Anticorrosion Performance of Magnesium Hydroxide Coatings on Steel Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coatings and Substrates
2.1.1. Coatings
2.1.2. Substrates
2.1.3. Coatings’ Application onto Steel Substrate
2.2. Sulfuric Acid Spraying Test
2.3. Evaluation Methods
2.3.1. Mass Measurements
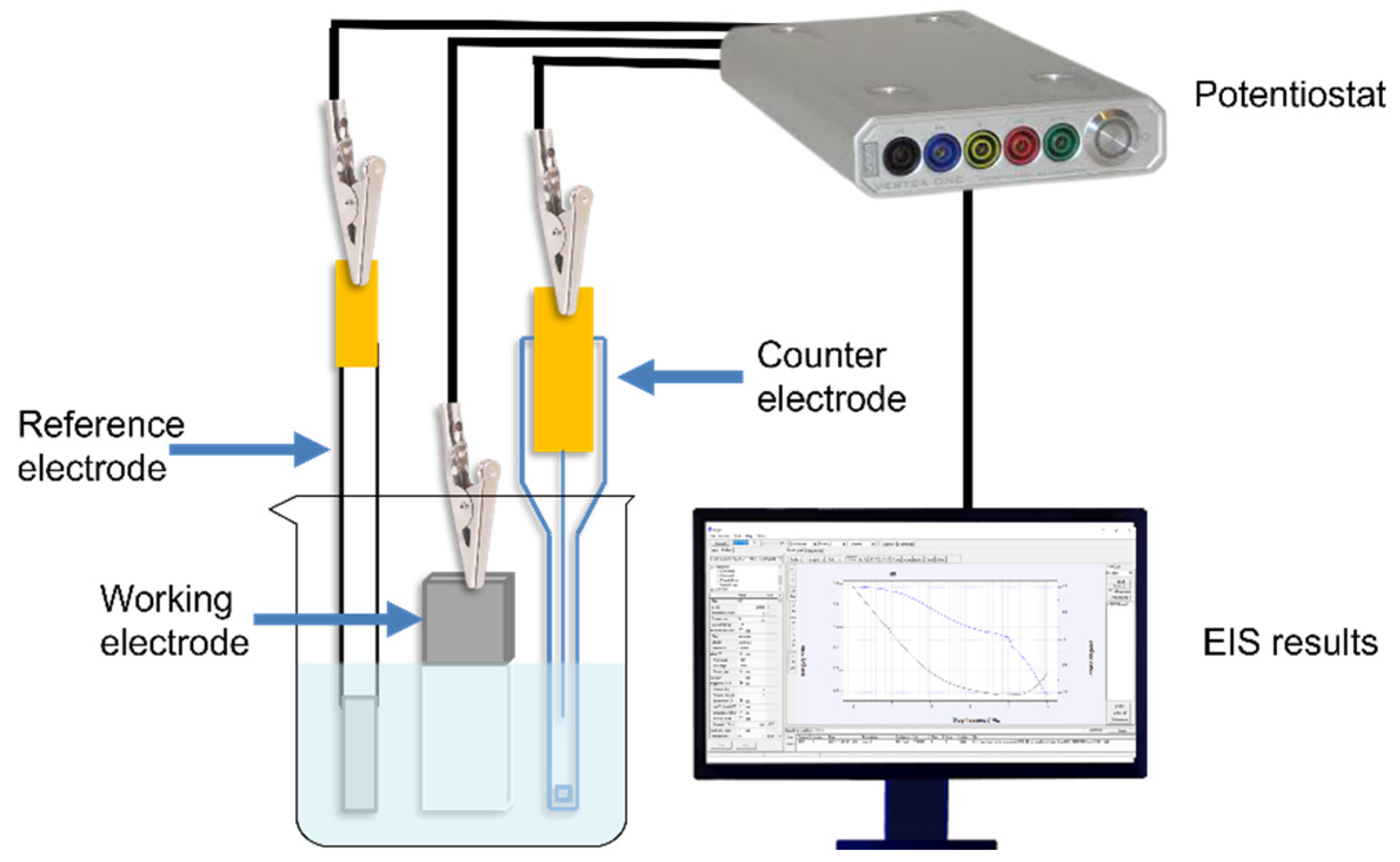
2.3.2. Electrochemical Impedance Spectroscopy (EIS)
3. Results
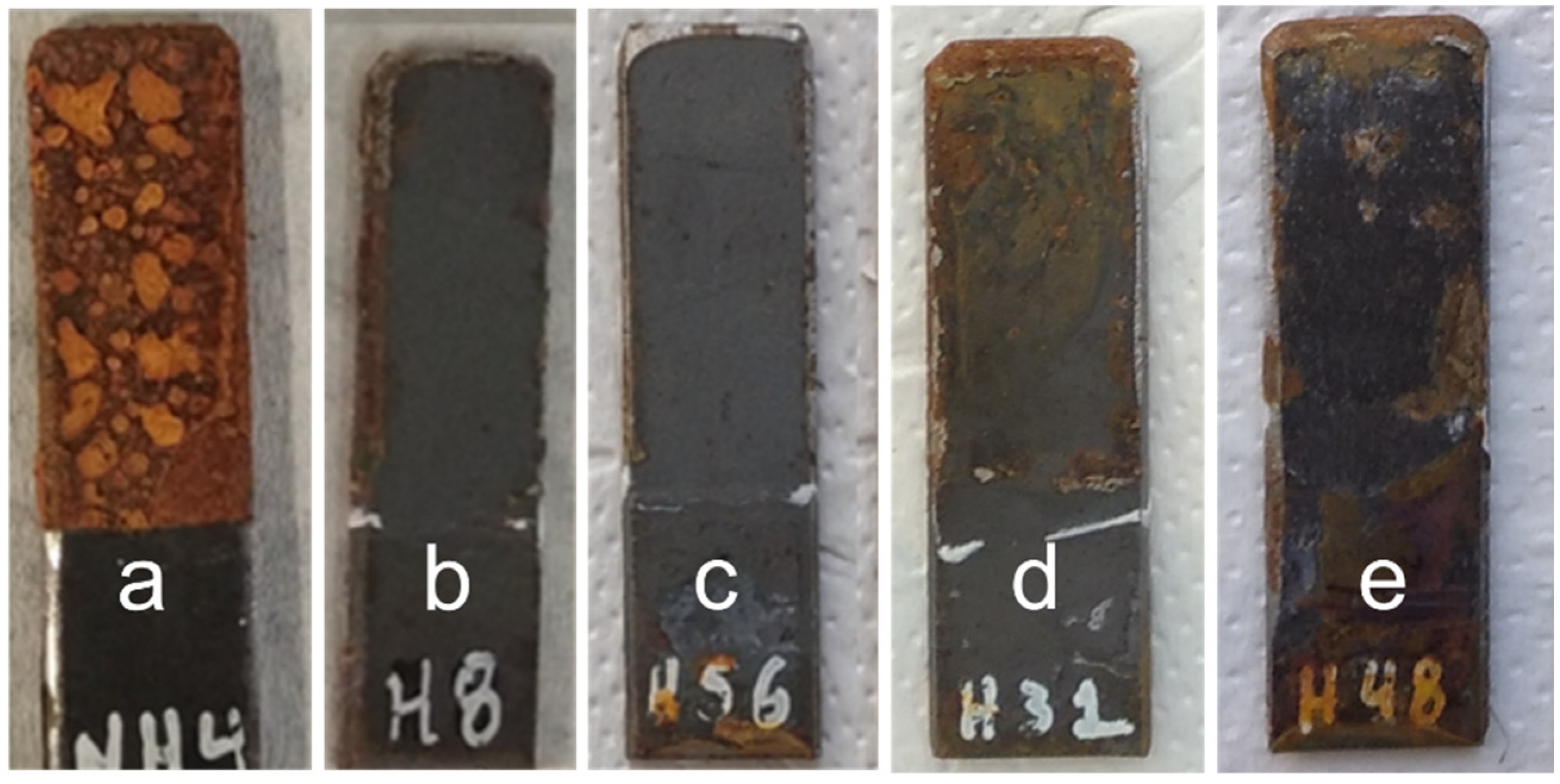
3.1. Optical Observation
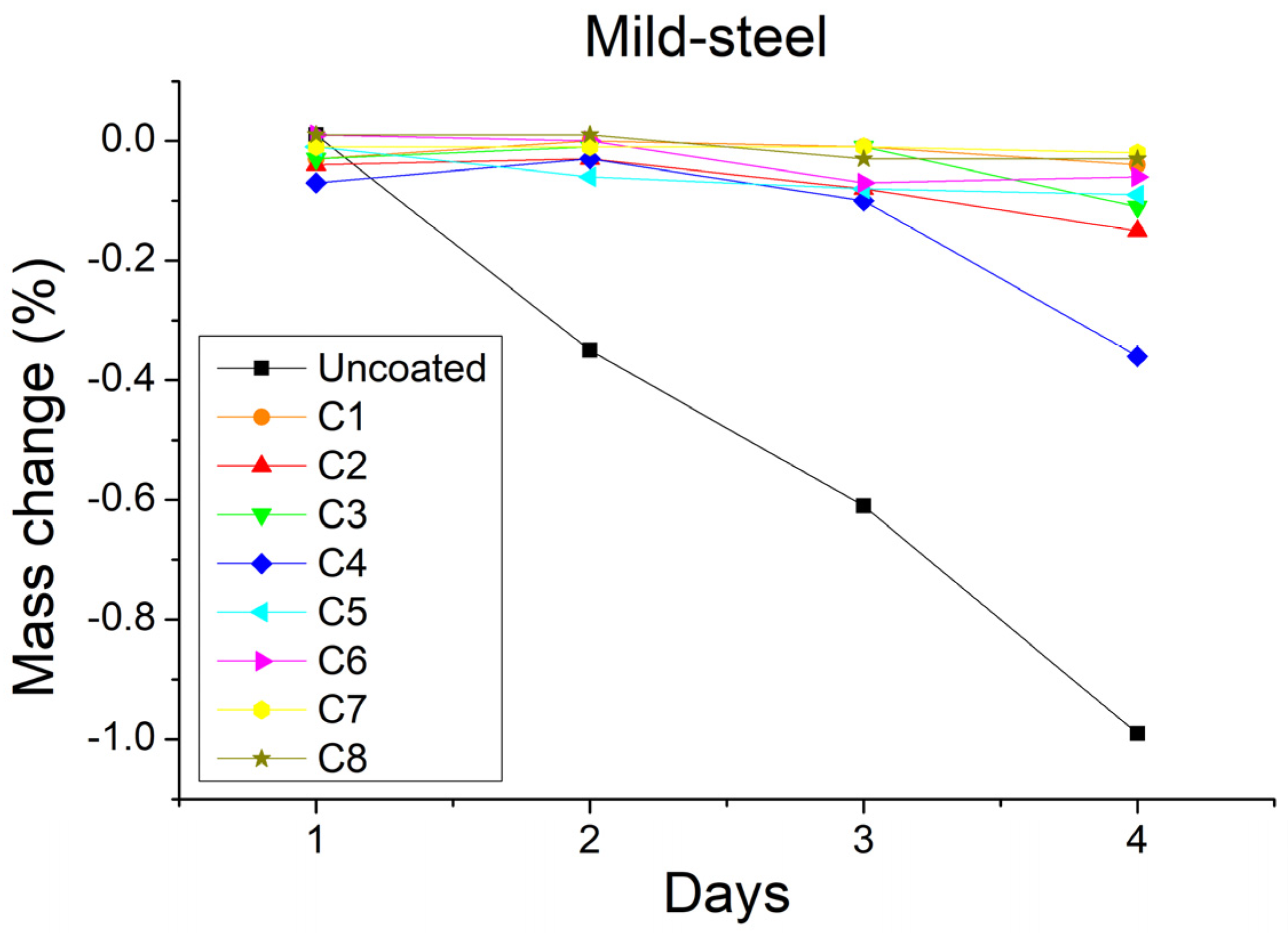
3.2. Mass Measurements
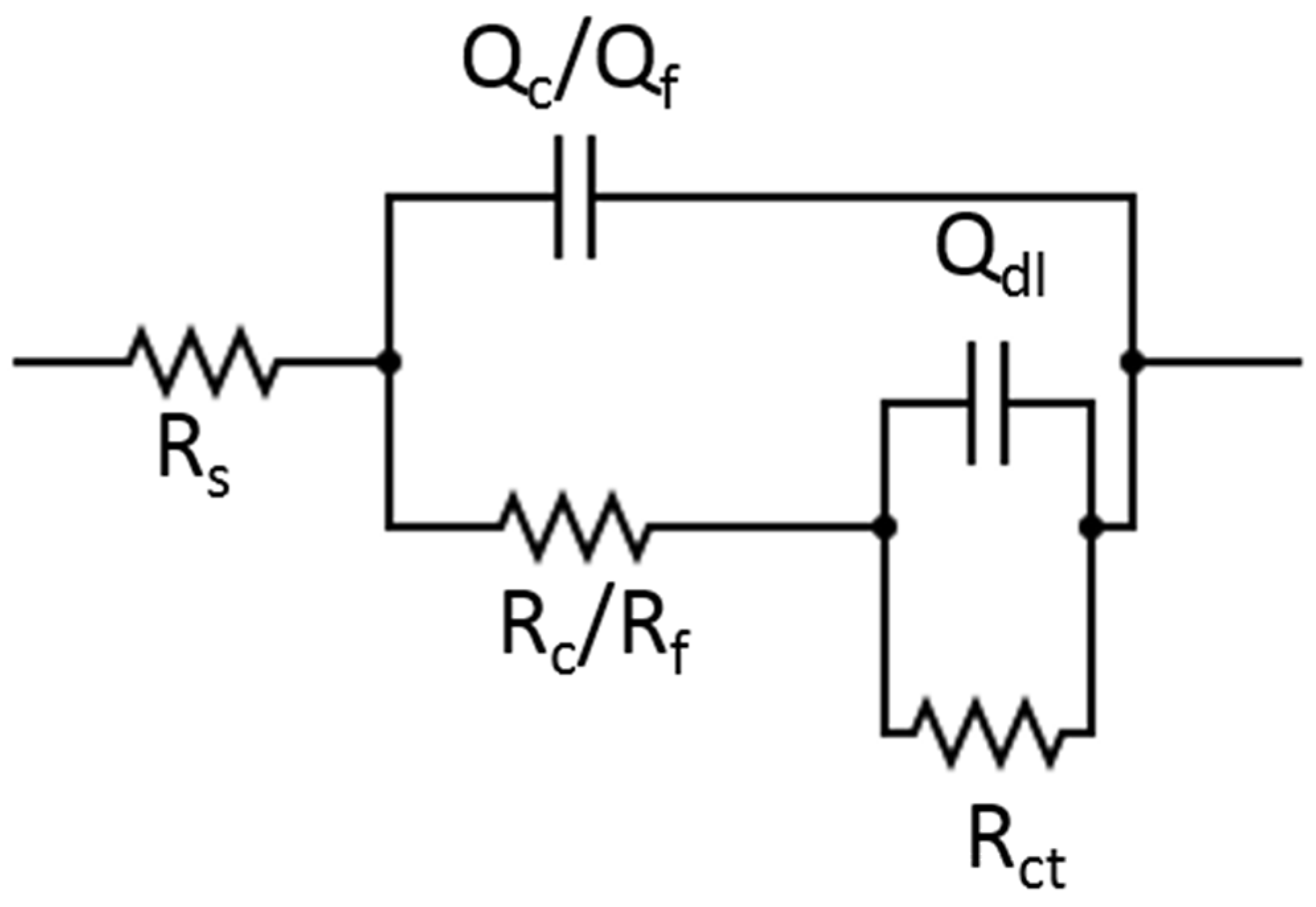
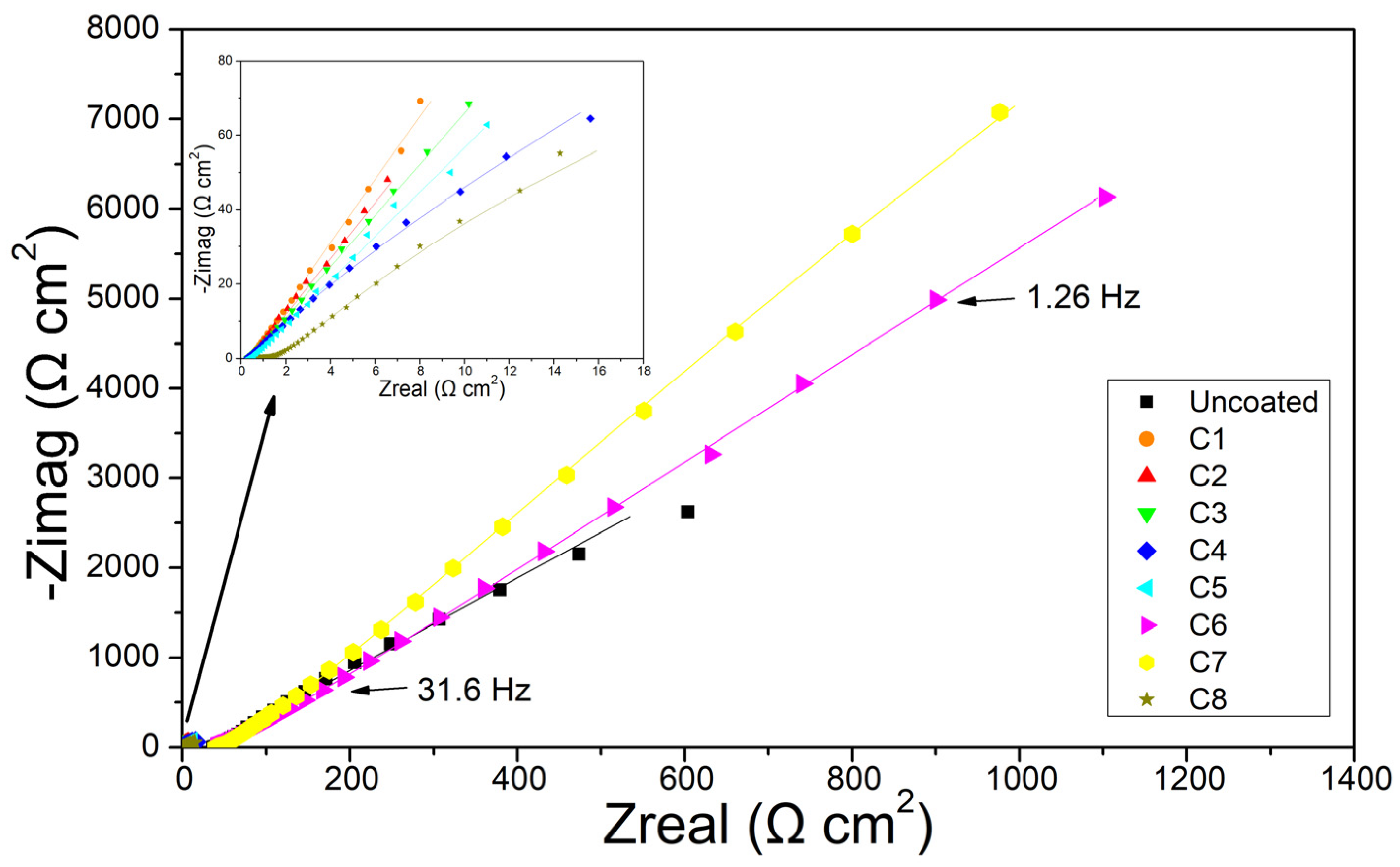
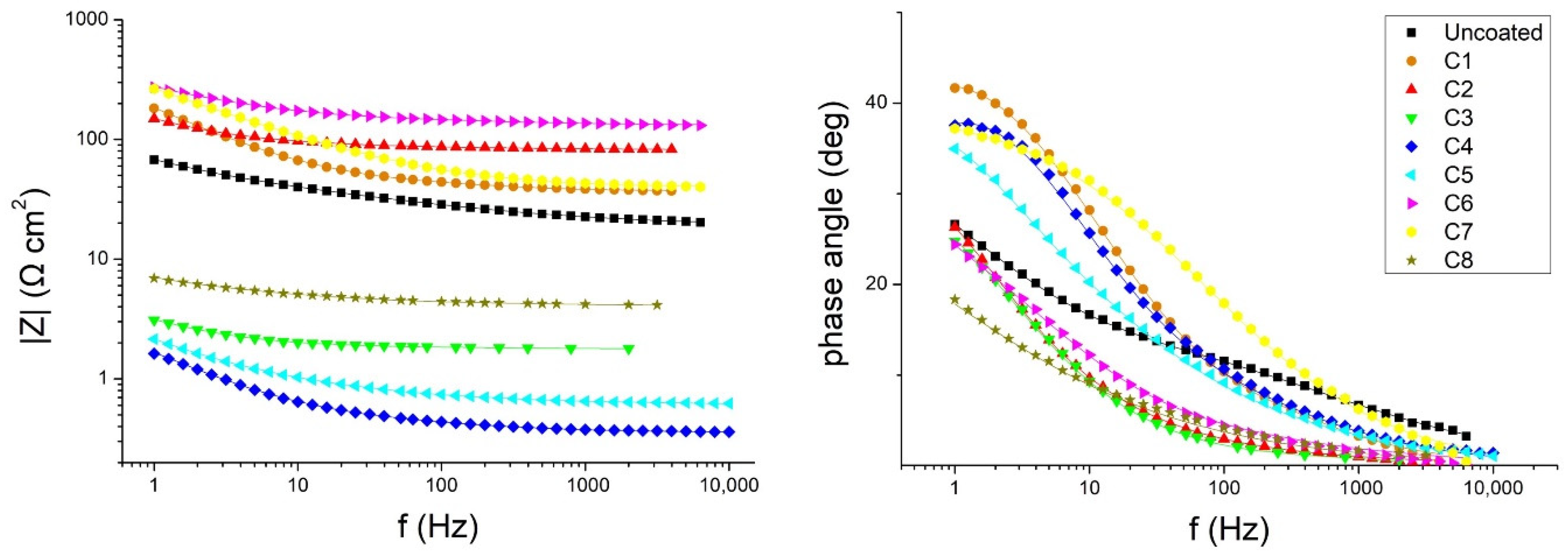
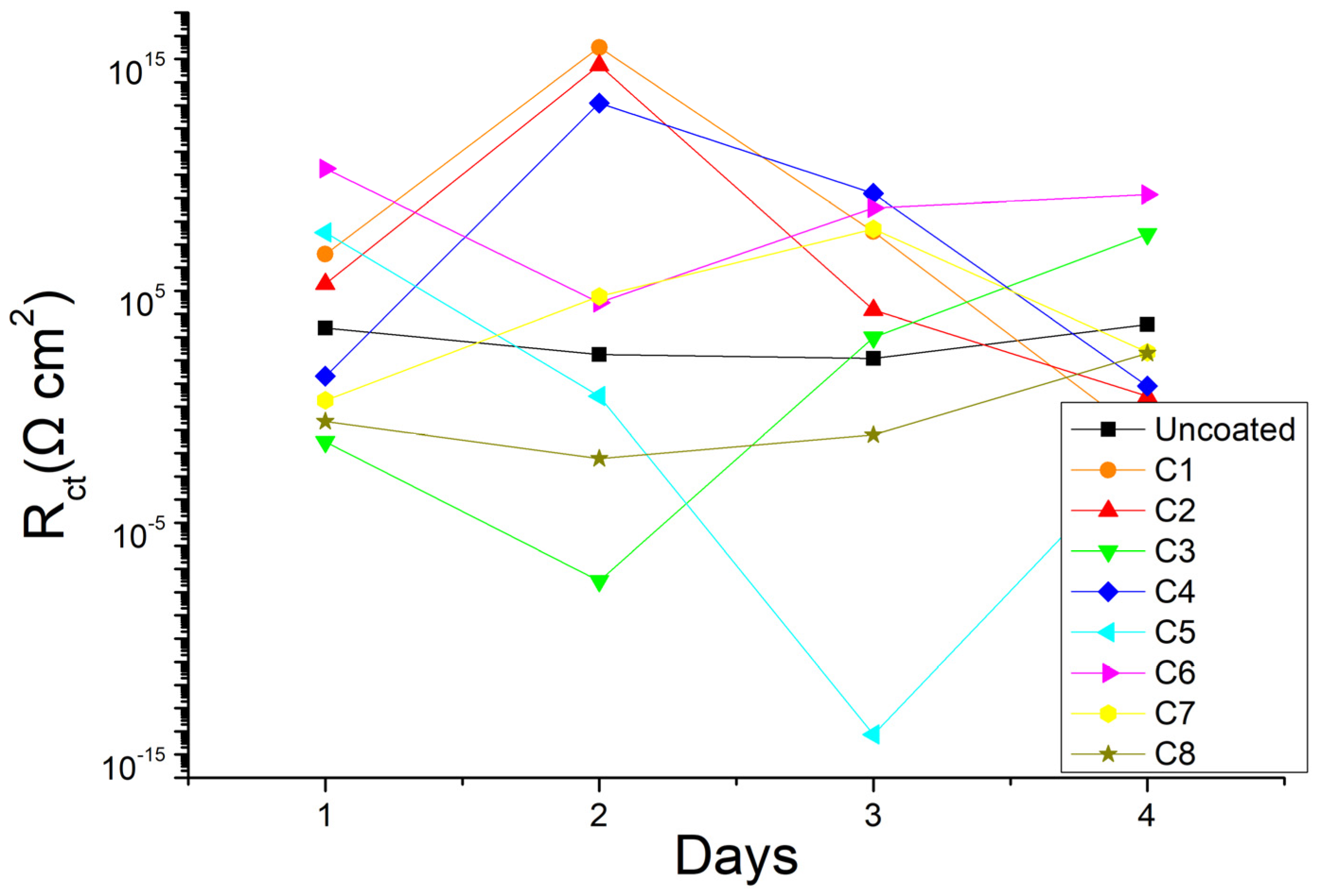
3.3. Electrochemical Impedance Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usher, K.M.; Kaksonen, A.H.; Cole, I.; Marney, D. Critical Review: Microbially Influenced Corrosion of Buried Carbon Steel Pipes. Int. Biodeterior. Biodegrad. 