Abstract
Mid-day maximum soil temperatures were measured at 10 study plots during different hot summer days in Haleakalā Crater, Maui, with thermocouple thermometers on five adjacent microsite types: bare surface soils, soils under black tephra, soils under reddish tephra, soils shaded by silverswords, and soils under plant litter. The main tephra morphologies and geomorphic environments, as well as their geoecological association with silversword rosettes (Argyroxiphium sandwicense), were also assessed; silversword density was substantially greater on reddish tephra-covered areas than under black tephra fragments. Silversword seeds are extremely sensitive to high temperatures and fail to germinate after a short exposure to soil temperatures ≥35 °C. Thermal data sets were statistically compared with parallel box plots; the ability of various microsites to provide safe sites for silversword growth was also assessed. Bare soils and black tephra reached the highest median temperatures, up to 48.7 °C and 40.3 °C, respectively; reddish tephra remained much cooler, with all median temperatures ≤30.8 °C. Rosette-shaded soils and soils under silversword litter were the coolest, with temperatures below 18.7 °C and 18.5 °C, respectively. Temperatures in all microsites, except those under black tephra, were significantly lower (p < 0.0001) than on contiguous bare ground. It was concluded that reddish tephra provides the ideal conditions for silversword regeneration.
Keywords:
albedo; box plots; nurse boulders; nurse plants; nurse rocks; rosette plants; soil color; volcanic tephra On a clear cloudless summer day by 9 AM surface [cinder] fragments can approach, and exposed sand can greatly exceed, the lethal germination temperature of 35 °C. By contrast, an abrupt decrease in temperature… [is] encountered only a few centimeters below the surface of rock fragments’. Herbert Kumeo Kobayashi, 1973 [1], p. 48.
1. Introduction: The Scope of the Project
1.1. Beneficial Effects of Nurse Rocks and Nurse Plants
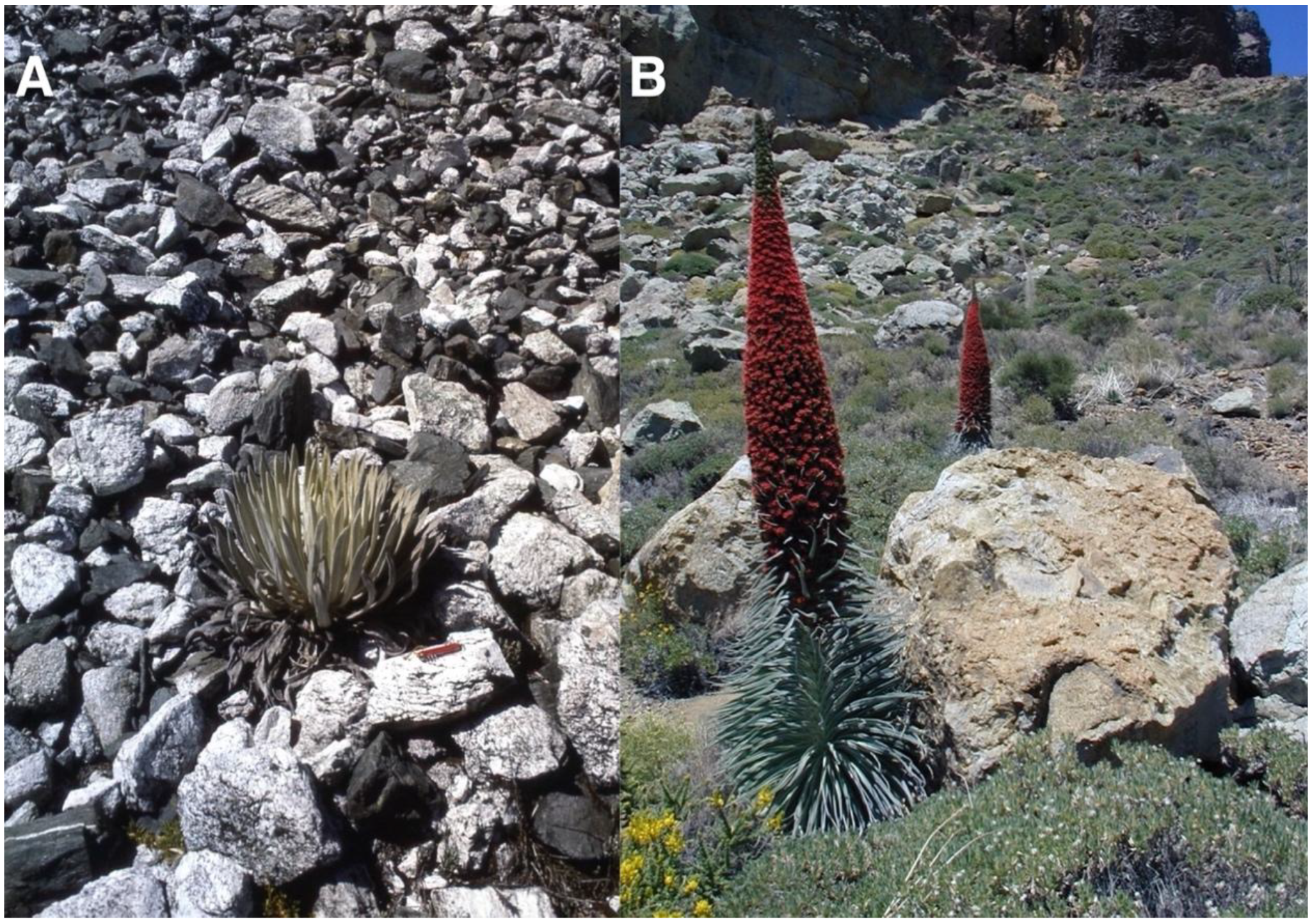
Many plants in extreme arid, alpine, and arctic environments experience significant germination difficulties caused by a host of adverse climatic, pedological, geomorphic, or biotic conditions and may benefit from the protection afforded by various substrate components in their native habitat. In the early 20th century, the botanist August Weberbauer [2,3]—possibly the first to comment on spatial correlations between plants and stones—observed how grasses and shrubs grew at greater elevations in the high Peruvian Andes on rock-covered areas; he ascribed this relationship to the thermal influence of stones on adjacent soil. Karl Olov Hedberg [4] noticed rocks on Mount Kenya, East Africa, that commonly provided shelter to rosette plants at high elevations, and referred to these stones as edafids, thus assuming pedological effects. In recent times, plant researchers have preferred to use the names nurse rocks, inanimate nurse objects, or simply nurse objects [5,6,7,8]. Massive nurse boulders [9,10,11,12], tall outcrops, and crevices may also shade and provide effective shelter from daily insolation for emergent seedlings for some period of time, depending on rock dimensions, slope inclination, and orientation [8,13,14,15]. Giant tropical rosette plants are known to particularly favor nurse rocks in several mountain areas of the world (Figure 1).

Figure 1.
(A). Coespeletia timotensis (Cuatrec.) Cuatrec. (Asteraceae) growing on a steep talus slope of granite and amphibolitic schist fragments; stones are ~25–35 cm long, rosette is ~42 cm tall; pocket knife for scale is 8 cm long. Parque Nacional Sierra de La Culata, equatorial Venezuelan Andes, 4355 m. Photo: 26 December 1982. This is the rosette species that grows at highest elevations in the world [16,17]. (B). Echium wildpretii H. Pearson ex. Hook. f. (Boraginaceae), growing in Roques de García, a group of volcanic pinnacles at Parque Nacional del Teide, Tenerife, Spain. The largest individual in front is rooted at the base of a ~1.6 m tall volcanic boulder, produced by a large rockfall, visible on the upper left—slope angle is 19–21°; 2060 m. Photo: Teide-08, 28 May 2008. Note the ≤2.7 m tall inflorescence stalks; compare these with those in silverswords Figure 3). All ground photographs, except when otherwise indicated, were taken by the author.
Ecologists working in grassland and forest environments have also found significant effects of nurse rocks on the establishment of woody plant [14,18]. Different plant species may also help generate favorable locations for seedling growth; these nurse plants have been extensively studied in the lowland deserts of North America [5,6,19,20,21,22,23,24] and in several alpine Andean areas in South America [25,26,27,28] and elsewhere (Figure 2).

Figure 2.
A ~24 cm tall kūpaoa, Dubautia menziesii (A. Gray) D.D. Keck (Asteraceae) seedling emerging through the dense canopy of a ~62 cm diameter pukiawe, Leptecophylla tameiameiae (Cham.) C.M. Weiller (Ericacea) nurse shrub on a 19°-slope, Haleakalā Crater, 2625 m. This is an infrequent example of nurse plants in the crater; the ruler is 15 cm long. Photo: Hk-05.497, 29 July 2005.
Several ecologists have specifically compared the level of protection provided by nurse rocks with that offered by nurse plants. Pérez [25] determined the spatial distribution of three alpine rosette species in high-Andean areas and found that seedlings were ~30% more common next to rocks than on cushion nurse plants. This relationship was ascribed to interspecific root competition between nurse plants and rosettes; nurse plants were also eventually negatively affected by the ground shading caused by rosettes. In addition, ~21% of rosette seedlings grew next to adult plants, suggesting that giant Andean rosettes could play a ‘self-nursing’ role in the high Andes [4]. Peters et al. [29] also concluded nurse rocks were more important in determining the distribution and establishment of small globose Mammillaria Haw. cacti in Mexican deserts, as shading by shrubs reduced photosynthetically active radiation needed for cactus growth, whereas rocks provided suitably cooler and moist environments without diminishing sunlight. Munguia-Rosas and Sosa [8] investigated columnar cacti in Veracruz, Mexico, and decided the mechanisms of plant facilitation were different from those of rock facilitation; nurse plants offered appropriate microclimatic conditions, while rocks protected seeds from ant predators. Haussmann et al. [30] analyzed the distribution of two Azorella Lam. cushion-plant species in sub-Antarctic Marion Island, South Africa, which positively affected the spatial patterns of their own seedlings and of perennial grasses; they thought nurse rocks might offer only mechanical substrate stability, whereas nurse cushions additionally offered crucial biological benefits, such as increased nutrient concentrations.
1.2. Significance of the Haleakalā Silversword and of Volcanic Tephra Covers in the Crater
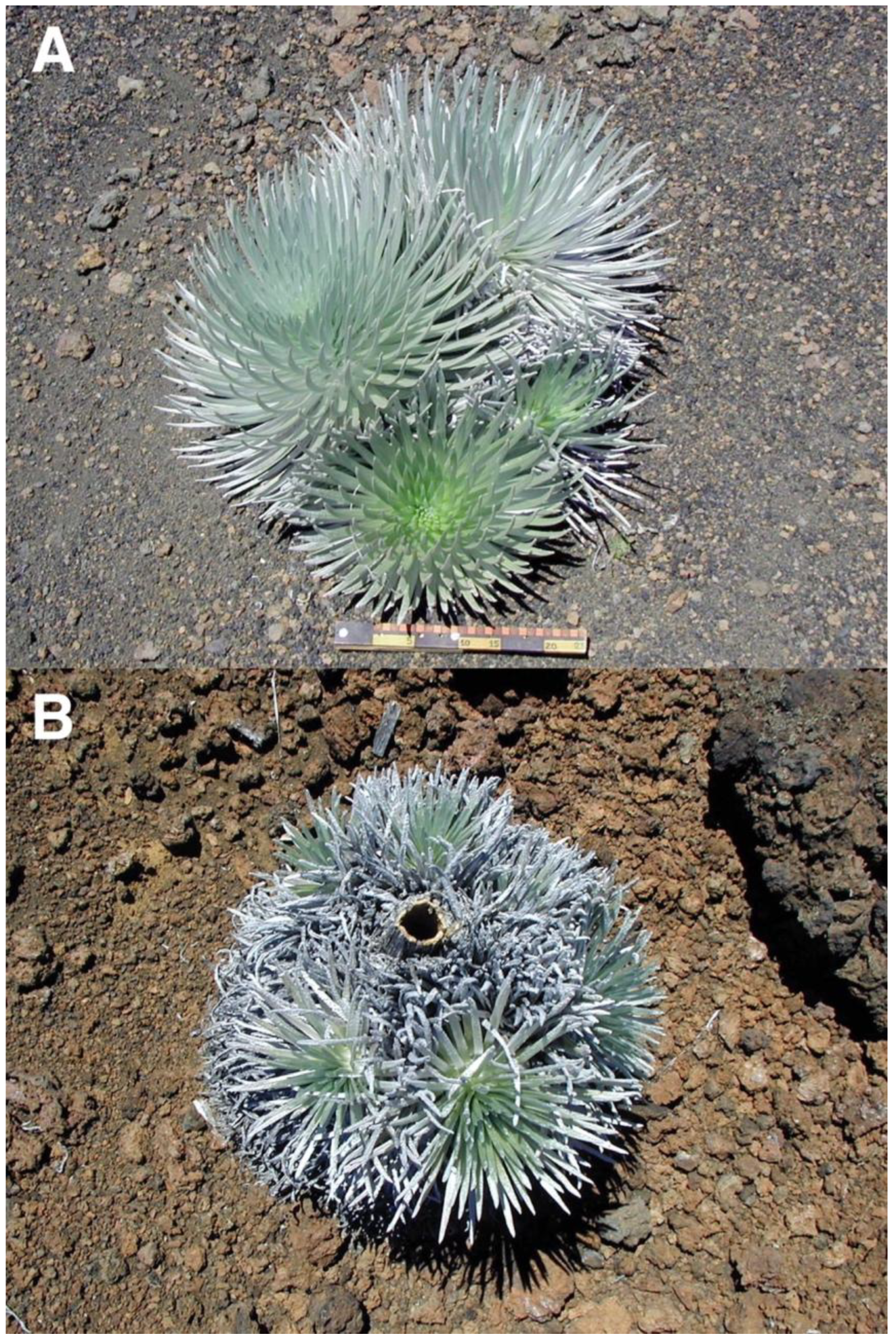
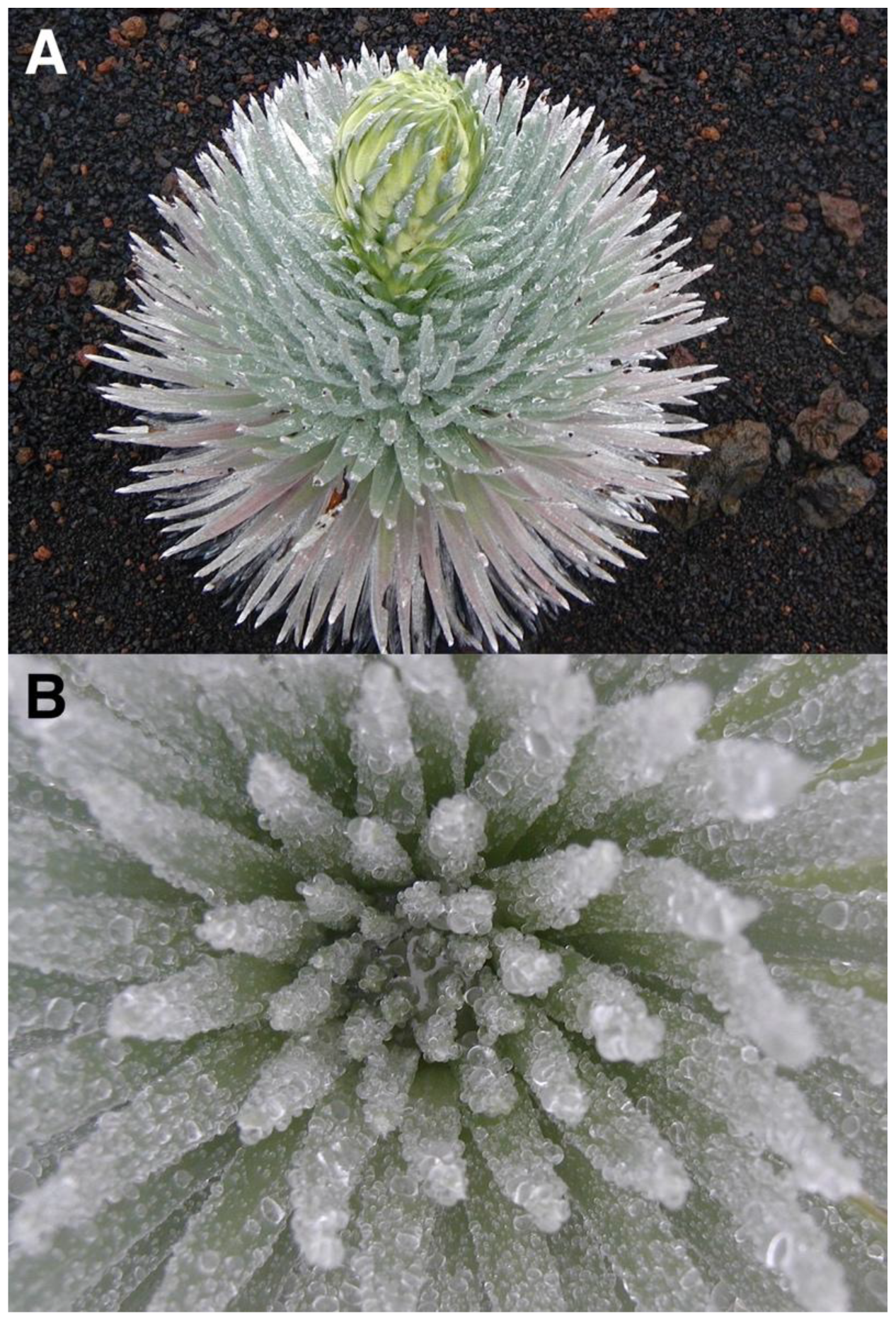
The Haleakalā silversword (Argyroxiphium sandwicense DC. subsp. macrocephalum (A. Gray) Meyrat)—’āhinahina in Hawaiian—is the most impressive plant found in Haleakalā National Park [HNP]. This giant, perennial, monocaulic (single-stemmed), rarely branched, rosette-plant has a short, ≤8 cm diameter woody stem that ends in a large (≤1 m diameter), dense crown of silvery, shiny, evergreen, long, narrow, sword-shaped, densely pubescent, floccose-silky leaves; the older, dry, shriveled, marcescent, 15–40 cm long leaves remain strongly attached to the stem until they finally decompose [31]. The silversword is dioecious and monocarpic and dies after flowering just once at the end of its life, perhaps after 39 to 63 years—although some individuals may survive nearly 90 years [32]—when the plant initiates bolting, and grows a spectacular, up to 250 cm long, flowering stalk with ~50–600 purple flower heads (Figure 3). An important characteristic of the silversword—relevant to this story—is that the shiny, light color and hair cover on its leaves confer it an unusually high degree of reflectance, i.e., high albedo (Figure 4).

Figure 4.
Silversword rosette with its leaf crown fully open, exposing its extended foliage—for some unclear reason—during an extremely sunny day. Note the high reflectance of this ~55 cm diameter individual growing on black fine ash and lapilli. Plot 9, Silversword Loop; plant grows on Pu’u o Māui’ lava flow, ~2170 m. Photo: Hk-02.304, 30 July 2002, 11:48 a.m. HST.

Figure 3.
Dense group of Haleakalā silverswords in the upper crater; near plots 6 and 7, 2495 m, ~4–6° slope. Photo taken during a mast year, when a high percentage of plants bloomed across the crater; at least 17 recently, 160 to 210 cm tall, and inflorescence stalks are seen in this view. The inflorescence on the forefront, right, is ~200 cm tall. Note the high spatial degree of aggregation the rosettes show, growing close to each other; the ground is covered by small gray and light-brown tephra particles. The crater rim (~2950 m) and the Kalahaku palis appear in the background. Photo: Hk-05.413, 4 August 2005.
Figure 3.
Dense group of Haleakalā silverswords in the upper crater; near plots 6 and 7, 2495 m, ~4–6° slope. Photo taken during a mast year, when a high percentage of plants bloomed across the crater; at least 17 recently, 160 to 210 cm tall, and inflorescence stalks are seen in this view. The inflorescence on the forefront, right, is ~200 cm tall. Note the high spatial degree of aggregation the rosettes show, growing close to each other; the ground is covered by small gray and light-brown tephra particles. The crater rim (~2950 m) and the Kalahaku palis appear in the background. Photo: Hk-05.413, 4 August 2005.

Silverswords were much more abundant, and covered a slightly larger geographical range, in the past. Even during the late 1860s, they grew copiously on the outer flanks of Haleakalā, as well as inside the crater [33]. Isabella Lucy Bird, a naturalist, visited Haleakalā Crater in May 1873, and remarked that silverswords covered the inner crater slopes and the cinder cones with ‘… thousands of silverswords, their cold, frosted silver gleam making the hill-side look like winter or moonlight’ [34], p. 338. Goats (Capra hircus), introduced to Maui in 1793 [35], gradually escaped farmsteads across the island and soon became feral, spreading rapidly as they invaded high shrublands and remote sites, such as deep gulches, steep ravines, and inaccessible mountain summits [36,37]. It was not until the late 20th century, when a 57 km long fence fully encircling HNP was completed in 1986, that goats were finally excluded from the crater [35]. Silversword populations were severely damaged and decimated during decades of attack by the animals. In 1938, an internal HNP report by H.A. Powers barely found ~4000 individual rosettes across the crater, and R. Badaracco mentioned in 1962 ‘perhaps 10,000’ plants for the entire HNP [1], p. 10. Incessant control and stern protection by HNP after its establishment in 1916 gradually brought silversword numbers to about 43,000 plants in 1969 and then to ~65,000 in 1991 [1,38,39]. Unfortunately, due to recent changes of regional weather patterns and global climatic conditions, this positive trend has been reversed, and a rapid, worrisome decline in Argyroxiphium numbers has been detected during the 21st century, with very sparse seedling recruitment in recent years [40,41]. Currently, the silversword is officially considered as ecologically fragile; thus, it is federally protected and listed as ‘threatened’ [42].
Early explorers were intrigued by the characteristics and distribution patterns of tephra covers across the crater. The first scientific party from the U.S. Exploring Expedition to venture down into the crater in 1841 referred to tephra deposits as follows: ‘…the gravel that occurred on the top [of Haleakalā] was composed of small angular pieces of cellular lava, resembling comminuted mineral coal… containing irregular cavities rather than vesicles’ [43], p. 254. William DeWitt Alexander [33], first Surveyor-General for the Kingdom of Hawaii’s Government, visited the crater in 1869 and noticed that silverswords were most abundant over tephra deposits. Kobayashi [1], pp. 43, 68, was the first ecologist to observe that silversword regeneration was better on slopes blanketed by red tephra than on those covered by black cinder. Hypothesizing there might be some variation in their chemical composition, he analyzed samples from both tephras and determined there were no significant differences between them, thus concluding that physical, rather than chemical differences in the substrate, influenced the spatial patterns of rosettes. Color variation in Hawaiian pyroclasts is actually affected by the degree of oxidation during, and immediately after, the eruptions depositing cinders [44,45], p. 50. Different Fe oxides may be identified by their specific mineral colors [46,47]; in fact, the intensity of redness in soils and tephra deposits is determined by the type of iron oxide—namely hematite—present in them [48,49].
Color greatly determines the reflectance (i.e., albedo) of a soil or surface deposit [47,50]; the color component most closely associated with albedo is the Munsell color value [51,52], which may account for up to 93% of albedo variation [47], as shown in Figure 1. In essence—as expected—lighter soils display higher albedo; the dark, black cinder deposits at Haleakalā should also show lower albedo than the reddish tephras. Soil moisture also affects its albedo; surface albedo drops with increasing soil moisture below the field capacity [53,54]. In addition, as a rough soil surface scatters light in multiple directions, soil albedo decreases with greater surface roughness [47,55]. The solar elevation angle could also affect soil reflectance; maximum albedo values are reached at the zenith and at solar angles no lower than 30° [54,55]. All soil and tephra measurements (see below) were taken during dry summer days at or nearly noon hours, and the two tephra types displayed similar surface roughness and irregularities; thus, the most significant edaphic variable influencing their albedo was color. All things considered, reddish tephras should be expected to have greater reflectance and absorb less solar radiation than black cinders, which might consequently reach higher temperatures and also allow for greater substrate evaporation.
Any reduction in substrate temperatures would be of great ecological significance for the establishment and survival of silversword seedlings. Laboratory experiments using Argyroxiphium seeds from Haleakalā, as well as from Mauna Loa (island of Hawai’i) [56], indicated that seeds were extremely sensitive to high temperatures and failed to germinate after only 8 h of exposure to a soil temperature of 35 °C; brief exposure periods of just 15 min to temperatures of 60 °C sufficed to kill all seeds. Laboratory and fieldwork research also showed that silverswords from Mauna Kea had a greater germination percentage, faster growth rates, and lower mortality in tephra-covered areas than on bare soils [57,58].
1.3. General Focus and Purpose of This Study
A previous report [12] contrasted diurnal soil temperatures under large boulders with those of a small number of red tephra locations in Haleakalā; the present geoecological project will examine a much more extensive data set of nurse objects potentially significant for silversword regeneration. The main goals of this study are as follows: (i) Characterize and graphically illustrate some of the most significant black and reddish tephra types and environments in the crater, explicitly focusing on the main volcanic and geomorphic processes involved in their genesis. (ii) Report on several comprehensive data sets of maximum daily substrate temperatures, as measured in several contrasting, adjacent, microsites, which may perform as nurse objects, thus facilitating rosette establishment: a—bare surface, exposed soils; b—soils under a cover of black tephra fragments; c—soils below a layer of reddish tephra particles; d—soils on shaded ground areas near and around adult silverswords; and e—soils beneath small heaps of decomposing organic matter, left by dead rosettes. (iii) Statistically analyze all microsite data, employing parallel box plots—see below—and compare these multiple sets of microsite temperatures with those of contiguous open soil spaces. (iv) Briefly evaluate whether the thickness of tephra covers, or the size of silverswords, has any effect on the maximum temperature readings of adjoining soils influenced by them. (v) Compare all the general traits of different microsite types that could influence silversword growth and survival.
2. Study Methods
2.1. Fieldwork Procedures and Observations
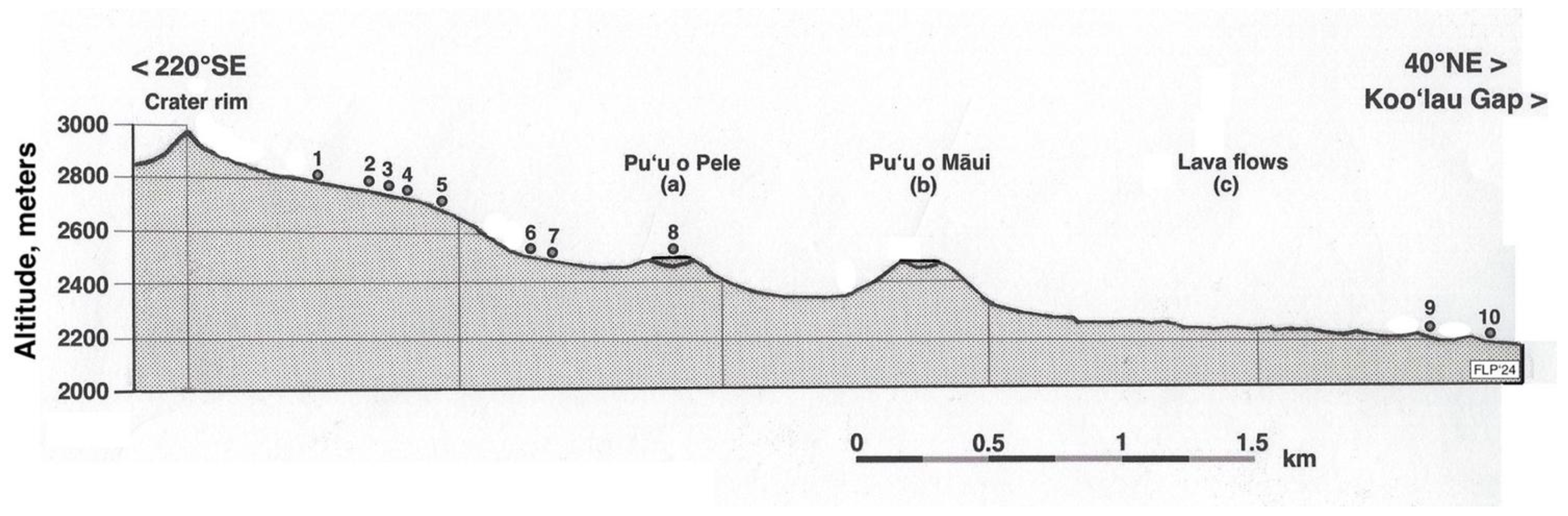
Fieldwork encompassed 19 years, from 1996 to 2014; ten summer field seasons included extensive reconnaissance of potential research sites in the western and central parts of Haleakalā Crater, during which a diversity of tephra environments and geomorphic sites was explored across the volcanic depression. Substrate temperature data sets—see below—were gathered at ten extensive study parcels, henceforth called measurement plots; thermal data were obtained at each plot during a single, sunny, cloud-free, clear-sky, warm, day, close to noon, during summer months (June, July, or August) between 2001 and 2011. Plots were located between 2165 and 2755 m; this elevation range encompasses most of the whole natural altitudinal span of the silversword (Figure 5 and Figure 6). Individual plots varied in area from ~100 to ≤500 m2; plots were consecutively numbered starting from the highest elevation and exhibited a NE aspect, except plot 8 inside Pu’u o Pele’s crater, a large cinder cone, which had a SE orientation. Plot altitude was assessed with a Thommen™ (Thommen, Muttenz, Switzerland) altimeter—expected error of ±10 m—and later verified on USGS topographic maps. Average slope inclination was determined with a Suunto™ (Suunto, Vantaa, Finland) clinometer; mean plot azimuthal orientation was measured with a geological Brunton™ (Brunton, Riverton, WY, USA) compass. Prior to sampling, plots were carefully examined to identify potential locations where readings for different substrate types could be obtained on closely adjacent positions. Data collection was designed afterward to compare consecutive readings of soil temperatures in contiguous (~100- to ~250 cm apart) locations, called sampling microsites here.
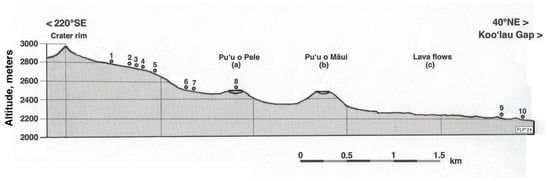
Figure 5.
Schematic topographic section along a 220° SE–40° NE azimuthal direction, showing the 10 temperature measurement plots, arranged by altitude (m) as numbered dots. Plots and cinder cones located along the 5 km long transect were laterally projected on a cross-section plane; scale is in km. Data were from base map, kindly allowed by U.S. Geological Survey: USGS Map I-1088 (1:24,000), Miscellaneous Investigations Series. Geologic map of the crater section of Haleakala National Park, Maui, Hawai’i, Gordon A. Macdonald, 1978 [59]. USGS is located at https://usgs.gov, accessed on 12 May 2015. Compared with a recent satellite photograph (Figure 6).

