Liquid Metal Nanoenergy Systems: Progress and Challenges
Abstract
1. Introduction
2. Fundamental Properties of Liquid Metals and Nano-Liquid Metals
2.1. Fundamental Properties of Liquid Metals
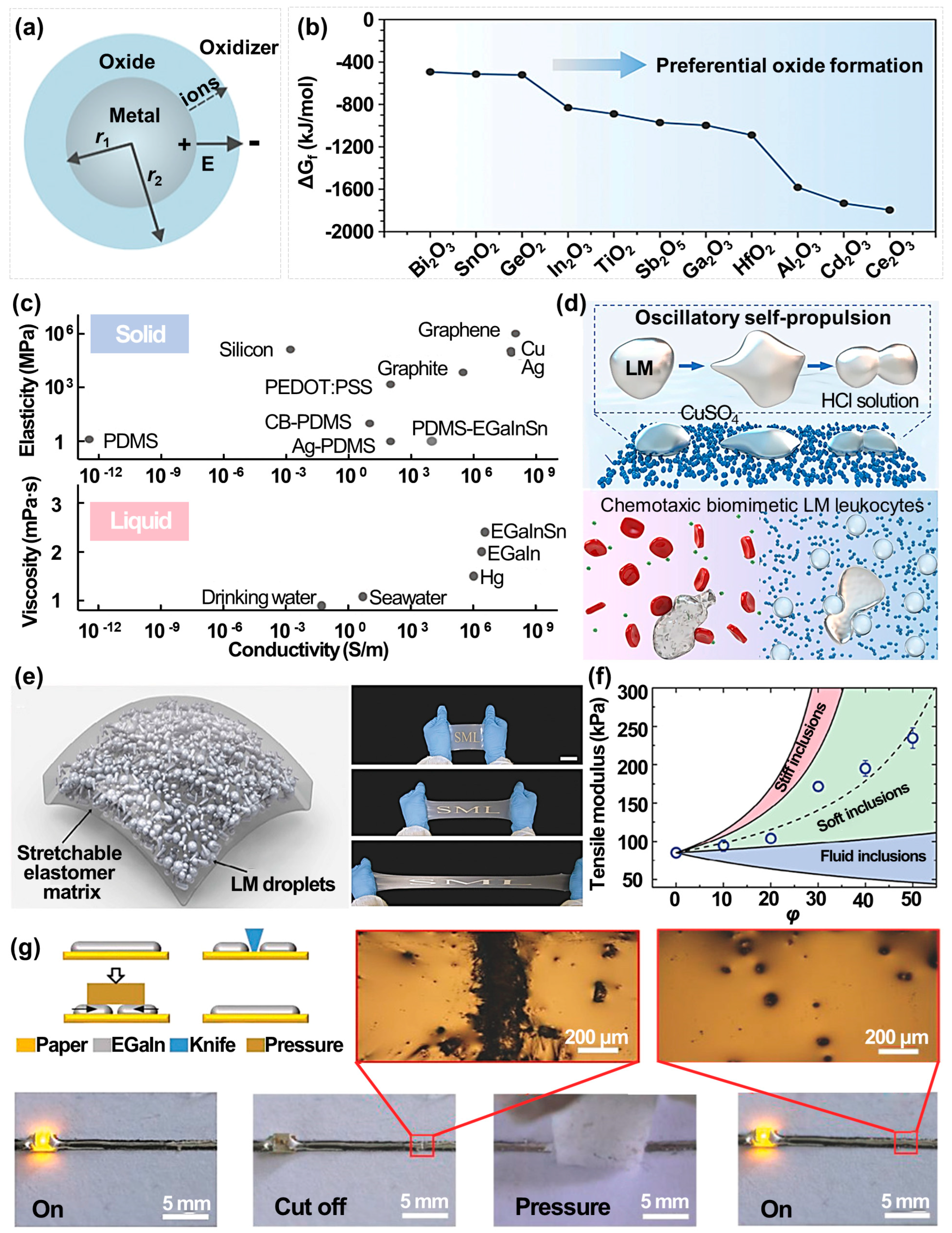
2.2. Novel Properties of Liquid Metals Imparted by the Nanoscale
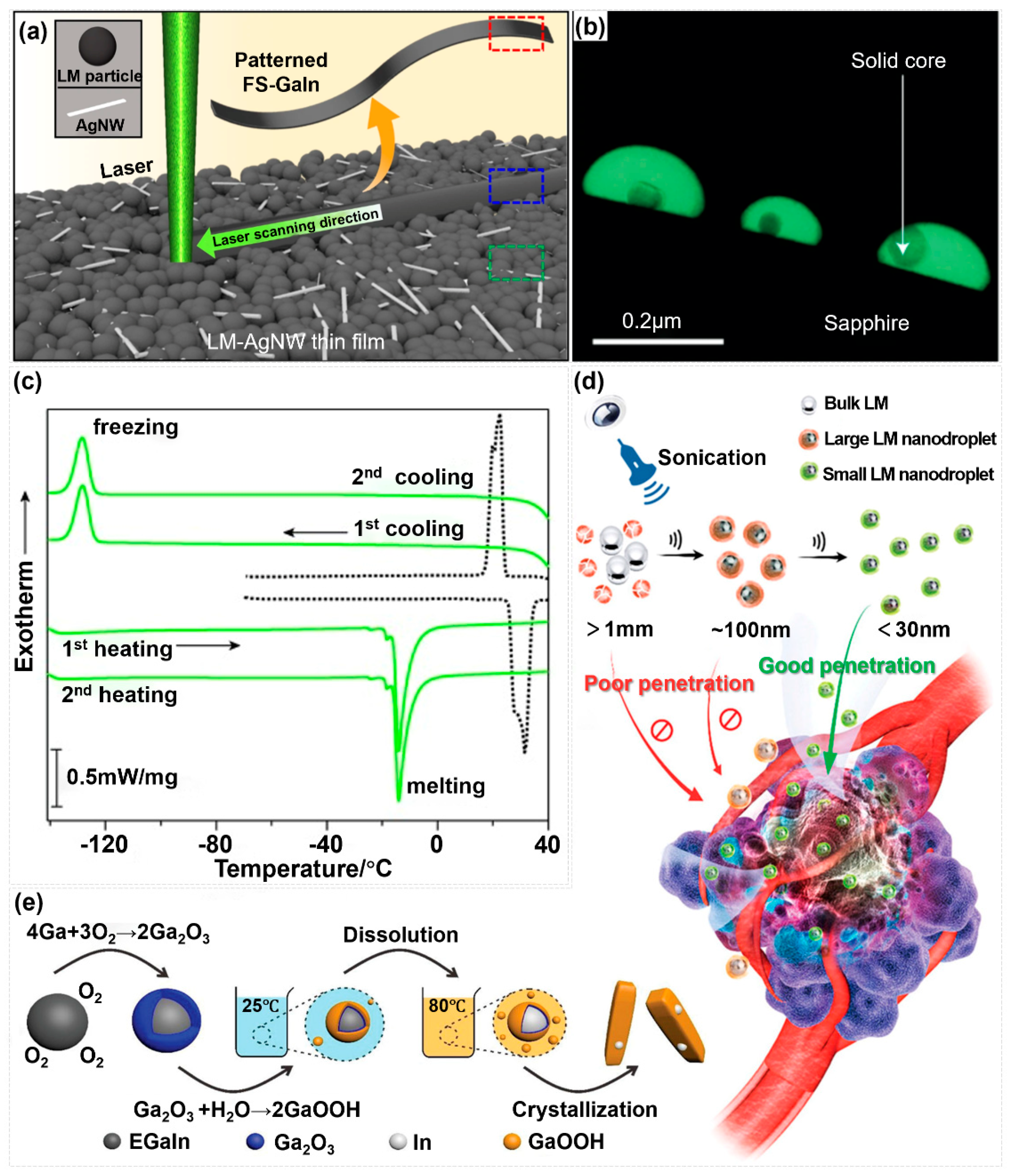
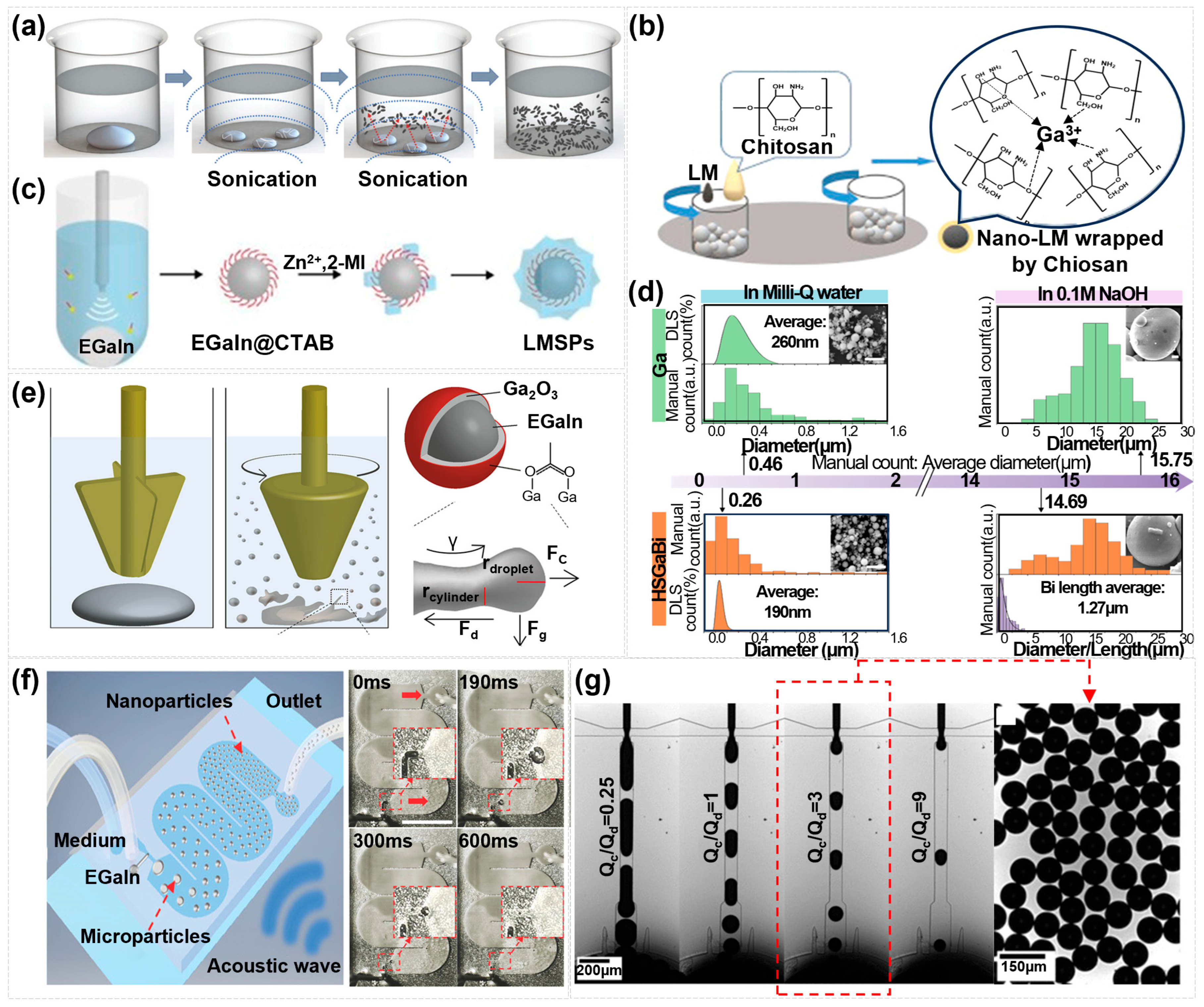
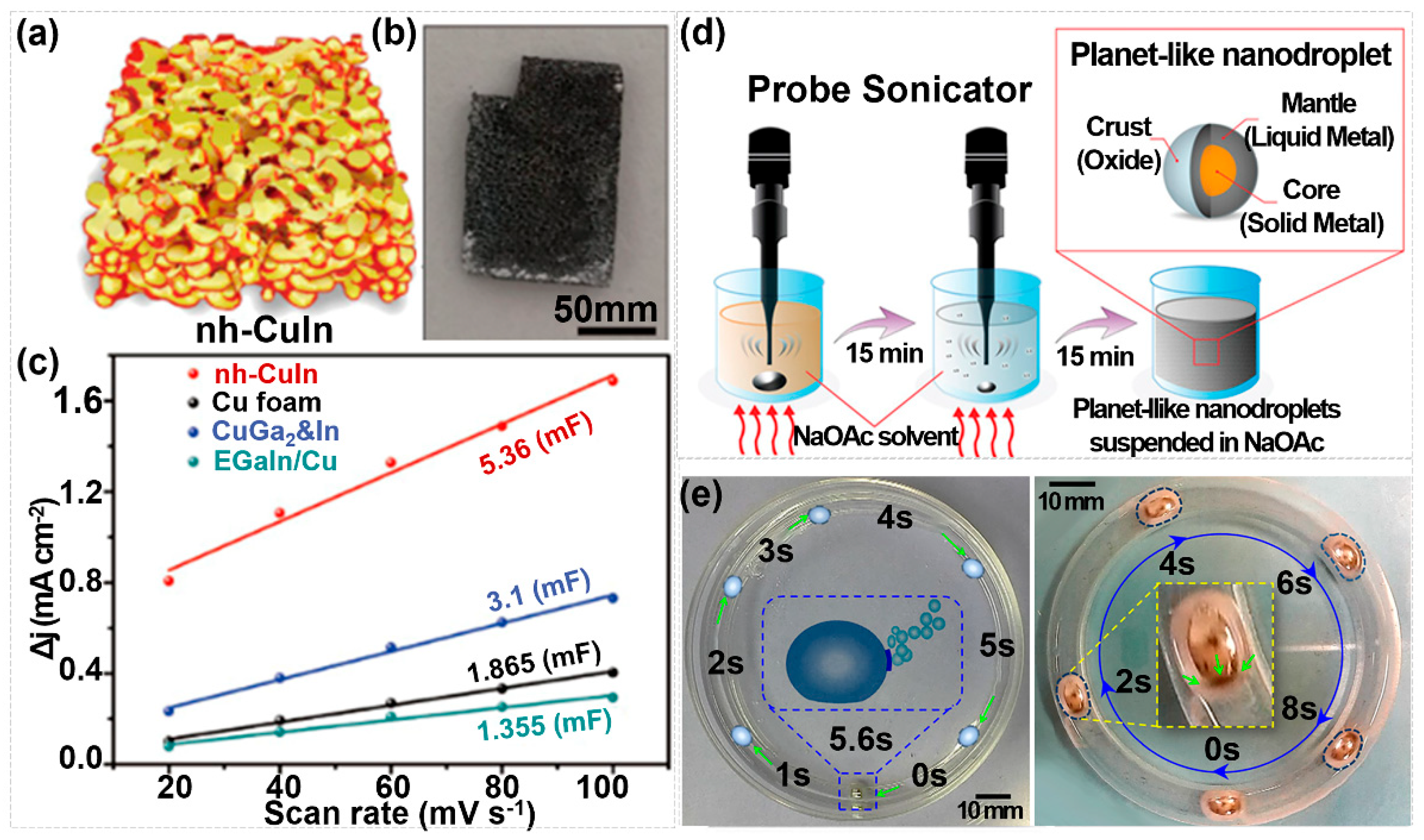
3. Synthesis Strategies for Nano-Liquid Metals
4. The Applications of Nano-Liquid Metals
4.1. Nano-Liquid Metals for Energy Storage Devices

4.2. Nano-Liquid Metals for Energy Harvesting
4.2.1. The Photothermal Effect of Nano-Liquid Metals

4.2.2. Liquid Metal Nanogenerators
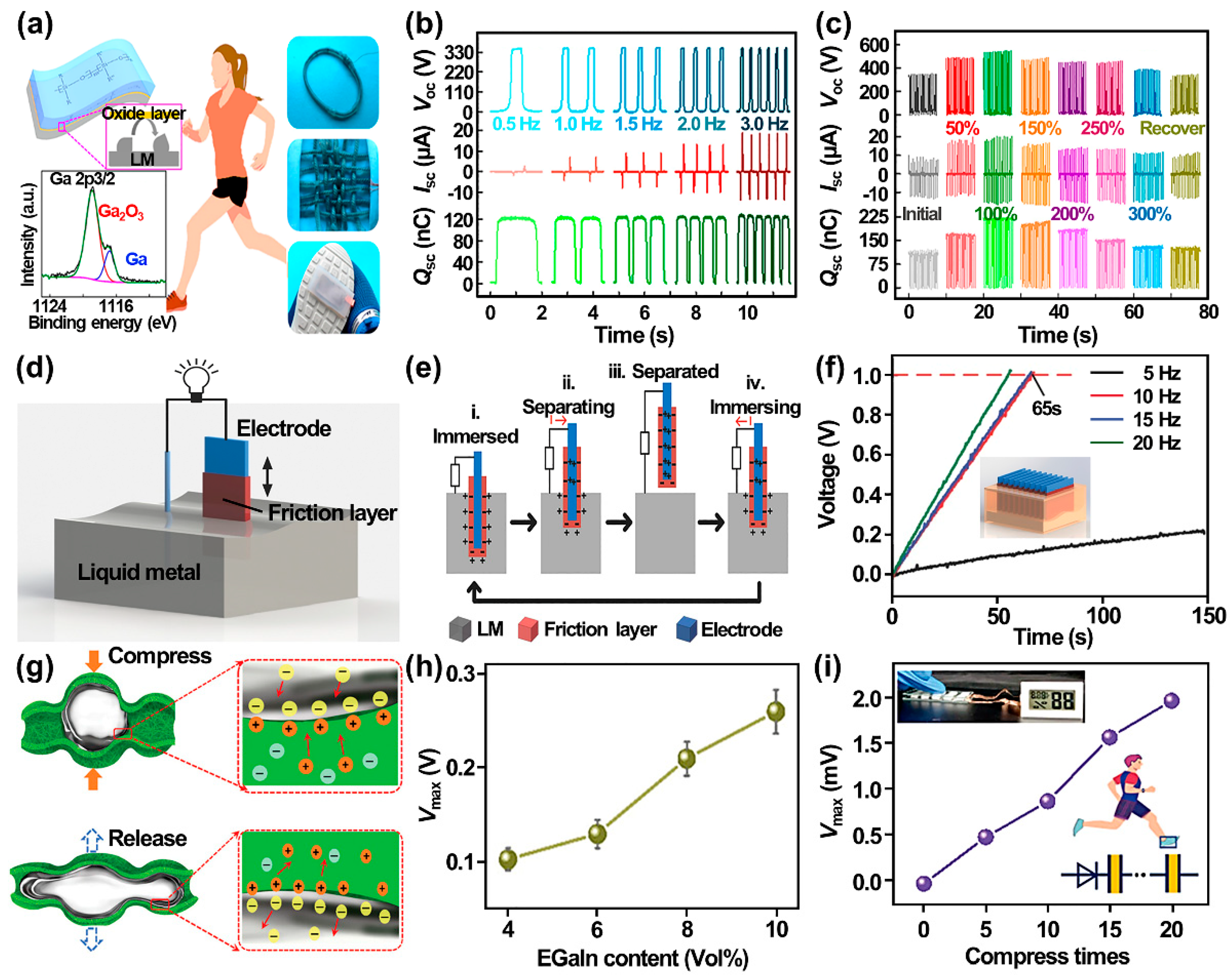
4.3. Nano-Liquid Metals for Catalysis and Energy Conversion
5. Discussion and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Wang, S.; Zhang, M.; Li, T.; Hu, G.; Kong, D. Fully solution processed liquid metal features as highly conductive and ultrastretchable conductors. npj Flex. Electron. 2021, 5, 25. [Google Scholar] [CrossRef]
- Qiao, M.W.; Xing, Z.R.; Fu, J.H.; Liu, J. Multiphase flow physics of room temperature liquid metals and its applications. Sci. China Technol. Sci. 2023, 66, 1483. [Google Scholar] [CrossRef]
- Li, X.; Hou, K.; Hao, D.; Long, Y.; Song, K. Liquid metal-gel (LM-Gel) with conductivity and deformability. J. Mater. Chem. C 2023, 11, 15008–15015. [Google Scholar] [CrossRef]
- Li, Y.; Feng, S.; Cao, S.; Zhang, J.; Kong, D. Printable liquid metal microparticle ink for ultrastretchable electronics. ACS Appl. Mater. Interfaces 2020, 12, 50852–50859. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, K.; Zhao, Y.; Ye, C. Highly conductive, ultratough, and adhesive eutectogels with environmental tolerance enabled by liquid metal composites. Small 2025, 21, 2410806. [Google Scholar] [CrossRef]
- Lopes, P.A.; Santos, B.C.; de Almeida, A.T.; Tavakoli, M. Reversible polymer-gel transition for ultra-stretchable chip-integrated circuits through self-soldering and self-coating and self-healing. Nat. Commun. 2021, 12, 4666. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J. Nano liquid metal as an emerging functional material in energy management, conversion and storage. Nano Energy 2013, 2, 863. [Google Scholar] [CrossRef]
- Bae, Y.; Wei, Y.; Bhuyan, P.; Mun, S.; Park, S. Soft and stretchable electromagnetic energy-harvesting devices with topographically wrinkled liquid-metal electrodes. ACS Appl. Polym. Mater. 2024, 6, 1992–2000. [Google Scholar] [CrossRef]
- Jin, B.; Ye, Z.; Xing, Z.; Li, N.; Guo, M.; Chen, X.; Li, Y.; Su, J.; Qiu, C.; Li, Z.; et al. Fabrication of flexible reconfigurable battery based on liquid metal. Int. J. Extrem. Manuf. 2025, 7, 065505. [Google Scholar] [CrossRef]
- Qin, C.; Song, P.; Sun, X.; Wang, R.; Wei, M.; Mao, M. Actuation technique of liquid metal in thermal management: A review. Appl. Therm. Eng. 2024, 248, 123290. [Google Scholar] [CrossRef]
- Guo, R.; Li, T.; Jiang, C.; Zong, H.; Li, X.; Wan, C.; Yu, H.; Huang, X. Pressure regulated printing of semiliquid metal on electrospinning film enables breathable and waterproof wearable electronics. Adv. Fiber Mater. 2023, 6, 354–366. [Google Scholar] [CrossRef]
- Suarez, F.; Parekh, D.P.; Ladd, C.; Vashaee, D.; Dickey, M.D.; Öztürk, M.C. Flexible thermoelectric generator using bulk legs and liquid metal interconnects for wearable electronics. Appl. Energy 2017, 202, 736–745. [Google Scholar] [CrossRef]
- Duan, L.; Zhou, T.; Mu, W.; Deng, Z.; Guo, M.; Wang, Q.; Yang, H.; Liu, J. Liquid-metal molecular scissors. ACS Appl. Mater. Interfaces 2024, 16, 4212. [Google Scholar] [CrossRef]
- Liang, S.T.; Wang, H.-Z.; Liu, J. Progress, mechanism and application of liquid metal catalysis system: A Review. Chem. Eur. J. 2018, 24, 17616. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Guan, T.; Tao, Y.; Guo, M.; Liu, J. Chemotaxic biomimetic liquid metallic leukocytes. Matter 2025, 8, 101991. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y.; Zhang, Q.; Wang, L.; Liu, J. Controllable dispersion and reunion of liquid metal droplets. Sci. China Mater. 2018, 62, 407–415. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, X.; Li, J.; Zhou, Y.; Liu, J. Liquid metal phagocytosis: Intermetallic wetting induced particle internalization. Adv. Sci. 2017, 4, 1700024. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Mehta, S.K. Selenium nanomaterials: An overview of recent developments in synthesis, properties and potential applications. Prog. Mater. Sci. 2016, 83, 270–329. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Hu, C.; He, G.; Yang, Y.; Wang, N.; Zhang, Y.; Su, Y.; Zhao, F.; Wu, J.; Wang, L.; Lin, Y.; et al. Nanomaterials regulate bacterial quorum sensing: Applications, mechanisms, and optimization strategies. Adv. Sci. 2024, 11, 2306070. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Shi, D.; Xiao, R.; Kang, H.; Li, F.; Ling, D. Stimuli-responsive nanomaterials for wireless and precise neuromodulation. Small Methods 2025, e01275. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-nanomaterial-based flexible batteries for wearable electronics. Adv. Mater. 2019, 31, e1800716. [Google Scholar] [CrossRef]
