Abstract
Background: Drug treatments for gait disorders in post-stroke patients aim to reduce muscular hyperactivity. The analysis of muscle activity is essential to help clinicians understand these disorders. This study aimed to quantify changes in muscle synergies before (PRE) and after (POST) a rectus femoris nerve block. Methods: Gait analysis of 8 post-stroke patients before and immediately after nerve block. Muscle synergies were quantified from electromyographic signals. We have selected the account for variance, which indicates the effectiveness of the synergies, the recruitment selectivity index, which indicates the degree of multiplexing of the synergies, and the recruitment consistency index, which indicates the variability of the synergy activations across gait cycles. Results: A decrease in Variance Account For (VAF) is observed, showing a lack of robustness of the underlying muscle synergies between PRE and POST. We also note that spatial and temporal primitives result in different Index of Recruitment Selectivity (IRS) but similar Index of Recruitment Consistency (IRC) for PRE vs. POST. This shows that the synergies’ activations remain consistent across cycles but are more distributed in POST than in PRE. Conclusions: The motor nerve block has not created new muscle synergies of the paretic limb during gait but indicates that there is flexibility in motor organization. This method of quantification may enable clinicians to assess the motor adaptation potential of their post-stroke patients.
1. Introduction
Muscle synergy analysis is becoming increasingly used to study gait patterns [1,2]. In accordance with Safavynia et al., muscle synergies represent a library of motor subtasks that the central nervous system can flexibly combine to produce complex and natural movements [3]. In fine, it is a group of muscles contracting together as part of a functional unit [4]. This method involves extracting muscle synergies from electromyography (EMG) signals and considers the muscle activity of the whole limb that underlies gait patterns, thereby simplifying the multidimensional analysis. Muscle synergies have been shown to be modified by different treatments (e.g., rehabilitation interventions, botulinum toxin type-A injections, and selective dorsal rhizotomy) in a range of pathologies (e.g., cerebral palsy, spinal cord injury, Parkinson’s disease) [4,5,6,7]. Nonetheless, some studies found mixed results. Indeed, Routson et al. found that 12 weeks of treadmill training with body weight support modified both the composition and timing of lower limb muscle synergies [2]. In contrast, Ambrosini et al. noted only minor changes in muscle synergies after 3 weeks of cycle training with functional electrical stimulation for the stroke participants [8].
The analysis of muscle synergy flexibility has rarely been studied in stroke patients. Moreover, the impact of an intervention that may modify muscle synergies or their activations immediately after remains largely unexplored. Gait disorders are a common sequel of stroke [9,10]. Although various gait deviations may occur, one gait pattern commonly observed after stroke is Stiff Knee Gait (SKG), which is defined by a reduction of peak knee flexion during the swing phase. This reduction of knee flexion has some consequences, among them decreased toe clearance, which compromises the stability of gait and increases the risk of falling [11]. Several mechanisms have been proposed as causes of SKG. For example, this includes an increase in forces generated by the vastii [12], a decrease of hip flexion moment [13], a decrease in ankle plantar flexion moment, or an over-activity of the rectus femoris muscle during the swing phase of gait [14,15]. Although SKG is often multifactorial, over-activity of the rectus femoris is frequently the predominant mechanism in stroke patients [16]. To help in the diagnosis of the main cause of stiff knee gait, anesthetic motor nerve block of the rectus femoris nerve, to temporarily stop nerve conduction and thus muscle activity, is commonly used to determine the role of this muscle in peak knee flexion alteration. The nerve block involves injecting an anesthetic around the rectus femoris nerve, reducing both sensory and motor activity for 30 to 60 min [17]. It has been shown that the anesthesic motor nerve block of the nerve branch of the rectus femoris improved peak knee flexion during the swing phase of the gait cycle when patients exhibited rectus femoris over-activity during the swing phase of the gait cycle [18]. However, the adaptability of the gait pattern of the other muscles involved during walking after such a nerve block is poorly studied. Indeed, to our knowledge, the extent to which the muscle synergies involved in the gait pattern of the paretic limb are modified by the decrease of rectus femoris muscle overactivity has never been studied.
The objective of this study is to quantify the robustness and variability of muscle synergies before and after a nerve block of the rectus femoris muscle in patients who have suffered a stroke and present a stiff-knee gait (SKG) due to hyperactivity of this muscle in the paretic lower limb during the swing phase of the gait cycle. To achieve this, muscle synergies were extracted before and after the nerve block and compared. We hypothesize that the robustness of muscle synergies is affected by a nerve block.
2. Materials and Methods
2.1. Participants
We recruited a convenience sample of eight adult patients with stroke who were attending a routine clinical consultation at the Raymond Poincaré University Hospital gait laboratory. Inclusion criteria were a diagnosis of stroke more than 12 months previously (ischaemic or haemorrhagic), the ability to walk 10 m barefoot with no walking aids, quadriceps spasticity of ≥1 on the modified Ashworth scale [19], and a stiff knee gait, defined as a peak of knee flexion during the swing of less than 45° [20]. Here, we analyze the results of the gait analyses, enabling the inclusion of participants in the study of gait disorders due to post-stroke muscle overactivity (NCT01973023).
