Spray-Coated Transition Metal Dichalcogenides as Hole Transport Layers in Inverted NFA-Based Organic Photovoltaics with Enhanced Stability under Solar and Artificial Light
Abstract
1. Introduction
2. Materials and Methods
3. Results
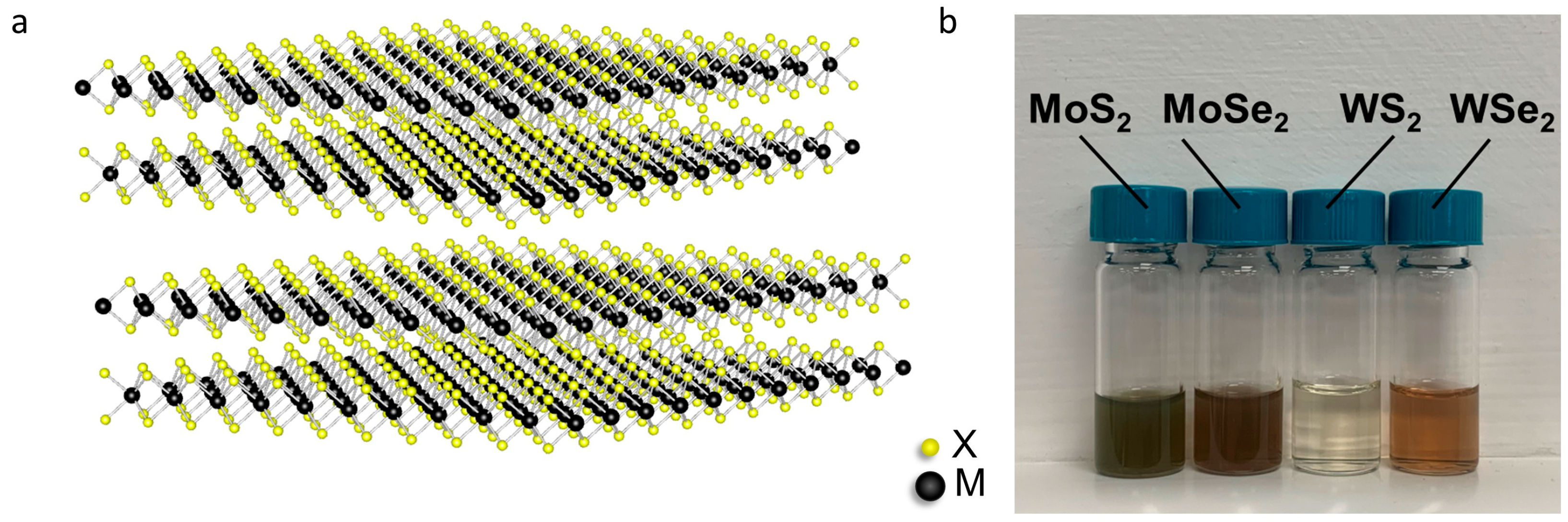
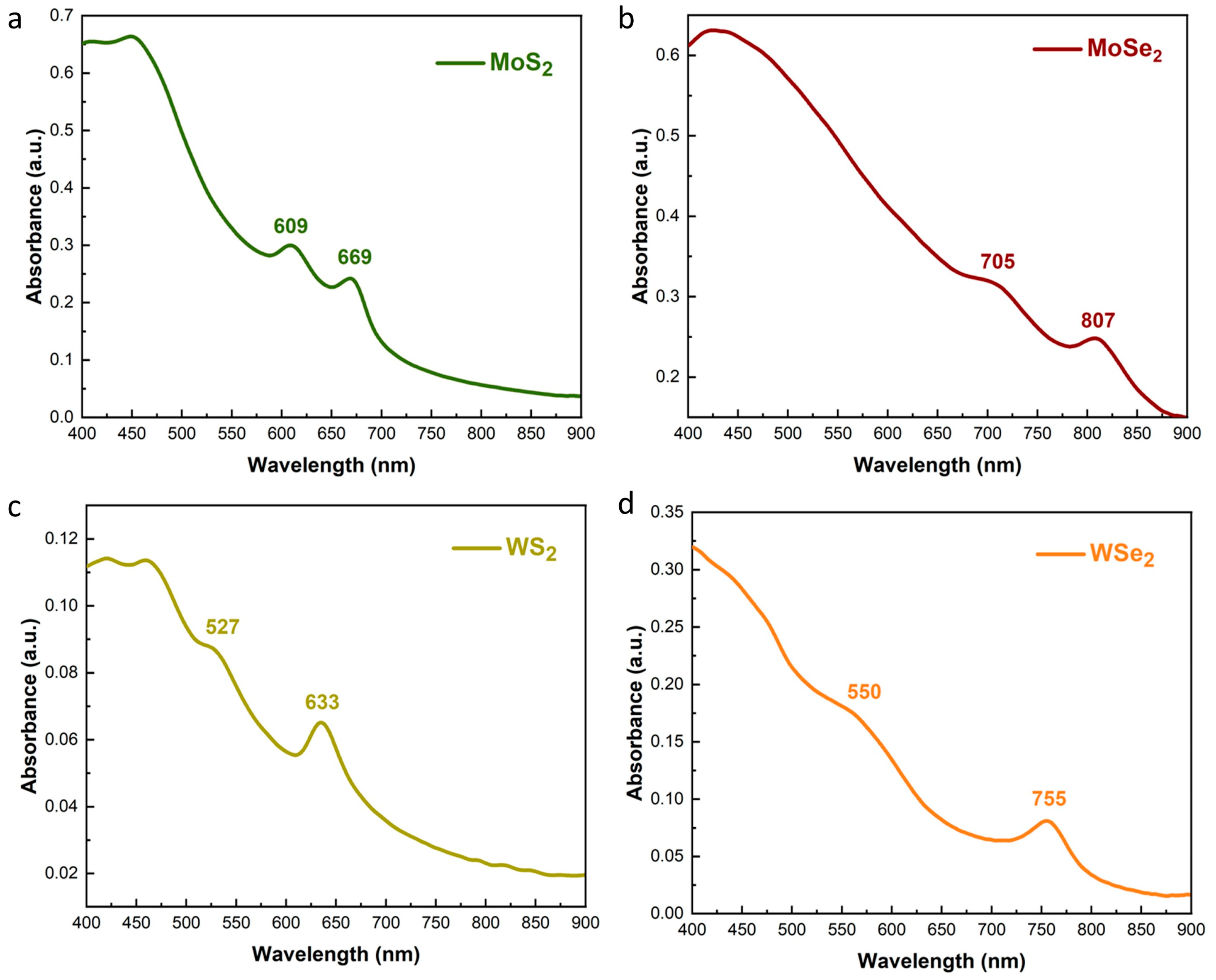
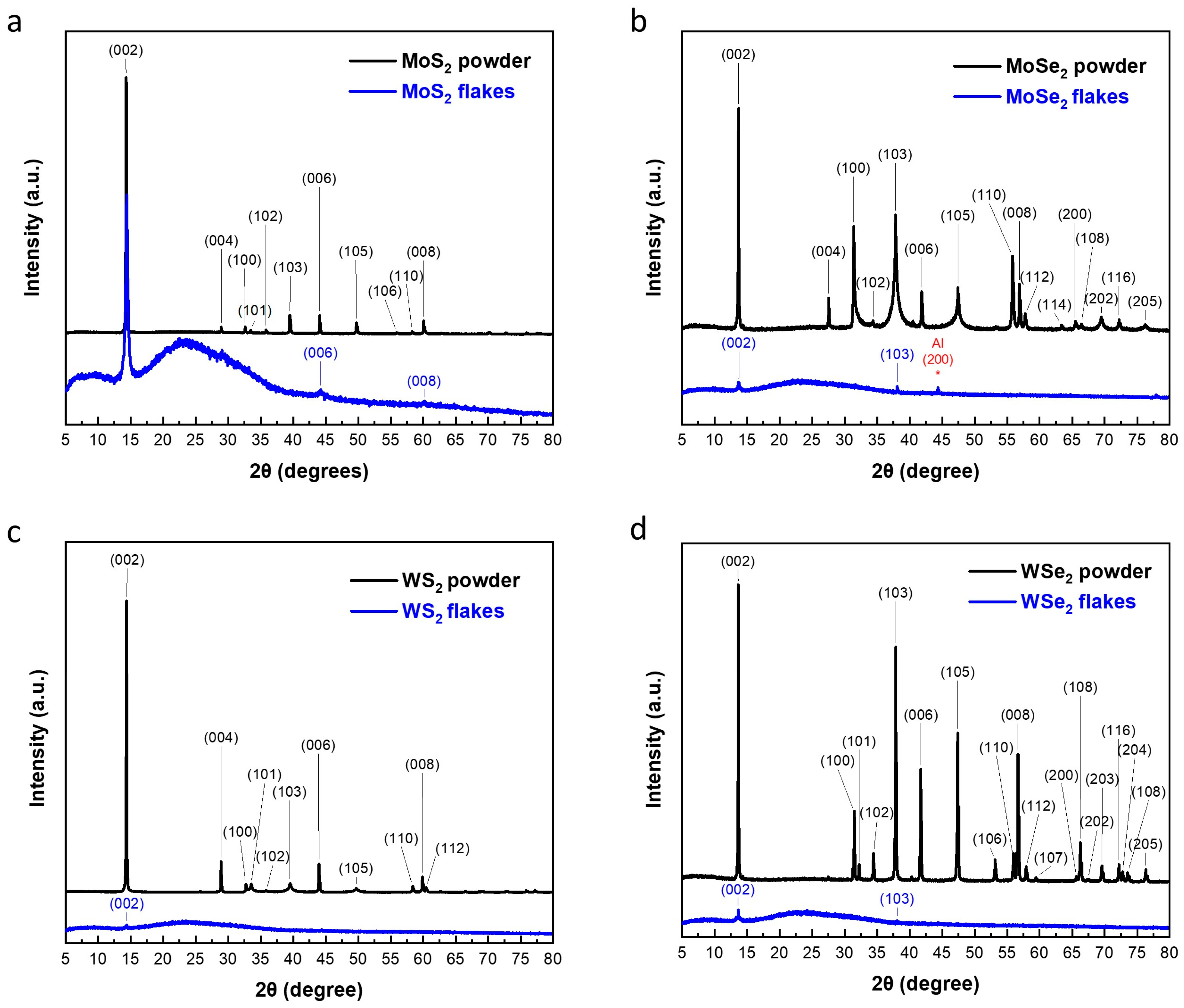
3.1. Characterization of Exfoliated TMDs
3.2. Device Fabrication and Characterization
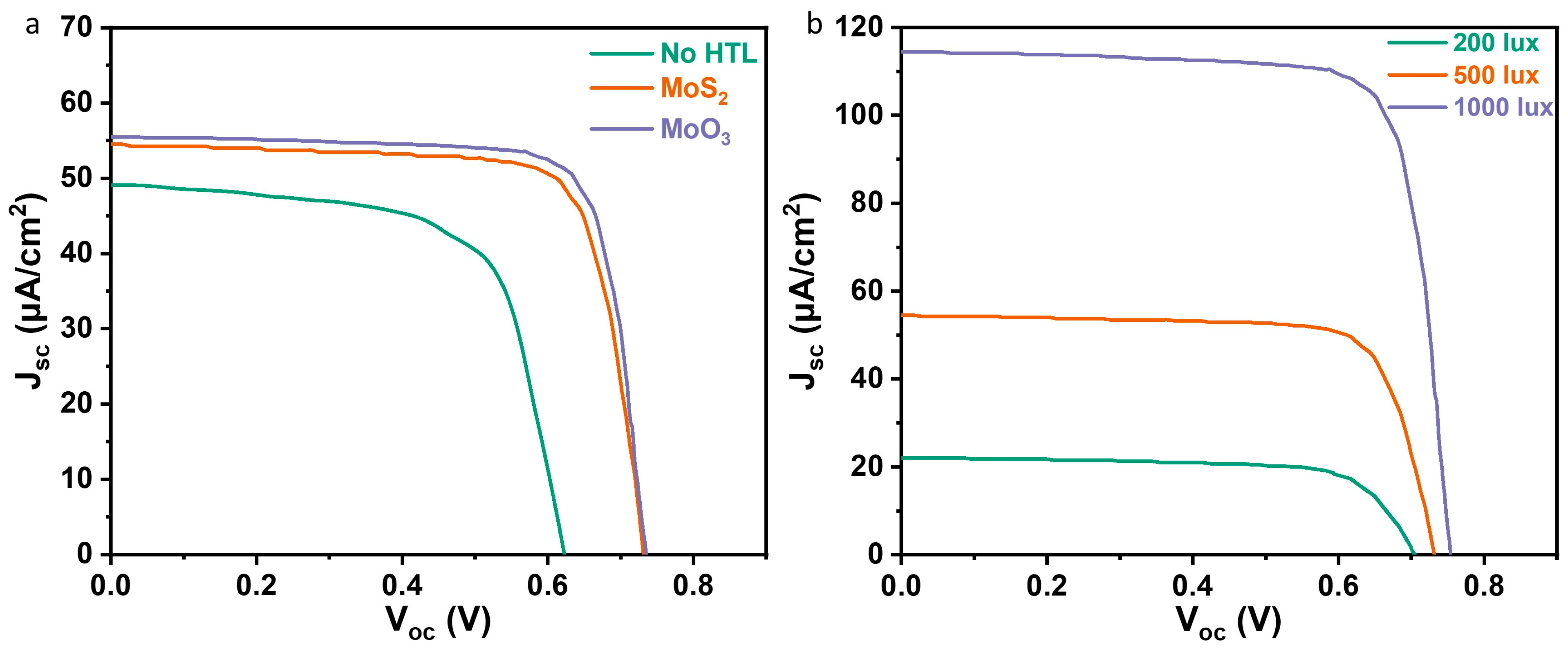
3.3. Indoor Devices Fabrication and Characterization
3.4. Device Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chamberlain, G.A. Organic Solar Cells: A Review. Sol. Cells 1983, 8, 47–83. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 3 December 2023).
- Chander, N.; Singh, S.; Iyer, S.S.K. Stability and Reliability of P3HT:PC61BM Inverted Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2017, 161, 407–415. [Google Scholar] [CrossRef]
- Solak, E.K.; Irmak, E. Advances in Organic Photovoltaic Cells: A Comprehensive Review of Materials, Technologies, and Performance. RSC Adv. 2023, 13, 12244–12269. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, Y.; Le Corre, V.M.; Khan, J.I.; Kan, Z.; Laquai, F.; Beaujuge, P.M.; Anthopoulos, T.D. Key Parameters Requirements for Non-Fullerene-Based Organic Solar Cells with Power Conversion Efficiency >20%. Adv. Sci. 2019, 6, 1802028. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, Q.; Zhang, S.; Zhang, D.; Wang, J.; Xie, S.; Wang, R.; Qin, Y.; Li, W.; Hong, L.; et al. A Highly Efficient Non-Fullerene Organic Solar Cell with a Fill Factor over 0.80 Enabled by a Fine-Tuned Hole-Transporting Layer. Adv. Mater. 2018, 30, 1801801. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Mai, C.-K.; Collins, S.D.; Nguyen, T.-Q.; Bazan, G.C.; Heeger, A.J. Conductive Conjugated Polyelectrolyte as Hole-Transporting Layer for Organic Bulk Heterojunction Solar Cells. Adv. Mater. 2014, 26, 780–785. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced Power-Conversion Efficiency in Polymer Solar Cells Using an Inverted Device Structure. Nat. Photon 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Hu, Z.; Xiao, J.; Zhao, W.; Li, H.-W.; Li, Q.-Y.; Tsang, S.-W.; Xu, Y.-X.; Zhang, K.; et al. Interface Design for High-Efficiency Non-Fullerene Polymer Solar Cells. Energy Environ. Sci. 2017, 10, 1784–1791. [Google Scholar] [CrossRef]
- Sun, Y.; Takacs, C.J.; Cowan, S.R.; Seo, J.H.; Gong, X.; Roy, A.; Heeger, A.J. Efficient, Air-Stable Bulk Heterojunction Polymer Solar Cells Using MoOx as the Anode Interfacial Layer. Adv. Mater. 2011, 23, 2226–2230. [Google Scholar] [CrossRef]