- Zhu, J.; Hersam, M.C. Assembly and electronic applications of colloidal nanomaterials. Adv. Mater. 2017, 29, 1603895. [Google Scholar] [CrossRef]
- Gohar, O.; Zubair Khan, M.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Ramzan Abdul Karim, M.; Ali, F.; Bilal Hanif, M.; Motola, M. Nanomaterials for advanced energy applications: Recent advancements and future trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- McDowell, M.T.; Xiong, H.; Nazemi, M.; Peng, J.; Lutkenhaus, J.L.; Wang, R.; Djire, A.; Sankaran, A.; Leem, J.; Gogotsi, Y. Nanomaterials in the future of energy research. Cell Rep. Phys. Sci. 2023, 4, 101605. [Google Scholar] [CrossRef]
- Rehman, W.U.; Manj, R.Z.A.; Ma, Y.; Yang, J. The promising potential of gallium based liquid metals for energy storage. Chempluschem 2024, 89, e202300767. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, Y.; Liu, J. Liquid metal technology in solar power generation—Basics and applications. Sol. Energy Mater. Sol. Cells 2021, 222, 110925. [Google Scholar] [CrossRef]
- Xu, B.; Peng, W.; He, J.; Zhang, Y.; Song, X.; Li, J.; Zhang, Z.; Luo, Y.; Meng, X.; Cai, C.; et al. Liquid metal-based triboelectric nanogenerators for energy harvesting and emerging applications. Nano Energy 2024, 120, 109107. [Google Scholar] [CrossRef]
- Fatima, S.S.; Zuraiqi, K.; Zavabeti, A.; Krishnamurthi, V.; Kalantar-Zadeh, K.; Chiang, K.; Daeneke, T. Current state and future prospects of liquid metal catalysis. Nat. Catal. 2023, 6, 1131–1139. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Wu, W.; Zhu, Y.; Li, K.; Su, X.; Xie, H.; Zhang, X.; Xu, H.; Wang, K.; et al. A novel strategy of fabricated flexible ITO electrode by liquid metal ultra-thin oxide film. J. Mater. 2022, 8, 1205–1212. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, J.; Fu, L. Liquid Metals: A novel possibility of fabricating 2D metal oxides. Adv. Mater. 2021, 33, 2005544. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, S.; Yu, J.; Zhang, J.; Sun, Y.; Kong, D. A facile and scalable patterning approach for ultrastretchable liquid metal features. Lab Chip 2022, 22, 4933. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, B.; Chen, Y.; Chen, Y.; Zhang, J.; Liu, H. Soft robots with plant-inspired gravitropism based on fluidic liquid metal. Adv. Sci. 2024, 11, 2306129. [Google Scholar] [CrossRef] [PubMed]
- Handschuh-Wang, S.; Gan, T.; Wang, T.; Stadler, F.J.; Zhou, X. Surface tension of the oxide skin of gallium-based liquid metals. Langmuir 2021, 37, 9017–9025. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, M.; Zhou, Y.; Liu, J. Liquid metal droplet dynamics. Droplet 2024, 3, e104. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, L.; Liu, J. Synthetically chemical-electrical mechanism for controlling large scale reversible deformation of liquid metal objects. Sci. Rep. 2014, 4, 7116. [Google Scholar] [CrossRef]
- Fassler, A.; Majidi, C. Liquid-phase metal inclusions for a conductive polymer composite. Adv. Mater. 2015, 27, 1928–1932. [Google Scholar] [CrossRef]
- Gu, W.; Guo, Q.; Zhang, Y.; Li, Y.; Zhang, Q.; Li, K.; Hou, C.; Wang, H. Activation-independent biphasic liquid metal conductor enables multilayer stretchable electronics. Mater. Today 2025, 88, 89–98. [Google Scholar] [CrossRef]
- Liu, X.; Wan, C.; Liu, J.; Xu, H.; Liu, Y.; Liu, Y.; Liu, Y.; Liu, J.; Wang, H.; Fan, H.; et al. Liquid metal-based dynamic conformal electrodes. Soft Sci. 2025, 5, 34. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Cui, Y.; Liu, J. Self-growing and serpentine locomotion of liquid metal induced by copper ions. ACS Appl. Mater. Interfaces 2018, 10, 22889–22895. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, J. A polarized liquid metal worm squeezing across a localized irregular gap. RSC Adv. 2017, 7, 11049–11056. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Yuan, B.; Liu, J.; Sun, X. Liquid metal transformable machines. Acc. Mater. Res. 2021, 2, 1227–1238. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Y.; Liu, J. Liquid metal enabled electrobiology: A new frontier to tackle disease challenges. Micromachines 2018, 9, 360. [Google Scholar] [CrossRef]
