Abstract
Nowadays, due to improvements in living standards, more attention is reserved to all-around disease prevention and health care. In particular, research efforts have been made for developing novel methods and treatments for anti-cancer therapy. Self-powered nanogenerators have emerged in recent years as an attractive cost-effective technology to harvest energy or for biosensing applications. Bioelectronic nanogenerators can be used for inducing tissue recovery and for treating human illness through electrical stimulation. However, there is still a lack of comprehensive cognitive assessment of these devices and platforms, especially regarding which requirements must be satisfied and which working principles for energy transduction can be adopted effectively in the body. This review covers the most recent advances in bioelectronic nanogenerators for anti-cancer therapy, based on different transducing strategies (photodynamic therapy, drug delivery, electrical stimulation, atomic nanogenerators, etc.), and the potential mechanisms for tissue repair promotion are discussed. The prospective challenges are finally summarized with an indication of a future outlook.
1. Introduction
In recent years, cancers, tumors, and oncogenic diseases have increases in frequency and leading millions of people to death [1,2,3]. The main current treatments to contrast this burden consist either of a surgical resection or chemotherapy and radiotherapy. In the first case, the removal of cancerous tumors does not provide a permanent solution to the disease; in fact, they recur in 30–50% of cases [4]. In the case of chemo/radio-therapies, the treatments can inhibit the recurrence of cancerogenic conditions, facilitating the cancer regression [5,6], but they have various levels of effectiveness and their impact affects also non-cancerous dividing cells, implying a non-specifically spread toxicity. Novel treatments are thus urgently needed with higher efficiency and accuracy, fewer side effects and permanent effectiveness. Recently, new therapies have been proposed, including drug delivery through nanocarriers, gas therapy [7], electrical stimulation [8], nanozyme-based catalytic therapy [9], photodynamic therapy [10,11,12], sonodynamic therapy [13], etc. The common element of these treatments consists of delivering anti-cancer agents in a localised and specific manner. To this purpose, microdevices or nanodevices that can be manipulated and controlled accurately are increasingly needed: these can take the form of nanocarriers (nanoparticles, nanoparticles-embedded liposomes, etc.) or smart bioelectronic patches that can deliver anti-cancer drugs or electrical signals for actuating biochemical effects against the propagation of cancers. All these strategies and unconventional therapeutic platforms need to be connected to a power source or ideally self-powered. Nanogenerators have emerged as a useful and effective technology for supplying electrical power to other systems: distributed networks of these devices harvesting energy provide a promising alternative that can complement standard power plants [14,15,16,17]. Invented in 2006 by Wang’s group [18], they can convert energy from the available sources (i.e., mechanical, thermal, biochemical, etc.) into electrical energy [18,19], due to intrinsic properties of their constituent materials and at the microscale/nanoscale, against conventional bulky systems for energy generation [15,20,21,22], especially chemical batteries which need periodic recharging, are unsuitable for being installed in hostile places and have a limited lifetime [23,24,25,26,27]. They are mostly employed to scavenge energy from the environments and natural resources of clean energy [14,15,20,22,28,29,30,31,32,33,34], e.g., environmental vibrations, wind flows, water waves, ocean currents, impacts of rainfalls, human biomechanics (voluntary movements, heartbeat, breathing, blood circulation, etc.) but also for sensing human motion [35,36,37], monitoring physiological parameters [35,38,39,40,41,42], for supplying implantable biomedical devices [38,43,44] or wearable systems for robotics [45,46,47]. The recent advancements in electronics, i.e., miniaturization, multifunctionality, property-tunability, softness, flexibility, conformality, and design adaptability, have fueled the rapid and continuous development of different types of nanogenerators, with novel materials [16,48,49] and applications [35,36,37,38,39,50,51,52,53,54,55,56]. The most common nanogenerators developed over the years are based on the piezoelectric, pyroelectric, and triboelectric effects [15,48,57], which intrinsically lead to charge generation upon the application of a mechanical strain, deformation, stress, temperature change, or friction between two dissimilar materials [15,58,59]. These devices have gained attractive properties, e.g., light weight, flexibility, simplicity of designs, durability, and cost-effectiveness. Additionally, their biocompatibility has made them suitable for being implanted and powering devices for stimulation or drug delivery for many diseases, including cancers [60].
In the context of cancer treatment, the word “nanogenerator” also refers to another strategy to specifically target and wipe out cancer cells without any side effects. Atomic nanogenerators have been proposed by McDevitt et al. [61], consisting of single atoms of a radioactive isotope housed inside a molecular cage which is attached to a monoclonal antibody targeted to specific forms of cancer. After injecting these constructs into the body and internalising them into cancer cells, tumors may be shrunk without toxicity owing to the decaying activity of the radioactive isotope, which releases ultimately high-energy alpha particles that destroy cancer cells.
In this review, different types of nanogenerators (bioelectronic and atomic) used for anti-cancer therapy are described and classified. Section 2 provides an overview of the main unconventional strategies for cancer therapies, with a highlight on the working principles that are aimed at killing cancer cells and reducing the propagation/size of the tumor (Figure 1). Atomic nanogenerators are presented as a specific category of molecular-size carriers that generate energy in the form of particles to bombard and kill cancer cells. State-of-the-art bioelectronic nanogenerators and self-powered platforms are instead described in Section 3, with a focus on the targeted application and on the respective operation modes and materials. Finally, the applicative perspectives and future challenges are discussed, with the aim to suggest improvements in the design and implementation of next-generation nanogenerators for the treatment of cancers.
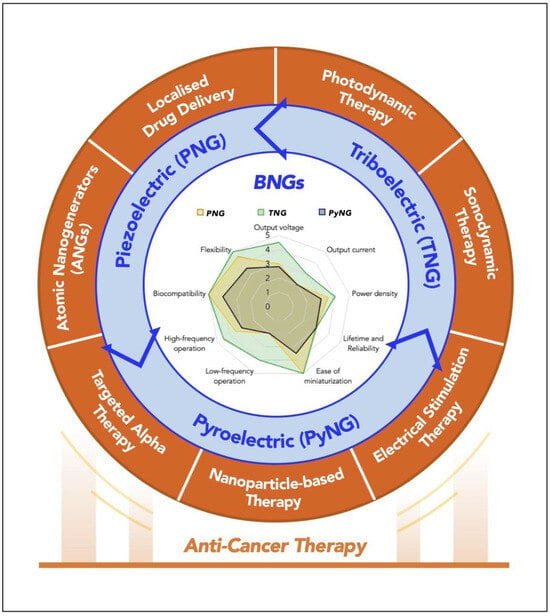
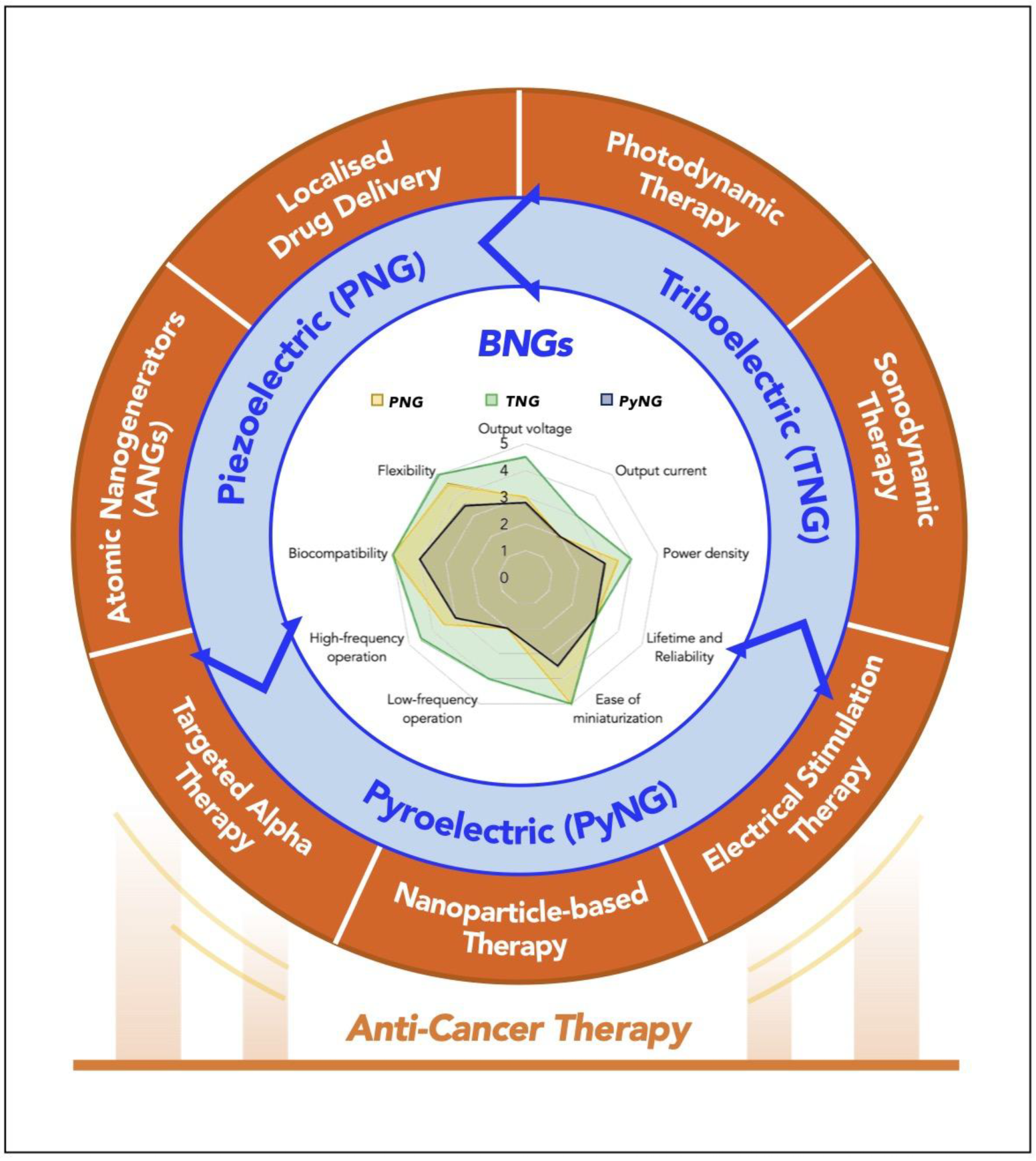
Figure 1.
Illustrations of the unconventional strategies for anti-cancer therapy: localized drug delivery, photodynamic therapy, sonodynamic therapy, electrical stimulation therapy, nanoparticle-based therapy, targeted alpha therapy, and atomic nanogenerators. BNGs: Bioelectronic nanogenerators.
2. Unconventional Strategies for Anti-Cancer Therapy
2.1. Localised Drug Delivery (LDD)
Localized drug delivery (LDD) has emerged as an unconventional yet highly effective strategy for anti-cancer therapies, offering a means to enhance therapeutic efficacy while minimizing systemic side effects [62] (Figure 2). This approach involves the direct administration of therapeutic agents to the tumor site, ensuring higher concentrations of the drug at the target location and reducing exposure to healthy tissues. Traditional systemic therapies often lead to significant adverse effects due to the non-specific distribution of drugs throughout the body. In contrast, localized drug delivery systems (LDDS) aim to provide a more targeted treatment modality, improving patient outcomes and quality of life. One of the primary advantages of localized drug delivery is its ability to stabilize embedded drug molecules and preserve their anticancer activity [63]. By directly administering drug-loaded implants or formulations at the tumor site, LDDS can achieve controlled and prolonged release of therapeutic agents. This sustained release ensures that adequate drug concentrations are maintained over time, allowing for effective diffusion into cancer cells as they divide. Various formulations have been developed for localized delivery, including hydrogels, nanoparticles, polymeric films, and wafers [64]. For instance, injectable hydrogels have gained popularity due to their ability to release drugs topically at the tumor site while minimizing systemic toxicity. These hydrogels can be engineered to respond to specific stimuli in the tumor microenvironment, such as pH or temperature changes, allowing for smart release mechanisms that further enhance therapeutic efficacy. Another significant aspect of localized drug delivery is its potential to load and release water-insoluble chemotherapeutics effectively. Many traditional chemotherapeutic agents suffer from poor solubility, limiting their effectiveness when administered systemically. However, by utilizing advanced delivery systems such as polymeric nanoparticles or liposomes, these drugs can be encapsulated and delivered directly to tumors [65]. This method not only improves the solubility of the drugs but also enhances their bioavailability at the target site. Brachytherapy exemplifies a successful application of localized drug delivery in cancer treatment [62,66]. This technique involves implanting radioactive seeds directly into or near a tumor, providing targeted radiation therapy while minimizing exposure to surrounding healthy tissues. Brachytherapy has shown efficacy in various cancers, including prostate and breast cancer, significantly reducing recurrence rates and improving patient survival outcomes. For example, studies have indicated that brachytherapy applied at surgical resection sites can decrease local recurrence rates in lung cancer patients from 19% to just 2%, demonstrating its effectiveness as a localized treatment option [67]. Recent advancements in LDDS technology have also focused on developing systems that allow for real-time monitoring and adjustment of drug release profiles [68]. These innovations include smart polymers that can alter their properties in response to environmental changes within the tumor microenvironment. Such systems enable clinicians to tailor treatment regimens based on individual patient needs, optimizing therapeutic outcomes. Despite its numerous advantages, localized drug delivery is not without challenges. One significant hurdle is ensuring adequate penetration of therapeutic agents into solid tumors, which often exhibit heterogeneous structures and varying degrees of vascularization [65,69]. Researchers are actively investigating methods to enhance drug diffusion within tumors, such as using combination therapies that incorporate mechanical or electrical stimulation alongside localized delivery systems. Moreover, regulatory approval processes for new LDDS technologies can be complex and time-consuming. Extensive preclinical and clinical trials are necessary to demonstrate safety and efficacy before these innovative treatments can be widely adopted in clinical practice. With ongoing advancements in formulation technologies and a deeper understanding of tumor biology, LDDS has the potential to revolutionize cancer treatment paradigms and improve patient outcomes significantly.
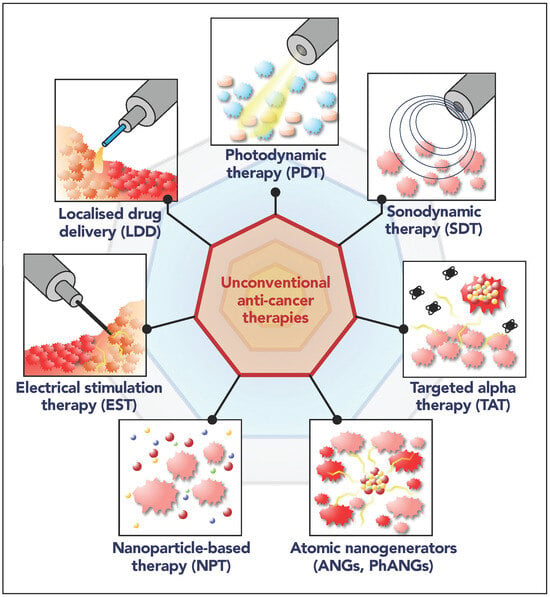
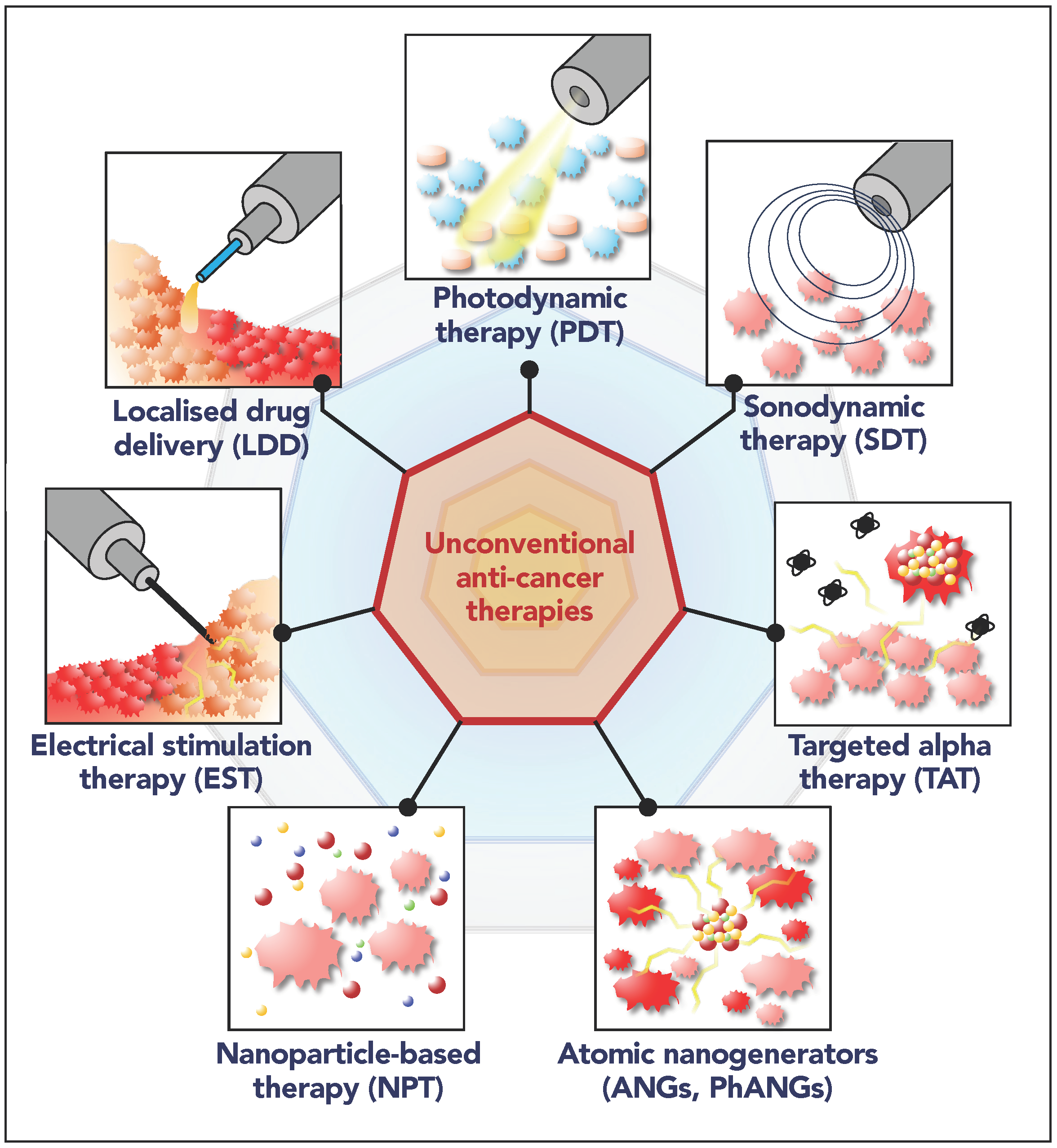
Figure 2.
Illustration of the unconventional anti-cancer therapies treated in this manuscript: localized drug delivery (LDD), photodynamic therapy (PDT), sonodynamic therapy (SDT), targeted alpha therapy (TAT), electrical stimulation therapy (EST), nanoparticle-based therapy (NPT), atomic nanogenerators (ANGs, PhANGs). The insets result from assembly of clipart images to illustrate the main working principle.
2.2. Photodynamic Therapy (PDT)
To induce photodynamic therapy (PDT), optical illumination is used to activate selectively some light-sensitive anti-cancer drugs (photosensitizers, PSs) [12,70,71,72,73] (Figure 2). PDT is characterized by its minimally invasive nature and high precision, allowing for localized treatment that minimizes damage to surrounding healthy tissues.
The fundamental mechanism of PDT involves the administration of a PS that preferentially accumulates in cancer cells [74]. Upon exposure to specific wavelengths of light, typically from lasers or light-emitting diodes (LEDs), the activated photosensitizer generates reactive oxygen species (ROS), including singlet oxygen and free radicals. These ROSs play a crucial role in inducing cellular damage, leading to apoptosis or necrosis of cancer cells. The effectiveness of PDT is contingent upon several factors, including the type of PS used, the light source’s wavelength and intensity, and the tumor’s oxygenation status [75]. PSs can be classified into first, second, and third generations based on their chemical properties and mechanisms of action. First-generation PSs, such as hematoporphyrin derivatives, have been widely used but are limited by poor solubility and inadequate tumor targeting [76]. In contrast, second-generation PSs have improved pharmacokinetics and selectivity for tumor tissues. Recent advancements in third-generation PSs leverage nanotechnology to enhance delivery and activation efficiency by utilizing nanocarriers that improve solubility, stability, and targeting capabilities. One significant advantage of PDT is its ability to induce not only direct cytotoxic effects on tumor cells but also to disrupt tumor vasculature. The generation of ROS can damage endothelial cells lining blood vessels within tumors, leading to thrombosis and reduced blood supply. This vascular disruption further contributes to tumor necrosis and enhances the overall therapeutic effect. Additionally, PDT has been shown to elicit an immune response against tumors [77,78]. The localized destruction of cancer cells can release tumor antigens into the surrounding tissue, stimulating an immune reaction that may help eliminate residual cancer cells even at distant sites.
Despite its advantages, PDT faces challenges that limit its clinical application. One major hurdle is the depth of light penetration in tissues; conventional light sources struggle to reach deeper tumors effectively [79]. Light delivery on the targeted cancerous area can be carried out through optical fibers or fiber-optic probes, similar to several other examples of implantable optical endoscopy probes for applications in optogenetics or laser therapy [80,81,82,83,84]. The main limitations for the long-term therapeutic applicability of this approach include the tight tethering, the risk of skin damage and the complexity of the operation [85]. To overcome these issues, novel miniaturized implantable devices have been proposed that can deliver light through integrated LEDs with spatial and temporal accuracy and, ideally, via wireless operation [86,87,88].
Furthermore, hypoxic conditions within tumors can significantly reduce the efficacy of PDT since oxygen is a critical component for ROS generation. Strategies to enhance oxygen delivery to tumors include using oxygen carriers such as perfluorocarbons or designing photosensitizers that can generate ROS under low-oxygen conditions. Clinical applications of PDT have expanded over the years. It has been approved for treating various cancers, including nonmelanoma skin cancer, oesophageal cancer, bladder cancer, and certain head and neck cancers. The versatility of PDT allows it to be used not only for curative purposes but also for palliative care to relieve symptoms associated with advanced cancers. Recent advancements in PDT research are focusing on integrating it with other treatment modalities, such as immunotherapy and targeted therapies. For instance, photoimmunotherapy combines PSs with immune checkpoint inhibitors or monoclonal antibodies to enhance anti-tumor immunity while leveraging the cytotoxic effects of PDT. In conclusion, PDT represents a significant advancement in cancer treatment strategies by offering a targeted approach that minimizes systemic toxicity while maximizing therapeutic efficacy. With ongoing research aimed at overcoming current limitations and improving treatment protocols through innovative technologies and combination therapies, PDT holds great promise as a vital tool in modern oncology.
2.3. Sonodynamic Therapy (SDT)
Sonodynamic therapy (SDT) is a cancer treatment based on low-intensity ultrasounds that are locally amplified by sono-sensitizers [89,90,91] (Figure 2). The propagation of ultrasounds is very efficient and compatible with biological tissue due to its non-radiative properties. Additionally, ultrasounds transfer energy in vivo independently from the environment [92,93,94,95,96].
This non-invasive approach offers significant advantages over traditional cancer therapies, particularly in its ability to penetrate deep tissues while minimizing systemic toxicity. The fundamental principle of SDT involves the administration of a sonosensitizing agent, which is a non-toxic chemical that becomes activated upon exposure to low-intensity ultrasound. When ultrasound waves are applied to the sensitized tissue, they generate reactive oxygen species (ROS) through various mechanisms, including cavitation, thermal effects, and mechanical stress. These ROSs are crucial for inducing cytotoxic effects within cancer cells, leading to apoptosis or necrosis.
Ultrasonic waves are usually delivered non-invasively using an ultrasound transducer placed on the skin or inserted endoscopically for deeper tumors. A coupling medium like ultrasound gel ensures efficient transmission. SDT typically uses low-intensity ultrasound in the 20 kHz-to-3 MHz range, depending on penetration depth and cavitation effects. Lower frequencies (20–500 kHz) enable deeper penetration and stronger cavitation, while higher frequencies (500 kHz–3 MHz) provide more localized effects. Frequencies around 0.5–1 MHz are commonly used to balance penetration and therapeutic efficiency in activating sonosensitizers for cancer treatment.
