COx-Free Hydrogen Production via CH4 Decomposition on Alkali-Incorporated (Mg, La, Ca, Li) Ni-Al Catalysts
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Synthesis
2.2. Catalytic Activity
2.3. Catalysts Characterization
3. Results and Discussion
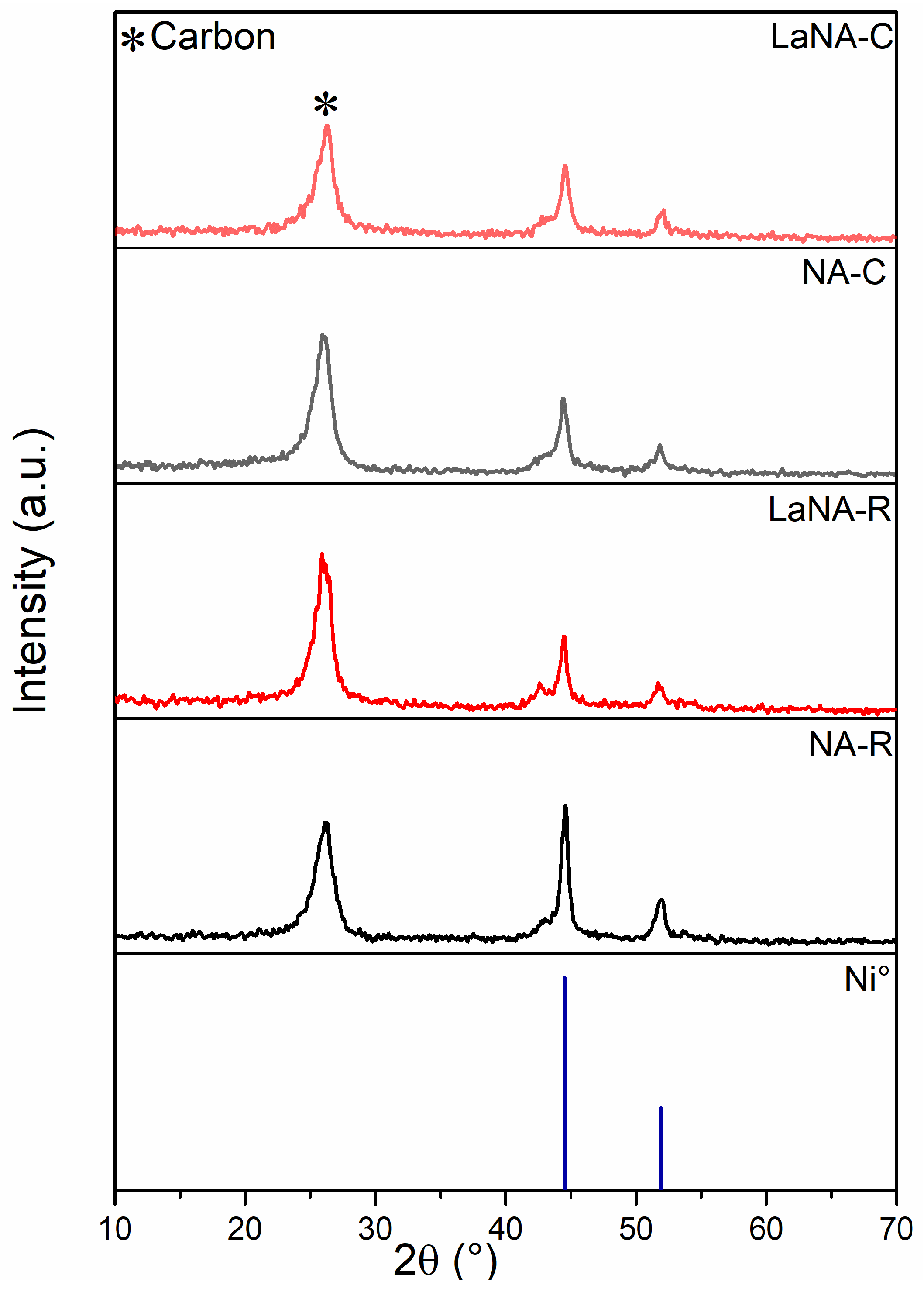
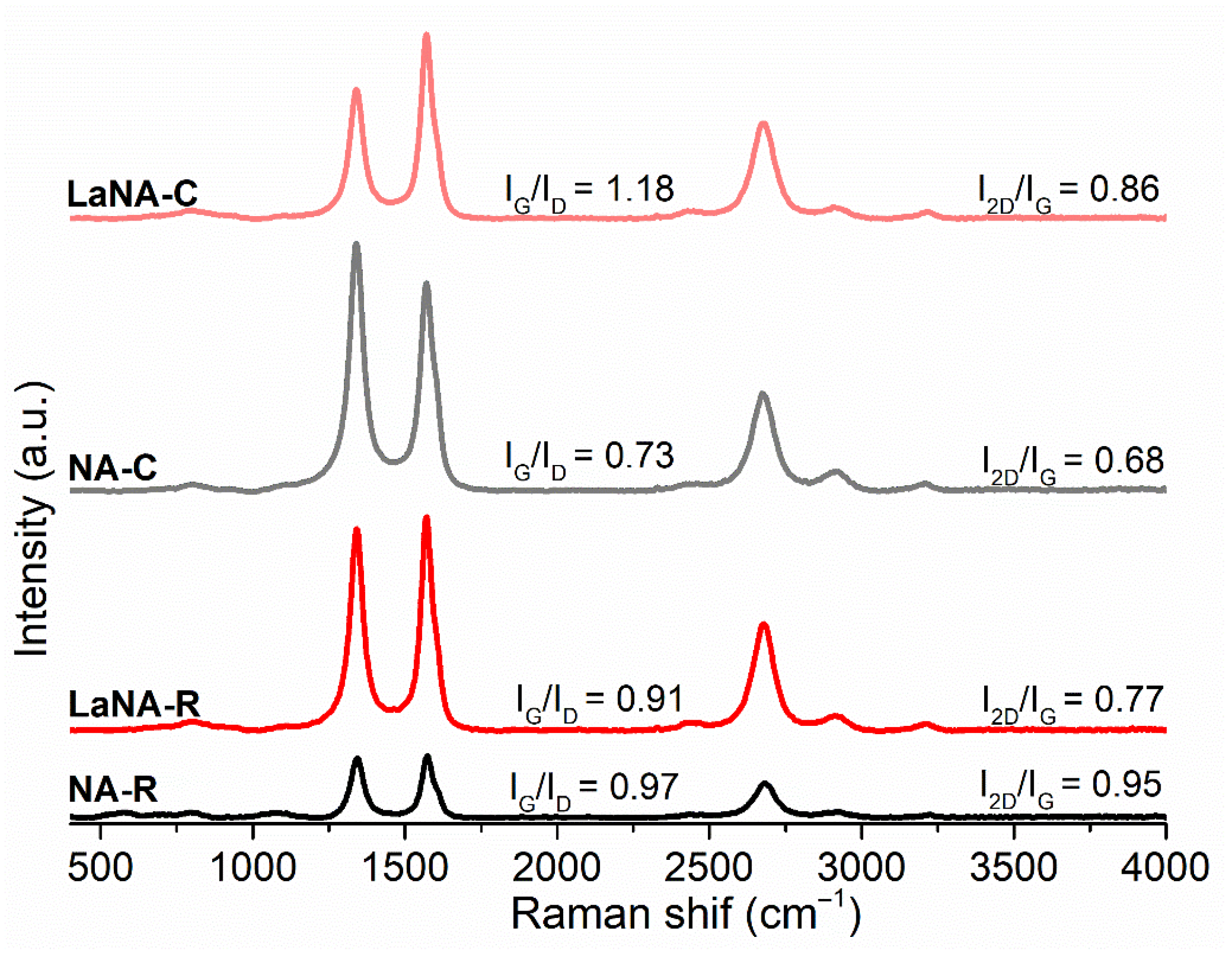
3.1. Catalyst Characterization
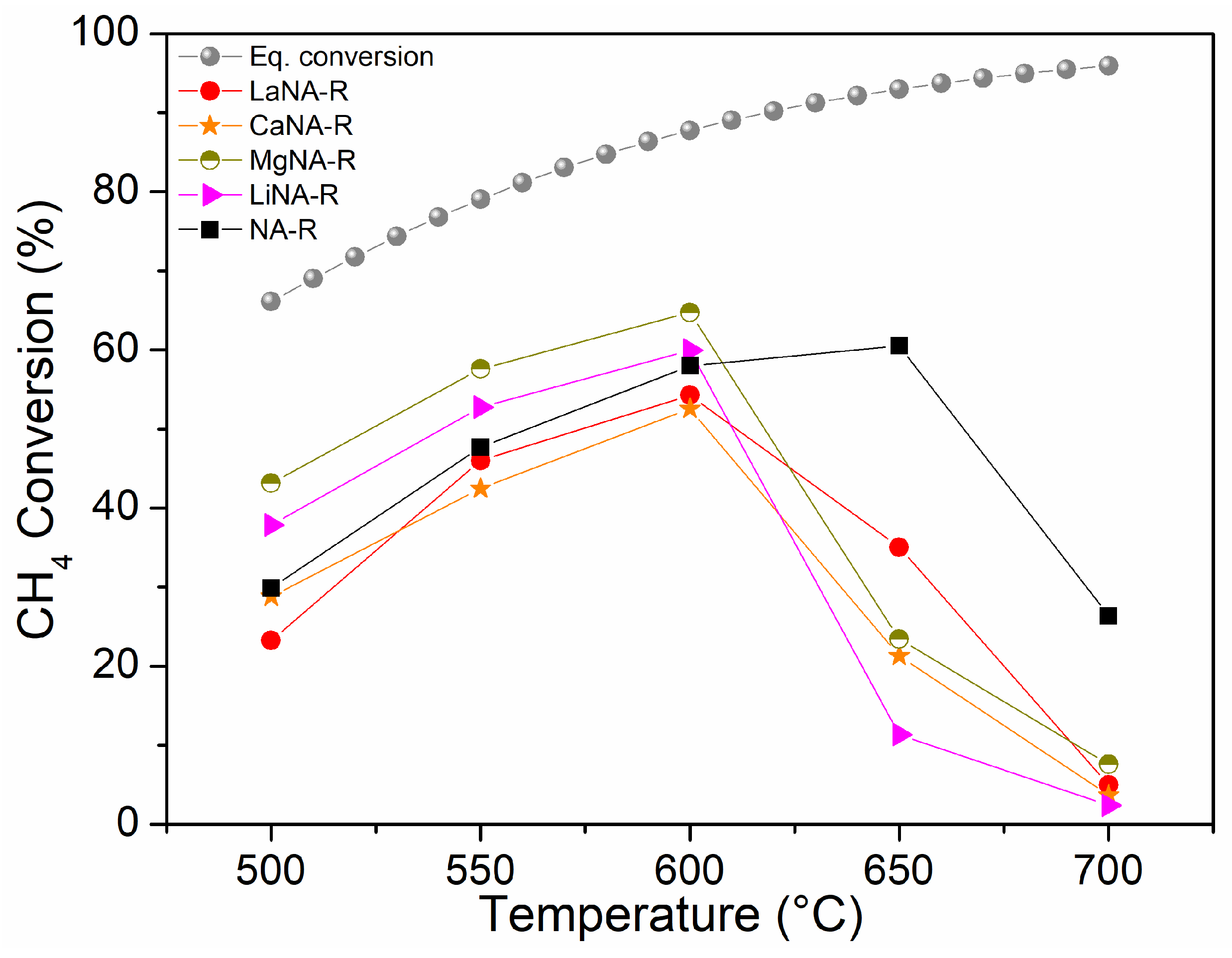
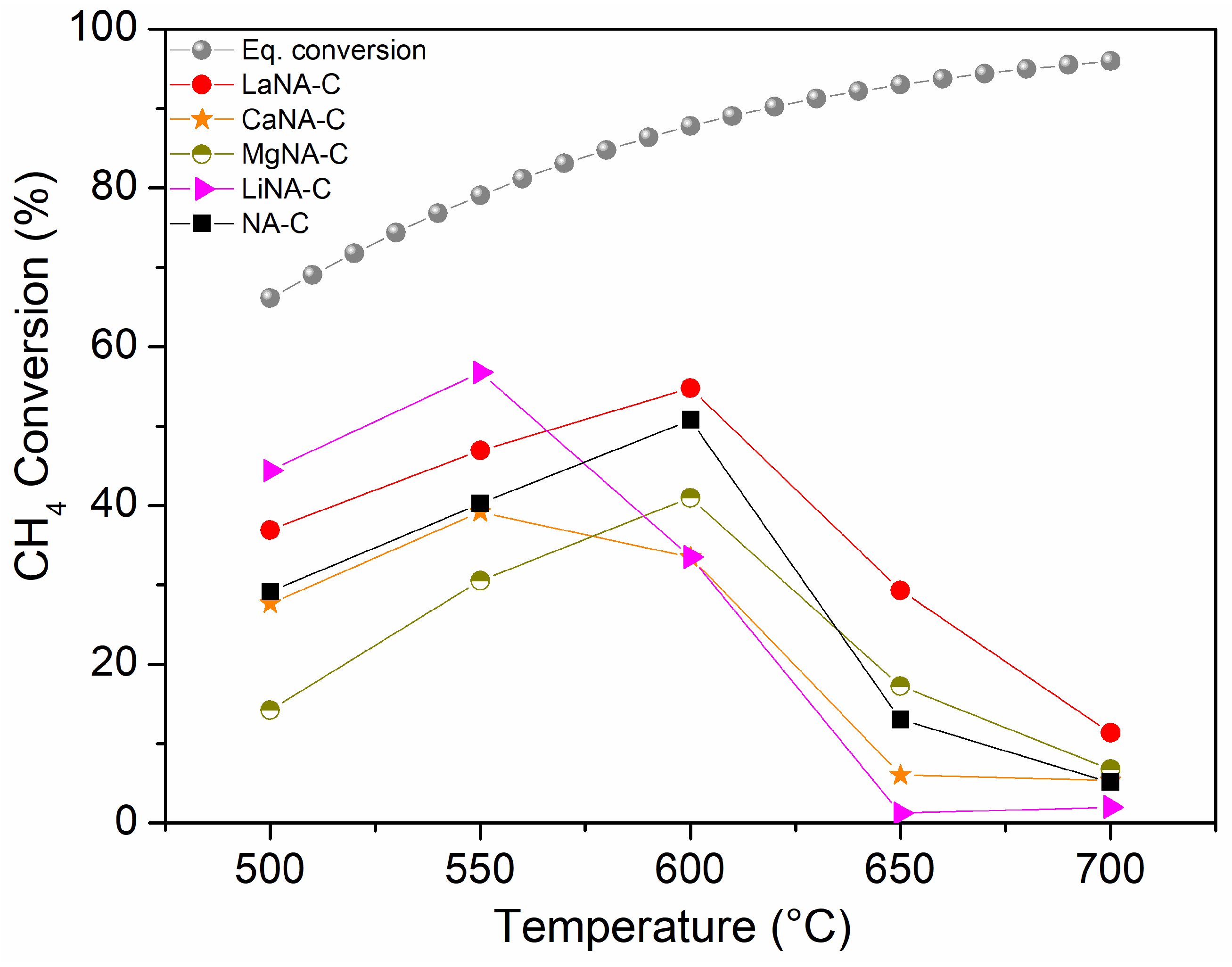
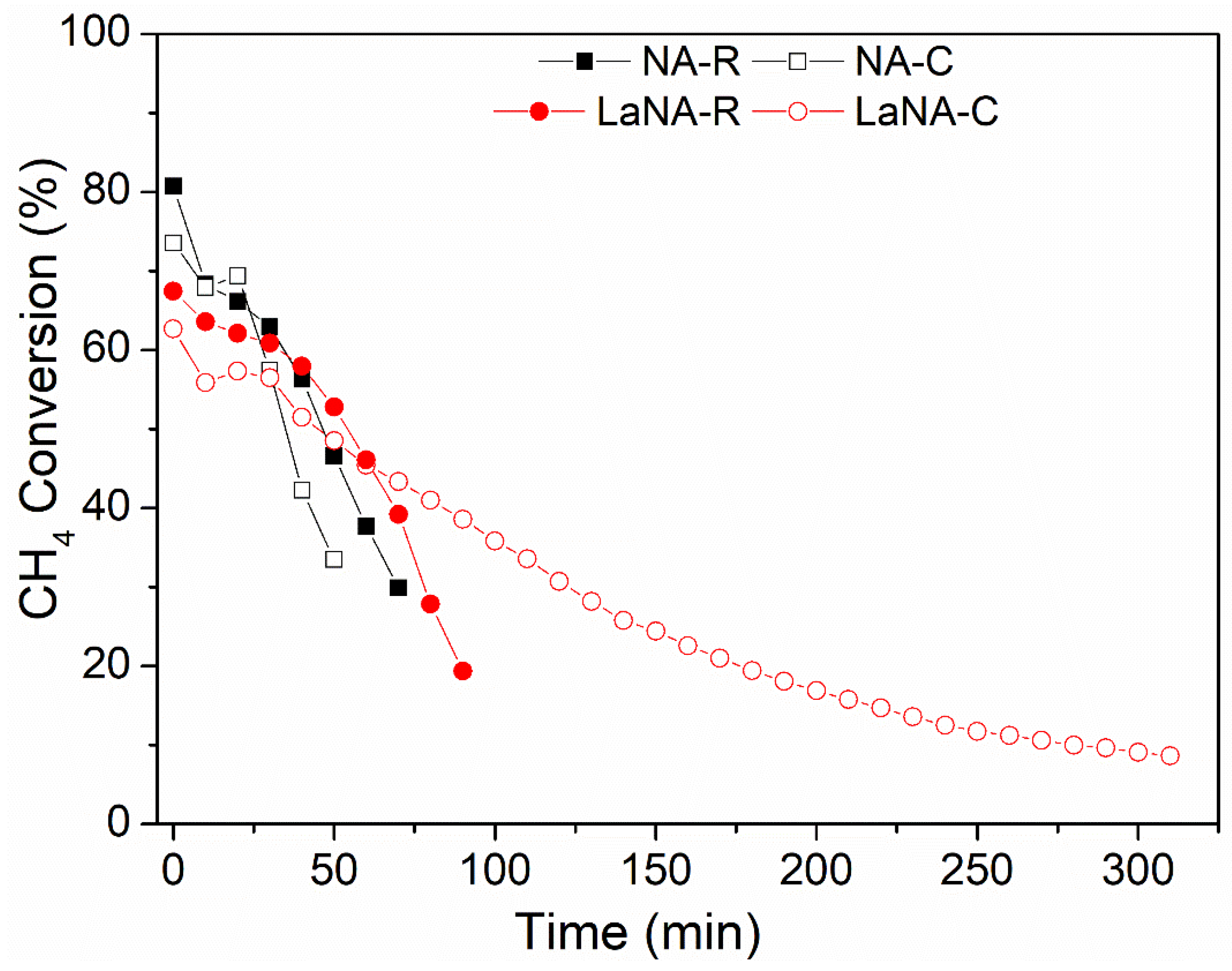
3.2. Catalytic Activity
Suggestion of the Reaction Mechanism of Catalytic Methane Decomposition (CMD)
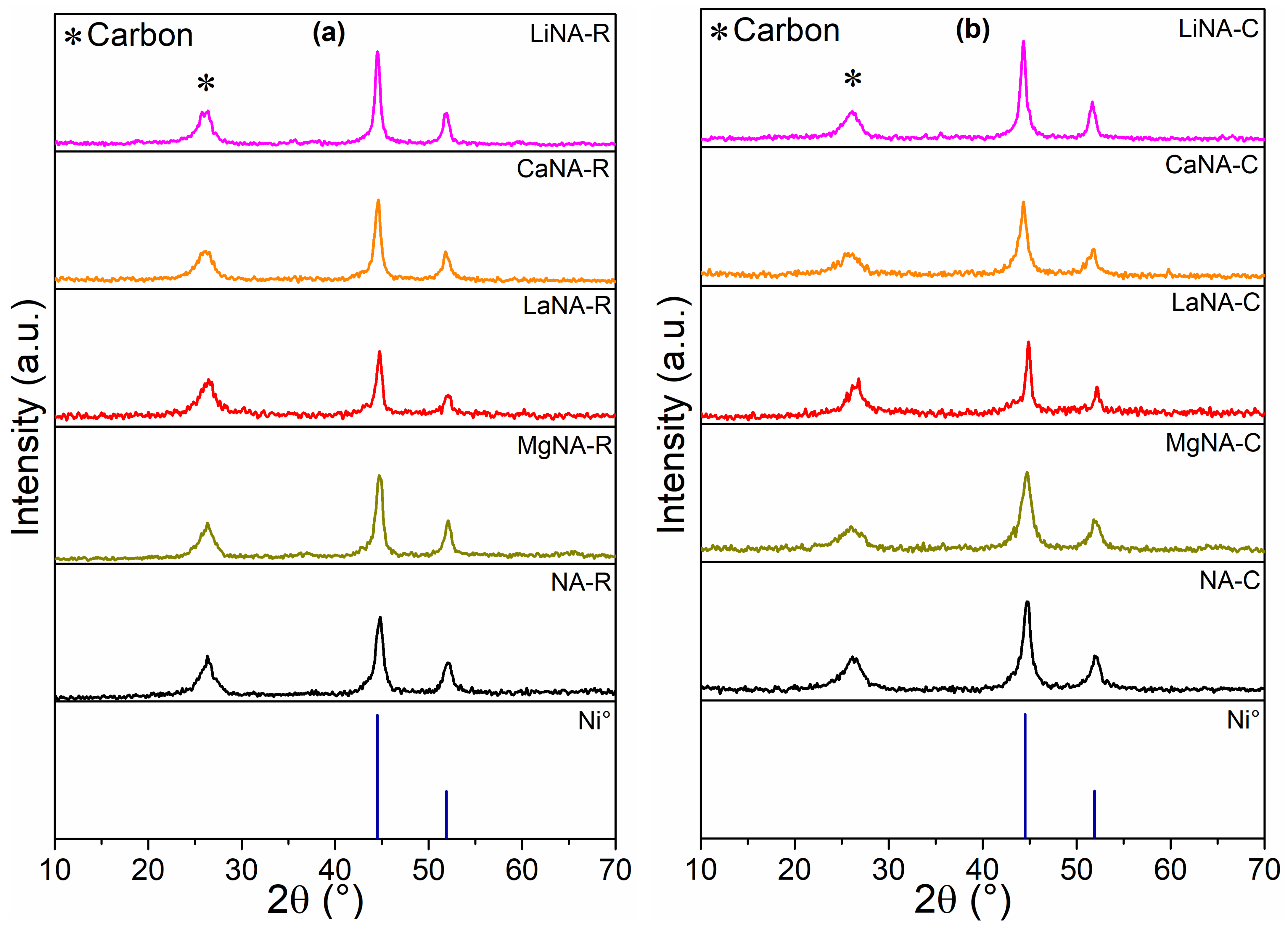
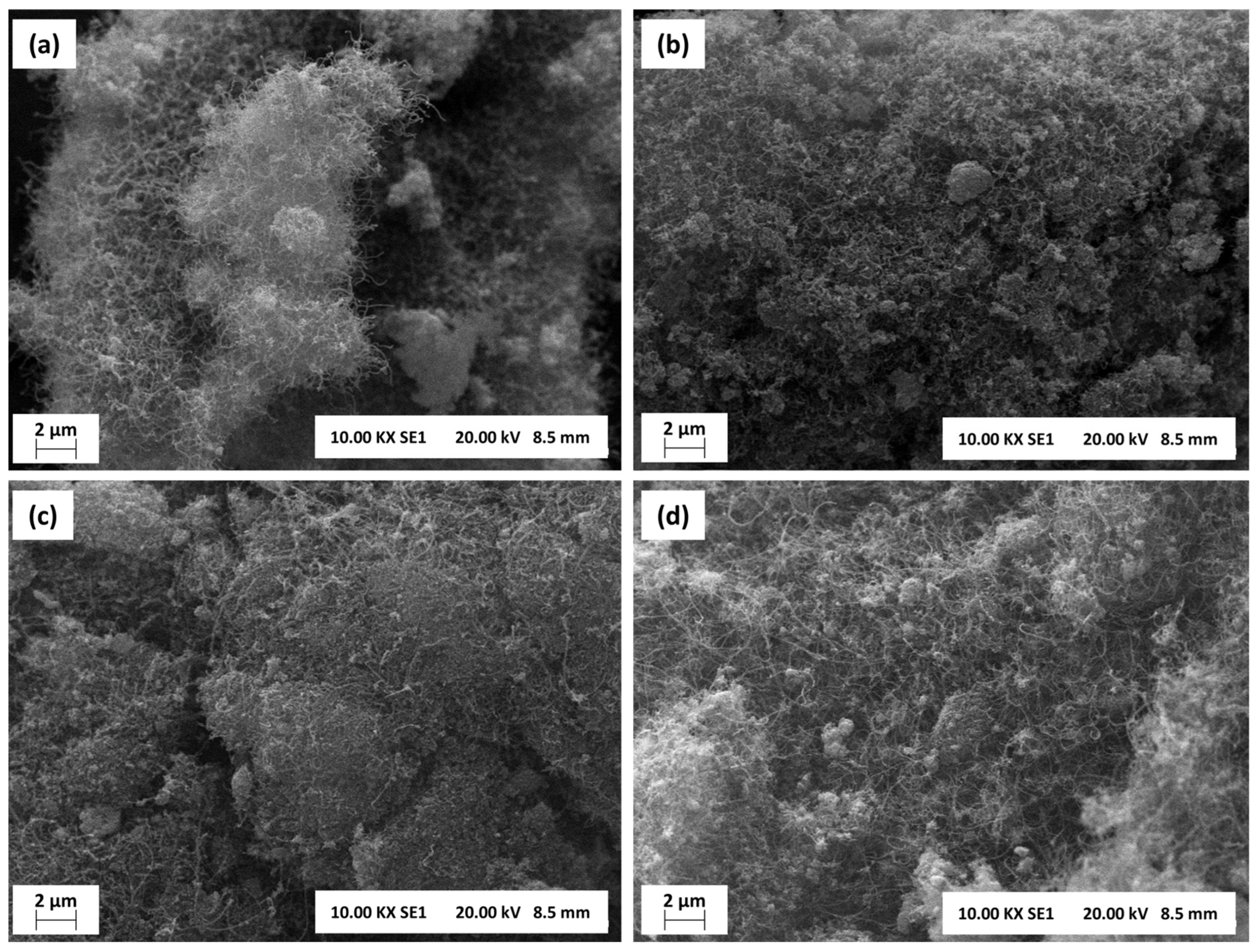
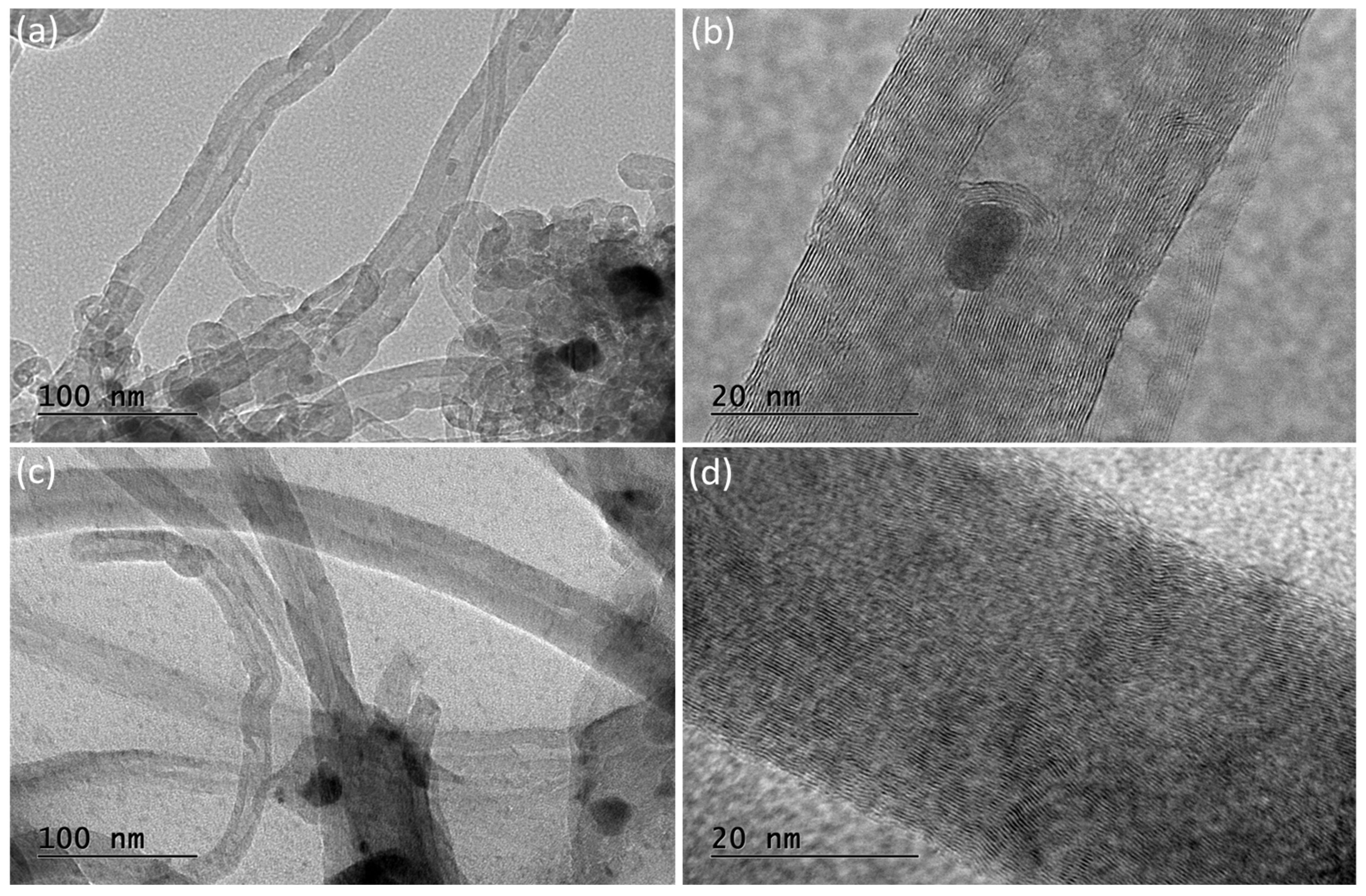
3.3. Characterization of Spent Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Li, X.; Chen, H.; Qi, M.; Zhang, G. Hydrogen production by catalytic methane decomposition: Carbon materials as catalysts or catalyst supports. Int. J. Hydrogen Energy 2017, 42, 19755–19775. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Daud, W.M.A.W.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane–A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef]
- Anjaneyulu, C.; Naveen Kumar, S.; Vijay Kumar, V.; Naresh, G.; Bhargava, S.K.; Chary, K.V.R.; Venugopal, A. Influence of La on reduction behaviour and Ni metal surface area of Ni-Al2O3 catalysts for COx free H2 by catalytic decomposition of methane. Int. J. Hydrogen Energy 2015, 40, 3633–3641. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.-M.; Zhou, L. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: A review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Abbas, H.F.; Ashik, U.P.M.; Mohammed, S.A.; Daud, W.M.A.W. Impact of reactor materials on methane decomposition for hydrogen production. Chem. Eng. Res. Des. 2021, 174, 127–136. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Hydrogen and carbon nanofibers synthesis by methane decomposition over Ni-Pd/Al2O3 catalyst. Int. J. Hydrogen Energy 2016, 41, 5494–5503. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Cho, H.; Kim, M.; Moon, I.; Kim, J. Multi-objective optimization of CO2 emission and thermal efficiency for on-site steam methane reforming hydrogen production process using machine learning. J. Clean. Prod. 2022, 359, 132133. [Google Scholar] [CrossRef]
- Zarei-Jelyani, F.; Salahi, F.; Farsi, M.; Reza Rahimpour, M. Synthesis and application of Ni-Co bimetallic catalysts supported on hollow sphere Al2O3 in steam methane reforming. Fuel 2022, 324, 124785. [Google Scholar] [CrossRef]