- Kim, S.; Oh, J.; Jeong, D.; Bae, J. Direct wiring of eutectic gallium-indium to a metal electrode for soft sensor systems. ACS Appl. Mater. Interfaces 2019, 11, 20557–20565. [Google Scholar] [CrossRef]
- Lin, Y.; Ladd, C.; Wang, S.; Martin, A.; Genzer, J.; Khan, S.A.; Dickey, M.D. Drawing liquid metal wires at room temperature. Extreme Mech. Lett. 2016, 7, 55–63. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Fassler, A.; Kazem, N.; Markvicka, E.J.; Mandal, P.; Majidi, C. Stretchable, high-k dielectric elastomers through liquid-metal inclusions. Adv. Mater. 2016, 28, 3726–3731. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Lu, L. Fatigue in metals and alloys. Nat. Mater. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.; Zan, G.; Song, M.J.; Park, C.; Oh, J.W.; Kim, Y.H.; Choi, S.G.; Shin, E.; Li, S.; et al. Graft copolymer-stabilized liquid metal nanoparticles for lithium-ion battery self-healing anodes. Adv. Funct. Mater. 2025, 35, 2508062. [Google Scholar] [CrossRef]
- Yu, M.; Cao, C.; Sa, Z.; Zhang, C.; Feng, J.; Sun, Q.; Ma, X.; Liang, J.; Sun, Y.; Yin, R.; et al. Liquid metal alchemy: Unlocking self-healing gallium-based materials for next-generation electronics. Mater. Sci. Eng. R Rep. 2025, 166, 101073. [Google Scholar] [CrossRef]
- Guo, R.; Tang, J.; Dong, S.; Lin, J.; Wang, H.; Liu, J.; Rao, W. One-step liquid metal transfer printing: Toward fabrication of flexible electronics on wide range of substrates. Adv. Mater. Technol. 2018, 3, 1800265. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, F.; Qin, C.; Wu, S.; Cao, M.; Yang, P.; Huang, L.; Wu, Y. A self-healing electrocatalytic system via electrohydrodynamics induced evolution in liquid metal. Nat. Commun. 2022, 13, 7625. [Google Scholar] [CrossRef]
- Chen, X.; Wang, B.; Duan, J.; Yang, B.; Wang, L.; Li, S.; Luo, Y.; Luo, S.; Sun, B.; Wang, C.; et al. Compression-durable soft electronic circuits enabled by embedding self-healing biphasic liquid-solid metal into microstructured elastomeric channels. Adv. Mater. 2025, 37, 2420469. [Google Scholar] [CrossRef]
- Ngoc Huu, N.; Pengfei, Z.; Fathima Shana Pattar, K.; Zhaoning, X.; Tien Thanh, N.; Wenshao, L.; Manh Tuong, N.; Chung Kim, N.; Duy Quang, P.; Thi Giang Tuyet, P.; et al. Multifunctional hydroxyapatite coated with gallium liquid metal-based silver nanoparticles for infection prevention and bone regeneration. Adv. Funct. Mater. 2025, 35, 2423496. [Google Scholar] [CrossRef]
- Sang, N.; Iwata, S.; Qi, Y.; Miyako, E. Bacterial-adjuvant liquid metal nanocomposites for synergistic photothermal immunotherapy. Adv. Compos. Hybrid Mater. 2025, 8, 353. [Google Scholar] [CrossRef]
- Xie, X.P.; Wang, J.; Xia, Z.Y.; Ren, L.; Shen, Z.H.; Nan, C.W. Conquering the adverse polarization-breakdown coupling in heat-resistant polymer nanocomposites by liquid metals. Small 2025, e03974. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, S.; Rao, W.; Liu, J. Transformable soft liquid metal micro/nanomaterials. Mater. Sci. Eng. R Rep. 2019, 138, 1–35. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Liang, J. Rupture stress of liquid metal nanoparticles and their applications in stretchable conductors and dielectrics. npj Flex. Electron. 2021, 5, 11. [Google Scholar] [CrossRef]
- Kim, M.; Cho, C.; Shin, W.; Park, J.J.; Kim, J.; Won, P.; Majidi, C.; Ko, S.H. Nanowire-assisted freestanding liquid metal thin-film patterns for highly stretchable electrodes on 3D surfaces. npj Flex. Electron. 2022, 6, 99. [Google Scholar] [CrossRef]
- Bo, G.; Ren, L.; Xu, X.; Du, Y.; Dou, S. Recent progress on liquid metals and their applications. Adv. Phys. X 2018, 3, 1446359. [Google Scholar] [CrossRef]
- Wang, D.; Ye, J.; Bai, Y.; Yang, F.; Zhang, J.; Rao, W.; Liu, J. Liquid metal combinatorics toward materials discovery. Adv. Mater. 2023, 35, 2303533. [Google Scholar] [CrossRef]
- Chen, C.; Feng, Z.; Yao, H.; Cao, F.; Lei, B.; Wang, Y.; Chen, Y.; Singh, D.; Zhang, Q. Intrinsic nanostructure induced ultralow thermal conductivity yields enhanced thermoelectric performance in Zintl phase Eu2ZnSb2. Nat. Commun. 2021, 12, 5718. [Google Scholar] [CrossRef]
- Fan, P.; Sun, Z.; Wang, Y.; Chang, H.; Zhang, P.; Yao, S.; Lu, C.; Rao, W.; Liu, J. Nano liquid metal for the preparation of a thermally conductive and electrically insulating material with high stability. RSC Adv. 2018, 8, 16232–16242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wang, Q.; Wang, Z.; Jing, L.; Garcia-Caraveo, A.V.; Li, Z.; Zhong, Y.; Liu, X.; Luo, X.; Huang, T.; et al. Liquid-infused nanostructured composite as a high-performance thermal interface material for effective cooling. Nat. Commun. 2025, 16, 794. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Yang, B.; Guo, Y.; Xu, K.; Zhang, Z.; Yang, R.; Zhang, J.; Zhu, B.; Qin, Y.; et al. Morphology-controllable liquid metal/diamond sandwich-structured thermal interface material toward high-efficiency thermal management. ACS Nano 2025, 19, 20956–20969. [Google Scholar] [CrossRef]
