Electronic Structure Regulation Enhances the Urea Oxidation Reaction Performance of the NiCo-MOF Catalyst
Abstract
1. Introduction
2. Experimental
2.1. Chemical Reagents
2.2. Pretreatment of Nickel Foam
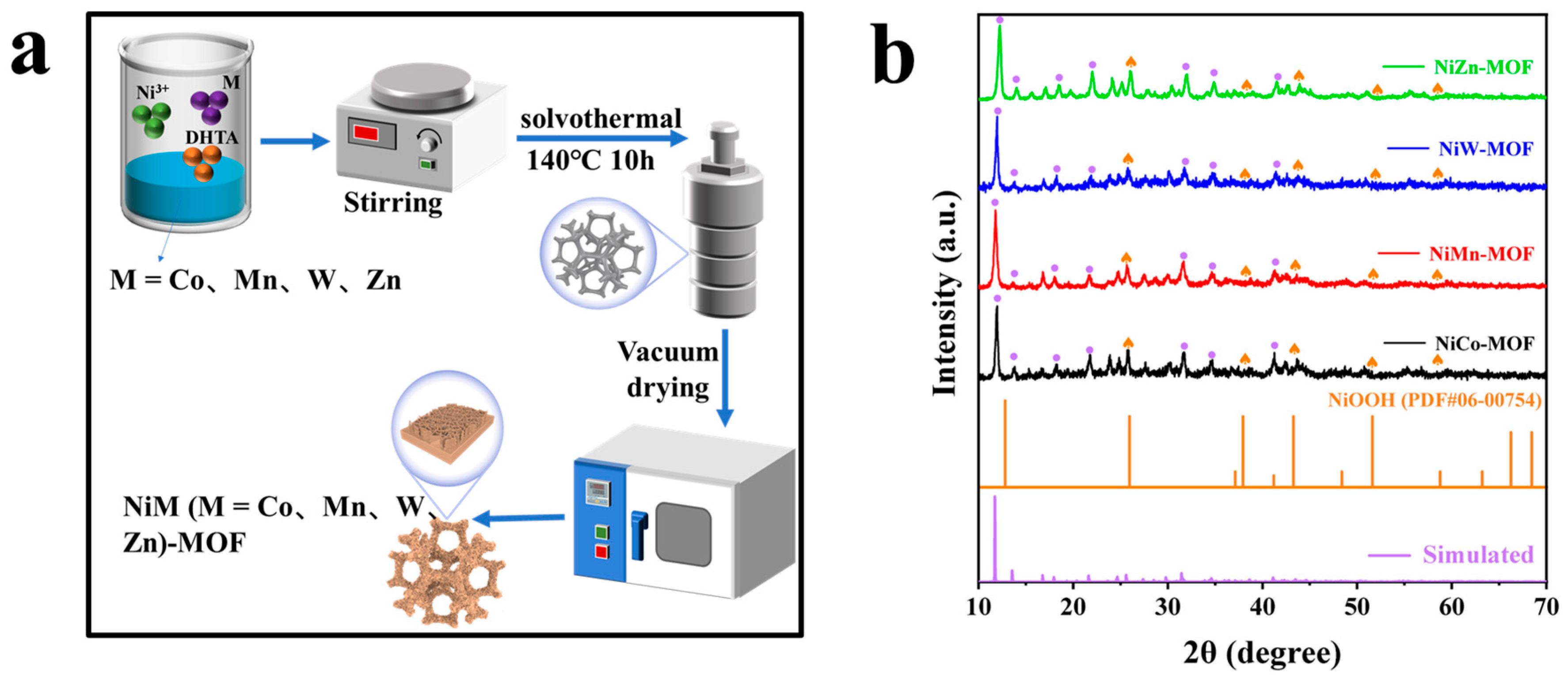
2.3. Preparation of NiM (M = Co, Mn, W, Zn)-MOF Catalyst
2.4. Characterization of Materials
2.5. Electrochemical Characterization
3. Results and Discussion
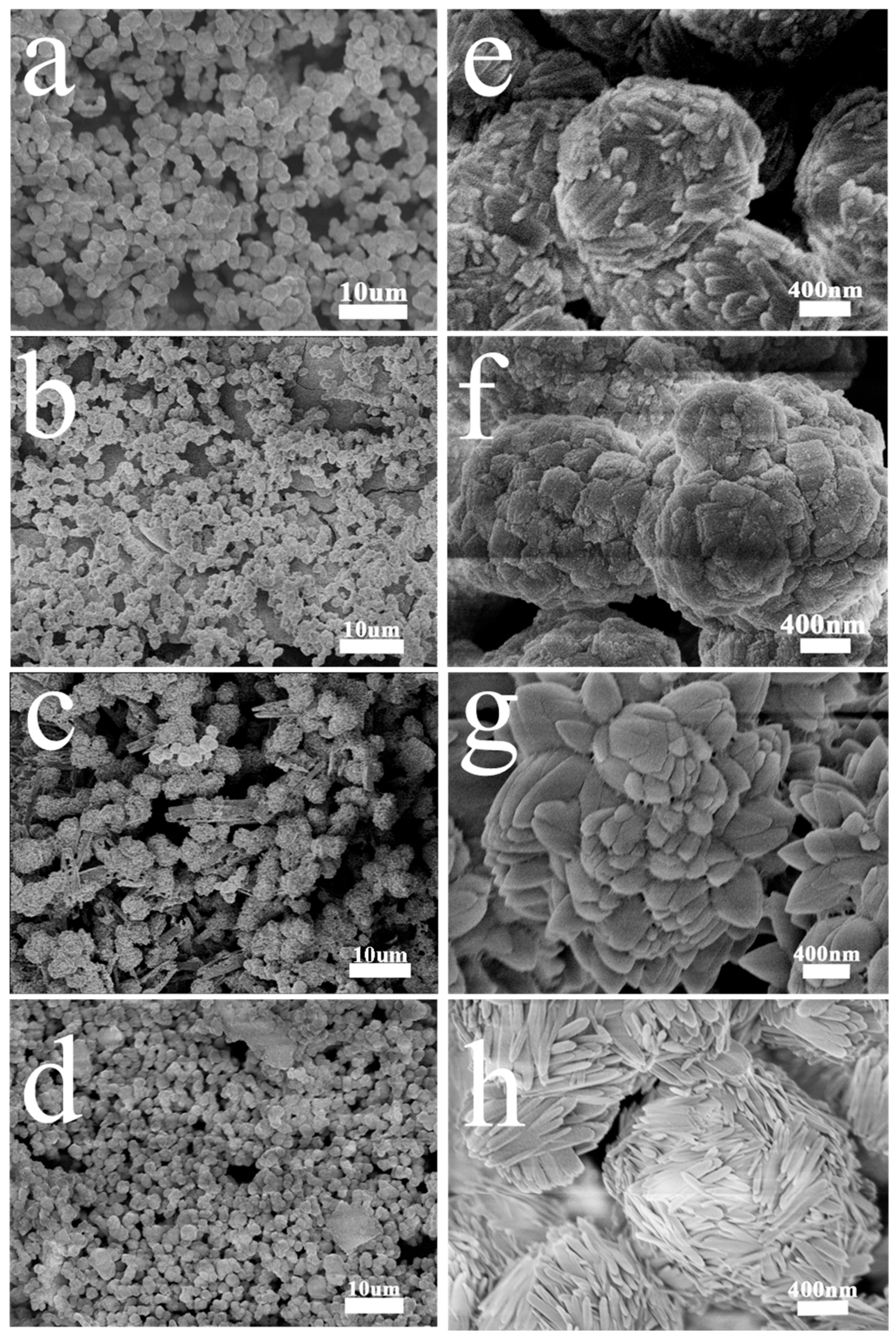
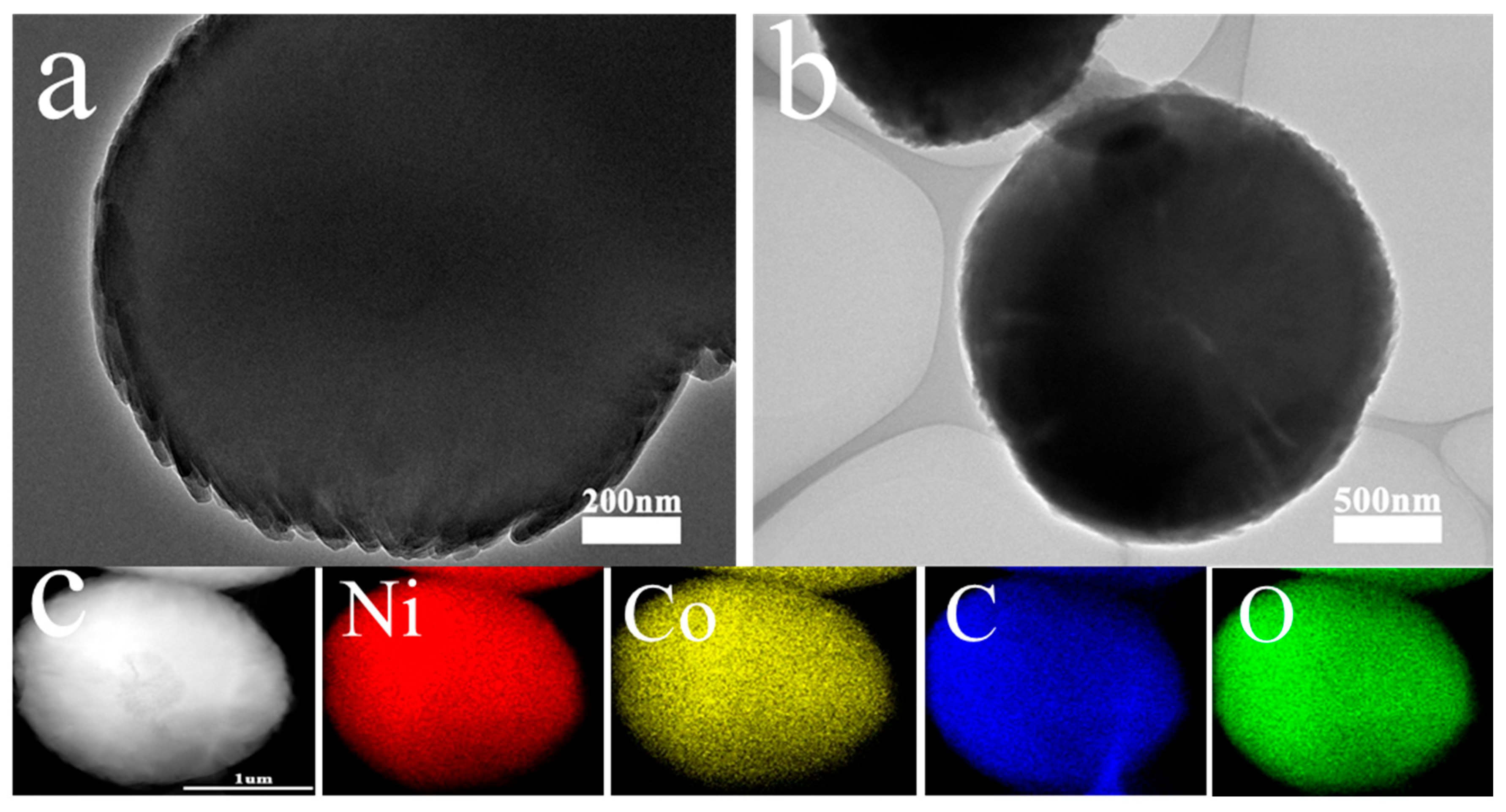
3.1. Characterization of the Sample
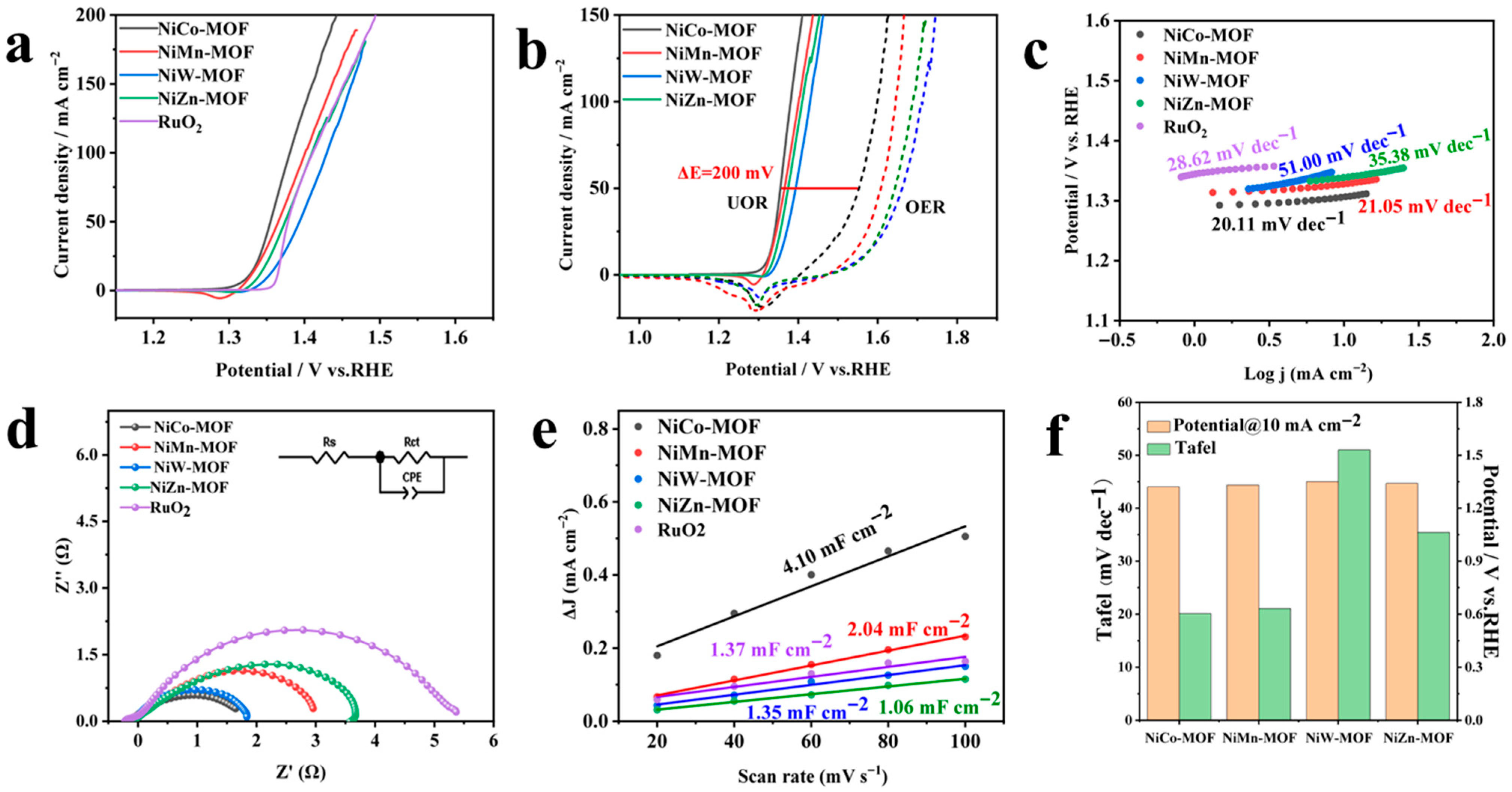
3.2. Electrochemical Characterization
3.2.1. OER and UOR Performance
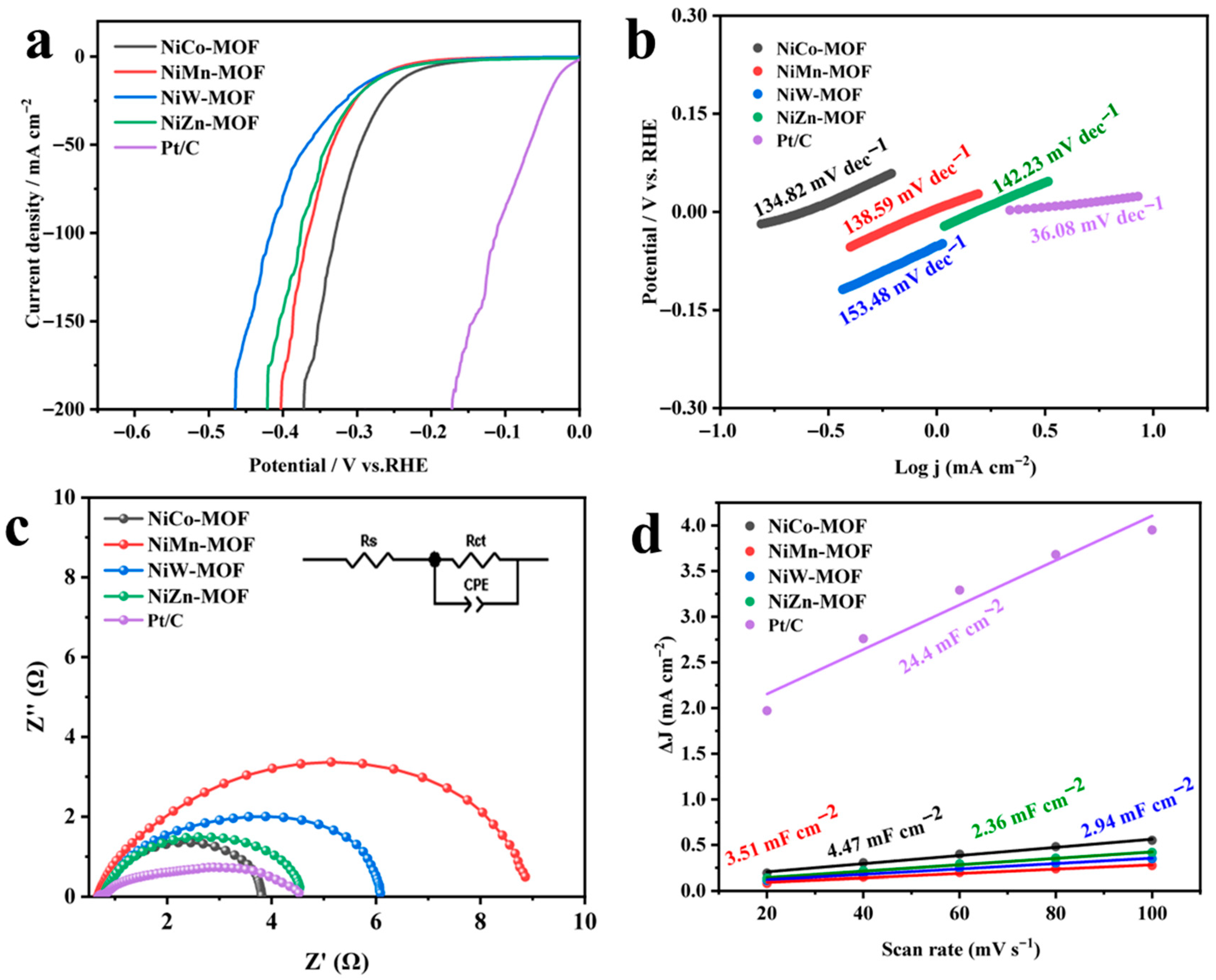
3.2.2. HER Performance
3.2.3. Performance Evaluation of NiCo(2:X)-MOF with Varied Cobalt Incorporation Levels
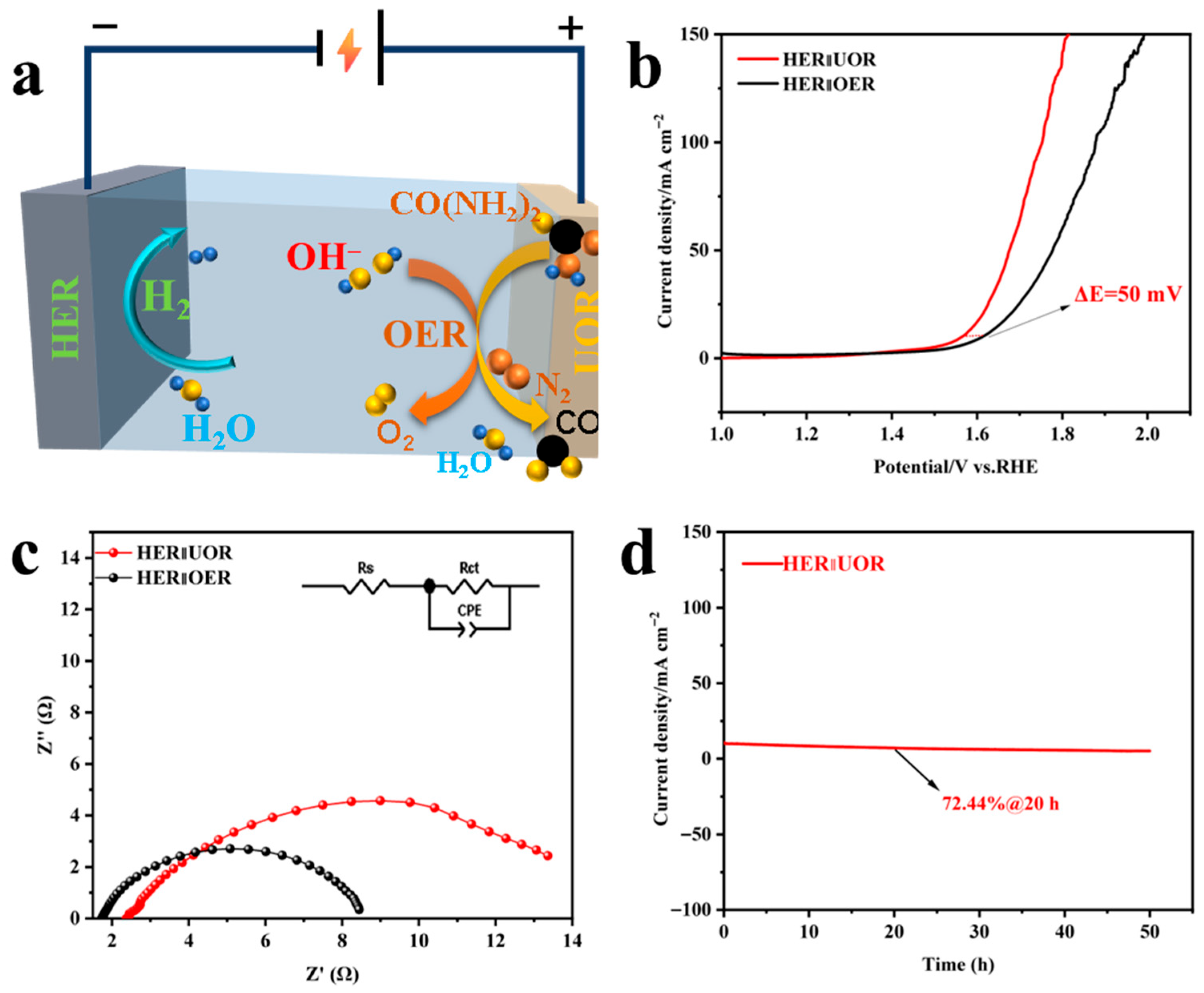
3.2.4. Urea-Assisted Overall Water Splitting
3.3. Characterization After UOR Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, X.; Xu, L.; Zeng, Q.; Zhang, Z.; Xu, Z.; Yin, C.; Wang, X. A NiMOF integrated with conductive materials for efficient bifunctional electrocatalysis of urea oxidation and oxygen evolution reactions. Dalton Trans. 2024, 53, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Shaddad, M.N.; Alharthi, A.I.; Aladeemy, S.A.; Arunachalam, P. Engineering oxygen vacancy on nickel-doped iron oxide nanorods as efficient bifunctional electrocatalysts for oxygen evolution and urea oxidation reaction. J. Taiwan Inst. Chem. Eng. 2024, 27, 105928. [Google Scholar] [CrossRef]
