Simultaneous Biocementation and Compaction of a Soil to Avoid the Breakage of Cementitious Structures during the Execution of Earthwork Constructions
Abstract
1. Introduction
2. Material and Methods
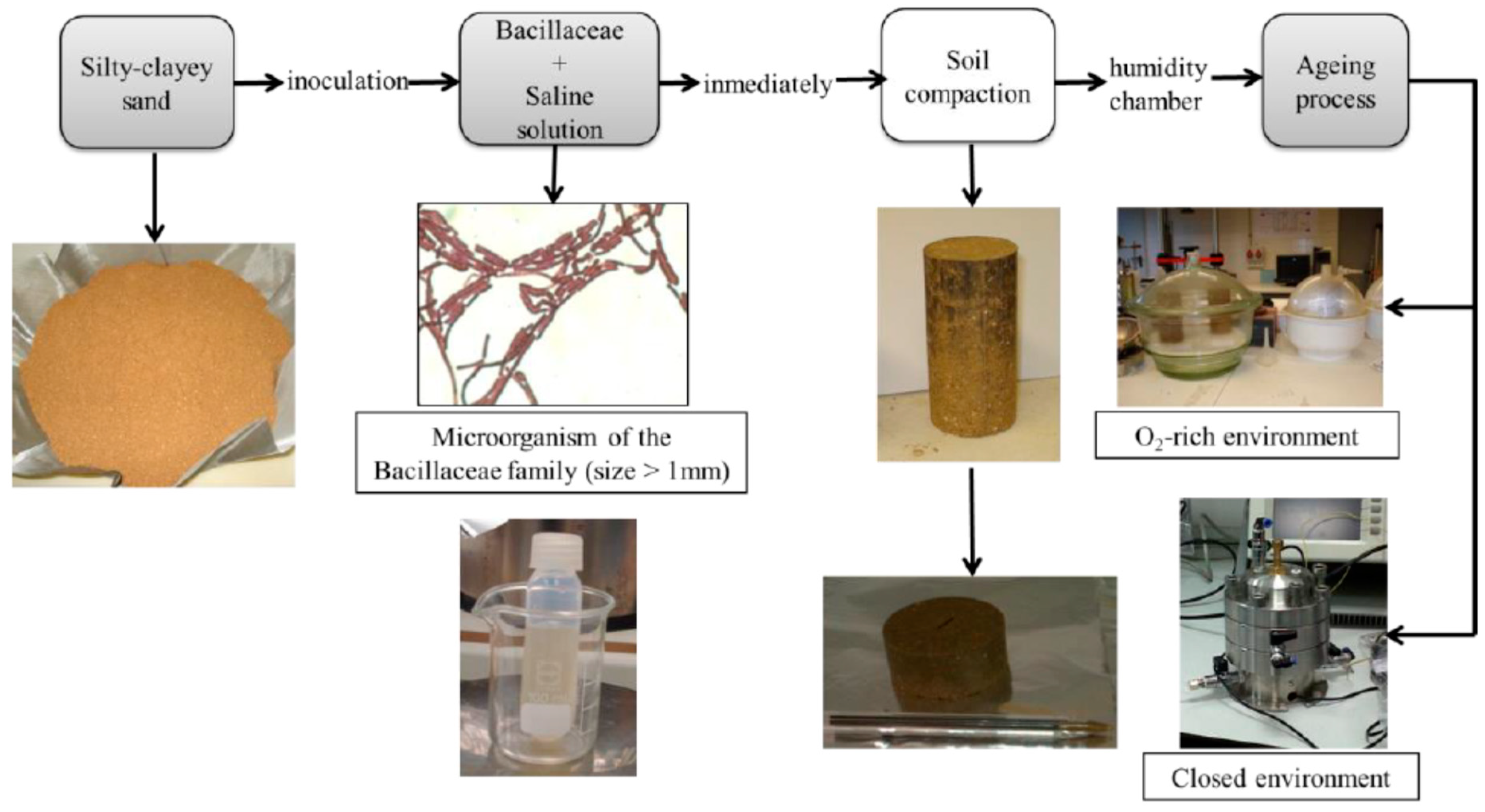
2.1. Material and Biotreatment
2.2. Mineralogical Characterization
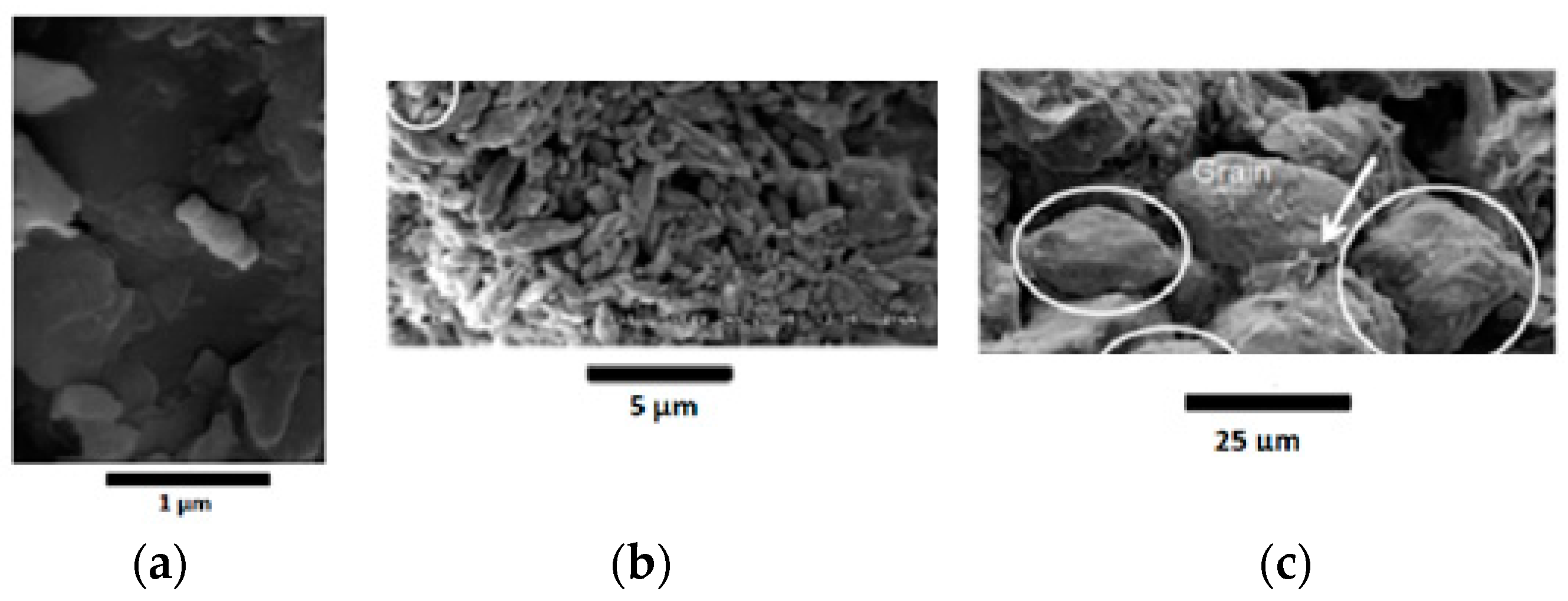
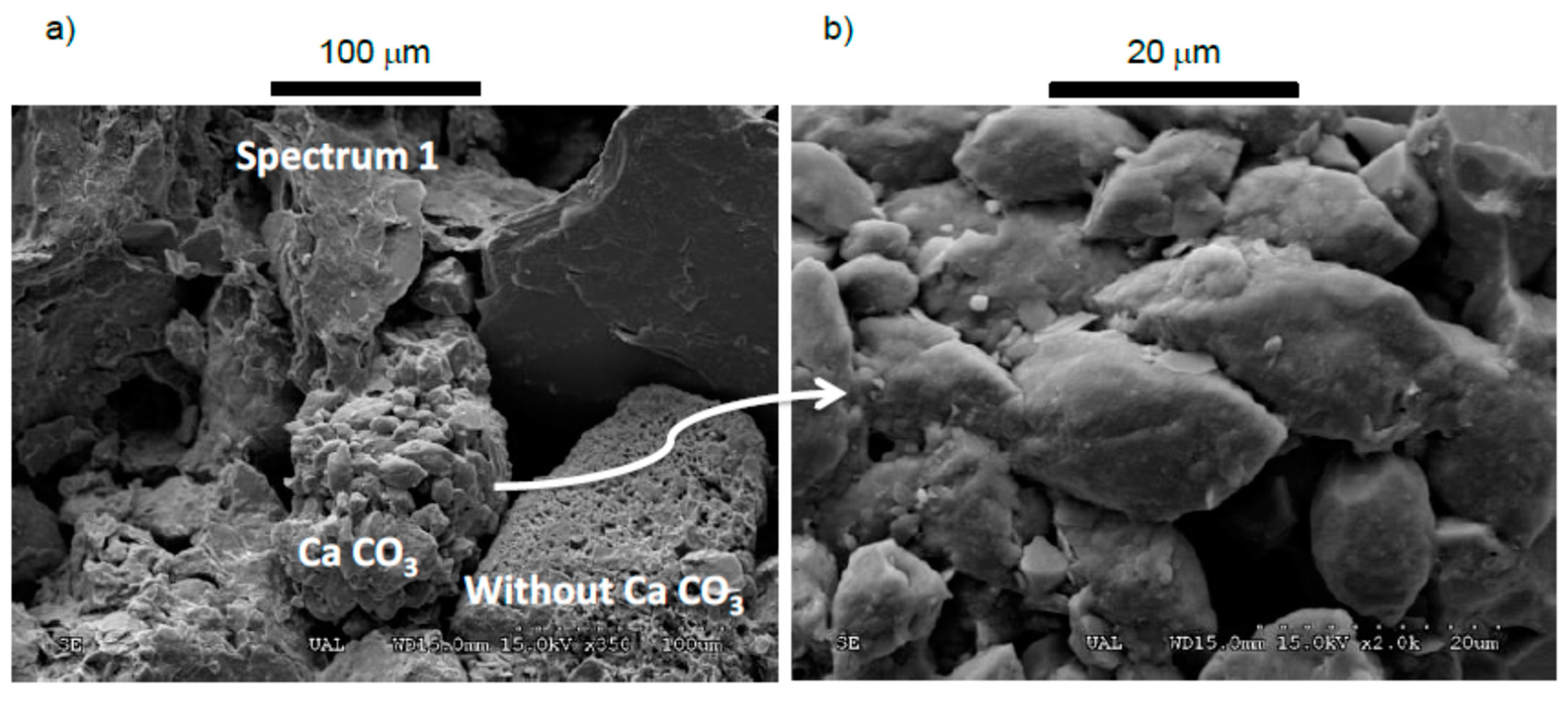
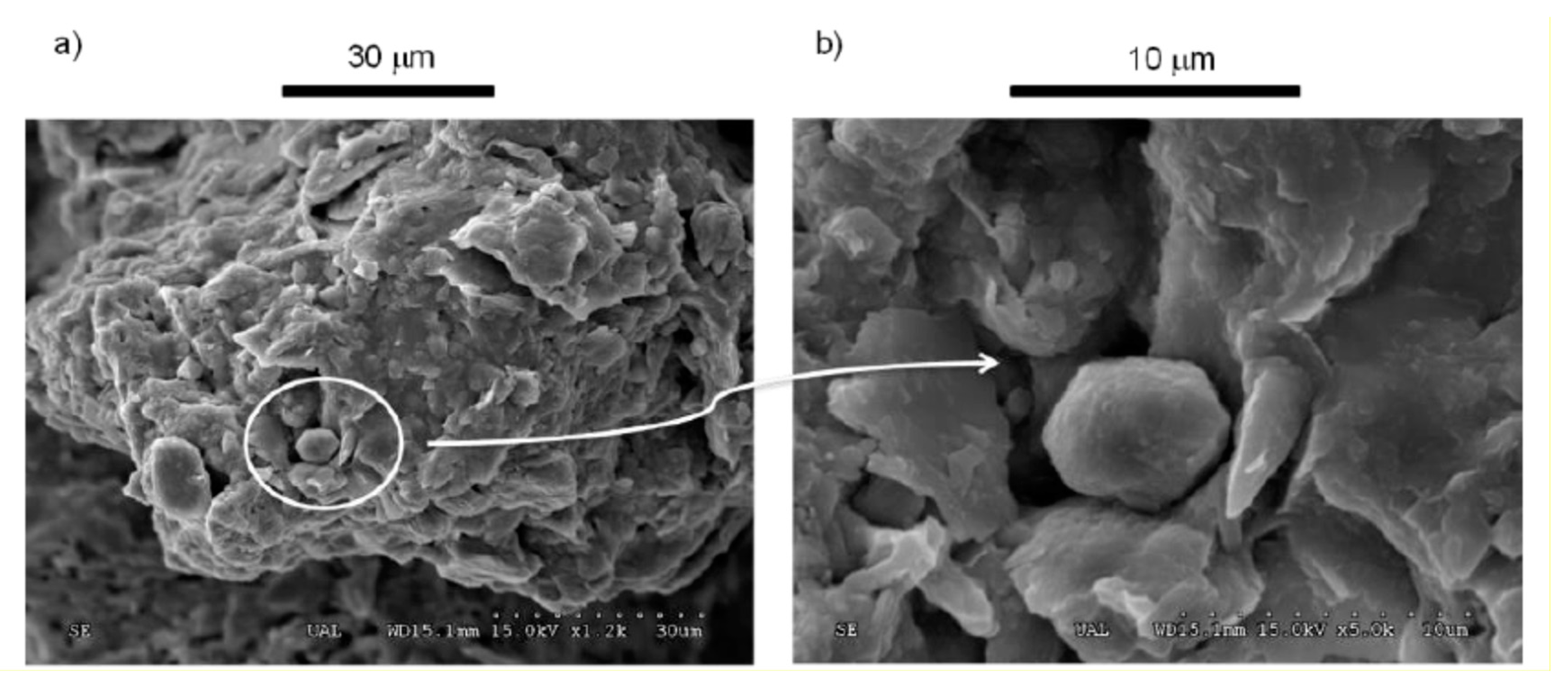
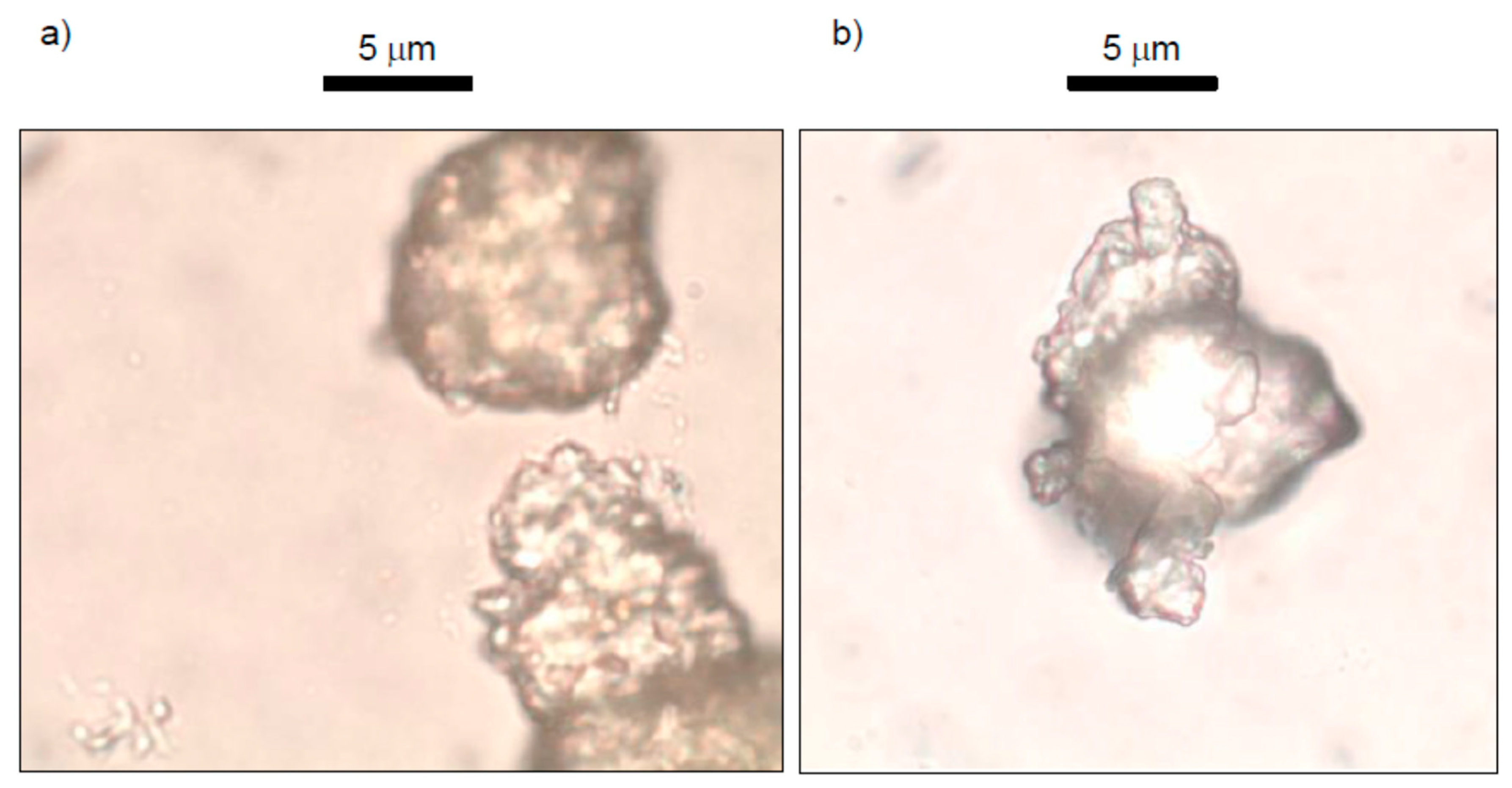
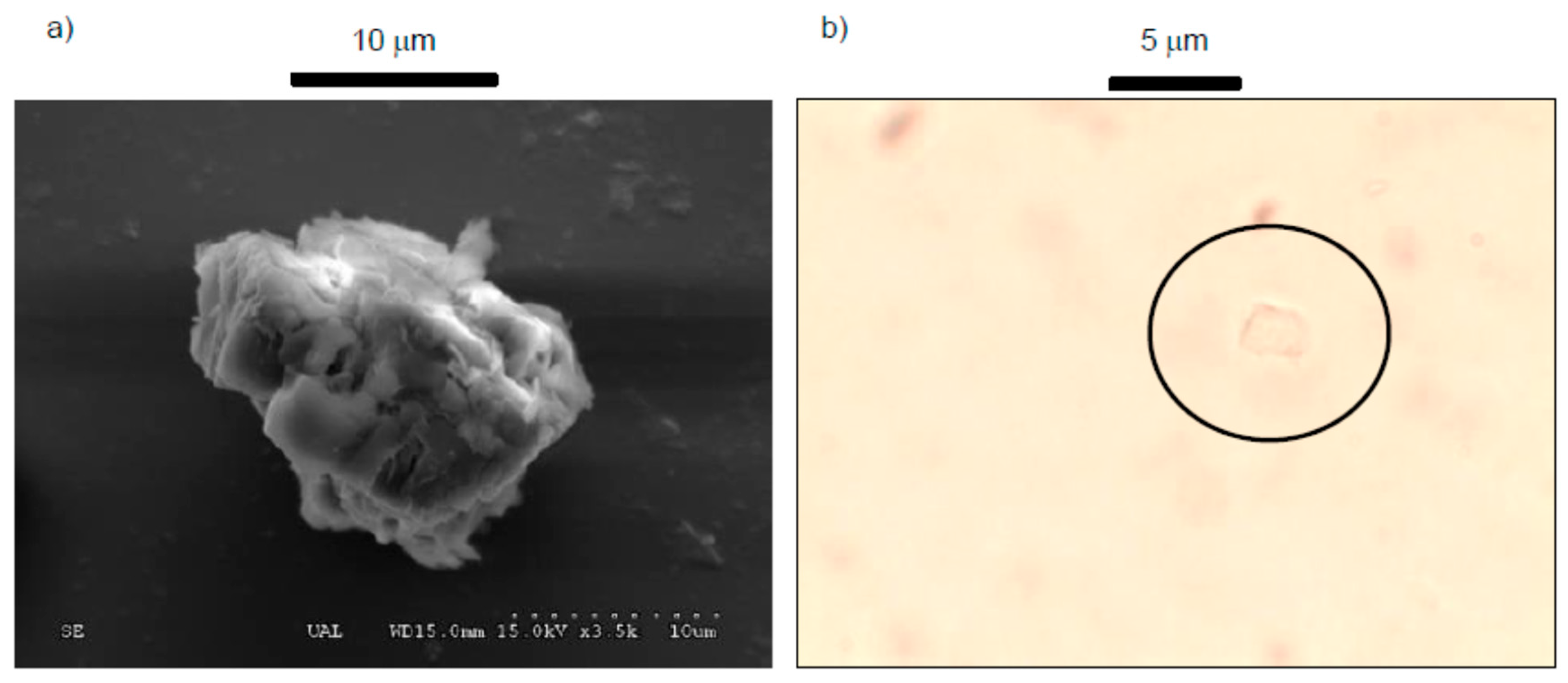
2.2.1. Scanning-Electron Microscopy (SEM)
2.2.2. Optical Microscope
2.3. Techniques Used to Study Aging Effects
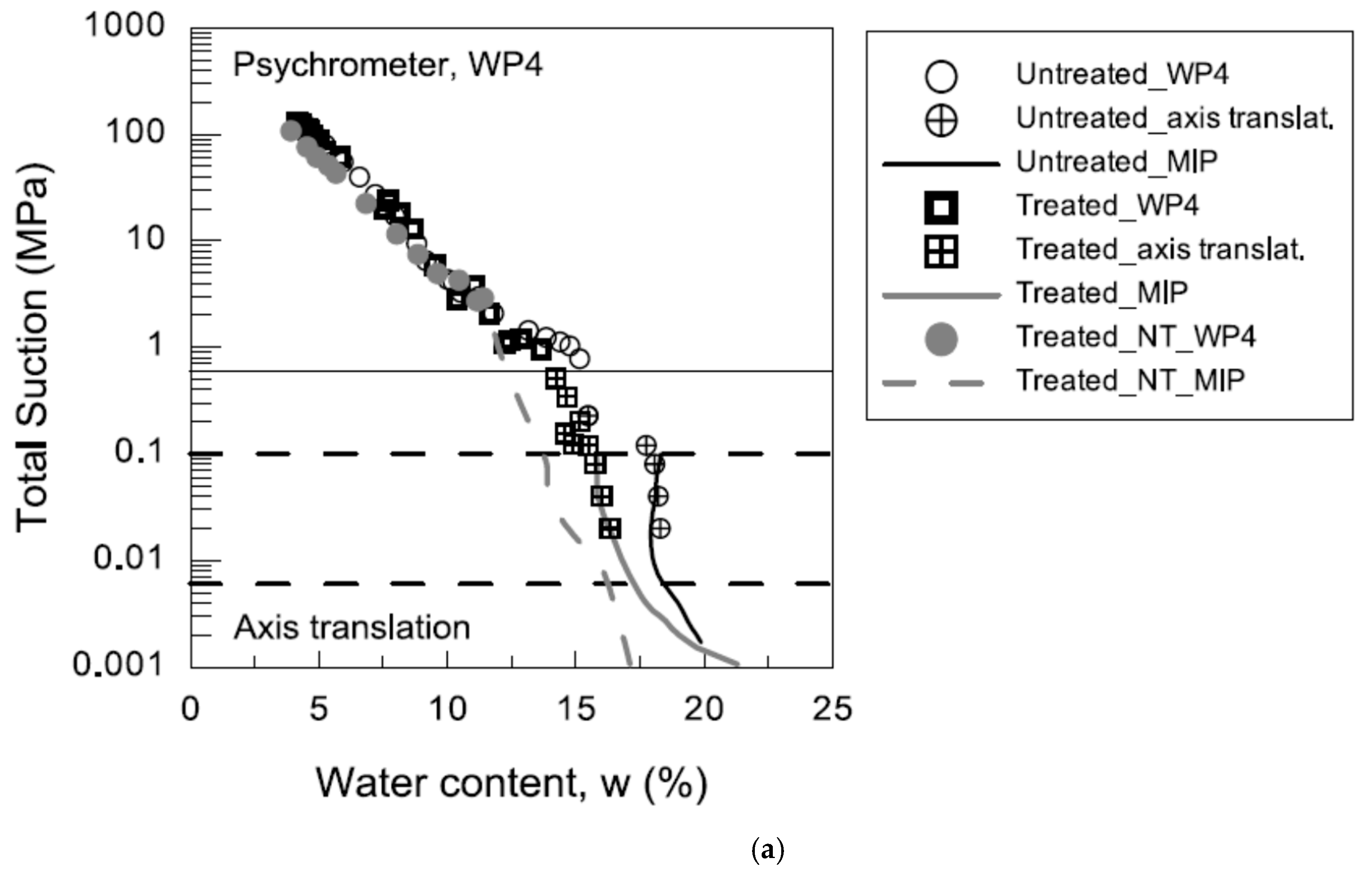
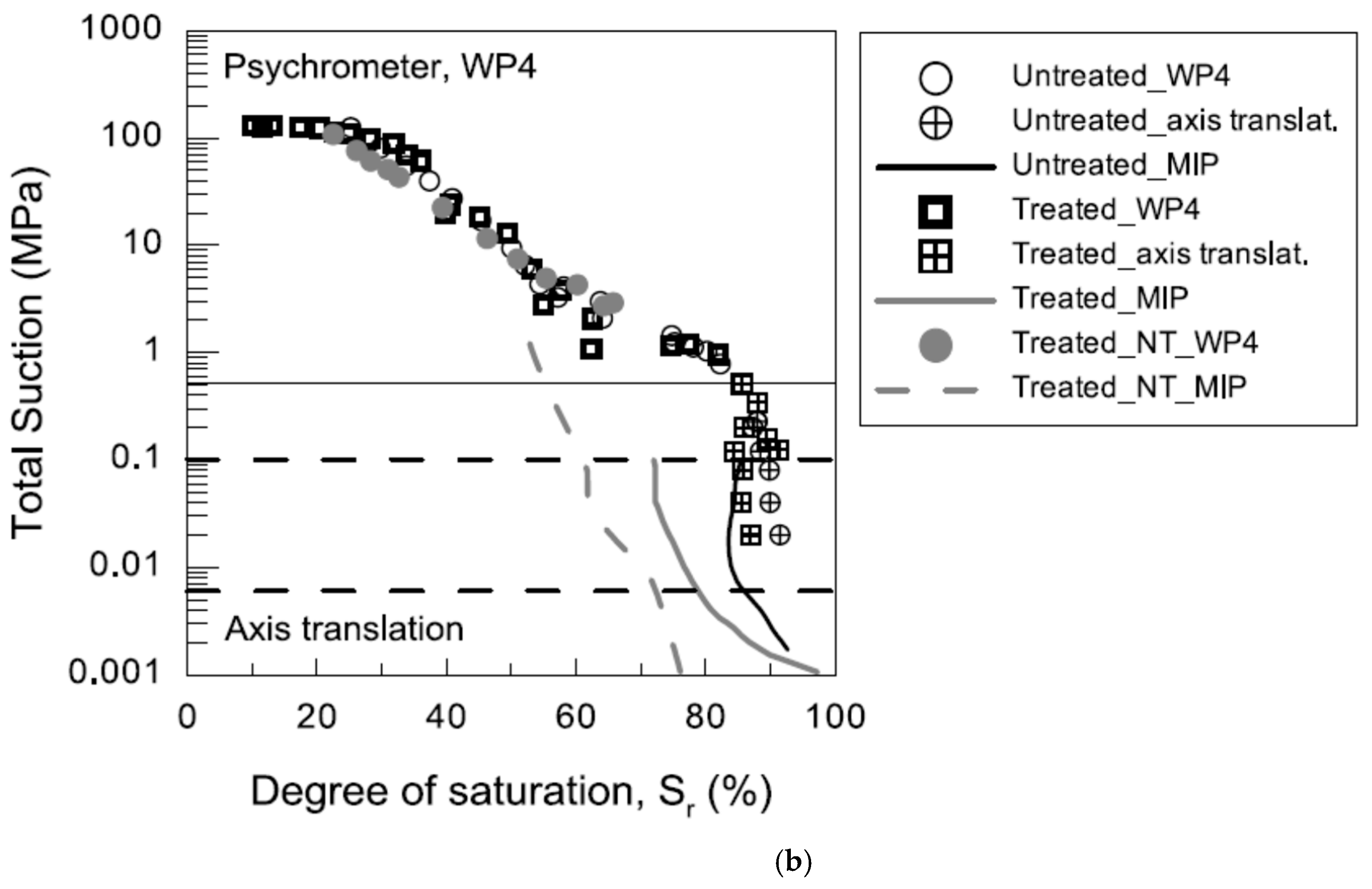
2.3.1. Water-Retention Curve
2.3.2. Mercury-Intrusion Permeability
2.3.3. Bender Elements
2.3.4. Ultrasonic-Pulse-Velocity Test (Pundit)
2.3.5. Unconfined Compression Test
2.3.6. Brazilian Test
2.3.7. Resonant Column (RC)
3. Results and Discussion
3.1. Mineralogical Characterization, Scanning-Electron Microscopy (SEM), and Optical Microscopy
3.2. Study of Aging Effects
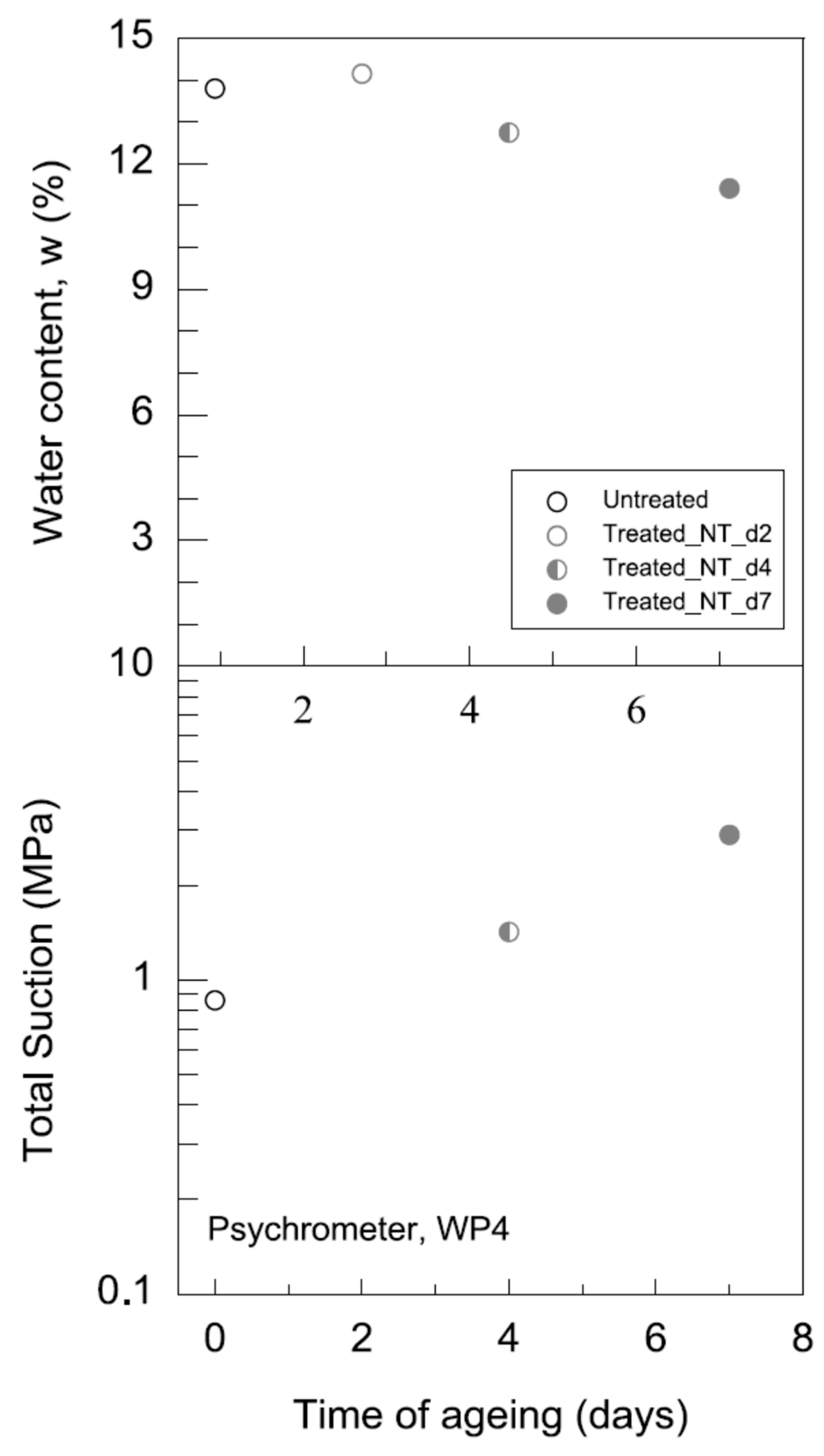
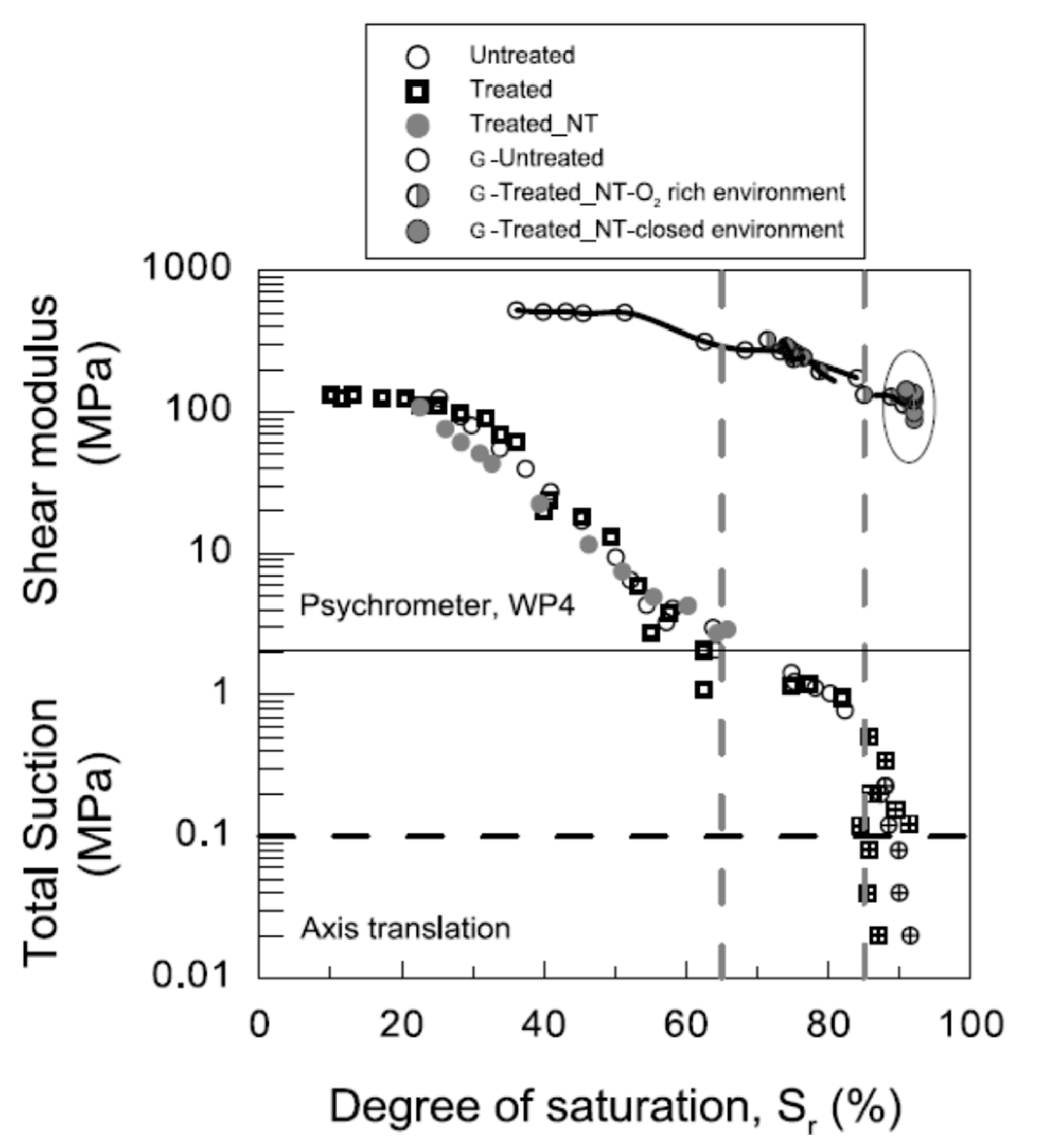
3.2.1. Water-Retention Curve (WRC)
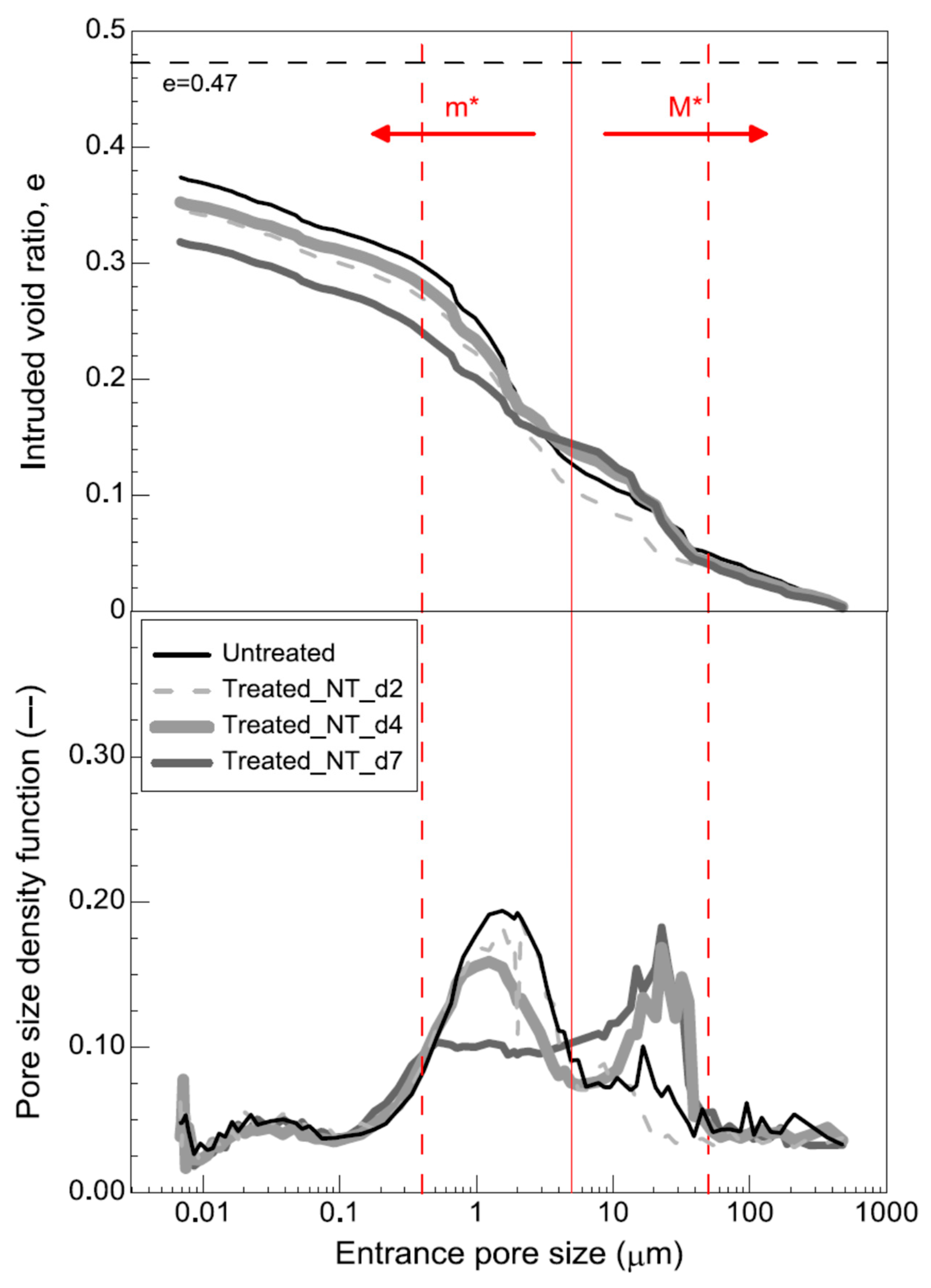
3.2.2. Mercury-Intrusion Permeability
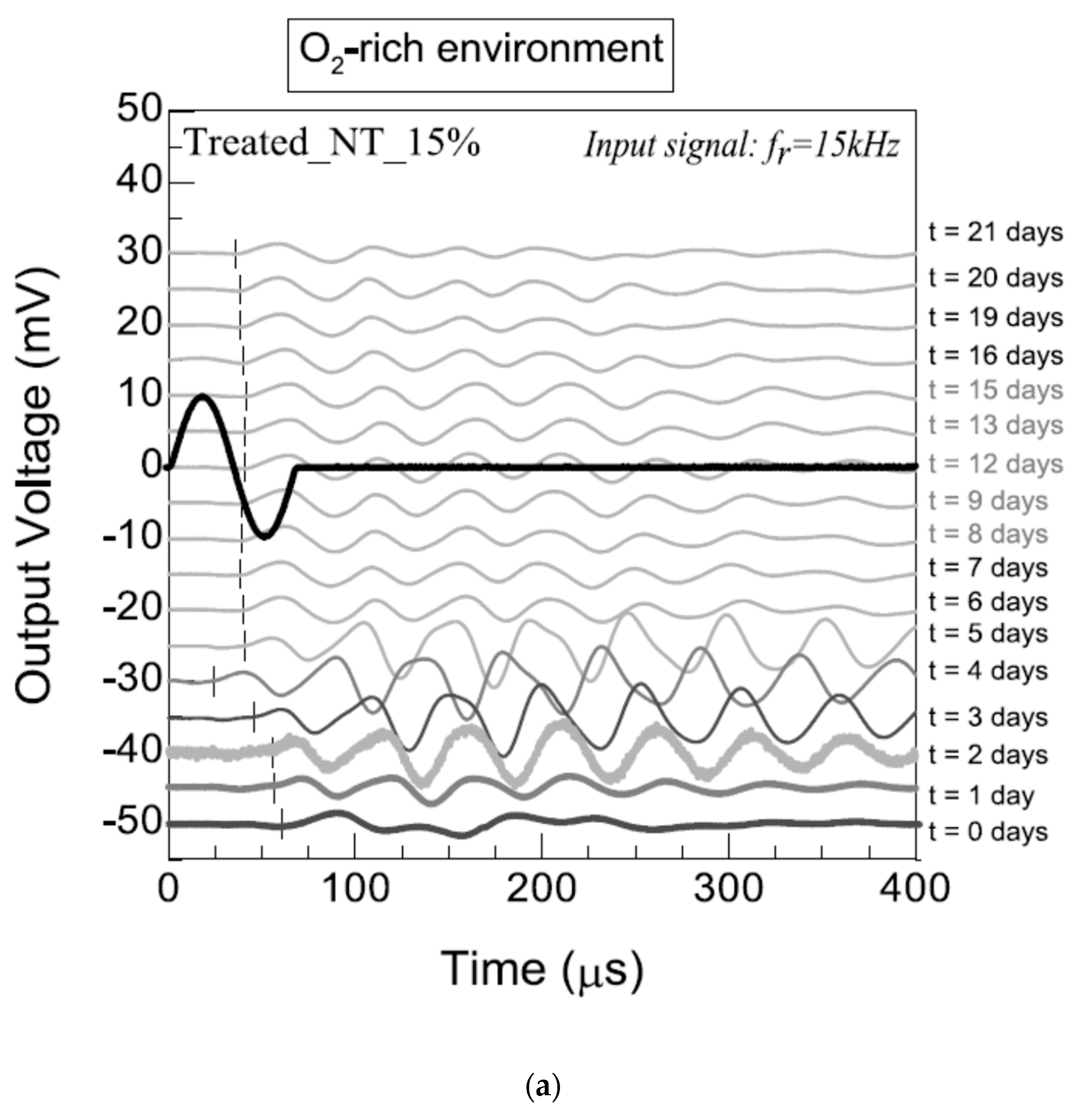
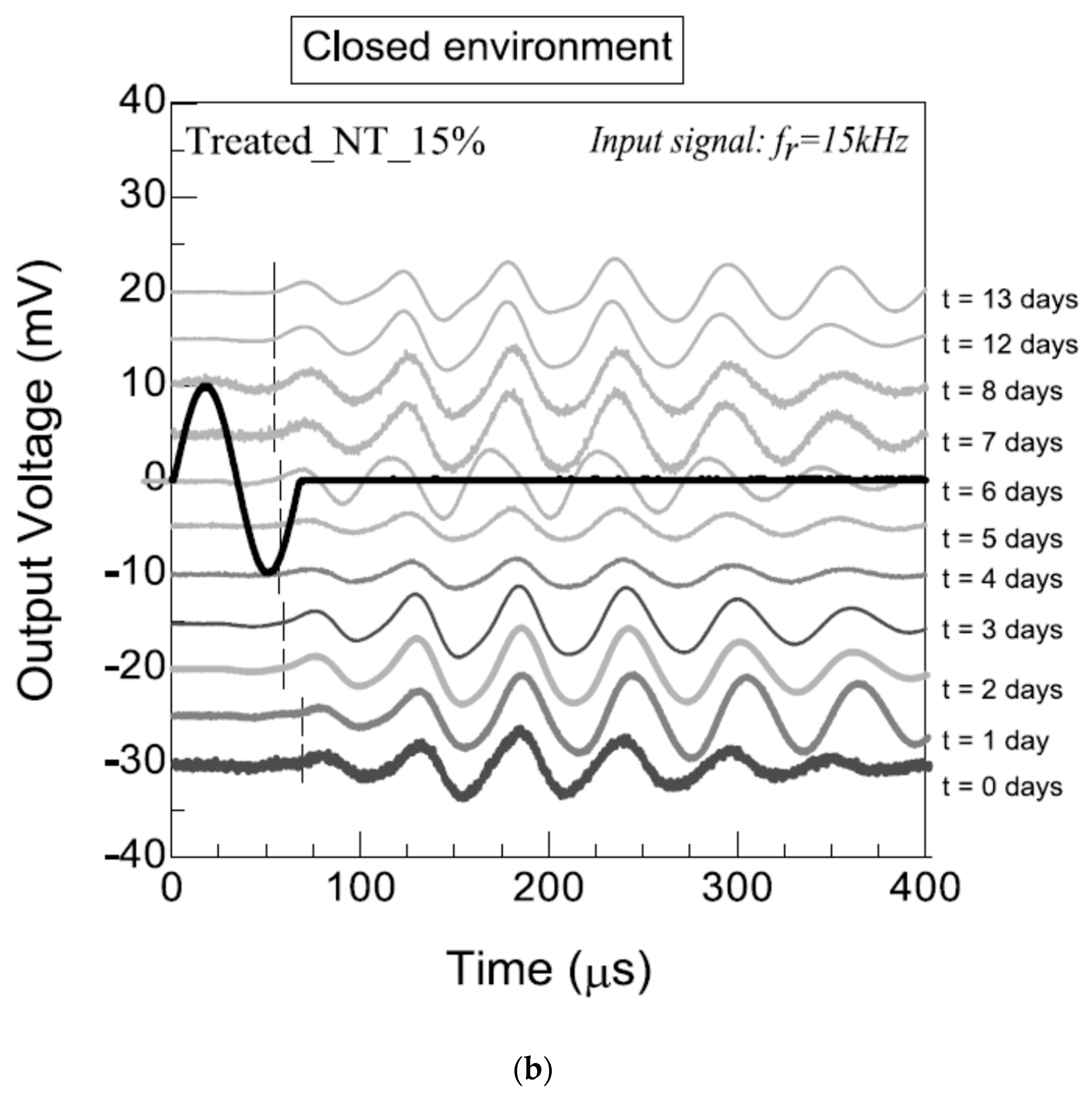
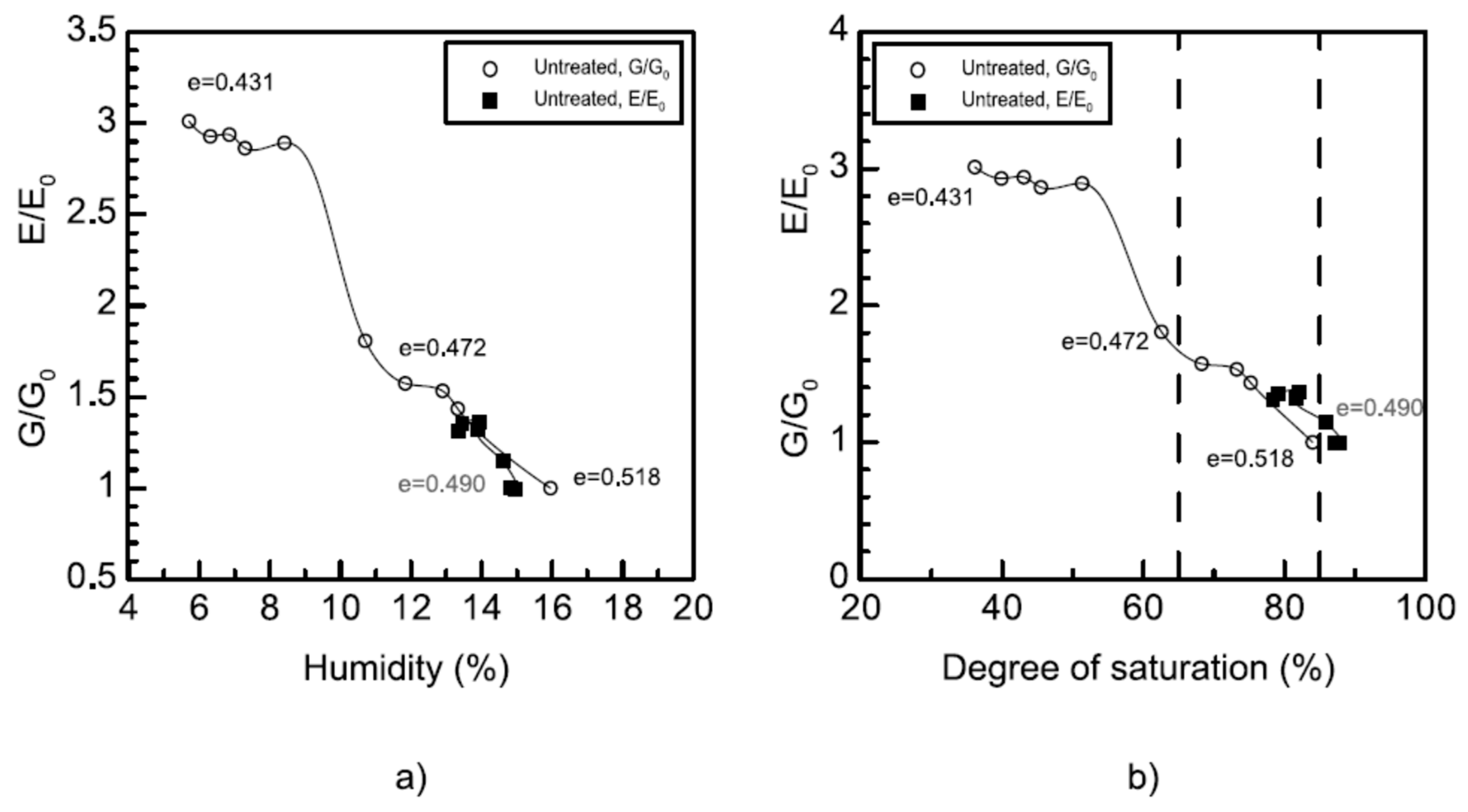
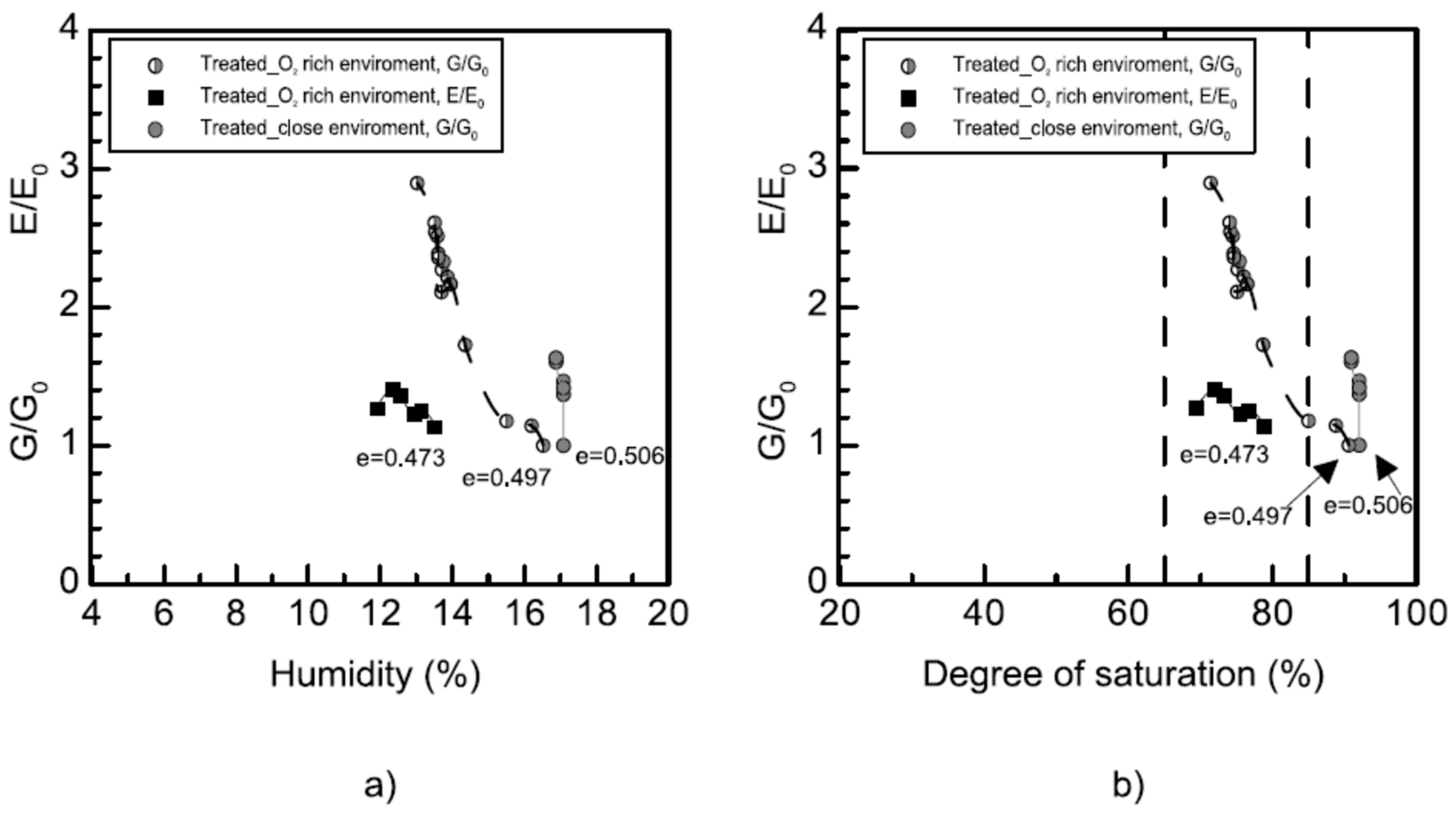
3.2.3. Bender Elements and Ultrasonic-Pulse-Velocity Test (Pundit)
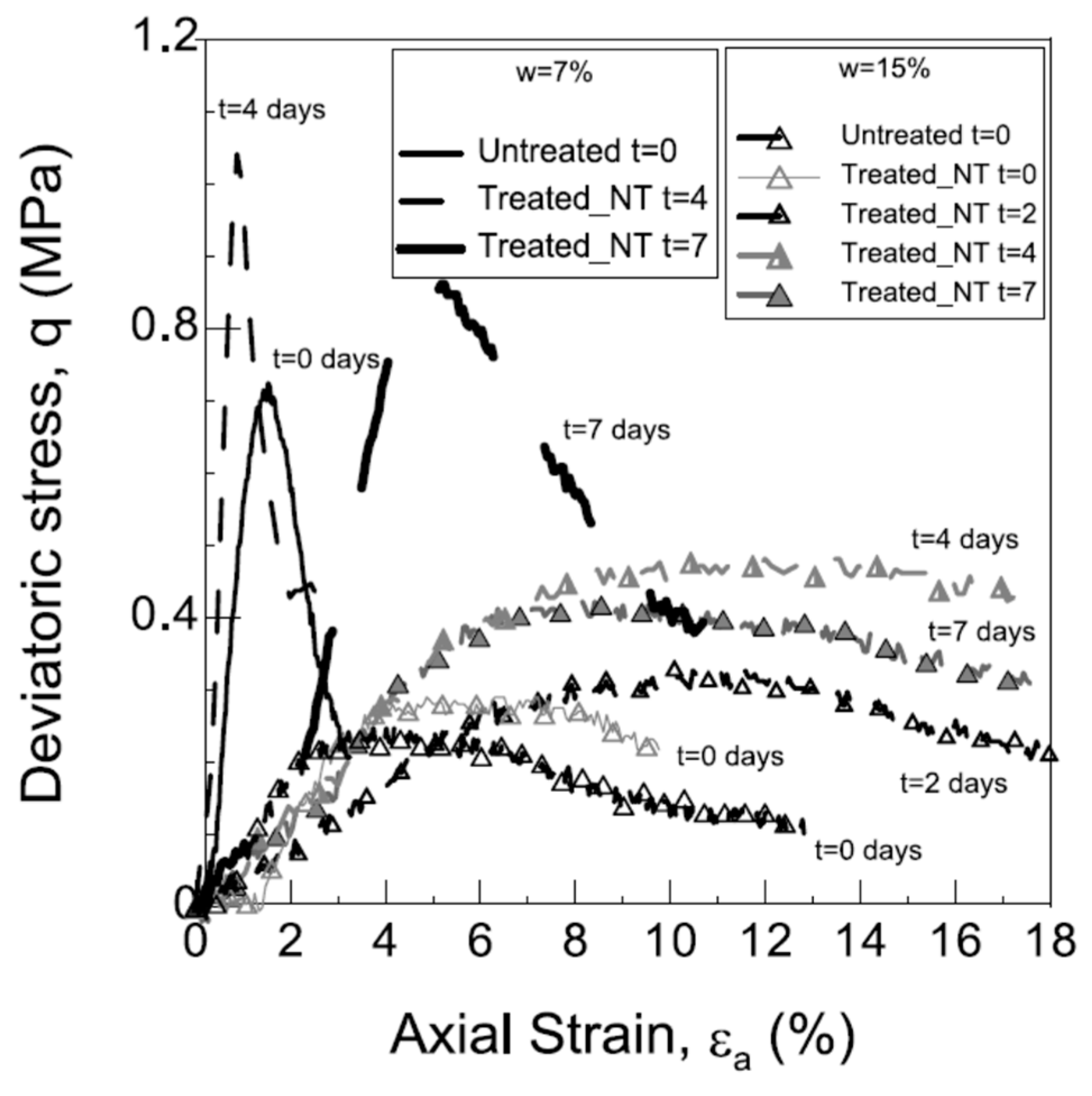
3.2.4. Unconfined Compression Test
