Glass-Ceramic Materials Obtained by Sintering of Vitreous Powders from Industrial Waste: Production and Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results
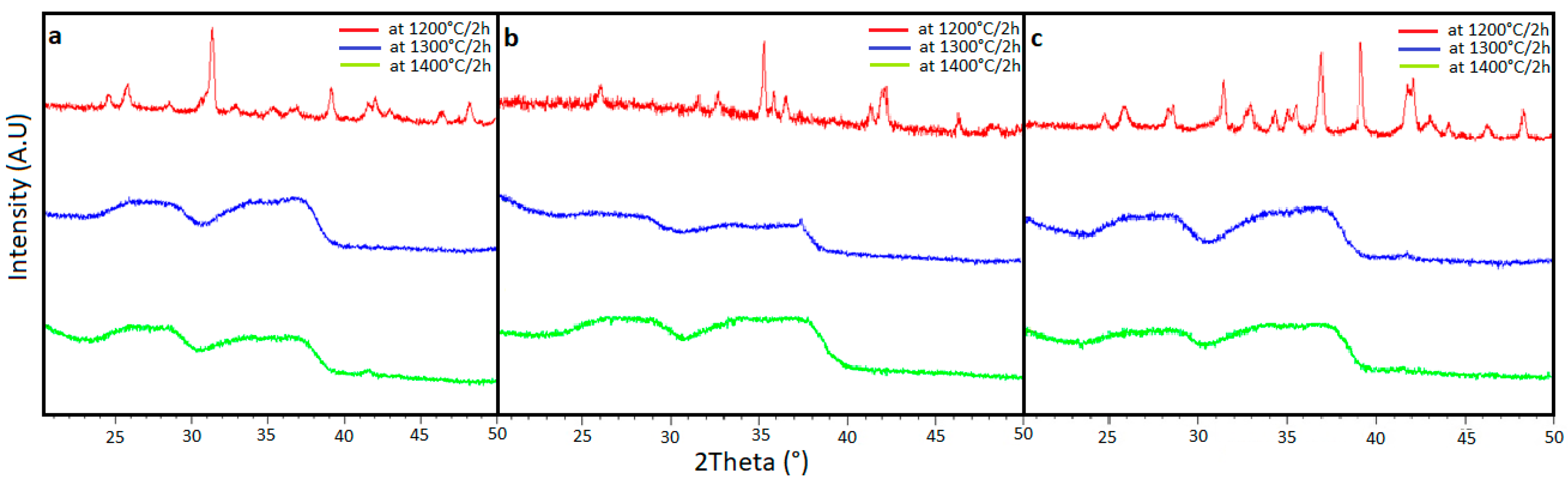
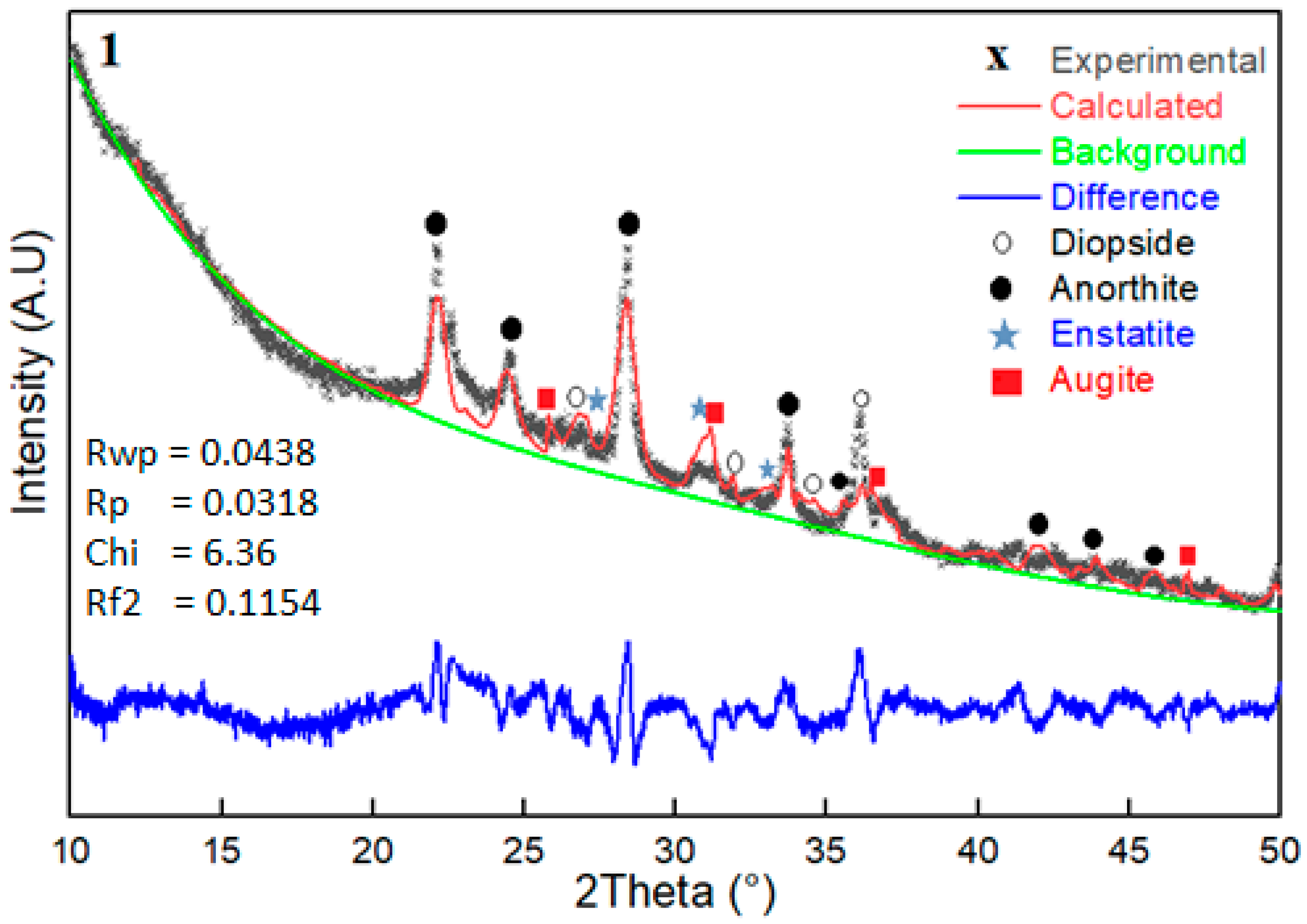
3.1. X-Ray Diffraction (XRD) Analysis
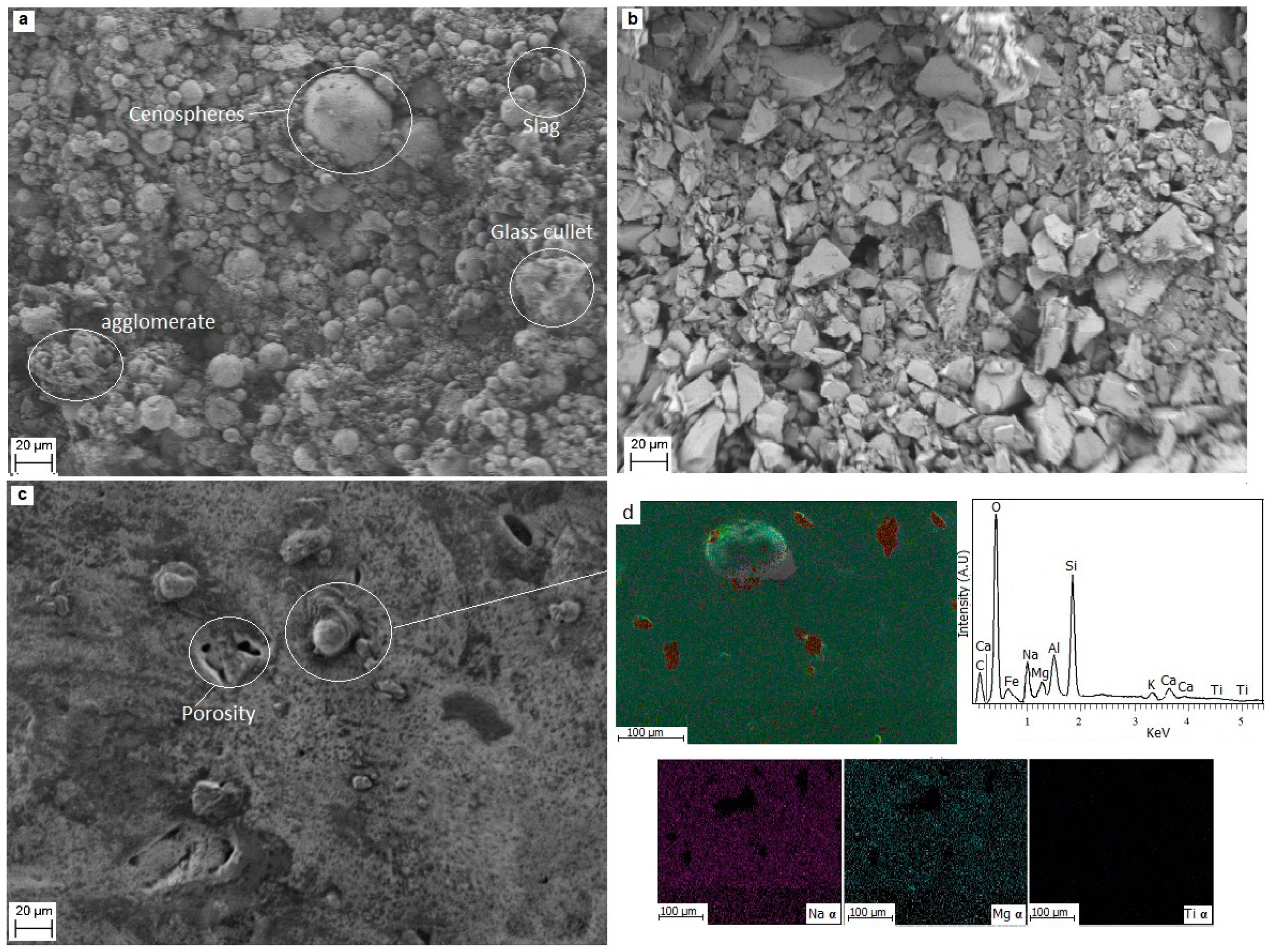
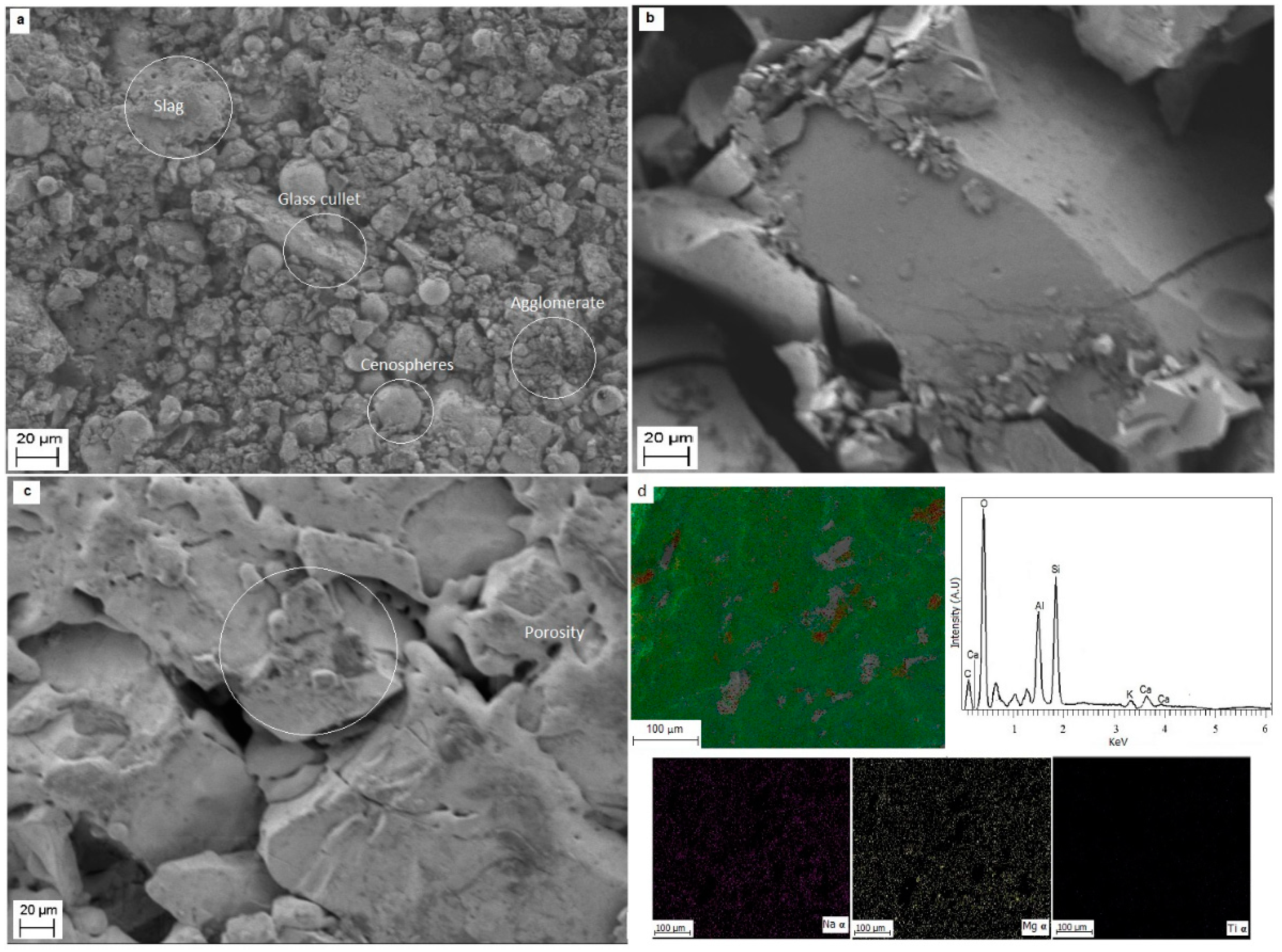
3.2. Scanning Electron Microscopy and Energy Dispersive of X-ray Spectroscopy (SEM-EDS) Analysis
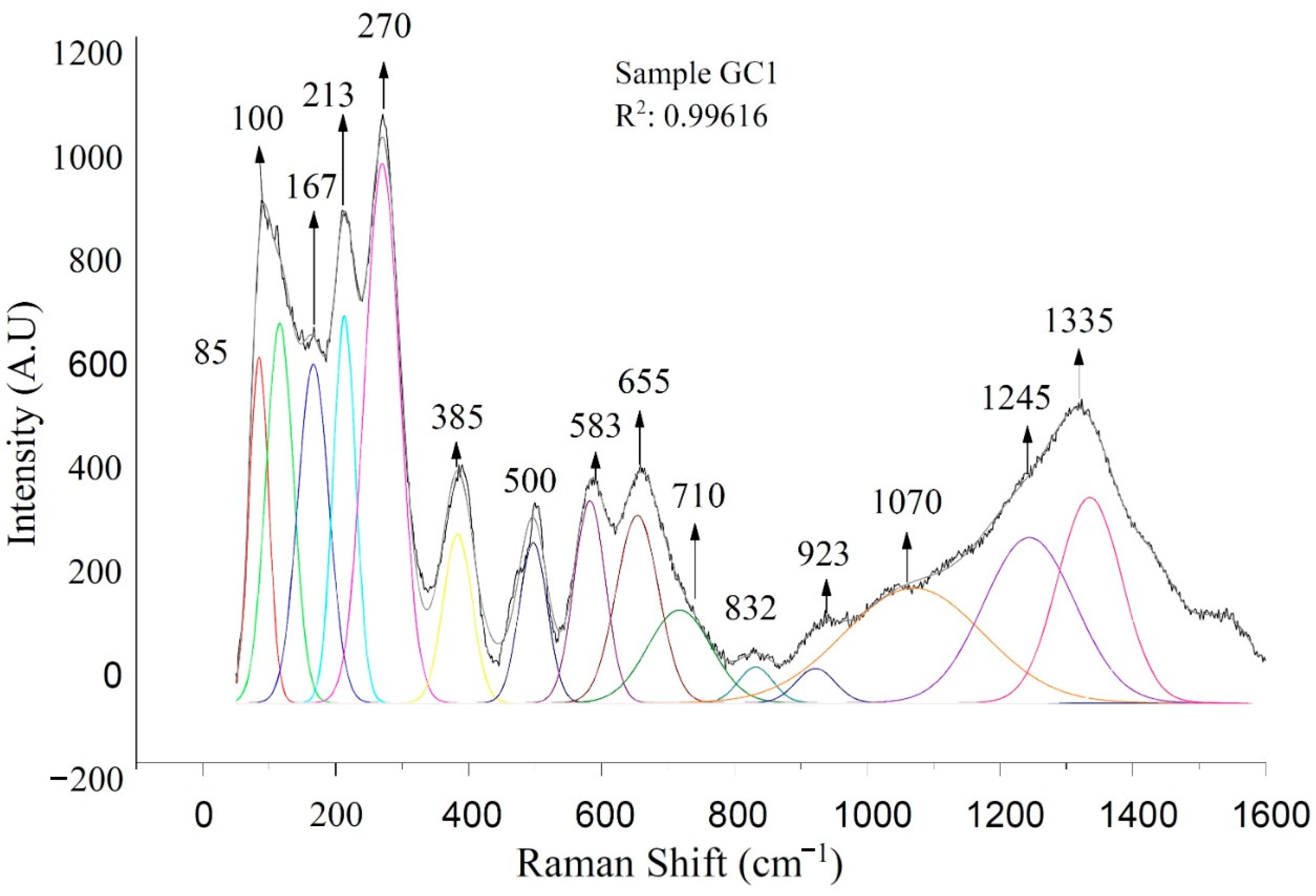
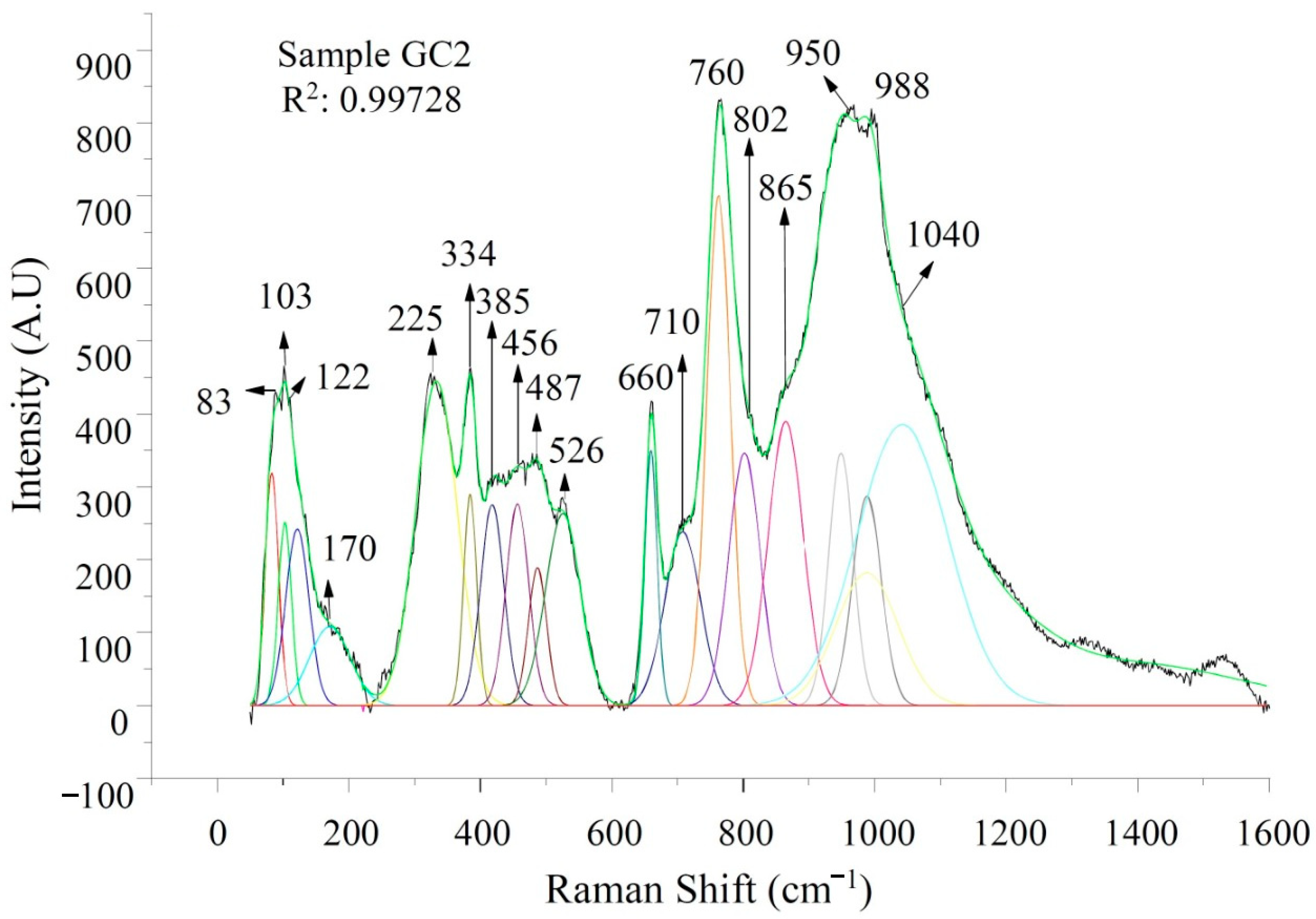
3.3. Raman Spectroscopy
3.4. Bulk Density, Water Absorption, and Apparent Porosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Leonelli, C.; Manfredini, T. Recycling of industrial wastes in ceramic manufacturing: State of art and glass case studies. Ceram. Int. 2016, 42, 13333–13338. [Google Scholar] [CrossRef]
- Chinnam, R.K.; Francis, A.A.; Will, J.; Bernardo, E.; Boccaccini, A.R. Functional glasses and glass-ceramics derived from iron rich waste and combination of industrial residues. J. Non-Cryst. Solids 2013, 365, 63–74. [Google Scholar] [CrossRef]
- Karayannis, V.; Moutsatsou, A.; Domopoulou, A.; Katsika, E.; Drossou, C.; Baklavaridis, A. Fired ceramics 100% from lignite fly ash and waste glass cullet mixtures. J. Build. Eng. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Höland, W.; Beall, G.H. Glass Ceramics Technology; The American Ceramic Society: Westerville, OH, USA, 2012. [Google Scholar]
- Rawlings, R.; Wu, J.; Boccaccini, A. Glass-ceramics: Their production from wastes—A Review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef] [Green Version]
- Dhir, R.; de Brito, J.; Ghataora, G.S.; Lye, C.Q. Use of Glass Cullet in Ceramics and Other Applications. Sustain. Constr. Mater. 2018, 327–387. [Google Scholar] [CrossRef]
- Cao, J.; Lu, J.; Jiang, L.; Wang, Z. Sinterability, microstructure and compressive strength of porous glass-ceramics from metallurgical silicon slag and waste glass. Ceram. Int. 2009, 42, 10079–10084. [Google Scholar] [CrossRef]
- Clark, T.J.; Reed, J.S. Kinetic Processes Involved in the Sintering and Crystallization of Glass Powders. J. Am. Ceram. Soc. 1986, 69, 837–846. [Google Scholar] [CrossRef]
- Yao, Z.; Ling, T.-C.; Sarker, P.K.; Su, W.; Liu, J.; Wu, W.; Tang, J. Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: A critical review. Renew. Sustain. Energy Rev. 2018, 81, 595–604. [Google Scholar] [CrossRef]
