Cement and Clinker Production by Indirect Mechanosynthesis Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Methods
2.2.1. Indirect Mechanosynthesis
- LSF (lime saturation factor) =
- SM (Silica Modulus) =
- AM (Alumina modulus) =
2.2.2. Characterization of Powders (Clinkers & Cements) and Cements Pastes
3. Results and discussions
3.1. Clinker Production
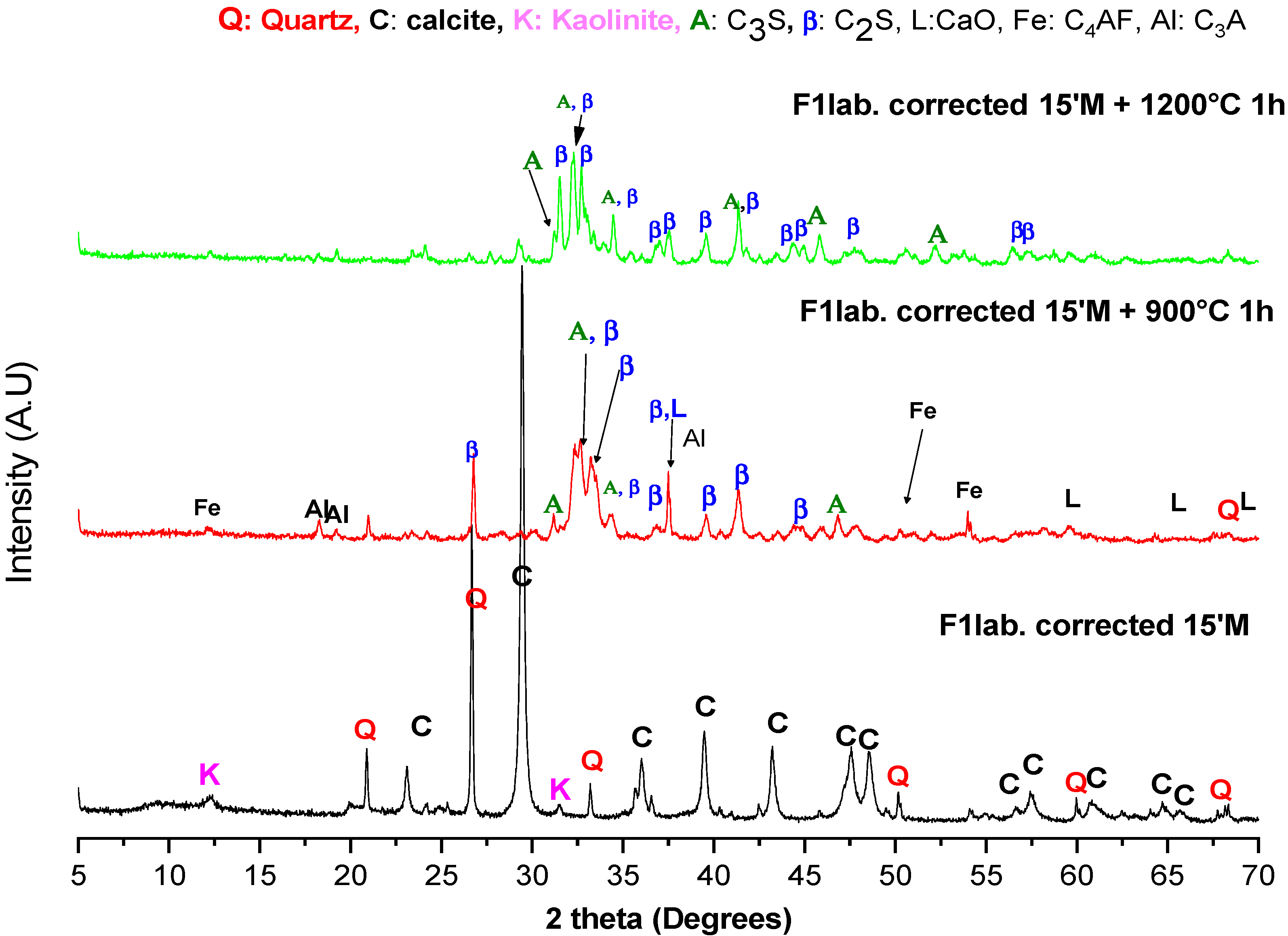
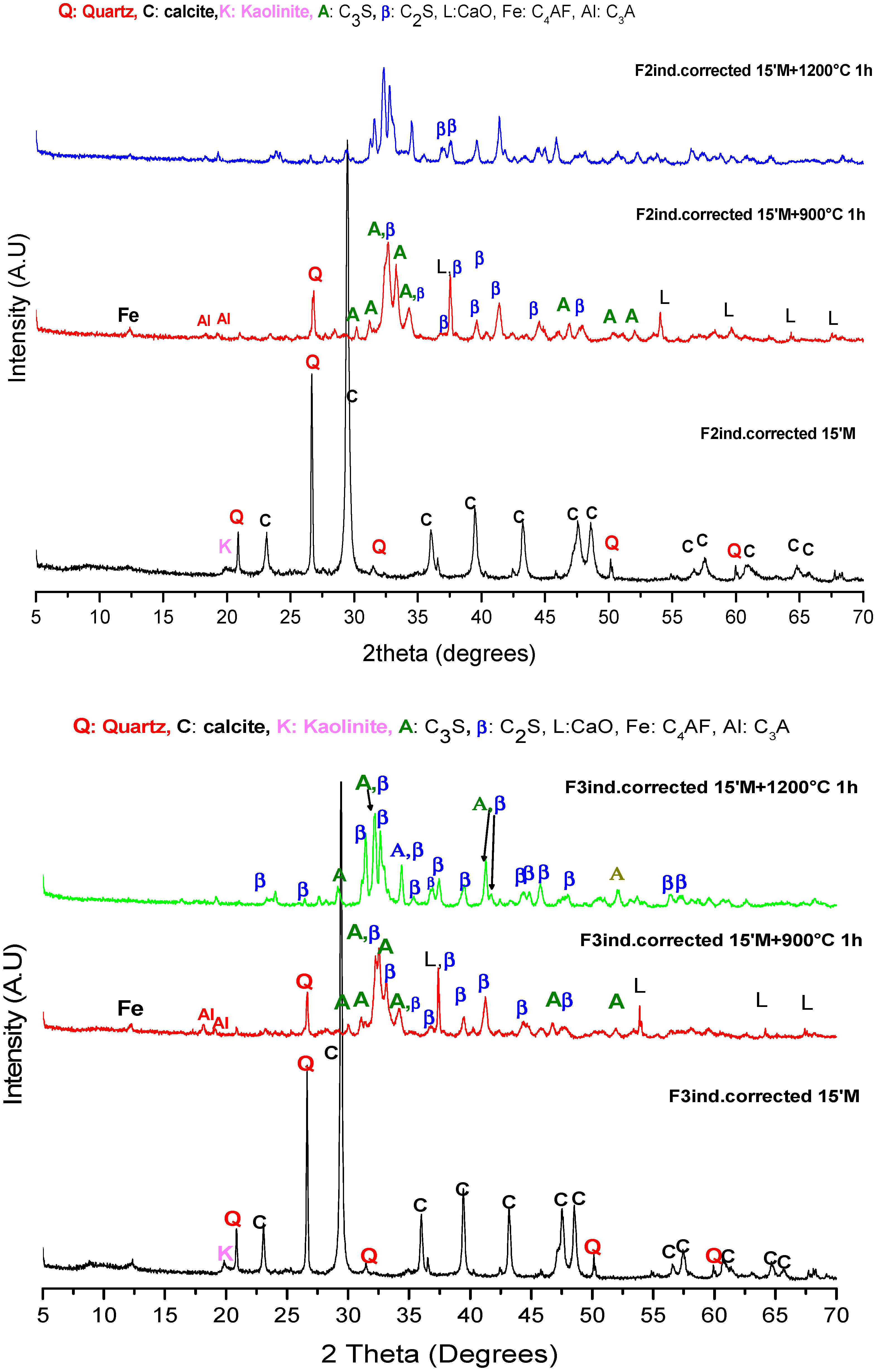
3.1.1. XRD
3.1.2. Particle Size Distribution (PSD)
3.2. Cement Production