2014, 93, 84–106. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Mohseni-Dargah, M.; Firoozirad, K.; Aryai, V.; Razmjou, A.; Abbassi, R.; Garaniya, V.; Beheshti, A.; Asadnia, M. Recent Advances in Sensing and Assessment of Corrosion in Sewage Pipelines. Process Saf. Environ. Prot. 2021, 147, 192–213. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Hernandez, M.T. Use of Sustainable Antimicrobial Aggregates for the In-Situ Inhibition of Biogenic Corrosion on Concrete Sewer Pipes. MRS Adv. 2019, 4, 2939–2949. [Google Scholar] [CrossRef]
- Chetty, K.; Xie, S.; Song, Y.; McCarthy, T.; Garbe, U.; Li, X.; Jiang, G. Self-Healing Bioconcrete Based on Non-Axenic Granules: A Potential Solution for Concrete Wastewater Infrastructure. J. Water Process Eng. 2021, 42, 102139. [Google Scholar] [CrossRef]
- Cabral, L.L.B.; Sousa, J.T.; Lopes, W.S.; Leite, V.D.; Barbosa, R.A. Performance of Anaerobic Hybrid Reactor with Post-Treatment in Intermittent Flow Sand Filter: A Sulfide-Oxidizing Bioprocess for the Treatment of Sanitary Sewage Using Nitrate as Electron Acceptor. Environ. Processes 2020, 7, 1095–1109. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, J.; Zhang, Z.; Wang, S.; Zhang, Z.; Lu, J. New Method for Efficient Control of Hydrogen Sulfide and Methane in Gravity Sewers: Combination of NaOH and Nitrite. Front. Environ. Sci. Eng. 2021, 16, 75. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Kan, L.; Wu, M. Current Understanding on Microbiologically Induced Corrosion of Concrete in Sewer Structures: A Review of the Evaluation Methods and Mitigation Measures. Constr. Build. Mater. 2020, 247, 118539. [Google Scholar] [CrossRef]
- Kong, L.; Fang, J.; Zhang, B. Effectiveness of Surface Coatings Against Intensified Sewage Corrosion of Concrete. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 1177–1186. [Google Scholar] [CrossRef]