We specifically focused on the impact of rectus femoris hyperactivity. Only eight patients were included in this study (Table 1). This selection allows us to better investigate the impact of this muscle’s hyperactivity on muscle synergies. Our results cannot be generalized to the broader population of stroke patients with SKG. To analyze our results, we calculated the effect size on biomechanical parameters of the gait cycle (Table 2). We observed a significant effect size on the peak knee flexion during the swing phase, indicating the positive impact of the nerve block and confirming that the condition required for muscle synergy extraction is met.

Table 1.
Anthropometric characteristics: M = male, F = female, Age (years), Height (cm), Weight (kg), and pathology of participants.

Table 2.
Summary of the kinematic and gait variables. ** denotes a statistical difference between the PRE and POST conditions (p < 0.01).
2.2. Intervention
A nerve block was performed according to the technique described by Sung et al. [17]. The effectiveness of the nerve block was tested by comparing rectus femoris EMG signals recorded before and after the intervention during maximal voluntary isometric hip flexion. A reduction of at least 50% of activity calculated using the root mean square was considered effective [18]. For both gait analyses, participants were asked to walk at a spontaneous gait speed.
2.3. Motion Analysis
A 3D gait analysis was performed in two conditions, before (PRE) and after (POST) nerve block, using a Motion Analysis system with eight cameras (100 Hz, Motion Analysis Corporation, Santa Rosa, CA, USA). The trajectories of 24 reflective markers placed on anatomical landmarks [21] were recorded. Surface electromyographic (EMG) signals (1000 Hz) (MA311, Motion Lab Systems, Lake Elsinore, CA, USA) were recorded from five muscles on the paretic leg: rectus femoris, vastus lateralis, semitendinosus soleus, and tibialis anterior. Participants were asked to walk at their own comfortable pace, barefoot, along a 10 m walkway. Eight trials were recorded before and after the rectus femoris motor nerve block. The following gait cycle phases were manually identified by a single operator: initial double contact, single support phase, final double contact, and swing phase.
2.4. Kinematic Variables
The marker displacement was filtered at 6 Hz using a 3rd-order Butterworth filter [22], and the following kinematic variables were calculated using Orthotrak 6.2.8 (Motion Analysis Corporation, Santa Rosa, CA, USA): maximum peak knee flexion in swing and gait speed. The mean of all the trials was calculated for each variable and used in the analysis.
2.5. EMG Variables
All EMG signals were analyzed using a custom-written MATLAB routine (version R2012b, The MathWorks, Natick, MA, USA). Before processing, EMG signals were visually checked to detect artifacts. EMG signals were band-pass filtered from 5 Hz to 500 Hz, centered, full-wave rectified, and low-pass filtered at 10 Hz. The amplitude of the EMG signals was normalized to the maximum activation recorded for each participant by condition [23,24]. To avoid time shifts induced by different gait speeds, we performed time normalization with respect to gait phase duration [25]: initial double contact = 20 time points, single support phase = 80 time points, final double contact = 20 time points, and swing phase = 80 time points. Thus, each gait cycle was time-normalized on 200 points. The number of points per phase was chosen in accordance with the literature and the relative duration of each cycle [26]. We applied these percentages in accordance with what is commonly described in the spatiotemporal parameters of the gait cycle: 10% of the duration of a gait cycle corresponded to the initial double contact, 40% to the single support phase, 10% to the final double contact, and 40% to the oscillation phase. In the continuation of the manuscript, we will call ‘processed EMGs’ the EMGs that have undergone the whole of the stages described above.
2.6. Muscle Synergy Extraction
Several methods have been proposed by different research teams to extract muscle synergies from surface electromyographic signals [27]. Three main methods can be identified: principal component analysis (PCA), independent component analysis (ICA), and non-negative matrix factorization (NMF). Although these methods are used in various contexts, the NMF method has superior relevance in studying muscle physiology (pull-only behavior of muscles) but has the drawback of being dependent on the choice of initial parameters. The selection of these initial parameters is detailed in the following sections. We opted for a method that unifies the main NMF-based approaches [28], allowing for the simultaneous identification of temporal and spatial primitives.
To extract the muscle synergies, we applied the space-by-time decomposition algorithm developed by Delis and colleagues [28], adapted from the non-negative matrix factorization algorithm [29]. This algorithm assumes that EMG envelopes from each muscle can be described as a linear combination of invariant temporal and spatial modules called primitives that are activated by scalar coefficients called combinators (Figure 1).