One of the key benefits of SDT is its capacity for deep tissue penetration, which allows it to target tumors that are otherwise difficult to reach with other therapies such as PDT. While PDT relies on light activation that can be limited by tissue opacity, ultrasound can effectively penetrate several centimeters into biological tissues, making SDT particularly suitable for treating solid tumors located deeper within the body. This characteristic not only enhances the therapeutic efficacy but also broadens the range of cancers that can be treated using this technique. The mechanisms by which SDT induces cancer cell death are multifaceted. One primary mechanism is the cavitation effect, where the rapid formation and collapse of microbubbles in response to ultrasound create shock waves and shear forces that disrupt cellular structures [90,97]. This mechanical damage can compromise cell membranes and organelles, leading to cell death. Additionally, the energy released from cavitating bubbles can facilitate the production of ROS, which further exacerbates cellular damage by oxidizing critical biomolecules such as lipids, proteins, and DNA [98]. Recent advancements in nanotechnology have significantly enhanced the effectiveness of SDT. Nanoparticles are being developed as sonosensitizers or carriers for existing sensitizers, improving their solubility, stability, and targeting capabilities [99,100,101]. For example, mesoporous silica nanoparticles can be loaded with chemotherapeutic agents alongside sonosensitizers, allowing for a dual-action approach where both drug delivery and sonodynamic effects occur simultaneously [102]. This combination not only increases the precision of treatment but also enhances overall therapeutic outcomes. Moreover, studies have indicated that SDT can elicit an immune response against tumors. The localized destruction of cancer cells releases tumor antigens into surrounding tissues, potentially stimulating systemic anti-tumor immunity. This phenomenon has been referred to as the “abscopal effect”, where treatment at a primary tumor site leads to the regression of distant metastases [103]. This aspect of SDT is particularly promising as it suggests that this therapy could not only target primary tumors but also help in controlling metastatic disease.
Several challenges remain in optimizing SDT for clinical use. One major hurdle is the need for effective sonosensitizers that can achieve high specificity and efficacy within tumor environments. Additionally, understanding the precise mechanisms by which ultrasound activates these sensitizers is crucial for maximizing therapeutic efficacy and minimizing side effects. Ongoing research aims to elucidate these mechanisms while developing new formulations and delivery systems that enhance the performance of SDT. In conclusion, sonodynamic therapy represents a cutting-edge strategy in cancer treatment that leverages ultrasound and sonosensitizers to induce targeted cell death with minimal side effects. Its ability to penetrate deep tissues and activate therapeutic agents selectively positions it as a valuable addition to existing cancer treatment modalities. As research continues to advance in this field, SDT holds great promise for improving patient outcomes and expanding treatment options for various malignancies.
2.4. Electrical Stimulation Therapy (EST)
Electrical stimulation represents a recent approach for numerous applications in the field of minimally invasive therapeutic devices. Electrical impulses are, in fact, the constituents of cognitive and skeletal functions for human beings and all animals [104,105]. Together with natural electrical impulses, the application of external impulses can be harnessed to address several medical disorders. Extracellular excitation (applied through electrodes) can generate or inhibit neural signals [106], or they can contribute to enhancing tissue regeneration processes for treatments of bone fractures [107,108,109], muscular dystrophy [110,111], hair loss [112,113,114,115], bladder control dysfunction [116,117,118,119,120,121], or skin wounds [122,123,124,125,126]. Bioelectronic nanogenerators or electronic skins are the most widely adopted devices for delivering electrical stimulation therapy (EST) for regenerative medicine applications [40,41,42,43,44,45,46,47,48,49], whereas neural dust platforms are generally used for other conditions, where peripheral nerves are considered [50,51,52,53,54,55,56,57,58]. The most recent devices for EST consist of electrodes patterned onto flexible or stretchable substrates, and they can be coupled with other functionalities, e.g., for aiding the delivery of drugs or of light with specific wavelengths.
In the context of anti-cancer therapy, EST has emerged as a promising approach, leveraging the application of electric fields to induce cell death in cancerous tissues (Figure 2). This method capitalizes on the unique responses of cancer cells to electrical inputs, which can disrupt cellular functions, promote apoptosis, and enhance the efficacy of conventional treatments.
One of the primary mechanisms through which electrical stimulation induces cancer cell death is by triggering apoptosis, a programmed cell death pathway that is often dysregulated in cancer cells. Recent studies have demonstrated that applying alternating current (AC) electric fields can activate bio-nanoantennae—nanostructures designed to facilitate electron transfer within cells. These bio-nanoantennae are typically created using conductive or semiconductive nanomaterials, such as gold, silver, carbon-based nanostructures, or piezoelectric nanocrystals, which can efficiently respond to external electric fields. They are often synthesized through chemical vapor deposition, electrodeposition, or self-assembly techniques to achieve optimal shape and conductivity. Once fabricated, these nanostructures are delivered to tumor sites via targeted nanoparticle carriers, functionalized with ligands or antibodies that ensure selective accumulation in cancerous tissues. Upon exposure to AC electric fields, the bio-nanoantennae enhance localized electron transfer, potentially modulating redox reactions, generating ROS, or interfering with cellular signaling pathways, ultimately contributing to cancer cell inhibition or death.
For instance, when redox mediators such as zinc porphyrin are used in conjunction with electrical stimulation, they can regulate electron transport and induce quantum biological tunneling, effectively triggering apoptosis in patient-derived cancer cells like glioblastoma (GBM) [127,128]. The application of a resonant electrical input has been shown to enhance mitochondrial activity and facilitate the release of cytochrome c, a key player in the apoptotic cascade. This process results in increased caspase activity, which is indicative of apoptosis, thereby selectively targeting and killing cancer cells while sparing normal tissues [129].
Electrical stimulation has also been found to significantly suppress the energy metabolism of cancer cells, leading to rapid cell death. By inducing changes in mitochondrial function and disrupting the electron transport chain (ETC), ES creates an energy crisis within cancer cells. This phenomenon has been described as a “domino effect”, where the initial electrical input leads to mitochondrial dysfunction and subsequent depletion of ATP levels [130]. Studies indicate that this disruption impedes critical metabolic pathways, resulting in cellular stress and eventual apoptosis or necrosis. The Young’s modulus of cancer cell membranes decreases due to cytoskeletal damage caused by electrical stimulation, further contributing to cell death by compromising membrane integrity [131].
Electroporation is another critical mechanism through which electrical stimulation induces cell death [132]. This process involves applying short bursts of high-voltage electric fields to create temporary pores in the cell membrane, allowing for increased permeability. In the context of cancer treatment, electroporation can facilitate the uptake of therapeutic agents or induce direct cell death by compromising cellular homeostasis.
Pulse streams, used in high-frequency irreversible electroporation (H-FIRE), improve tissue penetration while minimizing side effects. In contrast, AC electric fields, such as Tumor-Treating Fields (TTFields), are applied continuously at 100–500 kHz with low intensity (1–3 V/cm), disrupting cancer cell division without causing membrane poration, making them a non-invasive alternative to electroporation-based treatments. Nanosecond-pulsed electric fields (nsPEFs) extend this concept by generating numerous nanopores across all cellular membranes—a phenomenon known as supra-electroporation. This unique characteristic allows nsPEFs to induce significant cytotoxic effects across various tumor types by creating large numbers of pores that disrupt both plasma and intracellular membranes [133,134]. The rapid formation and subsequent collapse of these pores can lead to apoptosis or necrosis depending on the intensity and duration of the electric field applied [135].
In addition to direct effects on cancer cells, electrical stimulation can modulate the immune response against tumors. High-frequency electrical stimulation has been shown to enhance immune activity by promoting cytokine release and increasing the infiltration of immune cells into tumor sites. This dual action not only attacks cancer cells directly but also recruits the body’s immune system to recognize and eliminate tumor cells more effectively. For example, studies have demonstrated that combining electrical stimulation with immunotherapeutic agents can reduce tumor sizes in immune-competent mouse models while having minimal effects in immune-deficient models [136]. This suggests that ES may enhance the effectiveness of immunotherapies by improving drug delivery through electroporation or by stimulating an endogenous immune response.
The application of electrical stimulation for inducing cancer cell death holds significant promise for clinical translation. Several devices have been developed that utilize these principles, including bioelectronic nanogenerators that provide continuous bioelectrical stimulation at tumor sites. These devices can be designed for minimally invasive implantation and can operate autonomously without external power sources, making them suitable for long-term treatment regimens. Moreover, combining electrical stimulation with existing therapies such as chemotherapy or radiotherapy may enhance overall treatment efficacy while reducing side effects associated with systemic drug delivery. For instance, studies have indicated that applying electrical fields during chemotherapy can increase drug uptake in tumor cells while simultaneously inducing stress responses that sensitize them to treatment [137,138].
2.5. Nanoparticle-Based Therapy (NPT)
Nanoparticle-based therapy (NPT) has emerged as a revolutionary approach in the fight against cancer, offering innovative solutions that address the limitations of traditional treatment modalities such as chemotherapy, radiation, and surgery (Figure 2). The unique properties of nanoparticles (NPs)—including their size, surface area, and ability to be engineered for specific functions—enable them to enhance drug delivery, improve therapeutic efficacy, and reduce side effects [139,140,141,142,143].
Nanoparticles can be designed to deliver therapeutic agents directly to cancer cells with high specificity. The mechanisms of action primarily rely on two targeting strategies: passive targeting and active targeting [144]. Passive targeting exploits the enhanced permeability and retention (EPR) effect, which is a phenomenon observed in tumors due to their abnormal vasculature and poor lymphatic drainage [145,146]. This allows nanoparticles to accumulate preferentially in tumor tissues when administered systemically. The size of the nanoparticles plays a crucial role in this process; typically, particles ranging from 10 to 100 nanometers are optimal for effective accumulation in tumors. Active targeting, on the other hand, involves modifying the surface of nanoparticles with ligands that specifically bind to receptors overexpressed on cancer cells [147,148]. These ligands can include antibodies, peptides, or small molecules such as folic acid or transferrin. By functionalizing nanoparticles with these targeting moieties, researchers can enhance the selectivity of drug delivery systems, ensuring that therapeutic agents are released primarily at tumor sites while minimizing exposure to healthy tissues. Once the NPs reach the tumor microenvironment, they can release their payload through various mechanisms. For instance, stimuli-responsive nanoparticles can be engineered to release drugs in response to specific environmental cues such as pH changes, temperature fluctuations, or enzymatic activity prevalent in tumors. This controlled release enhances therapeutic efficacy while reducing systemic toxicity.
The use of NPT offers several significant advantages over conventional cancer treatments [149,150]. First, it provides improved pharmacokinetics: NPs can enhance the solubility and stability of poorly soluble drugs, improving their bioavailability and pharmacokinetics. This is particularly beneficial for hydrophobic chemotherapeutic agents that often face challenges in achieving therapeutic concentrations in plasma. Second, they help reduce side effects, in fact, by delivering drugs specifically to cancer cells, nanoparticle-based therapies can significantly reduce side effects associated with systemic chemotherapy. Traditional chemotherapy often affects rapidly dividing healthy cells, leading to adverse effects such as nausea, hair loss, and immunosuppression. Targeted delivery minimizes these effects by sparing normal tissues. Third, many cancers develop resistance to conventional therapies through various mechanisms such as drug efflux pumps or altered apoptotic pathways. Nanoparticles can be designed to circumvent these resistance mechanisms by co-delivering multiple therapeutic agents or using combination therapies that target different pathways simultaneously. Fourth, NPs can be engineered for various applications beyond drug delivery. They can be used for imaging purposes (theranostics), combining diagnostic and therapeutic functions within a single platform. This capability allows for real-time monitoring of treatment responses and tumor progression. Fifth, NPs have shown promise in enhancing immunotherapy by delivering immune-modulating agents directly to tumors or by acting as adjuvants that stimulate immune responses against cancer cells. By improving antigen presentation and T-cell activation, nanoparticle-based therapies can potentially increase the effectiveness of existing immunotherapies [151]. Furthermore, numerous studies have demonstrated the efficacy of NP formulations for delivering traditional chemotherapeutic agents such as doxorubicin and paclitaxel. For example, liposomal formulations of doxorubicin (Doxil) have been approved for treating breast cancer and Kaposi’s sarcoma due to their improved pharmacokinetics and reduced cardiotoxicity compared to free doxorubicin [152,153].
NPs are also employed in gene therapy applications where they serve as carriers for nucleic acids such as DNA or RNA. These carriers facilitate the delivery of therapeutic genes or RNA interference molecules directly into cancer cells, enabling targeted genetic modifications that can inhibit tumor growth [154,155,156].
Additionally, NPs can be used in conjunction with radiation therapy to enhance its effectiveness. For instance, gold nanoparticles have been shown to increase localized radiation dose deposition due to their high atomic number, resulting in improved tumor control while sparing surrounding healthy tissues [157,158,159,160]. Beyond their high-Z properties, AuNPs also possess a high surface area-to-volume ratio, leading to an enhanced density of free electrons at the nanoparticle interface. This contributes to increased secondary electron emission upon radiation exposure, amplifying localized energy deposition and DNA damage in cancer cells. While this effect is primarily due to physical dose enhancement, AuNPs also exhibit plasmonic properties, i.e., their conduction electrons collectively oscillate when exposed to specific electromagnetic fields, such as visible or near-infrared light. However, in the context of radiation therapy, plasmonic resonance plays a minor role compared to direct dose enhancement via secondary electron generation.
Besides regulatory hurdles, there are three main challenges for the extensive implementation of NPs in anti-cancer therapies. First, producing NPs at scale while maintaining consistent quality is a significant challenge. Standardized manufacturing processes need to be developed to ensure reproducibility and reliability across batches. Secondly, while many nanoparticles are designed with biocompatible materials, concerns regarding long-term toxicity and biodistribution remain. Comprehensive studies are essential to evaluate the safety profiles of these formulations over extended periods. Finally, the heterogeneous nature of tumors poses challenges for effective targeting using nanoparticles. Variability in receptor expression among different tumor cells may limit the effectiveness of targeted therapies; thus, strategies must be developed to account for this heterogeneity.
2.6. Targeted Alpha Therapy (TAT) and Atomic Nanogenerators (ANGs)
Targeted alpha therapy (TAT) is an innovative cancer treatment modality that utilizes the unique properties of alpha-emitting radionuclides, such as actinium-225 (225Ac), thorium-227 (227Th), and radium-223 (223Ra), to deliver localized cytotoxic effects directly to tumor cells (Figure 2). The fundamental principle behind atomic nanogenerators (ANGs) is their ability to emit high-energy alpha particles that have a short range in biological tissues, typically around 40 to 100 micrometers [61,161,162]. This limited range allows for the effective destruction of malignant cells while sparing surrounding healthy tissue, thereby minimizing systemic toxicity, a significant advantage over conventional radiation therapies.
The mechanism of action for ANGs involves the decay of radioactive isotopes, which emit alpha particles that cause direct damage to the DNA of cancer cells [163,164]. Alpha particles are highly ionizing and can create dense clusters of ionization events along their path, leading to substantial damage to cellular structures. This damage can lead to cell death through various pathways, including apoptosis and necrosis. The high linear energy transfer (LET) associated with alpha particles (approximately 80 keV/µm) results in a potent cytotoxic effect, making these radionuclides particularly effective against highly proliferative tumor cells. Research has shown that even minimal amounts of these radionuclides can achieve significant therapeutic outcomes, as they can kill cancer cells at very low activity levels, often measured in becquerels or picocuries [165,166].
One of the most compelling aspects of ANGs is their ability to be engineered for targeted delivery. By conjugating these radionuclides with monoclonal antibodies or peptides that specifically bind to tumor-associated antigens, researchers can enhance the selectivity of the treatment. This targeted approach ensures that the radioactive payload is delivered precisely to the tumor site, maximizing therapeutic efficacy while reducing off-target effects. For example, the use of 225Ac-labeled prostate-specific membrane antigen (PSMA) ligands has shown promising results in treating metastatic castration-resistant prostate cancer (mCRPC) [167,168]. Clinical studies have demonstrated impressive treatment responses in patients who had previously undergone extensive therapies, highlighting TAT as a salvage treatment option. In addition to antibody conjugation, NPs have been developed as carriers for radionuclides. These NPs can encapsulate alpha-emitting isotopes and facilitate their delivery directly to tumor cells. For instance, gold nanoparticles have been explored as carriers for 225Ac due to their biocompatibility and ability to enhance radiation effects through the photoelectric effect [169,170,171]. The combination of localized radiation from alpha emissions and enhanced absorption characteristics leads to improved therapeutic outcomes.
Moreover, ANGs can be combined with other therapeutic modalities to enhance their effectiveness further. For instance, integrating TAT with immunotherapy or chemotherapy could create a synergistic effect that improves overall treatment outcomes [172,173,174]. The localized destruction of tumor cells by alpha particles can release tumor antigens into the microenvironment, potentially stimulating an immune response that targets residual cancer cells. This dual approach not only addresses the primary tumor but also helps manage metastatic disease. Recent studies have shown that combining TAT with immune checkpoint inhibitors can lead to enhanced anti-tumor immunity. For example, when 225Ac-labeled antibodies are used alongside PD-1 inhibitors, there is an observed increase in T-cell activation and proliferation within the tumor microenvironment [175]. This not only enhances direct cytotoxic effects on cancer cells but also promotes systemic immune responses against distant metastases.
Clinical applications of ANGs have expanded over the years. They have been approved for treating various cancers, including non-Hodgkin lymphoma, prostate cancer, and certain types of neuroendocrine tumors. The versatility of TAT allows it to be used not only for curative purposes but also for palliative care to relieve symptoms associated with advanced cancers. For instance, 223Ra has been approved for treating bone metastases in prostate cancer patients. Clinical trials have demonstrated that patients receiving 223Ra experience significant reductions in pain and improved quality of life compared to those receiving standard care alone [176,177,178]. Similarly, 225Ac-PSMA therapy has shown remarkable efficacy in mCRPC patients with previously limited treatment options.
Despite their advantages, several challenges remain in the clinical application of ANGs. One significant hurdle is the limited availability and production of certain alpha-emitting radionuclides. The production process for isotopes like 225Ac is complex and often requires specialized facilities such as nuclear reactors or particle accelerators. Additionally, there are concerns regarding radiation safety and the management of radioactive waste associated with these therapies. Ongoing research is focused on optimizing production methods and developing new isotopes with favorable decay characteristics and availability. For example, researchers are exploring alternative production routes for 225Ac using neutron capture reactions or accelerator-based methods that may offer more sustainable production options [179]. Furthermore, understanding patient-specific factors that influence treatment responses is crucial for improving outcomes with atomic nanogenerators. Genetic mutations and variations in DNA repair mechanisms can affect how tumors respond to radiation therapy. Identifying biomarkers that predict sensitivity or resistance to TAT could help tailor treatments to individual patients, enhancing efficacy and minimizing adverse effects. Current research efforts are oriented towards addressing radionuclide availability and patient stratification.
2.7. Photoactivatable Atomic Nanogenerators (PhANGs)
Besides ANGs of alpha particles, other types of nanogenerators can be adopted in anti-cancer therapy to produce reactive species. The common nonmetal reactive species are based on oxygen, nitrogen, carbon, and sulfur, and they include a collective term of non-radical molecular forms (hydrogen peroxide (H2O2), CO, etc.) and radicals, i.e., atoms, ions, or molecules that contain unpaired electrons in the outermost electron orbitals, such as alkyl radical [180], nitric oxide (●NO) [181], peroxynitriteanion (ONOO–) [182], hydroxylradical (●OH) [183], chlorine radical (●Cl) [184], and superoxide radical anion (O●2−) [185].
Cancer cells exhibit elevated levels of reactive species, and, to maintain redox balance, they must produce higher amounts of endogenous antioxidants. This flexible ability to alter redox status can diminish the effectiveness of chemotherapy and radiotherapy against malignant tumor cells. Interestingly, some experimental findings indicate that reactive species may have a preferential inhibitory effect on cancer cells compared to normal cells due to the direct suppression of endogenous antioxidants by reactive species within cancer cells. Despite the inherent advantages of reactive species-based nanomedicine, therapies utilizing these species face several limitations [186]: (1) their dependence on oxygen restricts therapeutic efficacy in hypoxic tumors; (2) the short absorption wavelengths of photoactivatable nanogenerators hinder their ability to target deep-seated tumors; and (3) undesirable side effects resulting from the nonspecific distribution of nanogenerators continue to pose challenges. In light of these issues, researchers are focusing on designing and developing theranostic platforms that integrate reactive species-based monotherapy with synergistic therapies involving other treatment strategies. The generation of reactive species in cells can arise from internal stimuli (specific metabolic processes or pathological conditions) or external stimuli (physicochemical or biochemical reactions initiated by reactive species generators). Light-activated reactive species generators (or photoactivatable atomic nanogenerators, PhANGs) are promising therapeutic candidates due to their noninvasive nature and the ability to control their activity in a spatiotemporal manner for biomedical applications (Figure 2).
In the context of reactive species containing oxygen, PhANGs are often combined with PDT, offering two types of mechanisms [186]. The oxygen-dependent type-II PDT can transfer the photon energy to the PhANGs by exciting them from the ground state to the singlet state and to the triplet state, which eventually transfers the energy to the molecular oxygen for generating singlet oxygen. In contrast, the oxygen-independent type I PDT can generate superoxide radical anions and hydrogen peroxide without the involvement of molecular oxygen. Both types of PDT can induce both apoptotic and necrotic death of the tumor cells.
As an example, Liu et al. [187] developed an oxygen-independent radical nanogenerator, PI/FBC, through the co-assembly of iodized polymer PI and near-infrared (NIR) photosensitizer FBC. This system has been further evaluated as a remotely controllable platform for generating free radicals to enhance antitumor efficacy. The PI/FBC radical nanogenerator can be activated by NIR light to produce ROS by transferring energy to oxygen while also inducing the formation of toxic iodine radicals through electron transfer to PI. Importantly, this radical nanogenerator is controllable and unaffected by oxygen concentration, distinguishing it from traditional tumor treatments. Additionally, due to the strong NIR emission from FBC, the distribution of the PI/FBC radical nanogenerator can be monitored without the need for additional imaging agents.
Zheng et al. [186] proposed a comprehensive review of PhANGs and, more specifically, different examples of organic and inorganic photosensitizers for PDT.
Reactive nitrogen species (RNS), particularly nitric oxide (●NO) and its various metabolites, such as peroxynitrite anion (ONOO–), have also emerged as critical players in cancer therapy. As a vital biological regulatory molecule, ●NO is involved in numerous physiological and pathological processes, making it closely related to the development and progression of various diseases, including cancer. Similar to ROS, ●NO exhibits a concentration-dependent behavior that can be both beneficial and detrimental. For example, at concentrations below 0.2 μmol, ●NO can promote angiogenesis within solid tumors, facilitating their growth [186]. Conversely, at concentrations exceeding 1 μmol, ●NO can exert toxic effects on cells, leading to cell death and tumor regression when maintained at therapeutic levels. The antitumor mechanisms of ●NO are multifaceted. It influences the energy metabolism of cancer cells, which can result in tumor suppression. Additionally, ●NO can react with reactive oxygen radicals, generating nitrogen/oxygen-based free radicals that damage DNA structures. This damage is critical for inducing apoptosis in cancer cells. Furthermore, ●NO promotes macrophage activation to enhance the immune response against tumors and inhibits metastasis by downregulating platelet aggregation. It also plays a role in activating tumor suppressor genes like p53, which further contributes to cancer cell apoptosis and reduces the expression of P-glycoprotein (P-gp), a protein often associated with drug resistance. As a primary source of RNS in biological systems, ●NO possesses high reactivity in the presence of other radicals and can oxidize or nitrate local biomolecules. This characteristic allows for precise anti-cancer therapy through controlled release mechanisms at tumor sites. However, the creation of a precisely controllable NO generation system suitable for clinical use has proven challenging. Guo et al. [188] presented intelligent near-infrared (NIR) laser-triggered NO nanogenerators designed for the treatment of multidrug-resistant (MDR) cancer. These nanogenerators are created by integrating photothermal agents and heat-sensitive NO donors into a single nanoparticle. They can absorb 808 nm NIR photons and convert them into sufficient heat to trigger the release of NO. The produced NO molecules effectively reverse multidrug resistance by inhibiting the expression of P-glycoprotein, leading to increased intracellular accumulation of doxorubicin and heightened toxicity to MDR cancer cells in vitro. Additionally, through surface modification with targeting ligands, these nanoparticles selectively accumulate in tumor tissue. The therapeutic efficacy of the nanogenerators is confirmed in a humanized MDR cancer model, where in vivo experiments demonstrate their excellent tumor suppression capabilities with minimal side effects upon NIR laser exposure.
Various other strategies have been developed for the delivery of ●NO, including bonding methods such as R–O–NO, R–S–NO, R–N–NO (where R represents an alkyl group), and metal-NO complexes. These bonding strategies enable light-activated nanotherapeutic platforms to achieve controlled ●NO release through external light irradiation. The application of ●NO as monotherapy is relatively uncommon due to its concentration-dependent therapeutic effects. However, combination therapies involving ●NO have been extensively researched. Current studies on ●NO-based treatments primarily focus on four key pathways: (i) inducing cytotoxicity at high concentrations, (ii) chemotherapy sensitizer, (iii) radiotherapy sensitizer, and (iv) phototherapy sensitizer. The growing body of research on the integration of ●NO therapy with other treatment modalities underscores the importance of exploring innovative cancer treatment methods. For instance, Chen et al. [189] developed a tumor-specific nanogenerator for peroxynitrite (ONOO–) by encapsulating cisplatin and sodium nitroprusside within poly(d,l-lactide-co-glycolide) polymersomes to enhance drug delivery and improve chemotherapy outcomes. Following a series of catalysis involving nicotinamide adenine dinucleotide phosphate oxidases and glutathione reduction, this nanogenerator, referred to as PMCS, can selectively generate ONOO− within the tumor environment. The produced ONOO− not only significantly increases vascular permeability but also enhances the accumulation and penetration of PMCS into the tumor by activating matrix metalloproteinases that degrade the extracellular matrix. In addition to endocytosis, PMCS releases cisplatin to trigger apoptosis in tumor cells. Furthermore, free cisplatin released from dead cells can rapidly affect neighboring tumor cells through the ONOO-mediated upregulation of the copper transporter, thereby further enhancing the efficacy of chemotherapy.