- Muhammad, A.F.S.; Awad, A.; Saidur, R.; Masiran, N.; Salam, A.; Abdullah, B. Recent advances in cleaner hydrogen productions via thermo-catalytic decomposition of methane: Admixture with hydrocarbon. Int. J. Hydrogen Energy 2018, 43, 18713–18734. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen production by methane decomposition using bimetallic Ni–Fe catalysts. Int. J. Hydrogen Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Gómez-Pozuelo, G.; Pizarro, P.; Botas, J.A.; Serrano, D.P. Hydrogen production by catalytic methane decomposition over rice husk derived silica. Fuel 2021, 306, 121697. [Google Scholar] [CrossRef]
- Karaismailoglu, M.; Figen, H.E.; Baykara, S.Z. Hydrogen production by catalytic methane decomposition over yttria doped nickel based catalysts. Int. J. Hydrogen Energy 2019, 44, 9922–9929. [Google Scholar] [CrossRef]
- Keipi, T.; Tolvanen, H.; Konttinen, J. Economic analysis of hydrogen production by methane thermal decomposition: Comparison to competing technologies. Energy Convers. Manag. 2018, 159, 264–273. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Bayat, N.; Meshkani, F.; Rezaei, M. Thermocatalytic decomposition of methane to COx-free hydrogen and carbon over Ni–Fe–Cu/Al2O3 catalysts. Int. J. Hydrogen Energy 2016, 41, 13039–13049. [Google Scholar] [CrossRef]
- Kutteri, D.A.; Wang, I.W.; Samanta, A.; Li, L.; Hu, J. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3D transition metal catalysts. Catal. Sci. Technol. 2018, 8, 858–869. [Google Scholar] [CrossRef]
- Urdiana, G.; Valdez, R.; Lastra, G.; Valenzuela, M.; Olivas, A. Production of hydrogen and carbon nanomaterials using transition metal catalysts through methane decomposition. Mater. Lett. 2018, 217, 9–12. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrogen Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Rezaei, M.; Meshkani, F.; Zhang, K.; Zhao, X.; Pei, W.; Liu, Y.; Deng, J.; Arandiyan, H.; Dai, H. Influence of group VIB metals on activity of the Ni/MgO catalysts for methane decomposition. Appl. Catal. B 2019, 248, 515–525. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition over Pd promoted Ni/MgAl2O4 catalysts for the production of COx free hydrogen and multiwalled carbon nanotubes. Appl. Surf. Sci. 2015, 356, 1320–1326. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Meshkani, F.; Rezaei, M. Thermocatalytic decomposition of methane over mesoporous nanocrystalline promoted Ni/MgO·Al2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 16476–16488. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S. Production of hydrogen by methane catalytic decomposition over Ni-Cu-Fe/Al2O3 catalyst. Int. J. Hydrogen Energy 2009, 34, 2979–2985. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Wang, Q.; Ohare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Touahra, F.; Ketir, M.S.W.; Chebout, K.B.R. Effect of the Ni/Al ratio of hydrotalcite-type catalysts on their performance in the methane dry reforming process. Appl. Petrochem. Res. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Abdelsadek, Z.; Sehailia, M.; Halliche, D.; Gonzalez-delacruz, V.M.; Holgado, J.P. In-situ hydrogasi fi cation/regeneration of NiAl-hydrotalcite derived catalyst in the reaction of CO2 reforming of methane: A versatile approach to catalyst recycling. Journal of CO2 Utilization 2016, 14, 98–105. [Google Scholar] [CrossRef]
- Kumar, R.; Pant, K.K. Hydrotalcite-derived Ni-Zn-Mg-Al catalyst for Tri-reforming of methane: Effect of divalent to trivalent metal ratio and Ni loading. Fuel Process. Technol. 2020, 210, 106559. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Seshan, K.; Al Otaibi, R.L.; Al-Fatesh, A.S. Hydrogen production via catalytic methane decomposition over alumina supported iron catalyst. Arab. J. Chem. 2018, 11, 405–414. [Google Scholar] [CrossRef]
- Sikander, U.; Samsudin, M.F.; Sufian, S.; KuShaari, K.Z.; Kait, C.F.; Naqvi, S.R.; Chen, W.-H. Tailored hydrotalcite-based Mg-Ni-Al catalyst for hydrogen production via methane decomposition: Effect of nickel concentration and spinel-like structures. Int. J. Hydrogen Energy 2019, 44, 14424–14433. [Google Scholar] [CrossRef]
- Wan, C.; Shi, Z.; Huang, M.; Pan, J.; Luo, R.; Li, D.; Jiang, L. Influence of alloying on the catalytic performance of Ni–Al catalyst prepared from hydrotalcite-like compounds for methane decomposition. Int. J. Hydrogen Energy 2021, 46, 3833–3846. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Danish, M.; Shah, M. Synthesis and optimization of hydrotalcite derived Ni-Fe-Cu based catalysts for catalytic methane decomposition process using the design of experiment approach. J. Taiwan. Inst. Chem. Eng. 2021, 128, 370–379. [Google Scholar] [CrossRef]
- Anjaneyulu, C.; Naresh, G.; Kumar, V.V.; Tardio, J.; Rao, T.V.; Venugopal, A. Influence of Rare Earth (La, Pr, Nd, Gd, and Sm) Metals on the Methane Decomposition Activity of Ni-Al Catalysts. ACS Sustain. Chem. Eng. 2015, 3, 1298–1305. [Google Scholar] [CrossRef]
- Rosset, M.; Féris, L.A.; Perez-Lopez, O.W. Biogas dry reforming over Ni-Al catalyst: Suppression of carbon deposition by catalyst preparation and activation. Int. J. Hydrogen Energy 2020, 45, 6549–6562. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of Composition and Thermal pretreatment on properties of Ni–Mg–Al catalysts for CO2 reforming of methane. Appl. Catal. A Gen. 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Perez-Lopez, O.W. Biogas dry reforming for hydrogen production over Ni-M-Al catalysts (M = Mg, Li, Ca, La, Cu, Co, Zn). Int. J. Hydrogen Energy 2019, 44, 17750–17766. [Google Scholar] [CrossRef]