- Losurdo, M.; Suvorova, A.; Rubanov, S.; Hingerl, K.; Brown, A.S. Thermally stable coexistence of liquid and solid phases in gallium nanoparticles. Nat. Mater. 2016, 15, 995–1002. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Mashima, Y.; Iyoda, T. Reversible size control of liquid-metal nanoparticles under ultrasonication. Angew. Chem. Int. Ed. Engl. 2015, 54, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiao, R.; Davis, T.; Tang, S. Biomedical applications of liquid metal nanoparticles: A critical review. Biosensors 2020, 10, 196. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, B.; Wang, H.; Fan, L.; Duan, M.; Wang, X.; Guo, R.; Liu, J. Nano-biomedicine based on liquid metal particles and allied materials. Adv. NanoBiomed Res. 2021, 1, 2000086. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Wang, X.; Pan, D.; Su, C.; Wang, J.; Wang, M.; Xiong, J.; Chen, Y.; Wang, L.; et al. Activating tumor-selective liquid metal nanomedicine through galvanic replacement. Adv. Mater. 2024, 36, 2307817. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, N.; Feng, W.; Chen, S.; Zhu, X.; Shan, X.; Yuan, R.; Yuan, B.; Wang, H.; Zhou, G.; et al. Gallium nanostructure-based microneedle patch for multidrug-resistant bacterial wound healing: Enhanced metal release and NIR photothermal effect. Adv. Funct. Mater. 2024, 34, 2407934. [Google Scholar] [CrossRef]
- Song, C.; Tao, X.; Chen, Y.; Mao, K.; Tao, Y.; Ge, Z.; Wen, H.; Chen, G.; Li, B.; Xue, R.; et al. Electrocapillarity-induced hurricane-in-a-tube enables the generation and patterning of liquid metal droplets. Adv. Funct. Mater. 2024, 34, 2409341. [Google Scholar] [CrossRef]
- He, J.; Shi, F.; Wu, J.; Ye, J. Shape transformation mechanism of gallium-indium alloyed liquid metal nanoparticles. Adv. Mater. Interfaces 2021, 8, 2001874. [Google Scholar] [CrossRef]
- Kamarudheen, R.; Kumari, G.; Baldi, A. Plasmon-driven synthesis of individual metal@semiconductor core@shell nanoparticles. Nat. Commun. 2020, 11, 3957. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, J.; Jiang, S.; Panmai, M.; Li, P.; Xu, Y.; Dai, Q.; Tie, S.; Lan, S. Liquid gallium nanospheres emitting white light. Laser Photonics Rev. 2019, 13, 1800214. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chien, C.Y.; Wang, C.M.; Lin, R.S.; Chen, I.C. Plasmon tuning of liquid gallium nanoparticles through surface anodization. Materials 2022, 15, 2145. [Google Scholar] [CrossRef]
- Catalán-Gómez, S.; Redondo-Cubero, A.; Palomares, F.J.; Nucciarelli, F.; Pau, J.L. Tunable plasmonic resonance of gallium nanoparticles by thermal oxidation at low temperaturas. Nanotechnology 2017, 28, 405705. [Google Scholar] [CrossRef]
- Ye, S.; Chen, X.; Sun, X.; Patel, S.B.; Wu, Y.; Singler, T.J.; Zhang, P.; Zhou, G. Oxidation-induced oxide shell rupture and phase separation in eutectic gallium-indium nanoparticles. ACS Nano 2024, 18, 25107–25117. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Genzer, J.; Li, W.; Qiao, R.; Dickey, M.D.; Tang, S.-Y. Sonication-enabled rapid production of stable liquid metal nanoparticles grafted with poly(1-octadecene-alt-maleic anhydride) in aqueous solutions. Nanoscale 2018, 10, 19871–19878. [Google Scholar] [CrossRef] [PubMed]
- Syed, N.; Zavabeti, A.; Mohiuddin, M.; Zhang, B.; Wang, Y.; Datta, R.S.; Atkin, P.; Carey, B.J.; Tan, C.; van Embden, J.; et al. Sonication-assisted synthesis of gallium oxide suspensions featuring trap state absorption: Test of photochemistry. Adv. Funct. Mater. 2017, 27, 1702295. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Dong, G. Preparation of nanoscale liquid metal droplet wrapped with chitosan and its tribological properties as water-based lubricant additive. Tribol. Int. 2020, 148, 106349. [Google Scholar] [CrossRef]
- Li, J.; Fu, Z.; Liu, Y. Encapsulation of liquid metal nanoparticles inside metal-organic frameworks for hydrogel-integrated dual functional biotherapy. Chem. Eng. J. 2023, 457, 141302. [Google Scholar] [CrossRef]
- Ghodsi, V.; Jin, S.; Byers, J.; Pan, Y.; Radovanovic, P. Anomalous photocatalytic activity of nanocrystalline γ-phase Ga2O3 enabled by long-lived defect trap states. J. Phys. Chem. C 2017, 121, 9433–9441. [Google Scholar] [CrossRef]
- Orozco, S.; Rivero, M.; Montiel, E.; Espino Valencia, J. Gallium oxides photocatalysts doped with Fe ions for discoloration of rhodamine under UV and visible light. Front. Environ. Sci. 2022, 10, 884758. [Google Scholar] [CrossRef]