Carbon-containing reactive species (RCS), particularly carbon monoxide (CO) and various alkyl radicals, have garnered significant attention for their potential as ANGs in anti-cancer therapy. CO, a notable carbon-centric derivative, exhibits unique properties that make it an intriguing candidate for therapeutic applications. It is well-established that CO has a binding affinity for hemoglobin (Hb) that is 220 times greater than that of oxygen, which can disrupt the oxygen-carrying capacity of Hb in the bloodstream and lead to poisoning effects. Beyond its toxicological implications, CO also plays a role as an endogenous signaling molecule with diverse biomedical functions, including regulating blood pressure, reducing inflammation, and sensitizing tumor cells to chemotherapy while impairing mitochondrial respiration. Due to these multifaceted roles, the investigation of CO as a therapeutic agent has gained considerable momentum. However, to mitigate the inherent toxicity associated with CO, it is crucial to develop strategies for its targeted delivery to tumor sites, ensuring controlled release upon demand. Various nanocarriers have been engineered to support CO donors, such as Ru carbonyl, Fe carbonyl, and Mn carbonyl, enabling effective transport and release of CO specifically at the tumor location. For instance, Zhang et al. [190] utilized gold nanocages (AuNCs) as PhANG to encapsulate the CO donor iron pentacarbonyl (Fe(CO)5) under anaerobic conditions to prevent premature leakage and oxidation during circulation. Under 808 nm laser irradiation, the AuNCs converted light into heat energy, facilitating the decomposition of Fe(CO)5 into CO and iron. The released CO subsequently interfered with mitochondrial respiration, induced autophagy in cancer cells, and damaged lysosomes. Additionally, the iron produced from this reaction underwent a Fenton reaction in the acidic environment of lysosomes, generating cytotoxic hydroxyl radicals that further enhanced anti-cancer efficacy. Beyond its direct effects on mitochondrial function and tumor growth inhibition, CO can also enhance the efficacy of chemotherapy. Small molecule chemosensitizers are often employed to increase tumor cell sensitivity to chemotherapeutic agents. Research indicates that CO can act as a chemosensitizer while also exhibiting anti-inflammatory properties. For example, Zhang et al. [191] developed a multifunctional CO nanogenerator based on partially oxidized tin disulfide nanosheets (POS NSs), which were modified by targeting ligand-functionalized polymers to improve tumor specificity. The POS NSs effectively loaded doxorubicin (DOX), allowing for the generation of CO upon 561 nm laser irradiation through catalyzing CO2. This process not only sensitized tumor cells to DOX treatment but also induced hyperthermia upon laser exposure for photothermal ablation of tumors. The resultant CO release reduced inflammatory responses associated with photothermal therapy (PTT), demonstrating the potential of CO in enhancing therapeutic outcomes. Similarly, Shen et al. developed MCM@PEG–CO–DOX nanoparticles by oxidizing ferrocene compounds to create carbonized magnetic nanoparticles (MC), which were then modified with iron-based metal-organic frameworks (MOFs) to serve as carriers for both the CO donor Mn(CO)5Br and DOX. Upon 808 nm laser irradiation, these nanoparticles produced photothermal effects that triggered the release of both ●NO and DOX. The combination of these therapies elicited significant tumor suppression effects due to their synergistic action. Moreover, recent studies have shown that CO can enhance oxygen consumption in mitochondria, creating a hypoxic environment conducive to activating hypoxia-activated prodrugs. For instance, Li et al. [192] developed a theranostic nanoplatform using mesoporous Prussian blue nanoparticles (m-PB NPs) as carriers for tirapazamine (TPZ) and iron pentacarbonyl (Fe(CO)5). By modifying these nanoparticles with polyallylamine hydrochloride and polyacrylic acid to prolong blood circulation time and promote accumulation at tumor sites, they demonstrated that NIR light irradiation induced cleavage of coordination bonds in Fe(CO)5, releasing CO. The subsequent increase in aerobic respiration led to enhanced activation of TPZ within hypoxic tumor cells, resulting in improved antitumor efficacy. Furthermore, studies have indicated that multidrug resistance (MDR), often mediated by P-glycoprotein (P-gp)-dependent drug efflux mechanisms in cancer cells, poses significant challenges to chemotherapy efficacy. Various strategies have been proposed to overcome P-gp-mediated drug resistance. However, recent research has revealed that CO can effectively reverse this resistance by disrupting mitochondrial function and inhibiting ATP-dependent drug efflux mechanisms. In addition to enhancing chemotherapy effectiveness, CO has also been explored for optimizing PDT against hypoxic tumors. Wang et al. [193] synthesized a nano-photosensitizer based on the diketopyrrolopyrrole nucleus (DPP-NF), incorporating a light-responsive ●NO donor and pH-sensitive groups into its structure. The DPP-NF nanoparticles could be activated under weakly acidic lysosomal conditions to generate reactive oxygen species for PDT while enabling photothermal conversion under laser irradiation. Upon exposure to 660 nm light, released ●NO was able to induce DNA damage in tumor cells while overcoming the low efficiency of PDT in hypoxic environments. In conclusion, carbon-containing reactive species like carbon monoxide offer promising avenues for cancer treatment through their unique mechanisms of action and ability to synergize with existing therapies. By leveraging advanced nanotechnology platforms designed for controlled release and targeted delivery of CO, researchers aim to optimize therapeutic outcomes while minimizing side effects associated with traditional cancer treatments.
3. Bioelectronic Nanogenerators for Anti-Cancer Therapy
3.1. Bioelectronic Nanogenerators (BNGs): Materials and Mechanisms
Bioelectronic nanogenerators for implantable use against cancer are generally based on patches made of functional materials. Most commonly, these materials include insulators/dielectrics, conductive layers/electrodes, and/or electromechanically active materials, such as piezoelectrics. Piezoelectricity and triboelectricity are the most widely adopted mechanisms to generate charges, thus electrical energy, which can be directly used for electrical stimulation or for supplying other connected devices. In this section, the main principles of piezoelectric and triboelectric materials are outlined.
3.1.1. Piezoelectric Materials and Nanogenerators
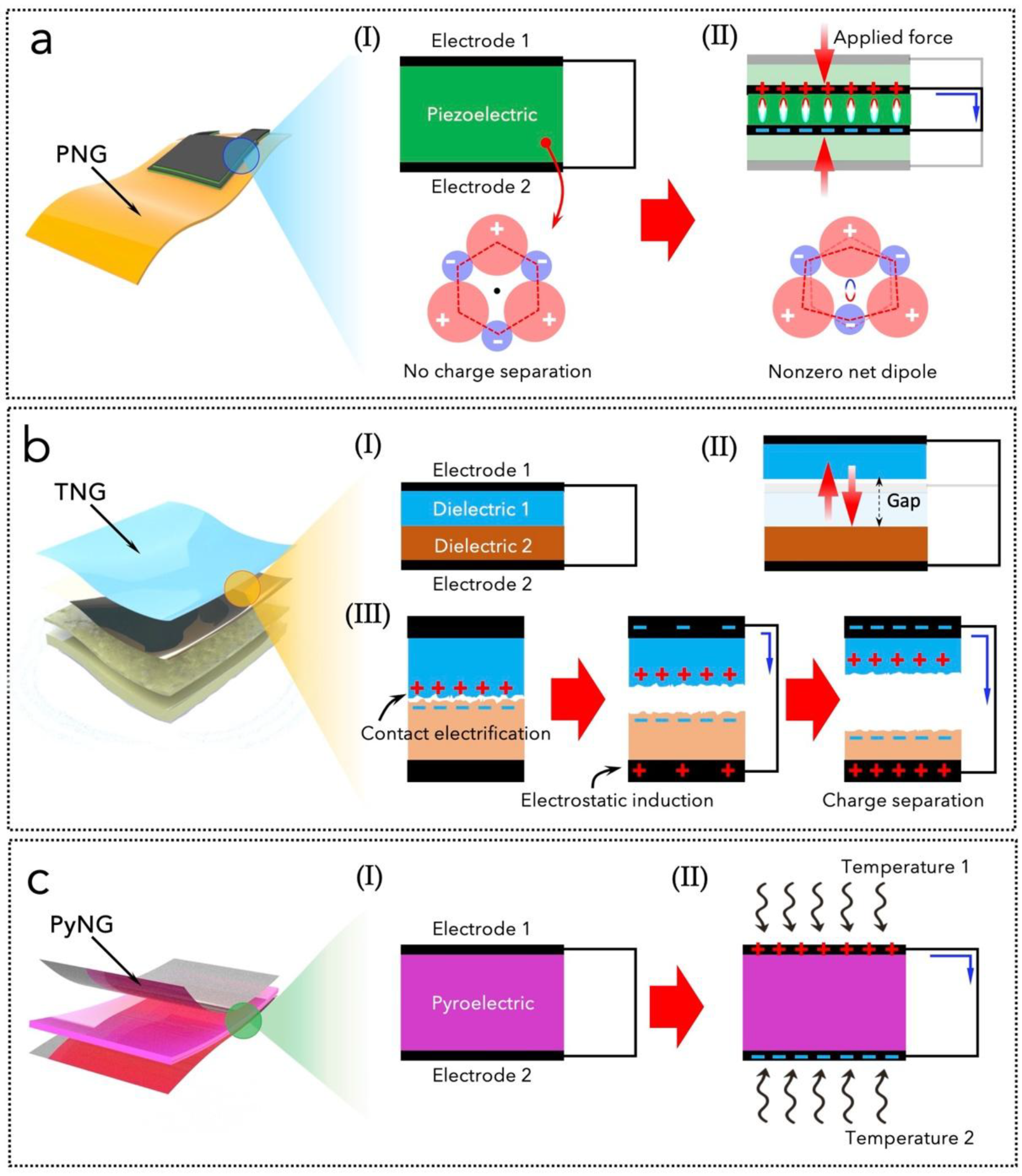
Piezoelectric nanogenerators are based on two electrodes and a piezoelectric material sandwiched between them (Figure 3a). The latter can directly convert applied strain energy into electrical energy, with high output power density [58,194,195]: under an applied mechanical strain, the center of negative and positive ions in the microstructure is no longer overlapped, resulting in a nonzero dipole moment that generates a piezoelectric potential and thus an electric current in an external circuit [196]. Conversely, an electric voltage applied onto a piezoelectric material will generate a displacement or a deformation, which is useful for actuation or to generate acoustic waves [197]. Piezoelectrics are usually produced in the form of nanostructured layers [198,199,200] or as thin films [201,202,203,204,205], and they can be ceramic or polymeric [196,197]. Thin-film ceramic piezoelectric materials can be deposited onto rigid and soft substrates, such as polyimide, poly(ethylene naphthalate), etc. [59]. There exist several choices for selecting a piezoelectric material: besides lead zirconate titanate (PbZr1−xTixO3, PZT) and its derivatives, which are highly performing but also toxic due to the presence of lead, other ceramic lead-free alternatives are potassium sodium niobite (KNN) [206], lithium niobite (LiNbO3) [207], barium titanate (BaTiO3) [208], zinc oxide (ZnO) [201,209], or aluminium nitride (AlN) [210,211]. For instance, ZnO has a wurtzite microstructure: under normal conditions, Zn2+ and O2− ions are arranged in a tetrahedron structure along the c-axis, but upon the application of outer stress, a non-centrosymmetric structure is formed and a piezoelectric potential is generated [212,213,214]. LiNbO3 is a cheap epitaxially grown piezoelectric material exploited for acoustic and optical devices [215], characterized by chemical inertness, heat resistance, lower dielectric constants than other piezo-ceramics [216], and thin-film processability [207]. AlN also emerged, together with ZnO, as a lead-free highly performing material [22,217,218,219,220,221]. It can be synthesized by sputtering deposition in the form of transparent micrometric films on flexible substrates [32,41,210,222,223] with attractive properties, e.g., temperature and humidity resistance [224], chemical stability [225,226], and biocompatibility [59,202]. Finally, piezo-polymers are an alternative solution to achieve flexibility and maintain piezoelectric performances. Poly(vinylidene fluoride) (PVDF) and its co-polymers [42,227] are widely used because they are lightweight, soft, and suitable for being employed in fluid flows or for wearable/implantable patches [42,228,229]. Although compared to other inorganic piezo-materials they have high dissipation factors, high dielectric constants, poor heat resistance, and damped performances [230], PVDF derivatives have high piezoelectric coefficient compared to other polymers, excellent chemical resistance, and ease of fabrication, such as electrospinning [42].
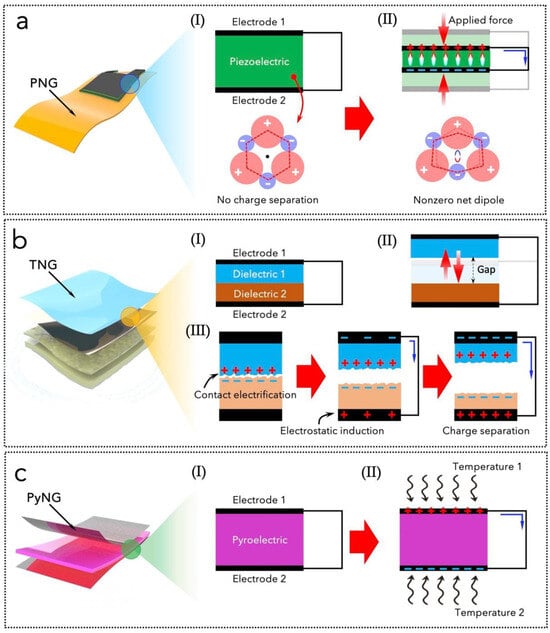
Figure 3.
Illustrations of the fundamental working principles of piezoelectric (a), triboelectric (b), and pyroelectric nanogenerators (c). (a,b) Reprinted from [19], MDPI, Creative Commons Attribution (CC BY) license.
3.1.2. Triboelectric Materials and Nanogenerators
Triboelectric nanogenerators are based on coupling the triboelectrification effect with the electrostatic induction effect. Triboelectricity, i.e., the generation of triboelectric surface charge densities, occurs during the relative contact (in vertical or horizontal mode) of two dissimilar materials [231,232,233]. Electrostatic induction induces the redistribution of charges from these materials to some conductive or metal electrodes, which allows for the generation of electrical power through an external circuit (Figure 3b) [234,235,236,237]. Triboelectric materials are classified based on their triboelectric polarity, i.e., their capability of attaining electrons or positive charges on the surface, which is responsible for the material’s surface charge density. The materials’ polarity depends on the electron affinity and the work function, as established in the triboelectric series [234,238,239]. Contact electrification consists of generating and redistributing surface charges at the contact interface between two materials. This process occurs at the interfaces, so it strongly depends on the materials’ morphology and surface properties, e.g., it is enhanced by different nanostructuring/patterning techniques that change the surface roughness, the porosity, and the wettability [240,241,242,243,244,245,246]. Common examples of flexible triboelectric materials are polyimide, polytetrafluoroethylene, polyethylenetereftalate, polydimethyl siloxane, and parylene C (poly(para-xylylene)) [22,41,247,248,249,250,251,252,253,254,255,256,257]. Triboelectric nanogenerators rely on the vertical or horizontal sliding contact between two materials connected to two electrodes: the charges generated during friction can be collected through an external circuit and produce current and voltage. Compared to electromagnetic generators, they are lightweight, low-density, cost-effective, and highly scalable, and they do not need bulky architectures, e.g., with coils or magnets [16,31,35,40,232,258,259,260,261]. Triboelectric nanogenerators can be divided into four basic operation modes. The first one is the lateral sliding mode, which consists of two electrode layers, two triboelectric materials, and the connecting wires. The second one is the vertical contact-separation mode, consisting of two triboelectric materials in front of each other with metal electrodes on the back of each one; the cycling contact and separation between the two materials generates surface charges that are transferred by induction to the electrodes. The third one is the single-electrode mode that presents a triboelectric layer with only one electrode to collect energy from a moving object without connecting leads. The fourth one is the freestanding triboelectric-layer mode, which includes a free-moving object and two electrode layers that are not in complete contact with the frictional object.
3.1.3. Pyroelectric Materials and Nanogenerators
Pyroelectric nanogenerators (PyNGs) convert thermal energy into electrical energy due to changes in the polarity of the material’s crystalline microstructure due to a change in temperature. This change in the internal spontaneous polarization strength is responsible for a flow of electrons that can be collected in an external circuit (Figure 3c). When the temperature of a pyroelectric material changes, it induces a variation in the polarization of the material. This change can be due to external heat sources or ambient temperature fluctuations. As the material’s polarization changes, bound charges are displaced within the material, leading to the generation of an electric field. This electric field drives charge carriers towards the electrodes of the nanogenerator, producing a measurable electric current. The generated electrical energy can be harnessed for powering small devices or stored for later use. The efficiency of energy conversion is influenced by factors such as material composition, temperature range, and structural design [262]. This property implies that these nanogenerators can be widely used in temperature-controlled closed-loop drug delivery systems. The development of PyNGs has garnered significant interest due to their potential applications in energy harvesting from ambient heat sources, such as body heat and environmental temperature variations. The materials employed in PyNGs can be broadly categorized into ceramics and polymers. Ceramic pyroelectrics include traditional lead-based ceramics, such as PZT, that are well-known for their high pyroelectric coefficients and energy density, making them effective for energy harvesting applications. However, environmental concerns regarding lead have prompted a shift towards lead-free alternatives. Recent advancements have focused on materials such as sodium bismuth titanate (NBT) and potassium niobate (KNbO3), which offer comparable pyroelectric properties with enhanced environmental compatibility [263,264]. For instance, NBT-based ceramics have demonstrated high energy density and stability under varying temperatures [264]. Among polymers, as for its piezoelectric properties, PVDF is widely used due to its excellent flexibility and high pyroelectric response. Variants such as P(VDF-TrFE) have been engineered to optimize their pyroelectric performance, achieving significant energy conversion efficiencies [265].
Combining polymers with nanostructured materials, such as metal oxides or carbon nanotubes, has been shown to enhance the overall performance of PyNGs, as well as of PNGs, by improving mechanical properties and increasing surface area for charge generation [266].
PyNGs are emerging as a promising power source for implantable medical devices, addressing the limitations of conventional battery systems. Recent advancements have also focused on hybrid systems that combine pyroelectric materials with piezoelectric elements to enhance energy harvesting efficiency. The flexibility and small size of these nanogenerators enable them to conform to the body’s contours, making them ideal for applications such as pacemakers and wearable health monitoring devices [267,268,269,270]. The development of PyNGs is accelerating the creation of self-powered biomedical systems that can monitor health conditions in real time while minimizing invasive procedures. As research progresses, these nanogenerators hold significant potential to revolutionize the field of implantable electronics by providing a reliable and sustainable power source that leverages the body’s inherent thermal energy.
3.2. BNGs for Localised Anti-Cancer Drug Delivery
When drugs are utilized for disease prevention, diagnosis, and treatment, they must be formulated into suitable dosage forms. The effectiveness of a drug is typically assessed based on the rationality, precision, safety, and efficacy of these dosage forms. Drug dosage forms have evolved through five generations. The first generation includes pastes or pills containing active pharmaceutical ingredients that can be administered either externally or orally. As clinical demands and administration methods expanded, the second generation emerged, comprising aerosols, capsules, tablets, and injections. The third generation introduced controlled drug delivery systems (DDS), which maintain effective drug concentrations in vivo over extended periods after one or more doses, overcoming limitations related to timing and frequency of administration. The fourth generation focuses on targeted DDS that enhance drug accumulation in specific organs, tissues, and cells, thereby minimizing toxicity and side effects while improving therapeutic outcomes. Targeted DDS are particularly prevalent in tumor therapy. The fifth generation includes pulsed DDS that automatically release drugs in sync with the body’s physiological rhythms during disease episodes. Self-powered technology based on nanogenerators BNGs offers practical solutions to these challenges [271].
A self-powered system operates independently of an external power source. In the intelligent-controlled DDS, self-powered devices can generate electric power to address the energy requirements for drug release control with output regulated by the design of the NG’s structure and function. Furthermore, biodegradable BNGs not only mitigate surgical risks but also reduce the overall size and complexity of drug delivery devices.
Chemotherapy is a commonly employed treatment for cancer. However, it is associated with limited therapeutic efficacy and significant side effects. As a result, there is a pressing need for drug delivery systems that can target specific sites, enhancing drug delivery to tumors while reducing off-target effects and overall drug dosages, thereby minimizing adverse reactions [272]. The integration of BNGs into localized anti-cancer drug delivery systems represents a significant advancement in anti-cancer therapy, enabling precise and controlled release of therapeutic agents directly at tumor sites. These devices harness biomechanical energy from the body to generate electricity, which can power drug delivery mechanisms, enhancing the efficacy of treatments while minimizing systemic side effects [273,274].
For these applications, BNGs typically operate based on the triboelectric effect or piezoelectric effect, converting mechanical energy from body movements or physiological processes into electrical energy. This electricity can be used to drive various drug delivery systems, allowing for real-time control over drug release. For instance, the generated electric field can stimulate the release of chemotherapeutic agents from encapsulated carriers, such as liposomes or hydrogels, directly into the tumor microenvironment. The ability to modulate drug release in response to external stimuli, such as changes in temperature or electric fields, enhances treatment precision and can significantly improve therapeutic outcomes [275,276].
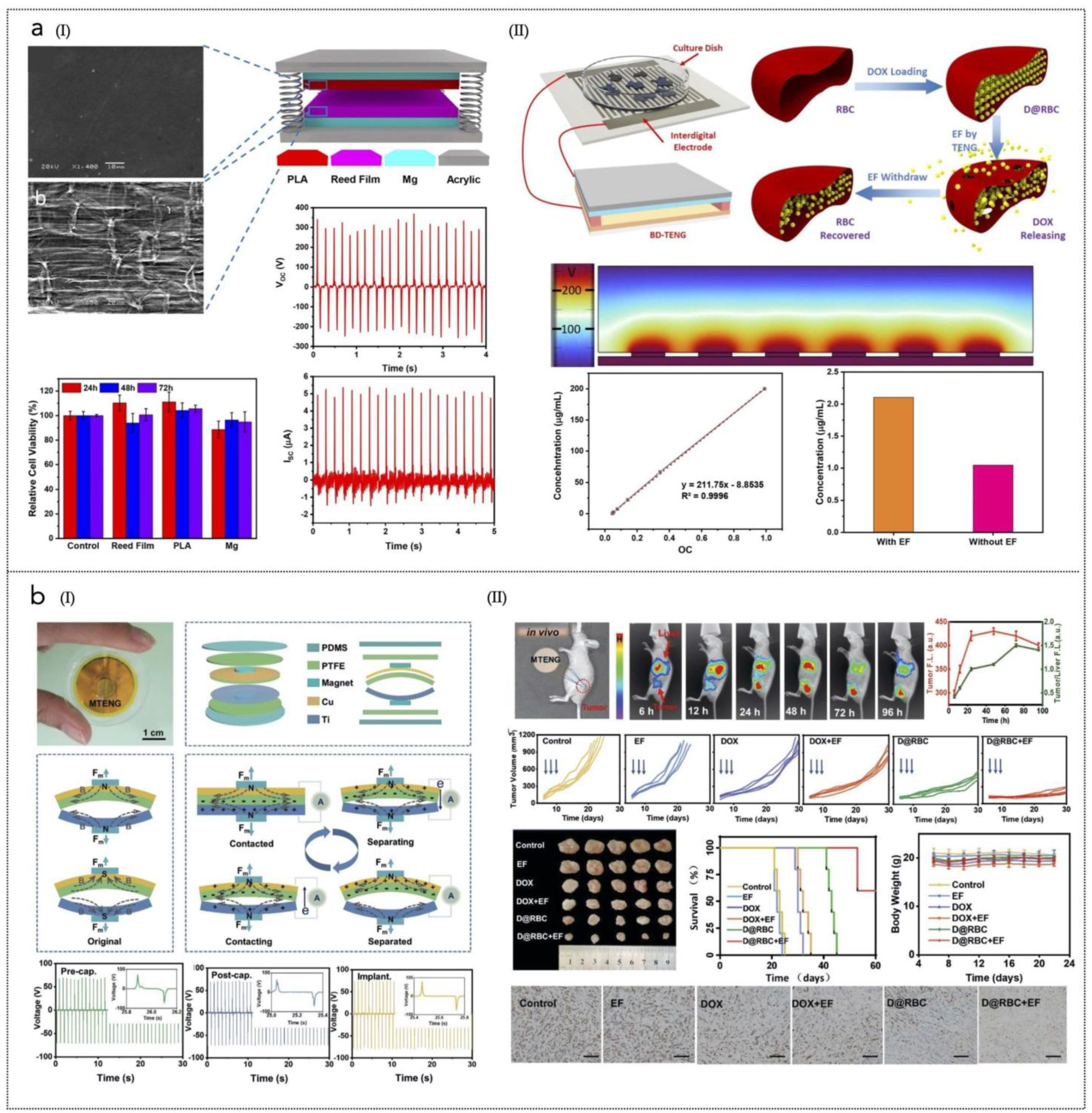
The application of BNGs in anti-cancer therapy is particularly promising due to their ability to deliver localized treatment while minimizing systemic exposure [277]. Recent studies have demonstrated that these devices can effectively enhance the therapeutic efficacy of chemotherapeutic agents like doxorubicin (DOX) by controlling their release rates through applied electric fields. Recently, red blood cells (RBCs) have gained attention as potential carriers for targeted drug delivery due to their biological origin, intrinsic biocompatibility, ease of access, long circulation time (approximately 120 days in humans), and stable, flexible membranes [278,279]. Notably, RBCs fall within the optimal size range for the enhanced permeation and retention (EPR) effect observed in tumors, facilitating their accumulation at tumor sites [280]. For example, a biodegradable triboelectric nanogenerator (BI-TENG) was shown to generate an electric field that accelerated DOX release from red blood cells, leading to improved targeting of tumour cells while reducing off-target effects [276] (Figure 4a).