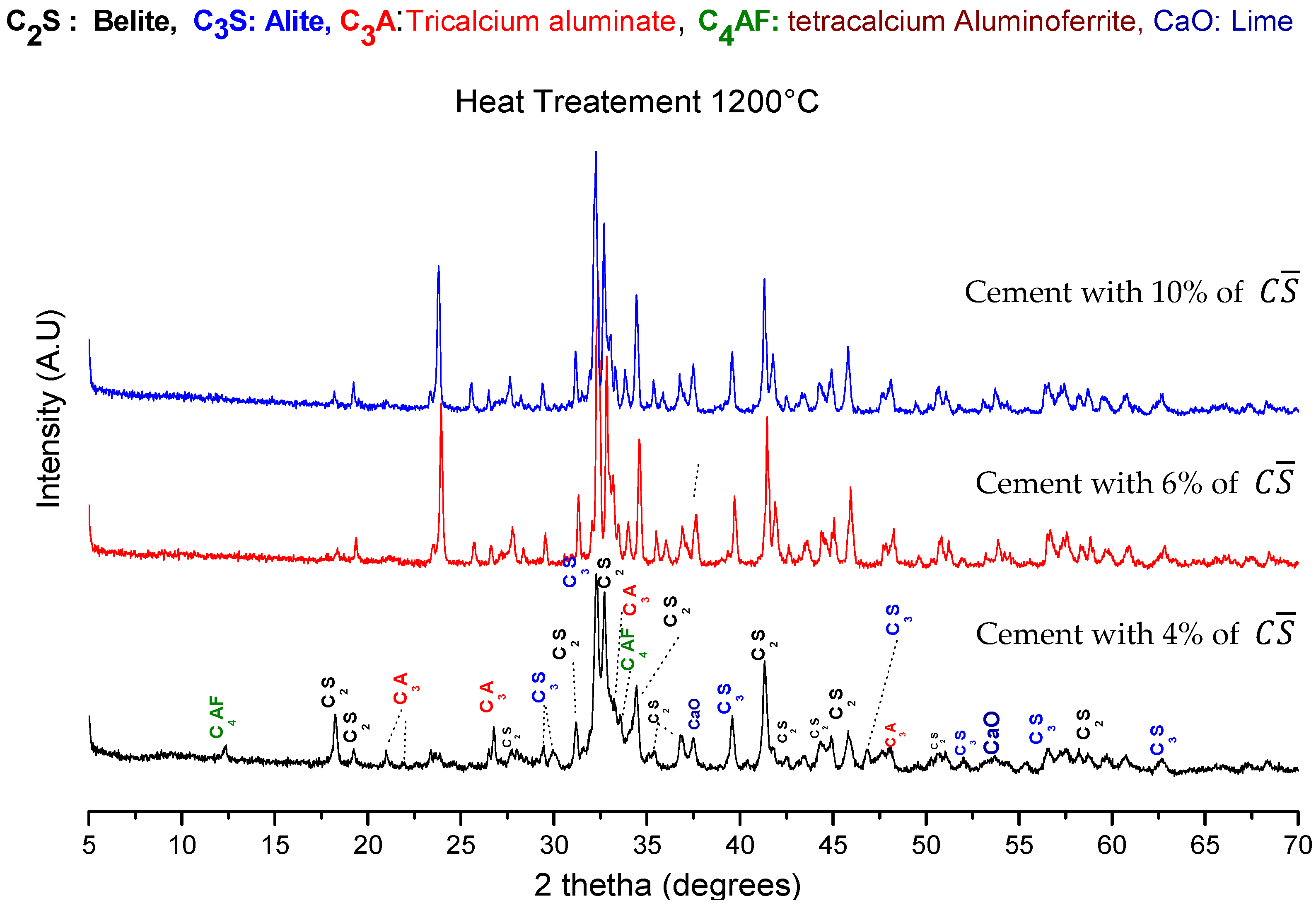
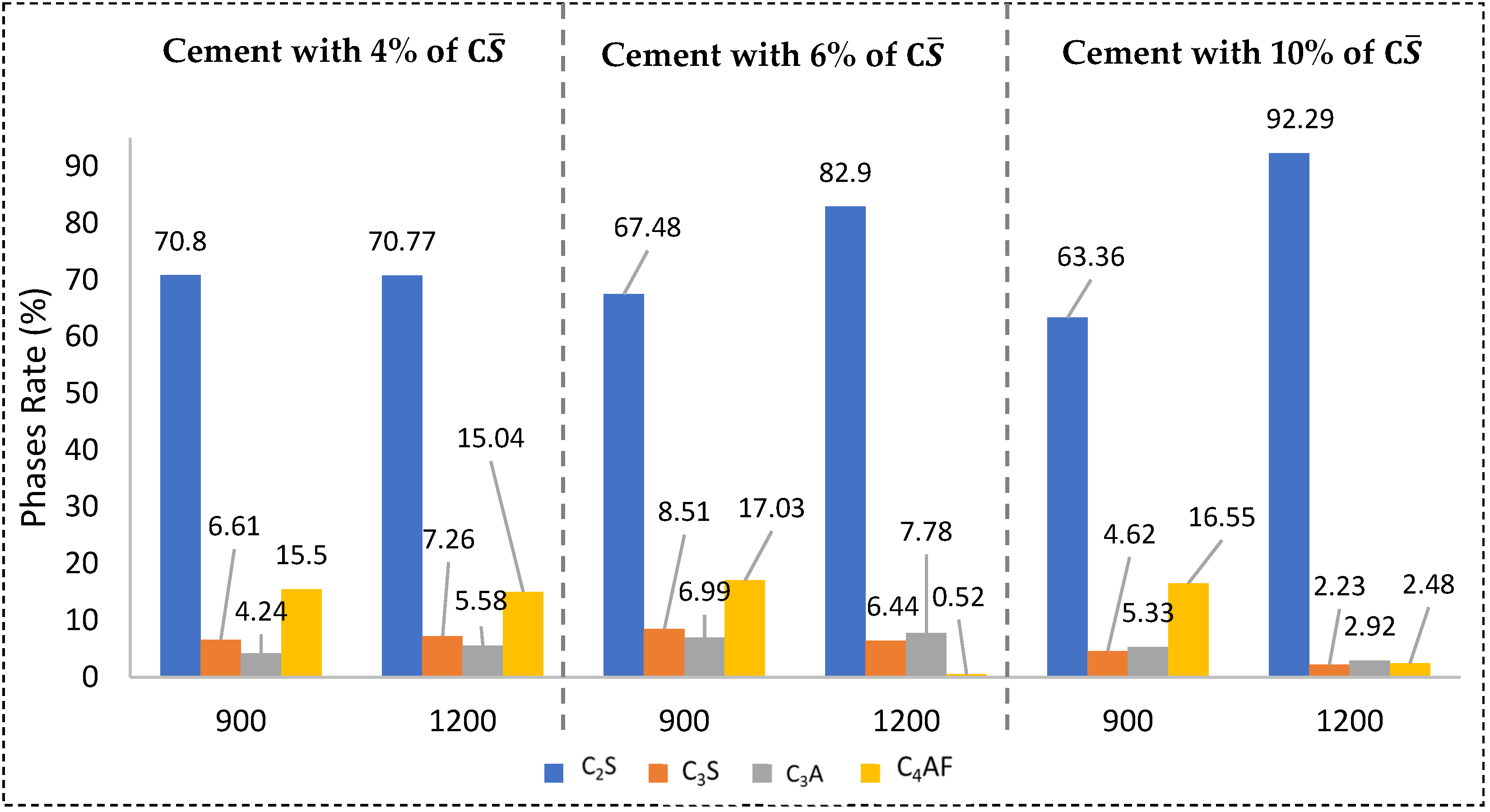
3.2.1. XRD
3.2.2. Particle Size Distribution of Cement
3.2.3. Compressive Strength (CS) of the Cement Paste
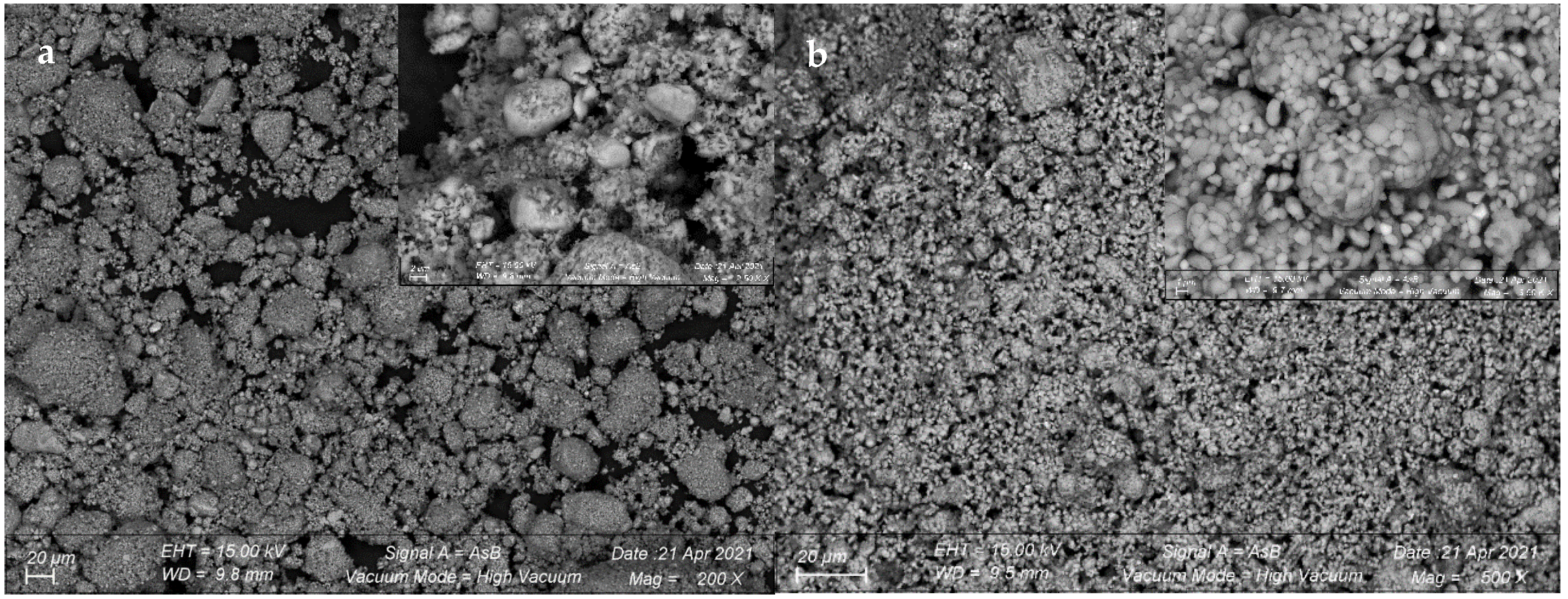
3.2.4. Morphology of the Cement Produced
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NF EN 197 1-Cement: Part 1: Composition, Specifications and Conformity Criteria for Common Cements; AFNOR: Saint-Denis, France, 2012.
