Association between the Strength of Flexor Hallucis Brevis and Abductor Hallucis and Foot Mobility in Recreational Runners
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Static Foot Posture
2.4. Flexor Hallux Strength
2.5. Foot Structure and Mobility
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| DiffFAH | Difference in foot arch height between weight- and non-weight-bearing conditions |
| DiffMFW | Difference in midfoot width between weight- and non-weight-bearing conditions |
| FAH | Foot arch height |
| FPI-6 | Foot Posture Index |
| ICC | Intraclass correlation coefficients |
| MFW | Midfoot width |
| NWB | Non-weight-bearing |
| WB | Weight-bearing |
References
- Lopes, A.D.; Costa, L.O.P.; Saragiotto, B.T.; Yamato, T.P.; Adami, F.; Verhagen, E. Musculoskeletal pain is prevalent among recreational runners who are about to compete: An observational study of 1049 runners. J. Physiother. 2011, 57, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Whatman, C.; Sheerin, K.; Hume, P.; Johnson, M.I. The proportion of lower limb running injuries by gender, anatomical location and specific pathology: A systematic review. J. Sports Sci. Med. 2019, 18, 21. [Google Scholar]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 49, 290. [Google Scholar] [CrossRef] [PubMed]
- Rottier, T.D.; Allen, S.J. The influence of swing leg technique on maximum running speed. J. Biomech. 2021, 126, 110640. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, X.; Deng, L.; Zhang, S.; Cui, K.; Fu, W. Relationships between foot morphology and foot muscle strength in healthy adults. Int. J. Environ. Res. Public Health 2020, 17, 1274. [Google Scholar] [CrossRef]
- Venkadesan, M.; Yawar, A.; Eng, C.M.; Dias, M.A.; Singh, D.K.; Tommasini, S.M.; Haims, A.H.; Bandi, M.M.; Mandre, S. Stiffness of the human foot and evolution of the transverse arch. Nature 2020, 579, 97–100. [Google Scholar] [CrossRef]
- Morita, N.; Yamauchi, J.; Kurihara, T.; Fukuoka, R.; Otsuka, M.; Okuda, T.; Ishizawa, N.; Nakajima, T.; Nakamichi, R.; Matsuno, S.; et al. Toe flexor strength and foot arch height in children. Med. Sci. Sports Exerc. 2015, 47, 350–356. [Google Scholar] [CrossRef]