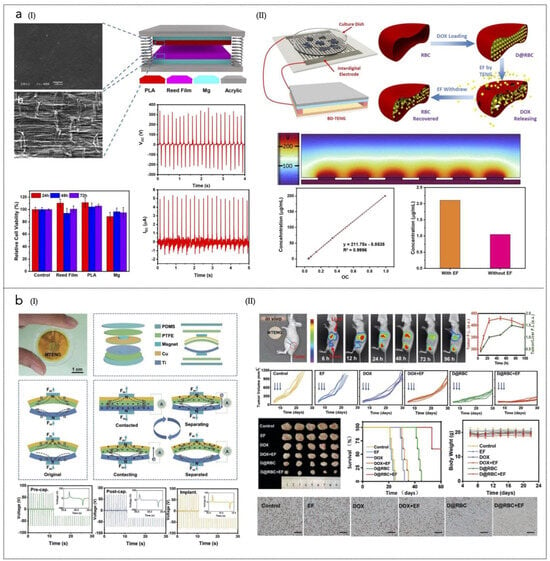
Figure 4.
(a) (I) Illustration of the prototype TNG with SEM images of the biodegradable film and PLA and reed film. The plots show the electrical signals with an outside force, Voc, Isc, and cell viability after 3 days of culture in media supplemented with several biodegradable films. (II) BI-TENG-controlled constructs used to control DOX release: DOX standard concentration curve and D&RBC before and after electric field stimulation are shown in the plots. Reprinted from [276], Springer Nature, Creative Commons Attribution 4.0 International License. (b) (I) Schematic diagram, device structure and output performance of the MTENG. Working principle of the MTENG; Voc of the MTENG before and after encapsulation; Voc of the MTENG after encapsulation and implanted subcutaneously in streaky pork. (II) MTENG-controlled RBC DDS in vivo. The images show blood circulation and accumulation to tumors of the D@RBCs obtained by in vivo imaging system at 6, 12, 24, 48, 72, and 96 h, respectively. The in vivo tumor growth curve is shown; blue arrows indicate the treatment time point: Day 6, Day 8, and Day 10. The other images of the harvested tumors in various groups after 1 month. At the bottom, the Ki67 immunohistochemistry images of the tumors in various groups are shown (scale bar: 200 µm), together with the survival curves of the mice in various groups, and the body weight of the mice. Reprinted with permission from [281]. Copyright 2019, Wiley-VCH GmbH.
Another triboelectric BNG (TNG)-supported RBC drug delivery system has been developed to enable controlled and localized delivery of DOX with improved efficiency [281] (Figure 4b). This system utilizes polytetrafluoroethylene (PTFE) and titanium as the friction layers in the encapsulated magnet-TNG (MTNG). The application of an electric field generated by the MTNG significantly enhances the release of DOX. In vivo studies demonstrated that when RBCs loaded with DOX (D@RBC) were injected into tumor-bearing mice, the DOX signal at the liver was initially high but decreased significantly over time. In contrast, the signal at the tumor site increased during the first 48 h and only slightly decreased at 72 and 96 h, indicating effective targeting. On day 30 post-treatment, tumors in the MTNG + D@RBC group exhibited significantly reduced volumes compared to other groups, highlighting the superior therapeutic efficacy of this combined approach in vivo.
Moreover, these devices can also be integrated with other therapeutic modalities such as photothermal therapy or immunotherapy. By combining localized drug delivery with additional treatment strategies, BNGs can create a synergistic effect that enhances overall treatment efficacy against tumors. Future research should focus on optimizing device design for better integration with existing cancer therapies and exploring novel materials that could enhance performance. Additionally, advancements in wireless technology may allow for remote control over drug delivery systems, providing clinicians with greater flexibility in managing patient care.
3.3. BNGs for Photodynamic Anti-Cancer Therapy
Despite its advantages, PDT faces several challenges, including inadequate localization of photosensitizers (PSs), limited light penetration in deep tissues, and the development of treatment resistance. PDT operates on the principle that when a photosensitizer is activated by specific wavelengths of light, it generates ROS, which can induce cell death in cancerous tissues. The effectiveness of PDT is contingent upon the precise delivery of light to the tumor site and the adequate concentration of PSs within the tumor. Traditional PDT methods often struggle with light penetration, especially in deep-seated tumors, leading to suboptimal therapeutic outcomes. Furthermore, the lack of targeted delivery can result in systemic toxicity and reduced efficacy due to off-target effects.
To address these limitations, the integration of BNGs into PDT systems offers a novel approach that enhances treatment efficacy [282,283]. In fact, BNGs can provide a self-powered system that ensures sustained illumination of PSs directly at the tumor site, thereby enhancing ROS production and improving treatment outcomes. Recent advancements in piezoelectric and triboelectric BNGs have demonstrated their potential to facilitate localized PDT by generating electrical energy from physiological movements or external stimuli.
A notable example is the development of an implantable micro-scale LED device that enables on-demand activation of PSs deep within tumor tissues. This device consists of multiple micro-LEDs arranged in a needle-like structure, allowing for direct implantation into tumor cores. By emitting mild visible light, this system minimizes inflammatory responses while promoting immunogenic cell death (ICD) in tumor cells. Studies have shown that this approach effectively enhances antitumor immunity while reducing adverse effects associated with intense light exposure [284].
Another innovative approach involves a fully implantable wireless optoelectronic system that integrates PDT with hyperthermia and real-time tumor size monitoring (Figure 5a). Utilizing micro inorganic LEDs (μ-LEDs), this system can emit programmable light intensities tailored to individual patient needs. The ability to monitor tumor size continuously allows for adaptive treatment strategies, optimizing therapeutic efficacy while minimizing damage to surrounding healthy tissues [285].
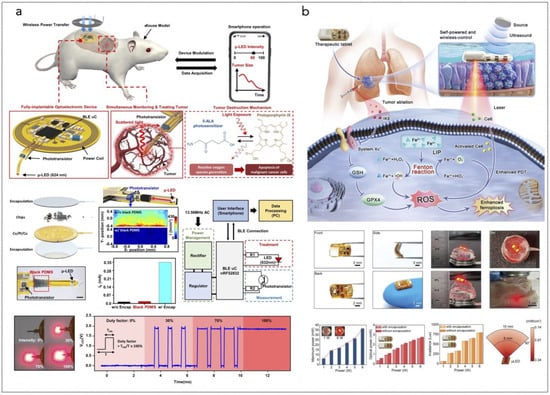
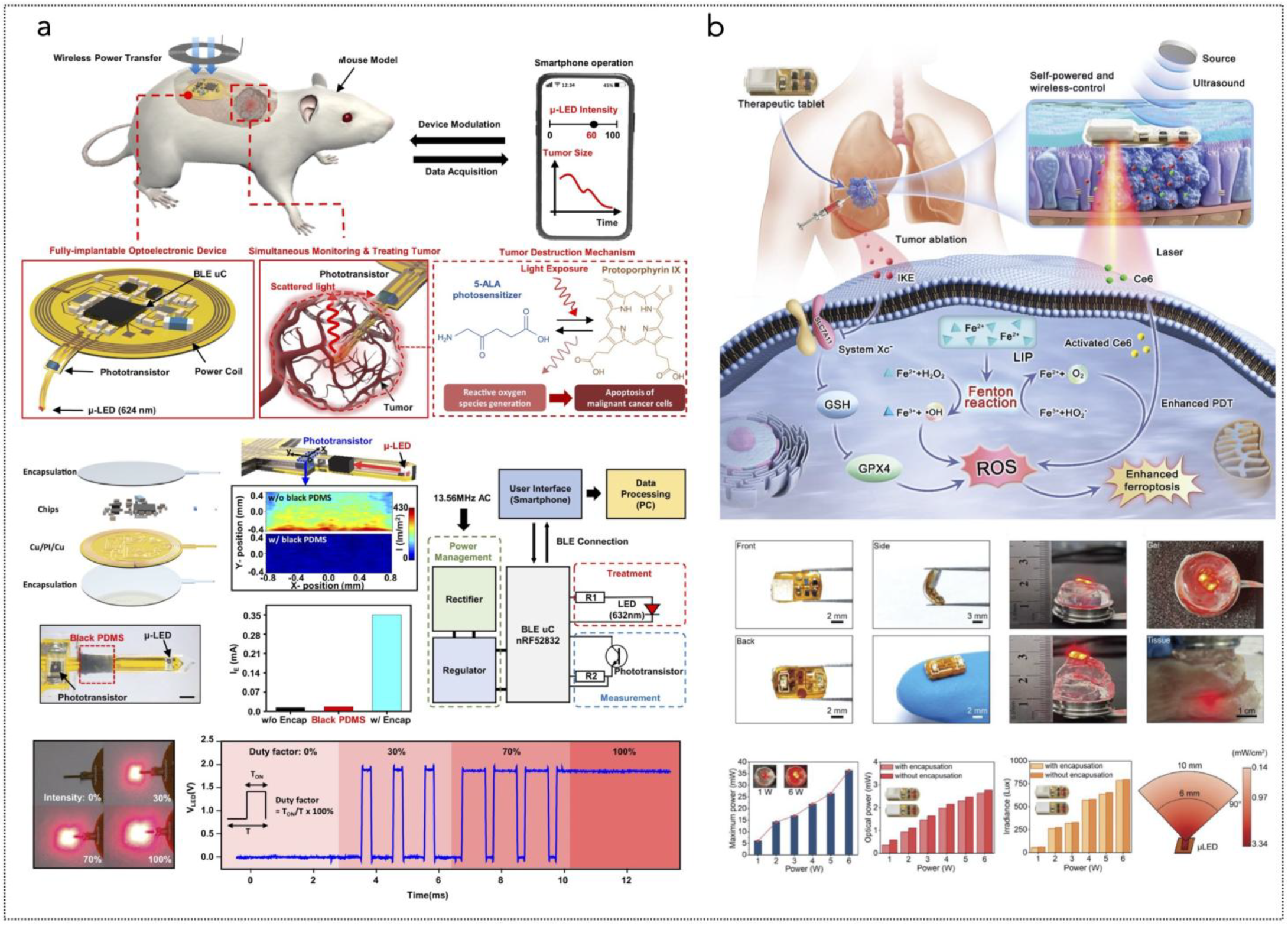
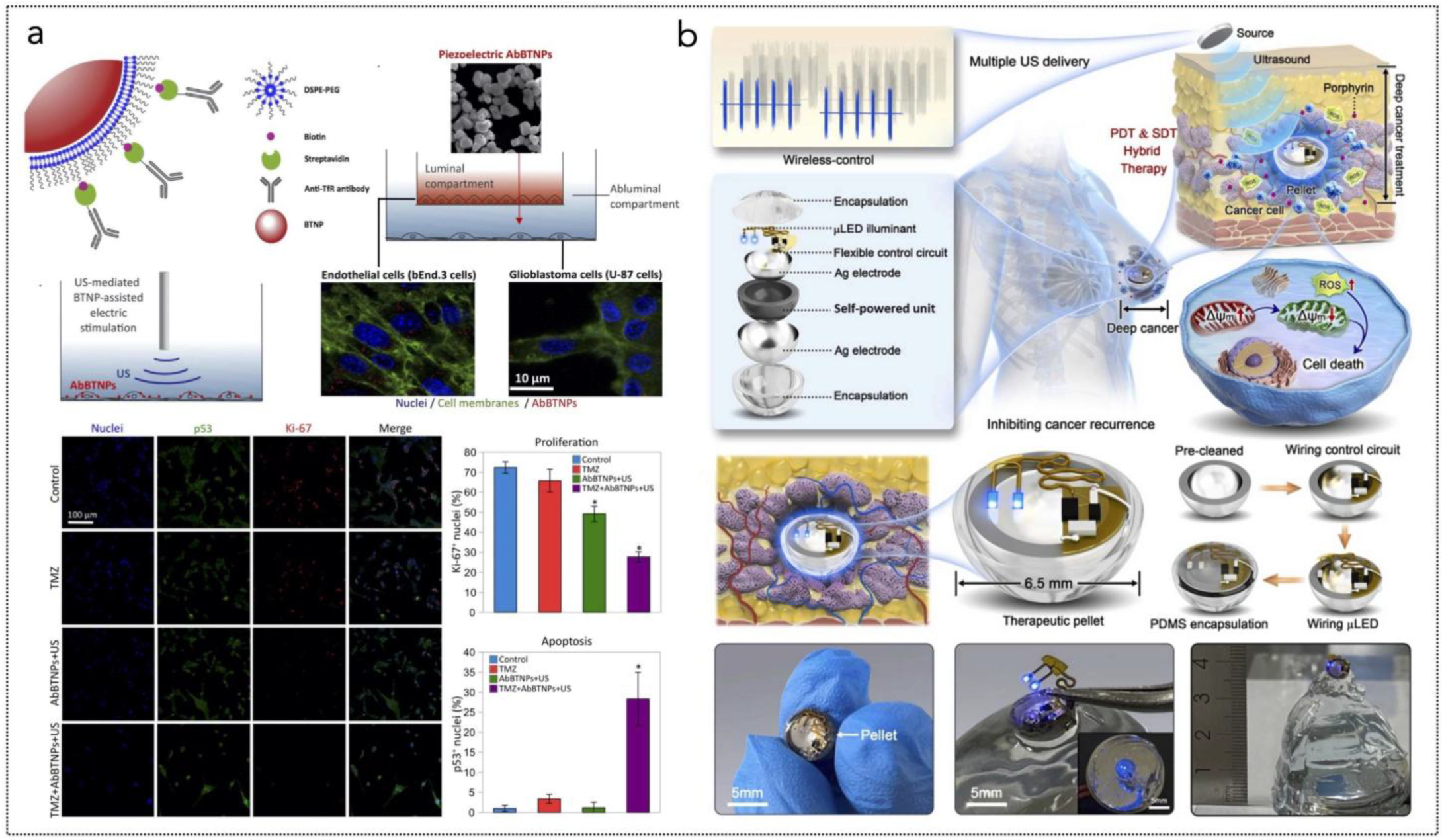
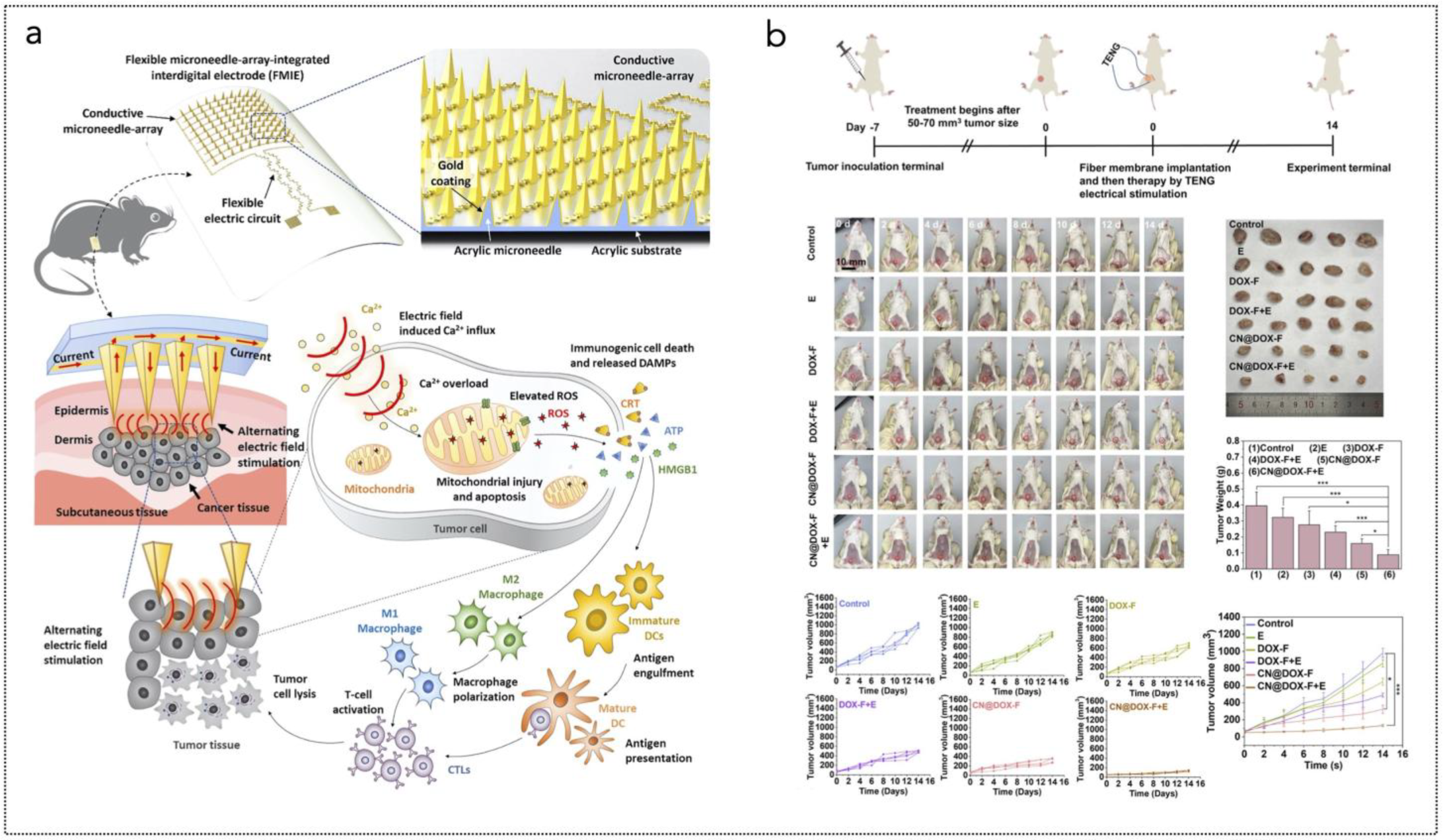
Figure 5.
(a) Overall concept of cancer treatment and monitoring system including device schematic, principle of device operation that enables fully programmable μ-LED intensity control and real-time monitoring of tumor size, and chemical mechanism of PDT. The device includes PDMS/SiO2/parylene C encapsulation layers and a probe part containing black PDMS for blocking internally reflected light; Scale bar, 1 mm. The block diagram of electronic components for wireless operation includes wireless power transfer, light delivery control, photocurrent measurement, and wireless communication with a smartphone. Pulse width modulation of the μ-LEDs with intensity of 0%, 30%, 70%, and 100% is shown, together with forward voltage of the μ-LED when duty factor is sequentially changed to 30%, 70%, and 100% from 0%. Reprinted from [285], Springer Nature, Creative Commons Attribution 4.0 International License. (b) Ferroptosis inducer IKE, when combined with photodynamic therapy, presents a novel approach to cancer treatment. IKE induces ferroptosis in tumor cells by locally inhibiting the SLC7A11-GSH-GPX4 signaling pathway. Concurrently, iron ions accumulate in the body and enhance the cellular hypoxic environment through the Fenton reaction. The therapeutic tablet is minimally invasively implanted into the primary lung cancer site, and ultrasound drives the µLEDs to activate the photosensitizer chlorin e6 (Ce6), utilizing oxygen supplied by IKE to enhance photodynamic therapy, generate reactive oxygen species (ROS), and deplete glutathione (GSH). The accumulation of ROS leads to ferroptosis in lung cancer cells due to the formation of lipid peroxides. Reprinted from [286], Wiley-VCH GmbH. Creative Commons Attribution 4.0 International License.
A self-powered PDT therapeutic tablet has been developed that synergizes with ferroptosis inducers to enhance cancer treatment outcomes (Figure 5b). This device leverages the Fenton reaction to generate additional ROS during PDT while simultaneously increasing oxidative stress within cancer cells. Experimental results indicate a significant tumor inhibition rate, showcasing the potential for combining NG technology with PDT to overcome challenges related to hypoxia and inadequate PS penetration [286].
Research has also explored human motion-driven self-powered PDT systems that autonomously manage irradiation modes based on patient activity levels. This approach allows for continuous treatment without requiring external power sources, making it particularly advantageous for outpatient settings where patient mobility is crucial [287].
While both BNGs for PDT and localized drug delivery systems aim to enhance therapeutic efficacy and minimize side effects, they operate through different mechanisms and have distinct advantages. Localized drug delivery aims to reduce systemic toxicity by concentrating drug action within the tumor environment. However, it may not address issues related to hypoxia or insufficient ROS generation inherent in traditional chemotherapy methods. On the other hand, ING-enhanced PDT specifically targets cancer cells through ROS generation while potentially stimulating immune responses against tumors.
Several challenges remain regarding the integration of BNGs into PDT, such as (i) ensuring that BNG materials are biocompatible to minimize adverse reactions upon implantation; (ii) guaranteeing durability over extended periods to maintain consistent therapeutic effects without requiring surgical replacement; (iii) navigating through extensive preclinical and clinical trials to demonstrate safety and efficacy.
3.4. BNGs for Sonodynamic Anti-Cancer Therapy
BNGs are revolutionizing SDT, which is an advantageous cancer treatment strategy over conventional therapies, owing to its minimally invasive approach, targeted tumor destruction, and reduced side effects. The effectiveness of SDT is often hindered by challenges such as insufficient penetration of ultrasound waves into deeper tissues and the need for effective delivery systems for sensitizers. Recent developments in BNG technology have shown great promise in enhancing SDT by providing a self-powered mechanism that improves the localization and activation of therapeutic agents directly at tumor sites.
Another promising application involves a nanoparticle formulation based on a cathepsin B-degradable co-polymer that carries hematoporphyrin (HPNP) [288]. This system has demonstrated significant improvements in cellular uptake and antitumor efficacy when used in conjunction with ultrasound exposure. In studies focused on pancreatic ductal adenocarcinoma (PDAC), the HPNP formulation exhibited enhanced sonodynamic effects, leading to considerable reductions in tumor volume within just 24 h post-treatment. The ability of this formulation to respond to specific conditions within the tumor microenvironment—such as elevated levels of proteolytic enzymes—facilitates targeted drug release and enhances overall treatment outcomes.
Moreover, recent investigations have explored liposomal nanosystems designed to function as sonodynamic immune adjuvants [289,290]. These systems aim to modulate the tumor microenvironment (TME) by increasing oxygen availability and inducing immunogenic cell death (ICD) alongside ferroptosis. By integrating these capabilities into a single platform, researchers have reported significant improvements in antitumor responses both in vitro and in vivo. The combination of BNGs with such advanced nanosystems allows for more effective modulation of the TME, addressing critical barriers to successful cancer treatment.
The incorporation of BNGs into SDT not only enhances therapeutic efficacy but also offers distinct advantages compared to localized drug delivery systems. While localized drug delivery primarily focuses on delivering chemotherapeutic agents directly to tumors, often utilizing carriers like liposomes or nanoparticles, BNG-enhanced SDT leverages ultrasound’s power to activate sensitizers and generate ROS on demand. By providing a self-powered solution, BNGs ensure that therapeutic agents remain active at the tumor site for extended periods, thereby enhancing their effectiveness while minimizing systemic toxicity [291,292,293].