- Choi, Y.-W.; Lee, H.; Song, Y.; Sohn, D. Colloidal stability of iron oxide nanoparticles with multivalent polymer surfactants. J. Colloid Interface Sci. 2015, 443, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Meconi, G.M.; Ballard, N.; Asua, J.M.; Zangi, R. Adsorption and desorption behavior of ionic and nonionic surfactants on polymer surfaces. Soft Matter 2016, 12, 9692–9704. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Ayan, B.; Nama, N.; Bian, Y.; Lata, J.P.; Guo, X.; Huang, T.J. On-chip production of size-controllable liquid metal microdroplets using acoustic waves. Small 2016, 12, 3861–3869. [Google Scholar] [CrossRef]
- Cassinath, Z.; Prasada Rao, A.K. Development of axial continuous metal expeller for melt conditioning of alloys. IOP Conf. Ser. Mater. Sci. Eng. 2016, 114, 012042. [Google Scholar] [CrossRef]
- Nor-Azman, N.-A.; Ghasemian, M.B.; Fuchs, R.; Liu, L.; Widjajana, M.S.; Yu, R.; Chiu, S.-H.; Idrus-Saidi, S.A.; Flores, N.; Chi, Y.; et al. Mechanism behind the controlled generation of liquid metal nanoparticles by mechanical agitation. ACS Nano 2024, 18, 11139–11152. [Google Scholar] [CrossRef] [PubMed]
- Tevis, I.D.; Newcomb, L.B.; Thuo, M. Synthesis of liquid core-shell particles and solid patchy multicomponent particles by shearing liquids into complex particles (SLICE). Langmuir 2014, 30, 14308–14313. [Google Scholar] [CrossRef]
- Lebon, G.S.B.; Tzanakis, I.; Pericleous, K.; Eskin, D.; Grant, P.S. Ultrasonic liquid metal processing: The essential role of cavitation bubbles in controlling acoustic streaming. Ultrason. Sonochem. 2019, 55, 243–255. [Google Scholar] [CrossRef]
- Bulychev, N.A.; Kazaryan, M.A.; Chaikov, L.L.; Burkhanov, I.S.; Krasovskii, V.I. Nanoscale metal oxide particles produced in the plasma discharge in the liquid phase upon exposure to ultrasonic cavitation. 1. Method for producing particles. Bull. Lebedev Phys. Inst. 2014, 41, 264–268. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Taladriz-Blanco, P.; de Oliveira, M.G.; Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 2018, 8, 2440. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, Y.; Zheng, X.; Hou, L.; Jiang, T.; Liu, W.; Tao, Y.; Jiang, H. A micro-needle induced strategy for preparation of monodisperse liquid metal droplets in glass capillary microfluidics. Microfluid. Nanofluid. 2019, 23, 13. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Hu, T.; Xuan, S.; Gong, X. A 3D-printed coaxial microfluidic device approach for generating magnetic liquid metal droplets with large size controllability. Microfluid. Nanofluid. 2020, 24, 30. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Qiao, R.; Yan, S.; Yuan, D.; Zhao, Q.; Yun, G.; Davis, T.P.; Li, W. Microfluidic mass production of stabilized and stealthy liquid metal nanoparticles. Small 2018, 14, 1800118. [Google Scholar] [CrossRef] [PubMed]
- Hutter, T.; Bauer, W.-A.C.; Elliott, S.R.; Huck, W.T.S. Formation of spherical and non-spherical eutectic gallium-indium liquid-metal microdroplets in microfluidic channels at room temperature. Adv. Funct. Mater. 2012, 22, 2624–2631. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Ma, B.; Zhang, D.; Zhao, Y. Liquid metal-integrated ultra-elastic conductive microfibers from microfluidics for wearable electronics. Sci. Bull. 2020, 65, 1752–1759. [Google Scholar] [CrossRef]
- Fan, L.W.; Wu, Y.Y.; Xiao, Y.Q.; Zeng, Y.; Zhang, Y.L.; Yu, Z.T. Transient performance of a thermal energy storage-based heat sink using a liquid metal as the phase change material. Appl. Therm. Eng. 2016, 109, 746–750. [Google Scholar] [CrossRef]
- Tao, Y.; Guan, T.; Ma, Y.; Sang, G.; Liu, J. Stretchable soft batteries: From structures to materials. Energy Storage Mater. 2025, 76, 104085. [Google Scholar] [CrossRef]
- Athair, A.S.; Armstrong, C.; Shahabuddin, M.; Datta, R.; Rao, P.M.; Teixeira, A.R. Preliminary investigation into the feasibility of an ambient-temperature gallium-based liquid metal-air battery. ACS Appl. Energy Mater. 2024, 7, 9715–9722. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y.; Huang, X.; Huang, L.; Cao, M.; Song, G.; Guo, X.; Sui, X.; Ren, R.; Chen, J. Self-healing liquid metal nanoparticles encapsulated in hollow carbon fibers as a free-standing anode for lithium-ion batteries. Nano Energy 2019, 62, 883–889. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, J.; Feng, X.; Xu, J.; Xu, C.; Wang, H.; Liu, H.; Hou, B.; Rui, X.; Gu, Y.; et al. The trade-off characteristic between battery thermal runaway and combustion. Energy Storage Mater. 2024, 69, 103380. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, W.; Li, Y.; Wu, Q.; Tang, S.; Yan, J.-W.; Zheng, M.; Wu, D.; Fan, C.; Hu, W.; et al. Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 2018, 9, 1339. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, T.; Wu, Y. New insights into the cooperativity and dynamics of dimeric enzymes. Chem. Rev. 2023, 123, 9940–9981. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ding, Y.; Xue, L.; Zhang, L.; Zhang, C.; Goodenough, J.B.; Yu, G. A self-healing room-temperature liquid-metal anode for alkali-ion batteries. Adv. Funct. Mater. 2018, 28, 1804649. [Google Scholar] [CrossRef]