- de Jong, M.P.; van IJzendoorn, L.J.; de Voigt, M.J.A. Stability of the Interface between Indium-Tin-Oxide and Poly(3,4-Ethylenedioxythiophene)/Poly(Styrenesulfonate) in Polymer Light-Emitting Diodes. Appl. Phys. Lett. 2000, 77, 2255–2257. [Google Scholar] [CrossRef]
- Wijeyasinghe, N.; Regoutz, A.; Eisner, F.; Du, T.; Tsetseris, L.; Lin, Y.-H.; Faber, H.; Pattanasattayavong, P.; Li, J.; Yan, F.; et al. Copper(I) Thiocyanate (CuSCN) Hole-Transport Layers Processed from Aqueous Precursor Solutions and Their Application in Thin-Film Transistors and Highly Efficient Organic and Organometal Halide Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1701818. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Sun, X.W.; Jiang, C.Y.; Lo, G.Q.; Zhao, D.W.; Kwong, D.L. An Inverted Organic Solar Cell Employing a Sol-Gel Derived ZnO Electron Selective Layer and Thermal Evaporated MoO3 Hole Selective Layer. Appl. Phys. Lett. 2008, 93, 221107. [Google Scholar] [CrossRef]
- Manders, J.R.; Tsang, S.-W.; Hartel, M.J.; Lai, T.-H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-Processed Nickel Oxide Hole Transport Layers in High Efficiency Polymer Photovoltaic Cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal Oxides for Optoelectronic Applications. Nat. Mater 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Cutting, C.L.; Bag, M.; Venkataraman, D. Indoor Light Recycling: A New Home for Organic Photovoltaics. J. Mater. Chem. C 2016, 4, 10367–10370. [Google Scholar] [CrossRef]
- Arai, R.; Furukawa, S.; Hidaka, Y.; Komiyama, H.; Yasuda, T. High-Performance Organic Energy-Harvesting Devices and Modules for Self-Sustainable Power Generation under Ambient Indoor Lighting Environments. ACS Appl. Mater. Interfaces 2019, 11, 9259–9264. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, J.-H.; Lee, H.H.; Nam, M.; Ko, D.-H. Over 30% Efficient Indoor Organic Photovoltaics Enabled by Morphological Modification Using Two Compatible Non-Fullerene Acceptors. Adv. Energy Mater. 2022, 12, 2200275. [Google Scholar] [CrossRef]
- Bi, P.; An, C.; Zhang, T.; Chen, Z.; Xu, Y.; Cui, Y.; Wang, J.; Li, J.; Wang, Y.; Ren, J.; et al. Achieving 31% Efficiency in Organic Photovoltaic Cells under Indoor Light Using a Low Energetic Disorder Polymer Donor. J. Mater. Chem. A 2023, 11, 983–991. [Google Scholar] [CrossRef]
- Polyzoidis, C.; Rogdakis, K.; Kymakis, E. Indoor Perovskite Photovoltaics for the Internet of Things—Challenges and Opportunities toward Market Uptake. Adv. Energy Mater. 2021, 11, 2101854. [Google Scholar] [CrossRef]