- Cumpston, B.; Shadman, F.; Risbud, S. Utilization of coal-ash minerals for technological ceramics. J. Mater. Sci. 1992, 27, 1781–1784. [Google Scholar] [CrossRef]
- Park, Y.J.; Moon, S.O.; Heo, J. Crystalline phase control of glass ceramics obtained from sewage sludge fly ash. Ceram. Int. 2003, 29, 223–227. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Chen, J.Q.; Qi, J.L.; Wang, Y.B.; Ai, Z.R. Preparation of glass-ceramics with low density and high strength using blast furnace slag, glass fiber and water glass. Ceram. Int. 2018, 44, 6044–6053. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, X.; Cang, D.; Zhao, L.; Wei, W. Synthesis of steel slag ceramics: Chemical composition and crystalline phases of raw materials. Int. J. Miner. Metall. Mater. 2015, 22, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Marinova, I.; Valencia, J.S.; Navarro, E.; Carda, J.B.; Quimicer, S.A.; Onda, D. Síntesis y caracterización de esmaltes de alto índice de refracción y dureza. Qualicer 2006, 2006, 249–260. [Google Scholar]
- Tabit, K.; Waqif, M.; Saâdi, L. Anorthite-cordierite based binary ceramics from coal fly ash and steel slag for thermal and dielectric applications. Mater. Chem. Phys. 2020, 254, 123472. [Google Scholar] [CrossRef]
- Li, B.; Guo, Y.; Fang, J. Effect of crystallization temperature on glass-ceramics derived from tailings waste. J. Alloy. Compd. 2020, 838, 155503. [Google Scholar] [CrossRef]
- Tabit, K.; Hajjou, H.; Waqif, M.; Saâdi, L. Effect of CaO/SiO2 ratio on phase transformation and properties of anorthite-based ceramics from coal fly ash and steel slag. Ceram. Int. 2020, 46, 7550–7558. [Google Scholar] [CrossRef]
- Monich, P.R.; Romero, A.R.; Rambaldi, E.; Bernardo, E. Case studies of up-cycling of partially crystallized ceramic waste in highly porous glass-ceramics. Constr. Build. Mater. 2020, 261, 119971. [Google Scholar] [CrossRef]
- Flesoura, G.; Monich, P.R.; Alarcón, R.M.; Desideri, D.; Bernardo, E.; Vleugels, J.; Pontikes, Y. Porous glass-ceramics made from microwave vitrified municipal solid waste incinerator bottom ash. Constr. Build. Mater. 2021, 270. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Liu, L.; Ma, J.; Shen, B. Preparation of additive-free glass-ceramics from MSW incineration bottom ash and coal fly ash. Constr. Build. Mater. 2020, 254, 119345. [Google Scholar] [CrossRef]