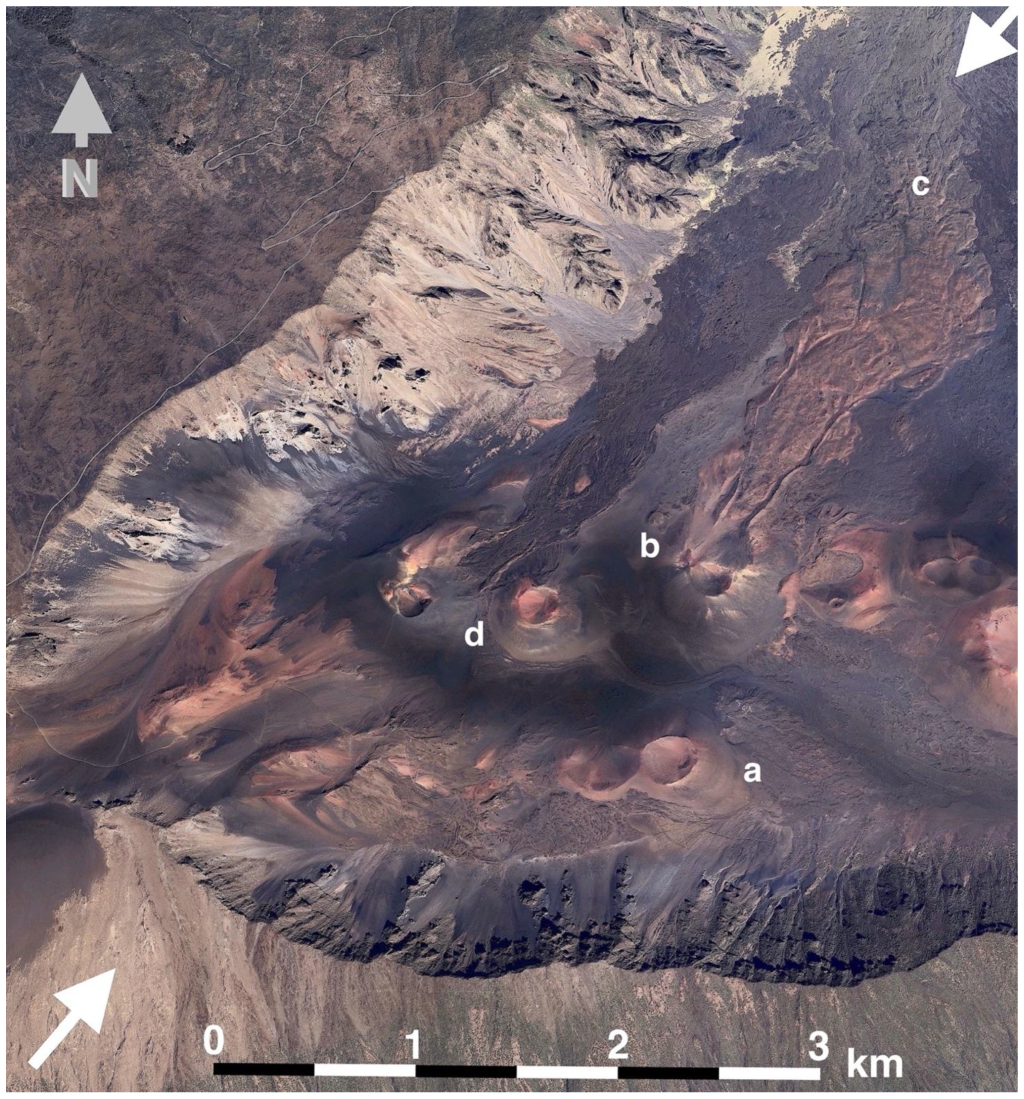
Figure 6.
Western Haleakalā Crater, showing the section that includes measurement plots; the W and SW crater rim appear on the left and lower sides, respectively. The highest end of the Haleakalā Highway is shown in the upper left. Two white arrows indicate the location of the topographic cross-section depicted on Figure 5; graphic scale in the foreground is ~3 km long. Key: (a)—Pu’u o Pele cinder cone; note its broad, saucer-shaped, crater; (b)—Pu’u o Māui cinder cone, with large lava flows issuing from its northern flank; (c)—distal end of Pu’u o Māui flows, where plots 9 and 10 are located; the Ko’olau Gap lies beyond these lava flows. (d)—Ka ma’o li’i cinder cone. North arrow, upper left, shown in dark gray. Image used with kind permission from GoogleMaps/Google Earth (Google Earth Pro 7.3.6.10201 (64-bit) Mac OS X (13.7.1) AMD Advanced Media Framework, Mountain View, CA, USA), following Universal Terms of Service. Imagery date: 18 January 2024. View centered at 20°45′11.92″ N, 156°12′58.28″ W. Elevation, 2092 m; eye altitude, 11.33 km.
Maximum diurnal soil temperatures were logged with thermocouple thermometers (Barnant-115™ 600-2810) (Barnant Company, Barrington, IL, USA) equipped with a 125 mm long, 3.5-mm-wide, depth-graduated thermoprobe [1,12,60] with a 0.1 °C precision and a ±0.1 °C accuracy and display resolution. Midday readings for individual microsites were obtained between ~1:30 and 3:30 p.m., as soil temperatures in the crater peak sometime between 2:00 and 3:45 p.m. [9,12]; the graduated probe was carefully inserted into the soil to a uniform 5 cm depth, and readings were logged in only after reaching full equilibrium in ~30–60 s [61]. Depth (mm) of tephra or organic matter layers (cm) above the soil was measured at each microsite with a vernier caliper; plant height and crown diameter—measured horizontally from leaf tip to opposite leaf tip [32,39]—were obtained for all silverswords on those plots where temperatures beneath adult rosette shades were assessed.
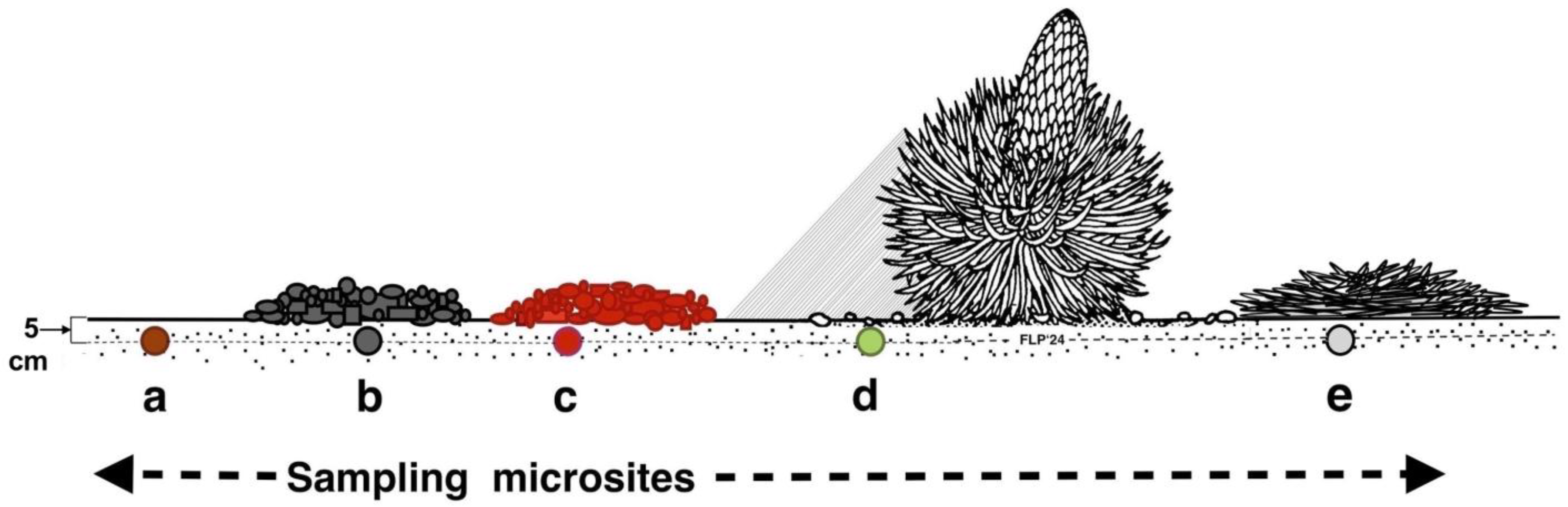
Sets of 10, 20, or 30 paired measurements were gathered at each microsite sequentially on various microsite substrates: (1) bare, uncovered soils, (2) soils covered by openwork—with empty voids—black tephra fragments, (3) soils covered by openwork reddish tephra clasts, (4) soils under the shade of tall silversword rosettes, and (5) soils covered by organic matter produced by dead rosettes (Figure 7).
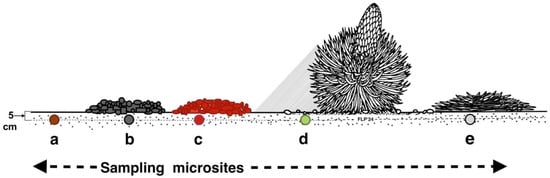
Figure 7.
Different substrate microsites sampled. Key: (a): bare soil, (b): black tephra, (c): reddish tephra, (d): shaded ground next to a silversword rosette, (e): dead silversword organic matter. All temperatures were measured at 5 cm depth within the soil.
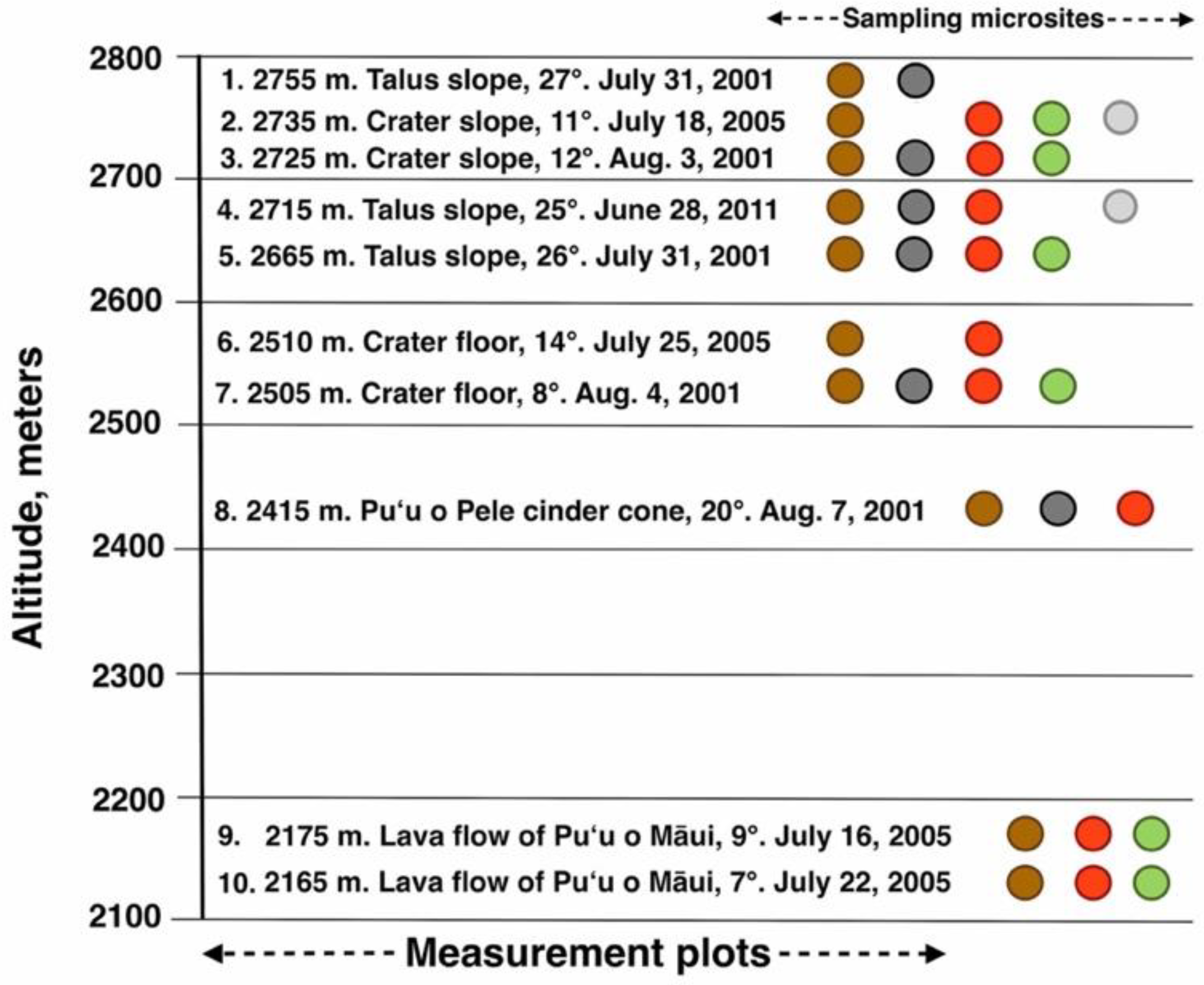
Bare soil maxima were sampled at all plots. Temperatures under tephra fragments were assessed wherever tephra covers were present; some sites (3, 4, 5, 7, and 8) showed both tephra types, other plots had only either black (1), or reddish (2, 6, 9, and 10) clasts; temperatures under the shade of rosettes were obtained at six plots (2, 3, 5, 7, 9, and 10); temperatures below rosette litter were gathered at two plots (2 and 4) only. Special care was taken in choosing microsites with a reasonably uniform cover of either black or reddish tephra, avoiding mixed-color tephras (Figure 8).
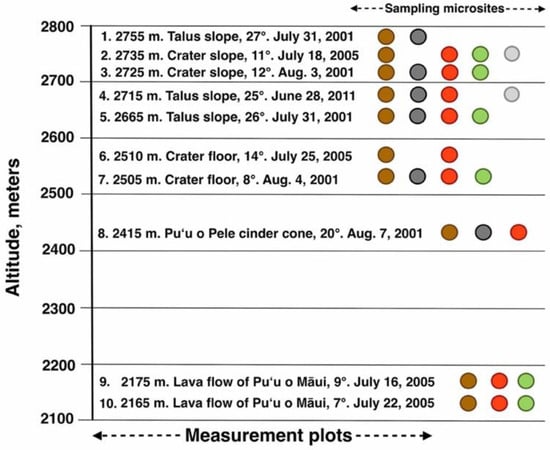
Figure 8.
Graphic chart depicting 10 measurement plots, arranged by altitude. Each plot is identified by its elevation (m), geomorphic environment, slope inclination, and date of temperature assessment. Sampling microsites for all plots follow graphic color key as in Figure 7: dark brown = bare soil; black = black tephra; red = reddish tephra; green = shaded ground near silverswords; light gray = dead silversword organic matter.
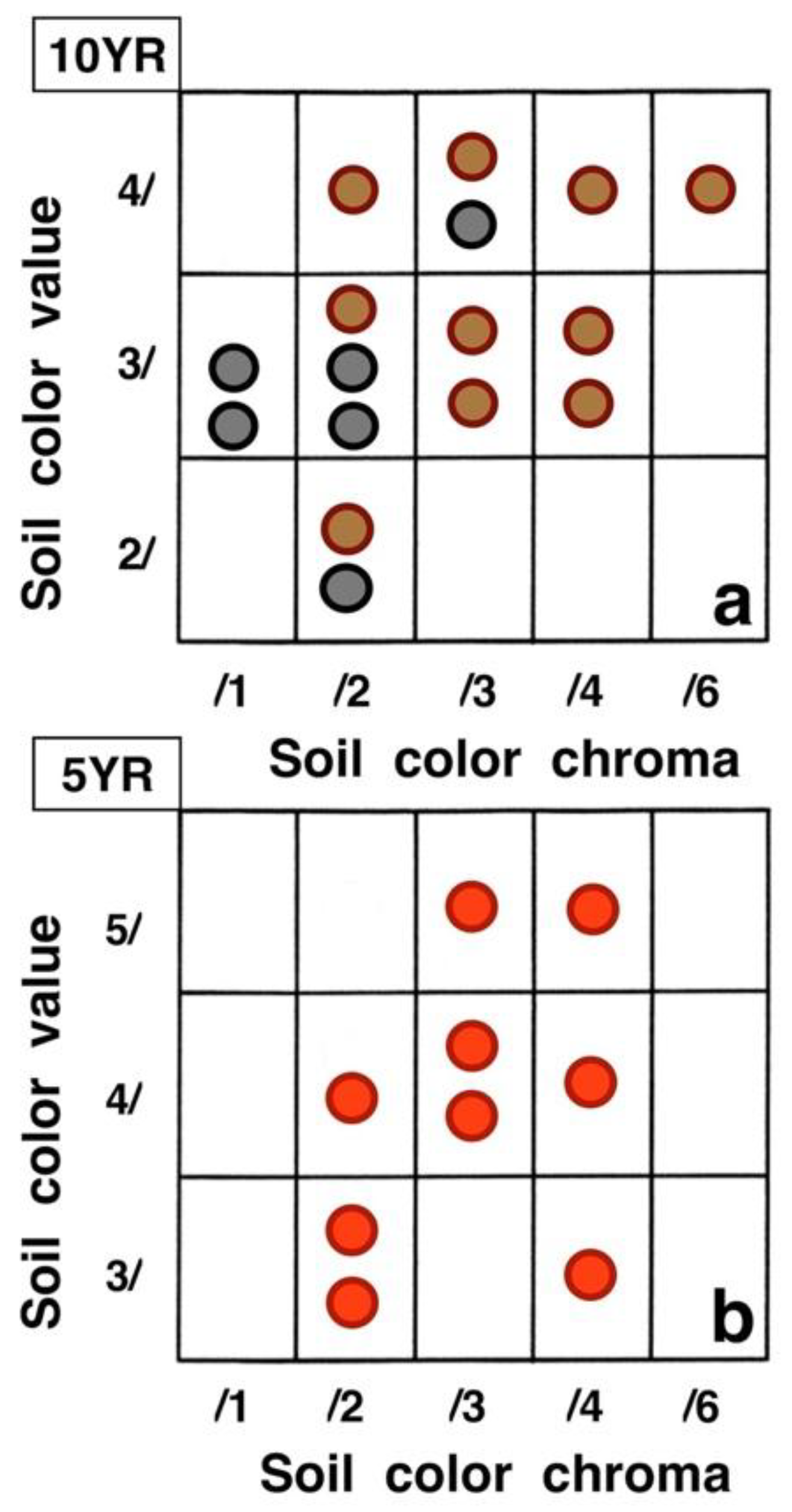
Colors of bare soil, tephra, and silversword organic matter were assessed in the field at each individual plot for multiple soil and tephra specimens with Munsell Soil Color Charts [51] and rock-color charts [62]; plant colors were determined with Munsell Soil Color Charts for Plant Tissues [63]; color mode—i.e., most common—is reported for each plot. All colors were determined on dry samples, as albedo significantly declines with increasing soil moisture content [47,53,54]. Thermocouple data were supplemented with relative humidity (RH) and air temperature readings, measured with digital, shielded, maximum/minimum electronic thermo-psychrometers, at all plots. Over the years, ~3400 photographs from tephra covers and environments, and from silversword habitats, were taken with various cameras, including two 35 mm Minolta™ (Chiyoda, Japan) SLR outfitted with 50 mm and 28 mm wide-angle lenses; color slides were cleaned and scanned at ≥600 dpi resolution on a flatbed Cannon™ (Ōta, Japan) scanner, then saved as JPEG files. Later, different Olympus™ (Shinjuku City, Japan) and Sony™ (Tokyo, Japan) electronic cameras, all with ≥10-megapixel capability, were also used. The codes included at the end of figure captions indicate the location, year, and photograph number; e.g., Figure 3: Hk-05.413, means Haleakalā—year 2005, photograph #413, followed by the actual date the photo was taken, 4 August 2005.
‘Exploratory data analysis isolates patterns and features of the data and reveals these forcefully to the analyst’. Hoaglin, Mosteller, and Tukey, 1983 [64], p. 1.
2.2. Analytical Methods
Soil temperatures for all sampled microsites were thoroughly evaluated, graphically and statistically, with Exploratory Data Analysis (EDA) [64,65,66,67,68]. EDA consists of a variety of visual and quantitative methods but, undoubtedly, the most valuable and often utilized tool in EDA are parallel box plots [67,68]. This powerful analytical technique was originally developed by John Wilder Tukey in 1970 [69]; among other concerns, Tukey considered histograms ‘old-fashioned’ and thought they were unable to adequately portray complex statistical data sets; thus, he devised box plots as a means of showing on a single summary graph several continuous data sets. Since then, box plots have proven extremely useful in the evaluation of voluminous data and for allowing comparisons across samples to help in the development of hypotheses [70]. Box plots can plainly portray the overall data range, the numerical extremes, and a measure of central tendency—usually the median—of a data population, thus providing immediate visual assessment of data dispersion [71]; they are particularly useful for indicating general sample symmetry of data distribution, showing whether a statistical distribution is skewed, and if there are unusual observations or presence of potential outliers within it. Box plots are non-parametric and—in contrast to histograms, which require a sample size of at least 30 to be useful—need only a minimum sample size of 5 and can also contain an unequal number of observations; box plots also provide more detail in the distribution tails and are easily compared across 3 or more sample data sets [72,73]. In fact, box plots may facilitate the identification of underlying patterns, relationships, and trends between data samples that might be concealed within unequal-size populations of numbers [74,75].
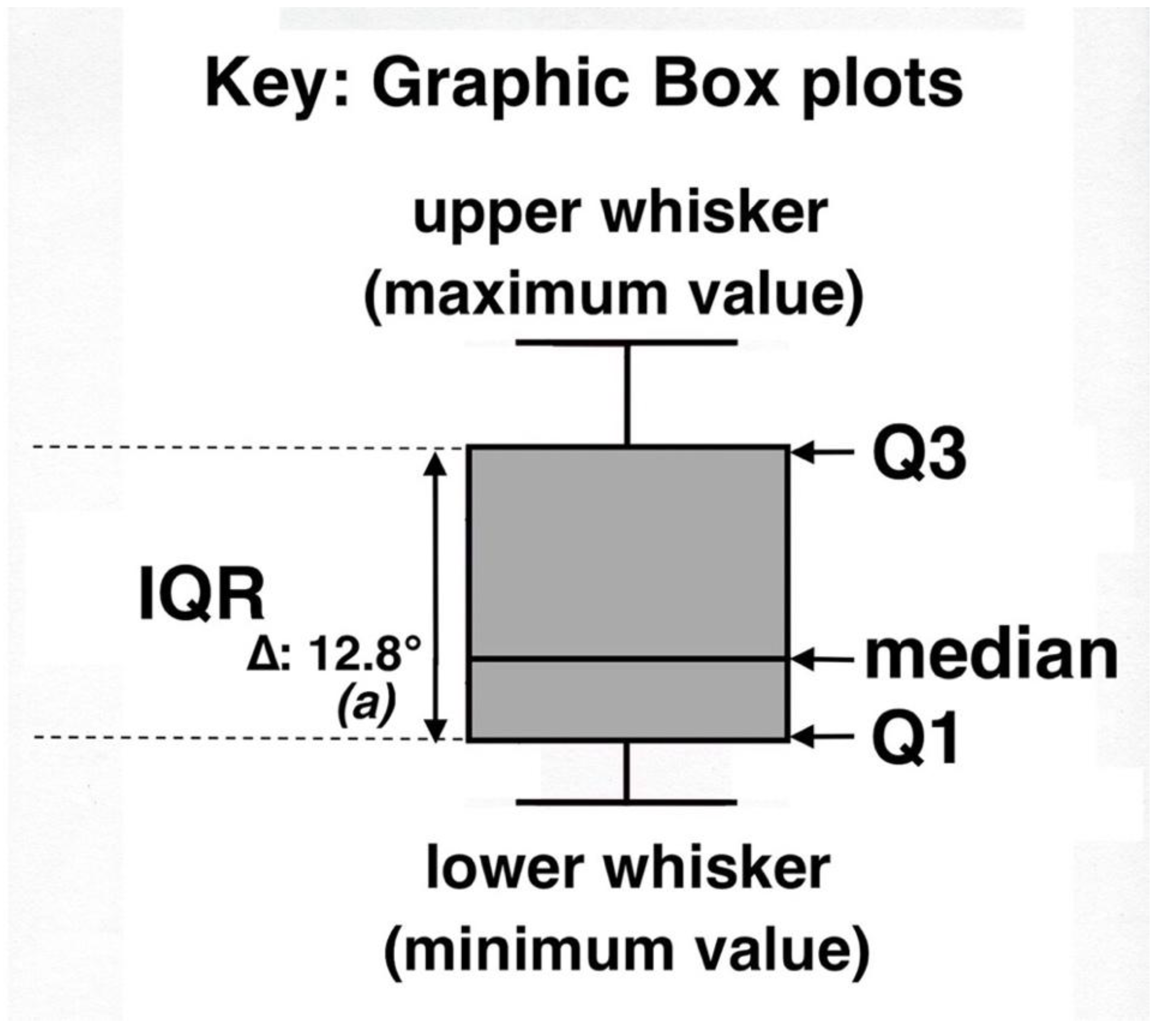
Several recent scientific studies have shown the usefulness of parallel box plots in such disparate disciplines as geography [76], geochemistry [77], geoecology [14,78], marine ecology [79], human genetics [71,80], and medical sciences [74,75]. Useful tutorials and computer programs for generating box plots are provided in several publications [66,71,81]. Specific characteristics of box plots used in this study are shown in Figure 9. The lower whisker shows the minimum temperature value, also known as Q0 = 0th percentile; Q1 = 25th percentile, also known as lower quartile; Q2 = the median, also known as 50th percentile, or middle value of a data set; Q3 = 75th percentile, also known as upper quartile; the upper whisker shows the maximum temperature value, also known as Q4. IQR (or F-spread) stands for the central, interquartile range of values (i.e., distance between Q1 and Q3).
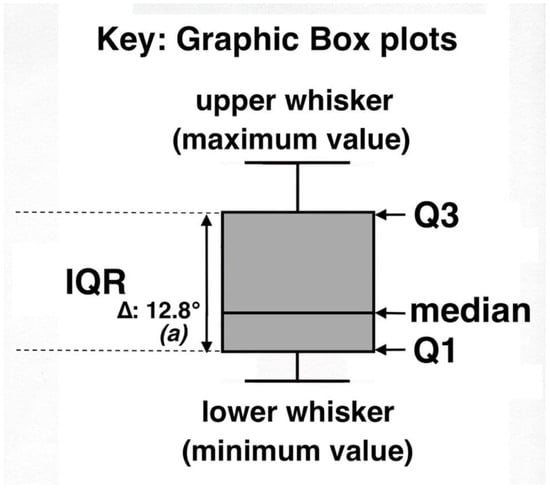
Figure 9.
Explanation key for statistical box plots displaying ground temperatures on 10 measurement plots; Tukey, 1977 [65]. In this graph, (a) indicates the statistical difference (p < 0.0001) between this box plot and the paired data set for bare soils. See text for further details.
Data in all box plots were carefully scrutinized for presence of outliers and/or far out values. Outliers are defined as observations seemingly inconsistent with the rest of a data set, whereas any unusual values highly discordant with the others in that data set are considered as far out values. These two kinds of values would normally lie outside the box plot; they were determined using cutoff points calculated with k = IQR; outliers had a k = ≥1.5 × IQR, and far out values a k = ≥3 × IQR [66,68,82] (Figure 9); presence of outliers is shown in the box plot diagrams with an asterisk (*). Delta symbols (∆) and temperature (°) values show the difference in median temperature between a given data set—in the example above, represented by a black tephra set—and the adjacent, paired data set for bare soil temperatures on the same measurement plot. For clarity, box plots do not indicate the arithmetic mean. As shown here, values greater or lower than the median may display significant graphic asymmetry, indicating positive—as in Figure 9—or negative skewness. It has been suggested [64,72] that lack of substantial visual overlap among individual parallel box plots from the same measurement plot might indicate statistically significant differences between medians. In any case, in this study, all temperature data sets included in the 10 individual plots were compared with non-parametric two-sample Kolmogorov–Smirnov (K-S) and Mann–Whitney–Wilcoxon (U) rank sum (MWW) tests [83]. These tests specifically compare all data sets for each one of the microsites—black tephra, reddish tephra, shaded ground under rosettes, and/or silversword organic matter—with the paired data set for bare soils; whenever a discrepancy between the two tests was detected, the most conservative results are given. In the box plot graphs, italic letters in parenthesis, (a), designate the statistical difference—if any—between these two data sets; levels of significance are as follows: (a): p < 0.0001; (b): p < 0.001; (c): p < 0.05; (n.d.): no significant difference. Data sets were also analyzed on some sampling plots with statistical regressions to ascertain the possible influence of (a) depth of black tephra or (b) silversword leaf crown diameter on sampled soil temperatures beneath them.
3. Study Area: Haleakalā Crater
Maui is located at 20°50′ N, 156°20′ W; East Maui, the tallest and most recent part of this island, reaches a 3055 m altitude at Haleakalā (Figure 10) The mountain summit is occupied by Haleakalā Crater, a vast erosional depression >900 m deep, ~12 × 4 km, and a ~42 km2 area. This misnamed ‘crater’ developed when two streams gradually incised and expanded their upper basins on opposite sides of Maui, and their valleys merged through the southern Kau-pō and the northern Ko’olau Gaps, creating a large summit depression [84,85]. The crater began developing ~0.23–0.15 Ma, and, by ~0.12 Ma, was well established [86].
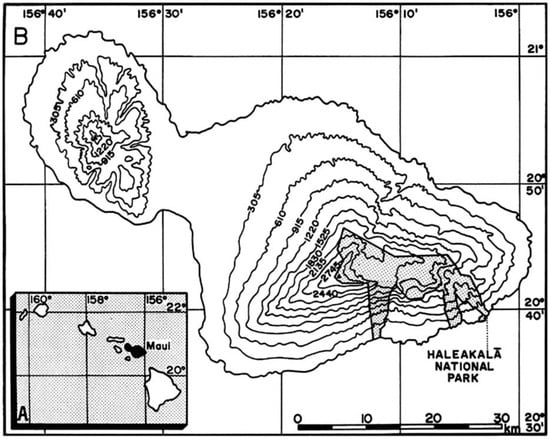
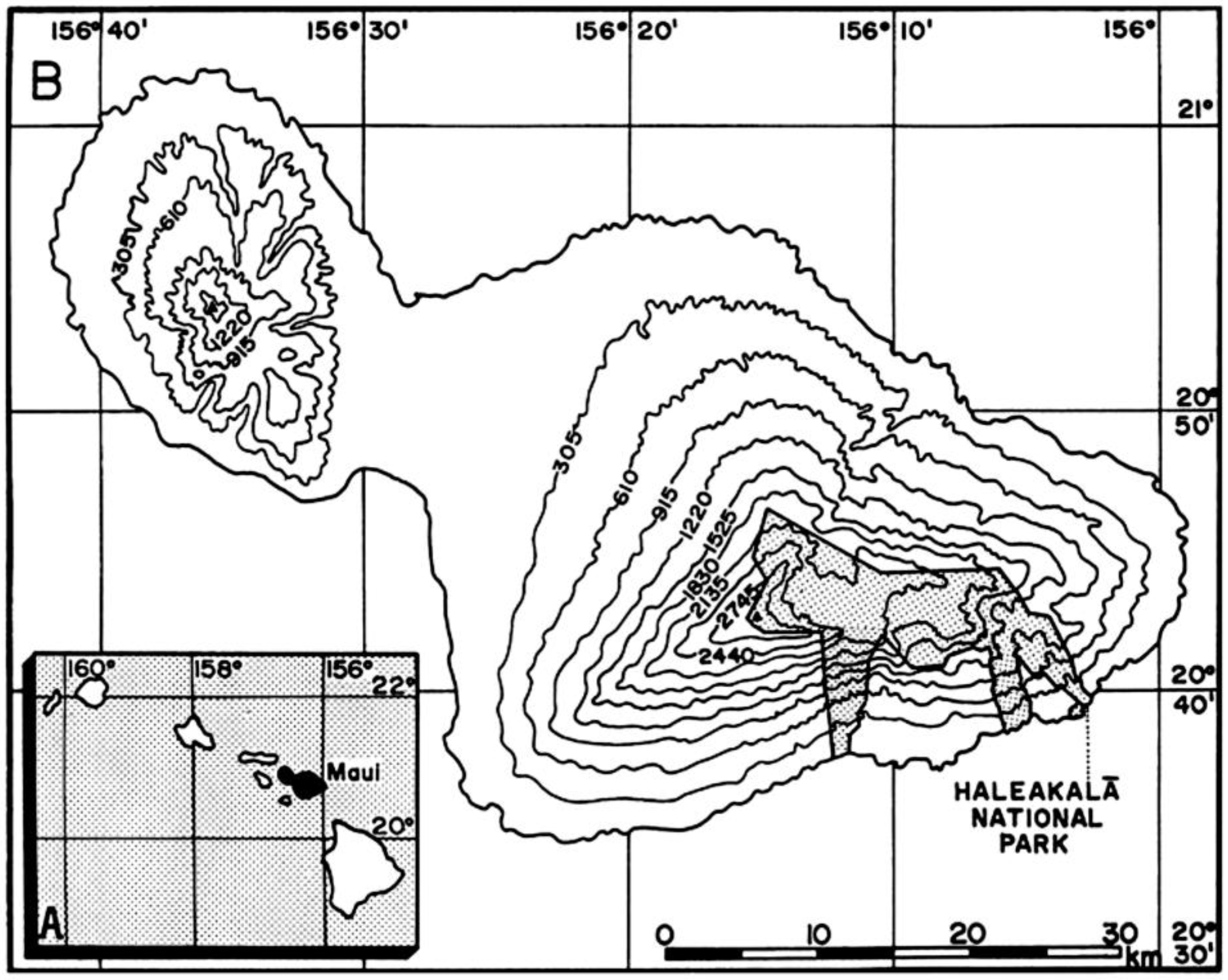
Figure 10.
Location of study area. (A) (inset). Hawaiian Island archipelago; the island of Maui is shown in black. (B). Maui; approximate altitudes are shown in meters (~305 m = 1000 feet); latitude (N) and longitude (W) are indicated. Stippled section indicates location of Haleakalā National Park in East Maui; map includes recent additions to the park. Scale in km. Base map: U.S.G.S., State of Hawai’i, Principal Islands, 1971; scale 1:500,000.
The crater floor, at ~2150–2510 m, is occupied by several spectacular cinder cones lined up along rift zones and lava flows formed after 11,000 BP during the Hāna period (Holocene) [59]. Two large cinder cones on its western section, Ka ma’o li’i and Pu’u o Pele, are the oldest in the crater, probably older than ~5000 BP [59,87,88] (Figure 5 and Figure 6). Pu’u o Maūi, the tallest cone, erupted later, between 1500 and 3000 BP—probably about 2700 BP [89,90]. Pu’u o Maūi’s eruptions also produced large reddish basalt ‘a’ā flows, which traveled north >3 km toward the Ko’olau Gap and the Silversword Loop [90,91] (Figure 5 and Figure 6). The Hawaiian Islands are affected by persistent NE trade winds and by orographic precipitation effects, which create spatially variable rain patterns; due to isolation from marine moisture sources and by a permanent subsidence inversion layer that forms at 1200–2400 m about 70% of the time, Maui’s uplands remain arid and experience a strong summer drought and insolation, with steadily clear, cloud-free skies [92,93]. High-altitude air is exceptionally dry, and relative humidity (RH) is normally <40% but frequently drops down to 5–10% [94,95]. Rainfall totals steadily increase from ~1037 mm/yr along the western crater to ~3404 mm/yr near its eastern edge [96]. As other tropical mountains, Haleakalā experiences a narrow yearly temperature range of just ~3.8 °C [94] but shows wide daily fluctuations of up to ~20–24 °C [32,97]. Air maxima at the study sites seldom exceed 33–34 °C, but surface soil temperatures at noon often reach 50 °C on dark, exposed soils [56]; I recorded summer maxima up to 51–56 °C [91], and readings over 60 °C are frequently reached on dark cinders [98]. Soils in the crater are coarsely textured, porous, sandy (~90 to 98% sand, ~2 to 10% silt) volcanic ash, with a trivial amount of clay, a high gravel content (≤92%) and very low (≤2%) organic matter component, largely provided by plant growth; as a result, soils show a scanty water-holding capacity and become quickly desiccated [88,91,99,100]. Numerous soil profiles examined in the crater [78,88,91,100,101,102] show a diversity of Inceptisols and Andisols with andic properties, developed on heterogeneous ash and tephra deposits and with a complex layer stratification, which shows the influence of episodic volcanic events and various geomorphic processes [103].
Owing to increasing aridity at high altitudes, vegetation in Haleakalā gradually forms a sparse alpine desert [94,99]; the crater extends completely within this desertic biome, associated with ~35 vascular plant species [88]. Common plants include several native grasses, such as piliuka, Trisetum glomeratum (Kunth) Trin. ex Steud., Hawaiian hairgrass, Deschampsia nubigena Hillebr., and Agrostis sandwicensis Hillebr. Dominant shrubs include Dubautia menziesii, Leptecophylla tameiameiae, catchfly, Silene struthioloides A. Gray, and Tetramolopium humile subsp. haleakalae Lowrey [31]; however, the iconic Haleakalā silversword remains the most remarkable plant in the crater (Figure 3).
4. Results
To minimize unnecessary subsequent repetition or the excessive fragmentation of information, some specific research results presented in this section will be concisely contrasted with the most salient findings of previous silversword population surveys or measurements, especially those by Kobayashi (1969–1972) and Powers (1938) [1]; these text portions are clearly identified with the italicized names of past researchers.
4.1. Diversity and Characteristics of Black Tephra Covers and Environments in Haleakalā
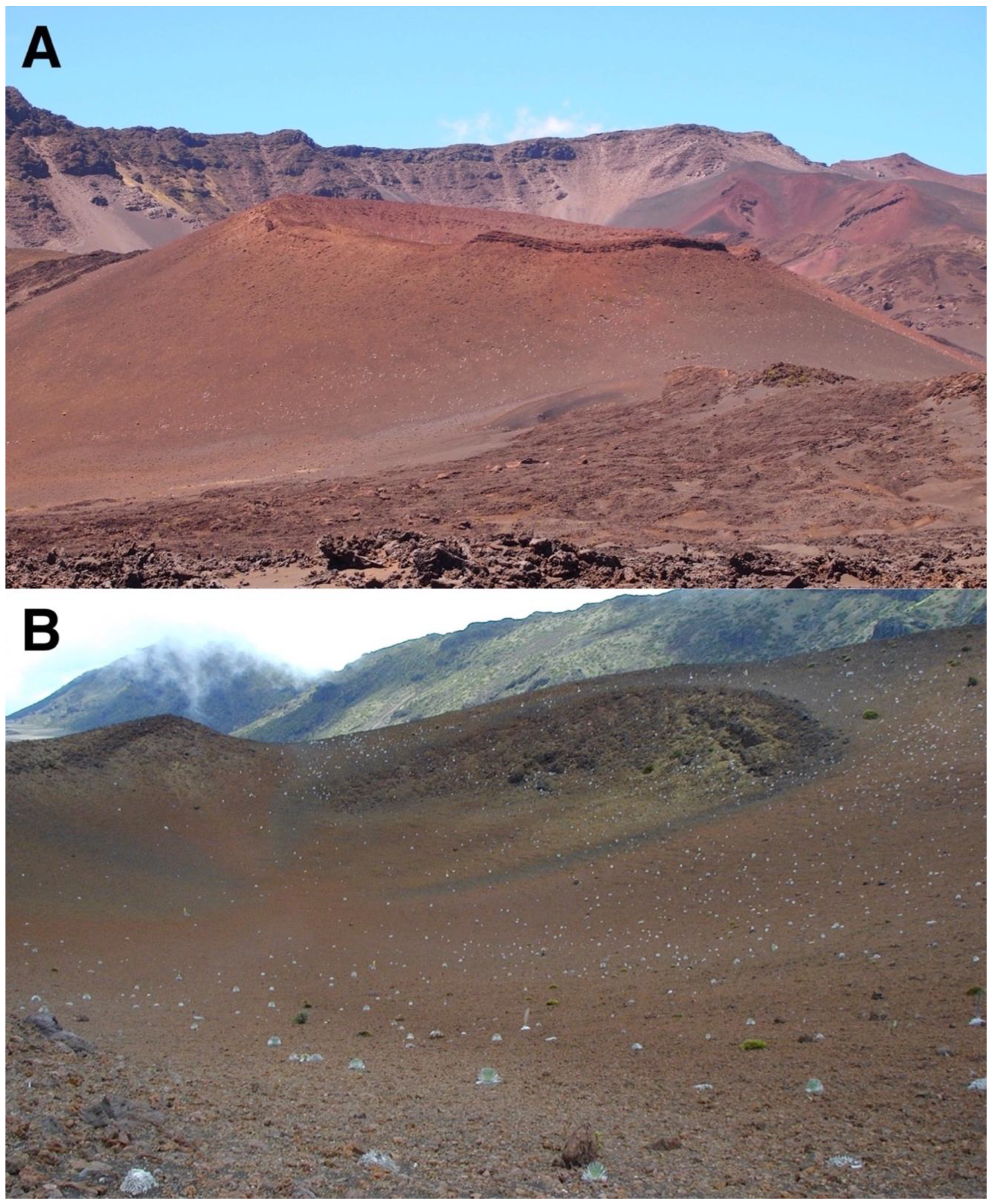
The uppermost site studied in the crater at 2755 m, plot 1, also harbors its highest natural silversword population (Table 1), but several isolated rosettes can be found nearby at greater elevations. This location is occupied by a steep, ~25–32°, talus slope that has developed along the upper section of the inner crater slope, right below its southern rim (Figure 11A). An extensive portion of this slope is mantled by large- and medium-size, predominantly black to dark-gray, mostly clastic angular fragments, derived from the weathering of upslope basaltic ‘a’ā lava outcrops, with some deposited cinder and spatter mixed with them. Some of these angular talus rocks were presumably dislodged from prominent lava outcrops a long time ago by—among other processes—gelifraction during colder stages in the Hāna period through the Holocene, or even during the Pleistocene (Kula period) [59], map. The clastic slope deposits show several surficial, gently undulating, steps parallel to contours, indicating this flank was, in the distant past, affected by widespread slope creep; very few, small, lava outcrops still protrude above the clastic mantle, which covers the underlying bedrock, and might possibly be rather thick. The silversword population supported by this talus area is relatively small, and extends as a narrow band parallel to slope contours, along with several large Dubautia menziesii shrubs, suggesting the presence of some substrate condition beneficial to plant growth (Figure 12). Kobayashi [1], p. 37 actually counted in 1969 a total of 180 rosettes at this site; that number seemed basically unchanged when this plot was last inspected in 2014. Additional geological and petrological data for this and other sample plots may be found in [44,45,59,85,102].