- Bian, H.; Wang, C.; Zhao, S.; Han, G.; Xie, G.; Qi, P.; Liu, X.; Zeng, Y.; Zhang, D.; Wang, P. Preparation of highly efficient high-entropy alloy catalysts with electrodeposition and corrosion engineering for OER electrocatalysis. Int. J. Hydrog. Energy 2024, 57, 651–659. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, K.; Zhang, B.; Xia, J.; Wu, J.; Zhang, Z.; Huang, Y. Corrosion engineering on AlCoCrFeNi high-entropy alloys toward highly efficient electrocatalysts for the oxygen evolution of alkaline seawater. Int. J. Miner. Metall. Mater. 2023, 30, 1922–1932. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhang, S.-F.; Zuo, L.-H.; Li, J.-K.; Guo, J.-X.; Wang, P.; Sun, J.-F.; Dai, L. Recent advances of high-entropy electrocatalysts for water electrolysis by electrodeposition technology: A short review. Rare Met. 2024, 1011, 178435. [Google Scholar] [CrossRef]
- Iqbal, W.; Hameed, A.; Tariq, I.; Shah, S.S.A.; Nadeem, M.A. Cobalt hydroxide supported nickel nanoparticles for an efficient electrocatalytic urea oxidation reaction. Electrochim. Acta 2023, 467, 143055. [Google Scholar] [CrossRef]