- Chaves, A.; Azadani, J.G.; Alsalman, H.; da Costa, D.R.; Frisenda, R.; Chaves, A.J.; Song, S.H.; Kim, Y.D.; He, D.; Zhou, J.; et al. Bandgap Engineering of Two-Dimensional Semiconductor Materials. Npj 2D Mater. Appl. 2020, 4, 29. [Google Scholar] [CrossRef]
- Tongay, S.; Zhou, J.; Ataca, C.; Lo, K.; Matthews, T.S.; Li, J.; Grossman, J.C.; Wu, J. Thermally Driven Crossover from Indirect toward Direct Bandgap in 2D Semiconductors: MoSe2 versus MoS2. Nano Lett. 2012, 12, 5576–5580. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chernikov, A.; Glazov, M.M.; Heinz, T.F.; Marie, X.; Amand, T.; Urbaszek, B. Colloquium: Excitons in Atomically Thin Transition Metal Dichalcogenides. Rev. Mod. Phys. 2018, 90, 021001. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and Optoelectronics of 2D Semiconductor Transition Metal Dichalcogenides. Nat. Photon 2016, 10, 216–226. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Pospischil, A.; Mueller, T. Optoelectronic Devices Based on Atomically Thin Transition Metal Dichalcogenides. Appl. Sci. 2016, 6, 78. [Google Scholar] [CrossRef]
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M.; et al. 17% Efficient Organic Solar Cells Based on Liquid Exfoliated WS2 as a Replacement for PEDOT:PSS. Adv. Mater. 2019, 31, 1902965. [Google Scholar] [CrossRef] [PubMed]
- Barrera, D.; Jawaid, A.; Daunis, T.B.; Cheng, L.; Wang, Q.; Lee, Y.-J.; Kim, M.J.; Kim, J.; Vaia, R.A.; Hsu, J.W.P. Inverted OPVs with MoS2 Hole Transport Layer Deposited by Spray Coating. Mater. Today Energy 2017, 5, 107–111. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; Ahn, Y.N.; Hur, J. Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries. Nanomaterials 2021, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Frindt, R.F. Single Crystals of MoS2 Several Molecular Layers Thick. J. Appl. Phys. 2004, 37, 1928–1929. [Google Scholar] [CrossRef]
- Dines, M.B. Lithium Intercalation via N-Butyllithium of the Layered Transition Metal Dichalcogenides. Mater. Res. Bull. 1975, 10, 287–291. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Mei, L.; Shin, H.S.; Voiry, D.; Lu, Q.; Li, J.; Zeng, Z. Synthesis of Atomically Thin Sheets by the Intercalation-Based Exfoliation of Layered Materials. Nat. Synth. 2023, 2, 101–118. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Samorì, P. Graphene via Sonication Assisted Liquid-Phase Exfoliation. Chem. Soc. Rev. 2013, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Bartolotta, A.; Coleman, J.N.; Backes, C. 2D-Crystal-Based Functional Inks. Adv. Mater. 