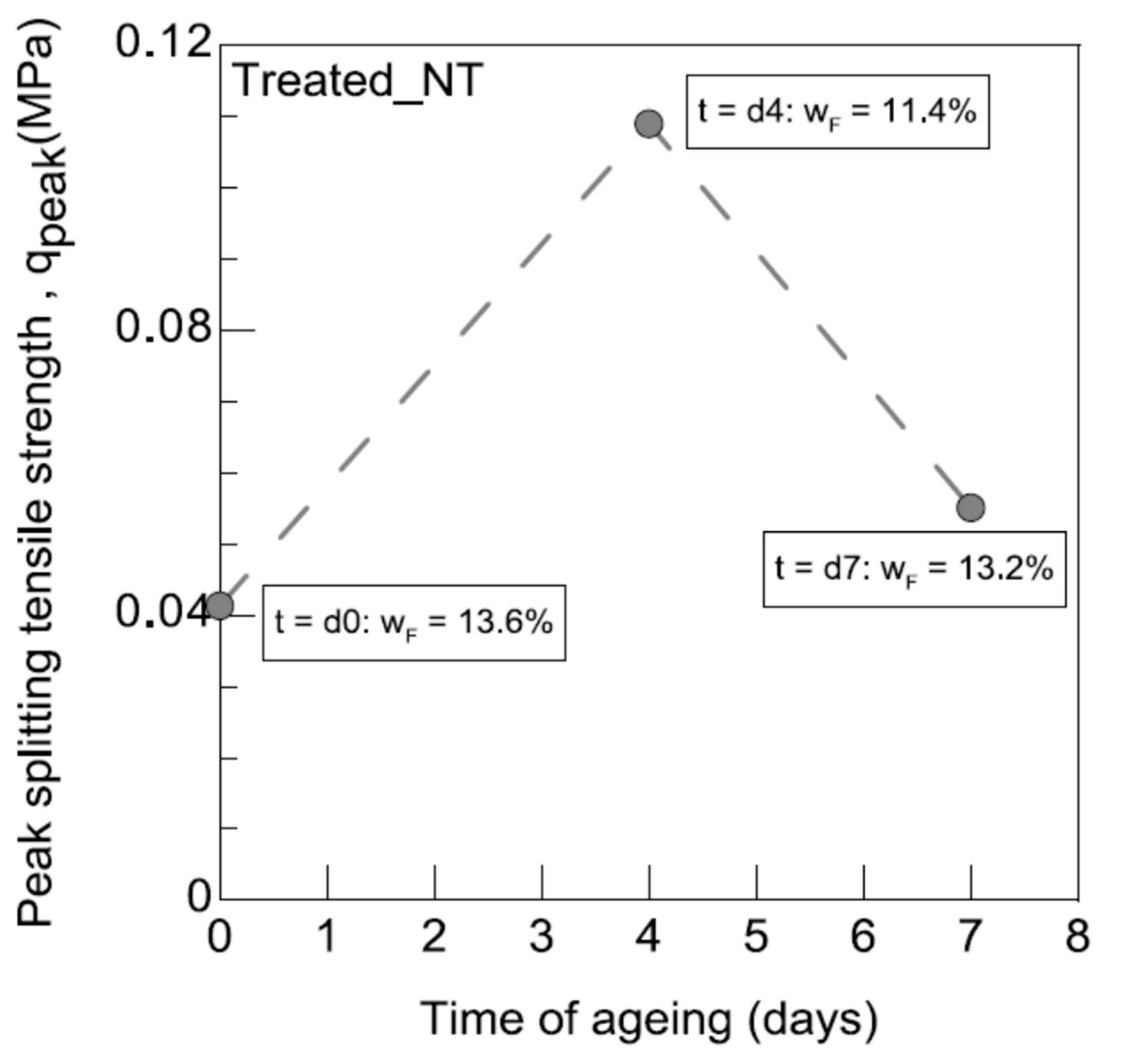
3.2.5. Brazilian Test (Splitting Tensile Strength)
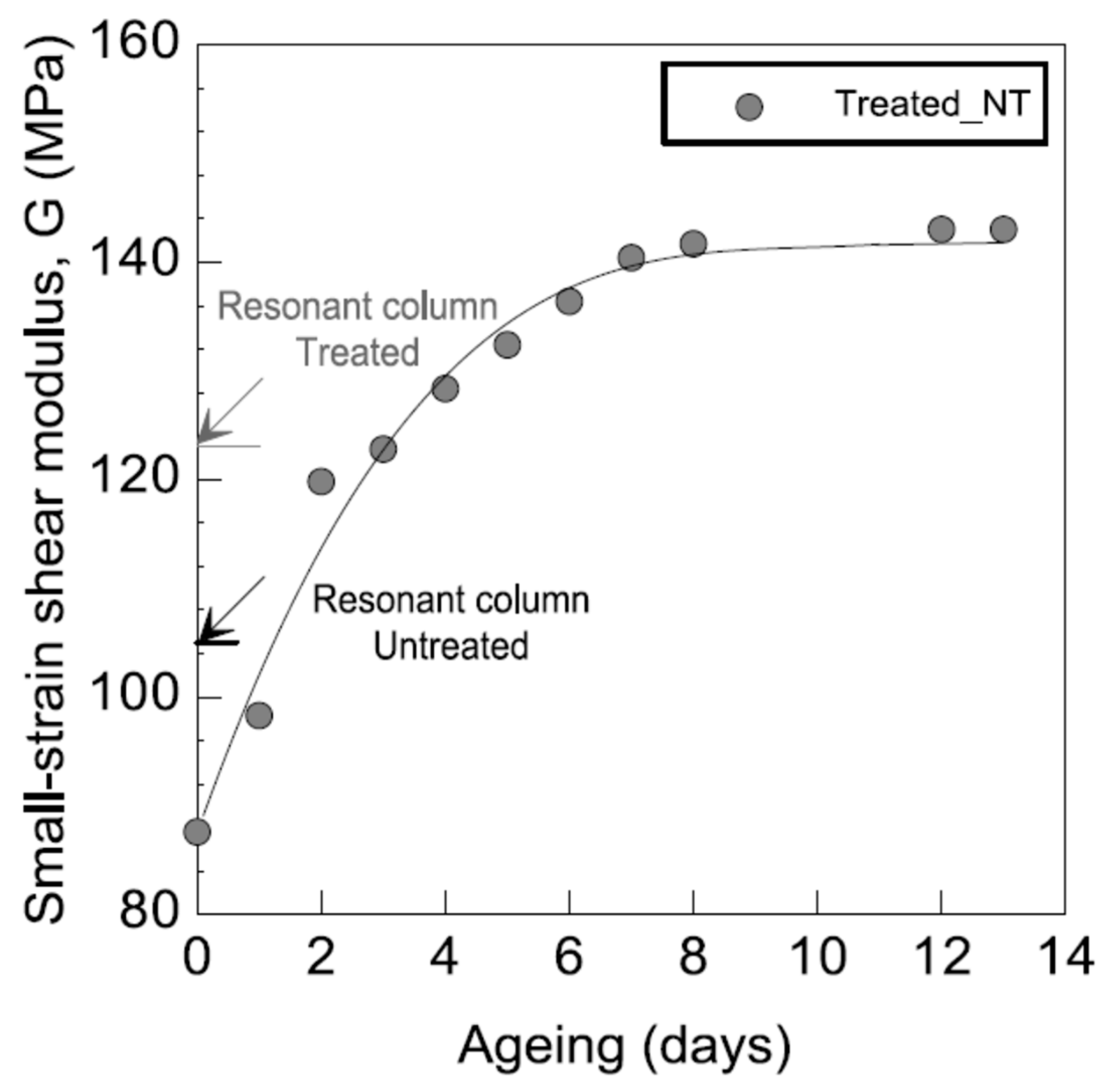
3.2.6. Resonant Column
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seagren, E.A.; Aydilek, A.H. Bioremediated Geomechanical Processes. In Environmental Microbiology; John Wiley and Sons: New York, NY, USA, 2010; pp. 319–348. [Google Scholar]
- Lian, B.; Hu, Q.; Chen, J.; Ji, J.; Teng, H.H. Carbonate Biomineralization Induced by Soil Bacterium Bacillus Megaterium. Geochim. Cosmochim. Acta 2006, 70, 5522–5535. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J. Ca-Carbonates Precipitation and Limestone Genesis—The Microbiogeologist Point of View. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, R.; del Moral, A.; Ferrer, M.R.; Ramos-Cormenzana, A. Precipitation of Calcium Carbonate by Vibrio Spp. from an Inland Saltern. FEMS Microbiol. Ecol. 1994, 13, 197–204. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum Conditions for Microbial Carbonate Precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ma, H.; Li, F.; Jin, Z.; Li, J.; Ma, F.; Wang, C. Citrobacter Sp. Strain GW-M Mediates the Coexistence of Carbonate Minerals with various Morphologies. Geomicrobiol. J. 2013, 30, 749–757. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Uad, I.; Rivadeneyra, A.; Vilchez, J.I.; Martin-Ramos, D.; González-López, J.; Rivadeneyra, M.A. Carbonate Precipitation of Bacterial Strains Isolated from Sediments and Seawater: Formation Mechanisms. Geomicrobiol. J. 2013, 30, 840–850. [Google Scholar] [CrossRef]