- Nourshargh, B.; Ghaffarinejad, A.; Barati Darband, G. Water splitting by CoFeLDH@NiFeS nanoelectrocatalyst assisted urea electrooxidation reaction. J. Environ. Chem. Eng. 2023, 12, 111629. [Google Scholar] [CrossRef]
- Yu, H.; Xu, W.; Chang, H.; Xu, G.; Li, L.; Zang, J.; Huang, R.; Zhu, L.; Yu, B. Electrocatalytic Ni-Co Metal Organic Framework for Efficient Urea Oxidation Reaction. Processes 2023, 11, 3035. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Naresh, B.; Prasad, K.; Kim, J.; Yoo, K. NiO/ATO as an efficient bifunctional electrocatalysts for oxygen evolution and urea oxidation reactions. Inorg. Chem. Commun. 2024, 170, 113315. [Google Scholar] [CrossRef]
- Guo, F.; Cheng, D.; Chen, Q.; Liu, H.; Wu, Z.; Han, N.; Ni, B.-J.; Chen, Z. Amorphous electrocatalysts for urea oxidation reaction. Prog. Nat. Sci. Mater. Int. 2024, 34, 362–375. [Google Scholar] [CrossRef]
- Cao, X.; Wang, T.; Qin, H.; Lin, G.; Zhao, L.; Jiao, L. Crystalline—Amorphous interfaces of NiO-CrOx electrocatalysts for boosting the urea oxidation reaction. Nano Res. 2022, 16, 3665–3671. [Google Scholar] [CrossRef]
- Diao, Y.; Liu, Y.; Hu, G.; Zhao, Y.; Qian, Y.; Wang, H.; Shi, Y.; Li, Z. NiFe nanosheets as urea oxidation reaction electrocatalysts for urea removal and energy-saving hydrogen production. Biosens. Bioelectron. 2022, 211, 114380. [Google Scholar] [CrossRef]