- Taylor, H.F.W. (Ed.) Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Hewlett, P.C.; Liska, M. (Eds.) Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier: Oxford, UK, 2019; ISBN 0750662565. [Google Scholar]
- IEA Technology Roadmap—Low-Carbon Transition in the Cement Industry. 2018. Available online: www.wbcsdcement.org. (accessed on 5 December 2018).
- Siddique, R.; Khan, M.I. Supplementary Cementing Materials; Springer: Berlin, Germany, 2011. [Google Scholar]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Peris, E.; González-López, E. Mechanical treatments of fly ashes. Part III: Studies on strength development of ground fly ashes (GFA)—Cement mortars. Cem. Concr. Res. 1997, 27, 1365–1377. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Peris-Mora, E.; Amahjour, F. Mechanical treatment of fly ashes—Part IV. Strength development of ground fly ash-cement mortars cured at different temperatures. Cem. Concr. Res. 2000, 30, 543–551. [Google Scholar] [CrossRef]
- Behim, M.; Cyr, M.; Clastres, P. Physical and chemical effects of El Hadjar slag used as an additive in cement-based materials. Eur. J. Environ. Civ. Eng. 2011, 15, 1413–1432. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Scrivener, K.L. SPECIAL ISSUE-Future Cements Options for the Future of Cement. 2014. Available online: http://www.lc3.ch/wp-content/uploads/2014/09/0851_ICJ_Article.pdf (accessed on 6 March 2019).
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- CEMBUREAU. Cementing the European Green Deal. 2020. Available online: https://cembureau.eu/green-deal/ (accessed on 5 June 2020).