- Jun Park, Y.; Heo, J. Conversion to glass-ceramics from glasses made by MSW incinerator fly ash for recycling. Ceram. Int. 2002, 28, 689–694. [Google Scholar] [CrossRef]
- Bernardo, E.; Castellan, R.; Hreglich, S. Sintered glass-ceramics from mixtures of wastes. Ceram. Int. 2007, 33, 27–33. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Production of glass-ceramics obtained from industrial wastes by means of controlled nucleation and crystallization. Chem. Eng. J. 2007, 132, 335–343. [Google Scholar] [CrossRef]
- Rabelo Monich, P.; Rincon Romero, A.; Höllen, D.; Bernardo, E. Porous glass-ceramics from alkali activation and sinter-crystallization of mixtures of waste glass and residues from plasma processing of municipal solid waste. J. Clean. Prod. 2018, 188, 871–878. [Google Scholar] [CrossRef]
- Ayala Valderrama, D.M.; Gomez Cuaspud, J.A.; Roether, J.A.; Boccaccini, A.R. Development and characterization of glass-ceramics from combinations of slag, fly ash, and glass cullet without adding nucleating agents. Materials 2019, 12, 2032. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Sinclair, H. On the determination of lattice parameters by the debye-scherrer method. Proc. Phys. Soc. 1945, 57, 126–135. [Google Scholar] [CrossRef]
- Han, W. Glass ceramic of high hardness and fracture toughness developed from iron-rich wastes. Acta Metall. Sin. Engl. Lett. 2009, 22, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Boccaccini, A.R.; Han, W.X.; Dimech, C.; Rawlings, R.D. Glass ceramics of high hardness and fracture toughness developed from steel fly ash. Mater. Sci. Technol. 2006, 22, 1148–1154. [Google Scholar] [CrossRef]
- Rozenstrauha, I.; Sosins, G.; Krage, L.; Sedmale, G. Elaboration of new ceramic composites containing glass fibre production wastes. Bol. Soc. Esp. Ceram. 2013, 52, 88–92. [Google Scholar] [CrossRef]
- Gralik, G.; Chinelatto, A.L.; Chinelatto, A.S.A.; Grossa, P. Effect of different sources of alumina on the microstructure and mechanical properties of the triaxial porcelain. Cerámica 2014, 60, 471–481. [Google Scholar] [CrossRef]
- Lara Viera, J.A. Estudio del Vitrocerámico (1 − x) LÍ2B407 − xBi2W06 (0 < x < 0.35) Usando el Método Rietveld; Universidad Autónoma de Nuevo León: San Nicolás de los Garza, Mexico, 2002. [Google Scholar]