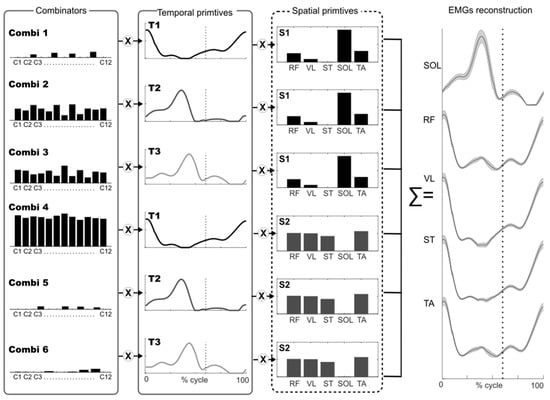
Figure 1.
Model of muscle synergies extracted using space-by-time-decomposition. The figure illustrates how muscle activity (EMG) can be reconstructed with a multiplexed approach, that is, linear combinations of all pairs of spatial (N = 2) and temporal (P = 3) primitives by scalar combinators. The interactions between the three spatial primitives and the two temporal primitives are modulated by combinators (black bars). Thus, each gait cycle is associated with a specific combinator (denoted by c1, c2…). The dotted line indicates toe off. Combi = combinators, T = temporal primitive, S = spatial primitive, EMG from 5 muscles across 12 gait cycles was used: RF = rectus femoris; VL = vastus lateralis; ST = semitendinosus; SOL= soleus; TA = tibialis anterior.
In this model, called space-by-time decomposition, each (non-negative) muscle pattern, corresponding to the gait cycle , is is expressed as follows:
where and are the temporal and spatial primitives respectively (and are invariant across gait cycles), is a scalar combinator that can vary across gait cycles, corresponds to the number of time-points ( = 200 in our study), and corresponds to the number of muscles ( = 5 in our study). The parameters and correspond to the number of temporal and spatial primitives, respectively. The index identifies a single gait cycle. For each gait cycle s, invariant spatial and temporal primitives are thus activated by a set of specific combinators. The aim of this algorithm is to iteratively minimize the total reconstruction error expressed as follows:
We computed the Variance Accounted For (VAF) to measure the accuracy of the muscle synergy decomposition for a fixed number of primitives. VAF was computed using the following formula [27], where is the grand mean value of the processed EMG data:
In addition, we used the VAF to select the number of primitives used throughout the study. We performed muscle synergy extraction on the matrix that grouped the EMG signals in PRE (Figure 2 matrix B1) until the VAF was greater than 75% [30,31].
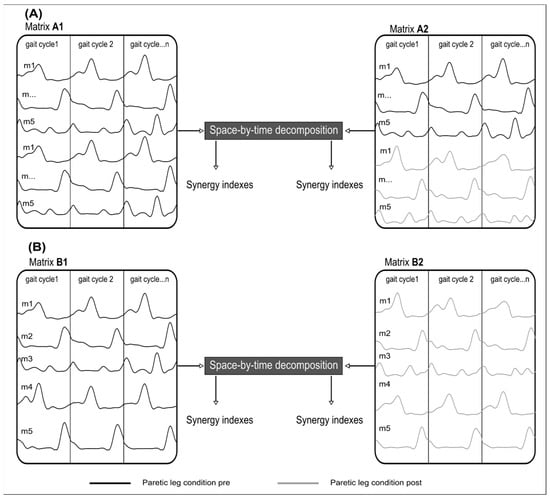
Figure 2.
Schematic illustration of two methods of muscle synergy extraction. (A) Illustration of the Similarity extraction. (Matrix A1) groups twice the EMGs of the paretic leg (black line) in PRE. In (Matrix A2), the EMGs of the paretic leg (black line) in PRE are grouped with the EMG of the paretic leg in POST (grey line). The same number of gait cycles was used in each matrix. (B) Recruitment extraction. (Matrix B1), concatenate all EMGs of the paretic leg (black line) for all cycles in PRE where concatenated. (Matrix B2) concatenates all EMGs of the paretic leg (grey line) for all cycles in POST. m = muscle.
The methodological choice of a 75% threshold limits the impact of participants’ specific muscle activity and facilitates the extraction of fundamental primitives for physiological interpretation. In this study, it is crucial that the fundamental primitives can be identified before and after a motor block to assess the impact of a commonly used medical intervention. This 75% threshold differs from the conventional 90% threshold because our method preserves inter-trial variability and naturally leads to lower variance accounted for (VAF) values than methods that average trials. This 75% threshold has been used in previous studies employing the same method [32]. We used the same number of gait cycles for each extraction to avoid an unbalanced number of gait cycles between conditions. To test the muscle synergy robustness between PRE and POST and muscle synergy selectivity/variability, we performed two separate muscle synergy extraction techniques. The first technique involved extracting synergies for each participant from a matrix that combined twice the EMG signals in PRE (A1 in Figure 2). Then, we built a matrix that pooled the EMG signals from PRE with the EMG signals from POST (A2 in Figure 2). Since we extract from a single matrix, temporal and spatial primitives are forced to be the same in PRE and POST. The EMG signals of matrix A1 have been doubled to have the same number of gait cycles between the two matrices and allow fair comparisons for the different metrics. If the VAF decreases when extracting from matrix A2, it means that the synergies are not robust between PRE and POST.