- Rodrigues, E.M.; Fernandes, C.M.; Alves, O.C.; Santos, E.C.S.; Garcia, F.; Xing, Y.; Ponzio, E.A.; Silva, J.C.M. MagnetoElectroCatalysis: A new approach for urea electro-oxidation reaction on nickel-iron oxide catalyst. Int. J. Hydrog. Energy 2023, 51, 1460–1470. [Google Scholar] [CrossRef]
- Song, S.; Huang, X.; Yang, Y.; Feng, L. Se self-doped Ni(OH)2 for an efficient urea oxidation reaction. Chem. Commun. 2024, 60, 10906–10909. [Google Scholar] [CrossRef]
- He, J. Role of Zr in promoted activity of urea oxidation reaction and oxygen evolution reaction for NiFe layered double hydroxide. Int. J. Hydrog. Energy 2024, 57, 929–938. [Google Scholar] [CrossRef]
- Liu, H.; Luo, J.; Zhu, S.; Cui, Z.; Liang, Y.; Yu, S.; Wei, J. Endowing nickel nitride with moderate amount of Ni0 species for the enhanced urea oxidation reaction reactivity. J. Electroanal. Chem. 2023, 948, 117821. [Google Scholar] [CrossRef]
- Li, J.; Hu, F.; Hei, J.; Liu, G.; Wei, H.; Wang, N.; Wei, H. Hierarchical Ni–Mo–P nanoarrays toward efficient urea oxidation reaction. Dalton Trans. 2022, 51, 18059–18067. [Google Scholar] [CrossRef]
- Liu, J.; Du, Y.; Zheng, D.; Wang, S.; Hou, Y.; Zhang, J.; Lu, X.F. Nickel-Based Anode Electrocatalysts for Hydrogen Production. ACS Mater. Lett. 2023, 6, 466–481. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea electrolysis: Direct hydrogen production from urine. Chem. Commun. 