- Potvin, E.; Brossard, L.; Larochelle, G. Corrosion Protective Performances of Commercial Low-VOC Epoxy/Urethane Coatings on Hot-Rolled 1010 Mild Steel. Prog. Org. Coat. 1997, 31, 363–373. [Google Scholar] [CrossRef]
- Ziadi, I.; Alves, M.M.; Taryba, M.; El-Bassi, L.; Hassairi, H.; Bousselmi, L.; Montemor, M.F.; Akrout, H. Microbiologically Influenced Corrosion Mechanism of 304L Stainless Steel in Treated Urban Wastewater and Protective Effect of Silane-TiO2 Coating. Bioelectrochemistry 2020, 132, 107413. [Google Scholar] [CrossRef]
- Chatzis, A.; Merachtsaki, D.; Zouboulis, A. Performance of Three Magnesium-Based Coatings for Corrosion Protection of Concrete against Sulfuric Acid. Environ. Processes 2022, 9, 12. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.; Zouboulis, A. Anti-Corrosion Properties of Magnesium Oxide/Magnesium Hydroxide Coatings for Application on Concrete Surfaces (Sewerage Network Pipes). Constr. Build. Mater. 2021, 312, 125441. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.; Zouboulis, A. Evaluation of the Protection Ability of a Magnesium Hydroxide Coating against the Bio-Corrosion of Concrete Sewer Pipes, by Using Short and Long Duration Accelerated Acid Spraying Tests. Materials 2021, 14, 4897. [Google Scholar] [CrossRef] [PubMed]
- Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.K.; Yiannoulakis, H.; Zouboulis, A. Comparison of Different Magnesium Hydroxide Coatings Applied on Concrete Substrates (Sewer Pipes) for Protection against Bio-Corrosion. Water 2021, 13, 1227. [Google Scholar] [CrossRef]
- Sydney, R.; Esfandi, E.; Surapaneni, S. Control Concrete Sewer Corrosion via the Crown Spray Process. Water Environ. Res. 1996, 68, 338–347. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, F.; Li, Y.; Song, L.; Jiang, D.; Zeng, R.C.; Tjong, S.C.; Chen, D.C. Corrosion Resistance of Dodecanethiol-Modified Magnesium Hydroxide Coating on AZ31 Magnesium Alloy. Appl. Phys. A Mater. Sci. Processing 2020, 126, 8. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Tsiaras, S.; Peleka, E.; Zouboulis, A. Selection of Magnesium Hydroxide Coatings for Corrosion Mitigation in Concrete Sewer Pipes by Using Multiple Criteria Decision Analysis. Environ. Sustain. Indic. 2022, 13, 100168. [Google Scholar] [CrossRef]
- Berndt, M.L. Evaluation of Coatings, Mortars and Mix Design for Protection of Concrete against Sulphur Oxidising Bacteria. Constr. Build. Mater. 2011, 25, 3893–3902. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.; Berra, M.; Pastore, T.; Ormellese, M. Effect of Polymer Modified Cementitious Coatings on Water and Chloride Permeability in Concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Fytianos, G.; Papastergiadis, E.; Samaras, P.; Yiannoulakis, H.; Zouboulis, A. Properties and Performance of Novel Mg(OH)2-Based Coatings for Corrosion Mitigation in Concrete Sewer Pipes. Materials 2020, 13, 5291. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Charitidis, C.A. Corrosion Protection Evaluation of Mild Steel: The Role of Hybrid Materials Loaded with Inhibitors. Appl. Sci. 2020, 10, 6594. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. EIS Comparison on Corrosion Performance of PVD TiN and CrN Coated Mild Steel in 0.5 N NaCl Aqueous Solution. Corros. Sci. 2001, 43, 1953–1961. [Google Scholar] [CrossRef]
- Mansfeld, F. Electrochemical Impedance Spectroscopy (EIS) as a New Tool for Investigating Methods of Corrosion Protection. Electrochim. Acta 1990, 35, 1533–1544. [Google Scholar] [CrossRef]
- ASTM B117-11; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM G85-11; Standard Practice for Modified Salt Spray (Fog) Test. ASTM International: West Conshohocken, PA, USA, 2011.