- Sanati, A.L.; Silva, A.F.; Maranha, M.; Tavakoli, M. Biphasic graphene-oxide liquid metal powder: Synthesis, characterization, and application in energy storage. Energy Environ. Mater. 2025, 8, e12890. [Google Scholar] [CrossRef]
- Gupta, A.; Al-Shamery, N.; Lv, J.; Thangavel, G.; Park, J.; Mandler, D.; Lee, P.S. Stretchable energy storage with eutectic gallium indium alloy. Adv. Energy Mater. 2025, 15, 2403760. [Google Scholar] [CrossRef]
- Yuan, Q.; Fang, H.; Wu, X.; Wu, J.; Luo, X.; Peng, R.; Xu, S.; Yan, S. Self-adhesive, biocompatible, wearable microfluidics with erasable liquid metal plasmonic hotspots for glucose detection in sweat. ACS Appl. Mater. Interfaces 2024, 16, 66810–66818. [Google Scholar] [CrossRef]
- Cai, Q.; Fu, X.; Zhang, X.; Cui, T.J. Liquid metal-based continuously tunable surface plasmon resonator for lab-on-chip sensor applications. IEEE Antennas Wirel. Propag. Lett. 2024, 23, 2506–2510. [Google Scholar] [CrossRef]
- Shao, B.; Lu, T.C.; Lu, M.H.; Chen, Y.T.; Wu, T.C.; Peng, W.C.; Ko, T.Y.; Chen, J.Y.; Sun, B.; Chen, C.Y.; et al. Efficient permeable monolithic hybrid tribo-piezo-electromagnetic nanogenerator based on topological-insulator-composite. Adv. Mater. 2024, 36, 2408936. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, Y.; Huang, H.; Sun, Z.; Xin, W.; Liu, W.; Zhao, X.; Long, S.; Xu, H. Ultrathin Ga2O3 photodetector with fast response and trajectory tracking capability fabricated by liquid metal oxidation. Nano Lett. 2024, 24, 13769–13774. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, J.; Zhuang, J.; Ren, L.; Du, Y. Native surface oxides featured liquid metals for printable self-powered photoelectrochemical device. Front. Chem. 2019, 7, 365. [Google Scholar] [CrossRef]
- Wang, P.-F.; Hu, Q.; Lv, B.; Zhu, J.-L.; Ma, W.; Dong, Z.; Wei, J.; Sun, J.-L. Facile fabrication of eutectic gallium-indium alloy nanostructure and application in photodetection. Nanotechnology 2020, 31, 145703. [Google Scholar] [CrossRef]
- Qi, X.; Yu, L.; Liu, Y.; Chen, L.; Li, X. Liquid metal powders wrapped with robust shell by bimetallic ions chelation strategy for energy harvesting and flexible electronics. Adv. Funct. Mater. 2024, 34, 2313960. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Bai, J.; He, Y.; Zhao, X.; Zhang, J. Integrating dual-interfacial liquid metal based nanodroplet architectures and micro-nanostructured engineering for high efficiency solar energy harvesting. ACS Nano 2022, 16, 15086–15099. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, J.; Pan, X.; Sánchez, S.; Yan, X.; Ma, X. Enzyme-powered liquid metal nanobots endowed with multiple biomedical functions. ACS Nano 2021, 15, 11543–11554. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Shang, W.; Handschuh-Wang, S.; Zhou, X. Light-induced shape morphing of liquid metal nanodroplets enabled by polydopamine coating. Small 2019, 15, 1804838. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, D.; Ford, M.J.; Majidi, C. Ultrastretchable, wearable triboelectric nanogenerator based on sedimented liquid metal elastomer composite. Adv. Mater. Technol. 2020, 5, 2000754. [Google Scholar] [CrossRef]
- Guo, J.; Duan, L.; Yang, W.; Wang, Q.; Feng, X.; Zhang, Y.; Zhang, Y.; Wang, Z.L.; Yang, P. Flexible triboelectric nanogenerator arrays for energy harvesting and direct current output via solid-liquid-gas interfaces involving liquid metals. Adv. Funct. Mater. 2025, e10233. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Seo, S. Amplifying the output of a triboelectric nanogenerator using an intermediary layer of gallium-based liquid metal particles. Nanomaterials 2023, 13, 1290. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, N.; Wen, Z.; Cheng, P.; Zheng, H.; Shao, H.; Xia, Y.; Chen, C.; Lan, H.; Xie, X.; et al. Liquid-metal-based super-stretchable and structure-designable triboelectric nanogenerator for wearable electronics. ACS Nano 2018, 12, 2027–2034. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Z.; Lin, Z.; Guo, H.; Chun, F.; Yang, W.; Wang, Z.L. All-in-one 3D acceleration sensor based on coded liquid-metal triboelectric nanogenerator for vehicle restraint system. Mater. Today 2021, 43, 37–44. [Google Scholar] [CrossRef]