- Barabesi, C.; Galizzi, A.; Mastromei, G.; Rossi, M.; Tamburini, E.; Perito, B. Bacillus Subtilis Gene Cluster Involved in Calcium Carbonate Biomineralization. J. Bacteriol. 2007, 189, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Lastrico, R.M. Static Properties of Lightly Cemented Sand. ASCE J. Geotech. Eng. Div. 1978, 104, 1449–1464. [Google Scholar] [CrossRef]
- Boquet, E.; Boronat, A.; Ramos Cormenzana, A. Production of Calcite (Calcium Carbonate) Crystals by Soil Bacteria is a General Phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Knorre, H.; Krumbein, W.E. Bacterial Calcification. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 25–31. [Google Scholar]
- McConnaughey, T.A.; Whelan, J.F. Calcification Generates Protons for Nutrient and Bicarbonate Uptake. Earth Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key Roles of pH and Calcium Metabolism in Microbial Carbonate Precipitation. Re. Environ. Sci. Bio/Technol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Ivanov, V.; Chu, J. Applications of Microorganisms to Geotechnical Engineering for Bioclogging and Biocementation of Soil in Situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, W.; Wu, H.; Liu, H. Fracture of Interparticle MICP Bonds under Compression. Int. J. Geomech. 2023, 23, 04022316. [Google Scholar] [CrossRef]
- Sutton, M.; Reis, S.; Baker, S. Atmospheric ammonia: Detecting emission changes and environmental impact. In Results of an Expert Workshop Under the Convention on Long-Range Transboundary Air Pollution; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, M.C.M. Potential Soil Reinforcement by Biological Denitrification. Ecol. Eng. 2010, 36, 168–175. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial Carbonate Precipitation in Construction Materials: A Review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Carretero, M.I.; Bernabé, J.M.; Galán, E. Application of Sepiolite-Cellulose Pastes for the Removal of Salts from Building Stones. Appl. Clay. Sci. 2006, 33, 43–51. [Google Scholar] [CrossRef]
- Rubio, A.; Escudero, A. Effect of Climate and Physiography on Occurrence and Intensity of Decarbonation in Mediterranean Forest Soils of Spain. Geoderma 2005, 125, 309–319. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-Mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- DeJong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; van Paassen, L.; Fragaszy, R.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; et al. Biogeochemical Processes and Geotechnical Applications: Progress, Opportunities, and Challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Ebina, K.; Kawasaki, S. Biocementation for Slope Soil Stabilization against Surface Erosion: A Bench-scale Preliminary Investigation. In 53rd US Rock Mechanics/Geomechanics Symposium; American Rock Mechanics Association (ARMA): Alexandria, VA, USA, 2019; pp. 19–406. [Google Scholar]
- Zhang, J.; Shi, X.; Chen, X.; Huo, X.; Yu, Z. Microbial-Induced Carbonate Precipitation: A Review on Influencing Factors and Applications. Adv. Civil Eng. 2021, 2021, 9974027. [Google Scholar] [CrossRef]