- Paucar Alvarez, C.G. Vitrocerámicos con bajo Coeficiente de Expansión Térmica Obtenidos por Sinterización con Cristalización Concurrente en Partículas Vítreas de Li2O.Al2O3.XSiO2; Universidad Autónoma de Madrid: Madrid, Spain, 2016. [Google Scholar]
- Daniel, I.; Gillet, P.; Ghose, S. A new high-pressure phase transition in anorthite (CaAl2Si2O8) revealed by Raman spectroscopy. Am. Miner. 1995, 80, 645–648. [Google Scholar]
- Cheng, X.; Ke, S.; Wang, Q.; Wang, H.; Shui, A.; Liu, P. Fabrication and characterization of anorthite-based ceramic using mineral raw materials. Ceram. Int. 2012, 38, 3227–3235. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Havlica, J.; Holešinský, R. Kinetics and mechanism of formation of gehlenite, Al-Si spinel and anorthite from the mixture of kaolinite and calcite. Solid State Sci. 2013, 26, 53–58. [Google Scholar] [CrossRef]
- Marques, V.M.F.; Tulyaganov, D.U.; Agathopoulos, S.; Gataullin, V.K.; Kothiyal, G.P.; Ferreira, J.M.F. Low temperature synthesis of anorthite based glass-ceramics via sintering and crystallization of glass-powder compacts. J. Eur. Ceram. Soc. 2006, 26, 2503–2510. [Google Scholar] [CrossRef]
- Si, W.; Li, S. Crystallization kinetics of diopside glass ceramics. J. Phys. Conf. Ser. 2020, 1676. [Google Scholar] [CrossRef]
- Reyes Ortiz, O.J.; Camacho Tauta, J.F. Efecto del desperdicio de una siderurgica en bases y subbases granulares. Rev. Cienc. Ing. Neogranadina 2003, 13, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Fernández Navarro, J. Constitución de los vidrios. Cons. Super. Investig. Cient. 1991, 1, 44–120. [Google Scholar]
- Ghosal, S.; Self, S.A. Particle size-density relation and cenosphere content of coal fly ash. Fuel 1995, 74, 522–529. [Google Scholar] [CrossRef]
- Liu, H.; Lu, H.; Chen, D.; Wang, H.; Xu, H.; Zhang, R. Preparation and properties of glass–ceramics derived from blast-furnace slag by a ceramic-sintering process. Ceram. Int. 2009, 35, 3181–3184. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Z.; Deng, Y.; Xing, X.; Qiu, K.; Long, J.; Li, J. International Journal of Mineral Processing Preparation of CaO–Al2O3–SiO2 glass ceramics from coal gangue. Int. J. Miner. Process. 2012, 102–103, 112–115. [Google Scholar] [CrossRef]
- Amstock, J. Manual del Vidrio en la Construcción; McGraw Hill: Mexico City, Mexico, 2011. [Google Scholar]