The second technique involved extracting muscle synergies for each participant from a matrix that pooled the EMG signals from PRE (B1 in Figure 2) as well as from a matrix that pooled the EMG signals from POST (name B2 in Figure 2). Here, the temporal and spatial primitives can be different in the PRE and POST conditions. This analysis allows us to assess the synergy structure in PRE and POST, with primitives specifically adapted to each condition.
2.7. Muscle Synergy Indexes
The main outcome was the VAF from the first muscle synergy extraction that provided an estimation of muscle synergy robustness in PRE and POST [7].
The secondary outcome was an Index of Recruitment Selectivity (IRS), which reflects the degree of multiplexing of muscle synergies [33], and an Index of Recruitment Consistency (IRC), which reflects variability (across cycles) of muscle synergy activations. The IRS and IRC were calculated from two separate muscle synergy extractions.
The IRS index evaluates the actual (in-use) dimensionality of muscle synergy space for the task under investigation. Basically, it evaluates how many pairs of spatial/temporal primitives are really used for the task with respect to the total number of available pairs in the decomposition. The formula is based on the sparseness measure proposed by Hoyer [34], applied to the combinatory values concatenated in a single vector of dimension N × P:
A large IRS value (i.e., large sparseness) would mean that only a few muscle synergies (less than all N × P activable muscle synergies) are recruited to reconstruct the original muscle patterns.
The IRC quantifies the variability of the combinators for each muscle synergy from one gait cycle to another. This can be compared between the PRE- and POST-conditions. The IRC is defined as the sum of the variances of the activation coefficients across gait cycles, normalized by the total number of activation coefficients:
A low IRC value would mean that the combinators are relatively invariant and consistent across gait cycles.
2.8. Statistical Analyses
All statistical tests were performed with MATLAB (version R2012b, The MathWorks, Natick, MA, USA). All data are reported as means ± standard deviations. A non-parametric Wilcoxon signed rank test was used to compare kinematic variables (the values of maximum peak knee flexion in swing and gait speed) and muscle synergy indexes (VAF, IRC, IRS) PRE and POST nerve block. The paired Student’s t-test and the Wilcoxon test are two statistical tests used to compare paired datasets. Although they are often used in similar contexts, we preferred the Wilcoxon test over the Student’s t-test due to the latter’s sensitivity to normality assumptions, especially in small samples. Additionally, we calculated Cohen’s d to quantify the effect size: <0.20 Small, 0.50 Medium, 0.80 Large, >1.2 Very Large.
3. Results
The mean age of the 8 participants was 41 ± 10 years, the mean height was 174 ± 11 cm, and the mean weight was 72 ± 10 kg (Table 1). Seven were men, and the average time since stroke was at least 12 months. Spasticity of the rectus femoris ranged from 1 to 3 on the modified Ashworth scale in PRE and decreased by at least one point on the modified Ashworth scale in POST.
3.1. Gait Variables
The results of the gait variables are presented in Table 2 and Figure 3. Maximum peak knee flexion increased significantly from PRE to POST (p = 0.007) for all participants, ranging from 26.68 ± 5.16° in PRE to 35.60 ± 7.07° in POST. This result confirmed the effect of the nerve block. There was no significant change in gait speed between conditions.
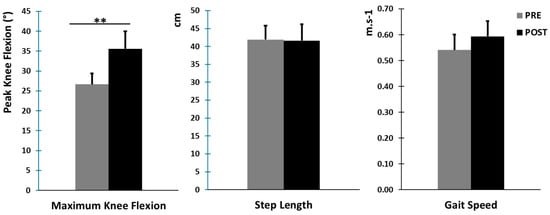
Figure 3.
Main kinematic gait variables (mean ± standard error). Left: maximum knee flexion in degrees in the swing phase for the paretic leg. Middle: step length in cm for the paretic leg. Right: gait speed in m·s−1. ** denotes a statistical difference between the PRE and POST conditions (p < 0.01).
All values are expressed as means ± standard deviations. Maximum Knee flexion in degree = Maximum peak knee flexion in swing phase for the paretic leg. The Gait Speed in m·s−1 and the values of Step Length in cm correspond to the paretic leg. ** denotes a statistical difference between the PRE and POST conditions (p < 0.01). Effect size (Cohen’s d).
3.2. Muscle Synergy Extraction
After extraction from the matrix (B1), the configuration with four spatial and temporal primitives was the closest VAF value to 75% (Figure 4, VAF = 74.5 ± 6.9% with four spatial and four temporal primitives).