- Ker, R.; Bennett, M.; Bibby, S.; Kester, R.; Alexander, R. The spring in the arch of the human foot. Nature 1987, 325, 147–149. [Google Scholar] [CrossRef]
- Lin, C.J.; Lai, K.A.; Kuan, T.S.; Chou, Y.L. Correlating factors and clinical significance of flexible flatfoot in preschool children. J. Pediatr. Orthop. 2001, 21, 378–382. [Google Scholar] [CrossRef]
- McPoil, T.G.; Warren, M.; Vicenzino, B.; Cornwall, M.W. Variations in foot posture and mobility between individuals with patellofemoral pain and those in a control group. J. Am. Podiatr. Med. Assoc. 2011, 101, 289–296. [Google Scholar] [CrossRef]
- Barton, C.J.; Bonanno, D.; Levinger, P.; Menz, H.B. Foot and ankle characteristics in patellofemoral pain syndrome: A case control and reliability study. J. Orthop. Sports Phys. Ther. 2010, 40, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pauel, R.; Deschamps, K.; Jonkers, I.; Vanwanseele, B. Differences in foot muscle morphology and foot kinematics between symptomatic and asymptomatic pronated feet. Scand. J. Med. Sci. Sports 2019, 29, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.; Rathleff, M.S.; Claus, A.; McPoil, T.; Nee, R.; Crossley, K.M.; Kasza, J.; Vicenzino, B.T. Does foot mobility affect the outcome in the management of patellofemoral pain with foot orthoses versus hip exercises? A randomised clinical trial. Br. J. Sports Med. 2020, 54, 1416–1422. [Google Scholar] [CrossRef]
- Fiolkowski, P.; Brunt, D.; Bishop, M.; Woo, R.; Horodyski, M. Intrinsic pedal musculature support of the medial longitudinal arch: An electromyography study. J. Foot Ankle Surg. 2003, 42, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Soysa, A.; Hiller, C.; Refshauge, K.; Burns, J. Importance and challenges of measuring intrinsic foot muscle strength. J. Foot Ankle Res. 2012, 5, 29. [Google Scholar] [CrossRef]
- Mann, R.; Inman, V.T. Phasic activity of intrinsic muscles of the foot. J. Bone Jt. Surg. 1964, 46, 469–481. [Google Scholar] [CrossRef]