- Hamzaoui, R.; Bouchenafa, O. Equivalent Cement Clinker Obtained by Indirect Mechanosynthesis Process. Materials 2020, 13, 5045. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying, Nanotechnology, Materials Science and Powder Metallurgy, 2nd ed.; William Andrew: Waltham, MA, USA, 2015. [Google Scholar]
- Gaffet, E.; Caër, G. Le Mechanical Milling. In Nanomaterials and Nanochemistry; Bréchignac, C., Houdy, P., Lahmani, M., Eds.; Springer: Berlin, Germany, 2008; pp. 455–471. [Google Scholar]
- Hamzaoui, R. Mécanosynthèse et Propriétés Magnétiques D’alliages Fe-Ni. Ph.D. Thesis, Université de Technologie de Belfort-Montbeliard, Belfort, France, 2004. [Google Scholar]
- Hamzaoui, R.; Bouchenafa, O.; Guessasma, S.; Leklou, N.; Bouaziz, A. The sequel of modified fly ashes using high energy ball milling on mechanical performance of substituted past cement. Mater. Des. 2016, 90, 29–37. [Google Scholar] [CrossRef]
- Bouchenafa, O.; Hamzaoui, R.; Bennabi, A.; Colin, J. PCA effect on structure of fly ashes and slag obtained by mechanosynthesis. Applications: Mechanical performance of substituted paste CEMI + 50% slag /or fly ashes. Constr. Build. Mater. 2019, 203, 120–133. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Bouchenafa, O.; BEN MAAOUIA, O.; Guessasma, S. Mechanosynthesis for kaolinite activation: The impact of the substitution on the mechanical performances of mortar. Powder Technol. 2019, 355, 340–348. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Mehrotra, S.P. Towards sustainable solutions for fly ash through mechanical activation. Resour. Conserv. Recycl. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Bouaziz, A.; Hamzaoui, R.; Guessasma, S.; Lakhal, R.; Achoura, D.; Leklou, N. Efficiency of high energy over conventional milling of granulated blast furnace slag powder to improve mechanical performance of slag cement paste. Powder Technol. 2017, 308, 37–46. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Badjena, S.; Mehrotra, S.P. Hydration of mechanically activated granulated blast furnace slag. Metall. Mater. Trans. B 2005, 36, 873–883. [Google Scholar] [CrossRef]
- Zhao, F.-Q.; Ni, W.; Wang, H.-J.; Liu, H.-J. Activated fly ash/slag blended cement. Resour. Conserv. Recycl. 2007, 52, 303–313. [Google Scholar] [CrossRef]
- Alex, T.C.; Kalinkin, A.M.; Nath, S.K.; Gurevich, B.I.; Kalinkina, E.V.; Tyukavkina, V.V.; Kumar, S. Utilization of zinc slag through geopolymerization: Influence of milling atmosphere. Int. J. Miner. Process. 2013, 123, 102–107. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Temuujin, J.; Rickard, W.; van Riessen, A. Characterization of various fly ashes for preparation of geopolymers with advanced applications. Adv. Powder Technol. 2013, 24, 495–498. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Guessasma, S.; Abahri, K.; Bouchenafa, O. Formulation of modified cement mortars using optimal combination of fly ashes, shiv and hemp fibres. J. Mater. Civ. Eng. 2020, 32, 04019354. [Google Scholar] [CrossRef]
- Malhouroux-Gaffet, N.; Gaffet, E. Solid state reaction induced by post-milling annealing in the FeSi system. J. Alloys Compd. 1993, 198, 143–154. [Google Scholar] [CrossRef]
- Öksüz, K.E.; Apaydın, F.; Bozdağ, A.E.; Çevik, M.; Özer, A. Phase and Morphological Evaluation of Mechanically Activated Sintered YAG Powders. Procedia Mater. Sci. 2015, 11, 44–48. [Google Scholar] [CrossRef]
- Farnè, G.; Ricciardiello, F.G.; Podda, L.K.; Minichelli, D. Innovative milling of ceramic powders: Influence on sintering zirconia alloys. J. Eur. Ceram. Soc. 1999, 19, 347–353. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling; Marcel Dekker. 2004. Available online: https://www.crcpress.com/Mechanical-Alloying-And-Milling/Suryanarayana/p/book/9780824741037 (accessed on 22 January 2019).