- Iqbal, D.M.; Wong, L.S.; Kong, S.Y. Bio-Cementation in Construction Materials: A Review. Materials 2021, 14, 2175. [Google Scholar] [CrossRef]
- Yu, T.; Souli, H.; Pechand, Y.; Fleureau, J.M. Review on Engineering properties of MICP-treated soils. Geomech. Eng. 2021, 27, 13–30. [Google Scholar]
- Valencia-Galindo, M.; Sáez, E.; Ovalle, C.; Ruz, F. Evaluation of the Effectiveness of a Soil Treatment Using Calcium Carbonate Precipitation from Cultivated and Lyophilized Bacteria in Soil’s Compaction Water. Buildings 2021, 11, 545. [Google Scholar] [CrossRef]
- Verma, H.; Ray, A.; Rai, R.; Gupta, T.; Mehta, N. Ground improvement using Chemical methods: A review. Heliyon 2021, 7, e07678. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, W.; Liang, W.; Dai, F.; Liu, H.; Xiao, Y. 3D DEM modeling of biocemented sand with fines as cementing agents. Int. J. Numer. Anal. Methods 2023, 47, 212–240. [Google Scholar] [CrossRef]
- Morales, L.; Romero, E.; Jommi, C.; Garzón, E.; Giménez, A. Feasibility of a soft biological improvement of natural soils used in compacted linear earth construction. Acta Geotech. 2013, 10, 157–171. [Google Scholar] [CrossRef]
- Morales, L.; Garzón, E.; Romero, E.; Jommi, C. Effects of a microbiological compound for the stabilisation of compacted soils on their microstructure and hydro-mechanical behaviour. In Proceedings of the 5th International Conference on Unsaturated Soils, Barcelona, Spain, 6–8 September 2010; pp. 573–578. [Google Scholar]
- Viggiani, G.; Atkinson, J.H. Stiffness of Fine-Grained Soil at very Small Strains. Geotechnique 1995, 45, 249–265. [Google Scholar] [CrossRef]