- Mulligan, E.P.; Cook, P.G. Effect of plantar intrinsic muscle training on medial longitudinal arch morphology and dynamic function. Man. Ther. 2013, 18, 425–430. [Google Scholar] [CrossRef]
- Mann, R.A.; Hagy, J.L. The function of the toes in walking, jogging and running. Clin. Orthop. Relat. Res. 1979, 142, 24–29. [Google Scholar] [CrossRef]
- Standring, S.; Ellis, H.; Healy, J.; Johnson, D.; Williams, A.; Collins, P.; Wigley, C. Gray’s anatomy: The anatomical basis of clinical practice. Am. J. Neuroradiol. 2005, 26, 2703. [Google Scholar]
- Farris, D.J.; Kelly, L.A.; Cresswell, A.G.; Lichtwark, G.A. The functional importance of human foot muscles for bipedal locomotion. Proc. Natl. Acad. Sci. USA 2019, 116, 1645–1650. [Google Scholar] [CrossRef]
- Kelly, L.A.; Cresswell, A.G.; Racinais, S.; Whiteley, R.; Lichtwark, G. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 2014, 11, 20131188. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.H.; Gross, M.T. Toe flexors strength and passive extension range of motion of the first metatarsophalangeal joint in individuals with plantar fasciitis. J. Orthop. Sports Phys. Ther. 2003, 33, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Aibast, H.; Okutoyi, P.; Sigei, T.; Adero, W.; Chemjor, D.; Ongaro, N.; Fuku, N.; Konstabel, K.; Clark, C.; Lieberman, D.E.; et al. Foot structure and function in habitually barefoot and shod adolescents in Kenya. Curr. Sports Med. Rep. 2017, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, J.P.; Sanno, M.; Willwacher, S.; Heinrich, K.; Brüggemann, G.P. The potential of toe flexor muscles to enhance performance. J. Sports Sci. 2013, 31, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Sulowska, I.; Mika, A.; Oleksy, Ł.; Stolarczyk, A. The influence of plantar short foot muscle exercises on the lower extremity muscle strength and power in proximal segments of the kinematic chain in long-distance runners. BioMed Res. Int. 2019, 2019, 6947273. [Google Scholar] [CrossRef]
- Kowalski, E.; Catelli, D.S.; Lamontagne, M. Side does not matter in healthy young and older individuals—Examining the importance of how we match limbs during gait studies. Gait Posture 2019, 67, 133–136. [Google Scholar] [CrossRef]