Table 1.
Site characteristics of black and reddish tephra covers, sampled rosettes, and organic silversword litter on 10 measurement plots. Plots are arranged by altitude; colors refer to Munsell Soil Color Charts [51].
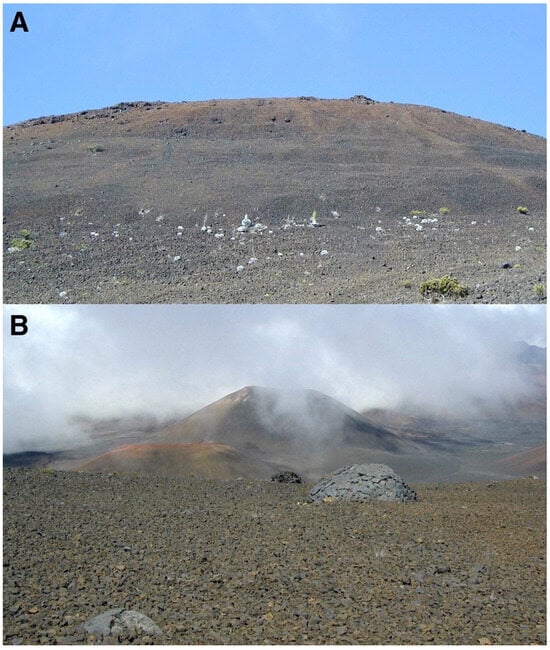
Figure 11.
(A) View of site 1, the highest surveyed plot in the crater, ~2755 m; this site shows good localized growth on black tephra. The crater rim, at ~2925 m, appears in the background. Photo: Hk-01.30, 25 July 2001. (B) Extensive black tephra area, downslope from site 1, with no regeneration of silverswords; ~2720 m. Note a few sizable breadcrust bombs scattered across this nearly level slope; the largest bomb seen is ~210 cm tall, ~370 cm wide. Two large cinder cones appear in the background, Ka ma’oli’i on the left, and the larger Pu’u o Māui in the center, far back. Photo: Hk-01.156, 1 August 2001.

Figure 12.
Silversword rosette with developing inflorescence on a steep, ~28° talus slope, completely covered by black and dark-brown angular clastic fragments; site 1, 2770 m. Rosette crown is ~48 cm wide; plant, including inflorescence, is ~80 cm tall. Photo: Hk-01.133, 29 July 2001.
A sizable, gently sloping, area directly downslope from plot 1 is associated with a continuous mantle of mostly black to gray cinder, intermingled with spatter and ash; some rocks here developed in situ from the weathering of alkali olivine basalt lava flows, but many of the angular, medium- to small-size clasts on this section were also derived from the large taluses above through downhill gravitational migration and water transport of fine soil material (Figure 11B); a wide, dense band of native grasses (Deschampsia nubigena and Trisetum glomeratum) across the basal talus clearly marks the point where subsoil water comes near the slope surface above this black tephra accumulation area. A few large, scattered breadcrust bombs, showing surface shrinkage cracks on their surface [44,45], are also present here. A surficial (≤10 cm thick) layer of scoriaceous tephra overlies deep (≥95-cm) coarse, gravelly sandy Vitrandic Haplustept soils with a low organic matter content [104]. Yet, despite the pervasive presence of thick soils, not a single adult rosette or seedling can be found in this extensive, ~8–10-Ha expanse (Figure 11B).
There are several other locations with widespread black tephra deposits on the upper crater. The impressive Pu’u o Māui cone is covered by ash, cinder, and coarse black clasts, produced during the eruptions that built the cone ~1500–3000 BP [89,90]. Owing to its enormous size, Pu’u o Māui has a large surface area, perhaps ~60–70 ha [59], map], so it is not surprising that Kobayashi [1], p. 37, 83 reported an actual count of 21,227 silverswords (1947 adults, plus 19,280 seedlings ≤20 cm diameter) in his 1969 rosette census of this cone. However, this massive rosette population exhibits a large spatial variation across this gigantic cone; Kobayashi’s [1] detailed survey maps reveal that the rosette population on the south flank (Figure 13A) contained only 4.7% of all the silverswords, with the remaining 95.3% spread across several populations on the NW and NE cone flanks. Loope and Crivellone (1986) also commented on the fact that silverswords are extremely abundant, and very closely spaced, on this north flank [105]. This considerable disparity can be explained by the fact that slopes facing to the north receive much precipitation from the moist trade winds arriving through the Ko’olau Gap; even more important, these flanks also intercept the incoming fog, which may account for an additional ~103–114% of water [1]. As fog and drizzle hits the NE-facing sides of blocks and outcrops, condensation on cool stone surfaces produces fog, which moistens soil microsites next to stones [97,99,106,107] (see Section 5.5 below).
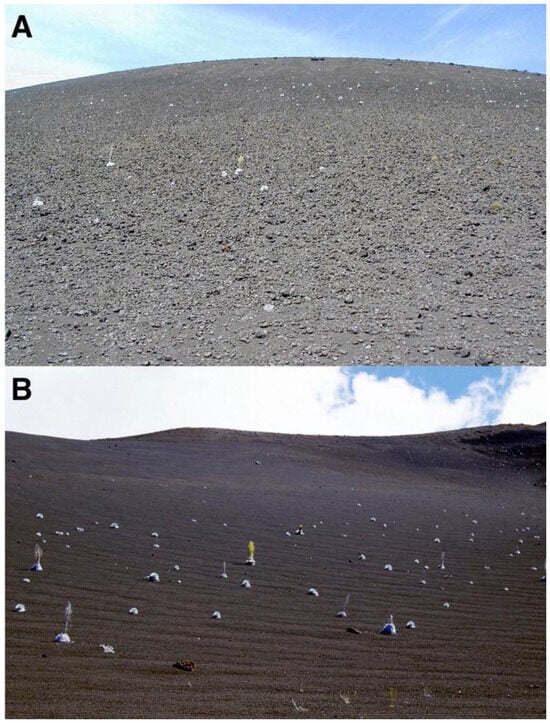
Figure 13.
(A). South-facing slope of Pu’u o Māui cinder cone, covered by ash and black tephra, likely emitted during Pu’u o Māui’s eruptions. Summit is located at 2479 m; the base of the slope is located at ~2340 m. Note the extremely sparse density of silverswords, concentrated on the upper and middle slope. Photo: Hk-02.467, 3 August 2002. (B). Northeast-facing slope of Ka ma’o li’i cinder cone; a layer of medium-sized black tephra completely covers its gentle (12–17°) basal area. The summit is located at 2489 m; cone base is located at ~2340 m. Note the significant concentration of silverswords along the footslope. Photo: Hk-02.324d, 28 July 2002.
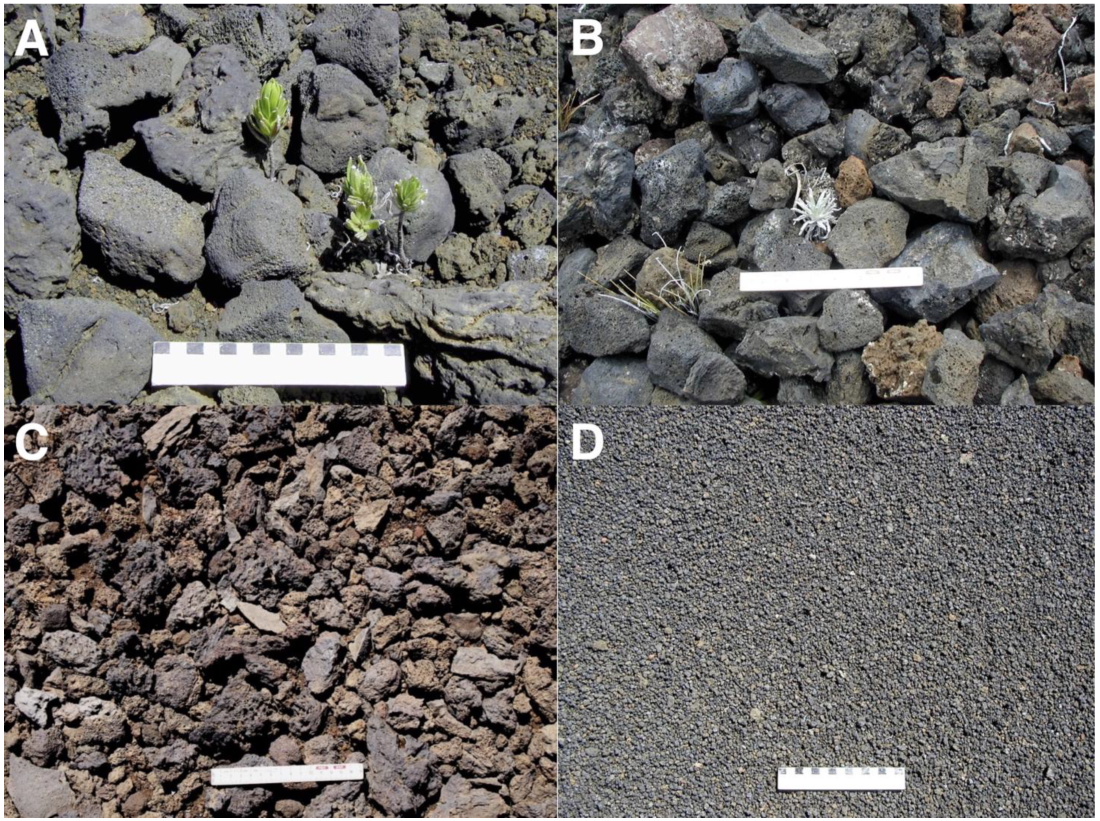
Ka ma’o li’i, another sizable cinder cone next to Pu’u o Māui, presents a continuous cover of black tephra fragments; its basal NE-facing slope supports a modest but dense rosette population—estimated at 95 plants in 1969 by Kobayashi—even if this cone partially lies in the rain shadow of Pu’u o Māui. It should be noted that silversword populations on this cone have experienced an astounding recovery in the past, as in 1938, Powers reported in his HNP census ‘none’ for the number of rosettes on this whole volcanic landform [1], p. 83. Interestingly, the cone footslope shows several prominent elongated rock steps and ridges parallel to contours; these indicate that the lower flank was subjected to substantial slope creep processes during the past. As this cone is one of the oldest in the crater, these presumed geomorphic events may have occurred prior to 5000 BP. Black tephra deposits present a variety of sizes and characteristics across the crater. Well-rounded, weathered, coarse blocks abound on gentle, ~15–20° slopes below the area with volcanic bombs described above. This section, characterized by either a thin layer of one or two blocks, or just isolated stones resting on a gravelly pebble surface, provides a favorable substrate for the regeneration of a large population of Dubautia menziesii shrubs [104,108] (Figure 14A). The higher-elevation, blocky talus areas with angular breccia, probably formed by gelifraction, above plot 1 (Figure 11A) also offer an advantageous substrate where isolated, sparse rosette seedlings appear to be expanding this population, which may continue slowly advancing upslope given the current regional climatic changes [40,41].