BNGs can also be used to enhance the effectiveness of low-intensity electric stimulation, which can diminish multidrug resistance in cancer and induce notable anti-proliferative effects by disrupting calcium (Ca2+) and potassium (K+) homeostasis and influencing the organization of mitotic spindles. Introducing piezoelectric materials may favor directly these electric stimuli specifically toward malignant cells to preserve the proliferation and function of healthy cells [294]. As demonstrated, Marino et al. [295] introduced a method based on ultrasound-sensitive piezoelectric NPs to remotely deliver electric stimulation to glioblastoma cells (Figure 6a). Barium titanate NPs have been functionalized with an antibody targeting the transferrin receptor (TfR) to achieve dual targeting of both the blood-brain barrier and glioblastoma cells. The remote ultrasound-mediated piezo-stimulation significantly reduced the proliferation of glioblastoma cells in vitro, and when combined with a sub-toxic concentration of temozolomide, it enhanced sensitivity to chemotherapy while producing remarkable anti-proliferative and pro-apoptotic effects. Cafarelli et al. [296] comprehensively reviewed the fundamental mechanisms of acoustically mediated piezoelectric stimulation, exploring the current challenges that prevent the full adoption of this technology in clinical practice, such as piezoelectric properties measurement, control of the ultrasound dose in vitro, modeling and measuring the piezo effects, knowledge on the triggered bioeffects, therapy targeting, biocompatibility studies, and control of the ultrasound dose delivered in vivo.
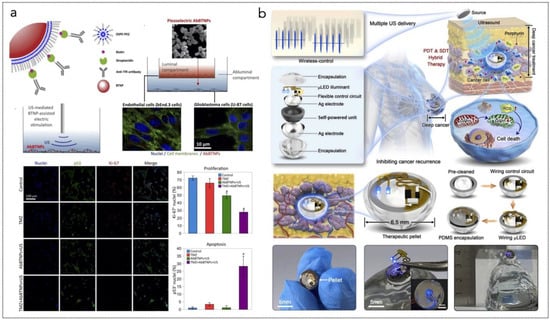
Figure 6.
(a) Illustration of barium titanate NP functionalization with antibody against transferrin receptor (TfR). The scheme shows the NP crossing through a static 3D model of the blood brain barrier and chronic piezoelectric stimulation of glioblastoma cells. At the bottom, CLSM imaging of Ki-67 and p53 expression in different experimental conditions for NP-assisted piezoelectric stimulation to improve anticancer efficacy of TMZ. Reprinted with permission from [295]. Copyright 2018, Elsevier. (b) Design and structure of implantable self-powered therapeutic pellet, with an integrated µLED with flexible serpentine electrode, with wireless PDT/SDT hybrid therapy for inhibiting cancer recurrence. Reprinted with permission from [297], Copyright 2022, Elsevier.
Hybrid systems have been recently introduced that combine SDT with PDT for cancer treatment. For instance, Guan et al. [297] proposed an innovative implantable self-powered therapeutic pellet designed for wireless hybrid therapy combining PDT and SDT to prevent cancer recurrence and reduce tumor size (Figure 6b). The pellet features a compact design that integrates a self-powered unit, an LED light source, and a control circuit, allowing it to be directly implanted at the tumor site. This self-powered unit captures ultrasound energy through the piezoelectric effect, enabling the delivery of therapeutic light doses to tissues for PDT while simultaneously facilitating direct SDT. In preclinical mouse models, this hybrid PDT/SDT approach has demonstrated significant efficacy in treating breast cancer, outperforming treatments that utilize either PDT or SDT alone, and shows promise for future clinical applications in humans. The system effectively addresses key limitations of current advanced technologies by achieving: (i) deep tissue treatment within the body; (ii) hybrid therapy that optimizes the activation of sensitizers; and (iii) wireless control over therapeutic dosimetry. Additionally, further research on this implantable self-powered therapeutic pellet for wireless PDT/SDT hybrid therapy, both in vitro and in vivo, could provide valuable insights into the interactions between established clinical cancer treatments and emerging precision therapies.
3.5. BNGs for Cancer Treatment Through Bioelectrical Stimulation
BNGs represent a promising frontier in cancer treatment, leveraging bioelectrical stimulation to directly kill cancer cells or inhibit their proliferation. These innovative devices are designed to harvest energy from the body’s own physiological movements, such as breathing, heartbeat, or muscle contractions, to power localized electrical stimulation. This approach is particularly advantageous as it eliminates the need for external power sources, enabling minimally invasive, self-sustained cancer therapies. The recent literature highlights several strategies and device architectures that have been explored to harness the potential of BNGs for oncological applications [298].
The mechanisms by which bioelectrical stimulation exerts anticancer effects are multifaceted. Electrical fields can disrupt the cell cycle, inhibit mitosis, and induce apoptosis in cancer cells. They can also alter the tumor microenvironment, reducing hypoxia and promoting immune cell infiltration. For instance, studies have shown that low-intensity electrical stimulation can enhance the efficacy of immunotherapies by increasing the expression of antigens on cancer cells, making them more recognizable to immune cells.
Pan et al. [136] presented an optically microprinted flexible interdigital electrode with a gold-plated polymer microneedle array, fabricated by combining optical 3D microprinting and electroless plating processes, to generate AC electric fields for cancer treatment (Figure 7a). EST induced necrotic cell death through mitochondrial Ca2+ overload and increased intracellular ROS production, leading to the release of damage-associated molecular patterns that activated the immune response and potentiated immunogenic cell death.
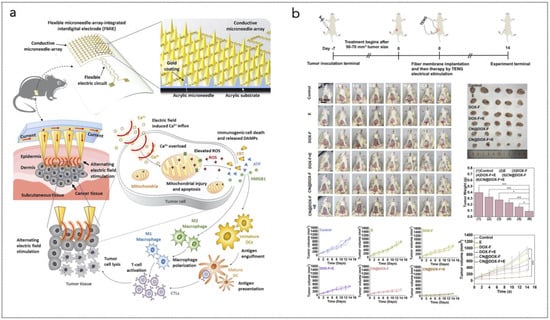
Figure 7.
(a) Schematic of the gold-plated polymer microneedle array for electrically stimulated cancer immunomodulation. Reprinted with permission from [136], Copyright 2023, Elsevier. (b) In vivo cancer therapy on the subcutaneous 4T1 tumor-bearing BALB/c mice: schematic illustration of the cancer therapy with the TENG-assisted catalytic and chemotherapeutic system, together with the photographs of the representative mice in different groups during the 14 days of treatment. The plots show the mean tumor weights after the excision on day 14, and the tumor volume change of the mice during the treatment. Reprinted with permission from [8], Copyright 2023, American Chemical Society.
Low-intensity electric stimulation offers an alternative treatment capable of inhibiting the proliferation of various tumor cell lines [299]. In more detail, this type of stimulation hinders cell division by impacting K+ channels, specifically through the overexpression of the inward rectifier K+ channel Kir3.2 [300], and by disrupting cytoskeletal structures during cell division, particularly through disorganization of the mitotic spindle [301]. Additionally, low-intensity and low-frequency AC stimulation has been shown to significantly enhance the effects of chemotherapy in glioblastoma treatment during clinical trials [302]. AC not only influences cancer cell proliferation without the use of drugs but also reduces multidrug resistance by interfering with the plasma membrane translocation of MDR1, a P-glycoprotein (P-gp) whose overexpression is linked to chemotherapy resistance [303]. However, since AC stimulation can also affect the proliferation of non-malignant cells, such as human astrocytes, it is crucial to specifically target electric stimuli to cancer cells. In this context, piezoelectric nanomaterials have emerged as promising tools for wireless, non-invasive, and targeted electric stimulation of cells and tissues. Owing to the remote ultrasound-mediated activation of these piezo-nanotransducers, various cell types can be successfully stimulated. Marino et al. [304] presented an innovative piezoelectric nanoplatform consisting of Barium titanate NPs functionalized with anti-HER2 antibodies (Ab-BTNPs), which specifically target HER2-positive cancer cells. The anti-proliferative effects of remote piezoelectric stimulation on SK-BR-3 breast cancer cells were assessed through cell cycle progression analysis, evaluation of proliferation markers, and measurement of metabolic activity in cell cultures. Additionally, intracellular Ca2+ levels, mitotic spindle development, and K+ channel expression at the gene level were assessed.
Moreover, bioelectrical stimulation can impair angiogenesis, depriving tumors of their blood supply and hindering growth. Angiogenesis is closely linked to inflammatory processes as it facilitates nutrient supply and waste removal to maintain physiological homeostasis. Conversely, pathological angiogenesis can occur due to dysregulated inflammatory processes, leading to chronic inflammation [305]. During inflammation, there is a significant interaction between immune and endothelial cells, with several studies indicating that these immune/endothelial cell interactions promote cancer development. In the tumor microenvironment, cancer cells secrete and activate various growth factors, including VEGF, FGFb, EGF, and TGFb, to stimulate angiogenesis [306,307].
Sen et al. [308] showed that the angiogenic behavior of human cerebral microvascular endothelial cells can be inhibited by using nudin-3a-loaded ApoE-functionalised polymeric piezoelectric NPs, remotely responsive to ultrasounds. The electrical stimulation favoured the inhibition of tumor-induced angiogenesis, specifically vessel formation, endothelial cell migration and invasion, and angiogenesis-related cytokines production.
These mechanisms underscore the potential of bioelectronic nanogenerators to serve as versatile tools in cancer treatment, combined with other therapies as well. For instance, Zheng et al. [8] presented a self-driven electrical stimulation-enhanced cancer catalytic therapy combined with chemotherapy by integrating a human-powered TNG with an implantable and biodegradable gelatin/polycaprolactone nanofibrous patch (Figure 7b). This patch is designed to incorporate DOX and graphitic carbon nitride (g-C3N4). Notably, the peroxidase (POD)-like activity of g-C3N4, which facilitates the generation of hydroxyl radicals, can be significantly amplified through self-driven electrical stimulation. Concurrently, DOX is released to enhance the therapeutic effect, particularly in the weakly acidic conditions typical of the tumor microenvironment.
Das et al. [131] described, in detail, different approaches of electrotherapy and electrochemotherapy for cancer treatment.
Table 1 reports some representative examples of BNGs for anti-cancer therapy, as proposed in previous works. It should be noted that, in many cases, it is challenging to categorize systems, devices, or approaches within the aforementioned groups of BNGs or ANGs. For example, some approaches rely on the ultrasound activation of piezoelectric nanomaterials for bioelectrical stimulation of cancer cells. These can fall into the category of EST but also NPT, and, anyway, they can be associated with the physical principle of PNGs, although the piezoelectric effect here is used in the reverse direction. Thus, Table 1 can serve as a reference for showcasing practical examples of approaches for anti-cancer therapies.

Table 1.
Representative examples of BNGs for anti-cancer therapy, proposed in previous works and classified in terms of the materials used, the targeted application, the underlying working principle. The colors correspond to different applications: green (localized drug delivery); yellow (photodynamic therapy); blue (sonodynamic therapy); orange (electrical stimulation therapy); grey (nanoparticle-based therapy); and pink (atomic nanogenerators). Some systems can deliver multiple synergistic therapies or can fall into multiple categories.
4. Conclusions: Challenges and Outlook
The advent of bioelectronic BNGs and ANGs has opened new frontiers in cancer treatment, offering innovative approaches that leverage the unique properties of these devices for therapeutic applications. These technologies have demonstrated significant potential in enhancing anti-cancer therapies through localized energy delivery and targeted treatment mechanisms. BNGs (PNGs, TNGs, and PyNGs) and ANGs differ significantly in their applicability, performance, and compatibility for anti-cancer therapy. Piezoelectric and triboelectric nanogenerators generate electricity from mechanical stimuli, making them well-suited for localized drug delivery, electrical stimulation therapy, and nanoparticle-based therapy, where controlled activation is beneficial. Pyroelectric nanogenerators, which convert thermal fluctuations into electrical energy, are more relevant for photodynamic and sonodynamic therapy, leveraging temperature variations for therapeutic activation. These BNGs are biocompatible, utilizing materials like polymers and ceramics, and are fabricated through scalable techniques such as electrospinning and nanolithography. However, they may suffer from limited stability and inconsistent energy output in dynamic biological environments. ANGs, in contrast, rely on nuclear decay, offering sustained energy generation for applications like targeted alpha-therapy and photodynamic therapy. Their stability and long-lasting power output make them advantageous for deep-tissue treatments, but their potential radioactive side effects and limited material options (radioisotopes) pose challenges in clinical translation. Fabrication is more complex, involving isotope enrichment and encapsulation strategies for safety. While BNGs provide versatile and safer alternatives, ANGs offer superior longevity and penetration depth, making them preferable for specific long-term or deeply embedded cancer therapies.
However, several challenges and bottlenecks must be addressed to realize the full potential of these technologies in clinical settings.
4.1. Material Limitations
One of the primary challenges facing the effective implementation of bioelectronic and atomic nanogenerators in anti-cancer therapy is the availability and performance of suitable materials. The materials used in the fabrication of BNGs must exhibit biocompatibility, mechanical robustness, and efficient energy conversion properties. For instance, while polymers such as polycaprolactone and gelatin are commonly used in the design of nanofibrous patches for drug delivery, their mechanical properties may not always meet the demands of long-term implantation or repeated mechanical stress. Additionally, the development of novel materials with enhanced piezoelectric or triboelectric properties is crucial for improving the output performance of these devices. Research into biodegradable materials that can withstand harsh biological environments while maintaining their functional integrity over time is essential for advancing these technologies.
4.2. Size Requirements
Another critical consideration for the successful application of bioelectronic and atomic nanogenerators in anti-cancer therapy is the optimization of system size to balance functionality and biocompatibility. BNGs must be small enough (<few cm2) to be minimally invasive while still generating sufficient energy to power therapeutic mechanisms, such as localized drug release or electrical stimulation. Miniaturization should not compromise structural integrity, requiring precise engineering to ensure durability under physiological conditions. Furthermore, the integration of nanogenerators with implantable or wearable platforms demands careful design to accommodate power requirements without increasing patient discomfort. Atomic nanogenerators, in particular, must be designed at the sub-nanometer to nanometer scale to harness anti-cancer effects at the cellular scale while maintaining stability within biological environments. Their ultra-small size allows for targeted delivery at the cellular level, but challenges arise in ensuring biosafety.
Achieving an optimal balance between size, efficiency, and stability is key to enhancing the clinical viability of these technologies.
4.3. Power Management Challenges
Power management remains a significant bottleneck for the effective use of bioelectronic nanogenerators in anti-cancer therapies. The output generated by these devices often involves low voltages and currents, which can complicate signal extraction and utilization. Designing efficient power management circuits that can optimize energy harvesting, storage, and distribution is critical for ensuring that these devices operate effectively within the biological context. Moreover, hybrid systems that combine different energy harvesting mechanisms (such as piezoelectric and triboelectric) can introduce additional complexity in circuit design, necessitating innovative solutions to maximize efficiency while minimizing losses.
4.4. Reliability and Durability
Reliability is another critical factor influencing the successful implementation of nanogenerators in cancer therapies. These devices are expected to undergo continuous mechanical deformation or friction during operation, which can lead to material fatigue and failure over time. Ensuring the durability of all components involved in the fabrication process is essential for long-term functionality. Furthermore, packaging solutions must be developed to protect these devices from environmental factors such as humidity, biofouling, and chemical exposure that could compromise their performance. Advanced encapsulation techniques that provide hermetic sealing while allowing for biocompatibility are necessary to enhance device longevity.
4.5. Integration with Existing Therapies
Integrating BNGs and ANGs with existing cancer treatment modalities poses another challenge. While these technologies hold promise for enhancing localized treatment efficacy, their successful integration into established therapeutic protocols requires extensive research to understand how they interact with other treatments such as chemotherapy or immunotherapy. For instance, combining TAT with nanogenerator technology could potentially enhance therapeutic outcomes by improving drug delivery or stimulating immune responses; however, understanding the timing, dosage, and potential interactions between therapies is essential for optimizing patient outcomes.
4.6. Regulatory Hurdles
Navigating regulatory pathways presents a significant barrier to the clinical translation of bioelectronic and atomic nanogenerators. The approval process for medical devices can be lengthy and complex, requiring extensive preclinical studies to demonstrate safety and efficacy before they can be tested in human trials. As these technologies are often at the intersection of multiple scientific disciplines—materials science, engineering, biology, and medicine—establishing clear regulatory guidelines tailored to their unique characteristics will be crucial for facilitating their development and adoption.
4.7. Cost-Effectiveness
Despite their advantages over conventional therapies, concerns regarding the cost-effectiveness of bioelectronic and atomic nanogenerators remain a significant challenge. While these technologies may offer long-term savings through improved patient outcomes and reduced side effects, initial development costs can be high due to complex fabrication processes and specialized equipment requirements. Developing scalable manufacturing techniques that reduce production costs while maintaining quality will be essential for making these technologies accessible to a broader range of healthcare settings.
4.8. Biocompatibility Issues
Biocompatibility is a crucial consideration when developing bioelectronic nanogenerators for cancer therapy. The materials used must not elicit adverse immune responses or toxicity when implanted in human tissues. Extensive biocompatibility testing is required to ensure that any materials used in device fabrication do not cause inflammation or other negative reactions within the body. Furthermore, as research progresses toward using biodegradable materials for temporary implants, understanding how these materials degrade over time without releasing harmful byproducts will be essential.
In summary, while BNGs and ANGs hold tremendous promise as unconventional strategies against cancer through enhanced localized treatment mechanisms, several challenges must be addressed before they can be effectively implemented in clinical practice. By focusing on material innovation, power management optimization, reliability studies, integration with existing therapies, regulatory pathways, cost-effectiveness considerations, and biocompatibility issues, researchers can pave the way for these advanced technologies to transform cancer treatment paradigms significantly. As advancements continue in this field, these technologies and approaches may become integral components of comprehensive cancer care strategies aimed at improving patient outcomes while minimizing side effects associated with traditional therapies.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
M.M. acknowledges the seminars held by the Ludwig Cancer Institute (Oxford) in 2024, whose topics were inspirational. This work is a development of the author’s studies on nanogenerators and is not related to the experimental projects carried out in his current institution.