- Blangy, J.P.; Strandenes, S.; Moos, D.; Nur, A. Ultrasonic velocities in sands; revisited. Geophysics 1993, 58, 344–356. [Google Scholar] [CrossRef]
- Pineda, J.A.; Mitaritonna, G.; Romero, E.; Arroyo, M. Effects of hydraulic cycling on the stiffness response of a rigid clay. In Proceedings of the 5th International Conference on Unsaturated Soils, Barcelona, Spain, 6–8 September 2010; pp. 1465–1470. [Google Scholar]
- Pineda, J.A. Swelling and Degradation of Argillaceous Rocks Induced by Relative Humidity Effects: An Experimental Study. Ph.D. Thesis, Universitat Politècnica de Catalunya (UPC), Barcelona, Spain, 2012. [Google Scholar]
- Anderson, D.G.; Stokoe, K.H. Shear Modulus: A Time Dependent Soil Property. In Dynamic Geotechnical Testing, ASTM STP 654; ASTM: Philadelphia, PA, USA, 1978; pp. 66–90. [Google Scholar]
- Das, B.M. Advanced Soil Mechanics; Hemisphere Publishing Corporation: Washington, DC, USA, 1983. [Google Scholar]
- Suriol, J. Medida de la deformabilidad de suelos mediante el equipo de columna resonante. Revista de Obras Públicas 1993, 3319, 57–66. [Google Scholar]
- Morales, L.; López-González, J.A.; Garzón, E.; Giménez, A.; Romero, E. Crecimiento de cristales de CaCO3 como resultado de la actividad microbiológica en suelos. Macla 2011, 15, 141–142. [Google Scholar]
- Romero, E.; Della Vecchia, G.; Jommi, C. An Insight into the Water Retention Properties of Compacted Clayey Soils. Geotechnique 2011, 61, 313–328. [Google Scholar] [CrossRef]
