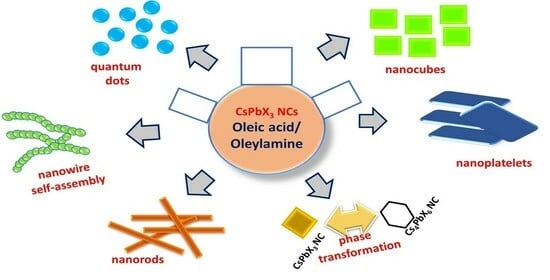
Influence of Binary Ligands in Designing Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals-Oleic Acid and Oleylamine
Abstract
:1. Introduction
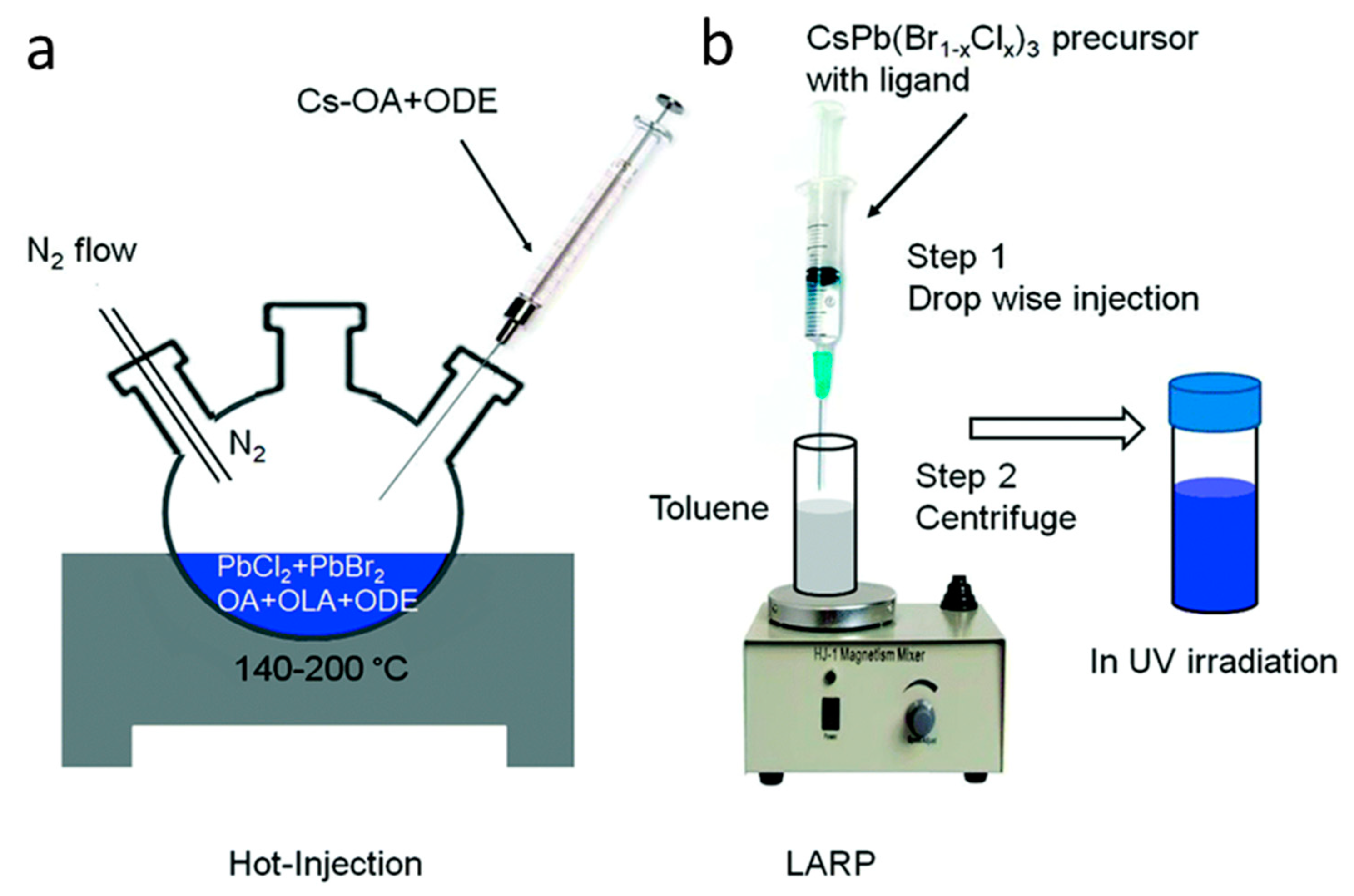
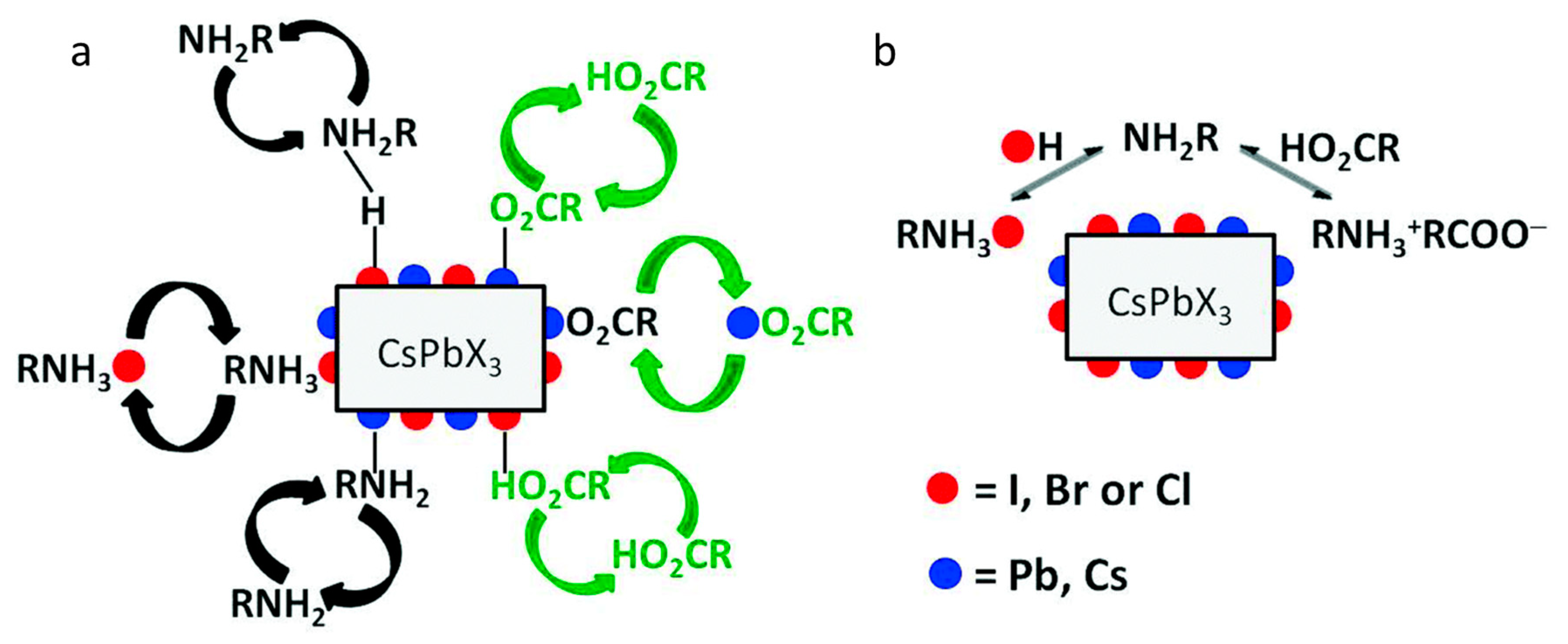
2. Significance of Oleic Acid (OA) and Oleylamine (OAm) in the Formation and Stabilization of Cs LHP NCs
2.1. Structural Modification of Cs LHP NCs Using OA/OAm Ligand Pair
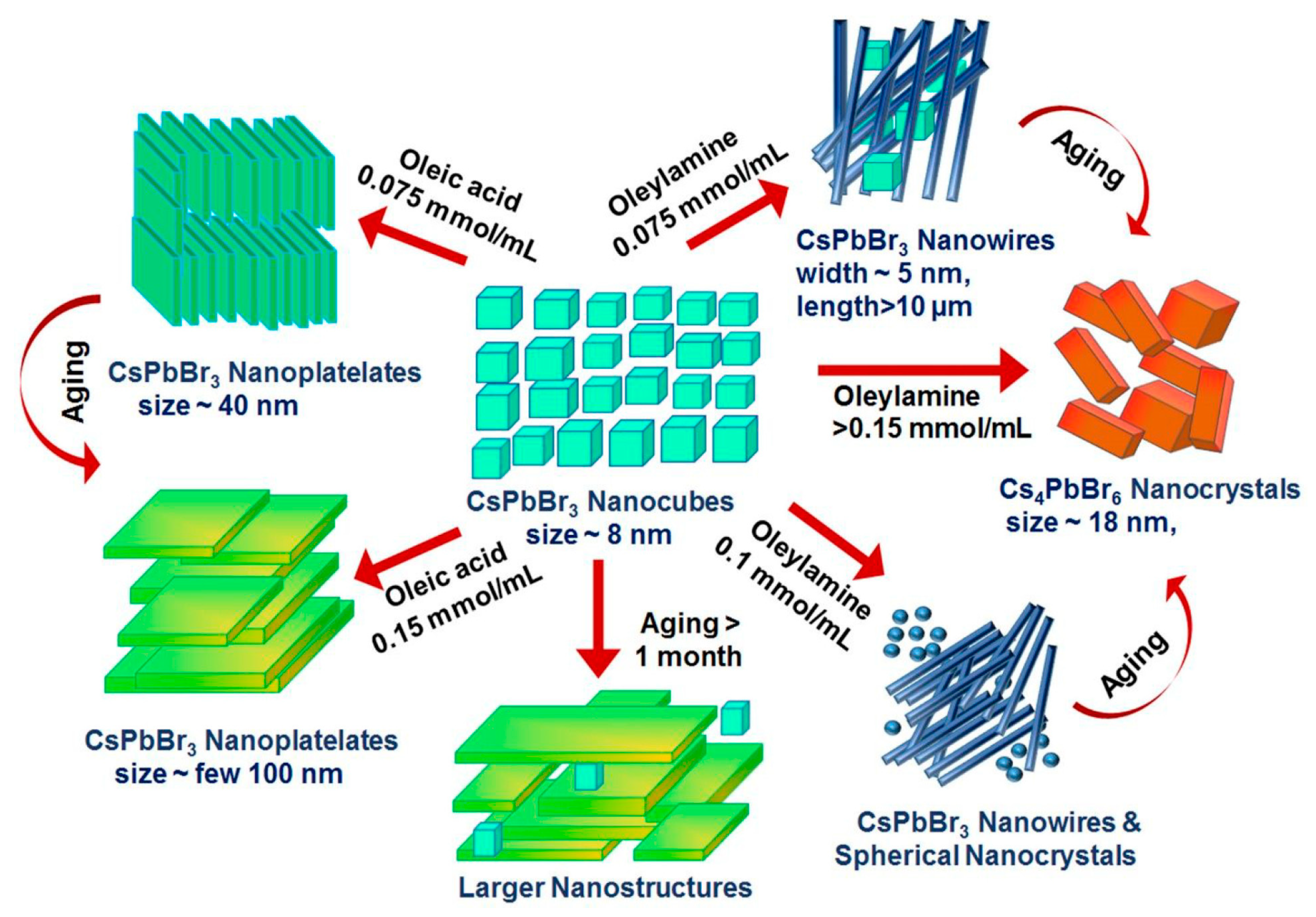
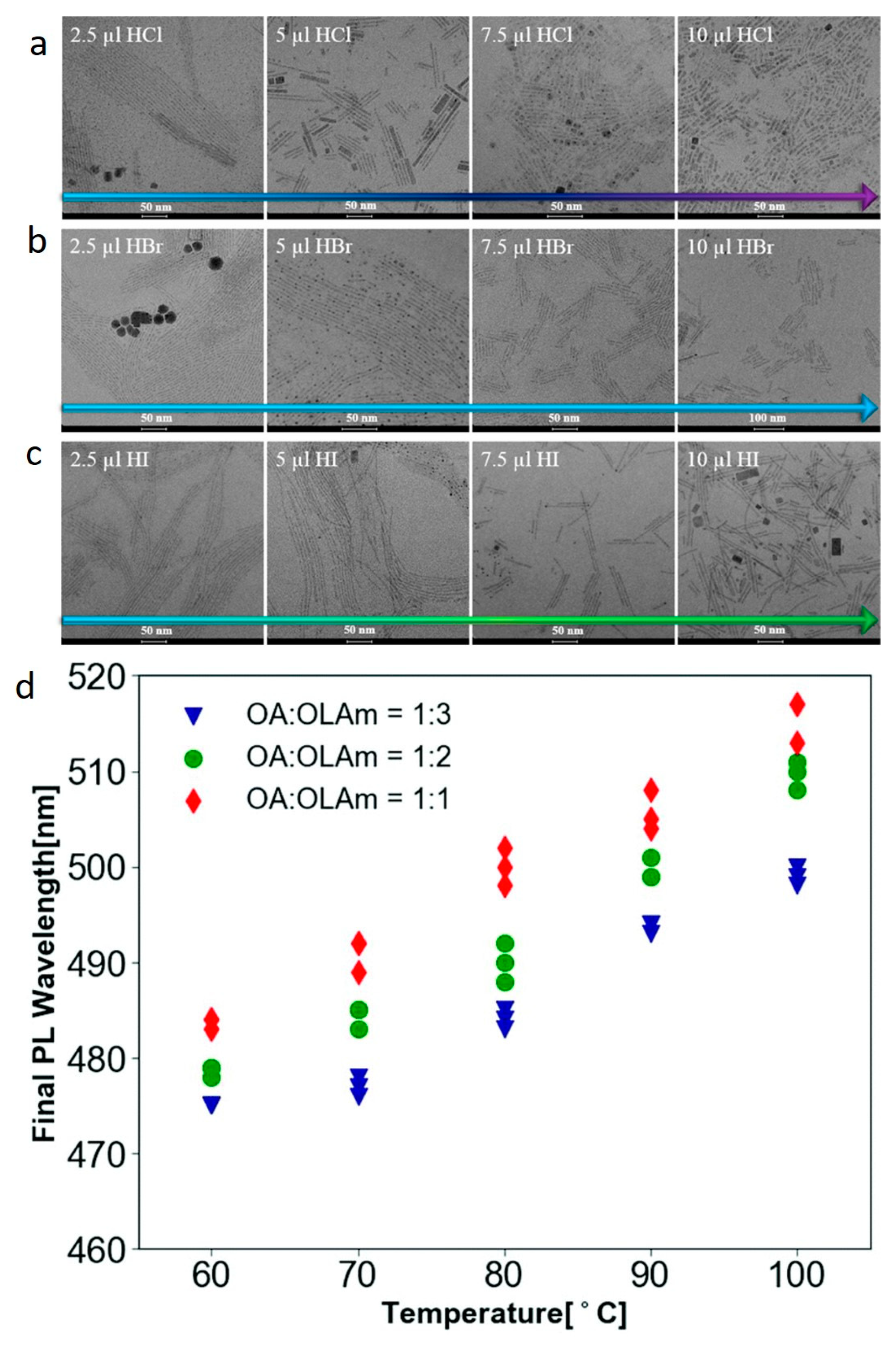