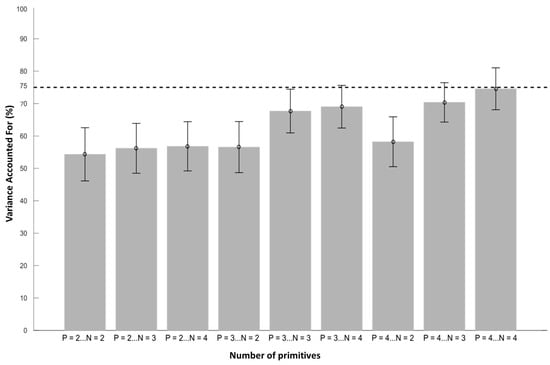
Figure 4.
Evolution of VAF values with respect to the number of spatial and temporal primitives. The letter N corresponds to the number of spatial primitives, and the letter P corresponds to the number of temporal primitives. The bars represent the mean values across participants with standard errors. A total of four spatial primitives and four temporal primitives are necessary to obtain a VAF close to the 75% threshold.
The mean VAF value decreased significantly from this reference value of 74.5 ± 6.9% in PRE to 70.3 ± 8.0% in POST (p = 0.015) when applying the extraction with matrices A1 and A2, respectively. This indicates that the same set of primitives cannot simultaneously account for the EMGs in PRE and POST as the VAF significantly decreases. This suggests a slight change in muscle synergies after the nerve block. Indeed, if the synergies underlying the PRE and POST conditions were identical, a VAF decrease would not be expected.
3.3. Muscle Synergy Indexes
Since the primitives can differ slightly between PRE and POST, we conducted the next analyses with extraction II, where the primitives are allowed to vary in PRE and POST. We then focused our analysis on the variability and selectivity of synergy recruitment between PRE and POST. The IRS quantifies selectivity. Mean IRS values decreased significantly from 0.48 ± 0.03 in PRE to 0.43 ± 0.07 in POST (p = 0.007), indicating an increase in interactions between spatial and temporal primitives and demonstrating that the degree of multiplexing of muscle synergies was increased by the nerve block (less sparseness, i.e., more complexity in synergy activations). The IRC quantifies variability in the recruitment of primitives across gait cycles. Mean IRC values increased from 3.93 ± 1.20 in PRE to 4.03 ± 2.00 in POST; however, this difference was not significant (p = 0.843), suggesting that the combinators were relatively invariant and consistent across gait cycles in both PRE and POST. In other words, once a strategy was found, it was quite consistent across gait cycles.
4. Discussion
The objective of this study was to quantify the robustness and variability of muscle synergies before and after a nerve block of the rectus femoris muscle in stroke patients presenting SKG due to hyperactivity of this muscle in the paretic lower limb during the swing phase of the gait cycle.
This study proposed a novel approach compared to previous studies evaluating modifications in muscle synergies [4]. Our study differs firstly by incorporating an additional analysis of joint kinematics, as this reflects the clinician’s functional objective in addressing gait disorders. Secondly, it quantifies the effect of reducing abnormal muscle tone, primarily responsible for gait disturbances, through a pharmacological intervention (lidocaine injection). Although the evaluation of this therapeutic approach is commonly used clinically, it differs from previous studies that focused on modifying lower limb kinematics and kinetics through rehabilitation [5]. Muscle synergies were extracted before and after the nerve block in muscles responsible for gait disturbances to analyze the immediate post-effects. We hypothesized that the robustness of muscle synergies would be affected by this nerve block.
The results of this study confirmed our hypothesis that the characteristics of the paretic limb’s muscle synergies during gait were modified after a rectus femoris nerve block. Spatial and temporal primitives are slightly different in PRE vs. POST, and their activation is more intertwined in POST compared to PRE.
4.1. Gait Variables
After the nerve block, our results showed that the maximum peak knee flexion in the swing phase was increased. In addition, there was no statistical change in speed. These findings confirm the efficiency of the nerve block. Moreover, they are in accordance with previous studies, which have not observed any changes in spatiotemporal parameters (spontaneous gait speed) after the nerve block [17,18].
4.2. Muscle Synergy Indexes
Four spatial and four temporal primitives were needed to obtain VAF values that were close to 75% in PRE. This is consistent with a recent systematic review that found that four primitives are common in stroke patients [4]. Furthermore, after a stroke, the number of primitives in the paretic limb is the same as healthy subjects or reduced during gait [4]. These four primitives have been associated with the biomechanical constraints of walking: body support, forward propulsion, and swing initiation [2]. These results suggest that the neural structures required for the activation of synergies were still intact [4]. Nevertheless, it is common for studies to specify a minimum VAF cut-off of 90% to identify the number of synergies. These cut-off differences can be explained by differences in the choice of the method used to extract muscle synergies. Most published studies extracted muscle synergies from averaged EMG signals [34,35], which was not the case here. If we used averaged EMG signals, the IRC and IRS could not have been calculated, but the VAF would be higher and similar to other reports. Therefore, the use of global indices such as VAF, IRS, and IRC avoids the need for more complex similarity analyses between synergies extracted using different methods and initial parameters, which would be less relevant for the clinical evaluation of gait muscle patterns. As a result, we had to use a more general method that allowed us to account for inter-cycle variability but hardly allowed us to reach 90% of VAF with few primitives.