- Rosset, M.; Féris, L.A.; Perez-Lopez, O.W. Biogas dry reforming using Ni–Al-LDH catalysts reconstructed with Mg and Zn. Int. J. Hydrogen Energy 2021, 46, 20359–20376. [Google Scholar] [CrossRef]
- Rosset, M.; Sfreddo, L.W.; Hidalgo, G.E.N.; Perez-Lopez, O.W.; Féris, L.A. Adsorbents derived from hydrotalcites for the removal of diclofenac in wastewater. Appl. Clay Sci. 2019, 175, 150–158. [Google Scholar] [CrossRef]
- Rosset, M.; Perez-Lopez, O.W. Catalytic properties of Cu–Mg–Al hydrotalcites, their oxides and reduced phases for ethanol dehydrogenation. React. Kinet. Mech. Catal. 2018, 123, 689–705. [Google Scholar] [CrossRef]
- Denardin, F.G.; Perez-Lopez, O.W. Methane dehydroaromatization over Fe-M/ZSM-5 catalysts (M = Zr, Nb, Mo). Microporous Mesoporous Mater. 2020, 295, 109961. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Perez-Lopez, O.W. Graphene and carbon nanotubes by CH4 decomposition over Co–Al catalysts. Mater. Chem. Phys. 2019, 226, 6–19. [Google Scholar] [CrossRef]
- Rosset, M.; Féris, L.A.; Perez-Lopez, O.W. Biogas dry reforming over Ni-M-Al (M = K, Na and Li) layered double hydroxide-derived catalysts. Catal Today 2021, 381, 96–107. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Bezdička, P.; Jirátová, K.; Obalová, L.; Pacultová, K.; Bastl, Z.; Grygar, T. Effect of hydrothermal treatment on properties of Ni-Al layered double hydroxides and related mixed oxides. J. Solid. State Chem. 2009, 182, 27–36. [Google Scholar] [CrossRef]
- Gousi, M.; Andriopoulou, C.; Bourikas, K.; Ladas, S.; Sotiriou, M.; Kordulis, C.; Lycourghiotis, A. Green diesel production over nickel-alumina co-precipitated catalysts. Appl. Catal. A Gen. 2017, 536, 45–56. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In situ investigation of the thermal decomposition of Co-Al hydrotalcite in different atmospheres. J. Mater. Chem. 2001, 11, 821–830. [Google Scholar] [CrossRef]
- Li, F.; Tan, Q.; Evans, D.G.; Duan, X. Synthesis of carbon nanotubes using a novel catalyst derived from hydrotalcite-like Co–Al layered double hydroxide precursor. Catal. Lett. 2005, 99, 151–156. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Biogas reforming of carbon dioxide to syngas production over Ni-Mg-Al catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar] [CrossRef]