- Morales, L.; Romero, E.; Pineda, J.A.; Garzón, E.; Giménez, A. Ageing effects on the stiffness behaviour of a microbiologically treated and compacted soil. In Unsaturated Soils: Research and Applications; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 371–376. [Google Scholar]
- Gallipoli, D.; Gens, A.; Sharma, R.; Vaunat, J. An Elasto-Plastic Model for Unsaturated Soil Incorporating the Effects of Suction and Degree of Saturation on Mechanical Behaviour. Geotechnique 2003, 53, 123–135. [Google Scholar] [CrossRef]
- Cho, G.C.; Santamarina, J.C. Unsaturated Particulate Materials-Particle-Level Studies. J. Geotech. Geoenviron. Eng. 2001, 127, 84–97. [Google Scholar] [CrossRef]









| Sample | Liquid Limit | Plasticity Index | Calcium Carbonate | Particle Size | Specific Surface | ||
|---|---|---|---|---|---|---|---|
| WL (%) | PI (%) | CaCO3 % dry mass | <75 mm (%) | <2 mm (%) | BET Ss(m2/g) | Langmuir Ss(m2/g) | |
| Untreated | 48 | 30 | 4.5 | 50 | 23 | 33 | 46 |
| Sample | Standard Proctor (SP) | Modified Proctor (MP) | Static Compaction | Soil Density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρd | W | Sr | Compact. Energy | ρd | W | Sr | Compact. Energy | ρd | W | Sr | Compact. Energy | ρs | |
| (Mg/m3) | (%) | - | (MJ/m3) | (Mg/m3) | (%) | - | (MJ/m3) | (Mg/m3) | (%) | - | (MJ/m3) | (Mg/m3) | |
| Untreated | 1.85 | 15.0 | 0.87 | 0.6 | 2.05 | 11.4 | 0.95 | 2.7 | 1.85 | 15.0 | 0.87 | 1.0 | 2.725 |
| 1.85 | 7.0 | 0.40 | 9.7 | ||||||||||
| Untreated | Treated_NT(O2 Rich Environment) | Treated_NT(Closed Environment) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W0 | Ψ0 | V0 | WF | ΨF | VF | W0 | Ψ0 | V0 | WF | ΨF | VF | W0 | Ψ0 | V0 | WF | ΨF | VF |