After the nerve block, the VAF value decreased from 74.50% (6.90) to 70.30% (8.00) (p = 0.015), suggesting a modification of the muscle synergies in order to adapt to the new biomechanical constraints. Kargo and Nitz showed that during a new motor task, the composition of muscle synergies was modulated until a stable state emerged with a new temporal profile [36]. So, the higher the VAF, the more the identified synergies explain the motor organization. Therefore, the formation of new muscle synergies is an adaptive process that is related to the experiences of each subject [37]. Thus, repeated movement practice could lead to the development of new muscle synergies or change the composition of existing ones [38]. In our case, the four spatial and temporal primitives explain part of the motor organization, but not all of it. The duration of the effect of the nerve block was likely too short for new muscle synergies to be created; thus, the changes that occurred were more likely due to the modification of existing muscle synergies.
In addition, previous studies have highlighted that during the learning of new motor tasks, subjects exhibited variability across muscle synergies [3,38]. This variability could be a ‘fine-tuning’ produced by the central nervous system to balance the opposing demands of achievement of the motor goal and energy efficiency. This fine-tuning is determined by ‘feed forward’ processes based on the knowledge of prior performance and ‘feedback’ processes. Finally, the increase in variability could be increased by the local anesthetic nerve block, which induces disturbances on motor nerves [39]. We hypothesize that in the spastic patient the use of the nerve block almost induces a new locomotor stain due to its disturbance [VAF from 74.50% to 70.30%].
The nerve block of the rectus femoris muscle induces a reduction in sensory afferents from the muscle spindle and a decrease in motor efferents. However, electrophysiologically, it is not possible to determine the respective contribution of each mechanism to the modifications observed in the extracted muscle synergies. Consequently, the short-term motor adaptation observed in the immediate post-effect may be explained either by a spinal phenomenon due to changes in the behavior of propriospinal interneurons or by a cortical mechanism such as the induction of short-term depression.
The nerve block did not affect the IRC values, showing an invariance and consistency of the combinators across gait cycles, despite the decrease in rectus femoris over-activity. After the nerve block, the central nervous system must adapt the muscle synergies. We hypothesize that the same slightly modified muscle synergies are at the origin of the modification of the paretic gait pattern and not the development of a new muscle synergy. Consequently, muscle synergies are not robust between PRE and POST, which may also explain the decrease in VAF. In fact, if stroke patients had created a new muscle synergies gait pattern, then the recruitment variability should have increased due to an exploration process. Indeed, learning new coordination is characterized by the continued experimentation enabling the development of new muscle synergy strategies, which will introduce some variability in the movement but might favor the optimization and the transfer of this motor behavior across other contexts [37]. In other words, IRC seems to reflect more a long-term adaptation of a subject after an intervention than a short one.
The analysis of the IRS values showed a lower level of muscle synergy selectivity after the nerve block (PRE = 0.48 ± 0.03 POST = 0.43 ± 0.07, p = 0.007), suggesting that the central nervous system recruited additional combinations of primitives to cope with the new biomechanical constraints. This likely suggests that the IRS reflects mainly short-term adaptations. This hypothesis is in accordance with other results. For example, Santuz et al. showed in healthy subjects that during gait on an uneven-surface treadmill, the central nervous system produced a temporal rearrangement of the shape of the motor primitives, but their structure was unaltered [40]. Another study in healthy subjects found that the same muscle synergies used for unperturbed gait were via supraspinal activity in challenging gait conditions [41]. Comparison of synergy behavior between healthy individuals and those with stroke showed a reduction in gait adaptability after stroke, with simplified movement strategies [42,43]. Muscle synergy in stroke patients was less complex [1,8]. This might indicate a reduction in cortical adaptability and flexible synergy recruitment. We conclude that the central nervous system flexibility controls the modification and activation of muscle synergies in case of peripheral disturbance.
4.3. Clinical Implications
Physiologically, this therapeutic intervention reduces both the Ia afferent inputs from the rectus femoris muscle spindle and the nerve conduction of alpha motoneurons innervating the rectus femoris. This short-term temporary nerve block induces modifications in muscle synergies, specifically a decrease in VAF and IRS. This quantification allows clinicians to consider the long-term impact of a nerve block beyond its temporary effect, such as neurotomy or neurolysis of the rectus femoris.
4.4. Limitations
Although our sample size is small and appears heterogeneous regarding kinematic and spatiotemporal parameters (Table 2), the observed effect size is sufficient to support our findings. Moreover, the heterogeneity of our sample also reflects the varying severity of stroke patients experiencing this specific gait disorder. Therefore, our results cannot be generalized to other gait disorders within this population.