- Nguyen-Phu, H.; Kim, T.; Kim, Y.; Kang, K.H.; Cho, H.; Kim, J.; Ro, I. Role of phase in NiMgAl mixed oxide catalysts for CO2 dry methane reforming (DRM). Catal. Today 2023, 411–412, 113894. [Google Scholar] [CrossRef]
- Bian, L.; Wang, W.; Li, Z. Ni-based catalyst derived from Ni/Al hydrotalcite- like compounds by the urea hydrolysis method for CO methanation. RSC Adv. 2015, 6, 677–686. [Google Scholar] [CrossRef]
- Jin, L.; Ma, B.; Zhao, S.; He, X.; Li, Y.; Hu, H.; Lei, Z. Ni/MgO–Al2O3 catalyst derived from modified [Ni,Mg,Al]-LDH with NaOH for CO2 reforming of methane. Int. J. Hydrogen Energy 2018, 43, 2689–2698. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The influence of nickel content on the performance of hydrotalcite-derived catalysts in CO2 methanation reaction. Int. J. Hydrogen Energy 2017, 42, 23548–23555. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, L.; Zhu, Z.; Li, Z. La-promoted Ni/Mg-Al catalysts with highly enhanced low-temperature CO2 methanation performance. Int. J. Hydrogen Energy 2018, 43, 2197–2206. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Kobzar, E.O.; Trenikhin, M.V.; Leont’eva, N.N.; Serkova, A.N.; Salanov, A.N.; Lavrenov, A.V. Catalysts Based on Ni(Mg)Al-Layered Hydroxides Prepared by Mechanical Activation for Furfural Hydrogenation. Catalysts 2023, 13, 497. [Google Scholar] [CrossRef]
- Chmielarz, L.; Jabłońska, M.; Strumiński, A.; Piwowarska, Z.; Węgrzyn, A.; Witkowski, S.; Michalik, M. Selective catalytic oxidation of ammonia to nitrogen over Mg-Al, Cu-Mg-Al and Fe-Mg-Al mixed metal oxides doped with noble metals. Appl. Catal. B 2013, 130–131, 152–162. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Shoro, D.; Murakami, K.; Honda, M.; Kawabata, T.; Takaki, K. Preparation of egg-shell type Ni-loaded catalyst by adopting “Memory Effect” of Mg-Al hydrotalcite and its application for CH4 reforming. Catal. Commun. 2004, 5, 209–213. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Pavel, O.D.; Tichit, D.; Marcu, I.C. Acido-basic and catalytic properties of transition-metal containing Mg-Al hydrotalcites and their corresponding mixed oxides. Appl. Clay Sci. 2012, 61, 52–58. [Google Scholar] [CrossRef]
- Cosimo JIDi Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 510, 499–510. [Google Scholar] [CrossRef]
- Kong, L.; Li, D.; Bi, J.; Fan, X.; Xie, Z.; Xiao, X.; Zhao, Z. Template-induced mesoporous Ni–Al oxide catalysts with tuned physico–chemical properties for the oxidative dehydrogenation of ethane. Chem. Eng. J. 2023, 452, 139247. [Google Scholar] [CrossRef]