Figure 14.
(A). Large, ~8–15 cm long black tephra blocks, with a Dubautia menziesii seedling emerging from beneath, 2660 m, north-facing flank of crater, ~16° slope. Photo: Hk-05.500, 20 July 2005. (B). Black talus blocks, ~6–10 cm long, upslope from site 1, 2795 m, ~28° slope, with an emergent ~7 cm tall silversword seedling, Photo: Hk-01.137, 29 July 2001. (C). Black tephra cover, Silverword Loop, site 9, 2180 m. Photo: Hk-14.501, 14 September 2014. (D). Fine black cinder and lapilli cover, presumably produced by Pu’u o Māui’s eruptions, on a gentle, ~5° slope, 2360 m. Photo: Hk-02.407, 3 August 2002. Ruler scale on all photos is 15 cm long.
(Figure 14B) Isolated small spots on the predominately reddish tephra flow from Pu’u o Māui display a uniform cover of black, clinkery, sharp, jagged-edge clasts with abundant small vesicles mixed with some reddish fragments derived from the lava flow (Figure 14C) (Table 1). South of Pu’u o Māui, an extensive, nearly level area, mantled by fine black cinder and lapilli, extends to the SW for more than 1.4 km; this plain is totally devoid of silverswords or any other vegetation, except for an unusual small patch of biological moss crusts near the cone’s base [78,88]; these very fine volcanic sediments were presumably deposited on this area during Pu’u o Māui’s eruptions 1500–3000 BP, as the NE trade winds blew fine pyroclastic materials in this direction (Figure 14D).
4.2. Diversity and Characteristics of Reddish Tephra Covers and Environments in Haleakalā
In contrast to black tephra sites, reddish tephra deposits across Haleakalā Crater often show high concentrations of silverswords, and judging by the numbers of seedlings, several locations appear to have become significantly more dense during the past few years. The most remarkable rosette site is probably plot 6, on the upper crater (Table 1), where transect and quadrat measurements by Pérez in 1998 [39] across an extensive area have previously determined a mean plant density of 283.6/100 m2, with an average distance between rosettes of 43.0 cm and a mean plant height of 6.9 cm; in other words, most plants here were closely-spaced seedlings (Figure 15). This plot seemed unchanged when last inspected in 2014. When Kobayashi examined this site in 1969, only 27 seedlings and 11 adult rosettes were present [1], p. 83; thus, silversword recovery during the following three decades, after goat extirpation, seems outstanding.

Figure 15.
Widespread, uniform reddish tephra cover, derived from weathered alkali olivine basalt lava flows, on a ~12–14° slope gradient, plot 6, 2510 m. This is the site with perhaps the best silversword regeneration on the upper crater; over 160 small- and medium-size rosettes cover the area in this view. Photo is ~4.5 m wide in the foreground. Photo: Hk-01.47, 25 July 2001.
Other reddish tephra crater landforms are associated with the presence of countless silverswords as well; Pu’u o Pele, a large cinder-and-spatter cone, is particularly impressive in this regard. Pu’u o Pele appears to be the only cone in Haleakalā crowned by a peculiar geological form, a circular ≤3 m tall, vertical stone rampart, called Pōhaku o Hanalei—wreath of stones—formed by welded spatter during late eruption stages, which becomes fused into a ruff-like mass of agglutinate spatter and driblet layers, sequentially accumulated around the crater rim [44,45,102] (Figure 16A). The surviving sections of this wreath are most prominent on the north and northeast cone sections, although remaining rocky segments along its east and southeast side indicate this rampart probably was formerly more extensive. Weathering of this rocky wall has produced numerous lava blocks and clastic fragments that accumulated inside this vast crater, as well as on the outer cone flanks, these rocks contributed to the development of a nearly continuous stone pavement where silverswords have proliferated (Figure 16B) (Table 1). In 1938, Powers reported just ‘a few good plants’ on the whole cone, [1], p. 37, whereas Kobayashi counted 3388 rosettes in 1969 [1], p. 8, yet even this number appears to have further increased during my reconnoitering visits in 2001, 2002, and 2014. The ubiquitous pavement of red clastic sediments inside Pu’u o Pele’s crater has also been subjected to long-term slope creep processes—probably solifluction—in the far past, as evidenced by several, ~5 to 9 cm high, parallel, sinuous, wavy ridges across the middle and lower sections of the crater (Figure 17C); as mentioned, this cinder-and-spatter cone is also one of the oldest in the crater, erupted ~5.000 BP or before.
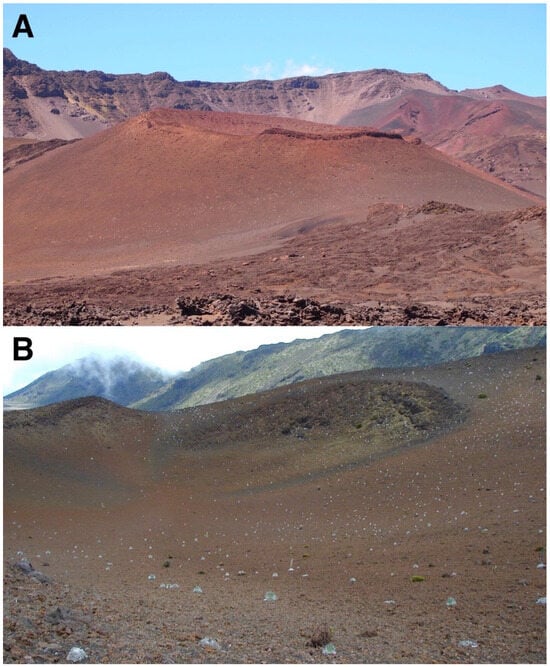
Figure 16.
(A). Pu’u o Pele’s east flank, showing substantial density of silverswords on its mid and lower slope; the right side of this basaltic cinder-and-spatter cone displays a prominent rock rampart along its rim at ~2450 m. The western rim of Haleakalā Crater appears in the background. Photo: Hk-14.540, 14 September 2014. (B). Pu’u o Pele’s broad crater, seen from its NW rim; this site supports one of the largest, more widespread rosette populations, within the crater. The south and west rim slopes of Haleakalā Crater appear in the background. Photo: Hk-01.90, 26 July 2001.
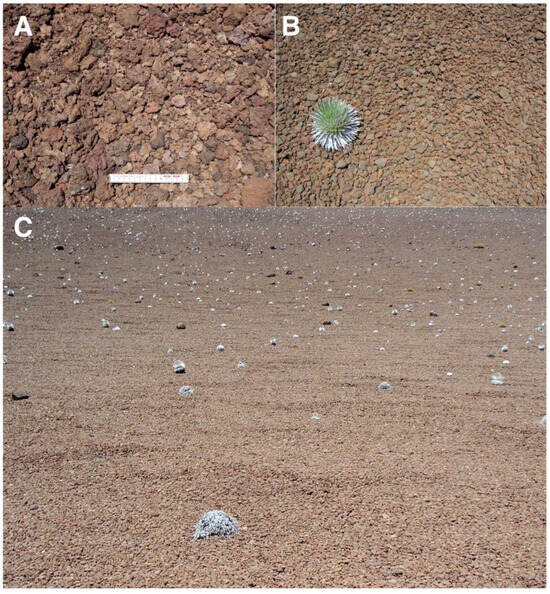
Figure 17.
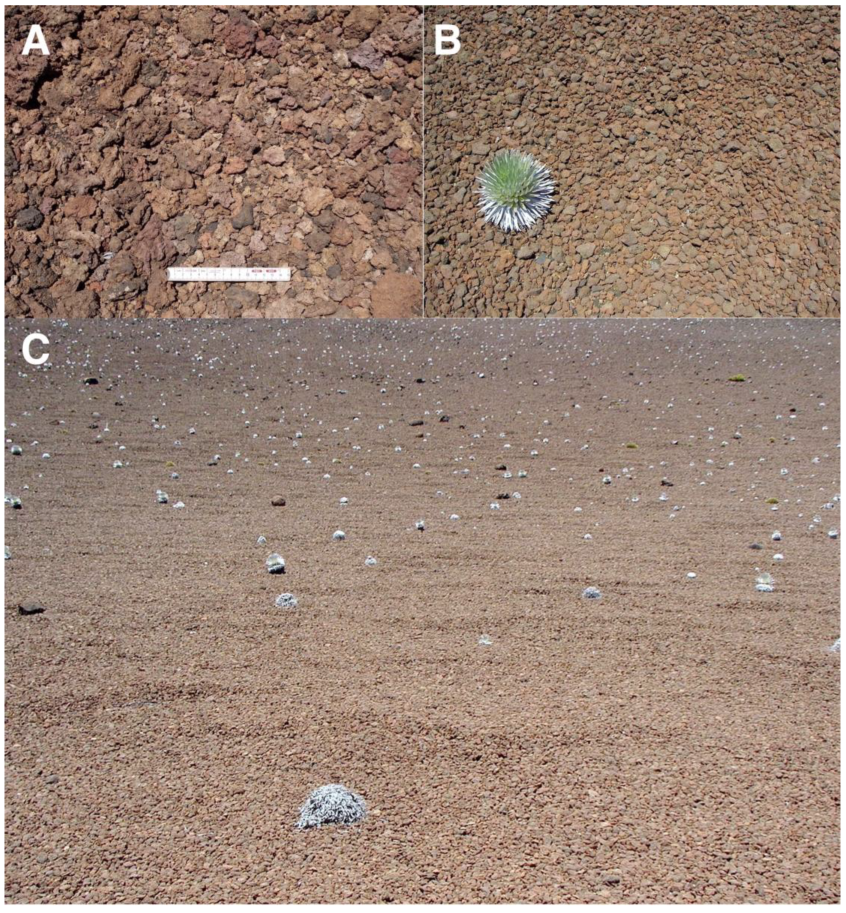
Representative views of reddish tephra covers. (A). Site covered by tephra in Silversword Loop, plot 9, 2175 m; the smaller fragments are 2.5- to 5 cm long, the larger ones are 5 to 7 cm. Scale is 15 cm long. Photo: Hk-14.318, 12 September 2014. (B). Reddish tephra of uniform, 6–8 cm long size and ellipsoidal shape, at crater floor of Pu’u o Pele, ~2–3° inclination, ~2400 m. The small rosette is ~30 cm wide; photo shows an area ~1.75-wide in the foreground. Photo: Hk-01.260, 7 August 2001. (C). Extensive reddish tephra pavement, covering the floor of Pu’u o Pele’s crater; ~2405 m, plot 8, ~7° gradient. These clastic sediments seem to have been affected by past slope creep movement. Photo covers an area ~6- to 7 m wide in the foreground. Photo: Hk-01.261, 7 August 2001.
Reddish tephra fragments show some interesting morphologies associated with different volcanic and geomorphic processes. An area totally covered by curiously homogeneous, 6–8 cm long, ellipsoidal clasts with many near-almond-shaped, subangular to subrounded clasts, was found at the bottom of Pu’u o Pele’s crater. The smoothed, weathered tephra rocks seemed to have gravitationally accumulated and become sorted as a continuous well-fitted unbroken mosaic across the cone floor (Figure 17B).
Across the lower crater, and all the way to the Silversword Loop, the vast red alkali olivine basalt ‘a’ā lava flows originating from Pu’u o Māui are largely covered by cinder and ash deposits of comparable composition to that of the cinder cone that released them; large portions of the cone were actually violently detached from it and rafted >2 km toward the Ko’olau Gap [59]. The intensely red deposits display multiple flow ridges, chiefly occupied by extensive basalt outcrops, with shallow intervenient troughs covered by ash and small tephra fragments (Figure 18). Rosettes on this lava flow grow primarily on rocky protrusions, coarse tephra, finer-debris areas next to nurse rocks, or thin soils over shallow bedrock [91].

Figure 18.
Panoramic view of Pu’u o Māui’s lava flow near the Ko’olau Gap, looking to the NE; plot 10, ~2160 m. Most of this area is associated with vividly colored reddish lava and tephra; note numerous ridges on the flow surface, occupied by extensive basalt outcrops, whereas intercalated troughs in between are covered by ash and smaller tephra. Photo: Hk-14.377, 12 September 2014.
Pu’u o Māui’s lava flows now support innumerable silverswords distributed on 11 subpopulations, which Kobayashi estimated at 1957 individuals in 1969 [1], p. 85. These flows cover >1 km2 of rugged, rolling terrain; it would be impossible to realistically quantify these rosette groups at present, unless a detailed census were conducted anew; but the general impression is that plant recovery on these reddish lava surfaces has been as significant as on the other red tephra landforms discussed above. Much of the surfaces examined on the flow were associated with small- to medium-size reddish fragments covered by irregular, spinose clinker particles with jagged edges (Figure 17A), an excellent substrate for silversword growth.
4.3. Additional Nurse Microsites: Ground Shading by Silverswords and Rosette Organic Matter
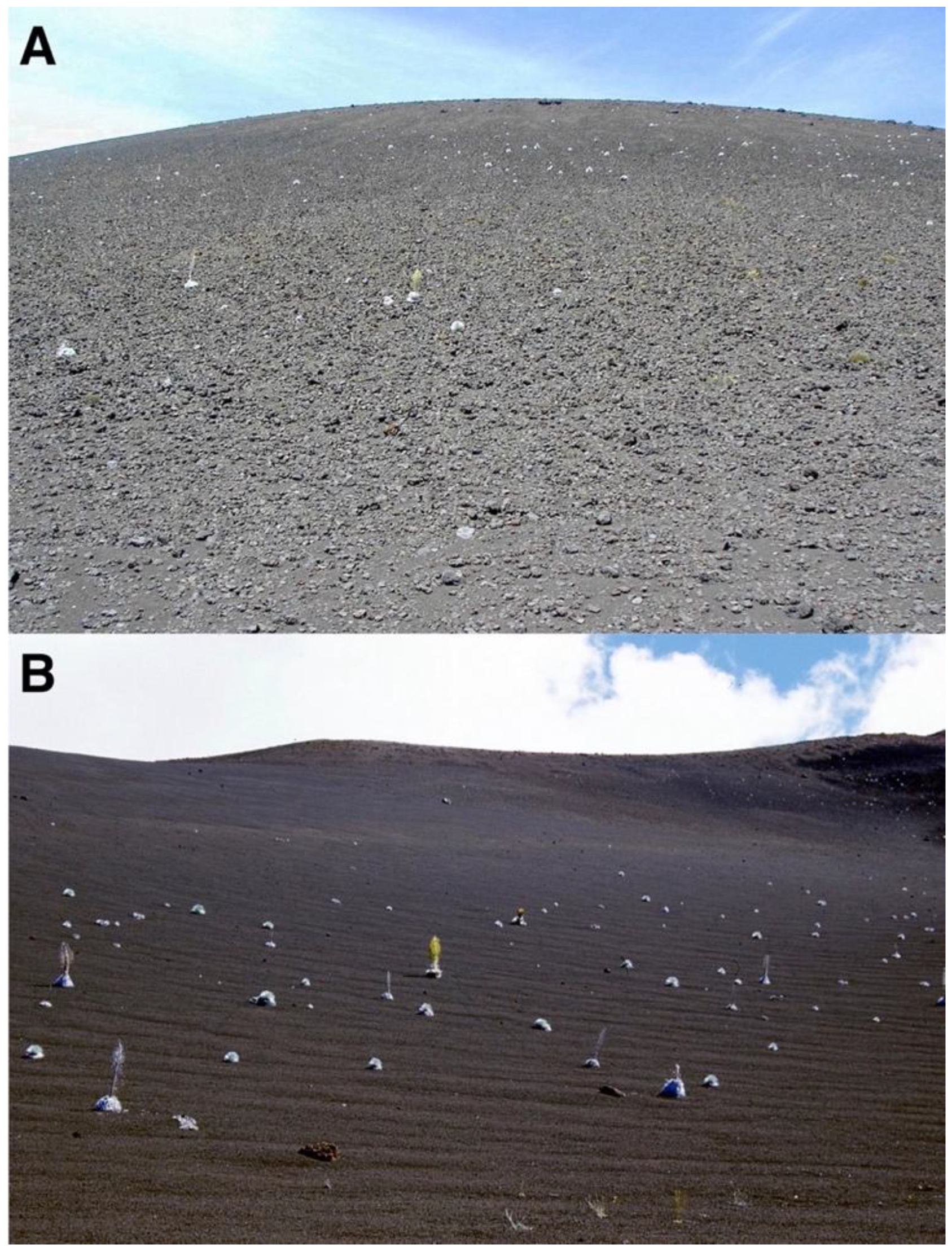
During early fieldwork, it was noticed that ground surface areas shaded by plants—including silverswords—were noticeably cooler than soils exposed to direct sunlight [8,15], so it was hypothesized that rosettes might possibly act as their own nurse plants in a thermal role, thus contributing to plant aggregation next to adult silverswords (Figure 3) [4,17]. In the bright sunshine that often characterizes Haleakalā, shadows cast by adult rosettes provide a stark contrast to surrounding unshaded ground (Figure 19) (Table 1). Not just tall rosettes, but smaller saplings also cast ample shaded areas that might be beneficial for seedling growth, especially on steep, north-facing slopes, where shadows are longer. Clustered silversword groups, which may conceivably be produced by seedlings sprouting near established adult rosettes, were encountered on several crater locations (Figure 20A). In most instances, the largest plant of the cluster was located on its upslope side, suggesting other, secondary, seedlings could have developed on the downslope edge. In some cases, remnants of the trunk of the original, oldest rosette were still present, unequivocally indicating other plants had sprouted around the periphery of the initial, larger one (Figure 20B). Alternative interpretations are considered in the Discussion Section.