- Li, Z.; Xu, B.; Han, J.; Tan, D.; Huang, J.; Gao, Y.; Fu, H. Surface-modified liquid metal nanocapsules derived multiple triboelectric composites for efficient energy harvesting and wearable self-powered sensing. Chem. Eng. J. 2023, 460, 141737. [Google Scholar] [CrossRef]
- Zheng, Q.; Jin, Y.; Liu, Z.; Ouyang, H.; Li, H.; Shi, B.; Jiang, W.; Zhang, H.; Li, Z.; Wang, Z.L. Robust multilayered encapsulation for high-performance triboelectric nanogenerator in harsh environment. ACS Appl. Mater. Interfaces 2016, 8, 26697–26703. [Google Scholar] [CrossRef]
- Du, B.; Hao, S.; Zhang, J.; Ren, W.; Wang, B.; Yang, J.; Wen, J.; Xiao, L.P.; Shao, C.; Sun, R. Demethylated lignin@liquid metal nanospheres enabling versatile conductive hydrogel for self-powered soft electronics. ACS Nano 2025, 19, 30072–30086. [Google Scholar] [CrossRef]
- Fan, H.; Zeng, Z.A.; Deng, C.; Chen, X.; Di, Z.; Lan, W.; He, K.; Lin, P.; Luo, Y.; Wang, W.; et al. High-performance solid-liquid triboelectric nanogenerator enabled by dual physicochemical modification for wearable sensing. Nano Energy 2025, 140, 111061. [Google Scholar] [CrossRef]
- Nayak, S.; Li, Y.; Tay, W.; Zamburg, E.; Singh, D.; Lee, C.; Koh, S.J.A.; Chia, P.; Thean, A.V.Y. Liquid-metal-elastomer foam for moldable multi-functional triboelectric energy harvesting and force sensing. Nano Energy 2019, 64, 103912. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, T.; Fan, F.R.; Yu, A.F.; Zhang, C.; Cao, X.; Wang, Z.L. Liquid-metal electrode for high-performance triboelectric nanogenerator at an instantaneous energy conversion efficiency of 70.6%. Adv. Funct. Mater. 2015, 25, 3718–3725. [Google Scholar] [CrossRef]
- Qin, H.; Xu, L.; Lin, S.; Zhan, F.; Dong, K.; Han, K.; Wang, H.; Feng, Y.; Wang, Z.L. Underwater energy harvesting and sensing by sweeping out the charges in an electric double layer using an oil droplet. Adv. Funct. Mater. 2022, 32, 2111662. [Google Scholar] [CrossRef]
- Zavabeti, A.; Daeneke, T.; Chrimes, A.F.; O’Mullane, A.P.; Zhen Ou, J.; Mitchell, A.; Khoshmanesh, K.; Kalantar-zadeh, K. Ionic imbalance induced self-propulsion of liquid metals. Nat. Commun. 2016, 7, 12402. [Google Scholar] [CrossRef]
- Che, X.; Yu, H.; Wang, T.; Zhang, B.; Zhai, Z.; Chen, Y.; Pei, D.; Li, M.; Li, C. Mechanically pulsating liquid metal within biologic porous ionogel for energy harvest. Adv. Funct. Mater. 2025, 35, 2415323. [Google Scholar] [CrossRef]
- Ouyang, H.; Tian, J.; Sun, G.; Zou, Y.; Liu, Z.; Li, H.; Zhao, L.; Shi, B.; Fan, Y.; Fan, Y.; et al. Self-powered pulse sensor for antidiastole of cardiovascular disease. Adv. Mater. 2017, 29, 1703456. [Google Scholar] [CrossRef]
- Jeong, J.; Noushin, T.; Lee, J.B. An enhanced liquid metal triboelectric nanogenerator (LM-TENG) using parallel placement of friction layer. In Proceedings of the 2023 IEEE SENSORS, Vienna, Austria, 29 October–1 November 2023; pp. 1–4. [Google Scholar]
- Cui, N.; Liu, J.; Lei, Y.; Gu, L.; Xu, Q.; Liu, S.; Qin, Y. High-performance triboelectric nanogenerator with a rationally designed friction layer structure. ACS Appl. Energy Mater. 2018, 1, 2891–2897. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X.; Li, S.; Wang, Z.L.; Wei, D. Ionic rectification by dynamic regulation of the electric double layer at the hydrogel interface. ACS Appl. Mater. Interfaces 2024, 16, 18236–18244. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, G.; Tan, X.; Liu, R.; Hu, K.; Ouyang, Z.; Wei, Y.; Guo, D. Enhanced electrical interfaces in flexible 2D material transistors via liquid metal and ionic liquid injection. Adv. Mater. 2025, 37, 2501501. [Google Scholar] [CrossRef]
- Long, R.; Kuang, Z.; Liu, Z.; Liu, W. Reverse electrodialysis in bilayer nanochannels: Salinity gradient-driven power generation. Phys. Chem. Chem. Phys. 2018, 20, 7295–7302. [Google Scholar] [CrossRef]
- Das, J.P.; Nardekar, S.S.; Ravichandran, V.; Kim, S.J. From friction to function: A high-voltage sliding triboelectric nanogenerator for highly efficient energy autonomous IoTs and Self-Powered Actuation. Small 2024, 20, 2405792. [Google Scholar] [CrossRef] [PubMed]
- Ruffman, C.; Steenbergen, K.G.; Gaston, N. An atomic-scale explanation for the high selectivity towards carbon dioxide reduction observed on liquid metal catalysts. Angew. Chem. Int. Ed. 2024, 63, e202407124. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; Gao, Y.; Wang, X.; Cai, R.; Chung, C.; Iftikhar, S.; Wang, W.; Li, F. Liquid metal shell as an effective iron oxide modifier for redox-based hydrogen production at intermediate temperatures. ACS Catal. 2021, 11, 10228–10238. [Google Scholar] [CrossRef]
- Okatenko, V.; Castilla-Amorós, L.; Stoian, D.C.; Vávra, J.; Loiudice, A.; Buonsanti, R. The native oxide skin of liquid metal Ga nanoparticles prevents their rapid coalescence during electrocatalysis. J. Am. Chem. Soc. 2022, 144, 10053–10063. [Google Scholar] [CrossRef]