2.2. Morphological Changes of Cs LHP NCs in the Presence of OA/OAm
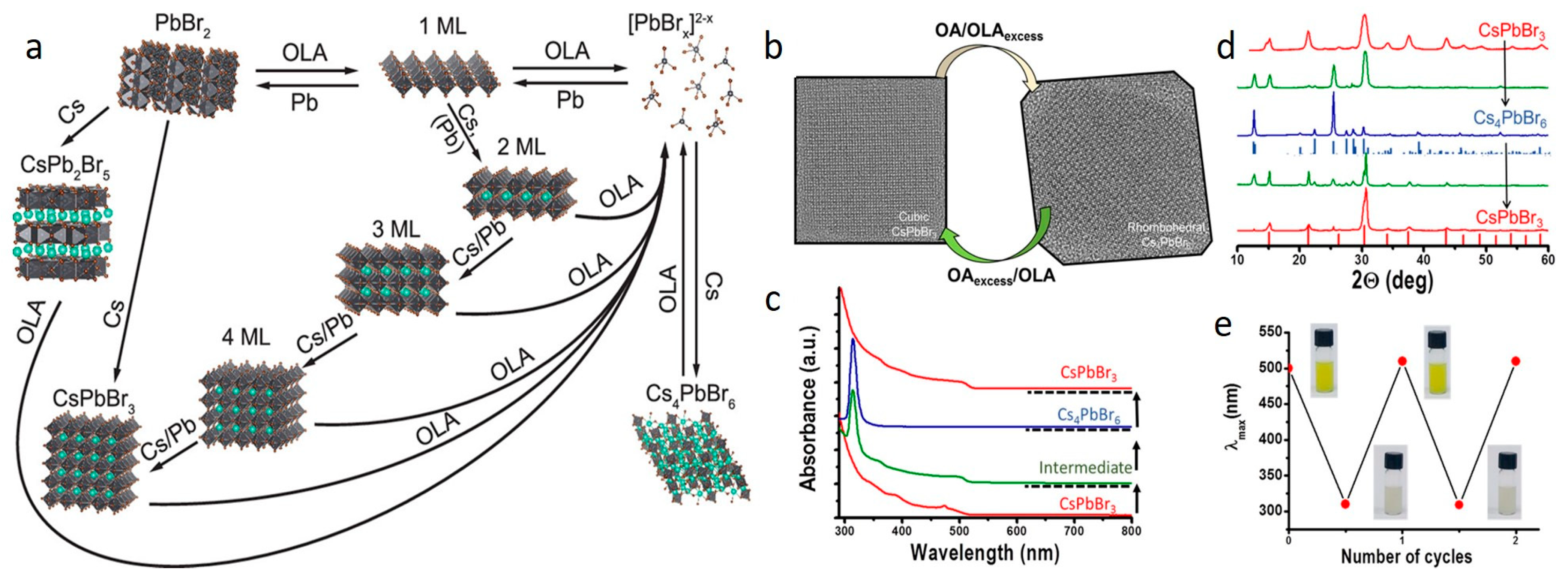
2.3. Phase Transformation of Cs LHP NCs in the Presence of OA/OAm
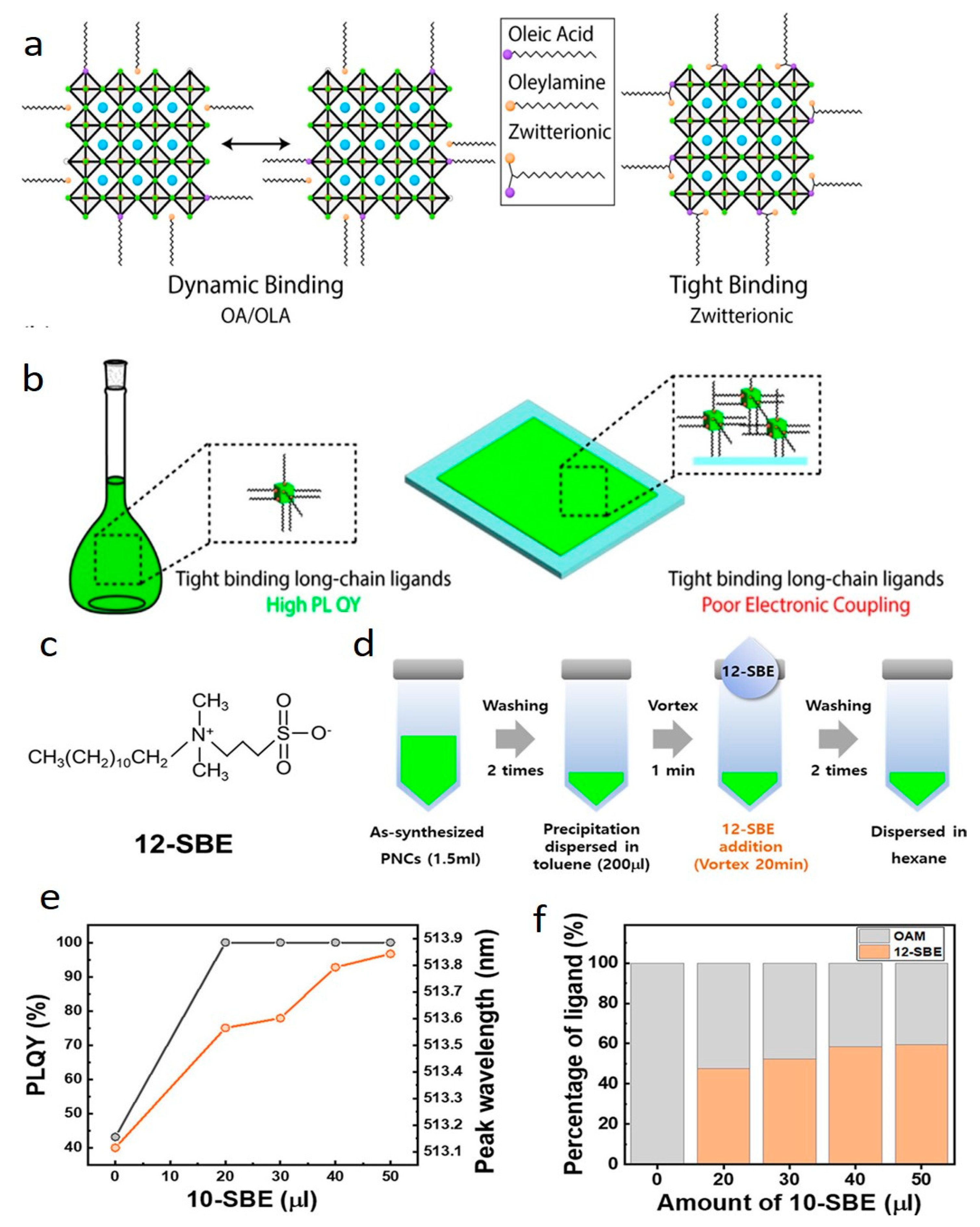
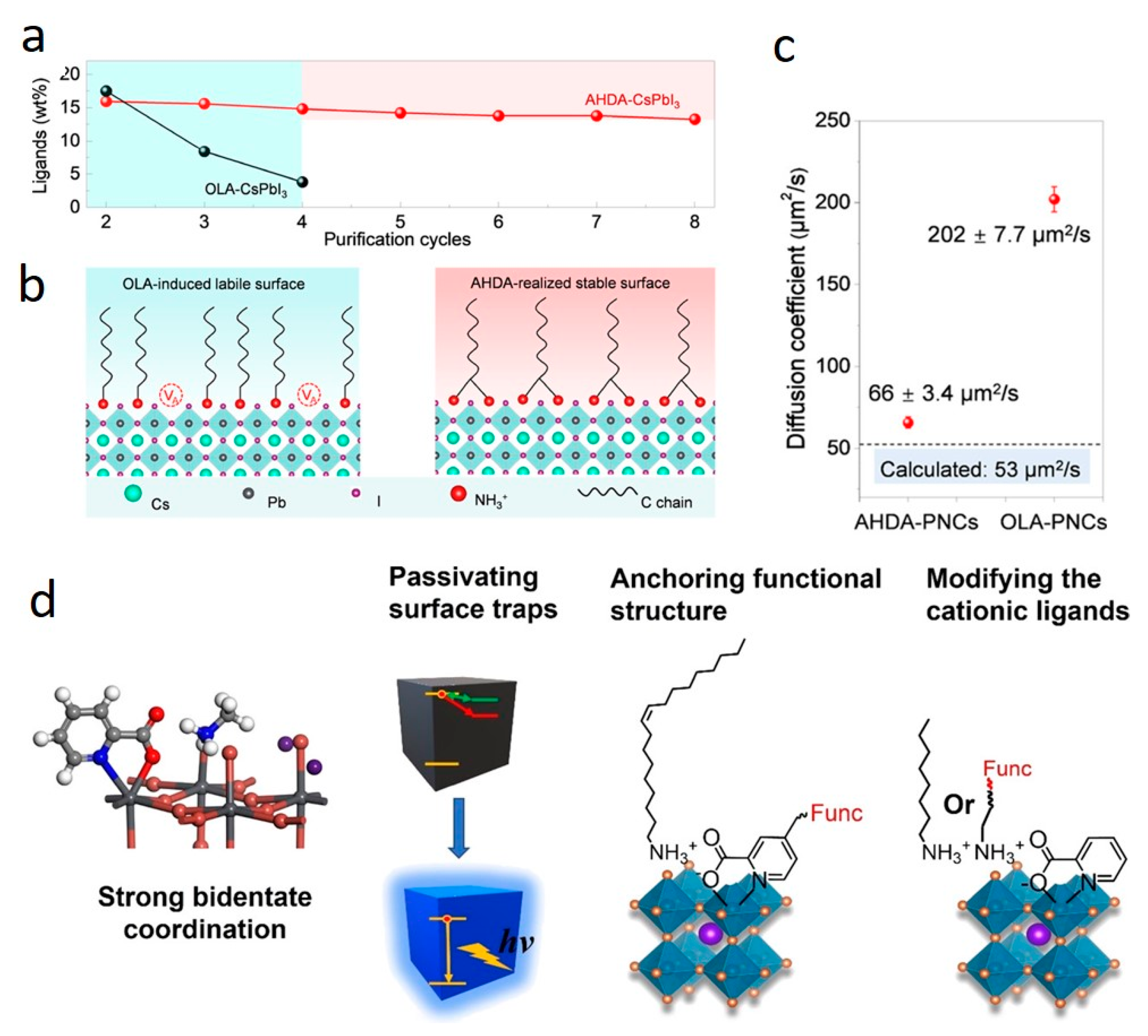
3. Potential Ligands and Solvents Replacing OA and OAm: A Step towards Enhancing Optical Properties and Stability of Cs LHP NCs
- (i)
- Encapsulation and passivation: One of the primary reasons for perovskite instability is exposure to