- Cornwall, M.W.; McPoil, T.G. Relationship between static foot posture and foot mobility. J. Foot Ankle Res. 2011, 4, 4. [Google Scholar] [CrossRef]
- Redmond, A. The Foot Posture Index: User guide and manual. Retrieved Sept. 2005, 29, 2014. [Google Scholar]
- Redmond, A.C.; Crosbie, J.; Ouvrier, R.A. Development and validation of a novel rating system for scoring standing foot posture: The Foot Posture Index. Clin. Biomech. 2006, 21, 89–98. [Google Scholar] [CrossRef]
- Quek, J.; Treleaven, J.; Brauer, S.G.; O’Leary, S.; Clark, R.A. Intra-rater reliability of hallux flexor strength measures using the Nintendo Wii Balance Board. J. Foot Ankle Res. 2015, 8, 48. [Google Scholar] [CrossRef]
- Endo, M.; Ashton-Miller, J.A.; Alexander, N.B. Effects of age and gender on toe flexor muscle strength. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2002, 57, M392–M397. [Google Scholar] [CrossRef] [PubMed]
- McPoil, T.G.; Vicenzino, B.; Cornwall, M.W.; Collins, N.; Warren, M. Reliability and normative values for the foot mobility magnitude: A composite measure of vertical and medial-lateral mobility of the midfoot. J. Foot Ankle Res. 2009, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; McClay, I.S. Measurements used to characterize the foot and the medial longitudinal arch: Reliability and validity. Phys. Ther. 2000, 80, 864–871. [Google Scholar] [CrossRef]
- Park, K.N.; Koh, E.K.; Jung, D.Y. The influence of age and gender on normalized foot arch height of Korean children and adolescents: A cross-sectional study. Footwear Sci. 2022, 14, 104–111. [Google Scholar] [CrossRef]
- Keenan, A.M.; Redmond, A.C.; Horton, M.; Conaghan, P.G.; Tennant, A. The Foot Posture Index: Rasch analysis of a novel, foot-specific outcome measure. Arch. Phys. Med. Rehabil. 2007, 88, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Latey, P.J.; Burns, J.; Nightingale, E.J.; Clarke, J.L.; Hiller, C.E. Reliability and correlates of cross-sectional area of abductor hallucis and the medial belly of the flexor hallucis brevis measured by ultrasound. J. Foot Ankle Res. 