- Matson, D.W.; Sharma, S.K.; Philpotts, J.A. Raman spectra of some tectosilicates and of glasses along the orthoclase-anorthite and nepheline-anorthite joins. Am. Mineral. 1986, 71, 694–704. [Google Scholar]
- Daniel, I.; Gillet, P.; Mcmillan, P.F.; Wolf, G.; Verhelst, M.A. High-pressure behavior of anorthite: Compression and amorphization is observed which transforms the Ii polymorph into. J. Geophys. Res. 1997, 102, 10313–10325. [Google Scholar] [CrossRef]
- Lucena, G.L.; de Lima, L.C.; Honório, L.M.C.; de Oliveira, A.L.M.; Tranquilim, R.L.; Longo, E.; de Souza, A.G.; Maia, A.d.S.; dos Santos, I.M.G. CaSnO3 obtained by modified Pechini method applied in the photocatalytic degradation of an azo dye. Cerámica 2017, 63, 536–541. [Google Scholar] [CrossRef]
- Starbird-Pérez, R.; Montero-Campos, V. Synthesis of magnetic iron oxide nanoparticles toward arsenic removal from drinking water. Tecnol. Marcha 2015, 28, 45–54. [Google Scholar]
- Le Parc, R.; Champagnon, B.; Dianoux, J.; Jarry, P.; Martinez, V. Anorthite and CaAl2Si2O8 glass: Low frequency Raman spectroscopy and neutron scattering. J. Non-Cryst. Solids 2003, 323, 155–161. [Google Scholar] [CrossRef]
- Sharma, S.K.; Simons, B.; Yoder, H.S. Raman study of anorthite, calcium Tschermak’s pyroxene, and gehlenite in crystalline and glassy states. Am. Mineral. 1983, 68, 1113–1125. [Google Scholar]
- Sánchez-Polo, A.; Briceño, S.; Jamett, A.; Galeas, S.; Campaña, O.; Guerrero, V.; Arroyo, C.R.; Debut, A.; Mowbray, D.J.; Zamora-Ledezma, C.; et al. An Archaeometric Characterization of Ecuadorian Pottery. Sci. Rep. 2019, 9, 2642. [Google Scholar] [CrossRef]
- Montoya-Quesada, E.; Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R.; Muñoz-Saldaña, J. Effect of ZnO content on the physical, mechanical and chemical properties of glass-ceramics in the CaO–SiO2–Al2O3 system. Ceram. Int. 2020, 46, 4322–4328. [Google Scholar] [CrossRef]
- Lu, S.G.; Kwok, K.W.; Chan, H.L.W.; Choy, C.L. Structural and electrical properties of BaTi4O9 microwa v e ceramics incorporated with glass phase. Mater. Sci. Eng. B 2003, 99, 6–9. [Google Scholar] [CrossRef]
- Solids, N.; Friebele, J.; Calcium, I. Glass formation and thermal properties of low-silica calcium aluminosilicate glasses. J. Non-Cryst. Solids 1990, 126, 209–215. [Google Scholar]