moisture, oxygen, and light. Encapsulation techniques involving protective coatings or capping ligands can shield Cs LHP NCs from these environmental factors. The passivation of surface defects can also improve stability [22].
- (ii)
- Composition engineering: Modifying the chemical composition of Cs LHP NCs by incorporating elements with higher stability, such as lead-free perovskites or mixed-halide perovskites, can enhance their resistance to degradation.
- (iii)
- Structural modifications: Researchers have explored alternative perovskite structures, such as double perovskites and 2D perovskites, which may exhibit improved stability compared to traditional 3D perovskites.
- (iv)
- Doping: Introducing suitable dopants into Cs LHP NCs can improve their stability and performance. For example, incorporating small amounts of certain metal ions can passivate defects and reduce degradation.
- (v)
- Stabilizing additives: The use of stabilizing additives or polymer matrices in perovskite NC films can improve their resistance to environmental factors and enhance long-term stability [174].
4. Conclusions and Summary
Author Contributions
Funding
Conflicts of Interest
References
- Pan, Y.; Zhang, Y.; Kang, W.; Deng, N.; Yan, Z.; Sun, W.; Kang, X.; Ni, J. Progress in the preparation and application of CsPbX3 perovskites. Mater. Adv. 2022, 3, 4053–4068. [Google Scholar] [CrossRef]
- Aznar-Gadea, E.; Sanchez-Alarcon, I.; Soosaimanickam, A.; Rodriguez-Canto, P.J.; Perez-Pla, F.; Martinez-Pastor, J.P.; Abargues, R. Molecularly imprinted