Figure 19.
(A). A ~62 cm tall silversword, growing on a ~9° slope gradient, plot 2, facing N28°NE, at ~2730 m. The rosette projects a dense shade in the downslope direction; sun is on the right side, south of the plant. Photo: Hk-01.14, 24 July 2001, ~11:30 a.m. HST. (B). A ~41 cm tall silversword growing on the outer, north-facing, flank of Pu’u o Pele cinder cone; slope gradient is ~26°. Photo: Hk-01.168, 1 August 2001, ~1:45 p.m. HST.
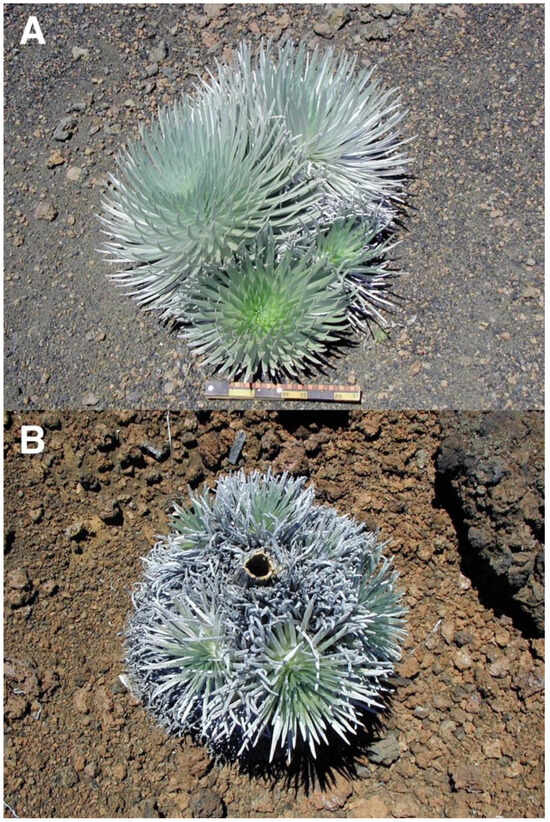
Figure 20.
(A). Cluster of four silverswords on the upper crater, near plots 6 and 7, on a 12° slope devoid of large tephra, 2490 m. The largest rosette in the back is ~48 cm high; the others (counterclockwise) are ~43, 32, and 23 cm tall. Downhill is toward the bottom; the ruler scale is ~29 cm long. Photo: Hk-05.438, 11 August 2005. (B). A ~68 cm wide cluster of 6–7 rosettes growing around the base of the stem of a larger—now dead—silversword; the hollow ~7 cm diameter stem of the dead plant is clearly visible at the cluster center. On a 7° lava ridge near plot 9, Silversword Loop, ~2185 m; downslope is toward the bottom. Photo: Hk-02.334, 1 August 2002.
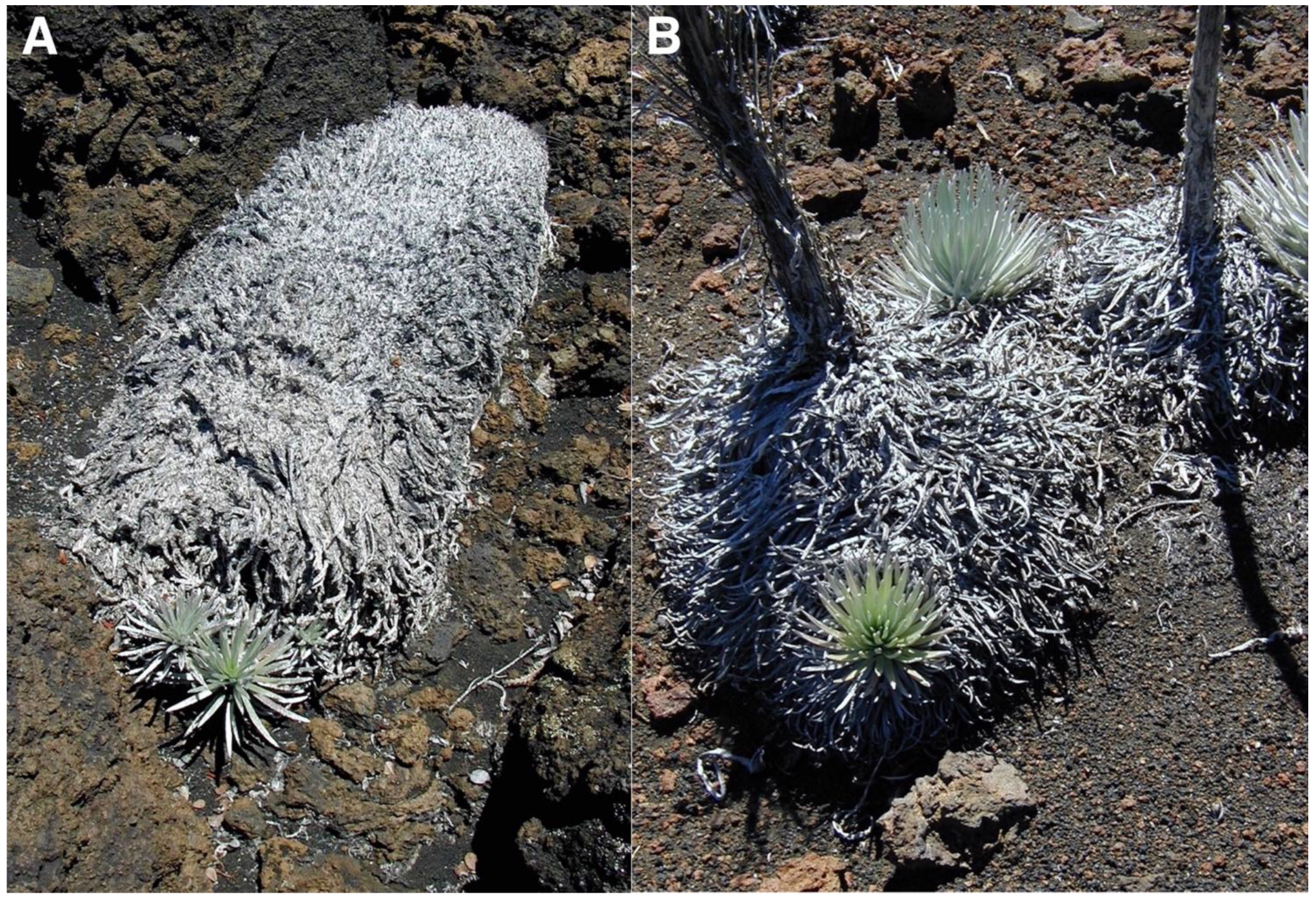
Piles of decomposing organic matter, produced by dead silverswords, offer another type of microsite advantageous for rosette development; some of these litter accumulations may be sizable, ranging from a whole fallen plant stem to miniature heaps (Figure 21). These remnants of plant litter, largely composed of white gray (8/1, Munsell color [51,63]), wilted, shriveled leaves, were common on several sites, including plots 2, 4, and 9. As a rule, colonizing plants became established around the edges of organic matter piles, where seeds transported by wind or water might become easily lodged; because some litter accumulations may be ~20 cm thick, seedlings were also more frequently found on thinner heap portions, where developing roots can reach the underlying soil more readily (Table 1). Occasionally, invading silversword seedlings might grow on the central litter area, formerly occupied by the now dead rosette stem.
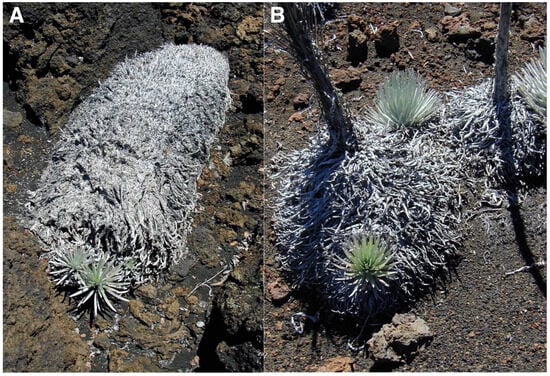
Figure 21.
(A). Three small silversword seedlings (~9, 12, and 5 cm tall) growing from underneath the edge of a decomposing, fallen rosette stem. Silversword Loop, plot 10, ~2170 m. Photo: Hk-02.339, 1 August 2002. (B). Two small silverswords (~18-, and ~23 cm tall) emerging from the periphery of the organic litter of two dead rosettes; their inner woody stems are still standing, and another small rosette grows on organic matter to the right side of the photo. Plot 7, 2505 m. Photo: Hk-01.198, 2 August 2001.
The significance of these litter piles as potential sheltered microsites is substantially increased by the fact that, even though they might not be considered permanent landscape elements, they are not ephemeral, either. Initial assessement suggested that organic matter heaps might last as much as 4–6 years before finally decomposing and disappearing [100], but detailed observations over three decades indicate they may remain available for seedling colonization for much longer, perhaps 15–20 years; this figure neatly agrees with previous estimates that ‘silverswords can persist in the field from several to over 17 years’. [40], p. 913 (Figure 22). Judging by their proximity and similar state of decomposition, rosettes depicted here may have died synchronously, perhaps following an extended period of extreme drought conditions [39].
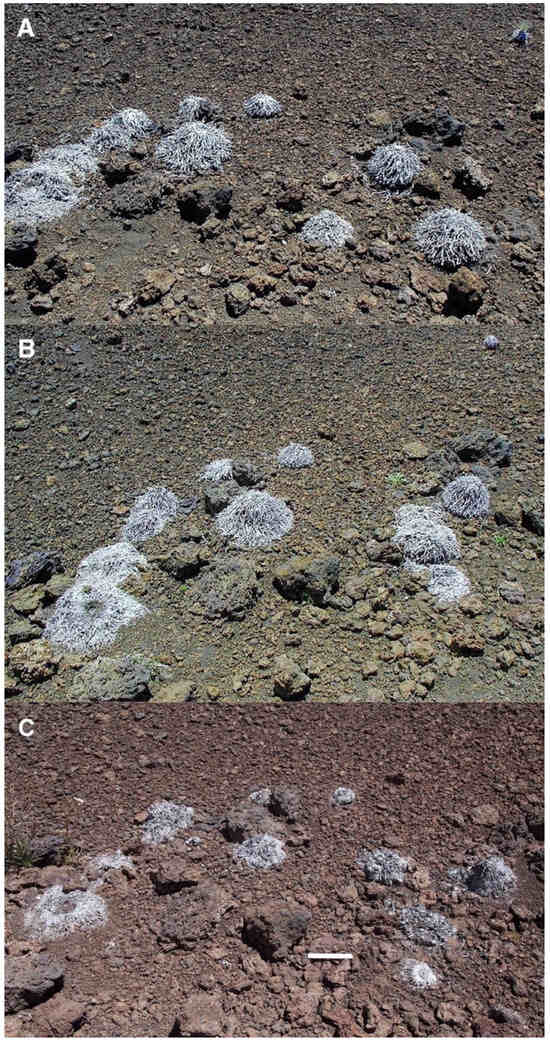
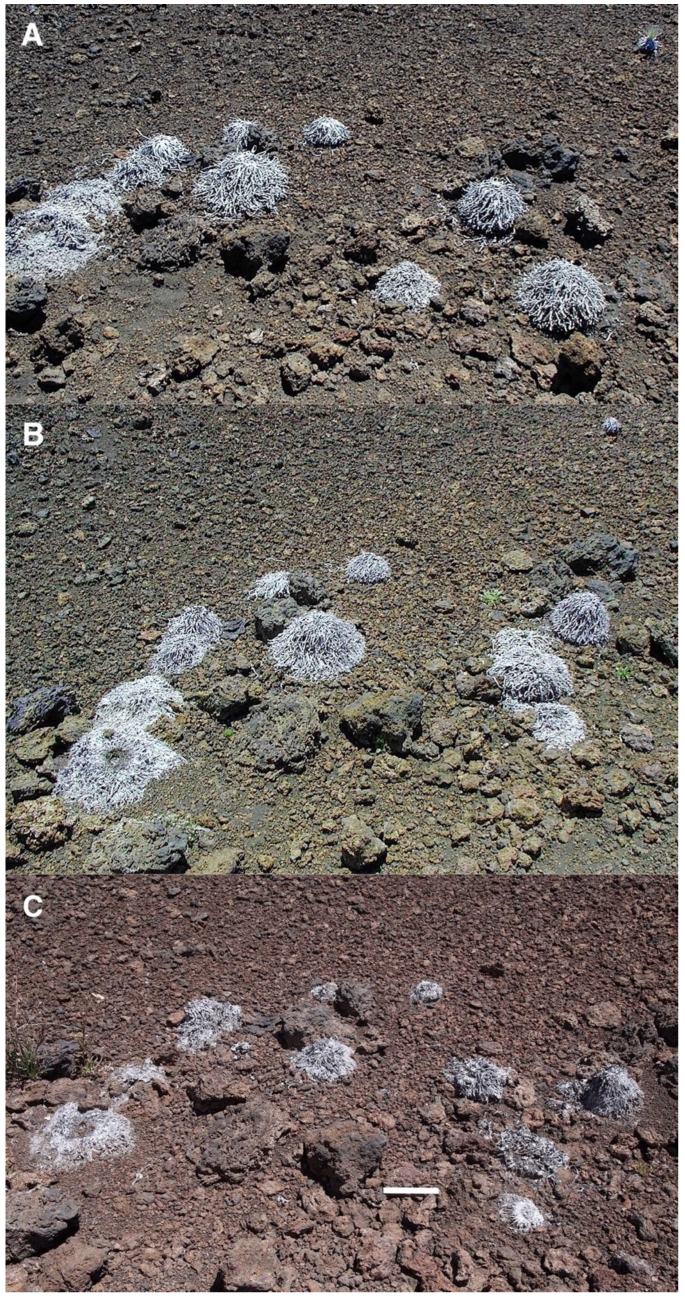
Figure 22.
Repeat photographs of ~11 dead rosettes on a 9° gradient lava ridge near plot 9, Silversword Loop, 2170 m. (A). Photo: Hk-01.227, 5 August 2001. (B). Photo: Hk-05.13, 2 August 2005. (C). Photo: Hk-14.336, 12 September 2014. Photorepeat series spans 4 years (B) and a total time of 13 years, 1 month (C); note: organic matter heaps have noticeably shrunk after this long time period. Ruler is 15 cm long.
4.4. Substrate Temperature Measurements in Different Microsites
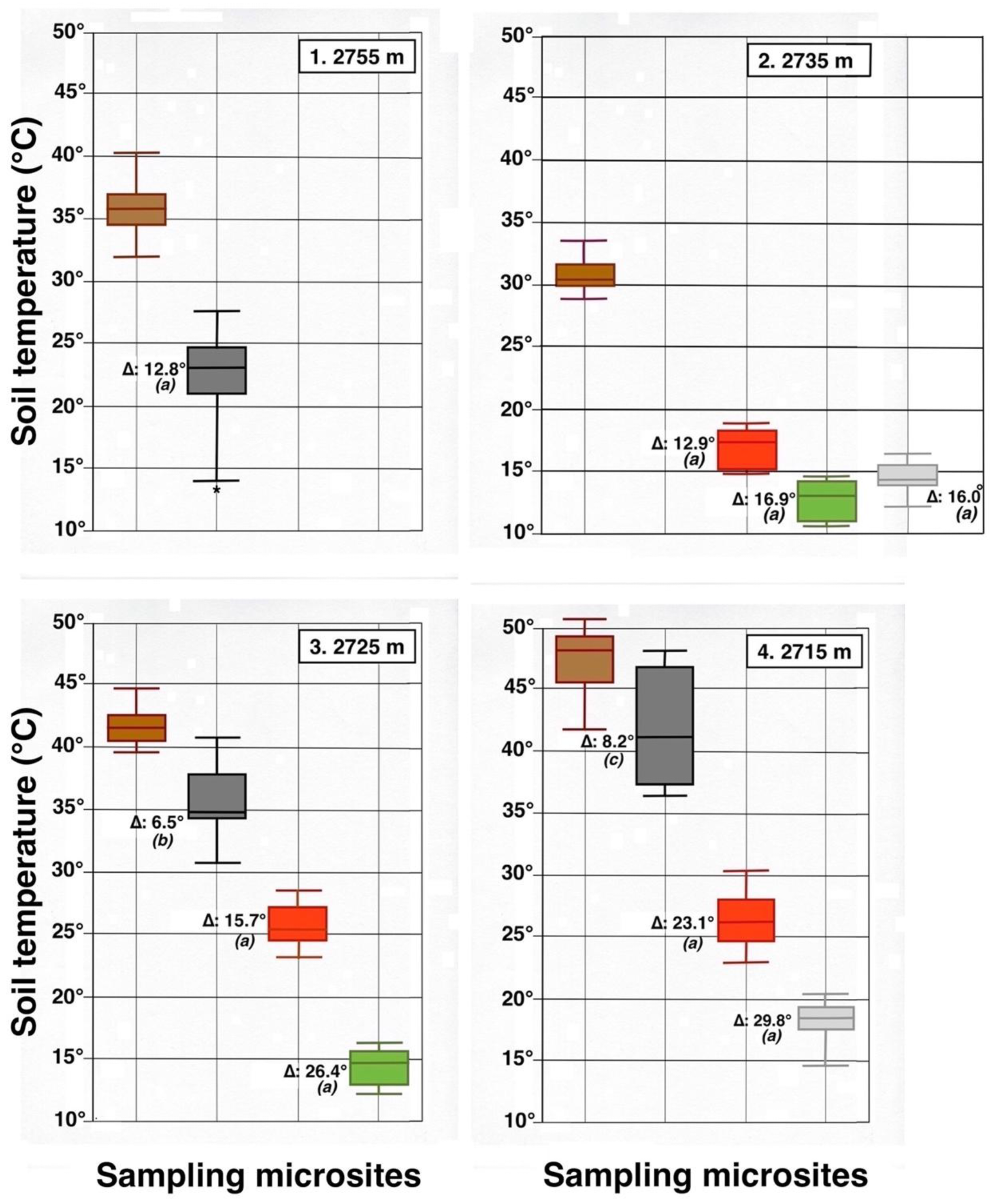
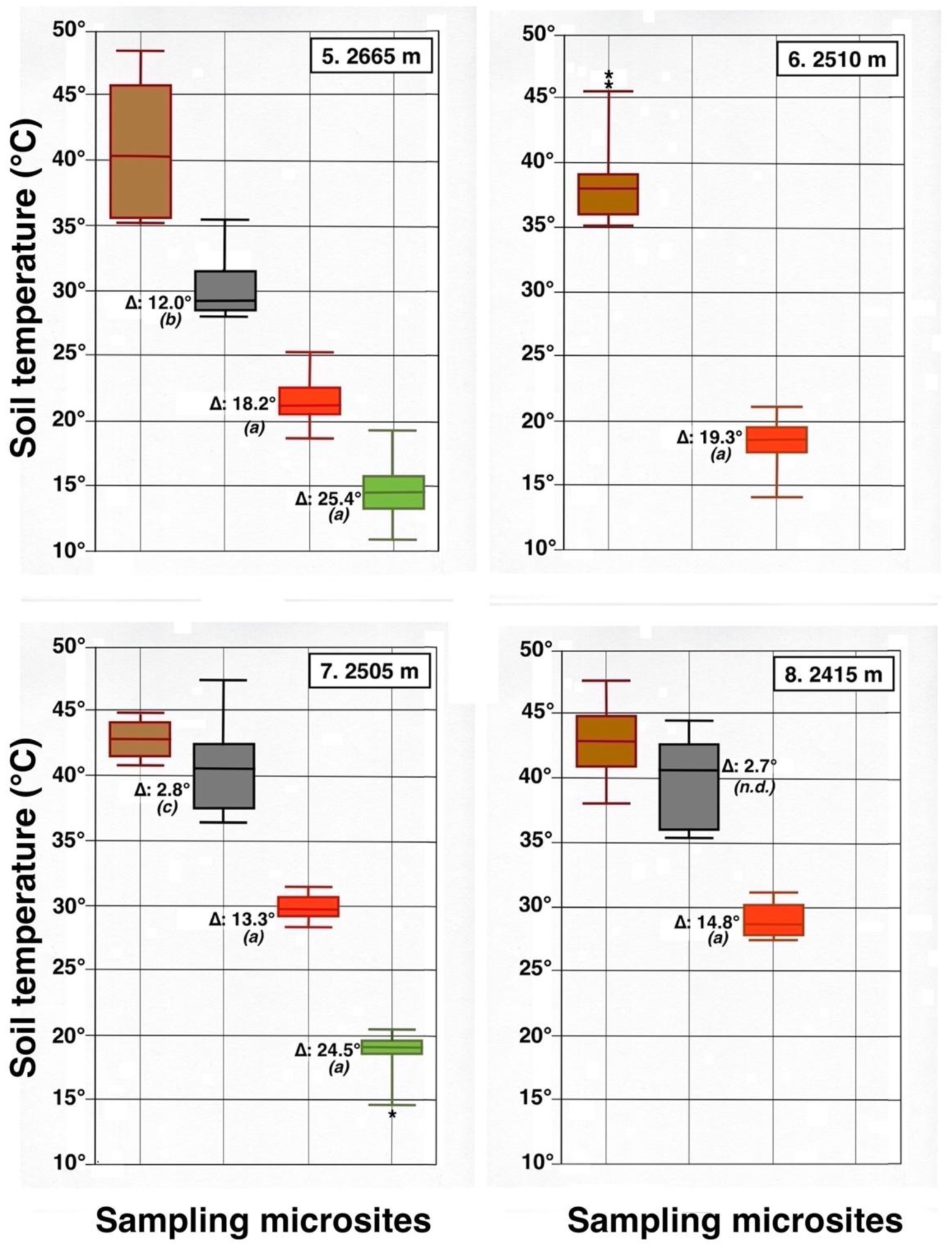
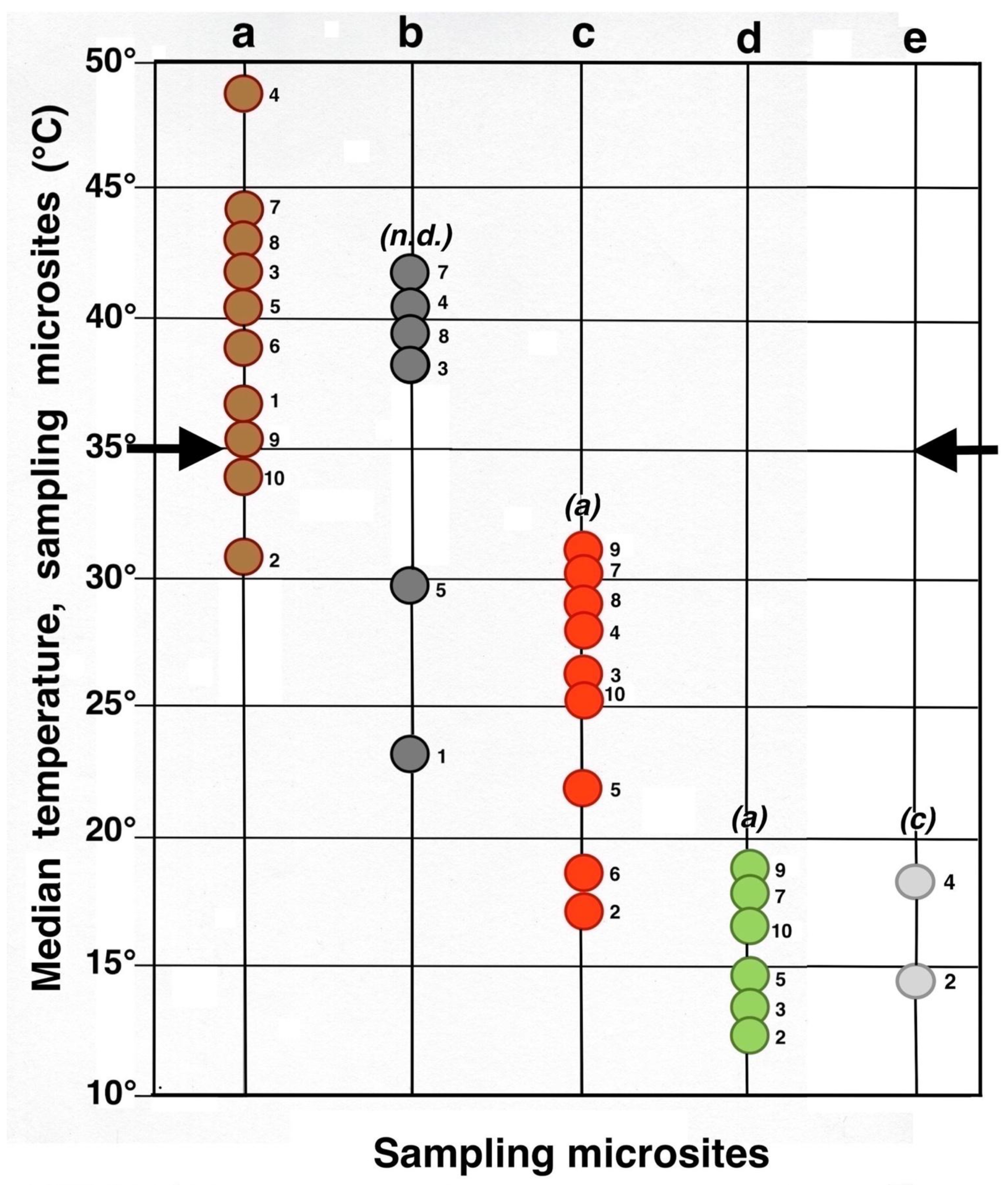
All days chosen for sampling were remarkably sunny and very warm. Air temperatures varied from 22.8 °C (plot 3) to 32.8 °C (plot 8), median temperature was 29.5 °C; RH fluctuated from 22% (plot 7) to 47% (plots 8 and 9), with a median value of 30%. Records of daily ground temperatures revealed substantial differences, not only among the four sheltered microsite types analyzed, but also between all locations and adjacent bare soil areas (Table 2). Temperature data are graphically summarized in 10 detailed box plot diagrams (Figure 23, Figure 24 and Figure 25); temperatures discussed below are median values. Undoubtedly, thermoprobe measurements, obtained on different calendar days, were not strictly comparable, and several factors, including minor color variance of soils and tephra, plot orientation, slope gradient, solar angle, amount of cloud cover, air temperature, and precise time of measurement, introduced some degree of variation to the thermal records (see Table 1).