- Skoufa, Z.; Xantri, G.; Heracleous, E.; Lemonidou, A.A. A study of Ni-Al-O mixed oxides as catalysts for the oxidative conversion of ethane to ethylene. Appl. Catal. A Gen. 2014, 471, 107–117. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, J.; Li, L.; Tian, M.; Li, X.; Pan, X.; Chen, Y.; Wang, X. Improving the selectivity of Ni-Al mixed oxides with isolated oxygen species for oxidative dehydrogenation of ethane with nitrous oxide. J. Catal. 2019, 377, 438–448. [Google Scholar] [CrossRef]
- Summa, P.; Gajewska, M.; Li, L.; Hu, C.; Samojeden, B.; Motak, M.; Da Costa, P. Solution combustion synthesis as an alternative synthesis route for novel Ni-Mg-Al mixed-oxide catalyst for CO2 methanation. J. CO2 Util. 2022, 60, 101983. [Google Scholar] [CrossRef]
- Wei, Q.; Gao, X.; Liu, G.; Yang, R.; Zhang, H.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Facile one-step synthesis of mesoporous Ni-Mg-Al catalyst for syngas production using coupled methane reforming process. Fuel 2018, 211, 1–10. [Google Scholar] [CrossRef]
- Dang, C.; Li, Z.; Long, J.; Yang, W.; Cai, W. Sorption-enhanced glycerol steam reforming over hierarchical hollow Ni-CaO-Ca12Al14O33 bi-functional catalyst derived from hydrotalcite-like compounds. Fuel 2022, 324, 124468. [Google Scholar] [CrossRef]
- Chatla, A.; Abu-Rub, F.; Prakash, A.V.; Ibrahim, G.; Elbashir, N.O. Highly stable and coke-resistant Zn-modified Ni-Mg-Al hydrotalcite derived catalyst for dry reforming of methane: Synergistic effect of Ni and Zn. Fuel 2022, 308, 122042. [Google Scholar] [CrossRef]
- Zhang, R.J.; Xia, G.F.; Li, M.F.; Wu, Y.; Nie, H.; Li, D.D. Effect of support on catalytic performance of Ni-based catayst in methane dry reforming. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Liu, B.; Ma, Q.; Zhao, T.S.; Zhang, J. Recent advances in thermal catalytic CO2 methanation on hydrotalcite-derived catalysts. Fuel 2022, 321, 124115. [Google Scholar] [CrossRef]
- Leal Pérez, B.J.; Medrano Jiménez, J.A.; Bhardwaj, R.; Goetheer, E.; van Sint Annaland, M.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrogen Energy 2021, 46, 4917–4935. [Google Scholar] [CrossRef]
- Escobar, C.; Perez-Lopez, O.W. Hydrogen Production by Methane Decomposition Over Cu–Co–Al Mixed Oxides Activated Under Reaction Conditions. Catal. Lett. 2014, 144, 796–804. [Google Scholar] [CrossRef]
- Zardin, L.; Perez-lopez, O.W. Hydrogen Production by methane decomposition over Co-Al mixed oxides derived from hydrotalcites: Effect of the catalyst activation with. Int. J. Hydrogen Energy 2017, 42, 7895–7907. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the effect of nickel particle size on carbon formation type in the methane decomposition reaction. Catalysts 2020, 10, 890. [Google Scholar] [CrossRef]