- Urquijo, J.P.; Casanova, H.; Morales, A.L. Engineering Iron oxide nanoparticles for biomedicine and bioengineering applications Diseño de nanopartículas magnéticas para aplicaciones en biomedicina y bioingeniería. Rev. Fac. Ing. Univ. Antoquia 2014, 71, 230–243. Available online: http://www.scielo.org.co/pdf/rfiua/n71/n71a21.pdf (accessed on 7 May 2021).
- Partyka, J. Effect of BaO ratio on the structure of glass–ceramic composite materials from the SiO2–Al2O3–Na2O–K2O–CaO system. Ceram. Int. 2015, 41, 9337–9343. [Google Scholar] [CrossRef]
- Jerez Delgado, D. Crecimiento y Caracterización de Micro y Nanoestructuras de Óxidos de Hierro y Estaño/Growth and Characterization of Iron and Tin Oxides Micro and Nanostructures. 2012. Available online: https://eprints.ucm.es/id/eprint/16499/ (accessed on 7 May 2021).
- Partyka, J.; Leśniak, M. Raman and infrared spectroscopy study on structure and microstructure of glass-ceramic materials from SiO2-Al2O3-Na2O-K2O-CaO system modified by variable molar ratio of SiO2/Al2O3. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.D.; Yang, Q.W.; Guo, B.; Liu, B.; Zhang, S.G. Crystallization mechanism of glass-ceramics prepared from stainless steel slag. Rare Met. 2018, 37, 413–420. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.U.; Ventura, J.M.G.; Kannan, S. Structural analysis and devitrification of glasses based on the CaO–MgO–SiO2 system with B2O3, Na2O, CaF2 and P2O5 additives. J. Non-Cryst. Solids 2006, 352, 322–328. [Google Scholar] [CrossRef]
- Chuvaeva, T.I.; Dymshits, O.S.; Petrov, V.I.; Tsenter, M.Y. Low-frequency Raman scattering of magnesium aluminosilicate glasses and glass-ceramics. J. Non-Cryst. Solids 2001, 282, 306–316. [Google Scholar] [CrossRef]
- Blake, R.L.; Hessevic, R.E.; Zoltai, K.T.; Finger, L.W. Refinement of the Hematite Structure. Am. Mineral. 1966, 51, 123–129. [Google Scholar]
- INEGI La Industria Minera Ampliada. p. 117, 2016. Available online: https://www.inegi.org.mx/contenido/productos/prod_serv/contenidos/espanol/bvinegi/productos/censos/economicos/2009/mineria/Mono_Industria_Minera.pdf (accessed on 7 May 2021).
- Haavik, C.; Stølen, S.; Fjellvåg, H.; Hanfland, M.; Häusermann, D. Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure. Am. Mineral. 2000, 85, 514–523. [Google Scholar] [CrossRef]
- ASTM C373-88—Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; ASTM International: Montgomery, PA, USA, 2006; pp. 1–2.