- Chen, X.; Zhen, C.; Qiu, J.; Li, N.; Jia, N.; Liu, G. Modulating band alignment at the 3D metal/semiconductor interface of liquid metal-embraced semiconductor photoelectrodes for water splitting. Sci. China Mater. 2024, 67, 1804–1811. [Google Scholar] [CrossRef]
- Chen, L.; Song, Z.; Zhang, S.; Chang, C.K.; Chuang, Y.C.; Peng, X.; Dun, C.; Urban, J.J.; Guo, J.; Chen, J.L.; et al. Ternary NiMo-Bi liquid alloy catalyst for efficient hydrogen production from methane pyrolysis. Science 2023, 381, 857–861. [Google Scholar] [CrossRef]
- Cao, G.; Liang, J.; Guo, Z.; Yang, K.; Wang, G.; Wang, H.; Wan, X.; Li, Z.; Bai, Y.; Zhang, Y.; et al. Liquid metal for high-entropy alloy nanoparticles synthesis. Nature 2023, 619, 73–77. [Google Scholar] [CrossRef]
- Ma, C.; Song, B.; Ma, Z.; Wang, X.; Tian, L.; Zhang, H.; Chen, C.; Zheng, X.; Yang, L.-M.; Wu, Y. A Supported palladium on gallium-based liquid metal catalyst for enhanced oxygen reduction reaction. Chem. Res. Chin. Univ. 2022, 38, 1219–1225. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Cui, M.; Huang, R.; Qi, W.; Su, R. Dynamic dual-atom synergistic catalysis boosted by liquid metal for direct seawater electroreduction. J. Mater. Chem. A 2024, 12, 13466–13473. [Google Scholar] [CrossRef]
- Ye, L.; Syed, N.; Wang, D.; Murdoch, B.J.; Zuraqi, K.; Alivand, M.S.; Xiao, P.; Singh, R.; Zu, L.; Mumford, K.A.; et al. CO2 reduction on the Li-Ga liquid metal surface. J. Mater. Chem. A 2023, 11, 8809–8816. [Google Scholar] [CrossRef]
- Ma, X.; Wang, F.; Jiao, D.; Zhang, D.; Zhao, X.; Singh, D.J.; Zhao, J.; Cui, X.; Zheng, W. Room-temperature liquid metal synthesis of nanoporous copper-indium heterostructures for efficient carbon dioxide reduction to syngas. Sci. China Mater. 2022, 65, 3504–3512. [Google Scholar] [CrossRef]
- Zhen, C.; Chen, X.; Chen, R.; Fan, F.; Xu, X.; Kang, Y.; Guo, J.; Wang, L.; Lu, G.Q.; Domen, K.; et al. Liquid metal-embraced photoactive films for artificial photosynthesis. Nat. Commun. 2024, 15, 1672. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.J.; Krishnamurthi, V.; Zuraiqi, K.; Nguyen, C.K.; Irfan, M.; Jabbar, F.; Yang, D.; Aukarasereenont, M.P.; Mayes, E.L.H.; Murdoch, B.J.; et al. Synthesis of planet-like liquid metal nanodroplets with promising properties for catalysis. Adv. Funct. Mater. 2024, 34, 2304248. [Google Scholar] [CrossRef]
- You, D.; Xu, C.; Wang, X.; Wang, J.; Su, W.; Wang, R.; Chen, T.; Shi, Z. Core@dual-shell nanorod array with cascading band configuration for enhanced photocatalytic properties and anti-photocorrosion. J. Mater. Chem. A 2020, 8, 3726–3734. [Google Scholar] [CrossRef]
- Raby, H.S.; Mohammed, M.G. Liquid metal-induced aluminum activation by thermal sintering for hydrogen production through water splitting reaction. Int. J. Hydrogen Energy 2025, 158, 150549. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Sheng, L.; Liu, J. Self-fueled biomimetic liquid metal mollusk. Adv. Mater. 2015, 27, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
| Properties | Liquid Metal Energy Storage Device | Conventional Energy Storage Device | Ref. |
|---|---|---|---|
| Thermal conductivity | 7~10 W/mK | Organic PCM: 0.1~0.3 W/mK | [98] |
| Volumetric latent heat | 217.8 MJ/m3 | Organic PCM: 214.3 MJ/m3 | |
| Electrical conductivity | Strain > 100%, conductivity remains stable | Graphene: strains > 10%, conductivity drastically decreases | [99] |
| Energy density | 1989 Wh/kg | Zinc-air battery: 1361 Wh/kg | [100] |
| Capacity retention rate | 91.3% (1500 cycles) | Graphite anode: <80% (500 cycles) | [101] |
| Safety | Flame-retardant | Flammable and prone to thermal runaway | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Gao, J.; Tao, Y.; Hua, C.; Guan, T.; Cheng, C.; Song, Y.; Liu, J. Liquid Metal Nanoenergy Systems: Progress and Challenges. Nanoenergy Adv. 2025, 5, 16. https://doi.org/10.3390/nanoenergyadv5040016
Ma Y, Gao J, Tao Y, Hua C, Guan T, Cheng C, Song Y, Liu J. Liquid Metal Nanoenergy Systems: Progress and Challenges. Nanoenergy Advances. 2025; 5(4):16. https://doi.org/10.3390/nanoenergyadv5040016
Chicago/Turabian StyleMa, Yibing, Jianye Gao, Yiyue Tao, Chen Hua, Tangzhen Guan, Cai Cheng, Yujia Song, and Jing Liu. 2025. "Liquid Metal Nanoenergy Systems: Progress and Challenges" Nanoenergy Advances 5, no. 4: 16. https://doi.org/10.3390/nanoenergyadv5040016
APA StyleMa, Y., Gao, J., Tao, Y., Hua, C., Guan, T., Cheng, C., Song, Y., & Liu, J. (2025). Liquid Metal Nanoenergy Systems: Progress and Challenges. Nanoenergy Advances, 5(4), 16. https://doi.org/10.3390/nanoenergyadv5040016