2009, 32, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Asiain-Mira, R.; Smith, C.; Zamora, P.; Monsalvo, V.M.; Torrente-Murciano, L. Hydrogen production from urea in human urine using segregated systems. Water Res. 2022, 222, 118931. [Google Scholar] [CrossRef]
- Kun, X.; Linjian, Y.; Yang, X.; Haidong, Z.; Jia, C.; Yuan, G. Cerium-incorporated Ni2P nanosheets for enhancing hydrogen production from overall water splitting and urea electrolysis. J. Alloys Compd. 2022, 912, 165234. [Google Scholar] [CrossRef]
- Zheng, J. Pt-free NiCo electrocatalysts for oxygen evolution by seawater splitting. Electrochim. Acta 2017, 247, 381–391. [Google Scholar] [CrossRef]
- Zemtsova, V.M.; Oshchepkov, A.G.; Savinova, E.R. Unveiling the Role of Iron in the Nickel-Catalyzed Urea Oxidation Reaction. ACS Catal. 2023, 13, 13466–13473. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Huang, M.; Meng, C.; Chang, L.; Xu, D.; Pei, W. Design and fabrication of intermetallic NiCo electrocatalysts for alkaline HER. Nanoscale 2024, 16, 15148. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Fang, Y.; Tang, C.; Meng, F.; Miao, N.; Sa, B.; Zhou, J.; Sun, Z. Rational design of bimetallic MBene for efficient electrocatalytic nitrogen reduction. J. Colloid Interface Sci. 2024, 670, 687–697. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, H.; Gu, Z.; Cao, M.; He, C.; Li, J.; Li, Z.; Tian, L. Modulation of the electronic structure of CoP by surface and interface codoping boosts electrocatalytic oxygen evolution reaction. Int. J. Hydrog. Energy 2023, 51, 914–921. [Google Scholar] [CrossRef]
- Yin, X.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.; Zhang, D.; Yao, J.; Wang, G. FeNi supported on carbon sponge for efficient urea oxidation in direct urea fuel cell. J. Colloid Interface Sci. 2023, 654, 36–45. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, K.; Zhong, B.; Yu, D.; Ye, F.; Liu, J.; Dutta, J.; Zhang, P. MOF-Derived Iron-Cobalt Phosphide Nanoframe as Bifunctional Electrocatalysts for Overall Water Splitting. Energy Environ. Mater. 2024, 7, 12747. [Google Scholar] [CrossRef]
- Kayode Adesina, A.; Nobanathi Wendy, M. Porous metal−organic framework (MOF)-based and MOF-derived electrocatalytic materials for energy conversion. Mater. Today Energy 2021, 21, 100816. [Google Scholar] [CrossRef]
- Wang, K.; Kang, J.; Jin, L.; Yang, L.; Liu, Y.; Li, Y.; Chen, G.; Xu, H. Defect engineering of MOF toward enhanced electrocatalytic water oxidation. Ionics 2023, 29, 5397–5403. [Google Scholar] [CrossRef]
- Marquez-Montes, R.A.; Kawashima, K.; Son, Y.J.; Weeks, J.A.; Sun, H.H.; Celio, H.; Ramos-Sánchez, V.H.; Mullins, C.B. Mass transport-enhanced electrodeposition of Ni–S–P–O films on nickel foam for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 7736–7749. [Google Scholar] [CrossRef]