- Nebot Diaz, I.; Marcharl, M.; Iran, M.; Carba, B.J. Nuevas Tecnologías Para el Sector Cerámico; Universitat Jaume: Valencia, Spain, 2000. [Google Scholar]
- Radeva, V. Adaptando el método de arquímedes para determinar las densidades y porosidad de muestras pequeñas de cerámica. Cienc. Soc. 2006, XXXI, 565–585. [Google Scholar] [CrossRef]
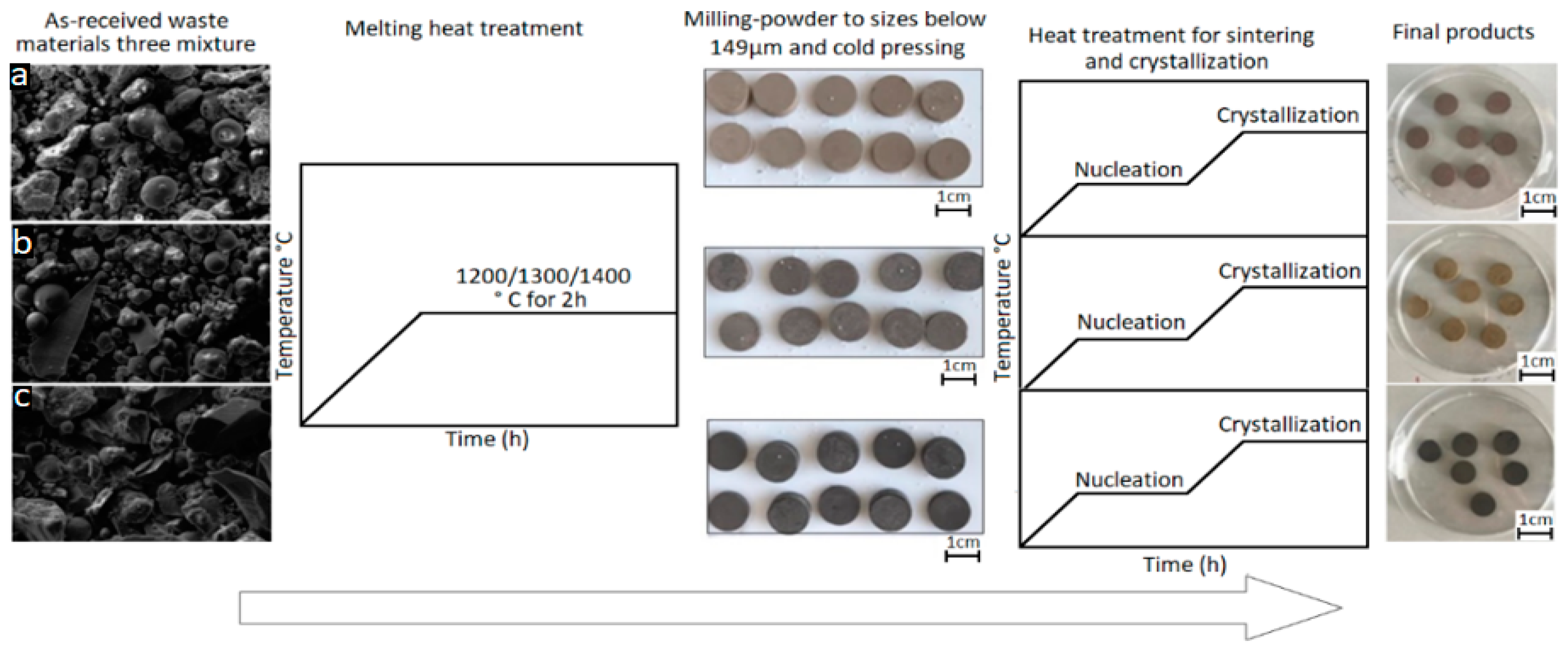




| Sample | wt.% | Composition of Mixtures Investigated (mole %). | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | Fly Ash | Glass Cullet | Na2O | MgO | Al2O3 | SiO2 | SO3 | K2O | CaO | TiO2 | Fe2O3 | Mn | Other | |
| GC1 | 10 | 70 | 20 | 3.627 | 1.85 | 13.64 | 66.3 | 0.8 | 0.7 | 7.1 | 1.09 | 3.924 | 0.7 | 0.285 |
| GC2 | 10 | 35 | 55 | 4.914 | 3.38 | 8.161 | 66.4 | 0.6 | 0.4 | 12 | 0.64 | 2.828 | 0.7 | 0.227 |
| GC3 | 55 | 35 | 10 | 1.813 | 3.12 | 11.24 | 46.9 | 0.7 | 0.5 | 20 | 1.03 | 9.686 | 3.7 | 0.927 |
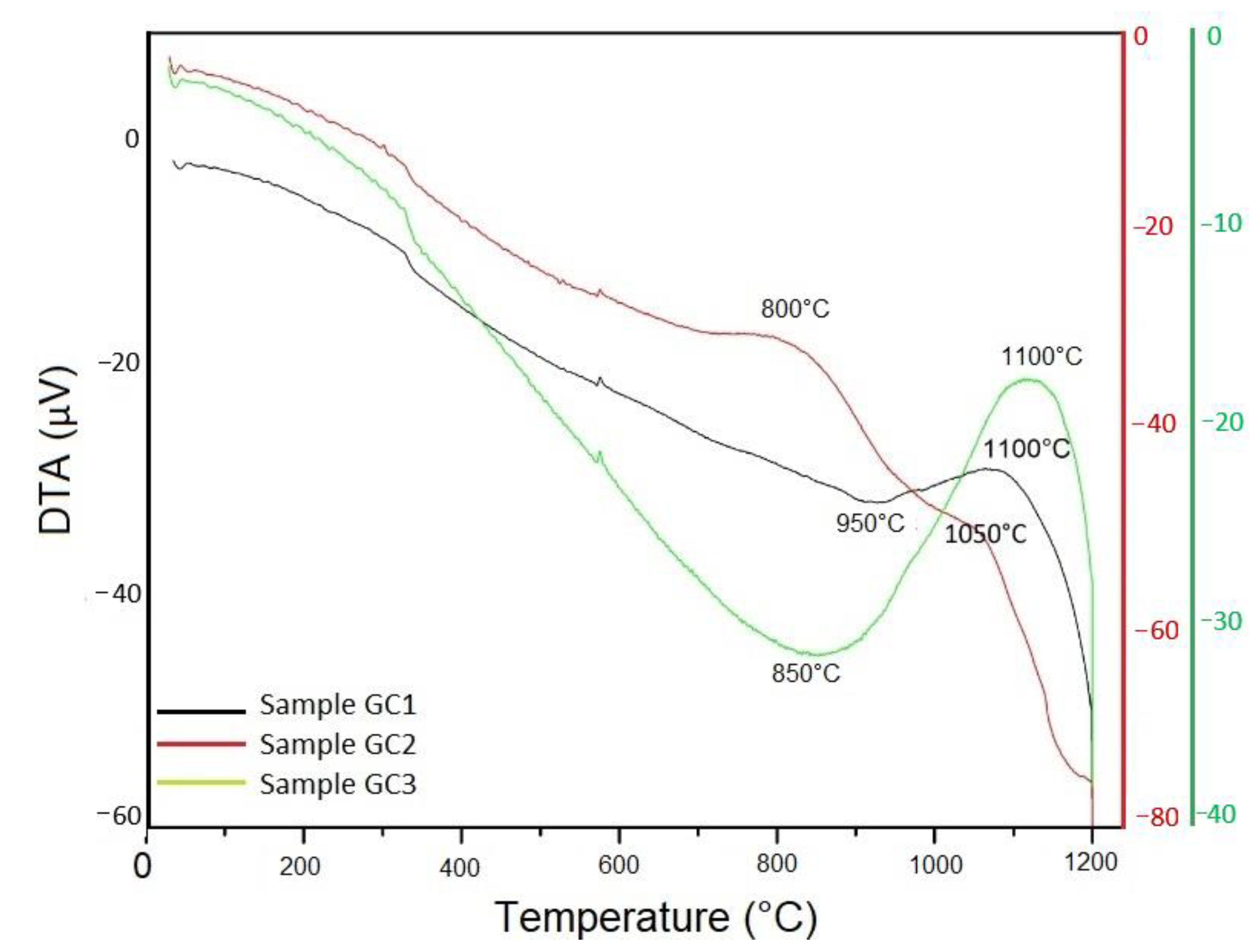
| Sample | Nucleation Temperatura | Crystallization Temperature |
|---|---|---|
| GC1 | 950 °C for 2 h | 1100 °C for 2 h |
| GC2 | 800 °C for 2 h | 1050 °C for 2 h |
| GC3 | 850 °C for 2 h | 1100 °C for 2 h |
| Crystalline Phase | Chemical Formula | Lattice Parameters (Å) | Composition Percentage (%) of Sample | ||||
|---|---|---|---|---|---|---|---|
| a | b | c | GC1 | GC2 | GC3 | ||
| Anorthite | (O64Ca8Si16Al16) | 8.173 | 12.869 | 12.894 | 59 | 63 | 43 |
| Diopside | (Mg4 Ca4 Si8 O24) | 9.681 | 8.849 | 5.218 | 7 | 15 | 5 |
| Enstatite | (Mg16Si16O48) | 5.181 | 18.251 | 8.814 | 7 | 16 | 21 |
| Augite | (Na0,36Ca2,46Mg3,61Fe0,84Al1,37Ti0,08Si7,28O24) | 9.699 | 8.844 | 5.272 | 27 | 6 | 31 |
| Sample | 2ϴL | 2ϴH | 2ϴ | ϴ | B | B(rad) | t (nm) | |
|---|---|---|---|---|---|---|---|---|
| GC1 | 23.03 | 23.68 | 23.40 | 11.698 | 0.36 | 0.006 | 0.59 | |
| GC2 | 27.36 | 27.48 | 27.57 | 13.785 | 0.21 | 0.003 | 1.91 | |
| GC3 | 27.39 | 27.87 | 27.69 | 13.845 | 0.30 | 0.005 | 1.60 | |
| 2ϴL: Left grain limit 2ϴH: Right side grade limit 2ϴ: Midpoint of grain size t = (kλ)/B(ϴ)·Cos ϴ | ϴ = 2ϴ/2 B = 2ϴH−2ϴL B(rad) = (B·π)/180 | |||||||
| Band Position (cm−1) | Possible Bond or Crystalline Phase | Ref. |
|---|---|---|
| 83,85 | Anorthite pase | [45,46] |
| 100–300 | Vibrations with a greater contribution of Ca2+ atoms and a small contribution of O2 | [47] |
| 225,270,272 | Hematite | [48] |
| 326 | Anorthite | [45,46] |
| 385 | Magnetite | [48] |