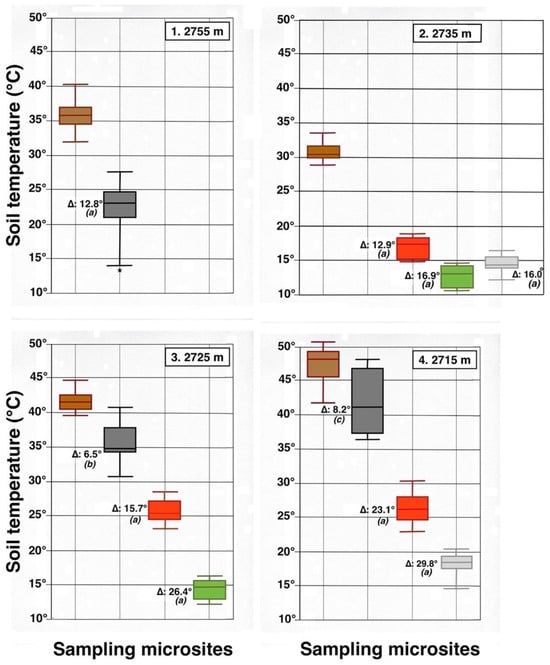
Figure 23.
Parallel box plots for maximum daily soil temperatures (°C, 5 cm depth) in adjacent microsites for 4 measurement plots on the NE-facing flank of Haleakalā Crater; plot number and altitude are indicated. Color Key is shown in Figure 7 and Figure 8: dark brown = bare soil; black = black tephra; red = reddish tephra; green = shaded ground next to silverswords; light gray = dead silversword organic matter. Italic letters in parenthesis show statistical differences (K-S or MWW tests) between data sets in bare soils and each microsite; levels of significance: (a): p < 0.0001; (b): p < 0.001; (c): p < 0.05. An asterisk shows presence of a statistical outlier. See Figure 9 for additional details.
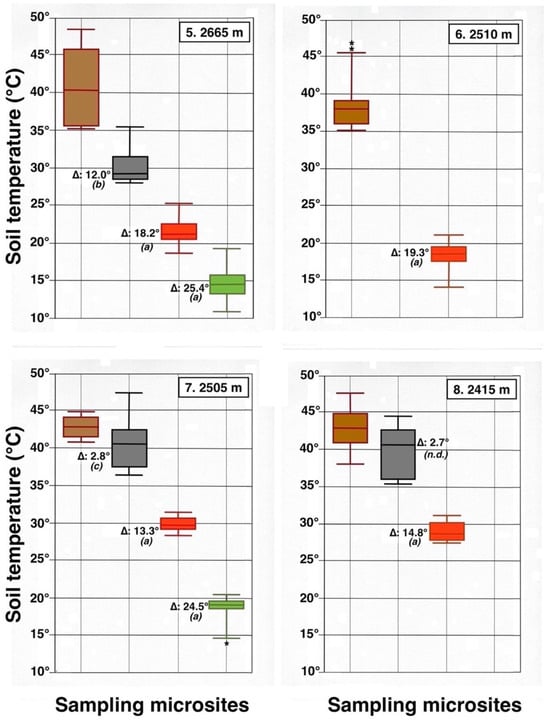
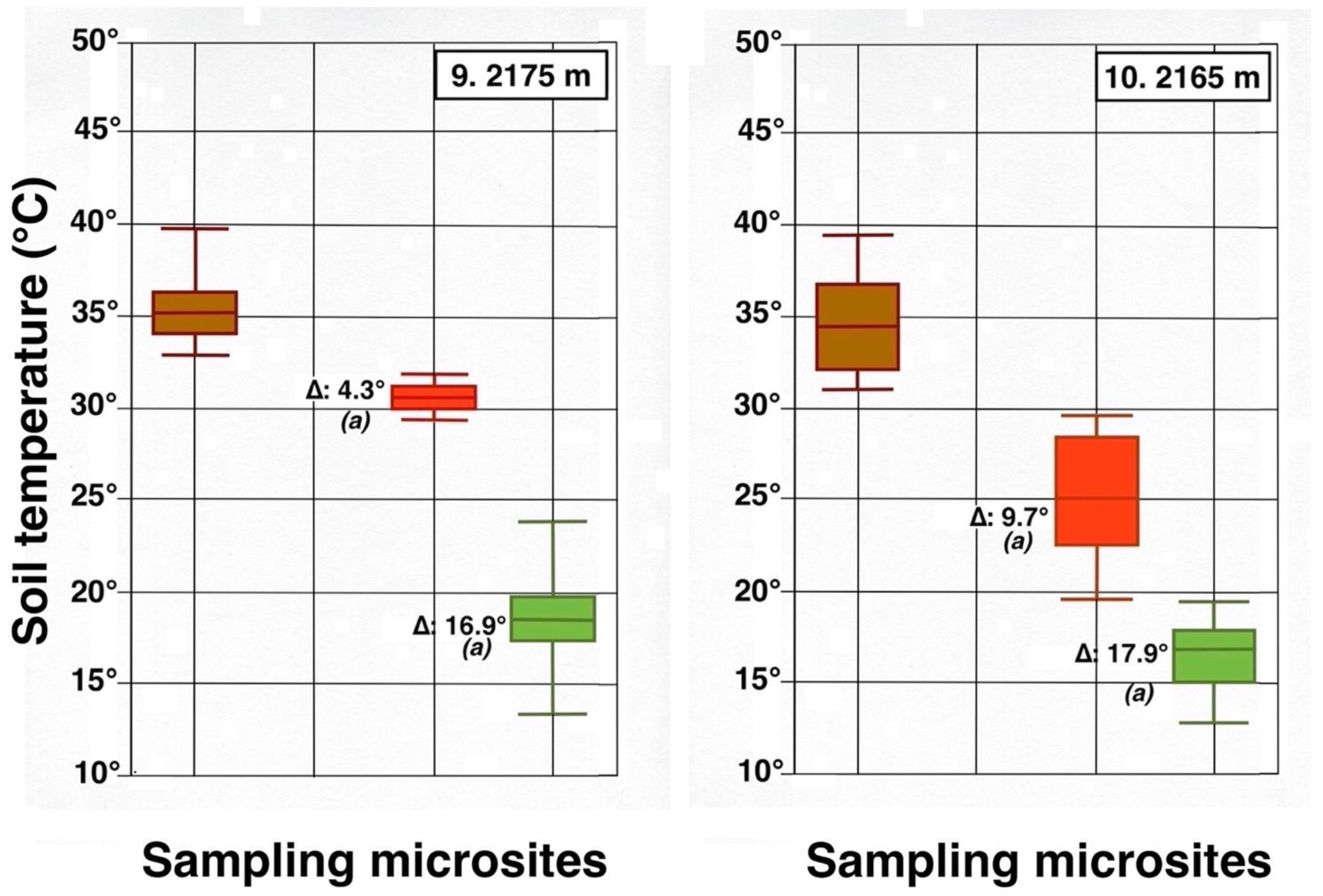
Figure 24.
Parallel box plots for maximum daily soil temperatures (°C, 5 cm depth) in adjacent microsites for 4 measurement plots on the upper Haleakalā Crater; details are shown in Figure 9 and Figure 23. Levels of statistical significance are: (a): p < 0.0001; (b): p < 0.001; (c): p < 0.05; (n.d.): no significant difference; asterisks show presence of statistical outliers.
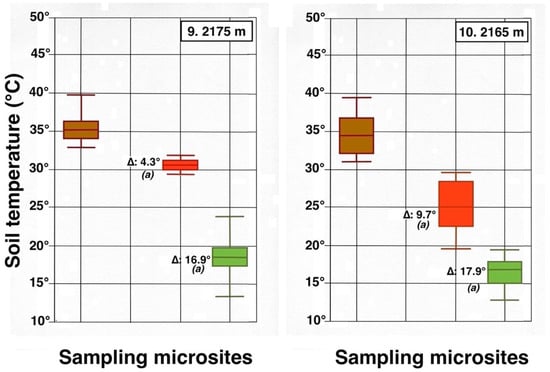
Contrasting these diagrams, it is apparent that all measurement plots show exactly the same ordering sequence of microsites: (i) bare soil temperatures are invariably the highest and show up on the upper left of all box plot diagrams; (ii) wherever black tephra are present on a plot, these temperatures are lower than, and are displayed to the right of, bare soils; (iii) reddish tephra locations, with even lower temperatures, now enter the picture along the temperature sequence; (iv) and these are, in turn, followed by the soils shaded under rosettes, and/or under organic matter piles, which virtually reached the same thermal levels. Recorded bare soil temperatures did not show any obvious trend with altitude; they were generally higher in upper crater plots (4, 5, 7, and 8: 2415–2715 m), but reached their lowest levels in both upper (1 and 2: 2735–2755 m) and lower (9 and 10: 2165–2175 m) crater locations. The analysis of temperatures focuses first on the differences between bare soils and other microsites; further considerations are presented in the discussion below. Bare soils and black tephra were much warmer at 2715 m (plot 4)—where they peaked at 48.7 °C and 40.3 °C, respectively—than at other sites, whereas the lowest bare soil value measured was 30.4 °C at 2735 m (plot 2) (Table 2). Black tephra measurements did not evince a clear relationship with altitude or other landscape variables either but seemed to vary in association with bare ground, as soils under black fragments, on plots 4, 7, and 8, were also the warmest (maxima of 40.3 °C, 40.4 °C, and 40.7 °C, respectively). Additionally, temperatures under dark fragments showed some dependence on particle size and tephra depth (see Section 4.5 below). Although microsites shielded by black tephra were always somewhat cooler than neighboring bare soils, some plots were nearly as warm as these; e.g., plots 7 and 8 were just 2.8 °C and 2.7 °C below exposed soils, and statistical differences between the two were either slight (p < 0.05) or undetectable (n.d.). As black tephra and bare soils had a relatively similar range of colors (Figure 26), their albedo was presumably not very different; thus, they warmed up at comparable rates. The substrate under reddish tephra fragments, however, did not reach such high temperatures: on all nine plots where reddish tephra were present, soils insulated by them were significantly (p < 0.0001) cooler than contiguous exposed ground (Figure 23, Figure 24 and Figure 25). Thermal differences between reddish tephra protected soils and nearby bare substrates—possibly influenced by a minor variability of tephra colors (Figure 26)—ranged moderately, from a minimum of 4.3 °C (plot 9, at 2175 m) to as much as 23.1 °C (plot 4, at 2715 m) and 19.3 °C (plot 6, at 2510 m); it may be recalled that plot 6 (Figure 15) showed an extremely high density, and excellent regeneration, of silverswords.
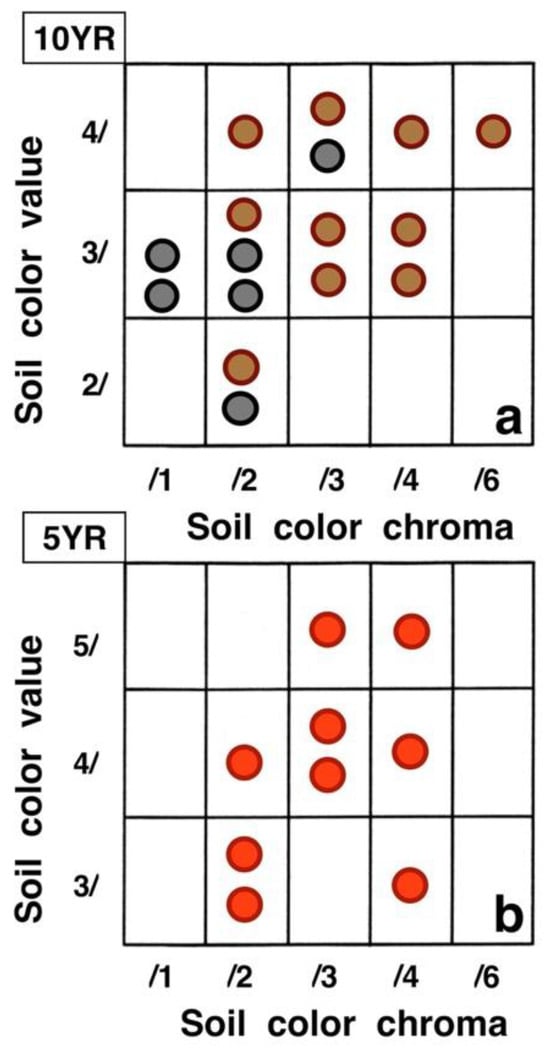
Figure 26.
Variation in (dry) color specimens for (a): bare soils (brown circles), black tephra (black circles), and (b): reddish tephra (red circles) on the sampling plots. Each circle represents one plot. Colors follow Munsell Color Charts [51]; hue names for the two charts used appear on their upper left. See Table 1 for additional details.
Other plots evinced comparable soil/reddish tephra temperature differences between 9.7 °C and 18.2 °C. Ample disparities between reddish and black tephra were equally striking on the six plots where both types were present, as all soils under the former were cooler by 6.2° to 14.9 °C; all these statistical differences were K-S significant, at p < 0.0001. Simply put, reddish tephra covers—even with comparable fragment thickness—were substantially more efficient insulators than black clasts.
All six microsites under the shade of silverwords were substantially cooler than either the exposed soils or sites under any type of tephra (K-S test, p < 0.0001). Thermal differentials between these locations and the ground immediately outside of the plant’s shade were astonishingly high, varying from a minimum of 16.9 °C at 2175 and 2735 m to a maximum of 26.4 °C at 2725 m; unmistakably, such areas were the coolest in all study plots (Figure 23, Figure 24 and Figure 25). Soils beneath rosette litter also remained cool. The statistical evaluation of all 33 box plots presented in these figures shows that temperature data variation—i.e., the vertical distance between the upper and lower whiskers—(S.D.)—was generally more prevalent in data populations with higher median values, like those under bare soils or black tephra, whereas data sets in cooler locations, especially under rosette-shaded areas or organic matter, were less variable.
4.5. Significance of Tephra Depth and Silversword Size for Ground Temperatures
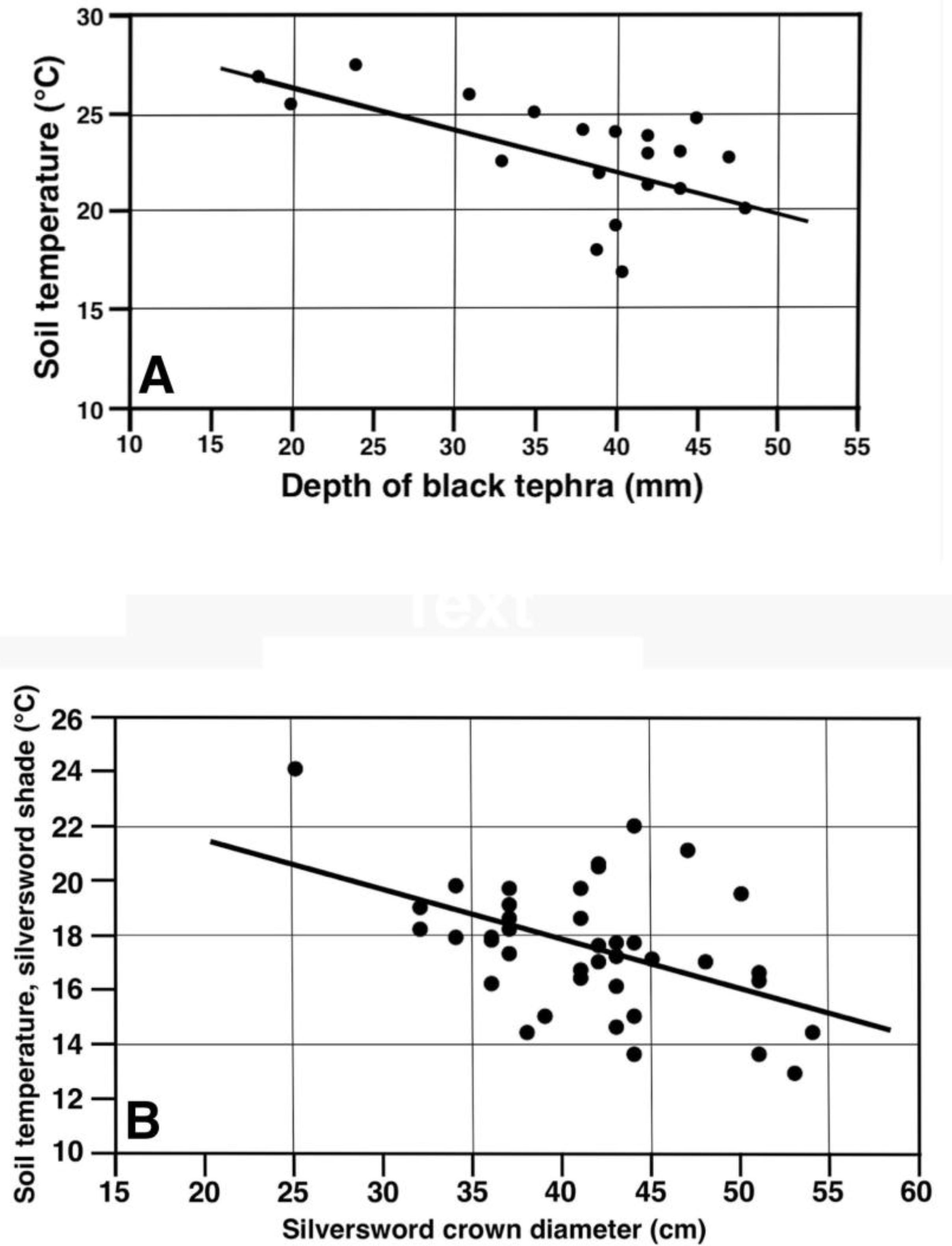
Temperatures under black tephra were analyzed on the highest plot, at 2755 m, with a linear regression to test the hypothesis that the soil temperature could be influenced by the tephra depth. Twenty data points, with a ~17 to 49 mm tephra thickness, yielded the equation (Y = −0.2128X + 30.53), with an R2 = 0.250, and p < 0.025; this indicated that the tephra depth could explain only ~25% of the observed temperature variability. Data were also tested with logarithmic (y = a + b × ln(x)) and exponential (y = a × bx) models, but the correlation coefficients explanatory of these variable interactions did not significantly improve; thus, only the linear equation is reported. This data set included 3 clear outliers located closely below the regression line (Figure 27A); after examining the original field data carefully, it became evident that the size of tephra fragments on these 3 parcels was much lower (1.5–2.2 cm, cf. Figure 14D) than on the other 17 measurement parcels (~6–10 cm, cf. Figure 14B). Exploratory data analysis omitting these outliers from the regression [66,68,82] increased the regression (n = 17) results to R2 = 0.590, p < 0.0005; thus, this procedure improved its explanatory ability to ~59%. It is concluded that, even though the thickness of the surface layers produced by these small tephra particles was comparable to the other samples, their smaller individual size generated fewer and/or smaller voids between them, which may have provided better temperature insulation than the larger empty, openwork voids prevalent in larger fragments. In any case, this analysis conclusively shows that a greater tephra depth results in a lower temperature of the underlying substrate.
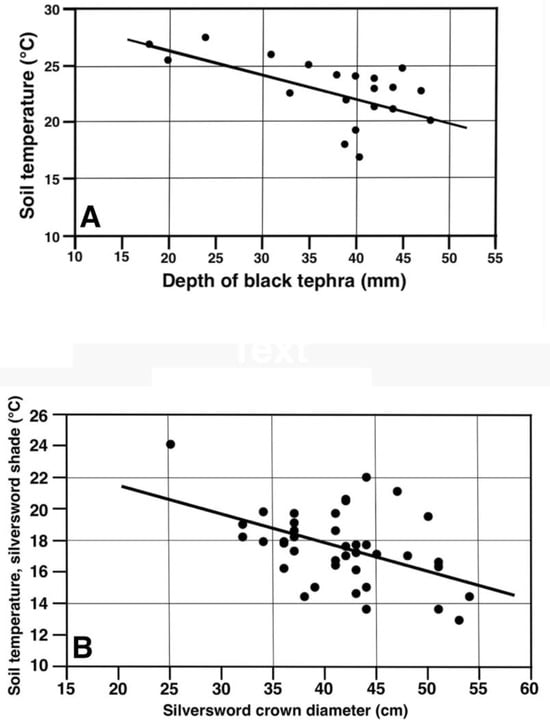
Figure 27.
Linear regressions for soil temperatures (°C, 5 cm depth) associated with (A) depth (mm) of black tephra on plot 1, 2755 m (n = 20). (B) Rosette crown diameter (cm), plots 9 and 10 combined, 2165–2175 m.
The effect of silversword size (crown diameter) and their shade on ground temperatures was also investigated with a linear regression (40 data points) for the combined data sets on plots 9 and 10—at 2165 and 2175 m—which were morphologically similar and were sampled under comparable conditions just 6 days apart. The resultant equation (Y = −0.1826X + 25.13), with an R2 = 0.242, and p < 0.0025, was able to explain ~24% of the temperature variance (Figure 27B). As all sampled plant shadows projected on the same general direction, it is reasoned that, as larger rosettes produced a greater and ostensibly more dense shade, they increased ground insulation in their vicinity and resulted in cooler surface soils (Figure 19).
5. Discussion
5.1. Features of Tephra Environments in Haleakalā
The concise assessment of tephra environments in the Haleakalā Crater above shows that volcaniclastic covers originated from several distinctive sources. Most of the tephra were produced during volcanic eruptions involving pyroclastic materials—ash, lapilli, cinder, and volcanic bombs—that generated cinder cones (Pu’u o Pele, Pu’u o Māui, and Ka ma’o li’i) and extensively mantled large slope areas (e.g., plots 2 and 3). On some cones (e.g., Pu’u o Pele), driblet and welded spatter, ejected during waning eruption phases, coalesced and accumulated around vents as hardened spatter ramparts; later, these rocky walls gradually weathered, and subsequent mass wasting allowed the extensive accumulation of tephra fragments below crater rims, both on the cinder-cone external flanks, as well as inside the crater (Figure 16A). On steep slopes, especially along the crater rim (plots 1, 4, and 5), long-term weathering of basalt outcrops, followed by mass wasting, built up a thick debris mantle on the slopes below. On ‘a’ā lava flows (plots 9 and 10) disaggregation of protruding rock outcrops and crags provided coarse tephra fragments on flow ridges, while the troughs in between remained covered by much finer ash and lapilli. Some of the oldest landforms, including extensive talus aprons below the crater rim (plot 1, Figure 11A), might be as old as the Kula period (Pleistocene) [59] and show obvious signs of pervasive slope movement. Two cinder cones (Pu’u o Pele and Ka ma’o li’i), probably 5000–11,000 years old, also bear signs of past episodic slope creep (Figure 13B and Figure 17C). A possible beneficial element of these latter mentioned geomorphic environments is that, regardless of tephra color, their greater age would have presumably produced more weathered soils, with higher contents of fine particles and organic matter and greater water-holding capacity, thus providing a more favorable substrate for silversword development [100].
5.2. Other Positive Effects of Tephra Covers
Tephra fragments are known to provide multiple additional beneficial roles for silversword growth. Volcaniclastic particles of different sizes act as nurse rocks and are able to affect water storage and input to the underlying soil. Infiltration into the ground is significantly influenced by a continuous rock cover; as water runs off impervious stone surfaces, it penetrates the substrate, becoming concentrated in the intervening interstitial spaces, filled with finer gravelly soil matrix, where ample water can be stored [95,97]. Afterward, the percolation front moves deeper into the underlying soil along the spaces between stones [109,110,111]; thus, a tephra cover increases the depth of infiltration. Rock fragments also decrease the rate of water evaporation from soils beneath them. This occurs because upward capillarity action is disrupted by tephra clasts covering the soil, which prevent water flow to the surface, and due to their efficacious reduction in high diurnal temperatures in the shielded soils [95,112,113,114,115].
Tephra clasts may often provide geomorphological refugia for seedlings and even for larger plants. On steep slopes, the loose volcanic debris episodically move downhill, propelled by gravity and local disturbances caused by other agents, including runoff, frost creep, surficial dry debris slides, miniature mudflows, rainsplash, rockfalls, thermal creep, animals, and/or hikers [1,10,11,101,108]. Descending debris is stopped by, and accumulates upslope from, a nurse rock; particles are then re-directed sideways and move downhill along rock edges, whereas stabilized sections below the stone offer favorable microhabitats for plant growth (Figure 28). Even on level substrates, frost and needle-ice formation may heave and vertically displace small- and medium-sized rocks, disrupting nearby plants and their root systems [1,4,25,108]. Nurse rocks may cause additional positive effects, as they are able to intercept seed and spore propagules transported by water and/or wind, which are easily lodged around rock edges [30,116,117,118]. In Haleakalā Crater, the roots of silverswords are symbiotically associated with arbuscular mycorrhizal fungi (AMF), which provide greater drought tolerance and are critical for increasing plant absorption of the scant phosphorus supply in volcanic ash soils [100,119]. As wind-blown AMF spores become readily wedged underneath rocks, the growth of mycorrhizae then facilitates the development of rosette seedlings [120]. These beneficial effects of stones have been amply investigated in Haleakalā and elsewhere, yet the influence of rock fragments on these processes is clearly associated with all types of tephra, not just restricted to fragments of any particular coloration. In contrast—as this study demonstrates—differently colored stones differ substantially in their thermal effects on soils [121,122].

Figure 28.
A ~5 cm tall silversword seedling growing downslope a ~28 cm long stone surrounded by fine, rapidly sliding gravel grains on a ~23° talus slope, plot 4, ~2715 m. Hk-98.108, 2 August 1998.
5.3. Thermal Effects of Tephra Covers and Other Nurse Objects
A meta-analysis comparing all termoprobe data points (Figure 29) plainly illustrates the following facts associated with the effects of tephra on soil temperatures: (i) Only 1/3 of the black tephra sampled populations were cooler than the 10 bare soil groups, and a as a result, the composite K-S tests between these two groups yielded no significant differences (n.d.). (ii). Eight uncovered soil plots were, on average, warmer than the critical 35 °C threshold presumably needed for survival of silversword seedlings; yet, this also suggests that rosette seedlings might have survived in ~20% of the sampled soil microsites even without the benefit of any tephra insulation (iii). Black tephra covers were not particularly effective in reducing soil temperatures as a whole, and only two plots under dark fragments may have been conducive to silversword development. (iv). In contrast, all nine plots where reddish tephra fragments were present provided good soil insulation and were significantly (K-S test, p < 0.0001) cooler than contiguous exposed ground. (v). All the reddish tephra plots were able to maintain underlying soils below the dangerous 35 °C boundary. (vi). All six microsites under a silverword shade were substantially cooler than either exposed soils or those covered by black tephra. (vii). About 78% of the rosette-shaded microsites were significantly cooler than those under reddish tephra. (viii). The two localities covered by small rosette organic matter mounds showed similarly cool conditions, and at the only plot where both shaded and litter-covered microsites were sampled (2, at 2735 m), the substrate under organic litter was a mere 0.9 °C warmer (K-S test, no statistical difference).
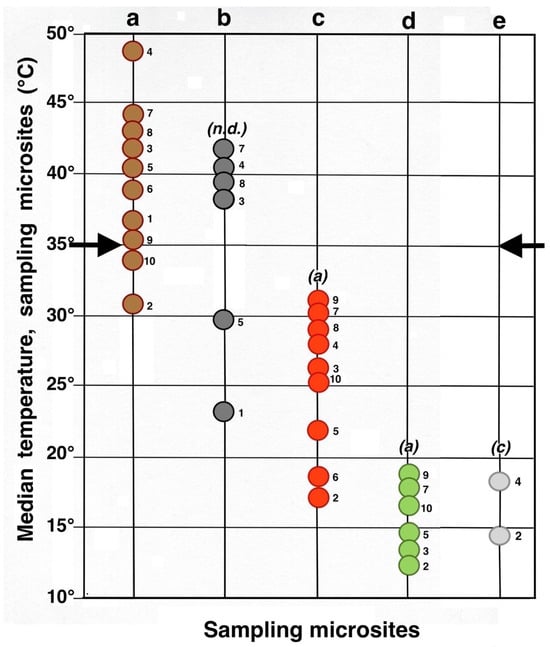
Figure 29.
Summary meta-analysis diagram of maximum daily soil temperatures (median, °C, 5 cm depth) in all microsites. Each circle represents an individual microsite; the adjacent number identifies the plot where it is located. Key: dark brown = bare soil; black = black tephra; red = reddish tephra; green = shaded ground next to silverswords; light gray = dead silversword organic matter. Italic letters in parenthesis show statistical differences (K-S or MWW tests) between complete data sets of bare soils and each microsite; levels of significance: (a): p < 0.0001; (c): p < 0.05; (n.d.): no significant difference. Black side arrows indicate the maximum temperature level critical for successful silversword reproduction [1,56,98].
5.4. Thermal Effects of Silversword Shade and Organic Litter
Microsites associated with rosette shade and/or its organic litter deserve some additional attention. Nurse plants protecting Dubautia menziesii seedlings under their cooling shade are only infrequently found at Haleakalā (Figure 2), but the striking reduction in ground temperatures that silverswords clearly provide led me early on to explore the possibility of a self-nursing role for these ubiquitous crater plants [25,30]. Other tropical alpine rosettes elsewhere significantly ameliorate the microclimate about them and provide safe shelter to their own juveniles under their leaf crown. In the Northern Andes, seedlings of several Espeletia Bonpl. and Coespeletia Cuatrec. giant-rosette species (Figure 1A) grow at the base of tall mature plants and show greater growth rates and survival than seedlings away from adult specimens, which may experience even 100% mortality [25,123,124,125]. In the East African Highlands, seedlings of Dendrosenecio keniodendron (R.E.Fr. & T.C.E.Fr.) B.Nord. (Asteraceae) and other giant rosettes are also considerably more abundant next to tall adults [4,126]. However, seedling proximity to adults in these high-altitude (≥4000 m) mountains causes climatic amelioration mainly during the night, as the presence of a canopy may raise nightly ground temperatures by up to 7 °C, thus reducing the freezing intensity [125,127].
Although diurnal soil temperatures in Haleakalā are, unquestionably, greatly reduced on all microsites under a silversword shade (Figure 27 and Figure 29), there is no definite evidence that its own seedlings actually take significant ecological advantage of this thermal relationship. The relatively few silversword clusters scattered across the crater might have been produced, as posited, by seeds germinating around the base of older, already established silverswords or, alternatively, might simply have some other cause: (i) rosette clusters were most often found in areas nearly devoid of surface tephra, suggesting the possibility that several individual monocaulic plants had grown around, and shared, an isolated nurse rock, buried or now hidden by the plants’ shriveled foliage around their bases [4,25,128,129]. (ii) The large, 7–15 mm long silversword seeds [31] have very limited dispersal; 61% of them are dropped ≤20 cm away from the parent plant, and only 21% travel ≥40 cm [57,130]. This limited transport distance acts as a positive safeguard, as it practically guarantees that most seeds actually land on contiguous favorable microsites, such as tephra or silversword litter (Figure 21). Restricted dispersal would also help explain the high degree of rosette aggregation observed at some sites (Figure 3 and Figure 15) [130]. Some rosette clusters might conceivably result from seedlings growing close to each other, then coming into contact as they grow larger afterwards, thus appearing as multistemmed plants. (iii). Or, indeed, the observed plant clusters might actually be produced by uncommon, multibranched silversword specimens. The actual frequency of multi-stemmed and/or polycarpic Haleakalā silverswords is not known, and careful excavation and examination of numerous plants is needed for such exploratory research; yet, severe disturbance and resultant destructive sampling of rosettes hardly seems an acceptable study strategy for a threatened plant, especially for one protected in a National Park.
Some studies report occasional polycarpy in Haleakalā silverswords. Forsyth [130], p. 226, mentioned that Haleakalā silverswords ‘nearly always’ develop a single rosette and that all individuals are monocarpic. Kobayashi [1], p. 65, found in 1972 a single young plant that had produced several lateral branches after its single apical meristem had been removed by goat browsing, but after building a fence around HNP in 1986, goats were not a danger to silverswords anymore. Axis deer (Axis axis), introduced to Maui from India in 1959, also seem to be largely controlled by this long enclosure, but as they are able to jump fences ≤2.6 m tall, supplementary control efforts are needed to keep them out of the park along its western boundary [130,131]. Other tropical–alpine monocaulic rosettes in the Northern Andes have been known to infrequently develop pleiochasial secondary branching after disturbance by rockfalls, cattle, or by spectacled bears (Tremarctos ornatus)—now nearly extinct—which may have eaten the succulent pith inside the rosette stems [124,132,133,134].
The Mauna Kea silversword (Argyroxiphium sandwicense DC. subsp. sandwicense) grows only in the island of Hawai’i; this is a polycarpic, multibranched rosette plant that normally produces a tight, ≤150 cm diameter, cluster of ~9 rosettes per individual; the plant grows one inflorescence per rosette, independently of the others; then, that rosette dies after flowering once [135]. A comparison of silversword species from the two islands indicates a number of important morphological differences, including the branching pattern, leaf width and length, and inflorescence dimensions [31,135,136,137] (Figure 30). The current morphology of the Mauna Kea silversword may partially have been caused by a significant genetic bottleneck the species went through, which reduced its genetic variability. Its original, natural populations extended above treeline over a large, high-elevation (2600–3800 m), area around the summit of Mauna Kea [58,135]; following the introduction of sheep (Ovis aries) and mouflon sheep (Ovis musimon) to Hawai’i, the plant was severely damaged by ungulate browsing, and by 14 September 1892, had nearly disappeared, as Alexander [138,139], p. 183 noted: ‘The beautiful Silver Sword (Argyroxiphium), once so abundant is nearly extinct, except in the most rugged and inaccessible localities’. By the late 20th century, only 38 individuals remained in natural populations [135]; an outplanting program was started in 1973 by the Hawai’i State Division of Forestry and, subsequently, about 500 established adults were censused in 1999 [58]. Unfortunately, inadvertent manipulation during the initial efforts to cultivate the species influenced its frequency of polycarpy and multiple branching; these might be either an effect of greenhouse cultivation during early seedling stages, or perhaps due to the fact that all outplants were produced by seeds from only one—or perhaps two—female plants used in the program. As a result, a high percentage of rosette outplants became multibranched. In essence, what seems like an extraordinarily high frequency of polycarpy actually resulted from accidental artificial selection for this morphological characteristic [137].

Figure 30.
Mauna Kea silversword, ~130 cm diameter cluster of ~11 polycarpic rosettes; note the rosette on the extreme left has a developing inflorescence. Mauna Kea Visitor Station, 2804 m. Photo: Mauna Kea-07.63, 30 June 2007.
Be that as it may, following two decades of fieldwork in Haleakalā, and after examining several thousands of silverswords there, I have never found any browsed and/or obviously multibranched plants. I have only come across perhaps fewer than eight silversword clusters in the whole crater, and these always displayed the same characteristic morphology of a few seemingly independent, unequally sized, gathered rosettes about a central point (Figure 20). Finally, with respect to rosettes growing on a silversword litter substrate, it would have been desirable for this study to measure additional microsites on more study plots (Figure 29); regrettably, although there were several small mounds of dead rosette plants present on practically all measurement plots (Figure 21), these were scarce on most locations and did not provide enough individuals for a thorough statistical analysis (Figure 23, Figure 24 and Figure 25).
5.5. Role of Additional Effects of Tephra and Silversword Plants
Even though there was no obvious evidence that tall rosettes facilitated the establishment of seedlings under their crown canopy, tephra covers and silverswords provide an additional, significant, effect to the substrates beneath them; this was noticed on several occasions during fieldwork. On cloudy, foggy days, dense fog often invades the west crater through the Ko’olau Gap (Figure 11B and Figure 18); on such occasions, copious amounts of fog and drizzle can be gradually intercepted by individual stones protruding above the uniform ground surface and by the rough, uneven tephra surfaces (Figure 14 and Figure 15). This process increases soil water content dramatically. Kobayashi [1], p. 4, reported that, during a 46-day period in late spring, two stations ‘in the most arid area of the crater’, Ka moa o Pele cinder cone and Pu’u Kauaua at 2210 m, received all their water—60 and 20 mm, respectively—exclusively from fog interception (Figure 31).