Conflicts of Interest
The author declares no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Buendía, R.; Jefferson, A. Global Burden of Disease 2019 Cancer Collaboration Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Yamagishi, K.; Kirino, I.; Takahashi, I.; Amano, H.; Takeoka, S.; Morimoto, Y.; Fujie, T. Tissue-Adhesive Wirelessly Powered Optoelectronic Device for Metronomic Photodynamic Cancer Therapy. Nat. Biomed. Eng. 2019, 3, 27–36. [Google Scholar] [CrossRef]
- Chandra, R.A.; Keane, F.K.; Voncken, F.E.M.; Thomas, C.R. Contemporary Radiotherapy: Present and Future. Lancet Lond. Engl. 2021, 398, 171–184. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation Therapy-Associated Toxicity: Etiology, Management, and Prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Yao, S.; Zheng, M.; Wang, Z.; Zhao, Y.; Wang, S.; Liu, Z.; Li, Z.; Guan, Y.; Wang, Z.L.; Li, L. Self-Powered, Implantable, and Wirelessly Controlled NO Generation System for Intracranial Neuroglioma Therapy. Adv. Mater. 2022, 34, 2205881. [Google Scholar] [CrossRef]
- Zheng, M.; Yao, S.; Zhao, Y.; Wan, X.; Hu, Q.; Tang, C.; Jiang, Z.; Wang, S.; Liu, Z.; Li, L. Self-Driven Electrical Stimulation-Promoted Cancer Catalytic Therapy and Chemotherapy Based on an Implantable Nanofibrous Patch. ACS Appl. Mater. Interfaces 2023, 15, 7855–7866. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, X.; Wang, X.; Huang, T.; Ding, Y.; Zhang, J.; Zhang, Z.; Wang, Z.L.; Li, L. Bioinspired Electron Polarization of Nanozymes with a Human Self-Generated Electric Field for Cancer Catalytic Therapy. Adv. Mater. 2022, 34, 2109568. [Google Scholar] [CrossRef]
- Kim, W.S.; Khot, M.I.; Woo, H.-M.; Hong, S.; Baek, D.-H.; Maisey, T.; Daniels, B.; Coletta, P.L.; Yoon, B.-J.; Jayne, D.G.; et al. AI-Enabled, Implantable, Multichannel Wireless Telemetry for Photodynamic Therapy. Nat. Commun. 2022, 13, 2178. [Google Scholar] [CrossRef]
- Karges, J.; Kuang, S.; Maschietto, F.; Blacque, O.; Ciofini, I.; Chao, H.; Gasser, G. Rationally Designed Ruthenium Complexes for 1- and 2-Photon Photodynamic Therapy. Nat. Commun. 2020, 11, 3262. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Qi, G.; Wang, B.; Yu, T.; Zhang, Y.; Li, H.; Kitte, S.A.; Jin, Y. Ultrasound-Activated Au/ZnO-Based Trojan Nanogenerators for Combined Targeted Electro-Stimulation and Enhanced Catalytic Therapy of Tumor. Nano Energy 2021, 87, 106208. [Google Scholar] [CrossRef]
- Wang, Z.L. Catch Wave Power in Floating Nets. Nat. News 2017, 542, 159. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; Todaro, M.T.; Desmaële, D.; De Vittorio, M. Nanogenerators for Harvesting Mechanical Energy Conveyed by Liquids. Nano Energy 2019, 57, 141–156. [Google Scholar] [CrossRef]
- Khan, U.; Kim, S.-W. Triboelectric Nanogenerators for Blue Energy Harvesting. ACS Nano 2016, 10, 6429–6432. [Google Scholar] [CrossRef]
- Viet, N.V.; Wu, N.; Wang, Q. A Review on Energy Harvesting from Ocean Waves by Piezoelectric Technology. J. Model. Mech. Mater. 2017, 1, 20160161. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Mariello, M. Recent Advances on Hybrid Piezo-Triboelectric Bio-Nanogenerators: Materials, Architectures and Circuitry. Nanoenergy Adv. 2022, 2, 64–109. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Yin, J.; Xu, Y.; Fei, W.; Xue, M.; Wang, Q.; Zhou, J.; Guo, W. Emerging Hydrovoltaic Technology. Nat. Nanotechnol. 2018, 13, 1109–1119. [Google Scholar] [CrossRef]
- Helseth, L.E.; Guo, X.D. Contact Electrification and Energy Harvesting Using Periodically Contacted and Squeezed Water Droplets. Langmuir 2015, 31, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; Madaro, F.; Mehdipour, I.; Todaro, M.T.; Rizzi, F.; De Vittorio, M. Chapter 13—Micro- and Nanodevices for Wind Energy Harvesting. In Nano Tools and Devices for Enhanced Renewable Energy; Devasahayam, S., Hussain, C.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 291–374. ISBN 978-0-12-821709-2. [Google Scholar]
- Wang, Z.L. Self-Powered Nanosensors and Nanosystems. Adv. Mater. 2012, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, T.; Wang, Z.L. Self-Powered Sensors and Systems Based on Nanogenerators. Sensors 2020, 20, 2925. [Google Scholar] [CrossRef]
- Wang, Z.L. Energy Harvesting for Self-Powered Nanosystems. Nano Res. 2008, 1, 1–8. [Google Scholar] [CrossRef]
- Pu, X.; Li, L.; Liu, M.; Jiang, C.; Du, C.; Zhao, Z.; Hu, W.; Wang, Z.L. Wearable Self-Charging Power Textile Based on Flexible Yarn Supercapacitors and Fabric Nanogenerators. Adv. Mater. 2016, 28, 98–105. [Google Scholar] [CrossRef]
- Sim, H.J.; Choi, C.; Kim, S.H.; Kim, K.M.; Lee, C.J.; Kim, Y.T.; Lepró, X.; Baughman, R.H.; Kim, S.J. Stretchable Triboelectric Fiber for Self-Powered Kinematic Sensing Textile. Sci. Rep. 2016, 6, 35153. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, K.Y.; Kumar, B.; Tien, N.T.; Lee, N.-E.; Kim, S.-W. Highly Sensitive Stretchable Transparent Piezoelectric Nanogenerators. Energy Environ. Sci. 2012, 6, 169–175. [Google Scholar] [CrossRef]
- Wu, C.; Liu, R.; Wang, J.; Zi, Y.; Lin, L.; Wang, Z.L. A Spring-Based Resonance Coupling for Hugely Enhancing the Performance of Triboelectric Nanogenerators for Harvesting Low-Frequency Vibration Energy. Nano Energy 2017, 32, 287–293. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Chen, J.; Jin, L.; Deng, W.; Tang, J.; Zhang, H.; Pan, H.; Zhu, M.; Yang, W.; et al. Lawn Structured Triboelectric Nanogenerators for Scavenging Sweeping Wind Energy on Rooftops. Adv. Mater. 2016, 28, 1650–1656. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Li, Z.; Fan, X.; Zi, Y.; Jing, Q.; Guo, H.; Wen, Z.; Pradel, K.C.; Niu, S.; et al. Networks of Triboelectric Nanogenerators for Harvesting Water Wave Energy: A Potential Approach toward Blue Energy. ACS Nano 2015, 9, 3324–3331. [Google Scholar] [CrossRef]
- Mariello, M.; Fachechi, L.; Guido, F.; De Vittorio, M. Multifunctional Sub-100 µm Thickness Flexible Piezo/Triboelectric Hybrid Water Energy Harvester Based on Biocompatible AlN and Soft Parylene C-PDMS-EcoflexTM. Nano Energy 2021, 83, 105811. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Wang, Z.L. Microfibre–Nanowire Hybrid Structure for Energy Scavenging. Nature 2008, 451, 809–813. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Hsiao, Y.-C.; Wu, H.-M.; Wang, Z.L. Waterproof Fabric-Based Multifunctional Triboelectric Nanogenerator for Universally Harvesting Energy from Raindrops, Wind, and Human Motions and as Self-Powered Sensors. Adv. Sci. 2019, 6, 1801883. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, K.; Chen, X.; Xue, C.; Wang, K. An Air-Cushion Triboelectric Nanogenerator Integrated with Stretchable Electrode for Human-Motion Energy Harvesting and Monitoring. Nano Energy 2018, 53, 108–115. [Google Scholar] [CrossRef]
- Mitcheson, P.D.; Yeatman, E.M.; Rao, G.K.; Holmes, A.S.; Green, T.C. Energy Harvesting From Human and Machine Motion for Wireless Electronic Devices. Proc. IEEE 2008, 96, 1457–1486. [Google Scholar] [CrossRef]
- Guido, F.; Qualtieri, A.; Algieri, L.; Lemma, E.D.; De Vittorio, M.; Todaro, M.T. AlN-Based Flexible Piezoelectric Skin for Energy Harvesting from Human Motion. Microelectron. Eng. 2016, 159, 174–178. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Shi, B.; Xue, X.; Liu, Z.; Jin, Y.; Ma, Y.; Zou, Y.; Wang, X.; An, Z.; et al. In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 2016, 10, 6510–6518. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, T.; Chen, H.; Wang, F.; Pu, Y.; Gao, C.; Li, S. Mini Review on Flexible and Wearable Electronics for Monitoring Human Health Information. Nanoscale Res. Lett. 2019, 14, 263. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.; Xu, L.; Wang, A.C.; Han, K.; Jiang, T.; Lai, Q.; Bai, Y.; Tang, W.; Fan, F.R.; et al. Flexible and Durable Wood-Based Triboelectric Nanogenerators for Self-Powered Sensing in Athletic Big Data Analytics. Nat. Commun. 2019, 10, 5147. [Google Scholar] [CrossRef]
- Mariello, M.; Fachechi, L.; Guido, F.; Vittorio, M.D. Conformal, Ultra-Thin Skin-Contact-Actuated Hybrid Piezo/Triboelectric Wearable Sensor Based on AlN and Parylene-Encapsulated Elastomeric Blend. Adv. Funct. Mater. 2021, 31, 2101047. [Google Scholar] [CrossRef]
- Mariello, M.; Qualtieri, A.; Mele, G.; De Vittorio, M. Metal-Free Multilayer Hybrid PENG Based on Soft Electrospun/-Sprayed Membranes with Cardanol Additive for Harvesting Energy from Surgical Face Masks. ACS Appl. Mater. Interfaces 2021, 13, 20606–20621. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-Y.; Lee, W.-H.; Hwang, H.-G.; Kim, D.-H.; Kim, J.-H.; Lee, S.-H.; Nahm, S. Resistive Switching Memory Integrated with Nanogenerator for Self-Powered Bioimplantable Devices. Adv. Funct. Mater. 2016, 26, 5211–5221. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Han, M.-D.; Wang, R.-X.; Zhu, F.-Y.; Li, Z.-H.; Wang, W.; Zhang, H.-X. Frequency-Multiplication High-Output Triboelectric Nanogenerator for Sustainably Powering Biomedical Microsystems. Nano Lett. 2013, 13, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, Z.; Chen, T.; Lee, C. Minimalist and Multi-Functional Human Machine Interface (HMI) Using a Flexible Wearable Triboelectric Patch. Nano Energy 2019, 62, 355–366. [Google Scholar] [CrossRef]
- O’Flynn, B.; Sanchez-Torres, J.; Tedesco, S.; Walsh, M. Challenges in the Development of Wearable Human Machine Interface Systems. In Proceedings of the 2019 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 7–11 December 2019; pp. 10.4.1–10.4.4. [Google Scholar]
- Sun, Z.; Zhu, M.; Lee, C. Progress in the Triboelectric Human–Machine Interfaces (HMIs)-Moving from Smart Gloves to AI/Haptic Enabled HMI in the 5G/IoT Era. Nanoenergy Adv. 2021, 1, 81–120. [Google Scholar] [CrossRef]
- Fan, F.R.; Tang, W.; Wang, Z.L. Flexible Nanogenerators for Energy Harvesting and Self-Powered Electronics. Adv. Mater. 2016, 28, 4283–4305. [Google Scholar] [CrossRef]
- Veeralingam, S.; Badhulika, S. Lead-Free Transparent Flexible Piezoelectric Nanogenerator for Self-Powered Wearable Electronic Sensors and Energy Harvesting through Rainwater. ACS Appl. Energy Mater. 2022, 5, 12884–12896. [Google Scholar] [CrossRef]
- Akaydin, H.D.; Elvin, N.; Andreopoulos, Y. Energy Harvesting from Highly Unsteady Fluid Flows Using Piezoelectric Materials. J. Intell. Mater. Syst. Struct. 2010, 21, 1263–1278. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Joe, P.; Tuzman, O.L.; Park, K.-I.; Lee, K.J.; Shi, Y.; Huang, Y.; Rogers, J.A. Recent Progress in Flexible and Stretchable Piezoelectric Devices for Mechanical Energy Harvesting, Sensing and Actuation. Extreme Mech. Lett. 2016, 9, 269–281. [Google Scholar] [CrossRef]
- Choi, A.Y.; Lee, C.J.; Park, J.; Kim, D.; Kim, Y.T. Corrugated Textile Based Triboelectric Generator for Wearable Energy Harvesting. Sci. Rep. 2017, 7, 45583. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Lin, Z.-H.; Zhou, Y.S.; Jing, Q.; Su, Y.; Yang, J.; Chen, J.; Hu, C.; Wang, Z.L. Human Skin Based Triboelectric Nanogenerators for Harvesting Biomechanical Energy and as Self-Powered Active Tactile Sensor System. ACS Nano 2013, 7, 9213–9222. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, H.; Wu, H.; Lee, C. Self-Powered Triboelectric Nanogenerator Buoy Ball for Applications Ranging from Environment Monitoring to Water Wave Energy Farm. Nano Energy 2017, 40, 203–213. [Google Scholar] [CrossRef]
- Olsen, M.; Zhang, R.; Örtegren, J.; Andersson, H.; Yang, Y.; Olin, H. Frequency and Voltage Response of a Wind-Driven Fluttering Triboelectric Nanogenerator. Sci. Rep. 2019, 9, 5543. [Google Scholar] [CrossRef]
- Chen, X.; Shao, J.; An, N.; Li, X.; Tian, H.; Xu, C.; Ding, Y. Self-Powered Flexible Pressure Sensors with Vertically Well-Aligned Piezoelectric Nanowire Arrays for Monitoring Vital Signs. J. Mater. Chem. C 2015, 3, 11806–11814. [Google Scholar] [CrossRef]
- Wang, A.C.; Wu, C.; Pisignano, D.; Wang, Z.L.; Persano, L. Polymer Nanogenerators: Opportunities and Challenges for Large-Scale Applications. J. Appl. Polym. Sci. 2018, 135, 45674. [Google Scholar] [CrossRef]
- Todaro, M.T.; Guido, F.; Mastronardi, V.; Desmaele, D.; Epifani, G.; Algieri, L.; De Vittorio, M. Piezoelectric MEMS Vibrational Energy Harvesters: Advances and Outlook. Microelectron. Eng. 2017, 183–184, 23–36. [Google Scholar] [CrossRef]
- Todaro, M.T.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Desmaële, D.; Epifani, G.; Vittorio, M.D. Biocompatible, Flexible, and Compliant Energy Harvesters Based on Piezoelectric Thin Films. IEEE Trans. Nanotechnol. 2018, 17, 220–230. [Google Scholar] [CrossRef]
- Ewii, U.E.; Onugwu, A.L.; Nwokpor, V.C.; Akpaso, I.; Ogbulie, T.E.; Aharanwa, B.; Chijioke, C.; Verla, N.; Iheme, C.; Ujowundu, C.; et al. Novel Drug Delivery Systems: Insight into Self-Powered and Nano-Enabled Drug Delivery Systems. Nano TransMed 2024, 3, 100042. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Ma, D.; Lai, L.T.; Simon, J.; Borchardt, P.; Frank, R.K.; Wu, K.; Pellegrini, V.; Curcio, M.J.; Miederer, M.; et al. Tumor Therapy with Targeted Atomic Nanogenerators. Science 2001, 294, 1537–1540. [Google Scholar] [CrossRef]
- Shaha, S.; Rodrigues, D.; Mitragotri, S. Locoregional Drug Delivery for Cancer Therapy: Preclinical Progress and Clinical Translation. J. Control. Release 2024, 367, 737–767. [Google Scholar] [CrossRef]
- Hariharan, A.; Tran, S.D. Localized Drug Delivery Systems: An Update on Treatment Options for Head and Neck Squamous Cell Carcinomas. Pharmaceutics 2023, 15, 1844. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local Drug Delivery Strategies for Cancer Treatment: Gels, Nanoparticles, Polymeric Films, Rods, and Wafers. J. Control. Release Off. J. Control. Release Soc. 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Woodring, R.N.; Gurysh, E.G.; Bachelder, E.M.; Ainslie, K.M. Drug Delivery Systems for Localized Cancer Combination Therapy. ACS Appl. Bio Mater. 2023, 6, 934–950. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, M.; Cai, C.; Ye, C.; Guo, T.; Yang, K.; Xiao, H.; Tang, X.; Liu, H. Recent Progress of Hydrogel-Based Local Drug Delivery Systems for Postoperative Radiotherapy. Front. Oncol. 2023, 13, 1027254. [Google Scholar] [CrossRef]
- Fernando, H.C.; Santos, R.S.; Benfield, J.R.; Grannis, F.W.; Keenan, R.J.; Luketich, J.D.; Close, J.M.; Landreneau, R.J. Lobar and Sublobar Resection with and without Brachytherapy for Small Stage IA Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2005, 129, 261–267. [Google Scholar] [CrossRef]
- Kang, M.; Quintana, J.; Hu, H.; Teixeira, V.C.; Olberg, S.; Banla, L.I.; Rodriguez, V.; Hwang, W.L.; Schuemann, J.; Parangi, S.; et al. Sustained and Localized Drug Depot Release Using Radiation-Activated Scintillating Nanoparticles. Adv. Mater. 2024, 36, 2312326. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon Dots as a Trackable Drug Delivery Carrier for Localized Cancer Therapy in Vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Wang, Y.; Wu, H.; Bao, K.; Sheng, R.; Li, X. Microenvironment-Triggered Dual-Activation of a Photosensitizer- Fluorophore Conjugate for Tumor Specific Imaging and Photodynamic Therapy. Sci. Rep. 2020, 10, 12127. [Google Scholar] [CrossRef]
- Teh, D.B.L.; Bansal, A.; Chai, C.; Toh, T.B.; Tucker, R.A.J.; Gammad, G.G.L.; Yeo, Y.; Lei, Z.; Zheng, X.; Yang, F.; et al. A Flexi-PEGDA Upconversion Implant for Wireless Brain Photodynamic Therapy. Adv. Mater. 2020, 32, 2001459. [Google Scholar] [CrossRef]
- Yao, C.; Wang, W.; Wang, P.; Zhao, M.; Li, X.; Zhang, F. Near-Infrared Upconversion Mesoporous Cerium Oxide Hollow Biophotocatalyst for Concurrent pH-/H2O2-Responsive O2-Evolving Synergetic Cancer Therapy. Adv. Mater. 2018, 30, 1704833. [Google Scholar] [CrossRef]
- Sando, Y.; Matsuoka, K.-I.; Sumii, Y.; Kondo, T.; Ikegawa, S.; Sugiura, H.; Nakamura, M.; Iwamoto, M.; Meguri, Y.; Asada, N.; et al. Author Correction: 5-Aminolevulinic Acid-Mediated Photodynamic Therapy Can Target Aggressive Adult T Cell Leukemia/Lymphoma Resistant to Conventional Chemotherapy. Sci. Rep. 2021, 11, 6420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An Updated Overview on the Development of New Photosensitizers for Anticancer Photodynamic Therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Dudzik, T.; Domański, I.; Makuch, S. The Impact of Photodynamic Therapy on Immune System in Cancer—An Update. Front. Immunol. 2024, 15, 1335920. [Google Scholar] [CrossRef]
- Huis in ‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Canales, A.; Jia, X.; Froriep, U.P.; Koppes, R.A.; Tringides, C.M.; Selvidge, J.; Lu, C.; Hou, C.; Wei, L.; Fink, Y.; et al. Multifunctional Fibers for Simultaneous Optical, Electrical and Chemical Interrogation of Neural Circuits in Vivo. Nat. Biotechnol. 2015, 33, 277–284. [Google Scholar] [CrossRef]
- Chin, A.L.; Jiang, S.; Jang, E.; Niu, L.; Li, L.; Jia, X.; Tong, R. Implantable Optical Fibers for Immunotherapeutics Delivery and Tumor Impedance Measurement. Nat. Commun. 2021, 12, 5138. [Google Scholar] [CrossRef]
- Nazempour, R.; Zhang, Q.; Fu, R.; Sheng, X. Biocompatible and Implantable Optical Fibers and Waveguides for Biomedicine. Materials 2018, 11, 1283. [Google Scholar] [CrossRef]
- Ung, K.; Arenkiel, B.R. Fiber-Optic Implantation for Chronic Optogenetic Stimulation of Brain Tissue. J. Vis. Exp. JoVE 2012, 68, 50004. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, K.; Gupta, N. Fiber-Optic Sensors. In Material-Integrated Intelligent Systems—Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 107–136. ISBN 978-3-527-67924-9. [Google Scholar]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in Hyperthermia Cancer Therapy: From Fundamental Principles to Advanced Applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, L.; Zhang, W.; Liu, H.; Li, J.; Jiang, L.; Zeng, K. Dynamics of HPV Viral Loads Reflect the Treatment Effect of Photodynamic Therapy in Genital Warts. Photodiagnosis Photodyn. Ther. 2018, 21, 86–90. [Google Scholar] [CrossRef]
- Bansal, A.; Yang, F.; Xi, T.; Zhang, Y.; Ho, J.S. In Vivo Wireless Photonic Photodynamic Therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 1469–1474. [Google Scholar] [CrossRef]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic Therapy—A Review of the Synergistic Effects of Drugs and Ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.-S.; Zhang, N. Recent Advances of Sonodynamic Therapy in Cancer Treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Fan, F.; Gao, F.; Ji, H.; Yang, L. Piezoelectric Materials as Sonodynamic Sensitizers to Safely Ablate Tumors: A Case Study Using Black Phosphorus. J. Phys. Chem. Lett. 2020, 11, 1228–1238. [Google Scholar] [CrossRef]
- Sonmezoglu, S.; Fineman, J.R.; Maltepe, E.; Maharbiz, M.M. Monitoring Deep-Tissue Oxygenation with a Millimeter-Scale Ultrasonic Implant. Nat. Biotechnol. 2021, 39, 855–864. [Google Scholar] [CrossRef]
- Hinchet, R.; Yoon, H.-J.; Ryu, H.; Kim, M.-K.; Choi, E.-K.; Kim, D.-S.; Kim, S.-W. Transcutaneous Ultrasound Energy Harvesting Using Capacitive Triboelectric Technology. Science 2019, 365, 491–494. [Google Scholar] [CrossRef]
- Kim, A.; Zhou, J.; Samaddar, S.; Song, S.H.; Elzey, B.D.; Thompson, D.H.; Ziaie, B. An Implantable Ultrasonically-Powered Micro-Light-Source (µLight) for Photodynamic Therapy. Sci. Rep. 2019, 9, 1395. [Google Scholar] [CrossRef]
- Kim, A.; Lee, S.K.; Parupudi, T.; Rahimi, R.; Song, S.H.; Park, M.C.; Islam, S.; Zhou, J.; Majumdar, A.K.; Park, J.S.; et al. An Ultrasonically Powered Implantable Microprobe for Electrolytic Ablation. Sci. Rep. 2020, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Neely, R.M.; Shen, K.; Singhal, U.; Alon, E.; Rabaey, J.M.; Carmena, J.M.; Maharbiz, M.M. Wireless Recording in the Peripheral Nervous System with Ultrasonic Neural Dust. Neuron 2016, 91, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.; Feng, Y.; Chen, Q.; Chen, L.; Liu, J. Microbubbles Ultrasonic Cavitation Regulates Tumor Interstitial Fluid Pressure and Enhances Sonodynamic Therapy. Front. Oncol. 2022, 12, 852454. [Google Scholar] [CrossRef]
- Araújo Martins, Y.; Zeferino Pavan, T.; Fonseca Vianna Lopez, R. Sonodynamic Therapy: Ultrasound Parameters and in Vitro Experimental Configurations. Int. J. Pharm. 2021, 610, 121243. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, W.; Lai, Z.; Qiu, G.; Bu, Z.; Zhu, X.; Wang, Q.; Yu, Y.; Liu, J. Nanotechnology-Enabled Sonodynamic Therapy against Malignant Tumors. Nanoscale Adv. 2024, 6, 1974–1991. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Kang, Y.; Zhen, X.; Zhou, Z.; Liu, C.; Chen, S.; Huang, X.; Liu, H.-J.; Koo, S.; et al. Nanosensitizer-Mediated Augmentation of Sonodynamic Therapy Efficacy and Antitumor Immunity. Nat. Commun. 2023, 14, 6973. [Google Scholar] [CrossRef]
- Yang, N.; Li, J.; Yu, S.; Xia, G.; Li, D.; Yuan, L.; Wang, Q.; Ding, L.; Fan, Z.; Li, J. Application of Nanomaterial-Based Sonodynamic Therapy in Tumor Therapy. Pharmaceutics 2024, 16, 603. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, Y.; Shao, Y. Mesoporous Silica Nanoparticles for Dual-Mode Chemo-Sonodynamic Therapy by Low-Energy Ultrasound. Materials 2018, 11, 2041. [Google Scholar] [CrossRef]
- Collins, V.G.; Hutton, D.; Hossain-Ibrahim, K.; Joseph, J.; Banerjee, S. The Abscopal Effects of Sonodynamic Therapy in Cancer. Br. J. Cancer 2024, 132, 409–420. [Google Scholar] [CrossRef]
- Rattay, F. The Basic Mechanism for the Electrical Stimulation of the Nervous System. Neuroscience 1999, 89, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.B. Which Elements Are Excited in Electrical Stimulation of Mammalian Central Nervous System: A Review. Brain Res. 1975, 98, 417–440. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.A.P.; Huda, N.; Farjana, S.H.; Asadnia, M.; Lang, C. Recent Advances in Nanogenerator-Driven Self-Powered Implantable Biomedical Devices. Adv. Energy Mater. 2018, 8, 1701210. [Google Scholar] [CrossRef]
- Doblaré, M.; García, J.M.; Gómez, M.J. Modelling Bone Tissue Fracture and Healing: A Review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone Fracture Healing in Mechanobiological Modeling: A Review of Principles and Methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Sabet, F.A.; Raeisi Najafi, A.; Hamed, E.; Jasiuk, I. Modelling of Bone Fracture and Strength at Different Length Scales: A Review. Interface Focus 2016, 6, 20150055. [Google Scholar] [CrossRef]