- Asiai, K.; Nagayasu, Y.; Takane, K.; Iwamoto, S.; Yagasaki, E.; Ishii, K.; Inoue, M. Mechanisms of Methane Decomposition over Ni Catalysts at High Temperatures. J. Jpn. Pet. Inst. 2008, 51, 42–49. [Google Scholar] [CrossRef][Green Version]
- Martins, R.L.; Baldanza, M.A.; Souza, M.M.; Schmal, M. The effect of support on methane activation over Pt catalysts in the presence of MoO3. Appl. Catal. A Gen. 2007, 318, 207–212. [Google Scholar] [CrossRef]
- Herrera, J.E.; Resasco, D.E. In situ TPO/Raman to characterize single-walled carbon nanotubes. Chem. Phys. Lett. 2003, 376, 302–309. [Google Scholar] [CrossRef]
- Hermes, N.A.; Lansarin, M.A.; Perez-lopez, O.W. Catalytic Decomposition of Methane Over M–Co–Al Catalysts (M = Mg, Ni, Zn, Cu). Catal. Lett. 2011, 141, 1018–1025. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Ver. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Phil Trans. R. Soc. Lond. A 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Sato, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Dong, L.; Du, Y.; Li, J.; Wang, H.; Yang, Y.; Li, S.; Tan, Z. The effect of CH4 decomposition temperature on the property of deposited carbon over Ni/SiO2 catalyst. Int. J. Hydrog. En. 2015, 40, 9670–9676. [Google Scholar] [CrossRef]
- Zhang, C.C.; Hartlaub, S.; Petrovic, I.; Yilmaz, B. Raman spectroscopy characterization of amorphous coke generated in industrial processes. ACS Omega 2022, 7, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Kim, M.S.; Deng, Y.; Kang, K.Y.; Choi, J.S.; Jang, E.; Bae, J.W. Chemical looping-based catalytic CH4 decomposition and successive coke gasification with CO2 on ordered mesoporous NiMCeOx (M = Co, Zr, La). Chem. Eng. J. 2024, 489, 151034. [Google Scholar] [CrossRef]
- Mansooripour, H.; Bibak, F.; Meshkani, F. Facile synthesis of Ni-M/MgO (M = Y2O3, Gd2O3) catalysts using the surfactant-assisted co-precipitation method and their applications in the methane decomposition reaction. Mol. Catal. 2024, 564, 114277. [Google Scholar] [CrossRef]
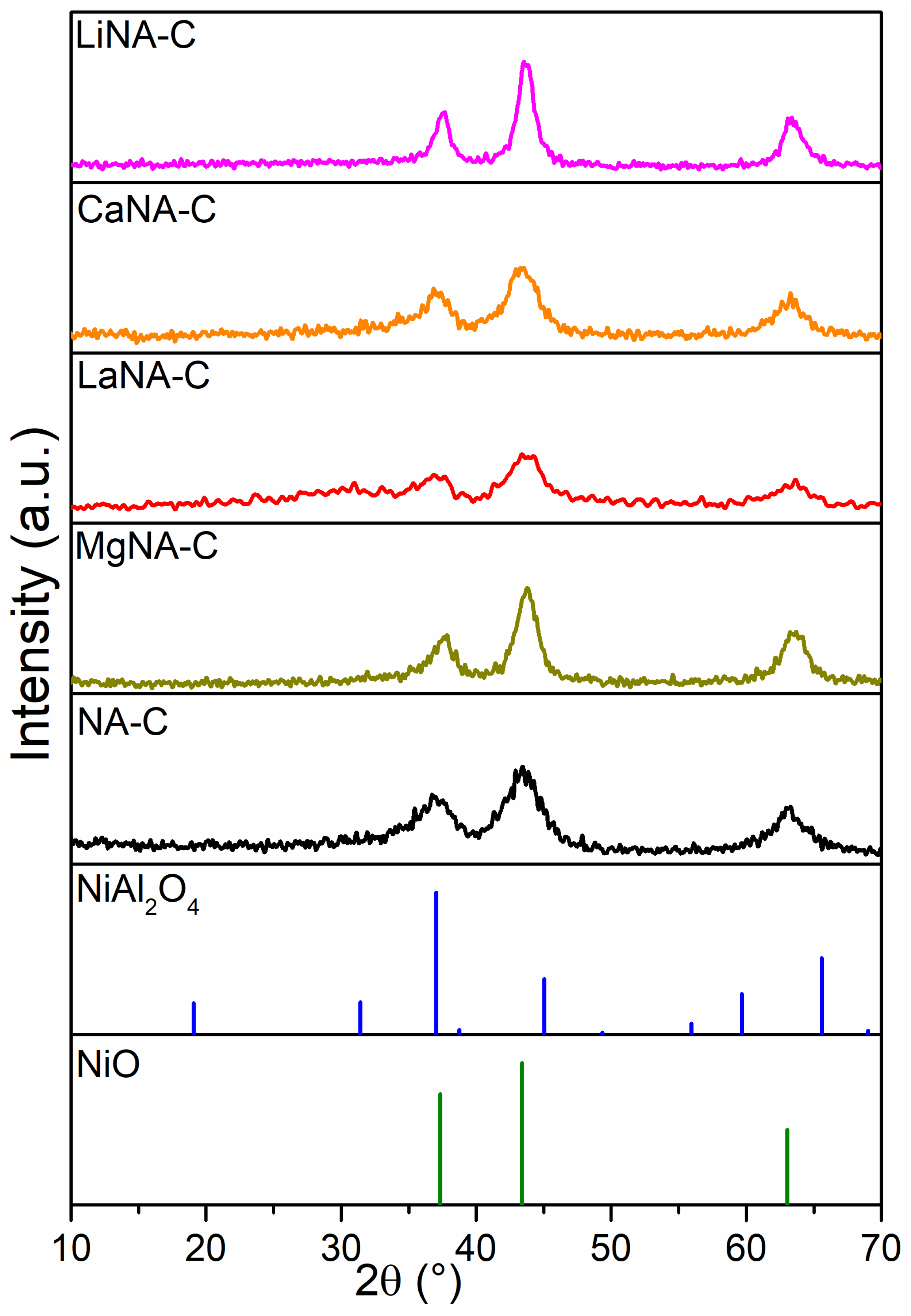
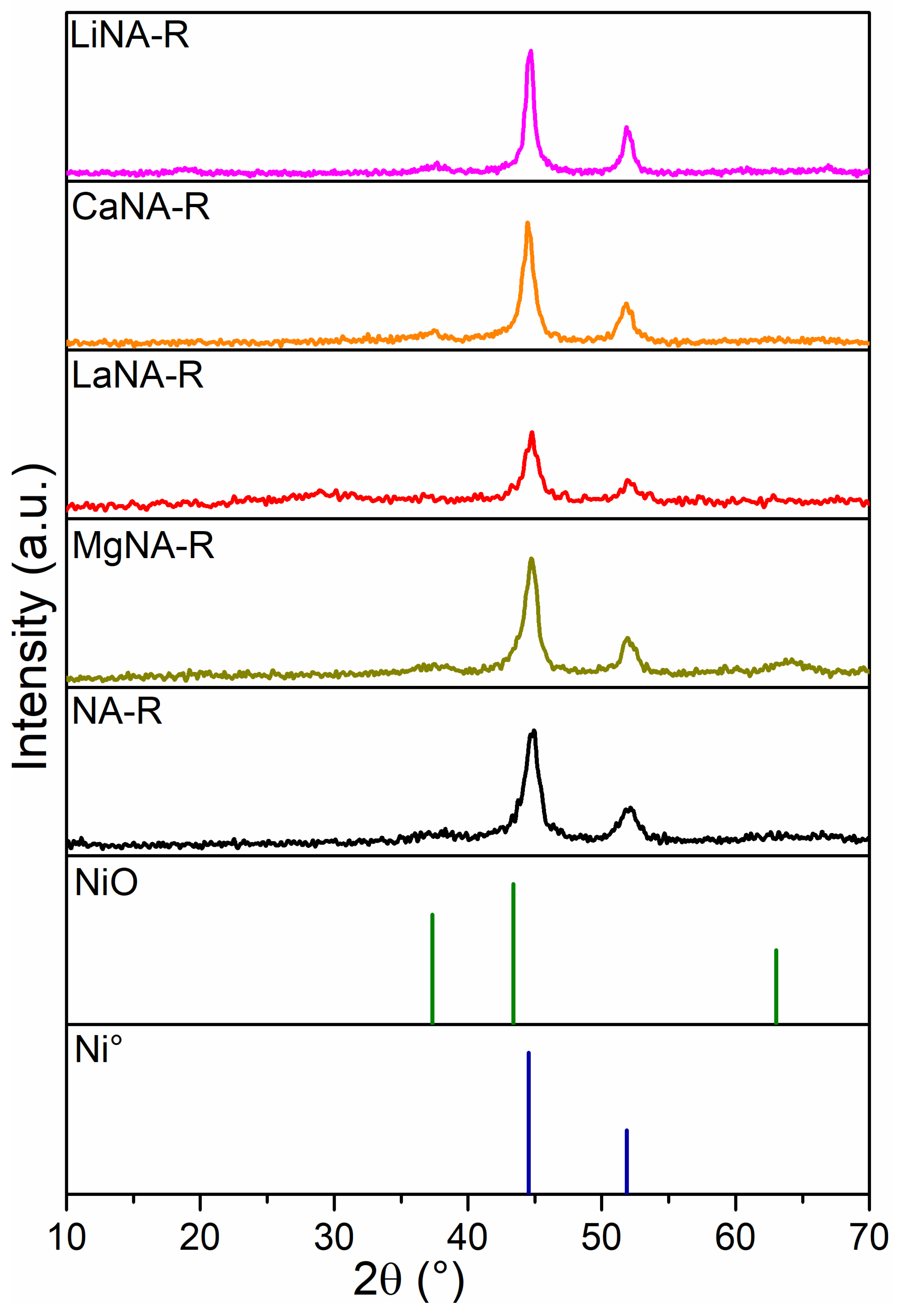
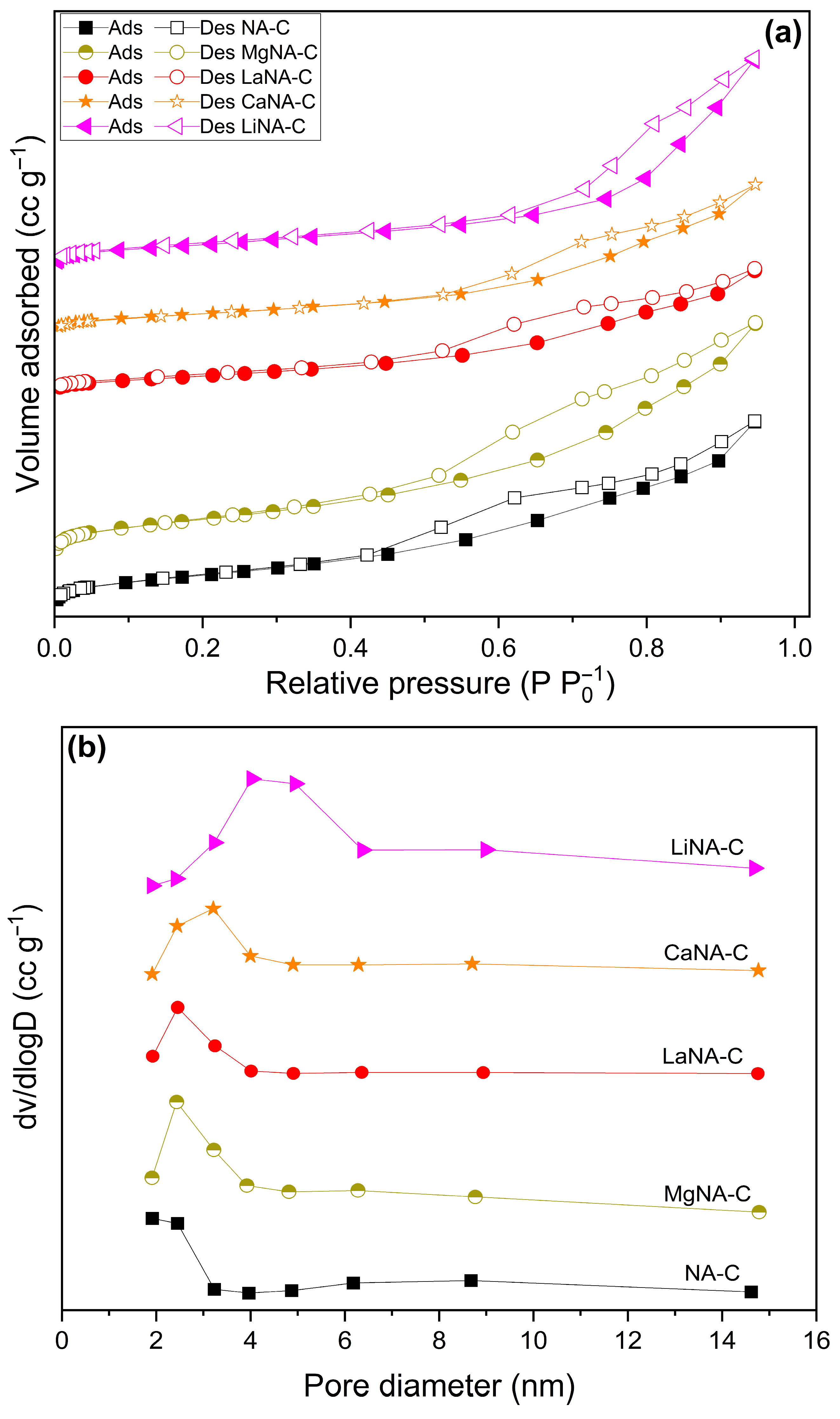

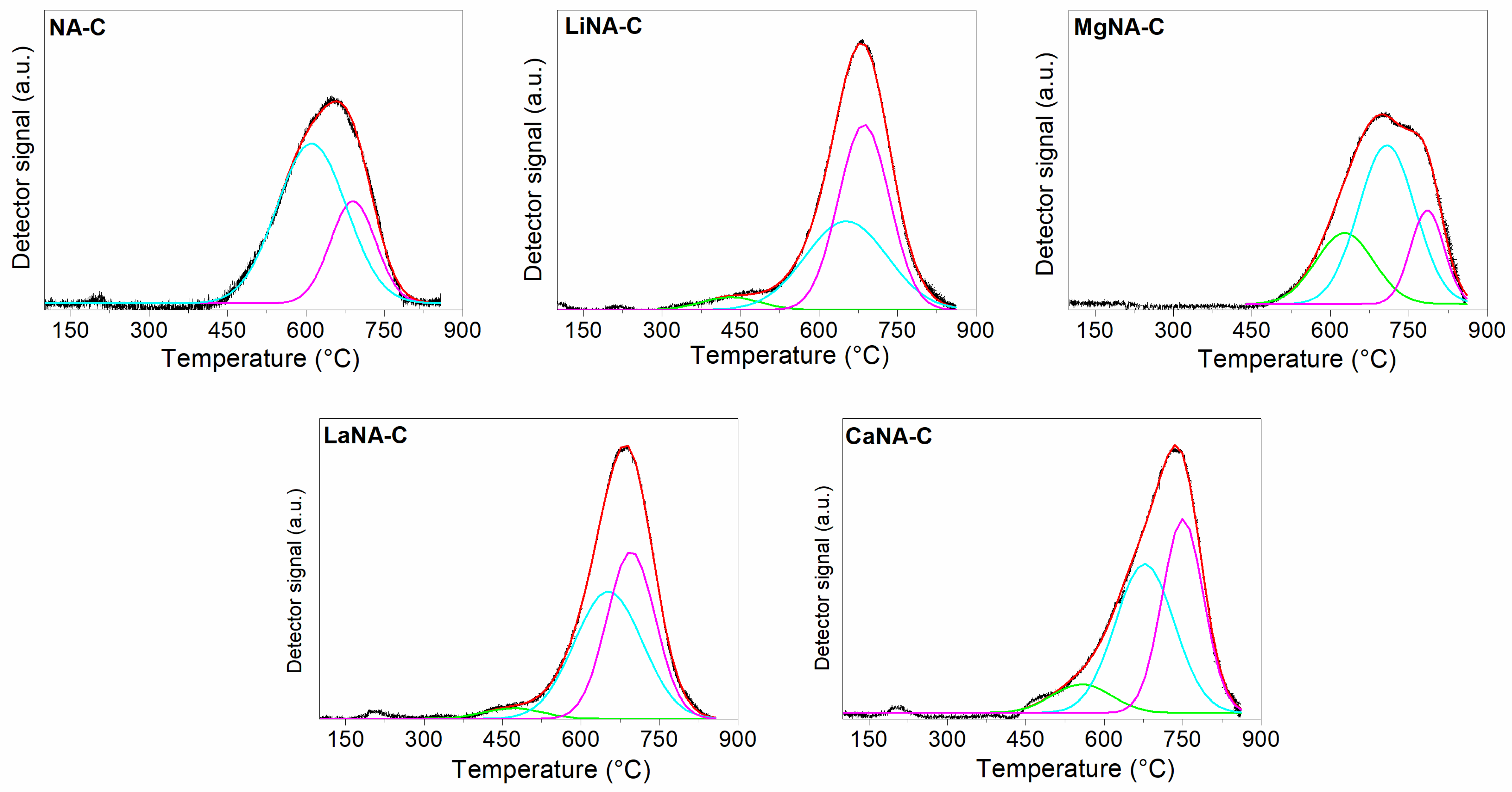


| Samples | SBET | Vpores | Dpores |
|---|---|---|---|
| (m2 gcat−1) a | (cm3 gcat−1) b | (nm) b | |
| NA-C | 174 | 0.323 | 3.8 |
| MgNA-C | 94 | 0.171 | 3.9 |
| LaNA-C | 100 | 0.216 | 4.9 |
| CaNA-C | 92 | 0.254 | 4.9 |
| LiNA-C | 118 | 0.344 | 8.1 |
| Samples | Temperature (°C) | Relative Basic Sites (mmol gcat−1) | Total Basic Sites | Total Area | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Peak | 2nd Peak | 3rd Peak | Weak | Medium | Strong | (mmol gcat−1) | (a.u.) | |