- Balaz, P. Applied Mechanochemistry. In Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 297–405. [Google Scholar] [CrossRef]
- Andini, S.; Bolognese, A.; Formisano, D.; Manfra, M.; Montagnaro, F.; Santoro, L. Mechanochemistry of ibuprofen pharmaceutical. Chemosphere 2012, 88, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Zerzouri, M.; Bouchenafa, O.; Hamzaoui, R.; Ziyani, L.; Alehyen, S. Physico-chemical and mechanical properties of fly ash based-geopolymer pastes produced from pre-geopolymer powders obtained by mechanosynthesis. Constr. Build. Mater. 2021, 288, 123–135. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Fukuhara, M.; Goto, S.; Asaga, K.; Daimon, M.; Kondo, R. Mechanisms and kinetics of C4AF hydration with gypsum. Cem. Concr. Res. 1981, 11, 407–414. [Google Scholar] [CrossRef]
- Campbell, D.H. Microscopical Examination and Interpretation of Portland Cement and Clinker, 2nd ed.; Portland Cement Association: Skokie, IL, USA, 1999. [Google Scholar]

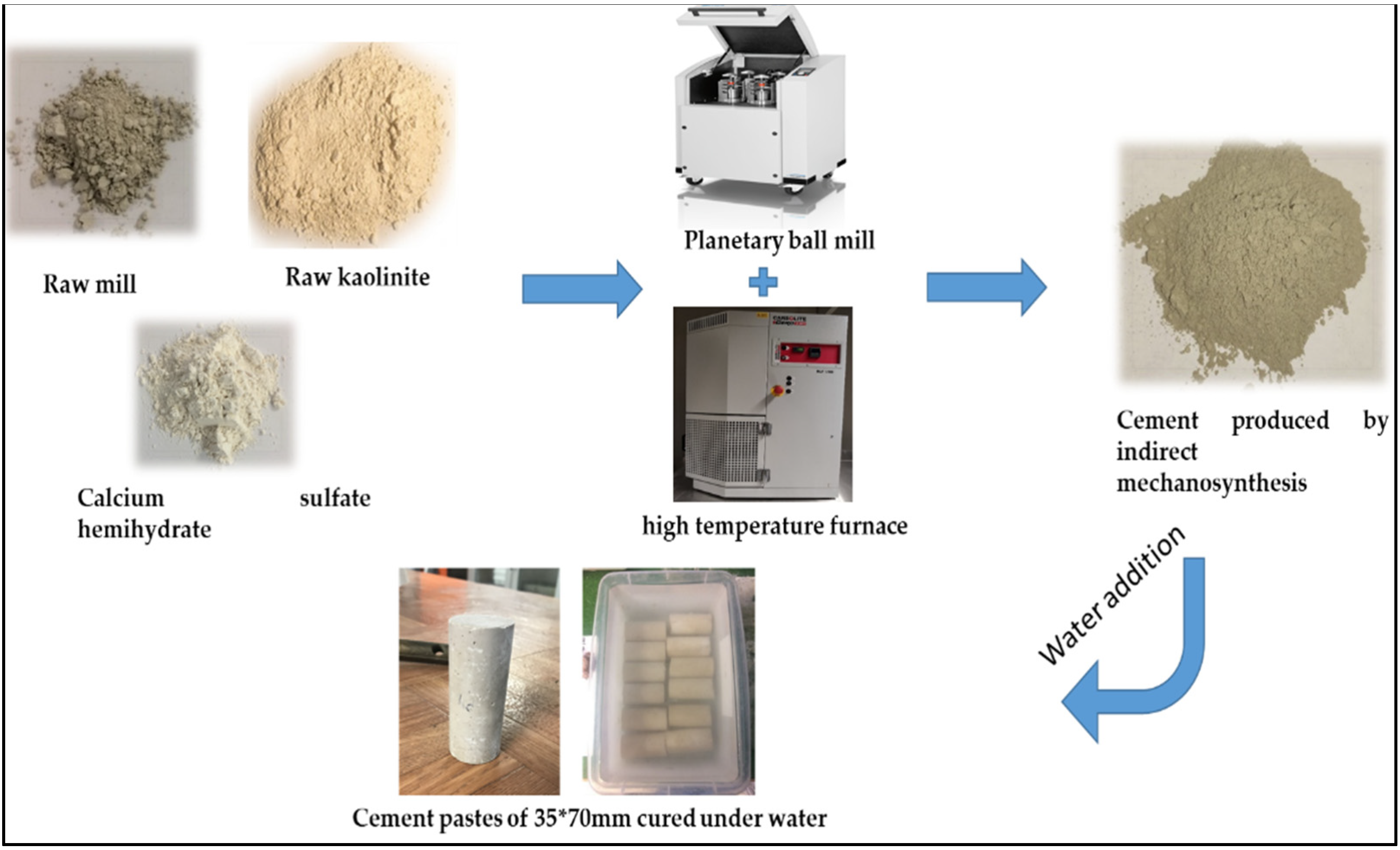






| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | TiO2 | SO3 | MnO | Na2O | LSF * | SM ** | AM *** | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Limestone | 96.79 | 1.3 | 0.63 | 0.22 | 0.5 | 0.16 | 0 | 0 | 0.07 | 0 | |||
| Kaolinite | 0.5 | 69.37 | 24.08 | 1.54 | 0.6 | 0.38 | 2.85 | 0.3 | 0 | 0 | |||
| F1lab. | 77.53 | 14.91 | 5.32 | 0.48 | 0.52 | 0.2 | 0.57 | 0.06 | 0.06 | 0 | 1.62 | 2.57 | 10.99 |
| F2ind. | 73.01 | 13.83 | 4.64 | 3.49 | 2 | 1.55 | 0.23 | 0.67 | 0.13 | 0 | 1.58 | 1.7 | 1.33 |
| F3ind. | 75.44 | 12.59 | 4.58 | 3.48 | 1.2 | 1.45 | 0 | 0.42 | 0.06 | 0 | 1.76 | 1.56 | 1.32 |
| Calcium sulfate hemihydrate | 40.21 | 1.41 | 0.67 | 0.06 | 0.7 | 0.08 | 0 | 51.03 | 0 | 5.3 |
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | TiO2 | SO3 | MnO | LSF * | SM ** | AM *** | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1lab. corrected | 66.93 | 20.71 | 7.3 | 2.99 | 0.52 | 0.23 | 0.81 | 0.1 | 0.05 | 0.98 | 2.01 | 2.44 |
| F2ind. corrected | 65.03 | 19.94 | 6.78 | 3.27 | 1.85 | 1.42 | 0.52 | 0.63 | 0.12 | 0.99 | 1.98 | 2.07 |