- Yan, X.; Hu, Q.-T.; Wang, G.; Zhang, W.-D.; Liu, J.; Li, T.; Gu, Z.-G. NiCo layered double hydroxide/hydroxide nanosheet heterostructures for highly efficient electro-oxidation of urea. Int. J. Hydrog. Energy 2020, 45, 19206–19213. [Google Scholar] [CrossRef]
- Kuang, M.; Han, P.; Huang, L.; Cao, N.; Qian, L.; Zheng, G. Electronic Tuning of Co, Ni-Based Nanostructured (Hydr)oxides for Aqueous Electrocatalysis. Adv. Funct. Mater. 2018, 28, 1804886. [Google Scholar] [CrossRef]
- Khalafallah, D.; Ouyang, C.; Zhi, M.; Hong, Z. Heterostructured Nickel-Cobalt Selenide Immobilized onto Porous Carbon Frameworks as an Advanced Anode Material for Urea Electrocatalysis. ChemElectroChem 2019, 6, 5191–5202. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Du, X.; Zhang, X. Electrocatalytic urea oxidation based on M (M = Fe, Cr, Mo)-Ni3S2/Co3S4: Performance optimization and mechanism analysis. Fuel 2024, 376, 132749. [Google Scholar] [CrossRef]
- Lu, X.; Wang, T.; Cao, M.; Cheng, W.; Yang, H.; Xu, H.; He, C.; Tian, L.; Li, Z. Homogeneous NiMoO4–Co(OH)2 bifunctional heterostructures for electrocatalytic oxygen evolution and urea oxidation reaction. Int. J. Hydrog. Energy 2023, 48, 34740–34749. [Google Scholar] [CrossRef]