- Hernández, H.H.; Reynoso, A.M.R.; González, J.C.T.; Morán, C.O.G.; Hernández, J.G.M.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. Electrochem. Impedance Spectrosc. 2020, 27, 137–144. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Xidas, P.; Giannakoudakis, P.; Triantafyllidis, K.; Spathis, P.; Merachtsaki, D.; Xidas, P.; Giannakoudakis, P.; Triantafyllidis, K.; Spathis, P. Corrosion Protection of Steel by Epoxy-Organoclay Nanocomposite Coatings. Coatings 2017, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Yan, D.; Tang, F.; Chen, G.; Liu, Y.; Chen, S. Effect of Sintering Temperature on the Microstructure, Corrosion Resistance and Crack Susceptibility of Chemically Reactive Enamel (CRE) Coating. Constr. Build. Mater. 2020, 238, 117720. [Google Scholar] [CrossRef]
- Gong, K.; Wu, M.; Xie, F.; Liu, G.; Sun, D. Effect of Dry/Wet Ratio and PH on the Stress Corrosion Cracking Behavior of Rusted X100 Steel in an Alternating Dry/Wet Environment. Constr. Build. Mater. 2020, 260, 120478. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The Electrochemical Behaviour of Stainless Steel AISI 304 in Alkaline Solutions with Different PH in the Presence of Chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Ziadi, I.; Akrout, H.; Hassairi, H.; El-Bassi, L.; Bousselmi, L. Investigating the Biocorrosion Mechanism of 304L Stainless Steel in Raw and Treated Urban Wastewaters. Eng. Fail. Anal. 2019, 101, 342–356. [Google Scholar] [CrossRef]
- Maocheng, Y.A.N.; Jin, X.U.; Libao, Y.U.; Tangqing, W.U.; Cheng, S.U.N.; Wei, K.E. EIS Analysis on Stress Corrosion Initiation of Pipeline Steel under Disbonded Coating in Near-Neutral PH Simulated Soil Electrolyte. Corros. Sci. 2016, 110, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Tsouli, S.; Lekatou, A.G.; Nikolaidis, C.; Kleftakis, S. Corrosion and Tensile Behavior of 316L Stainless Steel Concrete Reinforcement in Harsh Environments Containing a Corrosion Inhibitor. Procedia Struct. Integr. 2019, 17, 268–275. [Google Scholar] [CrossRef]






| MgO | SiO2 | CaO | Fe2O3 | Al2O3 | SO3 | LOI | |
|---|---|---|---|---|---|---|---|
| C1 | 63.49 | 8.77 | 2.30 | 0.15 | 0.15 | 0.11 | 25.03 |
| C2 | 63.15 | 8.73 | 2.29 | 0.15 | 0.15 | 0.11 | 25.42 |
| C3 | 66.54 | 3.05 | 1.48 | 0.07 | 0.07 | 0.09 | 28.70 |
| C4 | 61.91 | 8.80 | 2.31 | 0.15 | 0.15 | 0.11 | 26.57 |
| C5 | 65.00 | 3.32 | 1.52 | 0.08 | 0.05 | 0.14 | 29.90 |
| C6 | 62.81 | 4.25 | 2.46 | 0.25 | 0.10 | 0.02 | 30.11 |
| C7 | 66.76 | 0.40 | 0.30 | 0.03 | 0.05 | 0.10 | 32.06 |
| C8 | 82.34 | 11.70 | 3.07 | 0.20 | 0.20 | 0.15 | 2.34 |
| Material | SSA (m2/g) | PSD (μm) | |
|---|---|---|---|
| D50 | D90 | ||
| C1 | 13.1 | 17.8 | 69.1 |
| C2 | 18.7 | 8.4 | 29.5 |
| C3 | 11.2 | 10.5 | 39.9 |
| C4 | 13.2 | 9.5 | 40.8 |
| C5 | 32.3 | 9.9 | 38.1 |
| C6 | 7.0 | 3.8 | 13.1 |
| C7 | 16.6 | 6.0 | 25.5 |
| C8 | 17.7 | 19.5 | 51.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merachtsaki, D.; Toliopoulos, I.; Peleka, E.; Zouboulis, A. Anticorrosion Performance of Magnesium Hydroxide Coatings on Steel Substrates. Constr. Mater. 2022, 2, 166-180. https://doi.org/10.3390/constrmater2030012
Merachtsaki D, Toliopoulos I, Peleka E, Zouboulis A. Anticorrosion Performance of Magnesium Hydroxide Coatings on Steel Substrates. Construction Materials. 2022; 2(3):166-180. https://doi.org/10.3390/constrmater2030012
Chicago/Turabian StyleMerachtsaki, Domna, Ilias Toliopoulos, Efrosini Peleka, and Anastasios Zouboulis. 2022. "Anticorrosion Performance of Magnesium Hydroxide Coatings on Steel Substrates" Construction Materials 2, no. 3: 166-180. https://doi.org/10.3390/constrmater2030012
APA StyleMerachtsaki, D., Toliopoulos, I., Peleka, E., & Zouboulis, A. (2022). Anticorrosion Performance of Magnesium Hydroxide Coatings on Steel Substrates. Construction Materials, 2(3), 166-180. https://doi.org/10.3390/constrmater2030012