Figure 31.
Fog and drizzle interception by a large ~35 cm tall lava block, at ~2415 m, ≤1 km downwind from Pu’u o Māui, the presumed source of this fine black tephra (cf. Figure 14D). This isolated block lies on a gentle, ~10° slope; the right-side, wet surface, of the block—and that of the small cobble on the lower right—faces to the northeast trade winds. A small, ~20 cm diameter Leptecophylla tameiameiae seedling grows right below the NE edge of the boulder, precisely below its drip-line. Note the left block side, and the ground lapilli on that side, remain dry after a ~5 h exposure to incoming fog. Photo: Hk-02.615, 11 August 2002, ~1:45 p.m. HST.
Seedlings of several plant species, including silverswords, Dubautia menziesii and Leptecophylla tameiameiae and ferns, often take advantage of this microclimatic situation, as they grow on the windward side of such rock obstacles. I believe the main—if not the only—reason silverswords are able to successfully colonize extensive black tephra areas facing NE, e.g., Pu’u o Māui (Figure 13A) [105], Figure 1, is that substantial amounts of additional precious water can be captured by this climatological phenomenon.
Afterwards, once silversword seedlings receive such a significant boost to their growth, they grow taller and, in turn, also intercept an even greater percentage of the incoming moist fog-laden airstream, contributing to their own development [140]. The densely pubescent leaves of silverswords are uniquely adapted to extract water from the moving moist air, as the tiny, ≤0.2- to 0.5 mm diameter, floating droplets, collide with the hairy plant’s indumentum, allowing water droplets to coalesce and gradually drop into the cup-like plant crown, and later to the ground, on the plant’s windward side (Figure 32).
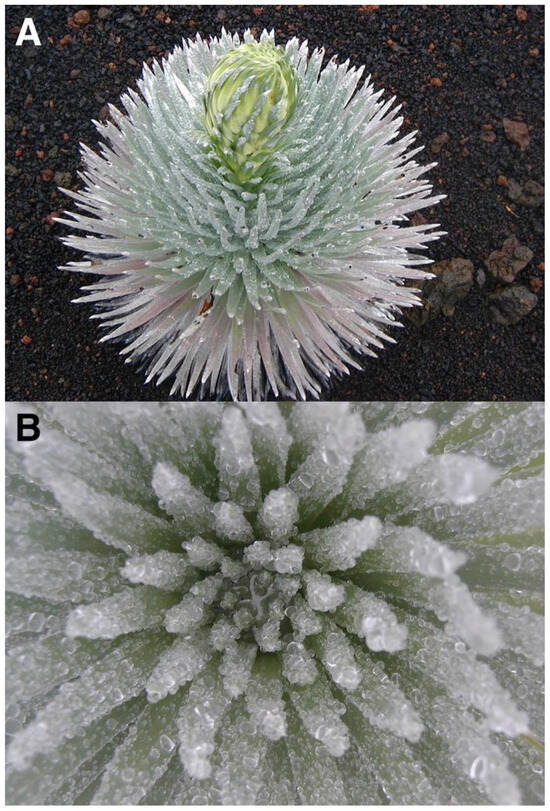
Figure 32.
Silversword plants after interception of abundant fog and drizzle during a ~5–6 h period; upper crater floor, near plot 7, ~2470 m. (A). Large, ~40 cm diameter rosette, with developing inflorescence, growing on fine black tephra. Photo: Hk-02.622, 11 August 2002, ~2:07 p.m. HST. (B). Close-up of a nearby silversword, showing a myriad water droplets adhering to the hairy new leaves at the rosette center; notice water pooling in the rosette center. Photo: Hk-02.625, 11 August 2002, ~2:10 p.m. HST.
Considering that silverswords are able to significantly lower ground temperatures below them (Figure 23, Figure 24 and Figure 25) and also appear to boost soil water, a relevant question is Why are silverswords not acting as their own nurse plants? Even more to the point: Why are there no examples of (other species) nurse plants helping the silverswords in Haleakalā? (Figure 2). As noted, some studies [25,29] suggest that nurse plants can negatively compete with developing seedlings through their ground shading, thus reducing much-needed photosynthetically active radiation, or perhaps competing for soil water, always in scarce supply at Haleakalā. These two options appear as reasonable possible answers for the observed lack of nurse plants.
5.6. Additional Biological and Environmental Factors Influencing Germination
Some specific biotic interactions may affect long-term silversword ecology. Silverswords are strongly self-incompatible, and flowers from a specific plant must receive pollen from a different individual in order to produce viable seeds [38,141,142,143]. In the crater, the number of plants flowering each year fluctuates greatly, from zero in 1970 to 6632 individuals in 1991; however, the triggering factor(s) for extreme flowering events are unknown [1,40,105]. This high annual variation, coupled with the wide disparity in population densities, produces a strong allee effect, and rosettes blooming during high-flowering years show a greater percentage of seed set than in low-flowering seasons [130,143]. In smaller populations, a scarcity of flowering individuals leads to lower reproductive success, which, in turn, may affect the future population size. As the allee effect is pollinator-mediated, reduced pollinator activity or abundance would worsen the situation and further diminish silversword population size. This chain of events might lead to a gradual shrinking of small rosette populations, whereas larger populations would be maintained or even expand in numbers.
More than 170 years ago, there was concern that silversword seeds were largely ‘destroyed by insects’ [144], p. 399, but as Kobayashi pointed out, many of these insects were a natural ecological factor necessary for plant pollination. Field observations have determined that some 19 insect taxa visit rosette flowers, but Hylaeus bees (Hylaeus spp., family Colletidae) are by far the main pollinators for Haleakalā silverswords [37,142,145]. A reduction or loss of pollinators might constitute the largest danger to the continued survival of Argyroxiphium [38,105,145]. The introduced predatory Argentine ant (Iridomyrmex humilis) reached HNP in 1967, and by 1985, it had spread to the crater rim at 2880 m [37,105,145,146]; this highly destructive insect significantly diminishes native arthropod abundance and diversity and has recently invaded silversword range, where ant occurrence has been linked to substantially lower rates of silversword floral visitors, including Hylaeus bees [145]. The introduced predatory western yellowjacket wasp (Vespula pensylvanica, Hymenoptera, family Vespidae) became established in HNP in 1978, where its populations rapidly expanded and reached a 2760 m elevation [37,147]. This wasp is an opportunistic generalist predator; prey items from six insect orders were collected from wasp workers. Vespula preferentially nests in plant litter under Styphelia tameiameia shrubs, and it is not yet abundant in silversword habitat, but it has been observed to probe silversword plants for insects, causing concern to HNP managers regarding its possible ecological impact on vulnerable native pollinators.
Notwithstanding the possible impact of introduced insects, 50–95% (average: 60%) of the annual silversword seed crop is actually eaten or damaged by native insects, significantly reducing the rosette’s reproductive success. The two insects involved are an endemic moth to Maui (Rhynchephestis rhabdotis) and a fruit fly (Trupanea cratericola) [1,38,148]. However, during years with widespread silverword flowering, the abundance of achenes causes overall seed predation to drop to less than 50% [1,37,130,143].
Additional factors affecting germination success include seed dispersal and dormancy. Shortly after silversword seeds ripen at the end of summer (September-November), they are dispersed solely by wind [105], p. 4; only one instance of downslope seed transport—8 m distance—by running water has been observed, in the Mauna Kea silversword [12,57], and no animal dispersal has ever been ascertained [130]. As a result, the poor achene dispersal of Argyroxiphium is extremely leptokurtic, and most seeds accumulate directly below, or close to, decaying rosettes; this should produce a clumped spatial pattern of seedlings near each other, which mostly share self-incompatible alleles [145]. As seen, the reproductive effectiveness of silverswords is influenced by a complex balance among plant population size and density, spatial patterns and the distance between flowering individual plants sharing pollen, the efficiency of pollinators and their survival against predator insects, and limited seed dispersal [143].
Seed dormancy has not been intensively studied for silverswords. Kobayashi [1], p. 16, reported a viability of less than two years in Haleakalā, whereas seed viability in the Mauna Kea silversword declines rapidly in the soil [57]. Siegel et al. [56] mentioned the presence of several chemical inhibitors, which contribute to poor field germination. However, more recent studies show the successful germination of seeds older than 4 years for Haleakalā silverswords [130], indicating that seeds can remain viable in the field for several years. In any case, unpublished population monitoring studies in HNP indicate that rosette recruitment is higher in years following massive flowering events [143].
Like the Mauna Kea silversword, the Haleakalā silversword may have gone through some genetic bottleneck following a severe reduction in population size in the crater in the late 19th and early 20th centuries, but the actual reduction in plant numbers is not precisely known. Botanist Otto Degener [149], p. 307, completed a survey at the Ko’olau Gap (Figure 18) in 1927 and reported to HNP’s superintendent that ‘barely 100 plants were left of the silversword population’ [1], p. 10, [105], p. 7. This incongruous number is difficult to accept when compared to subsequent assessments, including the aforementioned figures of 4000 silverswords in 1938, and 10,000 plants in 1962; unfortunately, all the surveys, except those by Kobayashi, seem to report just on crudely rounded estimates, which inspire little statistical confidence and make their literal interpretation risky. In any case, it seems that, whether silverswords were reduced to just 100, or 4000, or even 10,000 individuals, some loss of genetic heterogeneity may have taken place, albeit not at the same appalling scale as that affecting Mauna Kea’s silversword [135,150]. A drastic reduction and subsequent recovery of this last plant is known to have resulted in reduced seed set in the outplanted population; this low allelic diversity may also pose a threat to long-term population persistence [150], p. 1145.
A final, peculiar genetic aspect of the Haleakalā silversword has been known for a while; this rosette plant commonly hybridizes with the closely related Dubautia menziesii on shared sympatric tephra areas, where both plants frequently grow near each other [12,151,152]. These hybrids, found on several crater areas, are unmistakable due to their acutely lanceolate, vividly dark-green leaves, distinctly different inflorescences, and clusters of multistemmed small rosettes (Figure 33). Hybridization is believed to be an important evolutionary event in the geologically young Hawaiian islands [152]; tephra surfaces, by offering thermally sheltered habitats to the two associated plant species, should be considered important environmental elements helping plant adaptation to these volcanic areas.

