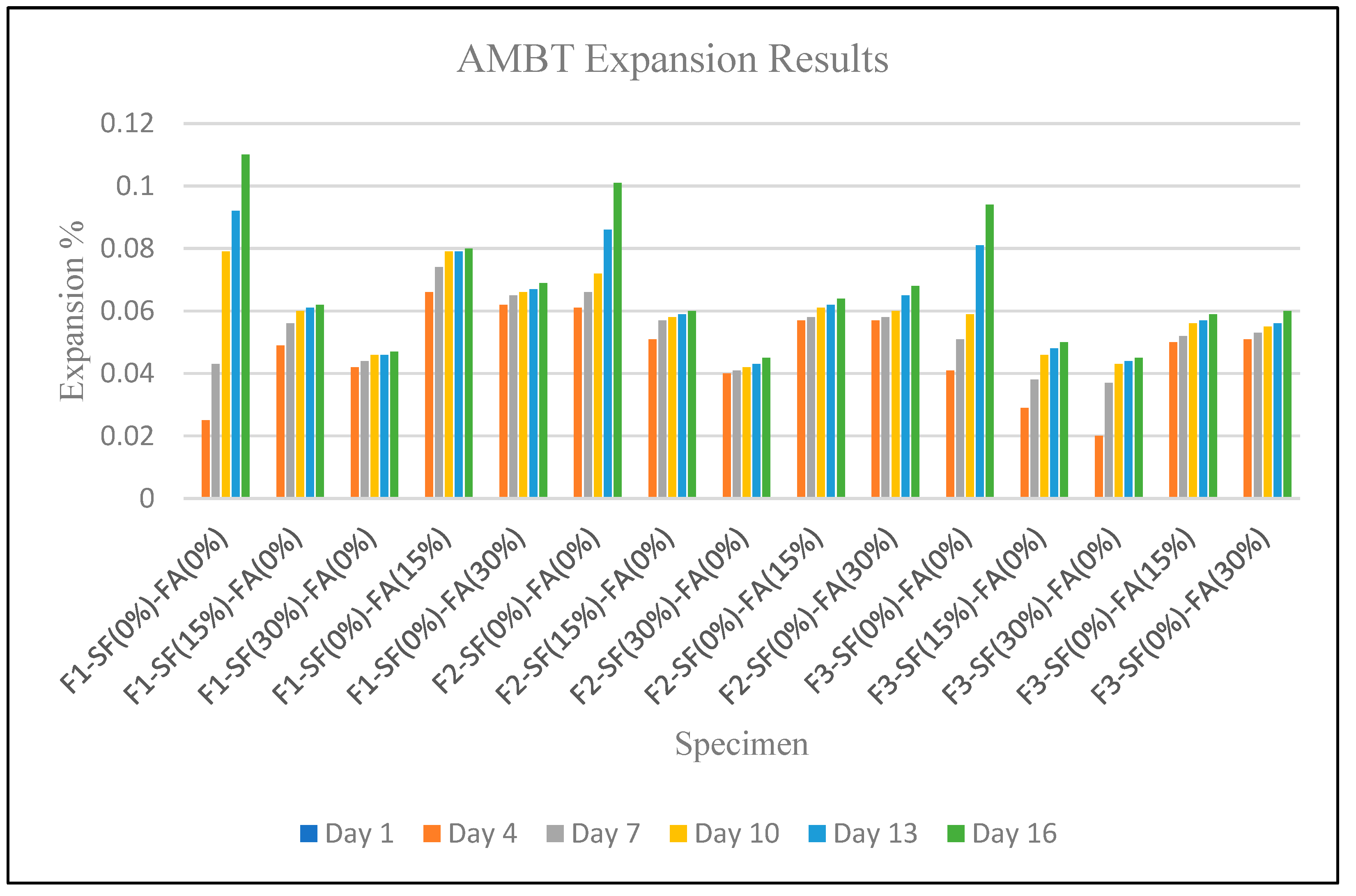
| Specimen | Day 1 | Day 4 | Day 7 | Day 10 | Day 13 | Day 16 |
|---|---|---|---|---|---|---|
| F1-SF(0%)-FA(0%) | 0.000 | 0.025 | 0.043 | 0.079 | 0.092 | 0.110 |
| F1-SF(15%)-FA(0%) | 0.000 | 0.049 | 0.056 | 0.060 | 0.061 | 0.062 |
| F1-SF(30%)-FA(0%) | 0.000 | 0.042 | 0.044 | 0.046 | 0.046 | 0.047 |
| F1-SF(0%)-FA(15%) | 0.000 | 0.066 | 0.074 | 0.079 | 0.079 | 0.080 |
| F1-SF(0%)-FA(30%) | 0.000 | 0.062 | 0.065 | 0.066 | 0.067 | 0.069 |
| F2-SF(0%)-FA(0%) | 0.000 | 0.061 | 0.066 | 0.072 | 0.086 | 0.101 |
| F2-SF(15%)-FA(0%) | 0.000 | 0.051 | 0.057 | 0.058 | 0.059 | 0.060 |
| F2-SF(30%)-FA(0%) | 0.000 | 0.040 | 0.041 | 0.042 | 0.043 | 0.045 |
| F2-SF(0%)-FA(15%) | 0.000 | 0.057 | 0.058 | 0.061 | 0.062 | 0.064 |
| F2-SF(0%)-FA(30%) | 0.000 | 0.057 | 0.058 | 0.060 | 0.065 | 0.068 |
| F3-SF(0%)-FA(0%) | 0.000 | 0.041 | 0.051 | 0.059 | 0.081 | 0.094 |
| F3-SF(15%)-FA(0%) | 0.000 | 0.029 | 0.038 | 0.046 | 0.048 | 0.050 |
| F3-SF(30%)-FA(0%) | 0.000 | 0.020 | 0.037 | 0.043 | 0.044 | 0.045 |
| F3-SF(0%)-FA(15%) | 0.000 | 0.050 | 0.052 | 0.056 | 0.057 | 0.059 |
| F3-SF(0%)-FA(30%) | 0.000 | 0.051 | 0.053 | 0.055 | 0.056 | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhnoukh, A.K. Improving Concrete Infrastructure Project Conditions by Mitigating Alkali–Silica Reactivity of Fine Aggregates. Constr. Mater. 2023, 3, 233-243. https://doi.org/10.3390/constrmater3020015
Akhnoukh AK. Improving Concrete Infrastructure Project Conditions by Mitigating Alkali–Silica Reactivity of Fine Aggregates. Construction Materials. 2023; 3(2):233-243. https://doi.org/10.3390/constrmater3020015
Chicago/Turabian StyleAkhnoukh, Amin K. 2023. "Improving Concrete Infrastructure Project Conditions by Mitigating Alkali–Silica Reactivity of Fine Aggregates" Construction Materials 3, no. 2: 233-243. https://doi.org/10.3390/constrmater3020015
APA StyleAkhnoukh, A. K. (2023). Improving Concrete Infrastructure Project Conditions by Mitigating Alkali–Silica Reactivity of Fine Aggregates. Construction Materials, 3(2), 233-243. https://doi.org/10.3390/constrmater3020015