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Hirokawa, M.; Yoshitaka, Y.; Yamauchi, J. Toe flexor muscle strength and morphological characteristics of the foot in judo athletes. Int. J. Sports Med. 2019, 40, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M.; Martini, T.F. Chronic effect of different types of stretching on ankle dorsiflexion range of motion: Systematic review and meta-analysis. Foot 2018, 34, 28–35. [Google Scholar] [CrossRef]
- Abd, A.T.; Singh, R.E.; Iqbal, K.; White, G. A Perspective on Muscle Synergies and Different Theories Related to Their Adaptation. Biomechanics 2021, 1, 253–263. [Google Scholar] [CrossRef]
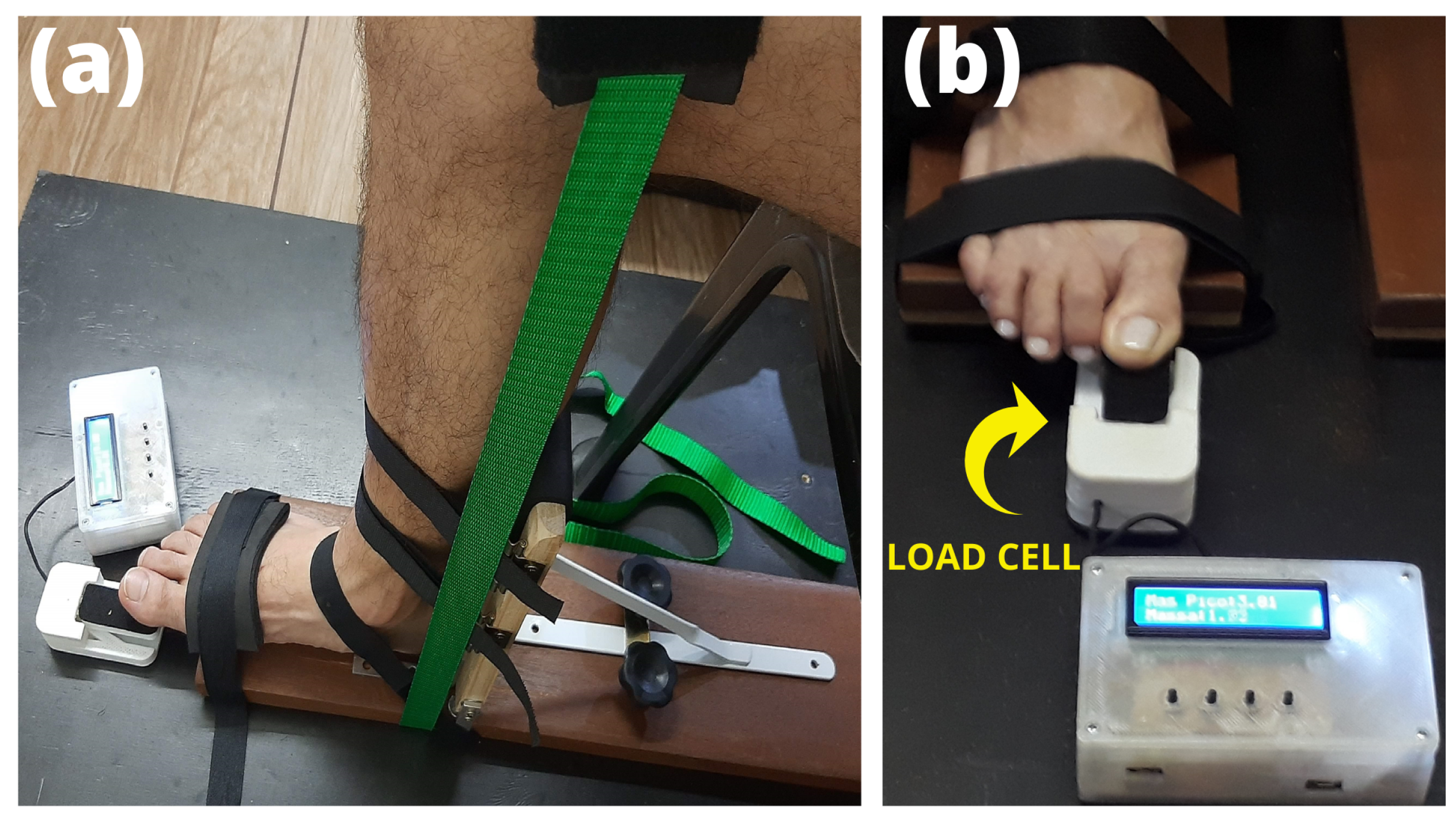
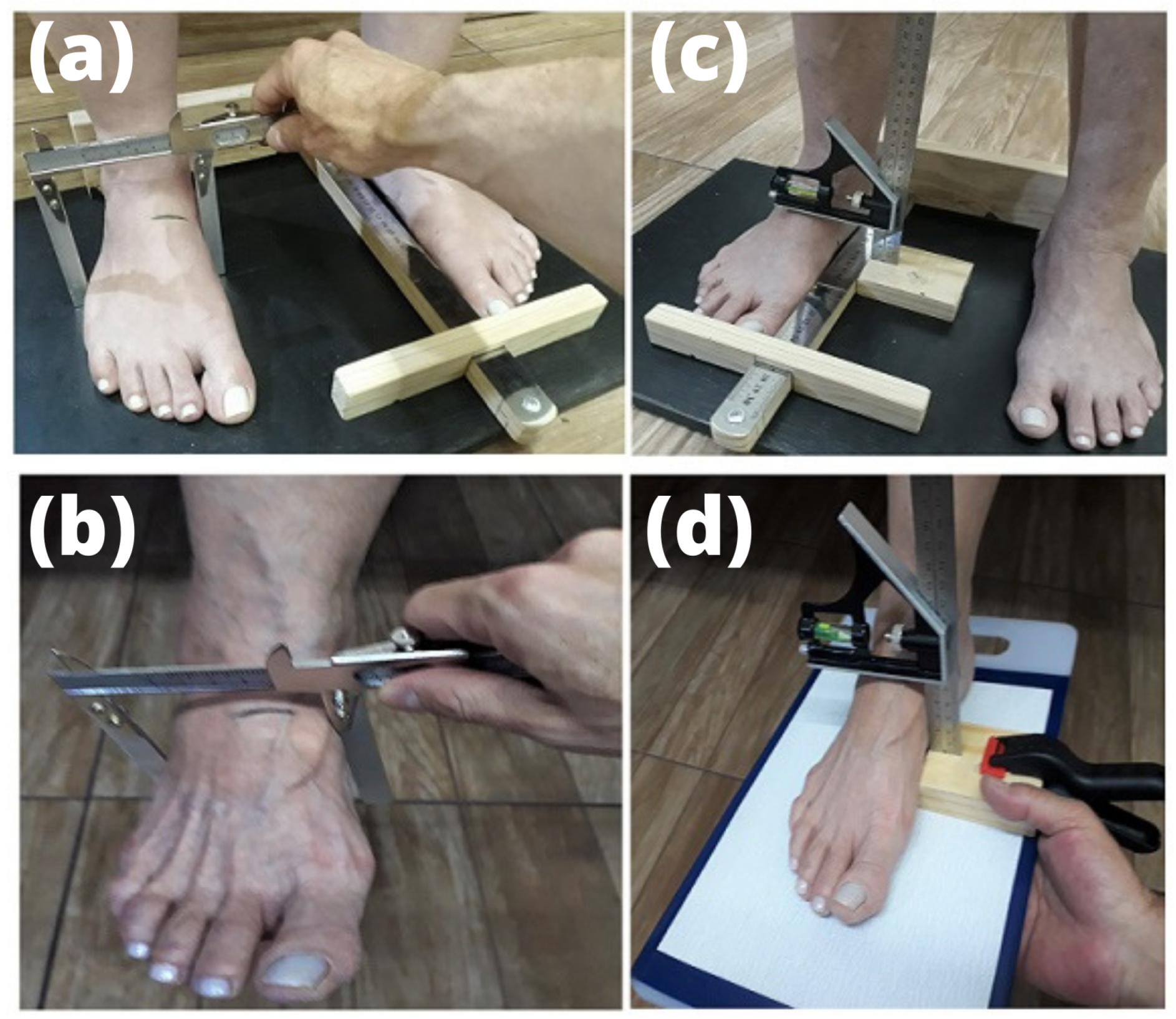
| Parameters | Participants (n = 24) |
|---|---|
| Age (years) | 33.58 (7.13) |
| Sex (female n (%)) | 6 (25%) |
| Height (m) | 1.71 (0.07) |
| Weight (kg) | 70.30 (10.28) |
| BMI (kg/m2) | 23.84 (2.54) |
| Training Experience (months) | 70 (60) |
| Leg dominance (right n (%)) | 19 (79.17) |
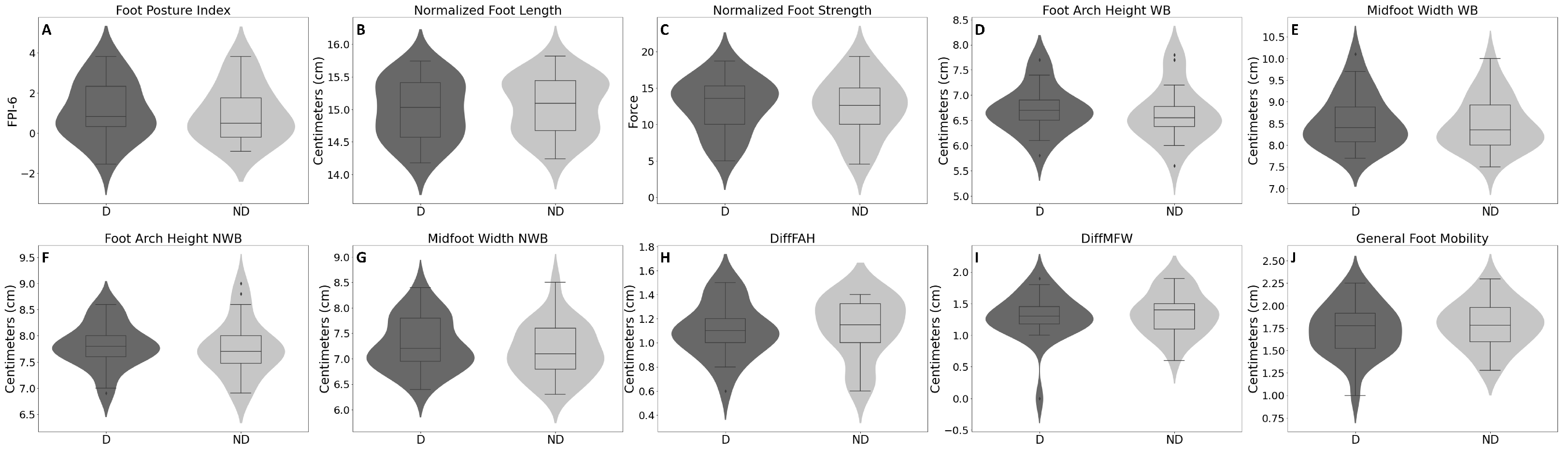
| Parameters | Dominant Mean (SD) | Non-Dominant Mean (SD) | p-Value | 95% CI [Lower, Upper] |
|---|---|---|---|---|
| Foot Posture Index (FPI-6) | 1.16 (1.45) | 1.00 (1.42) | 0.213 | [−0.098, 0.418] |
| Normalized Foot Length | 14.99 (0.47) | 15.06 (0.44) | 0.029 | [−0.129, −0.008] |
| Normalized Foot Strength | 12.63 (3.73) | 12.38 (3.90) | 0.201 | [−0.147, 0.658] |
| Foot Arch Height (cm)—WB | 6.72 (0.47) | 6.64 (0.55) | 0.127 | [−0.024, 0.183] |
| Midfoot Width (cm)—WB | 8.57 (0.62) | 8.50 (0.61) | 0.180 | [−0.035, 0.1771] |
| Foot Arch Height (cm)—NWB | 7.82 (0.42) | 7.77 (0.54) | 0.368 | [−0.063, 0.163] |
| Midfoot Width (cm)—NWB | 7.28 (0.52) | 7.16 (0.53) | 0.012 | [0.029, 0.212] |
| DiffFAH (cm) | 1.10 (0.23) | 1.13 (0.25) | 0.567 | [−0.133, 0.075] |
| DiffMFW (cm) | 1.29 (0.37) | 1.34 (0.34) | 0.494 | [−0.199, 0.099] |
| General Foot Mobility (cm) | 1.73 (0.29) | 1.79 (0.26) | 0.101 | [−0.135, 0.013] |