2016, 28, 6136–6166. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T.; Geisler, R.; Schanz, D.; Lindau, O. Acoustic Cavitation, Bubble Dynamics and Sonoluminescence. Ultrason. Sonochem. 2007, 14, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.J.; Lotya, M.; Cunningham, G.; Smith, R.J.; McCloskey, D.; Donegan, J.F.; Coleman, J.N. Inkjet Deposition of Liquid-Exfoliated Graphene and MoS2 Nanosheets for Printed Device Applications. J. Mater. Chem. C 2014, 2, 925–932. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lakshmi, K.V.; Huang, L. Eco-Friendly Synthesis of Metal Dichalcogenides Nanosheets and Their Environmental Remediation Potential Driven by Visible Light. Sci. Rep. 2015, 5, 15718. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Feng, Y.; Zhang, S.; Zhang, X.; Chang, C.; Fan, J.; Zhang, L.; Wang, J. Optical Limiting and Theoretical Modelling of Layered Transition Metal Dichalcogenide Nanosheets. Sci. Rep. 2015, 5, 14646. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Muscuso, L.; Cravanzola, S.; Cesano, F.; Scarano, D.; Zecchina, A. Optical, Vibrational, and Structural Properties of MoS2 Nanoparticles Obtained by Exfoliation and Fragmentation via Ultrasound Cavitation in Isopropyl Alcohol. J. Phys. Chem. C 2015, 119, 3791–3801. [Google Scholar] [CrossRef]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.-H.; Eda, G. Evolution of Electronic Structure in Atomically Thin Sheets of WS2 and WSe2. ACS Nano 2013, 7, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, Y.; Chang, C.; Zhan, J.; Wang, C.; Zhao, Q.; Coleman, J.N.; Zhang, L.; Blau, W.J.; Wang, J. Broadband Ultrafast Nonlinear Absorption and Nonlinear Refraction of Layered Molybdenum Dichalcogenide Semiconductors. Nanoscale 2014, 6, 10530–10535. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of Disorder on Raman Scattering of Single-Layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef]
- Korn, T.; Heydrich, S.; Hirmer, M.; Schmutzler, J.; Schüller, C. Low-Temperature Photocarrier Dynamics in Monolayer MoS2. Appl. Phys. Lett. 2011, 99, 102109. [Google Scholar] [CrossRef]
- Niu, Y.; Gonzalez-Abad, S.; Frisenda, R.; Marauhn, P.; Drüppel, M.; Gant, P.; Schmidt, R.; Taghavi, N.S.; Barcons, D.; Molina-Mendoza, A.J.; et al. Thickness-Dependent Differential Reflectance Spectra of Monolayer and Few-Layer MoS2, MoSe2, WS2 and WSe2. Nanomaterials 2018, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescence Emission and Raman Response of Monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908–4916. [Google Scholar] [CrossRef]