| NA-C | 220 | 286 | 446 | 0.24 | 0.29 | 0.38 | 0.91 | 401 |
| MgNA-C | 227 | 320 | 466 | 0.15 | 0.14 | 0.10 | 0.39 | 118 |
| LaNa-C | 214 | 273 | 384 | 0.15 | 0.17 | 0.21 | 0.53 | 237 |
| CaNA-C | 222 | 314 | 520 | 0.22 | 0.24 | 0.19 | 0.65 | 173 |
| LiNa-C | 202 | 280 | 384 | 0.07 | 0.06 | 0.10 | 0.23 | 99 |
| Samples | Peak Temperature (°C) | Relative Fraction of Peak Reduction Area (%) | Total Area (a.u.) | ||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | ||
| NA-C | - | 610 | 690 | - | 76 | 24 | 3218 |
| MgNA-C | 627 | 707 | 785 | 25 | 55 | 19 | 3306 |
| LaNA-C | 469 | 652 | 695 | 4 | 50 | 47 | 3437 |
| CaNA-C | 556 | 677 | 750 | 9 | 48 | 43 | 3727 |
| LiNA-C | 431 | 651 | 685 | 4 | 40 | 56 | 3389 |
| Samples | Average Crystallite Size by XRD (nm) a | ||||
|---|---|---|---|---|---|
| Reduced at 700 °C | After a Reaction Between 500 and 750 °C | After Reaction at 700 °C | |||
| Reduced with H2 | Heated with CH4 | Reduced with H2 | Heated with CH4 | ||
| NA | 13.2 | 7.3 | 7.7 | 15.6 | 9.3 |
| MgNA | 9.9 | 7.5 | 7.8 | - | - |
| LaNA | 7.2 | 13.1 | 16.3 | 15.3 | 11.2 |
| CaNA | 18.4 | 12.2 | 7.4 | - | - |
| LiNA | 16.8 | 14.7 | 16.6 | - | - |
| Sample | Weight Loss (%) | Carbon Formation Rate (mg gcat−1 h−1) | ||
|---|---|---|---|---|
| Reduced with H2 | Heated with CH4 | Reduced with H2 | Heated with CH4 | |
| NA | 72.62 | 77.98 | 864.52 | 666.50 |
| LaNA | 82.61 | 81.09 | 550.73 | 162.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosset, M.; Resing Dias, Y.; Amaral Féris, L.; Perez-Lopez, O.W. COx-Free Hydrogen Production via CH4 Decomposition on Alkali-Incorporated (Mg, La, Ca, Li) Ni-Al Catalysts. Nanoenergy Adv. 2025, 5, 10. https://doi.org/10.3390/nanoenergyadv5030010
Rosset M, Resing Dias Y, Amaral Féris L, Perez-Lopez OW. COx-Free Hydrogen Production via CH4 Decomposition on Alkali-Incorporated (Mg, La, Ca, Li) Ni-Al Catalysts. Nanoenergy Advances. 2025; 5(3):10. https://doi.org/10.3390/nanoenergyadv5030010
Chicago/Turabian StyleRosset, Morgana, Yan Resing Dias, Liliana Amaral Féris, and Oscar William Perez-Lopez. 2025. "COx-Free Hydrogen Production via CH4 Decomposition on Alkali-Incorporated (Mg, La, Ca, Li) Ni-Al Catalysts" Nanoenergy Advances 5, no. 3: 10. https://doi.org/10.3390/nanoenergyadv5030010
APA StyleRosset, M., Resing Dias, Y., Amaral Féris, L., & Perez-Lopez, O. W. (2025). COx-Free Hydrogen Production via CH4 Decomposition on Alkali-Incorporated (Mg, La, Ca, Li) Ni-Al Catalysts. Nanoenergy Advances, 5(3), 10. https://doi.org/10.3390/nanoenergyadv5030010