| Parameters | Normalized Strength (Dominant) Value | p-Value 95% | Normalized Strength (Non-Dominant) Value | p-Value 95% |
|---|---|---|---|---|
| General Foot Mobility (cm) | 0.063 (r) | 0.771 | 0.119 (r) | 0.579 |
| [−0.349, 0.455] | [−0.299, 0.499] | |||
| Normalized Foot Length | −0.076 (r) | 0.723 | 0.117 (r) | 0.587 |
| [−0.338, 0.465] | [−0.301, 0.497] | |||
| Foot Arch Height (WB) (cm) | −0.224 (rho) | 0.292 | −0.095 (rho) | 0.658 |
| [−0.575, 0.197] | [−0.480, 0.320] | |||
| Midfoot Width (WB) (cm) | 0.134 (rho) | 0.532 | 0.146 (rho) | 0.495 |
| [−0.285, 0.510] | [−0.273, 0.519] | |||
| DiffFAH (cm) | 0.158 (rho) | 0.461 | 0.145 (rho) | 0.500 |
| [−0.262, 0.528] | [−0.275, 0.518] | |||
| DiffMFW (cm) | 0.124 (rho) | 0.565 | −0.001 (rho) | 0.995 |
| [−0.294, 0.502] | [−0.404, 0.402] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, A.C.F.; Catelli, D.S.; Bedo, B.L.S.; Cesar, G.M.; Santos, T.F.; Junqueira, E.B.; Santiago, P.R.P. Association between the Strength of Flexor Hallucis Brevis and Abductor Hallucis and Foot Mobility in Recreational Runners. Biomechanics 2022, 2, 613-622. https://doi.org/10.3390/biomechanics2040048
Andrade ACF, Catelli DS, Bedo BLS, Cesar GM, Santos TF, Junqueira EB, Santiago PRP. Association between the Strength of Flexor Hallucis Brevis and Abductor Hallucis and Foot Mobility in Recreational Runners. Biomechanics. 2022; 2(4):613-622. https://doi.org/10.3390/biomechanics2040048
Chicago/Turabian StyleAndrade, Antonio C. F., Danilo S. Catelli, Bruno L. S. Bedo, Guilherme M. Cesar, Thiago F. Santos, Eduardo B. Junqueira, and Paulo R. P. Santiago. 2022. "Association between the Strength of Flexor Hallucis Brevis and Abductor Hallucis and Foot Mobility in Recreational Runners" Biomechanics 2, no. 4: 613-622. https://doi.org/10.3390/biomechanics2040048
APA StyleAndrade, A. C. F., Catelli, D. S., Bedo, B. L. S., Cesar, G. M., Santos, T. F., Junqueira, E. B., & Santiago, P. R. P. (2022). Association between the Strength of Flexor Hallucis Brevis and Abductor Hallucis and Foot Mobility in Recreational Runners. Biomechanics, 2(4), 613-622. https://doi.org/10.3390/biomechanics2040048