Methodologically, muscle synergy extraction methods are sensitive to the characteristics of recorded EMG signals. In our study, the immediate effect of the therapeutic intervention minimizes metrological biases related to sensor repositioning (EMGs and markers).
5. Conclusions
This study aimed to explore the muscle synergies’ flexibility before and after a motor nerve block. Results showed that an efficient lower limb motor nerve block in stroke patients induces a decrease of VAF, suggesting a lack of robustness of muscle synergies between PRE and POST. Our results also show a flexibility in muscle synergies and their activations after muscle nerve block in stroke patients, which seem to be more complex (i.e., multiplexed) in POST yet consistent across repeated cycles.
These results highlight the relevance of this methodology for assessing the potential impact of a long-term therapeutic intervention such as neurotomy. Furthermore, it would be valuable to compare these results with those obtained before and after botulinum toxin injections, which exclusively block the neuromuscular junction of the injected muscle. This difference in mechanism of action would provide better insight into the neurological substrates underlying neuromuscular synergy modifications in stroke patients.
Author Contributions
Conceptualization, A.S., D.P., N.R. and B.B.; methodology, A.S., D.P., N.R. and B.B.; software, A.S. and B.B.; formal analysis, A.S. and D.P.; investigation, A.S. and N.R.; resources, A.S., D.P. and B.B.; data curation, A.S. and B.B.; writing—original draft preparation, A.S.; writing—review and editing, A.S., B.B. and D.P.; visualization, A.S. and B.B.; supervision, D.P., N.R. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. NCT01973023.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We gratefully thank all the participants involved in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hashiguchi, Y.; Ohata, K.; Kitatani, R.; Yamakami, N.; Sakuma, K.; Osako, S.; Aga, Y.; Watanabe, A.; Yamada, S. Merging and Fractionation of Muscle Synergy Indicate the Recovery Process in Patients with Hemiplegia: The First Study of Patients after Subacute Stroke. Neural Plast. 2016, 2016, 5282957. [Google Scholar] [CrossRef] [PubMed]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture 2013, 38, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Safavynia, S.; Torres-Oviedo, G.; Ting, L. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Vermeulen, J.; Wagemans, K.; Schröder, J.; Embrechts, E.; Truijen, S.; Hallemans, A.; Saeys, W. Lower limb muscle synergies during walking after stroke: A systematic review. Disabil Rehabil. 2020, 42, 2836–2845. [Google Scholar] [CrossRef]
- Booth, A.T.C.; van der Krogt, M.M.; Harlaar, J.; Dominici, N.; Buizer, A.I. Muscle synergies in response to biofeedback-driven gait adaptations in children with cerebral palsy. Front. Physiol. 2019, 10, 1208. [Google Scholar] [CrossRef]
- Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle synergies in parkinson’s disease. Sensors 2020, 20, 3209. [Google Scholar] [CrossRef]
- Shuman, B.R.; Schwartz, M.H.; Steele, K.M. Electromyography data processing impacts muscle synergies during gait for unimpaired children and children with cerebral palsy. Front. Comput. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef]
- Ambrosini, E.; Parati, M.; Peri, E.; De Marchis, C.; Nava, C.; Pedrocchi, A.; Ferriero, G.; Ferrante, S. Changes in leg cycling muscle synergies after training augmented by functional electrical stimulation in subacute stroke survivors: A pilot study. J. Neuroeng. Rehabil. 2020, 27, 17–35. [Google Scholar] [CrossRef]
- Pollock, C.; Eng, J.; Garland, S. Clinical measurement of walking balance in people post stroke: A systematic review. Clin. Rehabil. 2011, 25, 693–708. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Weerdesteyn, V.; de Niet, M.; van Duijnhoven, H.J.; Geurts, A.C. Falls in individuals with stroke. J. Rehabil. Res. Dev. 2008, 45, 1195–1213. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Anderson, F.C.; Pandy, M.G.; Delp, S.L. Muscles that influence knee flexion velocity in double support: Implications for stiff-knee gait. J. Biomech. 2004, 37, 1189–1196. [Google Scholar] [CrossRef]
- Kerrigan, D.C.; Bang, M.S.; Burke, D.T. An algorithm to assess stiff-legged gait in traumatic brain injury. J. Head Trauma Rehabil. 1999, 14, 136–145. [Google Scholar] [CrossRef]
- Piazza, S.J.; Delp, S.L. The influence of muscles on knee flexion during the swing phase of gait. J. Biomech. 1996, 29, 723–733. [Google Scholar] [CrossRef]
- Riley, P.O.; Kerrigan, D.C. Torque action of two-joint muscles in the swing period of stiff-legged gait: A forward dynamic model analysis. J. Biomech. 1998, 31, 835–840. [Google Scholar] [CrossRef]