- Nam, D.; Lee, J.-U.; Cheong, H. Excitation Energy Dependent Raman Spectrum of MoSe2. Sci Rep 2015, 5, 17113. [Google Scholar] [CrossRef]
- Berkdemir, A.; Gutiérrez, H.R.; Botello-Méndez, A.R.; Perea-López, N.; Elías, A.L.; Chia, C.-I.; Wang, B.; Crespi, V.H.; López-Urías, F.; Charlier, J.-C.; et al. Identification of Individual and Few Layers of WS2 Using Raman Spectroscopy. Sci. Rep. 2013, 3, 1755. [Google Scholar] [CrossRef]
- McCreary, A.; Berkdemir, A.; Wang, J.; Nguyen, M.A.; Elías, A.L.; Perea-López, N.; Fujisawa, K.; Kabius, B.; Carozo, V.; Cullen, D.A.; et al. Distinct Photoluminescence and Raman Spectroscopy Signatures for Identifying Highly Crystalline WS2 Monolayers Produced by Different Growth Methods. J. Mater. Res. 2016, 31, 931–944. [Google Scholar] [CrossRef]
- Sahin, H.; Tongay, S.; Horzum, S.; Fan, W.; Zhou, J.; Li, J.; Wu, J.; Peeters, F.M. Anomalous Raman Spectra and Thickness-Dependent Electronic Properties of WSe2. Phys. Rev. B 2013, 87, 165409. [Google Scholar] [CrossRef]
- Harrington, G.F.; Santiso, J. Back-to-Basics Tutorial: X-Ray Diffraction of Thin Films. J. Electroceram. 2021, 47, 141–163. [Google Scholar] [CrossRef]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental Methods in Chemical Engineering: X-Ray Diffraction Spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.H.; Jang, H.W.; Kim, S.Y. Size-Dependent Properties of Two-Dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Wang, Y.; Sun, A. Preparation and Tribological Properties of MoS2 Nanosheets. Adv. Eng. Mater. 2010, 12, 534–538. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor Electrodes Based on Layered Tungsten Disulfide-Reduced Graphene Oxide Hybrids Synthesized by a Facile Hydrothermal Method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Luo, X.; Huang, J.; Li, J.; Cao, L.; Wang, Y.; Xu, Z.; Guo, L.; Cheng, Y.; Kajiyoshi, K.; Chen, S. Controlled WS2 Crystallinity Effectively Dominating Sodium Storage Performance. J. Energy Chem. 2020, 51, 143–153. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, V.; Upadhyay, S.; Koratkar, N.; Singh, F.; Kumar, S.; Mahajan, A.; Chandra, R.; Kumar, R. Defects Engineering and Enhancement in Optical and Structural Properties of 2D-MoS2 Thin Films by High Energy Ion Beam Irradiation. Mater. Chem. Phys. 2022, 276, 125422. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Veerasubramani, G.K.; Kim, S.J. Mechanically Delaminated Few Layered MoS2 Nanosheets Based High Performance Wire Type Solid-State Symmetric Supercapacitors. J. Power Sources 2016, 321, 112–119. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Chen, D. Sheet-like MoSe2/C Composites with Enhanced Li-Ion Storage Properties. J. Mater. Chem. A 2015, 3, 11857–11862. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xu, H.; Tan, H.; Ye, X. Preparation and Tribological Properties of WS2 Hexagonal Nanoplates and Nanoflowers. Nanomaterials 2019, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Zheng, B.; Qi, F.; He, J.; Li, Q.; Li, P.; Zhang, W. Graphene-like WSe2 Nanosheets for Efficient and Stable Hydrogen Evolution. J. Alloys Compd. 2017, 691, 698–704. [Google Scholar] [CrossRef]
- Rahul; Arora, S.K. Quantitative Evaluation of Nonlinear Temperature Dependence of Raman Shift in Exfoliated WSe2 Nanosheets. J. Electron. Mater. 2021, 50, 7126–7132. [Google Scholar] [CrossRef]
- Ni, P.; Dieng, M.; Vanel, J.-C.; Florea, I.; Bouanis, F.Z.; Yassar, A. Liquid Shear Exfoliation of MoS2: Preparation, Characterization, and NO2-Sensing Properties. Nanomaterials 2023, 13, 2502. [Google Scholar] [CrossRef]
- Chen, I.-W.P.; Lai, Y.-M.; Liao, W.-S. One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production. Nanomaterials 2021, 11, 2436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, M.; Yang, X.; Luo, G.; Yang, F. Hydrothermal Synthesis and Tribological Properties of MoSe2 Nanoflowers. Micro Nano Lett. 2015, 10, 339–342. [Google Scholar] [CrossRef]
- Ayieko, C.O.; Musembi, R.J.; Ogacho, A.A.; Aduda, B.O.; Muthoka, B.M.; Jain, P.K. Controlled Texturing of Aluminum Sheet for Solar Energy Applications. Adv. Mater. Phys. Chem. 2015, 5, 458–466. [Google Scholar] [CrossRef]
- Mikhalitsyna, E.A.; Kataev, V.A.; Larrañaga, A.; Lepalovskij, V.N.; Kurlyandskaya, G.V. Nanocrystallization in FINEMET-Type Fe73.5Nb3Cu1Si13.5B9 and Fe72.5Nb1.5Mo2Cu1.1Si14.2B8.7 Thin Films. Materials 2020, 13, 348. [Google Scholar] [CrossRef]
- Amjad, R.J.; Sahar, M.R.; Ghoshal, S.K.; Dousti, M.R.; Riaz, S.; Samavati, A.R.; Jamaludin, M.N.A.; Naseem, S. Plasmon-Enhanced Upconversion Fluorescence in Er3+:Ag Phosphate Glass: The Effect of Heat Treatment. Chin. Phys. Lett. 2013, 30, 027301. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Qin, J.; Wang, W.; Li, W.; He, D. Nanocrystalline MoS2 through Directional Growth along the (002) Crystal Plane under High Pressure. Mater. Chem. Phys. 2011, 130, 170–174. [Google Scholar] [CrossRef]
- Zsigmondy, R.; Scherrer, P. Kolloidchemie: Ein Lehrbuch—Primary Source Edition; NABU Press: Berlin, Germany, 2014; ISBN 978-1-294-87694-6. [Google Scholar]