- Snow, W.M.; Anderson, J.E.; Jakobson, L.S. Neuropsychological and Neurobehavioral Functioning in Duchenne Muscular Dystrophy: A Review. Neurosci. Biobehav. Rev. 2013, 37, 743–752. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic Developments for Duchenne Muscular Dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Cantelli, M.; Masarà, A.; Annunziata, M.C.; Marasca, C.; Cacciapuoti, S. Female Pattern Hair Loss: A Clinical, Pathophysiologic, and Therapeutic Review. Int. J. Womens Dermatol. 2018, 4, 203–211. [Google Scholar] [CrossRef]
- Gupta, M.; Mysore, V. Classifications of Patterned Hair Loss: A Review. J. Cutan. Aesthetic Surg. 2016, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lee, H.; Lee, J.; Lee, M.; Cho, S.; Kim, T.; Kim, H. Micro-Current Stimulation Has Potential Effects of Hair Growth-Promotion on Human Hair Follicle-Derived Papilla Cells and Animal Model. Int. J. Mol. Sci. 2021, 22, 4361. [Google Scholar] [CrossRef] [PubMed]
- Coolen, R.; Groen, J.; Blok, B. Electrical Stimulation in the Treatment of Bladder Dysfunction: Technology Update. Med. Devices 2019, 12, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Delivopoulos, E.; Chew, D.; Minev, I.; Fawcett, J.; Lacour, S. Concurrent Recordings of Bladder Afferents from Multiple Nerves Using a Microfabricated PDMS Microchannel Electrode Array. Lab. Chip 2012, 12, 2540–2551. [Google Scholar] [CrossRef]
- Li, X.; Liao, L.-M.; Chen, G.-Q.; Wang, Z.-X.; Lu, T.-J.; Deng, H. Clinical and Urodynamic Characteristics of Underactive Bladder. Medicine 2018, 97, e9610. [Google Scholar] [CrossRef]
- Suskind, A.M. The Aging Overactive Bladder: A Review of Aging-Related Changes from the Brain to the Bladder. Curr. Bladder Dysfunct. Rep. 2017, 12, 42–47. [Google Scholar] [CrossRef]
- Arkan, G.; Beser, A.; Ozturk, V. Experiences Related to Urinary Incontinence of Stroke Patients: A Qualitative Descriptive Study. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses 2018, 50, 42–47. [Google Scholar] [CrossRef]
- McGee, M.J.; Amundsen, C.L.; Grill, W.M. Electrical Stimulation for the Treatment of Lower Urinary Tract Dysfunction after Spinal Cord Injury. J. Spinal Cord Med. 2015, 38, 135–146. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Patel, S.; Ershad, F.; Zhao, M.; Isseroff, R.R.; Duan, B.; Zhou, Y.; Wang, Y.; Yu, C. Wearable Electronics for Skin Wound Monitoring and Healing. Soft Sci. 2022, 2, 9. [Google Scholar] [CrossRef]
- Portou, M.J.; Baker, D.; Abraham, D.; Tsui, J. The Innate Immune System, Toll-like Receptors and Dermal Wound Healing: A Review. Vascul. Pharmacol. 2015, 71, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-integrated Wound Dressing for Real-time Monitoring and On-demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef]
- Adapa, S.R.; Hunter, G.A.; Amin, N.E.; Marinescu, C.; Borsky, A.; Sagatys, E.M.; Sebti, S.M.; Reuther, G.W.; Ferreira, G.C.; Jiang, R.H. Porphyrin Overdrive Rewires Cancer Cell Metabolism. Life Sci. Alliance 2024, 7, e202302547. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gosling, J.; Liu, S.; Wang, H.; Stone, E.M.; Chakraborty, S.; Jayaraman, P.-S.; Smith, S.; Amabilino, D.B.; Fromhold, M.; et al. Wireless Electrical–Molecular Quantum Signalling for Cancer Cell Apoptosis. Nat. Nanotechnol. 2024, 19, 106–114. [Google Scholar] [CrossRef]
- Pessoa, J. Cytochrome c in Cancer Therapy and Prognosis. Biosci. Rep. 2022, 42, BSR20222171. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, M.; Tang, J.; Jin, Y. Molecular/Nanomechanical Insights into Electrostimulation-Inhibited Energy Metabolism Mechanisms and Cytoskeleton Damage of Cancer Cells. Adv. Sci. 2023, 10, e2207165. [Google Scholar] [CrossRef]
- Das, R.; Langou, S.; Le, T.T.; Prasad, P.; Lin, F.; Nguyen, T.D. Electrical Stimulation for Immune Modulation in Cancer Treatments. Front. Bioeng. Biotechnol. 2022, 9, 795300. [Google Scholar] [CrossRef]
- Aghayari, S. Electroporation Combined with Intelligent Drug Delivery Promising New and Clean Approach to Cancer Treatment. Results Eng. 2023, 19, 101379. [Google Scholar] [CrossRef]
- Nuccitelli, R. Application of Pulsed Electric Fields to Cancer Therapy. Bioelectricity 2019, 1, 30–34. [Google Scholar] [CrossRef]
- Szlasa, W.; Michel, O.; Sauer, N.; Novickij, V.; Lewandowski, D.; Kasperkiewicz, P.; Tarek, M.; Saczko, J.; Kulbacka, J. Nanosecond Pulsed Electric Field Suppresses Growth and Reduces Multi-Drug Resistance Effect in Pancreatic Cancer. Sci. Rep. 2023, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Gudvangen, E.; Kim, V.; Novickij, V.; Battista, F.; Pakhomov, A.G. Electroporation and Cell Killing by Milli- to Nanosecond Pulses and Avoiding Neuromuscular Stimulation in Cancer Ablation. Sci. Rep. 2022, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Shi, X.; Li, D.; Xu, X.; Xiao, B.; Piao, Y.; Xiang, J.; Shao, S.; Ho, F.C.-Y.; et al. Electrical Stimulation Induces Anti-Tumor Immunomodulation via a Flexible Microneedle-Array-Integrated Interdigital Electrode. Sci. Bull. 2023, 68, 2779–2792. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Venugopal, A.; Chandrasekhar, M.; Suriyanarayanan, A.; Balasubramani, K.; Sinthai Ilangovan, A.; Kamalakannan, S.; Gunaseelan, R.; Ayyadurai, N.; Valsala Gopalakrishnan, A.; et al. Electrical Based Cancer Therapy for Solid Tumours—Theranostics Approach. Biosens. Bioelectron. X 2022, 11, 100214. [Google Scholar] [CrossRef]
- Zhong, S.; Yao, S.; Zhao, Q.; Wang, Z.; Liu, Z.; Li, L.; Wang, Z.L. Electricity-Assisted Cancer Therapy: From Traditional Clinic Applications to Emerging Methods Integrated with Nanotechnologies. Adv. NanoBiomed Res. 2023, 3, 2200143. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Lan, H.; Jamil, M.; Ke, G.; Dong, N. The Role of Nanoparticles and Nanomaterials in Cancer Diagnosis and Treatment: A Comprehensive Review. Am. J. Cancer Res. 2023, 13, 5751–5784. [Google Scholar]
- Hajipour Keyvani, A.; Mohammadnejad, P.; Pazoki-Toroudi, H.; Perez Gilabert, I.; Chu, T.; Manshian, B.B.; Soenen, S.J.; Sohrabi, B. Advancements in Cancer Treatment: Harnessing the Synergistic Potential of Graphene-Based Nanomaterials in Combination Therapy. ACS Appl. Mater. Interfaces 2025, 17, 2756–2790. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Patel, A.P. Passive Targeting of Nanoparticles to Cancer. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 125–143. ISBN 978-3-030-06115-9. [Google Scholar]
- Bazak, R.; Houri, M.; Achy, S.E.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Huang, X.; He, T.; Liang, X.; Xiang, Z.; Liu, C.; Zhou, S.; Luo, R.; Bai, L.; Kou, X.; Li, X.; et al. Advances and Applications of Nanoparticles in Cancer Therapy. MedComm–Oncol. 2024, 3, e67. [Google Scholar] [CrossRef]
- Est-Witte, S.E.; Livingston, N.K.; Omotoso, M.O.; Green, J.J.; Schneck, J.P. Nanoparticles for Generating Antigen-Specific T Cells for Immunotherapy. Semin. Immunol. 2021, 56, 101541. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Misiak, P.; Niemirowicz-Laskowska, K.; Markiewicz, K.H.; Wielgat, P.; Kurowska, I.; Czarnomysy, R.; Misztalewska-Turkowicz, I.; Car, H.; Bielawski, K.; Wilczewska, A.Z. Doxorubicin-Loaded Polymeric Nanoparticles Containing Ketoester-Based Block and Cholesterol Moiety as Specific Vehicles to Fight Estrogen-Dependent Breast Cancer. Cancer Nanotechnol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Mohammed, K.A.; Nasreen, N. Nanoparticle-Based Targeted Gene Therapy for Lung Cancer. Am. J. Cancer Res. 2016, 6, 1118–1134. [Google Scholar]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.A. Nanoparticles for Cancer Gene Therapy and Imaging. Biomed. Mater. Devices 2024, 1–63. [Google Scholar] [CrossRef]
- Arif, M.; Nawaz, A.F.; Ullah Khan, S.; Mueen, H.; Rashid, F.; Hemeg, H.A.; Rauf, A. Nanotechnology-Based Radiation Therapy to Cure Cancer and the Challenges in Its Clinical Applications. Heliyon 2023, 9, e17252. [Google Scholar] [CrossRef]
- Haque, M.; Shakil, M.S.; Mahmud, K.M. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers 2023, 15, 1892. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in Radiation Therapy: A Summary of Various Approaches to Enhance Radiosensitization in Cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar] [CrossRef]
- Mostafavi, M.; Ghazi, F.; Mehrabifard, M.; Alivirdiloo, V.; Hajiabbasi, M.; Rahimi, F.; Mobed, A.; Taheripak, G.; Ramezani Farani, M.; Huh, Y.S.; et al. State-of-the-Art Application of Nanoparticles in Radiotherapy: A Platform for Synergistic Effects in Cancer Treatment. Strahlenther. Onkol. 2024. [Google Scholar] [CrossRef]
- Roscher, M.; Bakos, G.; Benešová, M. Atomic Nanogenerators in Targeted Alpha Therapies: Curie’s Legacy in Modern Cancer Management. Pharmaceuticals 2020, 13, 76. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nano-Generator Actinium-225-Lintuzumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef]
- Mulford, D.A.; Scheinberg, D.A.; Jurcic, J.G. The Promise of Targeted α-Particle Therapy. J. Nucl. Med. 2005, 46, 199S–204S. [Google Scholar]
- Pallares, R.M.; Abergel, R.J. Development of Radiopharmaceuticals for Targeted Alpha Therapy: Where Do We Stand? Front. Med. 2022, 9, 1020188. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’huyvetter, M.; Devoogdt, N. Targeted Alpha Therapy Using Short-Lived Alpha-Particles and the Promise of Nanobodies as Targeting Vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Toro-González, M.; Akingbesote, N.; Bible, A.; Pal, D.; Sanders, B.; Ivanov, A.S.; Jansone-Popova, S.; Popovs, I.; Benny, P.; Perry, R.; et al. Development of 225Ac-Doped Biocompatible Nanoparticles for Targeted Alpha Therapy. J. Nanobiotechnol. 2024, 22, 306. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- van der Doelen, M.J.; Mehra, N.; van Oort, I.M.; Looijen-Salamon, M.G.; Janssen, M.J.R.; Custers, J.A.E.; Slootbeek, P.H.J.; Kroeze, L.I.; Bruchertseifer, F.; Morgenstern, A.; et al. Clinical Outcomes and Molecular Profiling of Advanced Metastatic Castration-Resistant Prostate Cancer Patients Treated with 225Ac-PSMA-617 Targeted Alpha-Radiation Therapy. Urol. Oncol. 2021, 39, 729.e7–729.e16. [Google Scholar] [CrossRef]
- Dorsey, J.F.; Sun, L.; Joh, D.Y.; Witztum, A.; Kao, G.D.; Alonso-Basanta, M.; Avery, S.; Hahn, S.M.; Al Zaki, A.; Tsourkas, A. Gold Nanoparticles in Radiation Research: Potential Applications for Imaging and Radiosensitization. Transl. Cancer Res. 2013, 2, 280–291. [Google Scholar] [CrossRef]
- Wang, R.; Liu, H.; Antal, B.; Wolterbeek, H.T.; Denkova, A.G. Ultrasmall Gold Nanoparticles Radiolabeled with Iodine-125 as Potential New Radiopharmaceutical. ACS Appl. Bio Mater. 2024, 7, 1240–1249. [Google Scholar] [CrossRef]
- Daems, N.; Michiels, C.; Lucas, S.; Baatout, S.; Aerts, A. Gold Nanoparticles Meet Medical Radionuclides. Nucl. Med. Biol. 2021, 100–101, 61–90. [Google Scholar] [CrossRef]
- Allen, B.J.; Huang, C.-Y.; Clarke, R.A. Targeted Alpha Anticancer Therapies: Update and Future Prospects. Biol. Targets Ther. 2014, 8, 255–267. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Doligalski, M.L.; Tichacek, C.J.; Pandya, D.N.; Budzevich, M.M.; El-Haddad, G.; Khushalani, N.I.; Moros, E.G.; McLaughlin, M.L.; Wadas, T.J.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 24, 4314. [Google Scholar] [CrossRef]
- Winter, R.C.; Amghar, M.; Wacker, A.S.; Bakos, G.; Taş, H.; Roscher, M.; Kelly, J.M.; Benešová-Schäfer, M. Future Treatment Strategies for Cancer Patients Combining Targeted Alpha Therapy with Pillars of Cancer Treatment: External Beam Radiation Therapy, Checkpoint Inhibition Immunotherapy, Cytostatic Chemotherapy, and Brachytherapy. Pharmaceuticals 2024, 17, 1031. [Google Scholar] [CrossRef]
- Rodak, M.; Dekempeneer, Y.; Wojewódzka, M.; Caveliers, V.; Covens, P.; Miller, B.W.; Sevenois, M.B.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T.; et al. Preclinical Evaluation of 225Ac-Labeled Single-Domain Antibody for the Treatment of HER2pos Cancer. Mol. Cancer Ther. 2022, 21, 1835–1845. [Google Scholar] [CrossRef]
- Den, R.B.; George, D.; Pieczonka, C.; McNamara, M. Ra-223 Treatment for Bone Metastases in Castrate-Resistant Prostate Cancer. Am. J. Clin. Oncol. 2019, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- van der Zande, K.; Oyen, W.J.G.; Zwart, W.; Bergman, A.M. Radium-223 Treatment of Patients with Metastatic Castration Resistant Prostate Cancer: Biomarkers for Stratification and Response Evaluation. Cancers 2021, 13, 4346. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.E.; Etchebehere, E. Radium-223 as an Approved Modality for Treatment of Bone Metastases. Semin. Nucl. Med. 2020, 50, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Jalloul, W.; Ghizdovat, V.; Stolniceanu, C.R.; Ionescu, T.; Grierosu, I.C.; Pavaleanu, I.; Moscalu, M.; Stefanescu, C. Targeted Alpha Therapy: All We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide. Pharmaceuticals 2023, 16, 1679. [Google Scholar] [CrossRef]
- Shen, S.; Zhu, C.; Huo, D.; Yang, M.; Xue, J.; Xia, Y. A Hybrid Nanomaterial for the Controlled Generation of Free Radicals and Oxidative Destruction of Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017, 56, 8801–8804. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of Reactive Oxygen and Nitrogen Species with Nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Murugan, A.; Gorantla, K.R.; Mallik, B.S.; Sharada, D.S. Iron Promoted C3–H Nitration of 2H-Indazole: Direct Access to 3-Nitro-2H-Indazoles. Org. Biomol. Chem. 2018, 16, 5113–5118. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, Y.; Xiao, J.; Zhao, Z.-J.; Wang, Y.; Xu, Z.; Zhang, Y.; Yin, L.; Cao, H.; Gong, J. Single-Atom Mn–N4 Site-Catalyzed Peroxone Reaction for the Efficient Production of Hydroxyl Radicals in an Acidic Solution. J. Am. Chem. Soc. 2019, 141, 12005–12010. [Google Scholar] [CrossRef]
- Song, R.; Wang, H.; Zhang, M.; Liu, Y.; Meng, X.; Zhai, S.; Wang, C.; Gong, T.; Wu, Y.; Jiang, X.; et al. Near-Infrared Light-Triggered Chlorine Radical (·Cl) Stress for Cancer Therapy. Angew. Chem. Int. Ed. 2020, 59, 21032–21040. [Google Scholar] [CrossRef]
- Li, M.; Xiong, T.; Du, J.; Tian, R.; Xiao, M.; Guo, L.; Long, S.; Fan, J.; Sun, W.; Shao, K.; et al. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles’ Heels of Photodynamic Oncotherapy. J. Am. Chem. Soc. 2019, 141, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jin, Y.; Liu, X.; Liu, T.; Wang, W.; Yu, H. Photoactivatable Nanogenerators of Reactive Species for Cancer Therapy. Bioact. Mater. 2021, 6, 4301–4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, M.; Lan, M.; Zhang, W. Boosting Cancer Therapy Efficiency via Photoinduced Radical Production. Chem. Sci. 2021, 12, 9500–9505. [Google Scholar] [CrossRef]
- Guo, R.; Tian, Y.; Wang, Y.; Yang, W. Near-Infrared Laser-Triggered Nitric Oxide Nanogenerators for the Reversal of Multidrug Resistance in Cancer. Adv. Funct. Mater. 2017, 27, 1606398. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.-H.; Pan, P.; Zeng, R.-Y.; Zhang, X.-Z. Tumor-Specific ONOO− Nanogenerator for Improved Drug Delivery and Enhanced Chemotherapy of Tumor. ACS Nano 2021, 15, 11514–11525. [Google Scholar] [CrossRef]
- Wang, X.-S.; Zeng, J.-Y.; Li, M.-J.; Li, Q.-R.; Gao, F.; Zhang, X.-Z. Highly Stable Iron Carbonyl Complex Delivery Nanosystem for Improving Cancer Therapy. ACS Nano 2020, 14, 9848–9860. [Google Scholar] [CrossRef]
- Wang, S.-B.; Zhang, C.; Chen, Z.-X.; Ye, J.-J.; Peng, S.-Y.; Rong, L.; Liu, C.-J.; Zhang, X.-Z. A Versatile Carbon Monoxide Nanogenerator for Enhanced Tumor Therapy and Anti-Inflammation. ACS Nano 2019, 13, 5523–5532. [Google Scholar] [CrossRef]
- Li, Y.; Dang, J.; Liang, Q.; Yin, L. Thermal-Responsive Carbon Monoxide (CO) Delivery Expedites Metabolic Exhaustion of Cancer Cells toward Reversal of Chemotherapy Resistance. ACS Cent. Sci. 2019, 5, 1044–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Tang, Y.; Zou, J.; Wang, P.; Zhang, Y.; Si, W.; Huang, W.; Dong, X. A Light-Induced Nitric Oxide Controllable Release Nano-Platform Based on Diketopyrrolopyrrole Derivatives for pH-Responsive Photodynamic/Photothermal Synergistic Cancer Therapy. Chem. Sci. 2018, 9, 8103–8109. [Google Scholar] [CrossRef]
- Kim, S.-G.; Priya, S.; Kanno, I. Piezoelectric MEMS for Energy Harvesting. MRS Bull. 2012, 37, 1039–1050. [Google Scholar] [CrossRef]
- Li, P.; Ryu, J.; Hong, S. Piezoelectric/Triboelectric Nanogenerators for Biomedical Applications; IntechOpen: London, UK, 2019; ISBN 978-1-83881-060-3. [Google Scholar]
- Curie, J.; Curie, P. Phénomènes Électriques Des Cristaux Hémièdres à Faces Inclinées. J. Phys. Theor. Appl. 1882, 1, 245–251. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.-H.; Kim, J. A Review of Piezoelectric Energy Harvesting Based on Vibration. Int. J. Precis. Eng. Manuf. 2011, 12, 1129–1141. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Lin, T. Electrical Power Generator from Randomly Oriented Electrospun Poly(Vinylidene Fluoride) Nanofibre Membranes. J. Mater. Chem. 2011, 21, 11088–11091. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Yao, N.; Shi, Y. 1.6 V Nanogenerator for Mechanical Energy Harvesting Using PZT Nanofibers. Nano Lett. 2010, 10, 2133–2137. [Google Scholar] [CrossRef]
- Bai, S.; Xu, Q.; Gu, L.; Ma, F.; Qin, Y.; Wang, Z.L. Single Crystalline Lead Zirconate Titanate (PZT) Nano/Micro-Wire Based Self-Powered UV Sensor. Nano Energy 2012, 1, 789–795. [Google Scholar] [CrossRef]
- Wang, P.; Du, H. ZnO Thin Film Piezoelectric MEMS Vibration Energy Harvesters with Two Piezoelectric Elements for Higher Output Performance. Rev. Sci. Instrum. 2015, 86, 075002. [Google Scholar] [CrossRef]
- Algieri, L.; Todaro, M.T.; Guido, F.; Mastronardi, V.; Desmaële, D.; Qualtieri, A.; Giannini, C.; Sibillano, T.; De Vittorio, M. Flexible Piezoelectric Energy-Harvesting Exploiting Biocompatible AlN Thin Films Grown onto Spin-Coated Polyimide Layers. ACS Appl. Energy Mater. 2018, 1, 5203–5210. [Google Scholar] [CrossRef]
- Eom, C.-B.; Trolier-McKinstry, S. Thin-Film Piezoelectric MEMS. MRS Bull. 2012, 37, 1007–1017. [Google Scholar] [CrossRef]
- Wasa, K.; Matsushima, T.; Adachi, H.; Kanno, I.; Kotera, H. Thin-Film Piezoelectric Materials For a Better Energy Harvesting MEMS. J. Microelectromech. Syst. 2012, 21, 451–457. [Google Scholar] [CrossRef]
- Won, S.S.; Sheldon, M.; Mostovych, N.; Kwak, J.; Chang, B.-S.; Ahn, C.; Kingon, A.; Won Kim, I.; Kim, S.-H. Piezoelectric Poly(Vinylidene Fluoride Trifluoroethylene) Thin Film-Based Power Generators Using Paper Substrates for Wearable Device Applications. Appl. Phys. Lett. 2015, 107, 202901. [Google Scholar] [CrossRef]
- Kosec, M.; Malič, B.; Benčan, A.; Rojac, T. KNN-Based Piezoelectric Ceramics. In Piezoelectric and Acoustic Materials for Transducer Applications; Safari, A., Akdoğan, E.K., Eds.; Springer: Boston, MA, USA, 2008; pp. 81–102. ISBN 978-0-387-76540-2. [Google Scholar]
- Clementi, G.; Lombardi, G.; Margueron, S.; Suarez, M.A.; Lebrasseur, E.; Ballandras, S.; Imbaud, J.; Lardet-Vieudrin, F.; Gauthier-Manuel, L.; Dulmet, B.; et al. LiNbO3 Films—A Low-Cost Alternative Lead-Free Piezoelectric Material for Vibrational Energy Harvesters. Mech. Syst. Signal Process. MSSP 2021, 149, 107171. [Google Scholar] [CrossRef]
- Wang, Z.J.; Narita, F. Corona Poling Conditions for Barium Titanate/Epoxy Composites and Their Unsteady Wind Energy Harvesting Potential. Adv. Eng. Mater. 2019, 21, 1900169. [Google Scholar] [CrossRef]
- Singh, H.H.; Khare, N. Flexible ZnO-PVDF/PTFE Based Piezo-Tribo Hybrid Nanogenerator. Nano Energy 2018, 51, 216–222. [Google Scholar] [CrossRef]
- Qualtieri, A.; Rizzi, F.; Todaro, M.T.; Passaseo, A.; Cingolani, R.; De Vittorio, M. Stress-Driven AlN Cantilever-Based Flow Sensor for Fish Lateral Line System. Microelectron. Eng. 2011, 88, 2376–2378. [Google Scholar] [CrossRef]
- Caro, M.A.; Zhang, S.; Riekkinen, T.; Ylilammi, M.; Moram, M.A.; Lopez-Acevedo, O.; Molarius, J.; Laurila, T. Piezoelectric Coefficients and Spontaneous Polarization of ScAlN. J. Phys. Condens. Matter 2015, 27, 245901. [Google Scholar] [CrossRef]
- Jeronimo, K.; Koutsos, V.; Cheung, R.; Mastropaolo, E. PDMS-ZnO Piezoelectric Nanocomposites for Pressure Sensors. Sensors 2021, 21, 5873. [Google Scholar] [CrossRef]
- He, W.; Qian, Y.; Lee, B.S.; Zhang, F.; Rasheed, A.; Jung, J.-E.; Kang, D.J. Ultrahigh Output Piezoelectric and Triboelectric Hybrid Nanogenerators Based on ZnO Nanoflakes/Polydimethylsiloxane Composite Films. ACS Appl. Mater. Interfaces 2018, 10, 44415–44420. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Abdullah, A.M.; Hussain, I.; Lopez, J.; Cantu, D.; Gupta, S.K.; Mao, Y.; Danti, S.; Uddin, M.J. Lithium Doped Zinc Oxide Based Flexible Piezoelectric-Triboelectric Hybrid Nanogenerator. Nano Energy 2019, 61, 327–336. [Google Scholar] [CrossRef]
- Bartasyte, A.; Margueron, S.; Baron, T.; Oliveri, S.; Boulet, P. Toward High-Quality Epitaxial LiNbO3 and LiTaO3 Thin Films for Acoustic and Optical Applications. Adv. Mater. Interfaces 2017, 4, 1600998. [Google Scholar] [CrossRef]
- Kovacs, G.; Anhorn, M.; Engan, H.E.; Visintini, G.; Ruppel, C.C.W. Improved Material Constants for LiNbO3 and LiTaO3. In Proceedings of the IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 4–7 December 1990; Volume 1, pp. 435–438. [Google Scholar]
- Koblmueller, G.; Averbeck, R.; Geelhaar, L.; Riechert, H.; Hösler, W.; Pongratz, P. Growth Diagram and Morphologies of AlN Thin Films Grown by Molecular Beam Epitaxy. J. Appl. Phys. 2003, 93, 9591–9596. [Google Scholar] [CrossRef]
- Chen, D.; Colas, J.; Mercier, F.; Boichot, R.; Charpentier, L.; Escape, C.; Balat-Pichelin, M.; Pons, M. High Temperature Properties of AlN Coatings Deposited by Chemical Vapor Deposition for Solar Central Receivers. Surf. Coat. Technol. 2019, 377, 124872. [Google Scholar] [CrossRef]
- Mariello, M.; Blad, T.W.A.; Mastronardi, V.M.; Madaro, F.; Guido, F.; Staufer, U.; Tolou, N.; De Vittorio, M. Flexible Piezoelectric AlN Transducers Buckled through Package-Induced Preloading for Mechanical Energy Harvesting. Nano Energy 2021, 85, 105986. [Google Scholar] [CrossRef]
- Mariello, M. Heart Energy Harvesting and Cardiac Bioelectronics: Technologies and Perspectives. Nanoenergy Adv. 2022, 2, 344–385. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Qualtieri, A.; Pisanello, F.; De Vittorio, M. Microstructure and Electrical Properties of Novel Piezo-Optrodes Based on Thin-Film Piezoelectric Aluminium Nitride for Sensing. IEEE Trans. Nanotechnol. 2021, 20, 10–19. [Google Scholar] [CrossRef]