| 473–482 | Si-O-Si systems with bridge oxygen and Al-O vibrations with coordination number 4 | [33] |
| 500 | The movements of oxygen atoms along the union angles between T-O-T | [33] |
| 583 | Bending vibrations Si-O-Si, T-O-T | [49] |
| 526,660,750 | Characteristic bands of ceramic-ceramic materials | [45,46] |
| 660 | Si-O-Si vibrations in Q2 units related to the diopside phase | [50,51] |
| 611,705,872 | Stretching calcium carbonates | [51,52] |
| 710 | Indicates that there are aluminum-oxygen octahedrals | [53,54] |
| 712 | Pure magnetite, located in the band 710 cm−1 in this study | [49,55,56] |
| 760 | Characteristic bands of ceramic-ceramic materials - characteristic vibration of Si | [45,46] |
| 799 | FeO vibrations | [51] |
| 950 | Diopside phase given by Si/O vibrations (Q2) located in the band 958 cm-1 in this study | [48,57] |
| 958 | Diopside - Si/O Vibrations (Q2) | [49,50] |
| 997 | Si-O-Si vibrational modes | [51] |
| 1040 | Si-O-Al vibrations. | [49,50] |
| Sample | Dry Mass (D)(g) | Saturated Mass (W) (g) | Suspended Mass (S)(g) | Density (B) (g·cm−3) | Water Absorption (A) (%) | Apparent Porosity (P) (%) |
|---|---|---|---|---|---|---|
| GC1 | 0.7 | 3.0 | 2.4 | 3.1 | 7.2 | 22.3 |
| GC2 | 1.3 | 3.1 | 2.5 | 2.2 | 14.5 | 31.7 |
| GC3 | 1.9 | 3.4 | 2.7 | 2.3 | 12.9 | 30.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala Valderrama, D.M.; Cuaspud, J.A.G.; Taniolo, N.; Boccaccini, A.R. Glass-Ceramic Materials Obtained by Sintering of Vitreous Powders from Industrial Waste: Production and Properties. Constr. Mater. 2021, 1, 63-79. https://doi.org/10.3390/constrmater1010004
Ayala Valderrama DM, Cuaspud JAG, Taniolo N, Boccaccini AR. Glass-Ceramic Materials Obtained by Sintering of Vitreous Powders from Industrial Waste: Production and Properties. Construction Materials. 2021; 1(1):63-79. https://doi.org/10.3390/constrmater1010004
Chicago/Turabian StyleAyala Valderrama, Diana M., Jairo A. Gómez Cuaspud, Nicoletta Taniolo, and Aldo R. Boccaccini. 2021. "Glass-Ceramic Materials Obtained by Sintering of Vitreous Powders from Industrial Waste: Production and Properties" Construction Materials 1, no. 1: 63-79. https://doi.org/10.3390/constrmater1010004
APA StyleAyala Valderrama, D. M., Cuaspud, J. A. G., Taniolo, N., & Boccaccini, A. R. (2021). Glass-Ceramic Materials Obtained by Sintering of Vitreous Powders from Industrial Waste: Production and Properties. Construction Materials, 1(1), 63-79. https://doi.org/10.3390/constrmater1010004