- Shukla, R.K.; Chaturvedi, A.K.; Volla, C.M.R. Catalytic Cascade Cyclization and Regioselective Hydroheteroarylation of Unactivated Alkenes. ACS Catal. 2021, 11, 7750–7761. [Google Scholar] [CrossRef]
- Zhao, X.; Che, M.; Gong, Y. Synthesis and performance study of Fe2WO6@Ni3S2-WS2/NF for efficient electrolytic water to hydrogen. Sustain. Mater. Technol. 2025, 43, e01302. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Yang, Y.; Li, S.; Xiao, X. Electronic Structure Regulation Enhances the Urea Oxidation Reaction Performance of the NiCo-MOF Catalyst. Nanoenergy Adv. 2025, 5, 17. https://doi.org/10.3390/nanoenergyadv5040017
Yao L, Yang Y, Li S, Xiao X. Electronic Structure Regulation Enhances the Urea Oxidation Reaction Performance of the NiCo-MOF Catalyst. Nanoenergy Advances. 2025; 5(4):17. https://doi.org/10.3390/nanoenergyadv5040017
Chicago/Turabian StyleYao, Lang, Yanzhi Yang, Sirong Li, and Xuechun Xiao. 2025. "Electronic Structure Regulation Enhances the Urea Oxidation Reaction Performance of the NiCo-MOF Catalyst" Nanoenergy Advances 5, no. 4: 17. https://doi.org/10.3390/nanoenergyadv5040017
APA StyleYao, L., Yang, Y., Li, S., & Xiao, X. (2025). Electronic Structure Regulation Enhances the Urea Oxidation Reaction Performance of the NiCo-MOF Catalyst. Nanoenergy Advances, 5(4), 17. https://doi.org/10.3390/nanoenergyadv5040017