Figure 33.
A ~65 cm tall, multibranched, hybrid (Argyroxiphium sandwicense x Dubautia menziesii) individual with six small rosettes is in the foreground; three regular, monocaulic silverswords are seen in the background at 2520 m, near plot 7. Photo: Hk-01.43, 25 July 2001.
5.7. Potential Human Effects, and Management Considerations
In recent years, Haleakalā National Park has become the victim of its own success. HNP, now the most important tourist attraction in Maui, reached a peak of 1,963,187 visitors in 1999 [35,153]. Even though only an indeterminate minuscule percentage of these hike through the crater, their rising numbers may soon—if not already—become a problem for environmental conservation.
Along with goat browsing, vandalism and illegal collecting were a serious problem contributing to silversword decline during the 19th and early 20th centuries. Damage to silverswords by humans was noted even by the first climbing party of the mountain in August 20th, 1828: ‘…our guide, and servants made ornaments of it (silversword) for their hats, to demonstrate to those below, that they had been to the top of the mountain’ [154], p. 248, [38]; this practice seems to have been common, along with various types of illegal collection. In 1911, large amounts of silversword plants were gathered to decorate a float for Washington’s Birthday parade in Honolulu, where ‘a car was an immense silversword’; this tradition continued until 1914 [105], p. 7. In 1921, five years after HNP had already been established by an act of Congress, a magazine advertisement for the sale of silverswords offered ‘a specimen for fifty cents or a whole plant for One Hundred dollars’ [105], p. 7. General disregard for the crater landscape was widespread. Mark Twain, the famous author, visited Haleakalā in 1866, where he joyfully ‘…now and then tumbled rocks, half as large as a barrel… and saw them careering down the almost perpendicular sides’ of the crater [155], Ch LXXVI. This apparently was a popular pastime, as newspaper accounts often also mention such entertaining activity [105], p. 5, [156], p. 115. Vandalism and wanton collection were significantly reduced after the first ranger station was built in 1930 [38]. Willful plant destruction by hikers is probably minimal these days, but visitors need to be clearly made aware of the fragility of silverswords. Nevertheless, I have never seen any signs informing tourists, or encountered a ranger, while hiking across the crater, in two decades.
Trampling by backpackers may be a considerably more serious activity affecting silverswords [38,105]. Kobayashi [1], p. 78 noted that human traffic caused soil compaction and disturbance on the loose, non-cohesive cinder soils of the crater, especially on steep slopes (Figure 28) and cinder-cone flanks, which are studiously avoided even by HNP researchers. Kobayashi found that, after 4 years, most ≤10 cm tall seedlings in a 32-plant population in Kalahaku Overlook—one of the most disturbed areas in HNP—were missing or had their roots exposed and/or broken. Most trails across the crater carefully skirt around the edges of large rosette populations, but in some cases (e.g., plot 7, Figure 3), the hiking trail is lost among the silverswords, without any guidance or warning markings; under these conditions, even cautious visitors run the risk of stepping on small seedlings or trampling the substrate around larger plants. It would not be too expensive or difficult to install permanent, distinctive stone slabs marking the trails across these areas or to even relocate some fragile trail sections. Visitors should be instructed about the instability of volcanic-ash substrates and ought to be reminded not to stray away from established trails.
Although the majority of visitors to HNP are reasonably informed about the need to preserve live silverswords, I do not think most realize that dead plants should also be respected. Early climbers to the mountain gathered ‘…piles of withered silver-swords’ to warm themselves at night in the cold Hawaiian summits [157], p. 372, [158]. Gathering of rosette plants for fuel has also been reported both in the cold high-elevation of the South American Andes [134,159] and the East African Highlands [4,160], where this practice contributed to the destruction of vast rosette stands. I would hope hikers staying in the three Haleakalā Crater cabins do not collect silverswords for fuel, but HNP should inform visitors about the significance of withered piles of rosettes as potential microsites for seed germination, as well as their crucial role in enriching the meager volcanic ash soils with a range of nutrients and substantially increasing their low water-holding capacity [100]. In essence, the National Park Service should make visitors aware that all life stages of the silversword need to be protected [58].
Unchecked human refuse and trash do not seem to be a serious problem in the crater; however, as there are no bathroom facilities other than near the three cabins, hikers crossing this vast, 42 km2 depression must resort to using secluded locations behind dense shrubs of Dubautia menziesii or large boulders, as convenient toilet areas, where substrates and plant seedlings are heavily trampled, and rare mossball specimens are regularly damaged and destroyed. Some areas with dense silversword populations are probably similarly affected [12,101]. I have always avoided sampling soils and/or plant locations ≤3 m away from trails, as research elsewhere reveals that soils on restricted spots in heavily visited parks contain anomalously high levels of phosphorus, likely caused by contamination with human and/or dog urine [161]. Unfortunately, this is a problem without an obvious, easy solution.
In recent years, experimental ecological outplantings of silverswords have been initiated by University of Hawai’i researchers in the crater [35,145,162] to monitor the spatial patterns of rosette mortality and survival. It would be reasonable to expect that, if at some point in the future, it is determined that Haleakalā silverswords can benefit from outplantings, the areas selected for these are associated primarily with the tephra substrates most likely to allow rosette survival and prime growth. Hikers should also be informed about the crucial roles of such substrates and be discouraged from crossing or walking on them.
6. Conclusions: Influences of Different Microsite Types on Silversword Survival
This research project has specifically focused on the thermal characteristics of different microsites (Figure 7), already described in detail; in addition, a brief synopsis comparing the additional presumed roles that different nurse substrates may have on silversword growth and distribution reveals the following relevant ecological aspects:
- (i)
- Bare soils: These substrates seem the least adequate for rosette growth and survival. (a) Due to their overall surface uniformity and lack of rugosity, most soils have a limited ability to intercept incoming fog and thus do not receive much additional water beyond that provided by direct rainfall [1,11,12,95]; (b) owing to their high temperatures, exposed soils experience exceedingly high evaporation rates and subsequent drought, which may cause rapid plant wilting and/or death [11,12,41,95,97,113,114].
- (ii)
- Black tephra covers: In contrast to exposed soils, the rough, irregular tephra surfaces may help intercept much fog and drizzle, especially when facing the incoming trade winds; this would significantly increase the soil water content (see Section 5.5). (a) However, due to their high temperatures, substrates under black tephra fragments would still experience high evaporation rates, although these would be somewhat restrained by the clastic cover [95,97].
- (iii)
- Reddish tephra covers. These substrates undoubtedly provide the best locations for rosette survival, and areas with reddish fragments may achieve the highest silversword densities in the crater (see Section 4.2, and Figure 15). (a) The lower temperatures under reddish clasts would be associated with low evaporation rates [95]. (b) Like the black tephra, their uneven surface would allow significant fog interception and subsequent soil water storage.
- (iv)
- Shade of rosettes. (a) Due to the remarkably low temperatures in these substrates, evaporation rates would also be modest. These characteristics should presumably have made these locations conducive to satisfactory seed germination and survival; yet, it was not immediately clear why silverswords did not take advantage of such favorable substrates. (b) Plant competition for environmental resources—such as incoming light and soil water—might provide an explanation for this apparent inconsistency [8,25,29].
- (v)
- Silversword litter. (a) The low temperatures in soil patches covered by litter mounds would allow evaporation rates from insulated soils to remain low. (b) The long-term persistence of these organic matter piles, which can seemingly last 15 or 20 years before decomposing and disappearing, would make them stable, semi-permanent elements of the environment and desirable silversword locations. (c) However, the relative scarcity of these microsites in some plots limits their usefulness. (d) Seedling growth on litter mounds could also be restricted by their substantial thickness, which may reach 19.1 cm or perhaps a greater depth (Table 1), and the high water-retention capacity of litter limits effective water percolation to the soil beneath [95,97]. In any case, silversword seeds landing near the thinner edges of litter piles have a greater chance of survival and seem to be more common there (Figure 21). (e) A highly positive aspect of litter mounds is that decomposing rosettes gradually release comparatively large amounts of nutrients, including N, Mg, K, and P—normally in short supply in volcanic soils—raising their levels ~4 to 6 times in the soil, right next to seedlings, where these can readily benefit from such soil enrichment [100].
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in this article; further inquiries can be directed to the corresponding author.
Acknowledgments
I sincerely thank all personnel at Haleakalā National Park headquarters for their vital cooperation over three decades: former Park Superintendent Donald W. Reeser, Elizabeth Gordon, Ronald J. Nagata, and Patti Welton (Park Botanist) kindly provided annual research permits and much valuable information. I also appreciate the help of Lloyd L. Loope (1943–2017) (Research Scientist, USGS, Haleakalā Field Station, Maui) for advising me on research plans and for his essential support. I greatly value the expert help in the field of Alejandro G. Pérez-Bergquist and Martha M. Kowalak-Pérez; she also provided many editorial contributions and much cheery encouragement. She is my real nurse rock. The College of Liberal Arts (COLA), the University of Texas at Austin, contributed funding to my fieldwork, as well as three Special Research Grants toward the purchase of equipment. I am greatly indebted to Herbert Kumeo Kobayashi, exemplary trailblazer of ecological research in Haleakalā Crater and its silversword, for unwittingly inspiring me to follow in his footsteps. I want to express my gratitude to José Cuatrecasas Arumí (1903–1996) for transmitting to me, decades ago, his passion and obsession for tropical alpine rosettes. Muchas gracias por todo ‘lo nuestro’, José. I kindly thank two anonymous referees for their thoughtful, useful, methodical reviews of this manuscript. My sincere appreciation goes to all, but any study shortcomings are solely my fault.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Kobayashi, H.K. Ecology of the Silversword, Argyroxiphium sandwicense DC. (Compositae), Haleakala Crater, Hawaii. Ph.D. Dissertation, University of Hawaii, Manoa, HI, USA, 1973; p. 91. [Google Scholar]
- Weberbauer, A. Die Pflanzenwelt der peruanischen Anden. In Die Vegetation der Erde; Engler, A., Drude, O., Eds.; Wilhelm Engelmann: Leipzig, Germany, 1911; Volume 12, pp. 1–355. [Google Scholar]
- Weberbauer, A. El Mundo Vegetal de los Andes Peruanos. Estudio Fitogeográfico; Lumen, Ed.; Ministerio de Agricultura: Lima, Peru, 1945. [Google Scholar]
- Hedberg, O. Features of Afro-alpine plant ecology. Acta Phytogeogr. Suec. 1964, 49, 1–144. [Google Scholar]
- Parker, K.C. Site-related demographic patterns of organ pipe cactus populations in Southern Arizona. Bull. Torrey Bot. Club 1987, 114, 149–155. [Google Scholar] [CrossRef]
- Parker, K.C. Nurse plant relationships of columnar cacti. Phys. Geogr. 1989, 10, 322–335. [Google Scholar] [CrossRef]
- Flores, J.; Jurado, E. Are nurse-protégé interactions more common among plants from arid environments? J. Veg. Sci. 2003, 14, 911–916. [Google Scholar] [CrossRef]
- Munguia-Rosas, M.A.; Sosa, V.J. Nurse plants vs. nurse objects: Effects of woody plants and rocky cavities on the recruitment of the Pilosocereus leucocephalus columnar cactus. Ann. Bot. 2008, 101, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Peterson, C.R.; Arnold, S.J.; Porter, W.P. Hot rocks and not-so-hot rocks: Retreat-site selection by garter snakes and its thermal consequences. Ecology 1989, 70, 931–944. [Google Scholar] [CrossRef]
- Sedlacek, J. Demography and inbreeding depression in the rare, endemic Echium wildpretii (Boraginaceae). Msc. Thesis, Institute of Environmental Sciences, University of Zurich, Zurich, Switzerland, 2009; p. 38. [Google Scholar]
- Pérez, F.L. Phytogeomorphic influence of stone covers and boulders on plant distribution and slope processes in high-mountain areas. Geogr. Compass 2009, 3, 1774–1803. [Google Scholar] [CrossRef]
- Pérez, F.L. Hot soils and cooler stones: Geoecological influence of volcanic tephra and boulders on soil temperature, and significance for plant distribution in Haleakalā Crater (Maui, Hawaii). Catena 2017, 158, 9–19. [Google Scholar] [CrossRef]
- Noy-Meir, I. Ecology of wild emmer wheat in Mediterranean grasslands in Galilee. Israel. J. Plant Sci. 2001, 49, 43–52. [Google Scholar] [CrossRef]
- Fujita, T.; Mizuno, K. Role of nurse rocks on woody plant establishment in a South African grassland. Tropics 2015, 24, 57–64. [Google Scholar] [CrossRef]
- Conver, J.L.; Yarwood, E.; Hetherington, L.D.; Swann, D.E. Nurse rock microclimates significantly buffer exposure to freezing temperature and moderate summer temperature. J. Arid. Environ. 2020, 177, 104140. [Google Scholar] [CrossRef]
- Díazgranados, M. A nomenclator for the frailejones (Espeletiinae Cuatrec., Asteraceae). Phytokeys 2012, 16, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.L. Long-term growth rates of two acaulescent rosette species, Coespeletia timotensis (Cuatrec.) Cuatrec. and Espeletia schultzii Wedd., in an Andean páramo. Flora 2019, 252, 1–9. [Google Scholar] [CrossRef]
- Carlucci, M.B.; Duarte, L.; Pillar, V.D. Nurse rocks influence forest expansion over native grassland in southern Brazil. J. Veg. Sci. 2011, 22, 111–119. [Google Scholar] [CrossRef]
- Nobel, P.S. Environmental Biology of Agaves and Cacti; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Franco, A.C.; Nobel, P. Effect of nurse plants on the microhabitat and growth of cacti. J. Ecol. 1989, 77, 870–886. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Ezcurra, E. Shade as a cause of the association between the cactus Neobuxbamia tetetzo and the nurse plant Mimosa luisana in the Tehuacán Valley, Mexico. J. Ecol. 1991, 79, 961–971. [Google Scholar] [CrossRef]
- Nobel, P.S.; Miller, P.M.; Graham, E.A. Influence of rocks on soil temperature, soil water potential, and rooting patterns for desert succulents. Oecology 1992, 92, 90–96. [Google Scholar] [CrossRef]
- Reyes-Olivas, A.; García, E.; López, L. Cacti-shrub interactions in the coastal desert of northern Sinaloa Mexico. J. Arid. Environ. 2002, 52, 431–445. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Gómez, J.; Zamora, R.; Boettinger, J.L. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J. Veg. Sci. 2005, 16, 191–198. [Google Scholar]
- Pérez, F.L. Needle-ice activity and the distribution of stem-rosette species in a Venezuelan paramo. Arct. Alp. Res. 1987, 19, 135–153. [Google Scholar]
- Kleier, C.; Rundel, P. Microsite requirements, population structure and growth of the cushion plant, Azorella compacta, in the tropical Chilean Andes. Austral Ecol. 2004, 29, 461–470. [Google Scholar] [CrossRef]
- Kleier, C.; Rundel, P. Energy balance and temperature relations of Azorella compacta, a high-elevation cushion plant of the central Andes. Plant Biol. 2009, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kleier, C.; Lambrinos, J.G. The importance of nurse associations for three tropical alpine life forms. Arct. Antarct. Alp. Res. 2005, 37, 331–336. [Google Scholar] [CrossRef]
- Peters, E.M.; Martorell, C.; Ezcurra, E. Nurse rocks are more important than nurse plants in determining the distribution and establishment of globose cacti (Mammillaria) in the Tehuacán Valley, Mexico. J. Arid. Environ. 2008, 72, 593–601. [Google Scholar] [CrossRef]
- Haussmann, N.S.; McGeoch, M.A.; Boelhouwers, J.C. Contrasting nurse plants and nurse rocks: The spatial distribution of seedlings of two sub-Antarctic species. Acta. Oecol. 2010, 36, 299–305. [Google Scholar] [CrossRef]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Manual of the Flowering Plants of Hawaii. In B.P. Bishop Museum Special Publication 83; University Hawaii Press: Honolulu, HI, USA, 1990; Volume 2. [Google Scholar]
- Rundel, P.W.; Witter, M.S. Population dynamics and flowering in a Hawaiian alpine rosette plant, Argyroxiphium sandwicense. In Tropical Alpine Environments; Rundel, P.W., Smith, A.P., Meinzer, F.C., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 295–306. [Google Scholar]
- Alexander, W.D. On the crater of Haleakala, island of Maui, Hawaiian group. Am. J. Sci. Arts 1870, 49, 43–48. [Google Scholar] [CrossRef]
- Bird, I.L. The Hawaiian Archipelago: Six Months Among the Palm Groves, Coral Reefs, and Volcanoes of the Sandwich Islands; John Murray: London, UK, 1875. [Google Scholar]
- Pérez, F.L. Early uses by ancient Hawaiians, and environmental, geographical, and ecological history of Haleakalā Crater, East Maui. Geographies 2024, 4, 378–410. [Google Scholar] [CrossRef]
- Yocom, C.F. Ecology of feral goats in Haleakala National Park, Maui, Hawaii. Am. Midl. Nat. 1967, 77, 418–451. [Google Scholar] [CrossRef]
- Loope, L.L.; Medeiros, A.C. Biotic interactions in Hawaiian high elevation ecosystems. In Tropical Alpine Environments; Rundel, P.W., Smith, A.P., Meinzer, F.C., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 337–354. [Google Scholar]
- Bruegmann, M.M. Protecting habitat for silversword recovery. Endanger. Species Bull. 1995, 20, 6–7. [Google Scholar]
- Pérez, F.L. Spatial aggregation patterns and population structure of the Haleakalā silversword (Argyroxiphium sandwicense DC. subsp. macrocephalum), Maui, Hawaii. Phys. Geogr. 2014, 36, 34–59. [Google Scholar] [CrossRef]
- Krushelnycky, P.D.; Loope, L.L.; Giambelluca, T.W.; Starr, F.; Starr, K.; Drake, D.R.; Taylor, A.D.; Robichaux, R.H. Climate-associated population declines reverse recovery and threaten future of an iconic high-elevation plant. Glob. Change Biol. 2013, 19, 911–922. [Google Scholar] [CrossRef]
- Krushelnycky, P.D.; Felts, J.M.; Robichaux, R.H.; Barton, K.E.; Litton, C.M.; Brown, M.D. Clinal variation in drought resistance shapes past population declines and future management of a threatened plant. Ecol. Monogr. 2020, 90, e301398. [Google Scholar] [CrossRef]
- U. S. Fish and Wildlife Service. Final listing rules for 53 species, 1992. Endanger. Species Technol. Bull. 1992, 17, 15. [Google Scholar]
- Wilkes, C. Narrative of the United States Exploring Expedition During the Years 1838, 1839, 1840, 1841, 1842; Lea & Blanchard: Philadelphia, PA, USA, 1845; Volume 4. [Google Scholar]
- Wentworth, C.K.; MacDonald, G.A. Structures and forms of basaltic rocks in Hawaii. United States Geol. Surv. Bull. 1953, 994, 1–98. [Google Scholar]
- MacDonald, G.A. Forms and structures of extrusive basalt rocks. In Basalts: The Poldervaart Treatise on Rocks of Basaltic Composition; Hess, H.H., Poldervaart, A., Eds.; Wiley Interscience: New York, NY, USA, 1967; Volume 1, pp. 1–62. [Google Scholar]
- Schwertmann, U. Relations Between Iron Oxides, Soil Color, and Soil Formation. In Soil Color; Bigham, J.M., Ciolkosz, E.J., Eds.; Soil Science Society of America Special Publications: Madison, WI, USA, 1993; Volume 31, pp. 51–69. [Google Scholar]
- Post, D.F.; Fimbres, A.; Matthias, A.D.; Sano, E.E.; Accioly, L.; Batchily, A.K.; Ferreira, L.G. Predicting soil albedo from soil color and spectral reflectance data. Soil Sci. Soc. Am. J. 2000, 64, 1027–1034. [Google Scholar] [CrossRef]
- Bigham, J.M.; Golden, D.C.; Buol, S.W.; Weed, S.B.; Bowen, L.H. Iron oxide mineralogy of well drained Ultisols and Oxisols: II. Influence on color, surface area, and phosphate retention. Soil Sci. Soc. Am. J. 1978, 42, 825–830. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U. Influence of hematite on the color of red beds. J. Sediment. Petrol. 1987, 57, 682–686. [Google Scholar]
- USA Soil Survey Geographic Database (SSURGO). Soil Albedo; USDA, Dept. of Agriculture, Natural Resources Conservation Service, 2017. Available online: https://www.arcgis.com/home/item.html?id=e89fdc8e8b13417daa5ad232312f58cf#:~:text=This%20layer%20displays%20the%20ratio,and%20reflected%20by%20the%20soil (accessed on 14 November 2024).
- Munsell Soil Color Charts; Macbeth, Kollmorgen Instruments: Newburgh, NY, USA, 1992.
- Thompson, J.A.; Pollio, A.R.; Turk, P.J. Comparison of Munsell soil color charts and the GLOBE soil color book. Soil Sci. Soc. Am. J. 2013, 77, 2089–2093. [Google Scholar] [CrossRef]
- Graser, E.A.; van Bavel, C.H.M. The effect of soil moisture upon soil albedo. Agric. Meteorol. 1982, 27, 17–26. [Google Scholar] [CrossRef]
- Sugathan, N.; Biju, V.; Renuka, G. Influence of soil moisture content on surface albedo and soil thermal parameters at a tropical station. J. Earth Syst. 2014, 125, 1115–1128. [Google Scholar] [CrossRef]
- Cierniewski, J.; Ceglarek, J.; Karnieli, A.; Królewicz, S.; Kazmierowski, C.; Zagajewski, B. Predicting the diurnal blue-sky albedo of soils using their laboratory reflectance spectra and roughness indices. J. Quant. Spectrosc. Radiance Transf. 2017, 200, 25–31. [Google Scholar] [CrossRef]
- Siegel, S.M.; Carroll, P.; Corn, C.; Speitel, T. Experimental studies on the Hawaiian silverswords (Argyroxiphium spp.): Some preliminary notes on germination. Bot. Gaz. 1970, 131, 277–280. [Google Scholar] [CrossRef]
- Walker, L.R.; Powell, E.A. Factors affecting seed germination of the Mauna Kea silversword in Hawaii. Pac. Sci. 1995, 49, 205–211. [Google Scholar]
- Walker, L.R.; Powell, E.A. Regeneration of the Mauna Kea silversword Argyroxiphium sandwicense (Asteraceae) in Hawaii. Biol. Conserv. 1999, 89, 61–70. [Google Scholar] [CrossRef]
- Macdonald, G.A. Geologic Map of the Crater Section of Haleakala National Park, Maui, Hawaii, U.S. Geological Survey Miscellaneous Investigations Series Map I-1088 (s1:24,000), with Report: Summary of the Geology of Haleakala. 1–8 pp + map. 1978. Available online: https://www.usgs.gov/maps/geologic-map-crater-section-haleakala-national-park-maui-hawaii (accessed on 12 May 2015).
- Jury, W.A.; Bellantuoni, B. Heat and water movement under surface rocks in a field soil: I Thermal effects. Soil Sci. Soc. Am. J. 1976, 40, 505–509. [Google Scholar] [CrossRef]
- ASTM. Manual on the Use of Thermocouples in Temperature Measurement, 4th ed.; ASTM International Manual Series: MNL 12, Revision of Special Technical Publication 470B; ASTM: Baltimore, MD, USA, 1993. [Google Scholar]
- GSA. Rock-Color Chart, 7th ed.; Geological Society of America: Boulder, CO, USA, 1991. [Google Scholar]
- Munsell Color Charts for Plant Tissues; Macbeth, Kollmorgen Instruments: Baltimore, MD, USA, 1997.
- Hoaglin, D.C.; Mosteller, F.; Tukey, J.W. Understanding Robust and Exploratory Data Analysis; Wiley: New York, NY, USA, 1983. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Boston, MA, USA, 1977. [Google Scholar]
- Frigge, M.; Hoaglin, D.G.; Iglewicz, B. Some implementations of the Boxplot. Am. Stat. 1989, 43, 50–54. [Google Scholar] [CrossRef]
- Brillinger, D.R. Data Analysis, Exploratory; SAGE Publications: Statistics Department University of California Berkeley: Berkeley, CA, USA, 2011; pp. 530–537. Available online: https://stat.berkeley.edu (accessed on 16 July 2024).
- Iglewicz, B.; Hoaglin, D.G. Use of boxplots for process evaluation. J. Qual. Technol. 1987, 19, 180–190. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; John, W. Tukey and data analysis. Stat. Sci. 2003, 18, 311–318. [Google Scholar] [CrossRef]
- Reio, T.G.; Shuck, B. Exploratory Factor Analysis: Implications for theory, research, and practice. Adv. Dev. Hum. Resour. 2015, 17, 12–25. [Google Scholar] [CrossRef]
- Hu, K. Become competent within one day in generating boxplots and violin plots for a novice without prior R experience. Methods Protoc. 2020, 3, 64. [Google Scholar] [CrossRef]
- McGill, R.; Tukey, J.W.; Larsen, W.A. Variations of box plots. Am. Stat. 1978, 32, 12–16. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Visualizing samples with box plots. Nat. Methods 2014, 11, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.F.; Parker, R.A.; Kendricks, J.S. The box plot: A simple visual method to interpret data. Ann. Intern. Med. 1989, 110, 916–992. [Google Scholar] [CrossRef]
- Thirumalai, C.; Manickan, V.; Balaji, R. Data Analysis Using Box and Whisker Plot for Lung Cancer, Proceedings of the IEEE International Conference on Innovations in Power and Advancing Computing Technologies [i-PACT2017], Vellore, India, 21–22 April 2017; Institute of Electrical and Electronics Engineers: Piscataway Township, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Willmott, C.J.; Robeson, S.C.; Matsuura, K. Geographic box plots. Phys. Geogr. 2007, 28, 331–344. [Google Scholar] [CrossRef][Green Version]
- Perkins, R.B.; Mason, C.E. The relative mobility of trace elements from short-term weathering of a black shale. Appl. Geochem. 2015, 56, 67–79. [Google Scholar] [CrossRef]
- Pérez, F.L. Cryptogams build up a living microcosm: Geoecological effects of biocrusts on volcanic tephra (Haleakalā, Maui, Hawaii). Catena 2021, 203, 105320. [Google Scholar] [CrossRef]
- Choi, C.h.-Y.; Battley, P.F.; Potter, M.A.; Ma, Z.; Liu, W. Factors affecting the distribution patterns of benthic invertebrates at a major shorebird staging site in the Yellow Sea, China. Wetlands 2014, 34, 1085–1096. [Google Scholar] [CrossRef]
- Joshi, R.S.; Rigau, M.; García-Prieto, C.A.; de Moura, M.C.; Piñeyro, D.; Moran, S.; Davalos, V.; Carrión, P.; Ferrando-Bernal, M.; Olalde, I.; et al. Look-alike humans identified by facial recognition algorithms show genetic similarities. Cell Rep. 2022, 40, 111257. [Google Scholar] [CrossRef]
- Spitzer, M.; Wildenhain, J.; Rallsilber, J.; Tyers, M. BoxplotR: A web tool for generation of box plots. Nat. Methods 2014, 11, 121–122. [Google Scholar] [CrossRef]
- Iglewicz, B.; Hoaglin, D.G. How to Detect and Handle Outliers; American Association for Quality Control, Statistics Division: Milwaukee, IL, USA, 1993; Volume 16. [Google Scholar]
- Miller, R.L.; Kahn, J.S. Statistical Analysis in the Geological Sciences; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Stearns, H.T. Origin of the Haleakala crater, island of Maui, Hawaii. Geol. Soc. Am. Bull. 1942, 53, 1–14. [Google Scholar] [CrossRef]
- Macdonald, G.A.; Abbott, A.T.; Peterson, A.T. Volcanoes in the Sea. The Geology of Hawaii; University Hawaii Press: Honolulu, HI, USA, 1983. [Google Scholar]
- Sherrod, D.R.; Nishimitsu, Y.; Tagami, T. New K-Ar ages and the geologic evidence against rejuvenated-stage volcanism at Haleakalā, East Maui, a postshield volcano of the Hawaiian island chain. GSA Bull. 2003, 115, 683–694. [Google Scholar] [CrossRef]
- Sherrod, D.R.; Sinton, J.M.; Watkins, S.E.; Brunt, K.M. Geologic Map of the State of Hawaii. U.S. Geological Survey Scientific Investigations Map 3143. pamphlet 72 p., 5 sheets, scales 1:100,000 and 1:250,000. 2021. Available online: https://doi.org/10.3133/sim3143 (accessed on 22 April 2022).
- Pérez, F.L. Growth of Grimmia mosses on volcanic tephra: Geoecological processes of biocrust development in Haleakalā Crater (Maui, Hawaii). Catena 2020, 195, 104911. [Google Scholar] [CrossRef]
- USGS. The Age of Young Lava Flows on Haleakala’s Crater Floor, East Maui Volcano. USGS Hawaii Volcano Observatory. 2003. Available online: www.accuracyingenesis.com/haleak.html (accessed on 20 May 2020).
- Sherrod, D.R.; Hagstrum, J.T.; McGeehin, J.P.; Champion, D.E.; Trusdell, F.A. Distribution, 14C chronology, and paleomagnetism of latest Pleistocene and Holocene lava flows at Haleakalā volcano, Island of Maui, Hawaii: A revision of lava flow hazard zones. J. Geophys. Res. 2006, 111, B05205. [Google Scholar] [CrossRef]
- Pérez, F.L. Biogeomorphic influence of soil depth to bedrock, volcanic-ash soils, and surface tephra on silversword distribution, Haleakalā Crater (Maui, Hawaii). Geomorphology 2015, 243, 75–86. [Google Scholar] [CrossRef]
- Blumenstock, D.I.; Price, S. Climate of Hawaii. In Climates of the States; N° 60-51; Climatography of the United States, United States, US Department of Commerce: Washington, DC, USA, 1967; pp. 1–27. [Google Scholar]
- Giambelluca, T.W.; Nullet, D. Influence of the trade-wind inversion on the climate of a leeward mountain slope in Hawaii. Clim. Res. 1991, 1, 207–216. [Google Scholar] [CrossRef]
- Whiteaker, L.D. The vegetation and environment in the Crater District of Haleakala National Park. Pac. Sci. 1983, 37, 1–24. [Google Scholar]
- Pérez, F.L. The influence of surface volcaniclastic layers from Haleakala (Maui, Hawaii) on soil water conservation. Catena 2000, 38, 301–332. [Google Scholar] [CrossRef]
- Giambelluca, T.W.; Chen, Q.; Frazier, A.G.; Price, J.P.; Chen, Y.-L.; Chu, P.-S.; Eischeid, J.K.; Delparte, D.M. Online Rainfall Atlas of Hawaii. Bull. Am. Meteorol. Soc. 2013, 94, 313–316. [Google Scholar] [CrossRef]
- Pérez, F.L. The role of tephra covers on soil moisture conservation at Haleakala’s crater (Maui, Hawaii). Catena 2009, 76, 191–205. [Google Scholar] [CrossRef]
- Goldstein, G.; Drake, D.R.; Melcher, P.; Giambelluca, T.W.; Heraux, J. Photosynthetic gas exchange and temperature induced damage in seedlings of the tropical alpine species Argyroxiphium sandwicense. Oecologia 1996, 106, 298–307. [Google Scholar] [CrossRef]
- Leuschner, C.; Schulte, M. Microclimatological investigations in the tropical alpine scrub of Maui, Hawaii: Evidence for a drought-induced alpine timberline. Pacific Sci. 1991, 45, 152–168. [Google Scholar]
- Pérez, F.L. Geoecological alteration of surface soils by the Hawaiian silversword (Argyroxiphium sandwicense DC.) in Haleakala’s Crater, Maui. Plant Ecol. 2001, 157, 215–233. [Google Scholar] [CrossRef]
- Pérez, F.L. Biogeomorphic relationships between slope processes and globular Grimmia mosses in Haleakala’s Crater (Maui, Hawaii). Geomorphology 2010, 116, 218–235. [Google Scholar] [CrossRef]
- Pérez, F.L. Influence of substrate on the distribution of the Hawaiian silversword (Argyroxiphium sandwicense DC.) in Haleakala (Maui, HI). Geomorphology 2003, 55, 173–202. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Pérez, F.L. Plant organic matter really matters: Pedological effects of kūpaoa (Dubautia menziesii) shrubs in a volcanic alpine area, Maui, Hawaii. Soil Syst. 2019, 3, 31. [Google Scholar] [CrossRef]
- Loope, L.L.; Crivellone, C.F. Status of the Haleakala Silversword: Past and Present; Technical Report; Cooperative National Park Resources Studies Unit, Hawai’i, CNPRSU-58 University of Hawaii: Honolulu, HI, USA, 1986. [Google Scholar]
- Hendrix, L.B. Post-eruption succession on Isla Fernandina, Galápagos. Madroño 1981, 28, 242–254. [Google Scholar]
- Juvik, J.O.; Perreira, D.J. Fog interception on Mauna Loa, Hawaii. Proc. Assoc. Am. Geogr. 1974, 6, 22–25. [Google Scholar]
- Pérez, F.L. Steady as a rock: Biogeomorphic influence of nurse rocks and slope processes on kūpaoa (Dubautia menziesii) shrubs in Haleakalā Crater (Maui, Hawaii). Geomorphology 2017, 295, 631–644. [Google Scholar] [CrossRef]
- Yair, A.; Lavee, H. Areal contribution to runoff on scree slopes in an extreme arid environment. A simulated rainstorm experiment. Zeitschr. Für Geomorph. Suppl. 1974, 21, 106–121. [Google Scholar]
- Yair, A.; Lavee, H. Runoff generative process and runoff yield from arid talus mantled slopes. Earth Surf. Proc. 1976, 1, 235–247. [Google Scholar] [CrossRef]
- Poesen, J.W.; Lavee, H. Rock Fragments in Soils: Surface Dynamics. Catena 1994, 23, 1–198. [Google Scholar] [CrossRef]
- Poesen, J.W.; Bunte, K. The effects of rock fragments on desertification processes in Mediterranean environments. In Mediterranean Desertification and Land Use; Brandt, C.J., Thornes, J.B., Eds.; Wiley: London, UK, 1996; pp. 247–269. [Google Scholar]
- Poesen, J.W.; Lavee, H. Rock fragments in top soils: Significance and processes. Catena 1994, 23, 1–28. [Google Scholar] [CrossRef]
- Pérez, F.L. Conservation of soil moisture by different stone covers on alpine talus (Lassen, California). Catena 1998, 33, 155–177. [Google Scholar] [CrossRef]
- Pérez, F.L. Soil moisture and the distribution of giant Andean rosettes on talus slopes of a desert paramo. Clim. Res. 1991, 1, 217–231. [Google Scholar] [CrossRef]
- Del Moral, R.; Bliss, L.C. Mechanisms of primary succession: Insights resulting from the eruption of Mount St. Helens. Adv. Ecol. Res. 1993, 24, 1–66. [Google Scholar]
- Resler, L.M. Geomorphic controls of spatial pattern and process at alpine treeline. Prof. Geogr. 2006, 58, 124–138. [Google Scholar] [CrossRef]
- Titus, J.H.; del Moral, R. Seedling establishment in different microsites of Mount St. Helens, Washington, USA. Plant Ecol. 1998, 134, 13–26. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. Mycorrhizal status of two Hawaiian plant species (Asteraceae) in a tropical alpine habitat: The threatened Haleakalā silversword (Argyroxiphium sandwicense subsp. macrocephallum) and the endemic Dubautia menziesii. Pac. Sci. 2002, 56, 423–430. [Google Scholar]
- Gemma, J.N.; Koske, R.E. Mycorrhizae in recent volcanic substrates in Hawaii. Am. J. Bot. 1990, 77, 1193–1200. [Google Scholar] [CrossRef]
- Othieno, C.O.; Ahn, P.M. Effects of mulches on soil temperature and growth of tea plants in Kenya. Exp. Agric. 1980, 16, 287–294. [Google Scholar] [CrossRef]
- Kemper, W.D.; Nicks, A.D.; Corey, A.T. Accumulation of water in soils under gravel and sand mulches. Soil Sci. Soc. Am. J. 1994, 58, 56–63. [Google Scholar] [CrossRef]
- Smith, A.P. Population dynamics and life forms of Espeletia in the Venezuelan Andes. Ph.D. Dissertation, Duke University, Durham, NC, USA, 1974; p. 240. [Google Scholar]
- Smith, A.P. Population dynamics of Venezuelan Espeletia. Smithson. Contrib. Bot. 1981, 48, 1–45. [Google Scholar] [CrossRef]
- Pérez, F.L. Some effects of giant Andean stem-rosettes on ground microclimate, and their ecological significance. Int. J. Biometeorol. 1989, 33, 131–135. [Google Scholar] [CrossRef]
- Coe, M.J. The ecology of the alpine zone of Mount Kenya. Monogr. Biol. 1967, 17, 1–136. [Google Scholar]
- Goldstein, G.; Meinzer, F.C.; Rada, F. Environmental biology of a tropical treeline species, Polylepis sericea. In Tropical Alpine Environments; Rundel, P.W., Smith, A.P., Meinzer, F.C., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 129–149. [Google Scholar]
- Salt, G. A contribution to the ecology of upper Kilimanjaro. J. Ecol. 1964, 42, 375–423. [Google Scholar] [CrossRef]
- Pérez, F.L. Geobotanical influence of talus movement on the distribution of caulescent Andean rosettes. Flora 1994, 189, 353–371. [Google Scholar] [CrossRef]
- Forsyth, S.A. Demographic Modeling of Hawaiian Silverswords, and Its Implications for Conservation. Ph.D. Dissertation, University of Arizona, Tucson, AZ, USA, 2002; p. 240. [Google Scholar]
- Hess, S.C. Wild sheep and deer in Hawaii—A threat to fragile ecosystems. USGS Facts Sheet 2008, 2008–3102, 1–4. [Google Scholar]
- Cuatrecasas, J.; Torres-Barreto. Páramos; Villegas Editores: Bogotá Colombia, 1988. [Google Scholar]
- Pérez, F.L. Unusual stem branching in a caulescent Andean rosette. Flora 1991, 185, 385–390. [Google Scholar] [CrossRef]
- Cuatrecasas, J. A Systematic Study of the Subtribe Espeletiinae (Heliantheae, Asteraceae); Memoirs New York Botanical Garden: 107, 1-689; New York Botanical Garden Press: New York, NY, USA, 2013. [Google Scholar]
- Powell, E. Recovery Plan for the Mauna Kea Silversword (Argyroxiphium sandwicense DC. ssp. sandwicense); U.S. Fish and Wildlife Service, Pacific Region: Portland, OR, USA, 1994; pp. 1–44. [Google Scholar]
- Carr, G.D.; Meyrat, A. The status of the Mauna Kea silversword. In Proceedings of the 4th Conference in Natural Sciences, Hawaii Volcanoes National Park, Hawaii, HI, USA, 2–4 June 1982; University of Hawaii at Manoa, Deptartment of Botany: Honolulu, HI, USA, 1982; pp. 34–39. [Google Scholar]
- Hawaiian Silversword Alliance, UH Botany. Argyroxiphium sandwicence DC. ssp. sandwicense. Available online: https://www.manoa.hawaii.edu/lifesciences/faculty/carr/ass.htm (accessed on 1 December 2024).
- Alexander, W.D. The ascent of Mauna Kea, Hawaii. Friend 1892, 50, 74–75. [Google Scholar]
- Maly, K. Mauna Kea, Ka Piko Kaulana o Ka ʻĀina. Mauna Kea, The Famous Summit of the Land. A Collection of Native Traditions, Historical Accounts, and Oral History Interviews for: Mauna Kea, the Lands of Kaʻohe, Humuʻula and the ʻĀina Mauna on the Island of Hawaii; Kumu Pono Associates LLC: Hawaii, HI, USA, 2005. [Google Scholar]
- Sturm, H.; Rangel, O. Ecología de Los Páramos Andinos. Una Visión Preliminar Integrada; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, DE, Colombia, 1985. [Google Scholar]
- Carr, G.D.; Powell, E.A.; Kyhos, D.W. Self-incompatibility in the Hawaiian Madiinae (Compositae): An exception to Baker’s rule. Evolution 1986, 40, 430–434. [Google Scholar]
- Guisado-Chávez, J.F. Reproductive Ecology of Dubautia menziesii (the Haleakalā Kūpaoa) and Its Interaction with Pollinators in Haleakalā. M.S. Thesis, University of Hawaii, Manoa, HI, USA, 2024; p. 27. [Google Scholar]
- Forsyth, S.A. Density-dependent seed set in the Haleakala silversword: Evidence for an Allee effect. Oecologia 2003, 136, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.K. Preliminary investigations on insects affecting the reproductive stage of the silversword (Argyroxiphium sandwicense DC.) Compositae, Haleakala Crater, Maui, Hawaii. Proc. Hawaii. Entomol. Soc. 1974, 21, 397–402. [Google Scholar]
- Krushelnycky, P.D. Evaluating the interacting influences of pollination, seed predation, invasive species and isolation on reproductive success in a threatened alpine plant. PLoS ONE 2014, 9, e88948. [Google Scholar] [CrossRef] [PubMed]
- Cole, F.R.; Medeiros, A.C.; Loope, L.L.; Zuehlke, W.W. Effects of the Argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology 1992, 73, 1313–1322. [Google Scholar] [CrossRef]
- Gambino, P.; Medeiros, A.C.; Loope, L.L. Introduced vespids Paravespula pensylvanica prey on Mauiʻs endemic arthropod fauna. J. Trop. Ecol. 1987, 3, 169–170. [Google Scholar] [CrossRef]
- Federal Register. Rules and Regulations. Dly. J. United States Gov. Natl. Arch. 1986, 51, 9818. [Google Scholar]
- Degener, O. Ferns and Flowering Plants of Hawaii National Park with Descriptions of Ancient Hawaiian Customs and an Introduction to the Geologic History of the Islands; Honolulu Star-Bulletin Ltd. Press: Honolulu, HI, USA, 1930; p. 312. [Google Scholar]
- Robichaux, R.H.; Friar, E.A.; Mount, D.W. Molecular genetic consequences of a population bottleneck associated with reintroduction of the Mauna Kea silversword (Argyroxiphium sandwicense subsp. Sandwicense [Asteraceae]). Conserv. Biol. 1997, 11, 1140–1146. [Google Scholar] [CrossRef]
- Kobayashi, H.K. Putative generic hybrids of Haleakala’s silversword and kupaoa (Argyroxiphium sandwicense x Dubautia menziesii) Compositae. Pac. Sci. 1973, 27, 207–208. [Google Scholar]
- Carr, G.D. A fully fertile intergeneric hybrid derivative from Argyroxiphium sandwicense ssp. macrocephalum x Dubautia menziesii (Asteraceae) and its relevance to plant evolution in the Hawaiian islands. Am. J. Bot. 1995, 82, 1574–1581. [Google Scholar] [CrossRef]
- Haleakala Visitation per Year. Nationalparked.com. 2024. Available online: https://www.nationalparked.com/haleakala/visitation-statistics (accessed on 22 April 2024).
- Missionary Herald. Ascent of an Extinguished Volcano. Vol. 8; The Missionary Herald: Boston, MA, USA, 1829; Volume 25, pp. 247–248. [Google Scholar]
- Twain, M. Roughing It; American Publishing Company: Chicago, IL, USA, 1872. [Google Scholar]
- Lyon, H.C. Visit to Haleakala. Paradise Pac. 1896, 9, 115. [Google Scholar]
- Bates, G.W. Sandwich Island Notes by a Häolé; Harper & Brothers: New York, NY, USA, 1854. [Google Scholar]
- Culliney, J.L. Islands in a Far Sea. Nature and Man in Hawaii; Sierra Club: San Francisco, CA, USA, 1988. [Google Scholar]
- Pérez, F.L. Human impact on the high páramo landscape of the Venezuelan Andes. In Nature’s Geography. New Lessons for Conservation in Developing Countries; Zimmerer, K.S., Young, K.R., Eds.; University of Wisconsin Press: Madison, WI, USA, 1998; pp. 147–183. [Google Scholar]
- Mahaney, W.C. Environmental impact in the Afroalpine and subalpine belts of Mount Kenya, East Africa. Mt. Res. Dev. 1986, 6, 247–260. [Google Scholar] [CrossRef]
- Pérez, F.L. Geoecology of a granite dome: Spatial interactions between gnammas, rills, soils, and plant cover, Enchanted Rock (Texas USA). Catena 2023, 223, 106938. [Google Scholar] [CrossRef]
- Krushelnycky, P.D.; Berio Fortini, L.; Mallinson, J.; Felts, J.M. Empirical estimation of habitat suitability for rare plant restoration in an era of ongoing climatic shifts. Sci. Rep. 2023, 13, 19257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).