| F3ind. corrected | 65.32 | 20.26 | 7.21 | 3.2 | 1.12 | 1.31 | 0.38 | 0.4 | 0.05 | 0.97 | 1.94 | 2.24 |
| F1lab. Corrected | F2ind. Corrected | F3ind. Corrected | |
|---|---|---|---|
| Quartz | 16.50% | 15.26% | 15.52 |
| Calcite | 72.95% | 81.46% | 79.05 |
| Kaolinite | 10.75% | 3.28% | 5.43 |
| F1lab. 15′M | F2ind. 15′M | F3ind. 15′M | ||||
|---|---|---|---|---|---|---|
| 900 °C | 1200 °C | 900 °C | 1200 °C | 900 °C | 1200 °C | |
| α-C2S | 4.87% | 12.12% | 6.93% | 10.33% | 5.91% | 11.23% |
| β-C2S | 49.14% | 69.43% | 42.41% | 72.39% | 56.82% | 76% |
| C3S | 2.44% | 2.21% | 4.94% | 6.52% | 4.08% | 5.5% |
| C3A | 13.59% | 5.38% | 13.1% | 3.51% | 6.87% | 5.2% |
| C4AF | 19.3% | 9% | 20.69% | 5.79% | 16.18% | 0.31% |
| Lime | 3.31% | 1.62% | 5.5% | 1.33% | 4.79% | 1.76% |
| Quartz | 7.34% | 0.24% | 6.43% | 0.12% | 5.35% | 0 |
| D (10) | D (50) | D (90) | |
|---|---|---|---|
| CEMI | 4.25 | 14.47 | 32.88 |
| F2ind. | 1.36 | 6.35 | 80.22 |
| Clinker F2ind. 900 °C | 3.56 | 23.22 | 78.84 |
| Clinker F2ind. 1200 °C | 0.67 | 15.15 | 120.8 |
| Cement Paste | Compressive Strength (CS) (MPa) | |
|---|---|---|
| 900 °C | 1200 °C | |
| 3.60 | 7.60 | |
| 2.93 | 5.60 | |
| 3.67 | 3.59 | |
| CEM I | 23.03 | |
| CEM III | 19.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchenafa, O.; Hamzaoui, R.; Florence, C.; Mansoutre, S. Cement and Clinker Production by Indirect Mechanosynthesis Process. Constr. Mater. 2022, 2, 200-216. https://doi.org/10.3390/constrmater2040014
Bouchenafa O, Hamzaoui R, Florence C, Mansoutre S. Cement and Clinker Production by Indirect Mechanosynthesis Process. Construction Materials. 2022; 2(4):200-216. https://doi.org/10.3390/constrmater2040014
Chicago/Turabian StyleBouchenafa, Othmane, Rabah Hamzaoui, Céline Florence, and Sandrine Mansoutre. 2022. "Cement and Clinker Production by Indirect Mechanosynthesis Process" Construction Materials 2, no. 4: 200-216. https://doi.org/10.3390/constrmater2040014
APA StyleBouchenafa, O., Hamzaoui, R., Florence, C., & Mansoutre, S. (2022). Cement and Clinker Production by Indirect Mechanosynthesis Process. Construction Materials, 2(4), 200-216. https://doi.org/10.3390/constrmater2040014