| % | MPa | mm/s | % | MPa | mm/s | % | MPa | mm/s | % | MPa | mm/s | % | MPa | mm/s | % | MPa | mm/s |
| 15.1 | 1 | 275 | 11.1 | 3 | 424 | 16.5 | 0.003 | 230 | 13.0 | 0.22 | 396 | 17.1 | 0.001 | 203 | 16.9 | 0.001 | 260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, L.M.; Garzón, E.G.; Sánchez-Soto, P.J.; Morales, E.R. Simultaneous Biocementation and Compaction of a Soil to Avoid the Breakage of Cementitious Structures during the Execution of Earthwork Constructions. Geotechnics 2023, 3, 224-253. https://doi.org/10.3390/geotechnics3020014
Hernández LM, Garzón EG, Sánchez-Soto PJ, Morales ER. Simultaneous Biocementation and Compaction of a Soil to Avoid the Breakage of Cementitious Structures during the Execution of Earthwork Constructions. Geotechnics. 2023; 3(2):224-253. https://doi.org/10.3390/geotechnics3020014
Chicago/Turabian StyleHernández, Laura Morales, Eduardo Garzón Garzón, Pedro J. Sánchez-Soto, and Enrique Romero Morales. 2023. "Simultaneous Biocementation and Compaction of a Soil to Avoid the Breakage of Cementitious Structures during the Execution of Earthwork Constructions" Geotechnics 3, no. 2: 224-253. https://doi.org/10.3390/geotechnics3020014
APA StyleHernández, L. M., Garzón, E. G., Sánchez-Soto, P. J., & Morales, E. R. (2023). Simultaneous Biocementation and Compaction of a Soil to Avoid the Breakage of Cementitious Structures during the Execution of Earthwork Constructions. Geotechnics, 3(2), 224-253. https://doi.org/10.3390/geotechnics3020014