- Muniz, F.T.L.; Miranda, M.A.R.; Morilla dos Santos, C.; Sasaki, J.M. The Scherrer Equation and the Dynamical Theory of X-ray Diffraction. Acta Cryst. A 2016, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, L.; Sha, J.; Chen, Y. Size-Dependent Piezoelectricity of Molybdenum Disulfide (MoS2) Films Obtained by Atomic Layer Deposition (ALD). Appl. Phys. Lett. 2017, 111, 063902. [Google Scholar] [CrossRef]
- Saisopa, T.; Jitapunkul, K.; Bunpheng, A.; Nakajima, H.; Supruangnet, R.; Busayaporn, W.; Sukprom, T.; Hirunpinyopas, W.; Seubsai, A.; Songsiriritthigul, P.; et al. The Structure Analysis and Chemical Properties Probing during Recycling Processes of Transition Metal Dichalcogenides Exfoliation. Electrochim. Acta 2023, 449, 142171. [Google Scholar] [CrossRef]
- Chowdhury, T.; Kim, J.; Sadler, E.C.; Li, C.; Lee, S.W.; Jo, K.; Xu, W.; Gracias, D.H.; Drichko, N.V.; Jariwala, D.; et al. Substrate-Directed Synthesis of MoS2 Nanocrystals with Tunable Dimensionality and Optical Properties. Nat. Nanotechnol. 2020, 15, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent Strategies for Improving the Catalytic Activity of 2D TMD Nanosheets Toward the Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef]
- Kadiev, K.M.; Maximov, A.L.; Kadieva, M.K. The Effect of MoS2 Active Site Dispersion on Suppression of Polycondensation Reactions during Heavy Oil Hydroconversion. Catalysts 2021, 11, 676. [Google Scholar] [CrossRef]
- Mukherjee, S.; Biswas, S.; Ghorai, A.; Midya, A.; Das, S.; Ray, S.K. Tunable Optical and Electrical Transport Properties of Size- and Temperature-Controlled Polymorph MoS2 Nanocrystals. J. Phys. Chem. C 2018, 122, 12502–12511. [Google Scholar] [CrossRef]
- Mukherjee, S.; Maiti, R.; Midya, A.; Das, S.; Ray, S.K. Tunable Direct Bandgap Optical Transitions in MoS2 Nanocrystals for Photonic Devices. ACS Photonics 2015, 2, 760–768. [Google Scholar] [CrossRef]
- Fang, L.J.; Chen, J.H.; Wang, J.M.; Lin, W.W.; Lin, X.G.; Lin, Q.J.; He, Y. Hydrophobic Two-Dimensional MoS2 Nanosheets Embedded in a Polyether Copolymer Block Amide (PEBA) Membrane for Recovering Pyridine from a Dilute Solution. ACS Omega 2021, 6, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.C.; Kim, C.; Le, Q.V.; Gim, S.; Jeon, J.-M.; Ham, J.Y.; Lee, J.-L.; Jang, H.W.; Kim, S.Y. Synthesis of Atomically Thin Transition Metal Disulfides for Charge Transport Layers in Optoelectronic Devices. ACS Nano 2015, 9, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
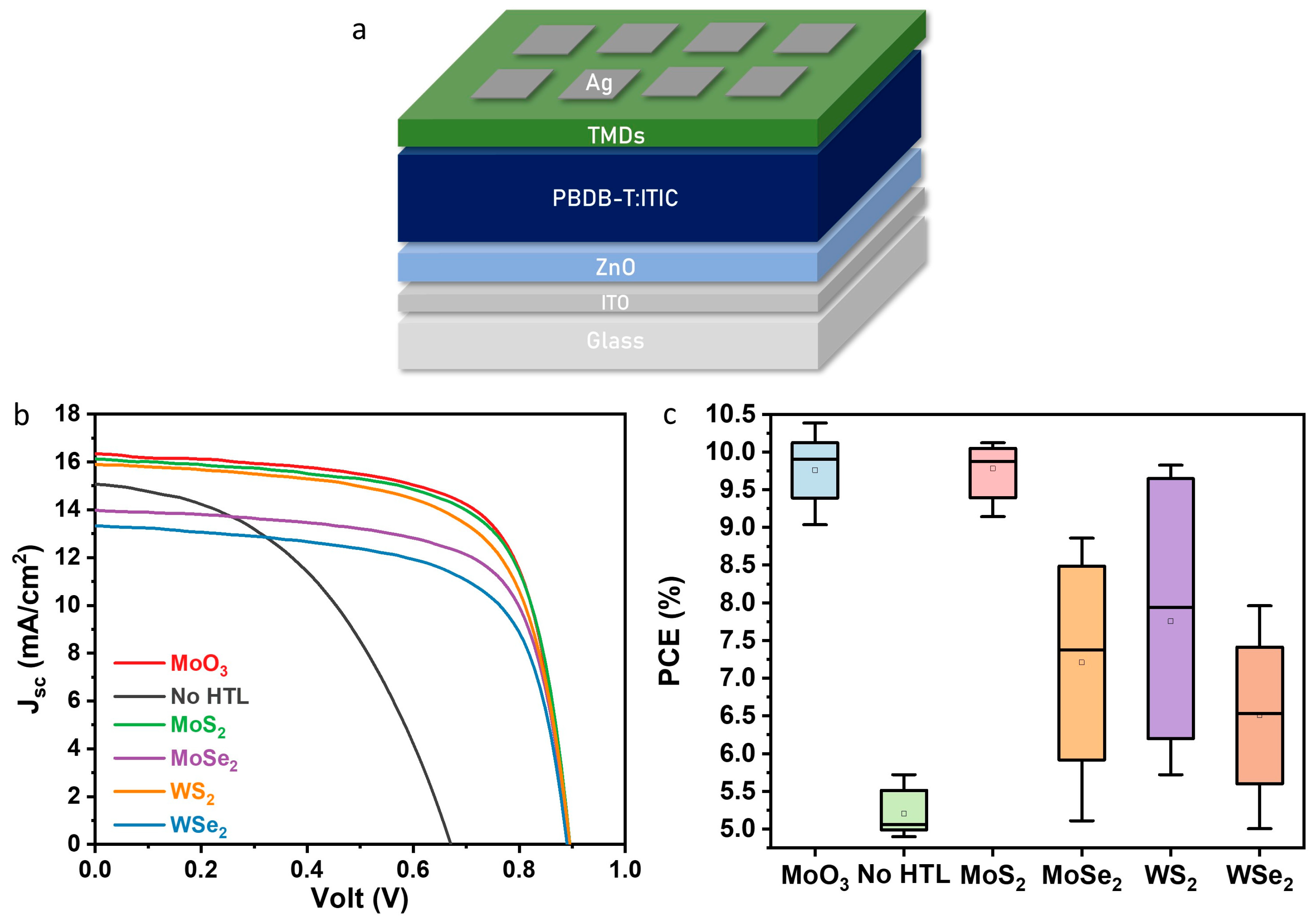