- Roche, N.; Bonnyaud, C.; Geiger, M.; Bussel, B.; Bensmail, D. Relationship between hip flexion and ankle dorsiflexion during swing phase in chronic stroke patients. Clin. Biomech. 2015, 30, 219–225. [Google Scholar] [CrossRef]
- Sung, D.H.; Bang, H.J. Motor branch block of the rectus femoris: Its effectiveness in stiff-legged gait in spastic paresis. Arch. Phys. Med. Rehabil. 2000, 81, 910–915. [Google Scholar] [CrossRef]
- Robertson, J.V.G.; Pradon, D.; Bensmail, D.; Fermanian, C.; Bussel, B.; Roche, N. Relevance of botulinum toxin injection and nerve block of rectus femoris to kinematic and functional parameters of stiff knee gait in hemiplegic adults. Gait Posture 2009, 29, 108–112. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Merlo, A.; Campanini, I. Impact of instrumental analysis of stiff knee gait on treatment appropriateness and associated costs in stroke patients. Gait Posture 2019, 72, 195–201. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Devarajan, K.; Cheung, V.C.K. On non-negative matrix factorization algorithms for signal-dependent noise with application to electromyography data. Neural Comput. 2014, 26, 1128–1168. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. The Influence of Footwear on the Modular Organization of Running. Front. Physiol. 2017, 8, 958. [Google Scholar] [CrossRef]
- Hebenstreit, F.; Leibold, A.; Krinner, S.; Welsch, G.; Lochmann, M.; Eskofier, B.M. Effect of walking speed on gait sub phase durations. Hum. Mov. Sci. 2015, 43, 118–124. [Google Scholar] [CrossRef]
- Perry, J.; Burnfield, J.M. Gait Analysis: Normal and Pathological Function, 2nd ed.; SLACK: Thorofare, NJ, USA, 1992. [Google Scholar]
- Tresch, M.C.; Cheung, V.C.; D’Avella, A. Matrix factorization algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef]
- Delis, I.; Panzeri, S.; Pozzo, T.; Berret, B. A unifying model of concurrent spatial and temporal modularity in muscle activity. J. Neurophysiol. 2014, 111, 675–693. [Google Scholar] [CrossRef]
- Lee, D.D.; Seung, H.S. Algorithms for non-negative matrix factorization. In Advances in Neural Information Processing Systems 13—Proceedings of the 2000 Conference, Neural Information Processing Systems Foundation, Denver, CO, USA, 27–30 November 2000; MIT Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Hayes, H.B.; Chvatal, S.A.; French, M.A.; Ting, L.H.; Trumbower, R.D. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin. Neurophysiol. 2014, 125, 2024–2035. [Google Scholar] [CrossRef]
- Hinnekens, E.; Berret, B.; Morard, E.; Do, M.C.; Barbu-Roth, M.; Teulier, C. Optimization of modularity during development to simplify walking control across multiple steps. Front. Neural Circuits 2024, 17, 1340298. [Google Scholar] [CrossRef]
- Hinnekens, E.; Barbu-Roth, M.; Do, M.C.; Berret, B.; Teulier, C. Generating variability from motor primitives during infant locomotor development. Elife 2023, 12, e87463. [Google Scholar] [CrossRef]
- Hoyer, P.O. Non-negative matrix factorization with sparseness constraints. J. Mach. Learn. Res. 2004, 5, 1457–1469. [Google Scholar]
- Oliveira, A.S.; Gizzi, L.; Farina, D.; Kersting, U.G. Motor modules of human locomotion: Influence of EMG averaging, concatenation, and number of step cycles. Front. Hum. Neurosci. 2014, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Kargo, W.J.; Nitz, D.A. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J. Neurosci. 2003, 23, 11255–11269. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Bastian, A.J. Natural error patterns enable transfer of motor learning to novel contexts. J. Neurophysiol. 2012, 107, 346–356. [Google Scholar] [CrossRef]
- Frère, J.; Hug, F. Between-subject variability of muscle synergies during a complex motor skill. Front. Comput. Neurosci. 2012, 6, 99. [Google Scholar] [CrossRef]
- Ririe, D.G.; Walker, F.O.; James, R.L.; Butterworth, J. Effect of alkalinization of lidocaine on median nerve block. Br. J. Anaesth. 2000, 84, 163–168. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging human locomotion: Stability and modular organisation in unsteady conditions. Sci. Rep. 2018, 8, 2740. [Google Scholar] [CrossRef]
- Oliveira, A.S.C.; Gizzi, L.; Kersting, U.G.; Farina, D. Modular organization of balance control following perturbations during walking. J. Neurophysiol. 2012, 108, 1895–1906. [Google Scholar] [CrossRef]
- Den Otter, A.R.; Geurts, A.C.H.; De Haart, M.; Mulder, T.; Duysens, J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp. Brain Res. 2005, 161, 180–192. [Google Scholar] [CrossRef]
- van Swigchem, R.; van Duijnhoven, H.J.R.; den Boer, J.; Geurts, A.C.; Weerdesteyn, V. Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabil. Neural Repair. 2013, 27, 230–239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).