- Akiyama, M.; Morofuji, Y.; Kamohara, T.; Nishikubo, K.; Tsubai, M.; Fukuda, O.; Ueno, N. Flexible Piezoelectric Pressure Sensors Using Oriented Aluminum Nitride Thin Films Prepared on Polyethylene Terephthalate Films. J. Appl. Phys. 2006, 100, 114318. [Google Scholar] [CrossRef]
- Signore, M.A.; Rescio, G.; De Pascali, C.; Iacovacci, V.; Dario, P.; Leone, A.; Quaranta, F.; Taurino, A.; Siciliano, P.; Francioso, L. Fabrication and Characterization of AlN-Based Flexible Piezoelectric Pressure Sensor Integrated into an Implantable Artificial Pancreas. Sci. Rep. 2019, 9, 17130. [Google Scholar] [CrossRef]
- Jackson, N.; Keeney, L.; Mathewson, A. Flexible-CMOS and Biocompatible Piezoelectric AlN Material for MEMS Applications. Smart Mater. Struct. 2013, 22, 115033. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.; Guo, M.; Pei, J.; Zhao, H.; Wang, Y. Fabrication of Polyimide Microfluidic Devices by Laser Ablation Based Additive Manufacturing. Microsyst. Technol. 2020, 26, 1573–1583. [Google Scholar] [CrossRef]
- Sun, Y.; Lacour, S.; Brooks, R.; Rushton, N.; Fawcett, J.; Cameron, R.E. Assessment of the Biocompatibility of Photosensitive Polyimide for Implantable Medical Device Use. J. Biomed. Mater. Res. A 2009, 90, 648–655. [Google Scholar] [CrossRef]
- Pi, Z.; Zhang, J.; Wen, C.; Zhang, Z.; Wu, D. Flexible Piezoelectric Nanogenerator Made of Poly(Vinylidenefluoride-Co-Trifluoroethylene) (PVDF-TrFE) Thin Film. Nano Energy 2014, 7, 33–41. [Google Scholar] [CrossRef]
- Chiu, Y.-Y.; Lin, W.-Y.; Wang, H.-Y.; Huang, S.-B.; Wu, M.-H. Development of a Piezoelectric Polyvinylidene Fluoride (PVDF) Polymer-Based Sensor Patch for Simultaneous Heartbeat and Respiration Monitoring. Sens. Actuators Phys. 2013, 189, 328–334. [Google Scholar] [CrossRef]
- Bae, J.-H.; Chang, S.-H. PVDF-Based Ferroelectric Polymers and Dielectric Elastomers for Sensor and Actuator Applications: A Review. Funct. Compos. Struct. 2019, 1, 012003. [Google Scholar] [CrossRef]
- Liu, J.; Yu, D.; Zheng, Z.; Huangfu, G.; Guo, Y. Lead-Free BiFeO3 Film on Glass Fiber Fabric: Wearable Hybrid Piezoelectric-Triboelectric Nanogenerator. Ceram. Int. 2021, 47, 3573–3579. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible Triboelectric Generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, L.; Chen, J.; Niu, S.; Zi, Y. Triboelectric Nanogenerators; Green Energy and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-40038-9. [Google Scholar]
- Luo, J.; Wang, Z.L. Recent Progress of Triboelectric Nanogenerators: From Fundamental Theory to Practical Applications. EcoMat 2020, 2, e12059. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.L. Theoretical Systems of Triboelectric Nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, Z.L. Triboelectric Nanogenerators as Flexible Power Sources. npj Flex. Electron. 2017, 1, 10. [Google Scholar] [CrossRef]
- Niu, S.; Liu, Y.; Wang, S.; Lin, L.; Zhou, Y.S.; Hu, Y.; Wang, Z.L. Theoretical Investigation and Structural Optimization of Single-Electrode Triboelectric Nanogenerators. Adv. Funct. Mater. 2014, 24, 3332–3340. [Google Scholar] [CrossRef]
- Henniker, J. Triboelectricity in Polymers. Nature 1962, 196, 474. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, Y.; Guo, L.; Wang, P.; He, X.; Dai, G.; Zheng, H.; Chen, C.; Wang, A.; Xu, C.; et al. Quantifying the Triboelectric Series. Nat. Commun. 2019, 10, 1427. [Google Scholar] [CrossRef]
- Ibrahim, M.; Jiang, J.; Wen, Z.; Sun, X. Surface Engineering for Enhanced Triboelectric Nanogenerator. Nanoenergy Adv. 2021, 1, 58–80. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, L.; Quan, L.; Zheng, X. Theories for Triboelectric Nanogenerators: A Comprehensive Review. Nanotechnol. Rev. 2020, 9, 610–625. [Google Scholar] [CrossRef]
- Arcot Narasimulu, A.; Zhao, P.; Soin, N.; Kovur, P.; Ding, P.; Chen, J.; Dong, S.; Chen, L.; Zhou, E.; Montemagno, C.; et al. Significant Triboelectric Enhancement Using Interfacial Piezoelectric ZnO Nanosheet Layer. Nano Energy 2017, 40, 471–480. [Google Scholar] [CrossRef]
- Ravichandran, A.N.; Ramuz, M.; Blayac, S. Increasing Surface Charge Density by Effective Charge Accumulation Layer Inclusion for High-Performance Triboelectric Nanogenerators. MRS Commun. 2019, 9, 682–689. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yim, E.-C.; Kim, J.-H.; Kim, S.-J.; Park, J.-Y.; Oh, I.-K. Bacterial Nano-Cellulose Triboelectric Nanogenerator. Nano Energy 2017, 33, 130–137. [Google Scholar] [CrossRef]
- Wen, R.; Guo, J.; Yu, A.; Zhang, K.; Kou, J.; Zhu, Y.; Zhang, Y.; Li, B.-W.; Zhai, J. Remarkably Enhanced Triboelectric Nanogenerator Based on Flexible and Transparent Monolayer Titania Nanocomposite. Nano Energy 2018, 50, 140–147. [Google Scholar] [CrossRef]
- Kil Yun, B.; Soo Kim, H.; Joon Ko, Y.; Murillo, G.; Hoon Jung, J. Interdigital Electrode Based Triboelectric Nanogenerator for Effective Energy Harvesting from Water. Nano Energy 2017, 36, 233–240. [Google Scholar] [CrossRef]
- Mariello, M.; Scarpa, E.; Algieri, L.; Guido, F.; Mastronardi, V.M.; Qualtieri, A.; De Vittorio, M. Novel Flexible Triboelectric Nanogenerator Based on Metallized Porous PDMS and Parylene C. Energies 2020, 13, 1625. [Google Scholar] [CrossRef]
- Calcagnile, P.; Blasi, L.; Rizzi, F.; Qualtieri, A.; Athanassiou, A.; Gogolides, E.; De Vittorio, M. Parylene C Surface Functionalization and Patterning with pH-Responsive Microgels. ACS Appl. Mater. Interfaces 2014, 6, 15708–15715. [Google Scholar] [CrossRef]
- Scarpa, E.; Dattoma, T.; Calcagnile, P.; Blasi, L.; Qualtieri, A.; Rizzi, F.; Vittorio, M.D. Surface-Tension-Confined Fluidics on Parylene C Coated Paper Substrate. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 259–262. [Google Scholar]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; De Donato, F.; Salbini, M.; Brunetti, V.; Qualtieri, A.; Rizzi, F.; De Vittorio, M. Captive-Air-Bubble Aerophobicity Measurements of Antibiofouling Coatings for Underwater MEMS Devices. Nanomater. Nanotechnol. 2019, 9, 1847980419862075. [Google Scholar] [CrossRef]
- Genter, S.; Langhof, T.; Paul, O. Electret-Based Out-Of-Plane Micro Energy Harvester with Parylene-C Serving as the Electret and Spring Material. Procedia Eng. 2015, 120, 341–344. [Google Scholar] [CrossRef][Green Version]
- Lo, H.; Tai, Y.-C. Parylene-Based Electret Power Generators. J. Micromech. Microeng. 2008, 18, 104006. [Google Scholar] [CrossRef]
- Mariello, M.; Guido, F.; Mastronardi, V.M.; Giannuzzi, R.; Algieri, L.; Qualteri, A.; Maffezzoli, A.; De Vittorio, M. Reliability of Protective Coatings for Flexible Piezoelectric Transducers in Aqueous Environments. Micromachines 2019, 10, 739. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Brzychczy-Wloch, M.; Engvall, K.; Aminlashgari, N.; Hakkarainen, M.; Kotarba, A. Microbiological Investigations of Oxygen Plasma Treated Parylene C Surfaces for Metal Implant Coating. Mater. Sci. Eng. C 2015, 52, 273–281. [Google Scholar] [CrossRef]
- Chen, T.-N.; Wuu, D.-S.; Wu, C.-C.; Chiang, C.-C.; Chen, Y.-P.; Horng, R.-H. Improvements of Permeation Barrier Coatings Using Encapsulated Parylene Interlayers for Flexible Electronic Applications. Plasma Process. Polym. 2007, 4, 180–185. [Google Scholar] [CrossRef]
- Applerot, G.; Abu-Mukh, R.; Irzh, A.; Charmet, J.; Keppner, H.; Laux, E.; Guibert, G.; Gedanken, A. Decorating Parylene-Coated Glass with ZnO Nanoparticles for Antibacterial Applications: A Comparative Study of Sonochemical, Microwave, and Microwave-Plasma Coating Routes. ACS Appl. Mater. Interfaces 2010, 2, 1052–1059. [Google Scholar] [CrossRef]
- Cieślik, M.; Engvall, K.; Pan, J.; Kotarba, A. Silane–Parylene Coating for Improving Corrosion Resistance of Stainless Steel 316L Implant Material. Corros. Sci. 2011, 53, 296–301. [Google Scholar] [CrossRef]
- Gorham, W.F. A New, General Synthetic Method for the Preparation of Linear Poly-p-Xylylenes. J. Polym. Sci. 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, H.S.; Kong, D.S.; Choi, M.; Kim, H.B.; Lee, J.-H.; Murillo, G.; Lee, M.; Kim, S.S.; Jung, J.H. Floating Buoy-Based Triboelectric Nanogenerator for an Effective Vibrational Energy Harvesting from Irregular and Random Water Waves in Wild Sea. Nano Energy 2018, 45, 247–254. [Google Scholar] [CrossRef]
- Wang, Z.L.; Jiang, T.; Xu, L. Toward the Blue Energy Dream by Triboelectric Nanogenerator Networks. Nano Energy 2017, 39, 9–23. [Google Scholar] [CrossRef]
- Xiong, J.; Lin, M.-F.; Wang, J.; Gaw, S.L.; Parida, K.; Lee, P.S. Wearable All-Fabric-Based Triboelectric Generator for Water Energy Harvesting. Adv. Energy Mater. 2017, 7, 1701243. [Google Scholar] [CrossRef]
- Luo, J.; Tang, W.; Fan, F.R.; Liu, C.; Pang, Y.; Cao, G.; Wang, Z.L. Transparent and Flexible Self-Charging Power Film and Its Application in a Sliding Unlock System in Touchpad Technology. ACS Nano 2016, 10, 8078–8086. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, H.; Bowen, C.R.; Yang, Y. Recent Advances in Pyroelectric Materials and Applications. Small 2021, 17, 2103960. [Google Scholar] [CrossRef]
- Ryu, H.; Kim, S.-W. Emerging Pyroelectric Nanogenerators to Convert Thermal Energy into Electrical Energy. Small 2021, 17, e1903469. [Google Scholar] [CrossRef]
- Thakre, A.; Kumar, A.; Song, H.-C.; Jeong, D.-Y.; Ryu, J. Pyroelectric Energy Conversion and Its Applications—Flexible Energy Harvesters and Sensors. Sensors 2019, 19, 2170. [Google Scholar] [CrossRef]
- Indira, S.S.; Vaithilingam, C.A.; Oruganti, K.S.P.; Mohd, F.; Rahman, S. Nanogenerators as a Sustainable Power Source: State of Art, Applications, and Challenges. Nanomaterials 2019, 9, 773. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Song, H.; Jo, J.; Kim, N.; Hwang, S.; Choi, Y.; Kim, H.G.; Kim, H.J.; Mishra, Y.K. Pyroelectric Based Energy Harvesting Devices: Hybrid Structures and Applications. Sustain. Energy Fuels 2023, 7, 5319–5335. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, K.Y.; Gupta, M.K.; Kim, T.Y.; Lee, D.-Y.; Oh, J.; Ryu, C.; Yoo, W.J.; Kang, C.-Y.; Yoon, S.-J.; et al. Highly Stretchable Piezoelectric-Pyroelectric Hybrid Nanogenerator. Adv. Mater. 2014, 26, 765–769. [Google Scholar] [CrossRef]
- Xue, H.; Yang, Q.; Wang, D.; Luo, W.; Wang, W.; Lin, M.; Liang, D.; Luo, Q. A Wearable Pyroelectric Nanogenerator and Self-Powered Breathing Sensor. Nano Energy 2017, 38, 147–154. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhang, Y.; Wang, Z.L. Pyroelectric Nanogenerators for Driving Wireless Sensors. Nano Lett. 2012, 12, 6408–6413. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Ryu, H.; Lee, J.-H.; Khan, U.; Kim, H.; Kwak, S.S.; Kim, S.-W. High-Performance Piezoelectric, Pyroelectric, and Triboelectric Nanogenerators Based on P(VDF-TrFE) with Controlled Crystallinity and Dipole Alignment. Adv. Funct. Mater. 2017, 27, 1700702. [Google Scholar] [CrossRef]
- Zhao, C.; Cui, X.; Wu, Y.; Li, Z. Recent Progress of Nanogenerators Acting as Self-Powered Drug Delivery Devices. Adv. Sustain. Syst. 2021, 5, 2000268. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Wang, M.D.; Shin, D.M.; Simons, J.W.; Nie, S. Nanotechnology for Targeted Cancer Therapy. Expert Rev. Anticancer Ther. 2007, 7, 833–837. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, J.K.; Milane, L.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Targeted Cancer Therapy; Nanotechnology Approaches for Overcoming Drug Resistance. Curr. Med. Chem. 2015, 22, 1335–1347. [Google Scholar] [CrossRef]
- Yao, S.; Wang, S.; Zheng, M.; Wang, Z.; Liu, Z.; Wang, Z.L.; Li, L. Implantable, Biodegradable, and Wireless Triboelectric Devices for Cancer Therapy through Disrupting Microtubule and Actins Dynamics. Adv. Mater. 2023, 35, e2303962. [Google Scholar] [CrossRef]
- Jian, G.; Zhu, S.; Yuan, X.; Fu, S.; Yang, N.; Yan, C.; Wang, X.; Wong, C.-P. Biodegradable Triboelectric Nanogenerator as a Implantable Power Source for Embedded Medicine Devices. NPG Asia Mater. 2024, 16, 12. [Google Scholar] [CrossRef]
- Li, X.; Tat, T.; Chen, J. Triboelectric Nanogenerators for Self-Powered Drug Delivery. Trends Chem. 2021, 3, 765–778. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Romero, M.R.; Blazquez, A.G.; Herraez, E.; Keck, E.; Briz, O. Importance and Limitations of Chemotherapy among the Available Treatments for Gastrointestinal Tumours. Anticancer Agents Med. Chem. 2009, 9, 162–184. [Google Scholar] [CrossRef]
- Biagiotti, S.; Canonico, B.; Tiboni, M.; Abbas, F.; Perla, E.; Montanari, M.; Battistelli, M.; Papa, S.; Casettari, L.; Rossi, L.; et al. Efficient and Highly Reproducible Production of Red Blood Cell-Derived Extracellular Vesicle Mimetics for the Loading and Delivery of RNA Molecules. Sci. Rep. 2024, 14, 14610. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA Drug Delivery Using Red Blood Cell Extracellular Vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, H.; Zhang, L.; Li, Z.; Zou, Y.; Tan, P.; Ouyang, H.; Jiang, D.; Yu, M.; Wang, C.; et al. Highly Efficient In Vivo Cancer Therapy by an Implantable Magnet Triboelectric Nanogenerator. Adv. Funct. Mater. 2019, 29, 1808640. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, P.; Chen, X.; Chen, P.; Shi, M.; Lang, J.; Chen, M. Overcoming Barriers in Photodynamic Therapy Harnessing Nanogenerators Strategies. Int. J. Biol. Sci. 2024, 20, 5673–5694. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, X.; Zheng, Y.; Zhang, Y.; Li, Z.; Zhu, S.; Jiang, H.; Cui, Z.; Wu, S. Wireless Powered Microwave-Light Conversion Platform with Dual-Stimulus Nanoresponder Coating for Deep-Seated Photodynamic Therapy. ACS Nano 2024, 18, 17086–17099. [Google Scholar] [CrossRef]
- Choi, J.; Lee, I.S.; Lee, J.S.; Jeon, S.; Yun, W.S.; Yang, S.; Moon, Y.; Kim, J.; Kim, J.; Choy, S.; et al. Implantable Micro-Scale LED Device Guided Photodynamic Therapy to Potentiate Antitumor Immunity with Mild Visible Light. Biomater. Res. 2022, 26, 56. [Google Scholar] [CrossRef]
- Kim, K.; Min, I.S.; Kim, T.H.; Kim, D.H.; Hwang, S.; Kang, K.; Kim, K.; Park, S.; Lee, J.; Cho, Y.U.; et al. Fully Implantable and Battery-Free Wireless Optoelectronic System for Modulable Cancer Therapy and Real-Time Monitoring. npj Flex. Electron. 2023, 7, 41. [Google Scholar] [CrossRef]
- Zou, P.; Lin, R.; Fang, Z.; Chen, J.; Guan, H.; Yin, J.; Chang, Z.; Xing, L.; Lang, J.; Xue, X.; et al. Implanted, Wireless, Self-Powered Photodynamic Therapeutic Tablet Synergizes with Ferroptosis Inducer for Effective Cancer Treatment. Adv. Sci. 2023, 10, e2302731. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, L.; Zheng, Q.; Kang, Y.; Shi, B.; Jiang, D.; Li, H.; Qu, X.; Fan, Y.; Wang, Z.L.; et al. Human Motion Driven Self-Powered Photodynamic System for Long-Term Autonomous Cancer Therapy. ACS Nano 2020, 14, 8074–8083. [Google Scholar] [CrossRef]
- Hadi, M.M.; Farrell, S.; Nesbitt, H.; Thomas, K.; Kubajewska, I.; Ng, A.; Masood, H.; Patel, S.; Sciscione, F.; Davidson, B.; et al. Nanotechnology-Augmented Sonodynamic Therapy and Associated Immune-Mediated Effects for the Treatment of Pancreatic Ductal Adenocarcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 5007–5023. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, J.; Huang, W.; Gong, P.; Shi, F.; Xu, X.; Fu, C.; Wang, X.; Wong, Y.K.; Long, Y.; et al. Antitumor Effects of a Distinct Sonodynamic Nanosystem through Enhanced Induction of Immunogenic Cell Death and Ferroptosis with Modulation of Tumor Microenvironment. JACS Au 2023, 3, 1507–1520. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, Z.; Xiang, X.; Wang, L.; Du, F.; Xiao, X.; Zhu, B.; Rong, X.; Qiu, L. Progress in Nanomedicine for Sonodynamic Immunotherapy of Tumors. EngMedicine 2024, 1, 100027. [Google Scholar] [CrossRef]
- Han, Q.; Fang, Z.; Lin, R.; Chen, J.; Wei, X.; Gong, C.; Yang, Z.; Zou, P.; Zhu, J.; Xing, L.; et al. Piezo-Photodynamic Therapy of Au@PEG-ZnO Nanostructures Enabled with a Battery-Free Wireless Cancer Therapeutic Dot. Nano Energy 2024, 125, 109530. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, T.; Zhang, X.; Cui, Y.; Zhang, L.; Li, L.; Wang, Z.L. Piezotronic and Piezo-Phototronic Effects on Sonodynamic Disease Therapy. BMEMat 2023, 1, e12006. [Google Scholar] [CrossRef]
- Jean, F.; Khan, M.U.; Alazzam, A.; Mohammad, B. Advancement in Piezoelectric Nanogenerators for Acoustic Energy Harvesting. Microsyst. Nanoeng. 2024, 10, 197. [Google Scholar] [CrossRef]
- Huang, H.; Miao, Y.; Li, Y. Recent Advances of Piezoelectric Materials Used in Sonodynamic Therapy of Tumor. Coord. Chem. Rev. 2025, 523, 216282. [Google Scholar] [CrossRef]
- Marino, A.; Almici, E.; Migliorin, S.; Tapeinos, C.; Battaglini, M.; Cappello, V.; Marchetti, M.; de Vito, G.; Cicchi, R.; Pavone, F.S.; et al. Piezoelectric Barium Titanate Nanostimulators for the Treatment of Glioblastoma Multiforme. J. Colloid Interface Sci. 2019, 538, 449–461. [Google Scholar] [CrossRef]
- Cafarelli, A.; Marino, A.; Vannozzi, L.; Puigmartí-Luis, J.; Pané, S.; Ciofani, G.; Ricotti, L. Piezoelectric Nanomaterials Activated by Ultrasound: The Pathway from Discovery to Future Clinical Adoption. ACS Nano 2021, 15, 11066–11086. [Google Scholar] [CrossRef]
- Guan, H.; Zou, P.; Lin, R.; Xiao, L.; Fang, Z.; Chen, J.; Lin, T.; Wang, Y.; Peng, Y.; Zhong, T.; et al. Implantable Self-Powered Therapeutic Pellet for Wireless Photodynamic/Sonodynamic Hybrid Therapy of Cancer Recurrence Inhibition and Tumor Regression. Nano Energy 2023, 105, 108002. [Google Scholar] [CrossRef]
- Conta, G.; Libanori, A.; Tat, T.; Chen, G.; Chen, J. Triboelectric Nanogenerators for Therapeutic Electrical Stimulation. Adv. Mater. 2021, 33, 2007502. [Google Scholar] [CrossRef]
- Janigro, D.; Perju, C.; Fazio, V.; Hallene, K.; Dini, G.; Agarwal, M.K.; Cucullo, L. Alternating Current Electrical Stimulation Enhanced Chemotherapy: A Novel Strategy to Bypass Multidrug Resistance in Tumor Cells. BMC Cancer 2006, 6, 72. [Google Scholar] [CrossRef]
- Cucullo, L.; Dini, G.; Hallene, K.L.; Fazio, V.; Ilkanich, E.V.; Igboechi, C.; Kight, K.M.; Agarwal, M.K.; Garrity-Moses, M.; Janigro, D. Very Low Intensity Alternating Current Decreases Cell Proliferation. Glia 2005, 51, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Marino, A.; Genchi, G.G.; Sinibaldi, E.; Ciofani, G. Piezoelectric Effects of Materials on Bio-Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 17663–17680. [Google Scholar] [CrossRef]
- Marino, A.; Battaglini, M.; De Pasquale, D.; Degl’Innocenti, A.; Ciofani, G. Ultrasound-Activated Piezoelectric Nanoparticles Inhibit Proliferation of Breast Cancer Cells. Sci. Rep. 2018, 8, 6257. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Loizzi, V.; Del Vecchio, V.; Gargano, G.; De Liso, M.; Kardashi, A.; Naglieri, E.; Resta, L.; Cicinelli, E.; Cormio, G. Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1967. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Hu, Y.; He, G.; Huang, L.; Liu, Y.; Wang, Z.L.; Jiang, P. Enhancing CAR-T Cell Therapy against Solid Tumor by Drug-Free Triboelectric Immunotherapy. Biomaterials 2025, 314, 122871. [Google Scholar] [CrossRef]
- Şen, Ö.; Marino, A.; Pucci, C.; Ciofani, G. Modulation of Anti-Angiogenic Activity Using Ultrasound-Activated Nutlin-Loaded Piezoelectric Nanovectors. Mater. Today Bio 2022, 13, 100196. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, W.; He, J.; Cheng, L.; Ren, P.-G.; Xu, Y. Enhancing Drug Utilization Efficiency via Dish-Structured Triboelectric Nanogenerator. Front. Bioeng. Biotechnol. 2022, 10, 950146. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Kong, X.; Ren, X.; Xuan, F. Drug-Device-Field Integration for Mitochondria-Targeting Dysfunction and Tumor Therapy by Home-Tailored Pyroelectric Nanocomposites. Biomaterials 2025, 316, 122990. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zou, P.; Lin, R.; Guan, H.; Fang, Z.; Chen, J.; Long, Z.; Zhang, Y.; Xing, L.; Qi, F.; et al. A Self-Powered Wireless Detachable Drug/Light Injector for Metronomic Photodynamic Therapy in Cancer Treatment. Nano Energy 2023, 116, 108826. [Google Scholar] [CrossRef]
- Truong Hoang, Q.; Kim, D.Y.; Park, H.S.; Jang, W.; Nguyen Cao, T.G.; Kang, J.H.; Ko, Y.T.; Mun, S.J.; Bhang, S.H.; Shim, M.S.; et al. Oxygen-Supplying Piezocatalytic Therapy of Hypoxic Tumors by Intratumoral Delivery of pH-Responsive Multicompartmental Carriers with Sequential Drug Release Capability. Adv. Funct. Mater. 2024, 34, 2306078. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, Y.; Yao, S.; Wang, S.; Wan, X.; Hu, Q.; Li, L. Enhanced Sonodynamic Cancer Therapy through Iron-Doping and Oxygen-Vacancy Engineering of Piezoelectric Bismuth Tungstate Nanosheets. Small 2023, 19, 2300327. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Chen, J.; Wu, Y.; Zhang, J.; Wang, J. Nanosecond Pulsed Electric Field Inhibits Malignant Melanoma Growth by Inducing the Change of Systemic Immunity. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e555–e561. [Google Scholar] [CrossRef]
- Ge, M.; Xu, D.; Chen, Z.; Wei, C.; Zhang, Y.; Yang, C.; Chen, Y.; Lin, H.; Shi, J. Magnetostrictive-Piezoelectric-Triggered Nanocatalytic Tumor Therapy. Nano Lett. 2021, 21, 6764–6772. [Google Scholar] [CrossRef]
- Fan, Y.; Ye, J.; Kang, Y.; Niu, G.; Shi, J.; Yuan, X.; Li, R.; Han, J.; Ji, X. Biomimetic Piezoelectric Nanomaterial-Modified Oral Microrobots for Targeted Catalytic and Immunotherapy of Colorectal Cancer. Sci. Adv. 2024, 10, eadm9561. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering Nanoparticles to Locally Activate T Cells in the Tumor Microenvironment. Sci. Immunol. 2019, 4, eaau6584. [Google Scholar] [CrossRef]
- Su, C.; Lin, J.; Li, C.; Wang, X.; Pan, D.; Wang, L.; Xu, Y.; Chen, C.; Ji, K.; Wang, J.; et al. Tumor-Specific Liquid Metal Nitric Oxide Nanogenerator for Enhanced Breast Cancer Therapy. Asian J. Pharm. Sci. 2025, 101018. [Google Scholar] [CrossRef]
- Fu, L.-H.; Li, C.; Yin, W.; Hu, Y.-R.; Sun, T.; Wan, Y.; Lin, J.; Li, Z.; Huang, P. A Versatile Calcium Phosphate Nanogenerator for Tumor Microenvironment-Activated Cancer Synergistic Therapy. Adv. Healthc. Mater. 2021, 10, e2101563. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Liu, X.; Hu, X.; Li, Y.; Sun, J.; Wang, P.; Wu, H.; Kim, H.; Ramalingam, M.; et al. Ultrasound and Laser-Promoted Dual-Gas Nano-Generator for Combined Photothermal and Immune Tumor Therapy. Front. Bioeng. Biotechnol. 2022, 10, 1005520. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tong, G.; Song, Q.; Zhu, C.; Zhang, H.; Shi, J.; Zhang, Z. Enhanced Intracellular Ca2+ Nanogenerator for Tumor-Specific Synergistic Therapy via Disruption of Mitochondrial Ca2+ Homeostasis and Photothermal Therapy. ACS Nano 2018, 12, 6806–6818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).