| HTL | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| No HTL | 0.73 ± 0.02 (0.74) | 14.35 ± 0.31 (14.63) | 48.87 ± 0.32 (49.15) | 5.2 ± 0.14 (5.34) |
| MoO3 | 0.88 ± 0.01 (0.89) | 15.71 ± 0.1 (16.36) | 69.27 ± 0.52 (71.13) | 9.68 ± 0.12 (10.38) |
| MoS2 | 0.89 ± 0.01 (0.90) | 15.88 ± 0.09 (16.07) | 69.08 ± 0.45 (70.31) | 9.78 ± 0.10 (10.12) |
| MoSe2 | 0.77 ± 0.05 (0.89) | 14.25 ± 0.44 (13.94) | 62.73 ± 3.34 (71.04) | 7.21 ± 0.41 (8.86) |
| WS2 | 0.86 ± 0.02 (0.89) | 14.84 ± 0.55 (16.06) | 60.42 ± 3.43 (68.46) | 7.76 ± 0.64 (9.82) |
| WSe2 | 0.8 ± 0.05 (0.89) | 13.81 ± 0.56 (13.29) | 59.03 ± 4.09 (67.28) | 6.51 ± 0.62 (7.96) |
| HTL | Light Source (lux) | Pin (μW/cm2) | Voc (V) | Jsc (μA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| No HTL | 200 | 61 | 0.58 | 20.04 | 44.51 | 8.55 |
| 500 | 151 | 0.61 | 49.59 | 48.55 | 9.68 | |
| 1000 | 303 | 0.62 | 104.49 | 50.31 | 10.83 | |
| MoO3 | 200 | 61 | 0.71 | 22.41 | 68.90 | 17.97 |
| 500 | 151 | 0.73 | 55.45 | 74.74 | 20.02 | |
| 1000 | 303 | 0.75 | 116.84 | 77.28 | 22.34 | |
| 200 | 61 | 0.70 | 22.01 | 67.99 | 17.29 | |
| MoS2 | 500 | 151 | 0.72 | 54.47 | 73.77 | 19.16 |
| 1000 | 303 | 0.75 | 114.79 | 78.09 | 22.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tountas, M.; Anagnostou, K.; Sotiropoulos, E.; Polyzoidis, C.; Kymakis, E. Spray-Coated Transition Metal Dichalcogenides as Hole Transport Layers in Inverted NFA-Based Organic Photovoltaics with Enhanced Stability under Solar and Artificial Light. Nanoenergy Adv. 2024, 4, 221-234. https://doi.org/10.3390/nanoenergyadv4030014
Tountas M, Anagnostou K, Sotiropoulos E, Polyzoidis C, Kymakis E. Spray-Coated Transition Metal Dichalcogenides as Hole Transport Layers in Inverted NFA-Based Organic Photovoltaics with Enhanced Stability under Solar and Artificial Light. Nanoenergy Advances. 2024; 4(3):221-234. https://doi.org/10.3390/nanoenergyadv4030014
Chicago/Turabian StyleTountas, Marinos, Katerina Anagnostou, Evangelos Sotiropoulos, Christos Polyzoidis, and Emmanuel Kymakis. 2024. "Spray-Coated Transition Metal Dichalcogenides as Hole Transport Layers in Inverted NFA-Based Organic Photovoltaics with Enhanced Stability under Solar and Artificial Light" Nanoenergy Advances 4, no. 3: 221-234. https://doi.org/10.3390/nanoenergyadv4030014
APA StyleTountas, M., Anagnostou, K., Sotiropoulos, E., Polyzoidis, C., & Kymakis, E. (2024). Spray-Coated Transition Metal Dichalcogenides as Hole Transport Layers in Inverted NFA-Based Organic Photovoltaics with Enhanced Stability under Solar and Artificial Light. Nanoenergy Advances, 4(3), 221-234. https://doi.org/10.3390/nanoenergyadv4030014