nanocomposites of CsPbBr3 nanocrystals: An approach towards fast and selective gas sensing of explosive taggants. J. Mater. Chem. C 2022, 10, 1754–1766. [Google Scholar] [CrossRef]
- Dong, H.; Ran, C.; Gao, W.; Li, M.; Xia, Y.; Huang, W. Metal halide perovskite for next-generation optoelectronics: Progresses and prospects. eLight 2023, 3, 3. [Google Scholar] [CrossRef]
- De Trizio, L.; Infante, I.; Manna, L. Surface chemistry of lead halide perovskite colloidal nanocrystals. Acc. Chem. Res. 2023, 56, 1815–1825. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Kim, Y.; Sihn, M.R.; Jeon, M.-G.; Jeong, J.-R.; Choi, J. Solubility-controlled room-temperature synthesis of cesium lead halide perovskite nanocrystals. ChemNanoMat 2020, 6, 1863–1869. [Google Scholar] [CrossRef]
- Navarro-Arenas, J.; Suarez, K.; Chirvony, V.S.; Gualdron-Reyes, A.F.; Mora-Sero, I.; Martinez-Pastor, J. Sinlge-exciton amplified spontaneous emission in thin films of CsPbX3 (X = Br, I) perovskite nanocrystals. J. Phys. Chem. Lett. 2019, 10, 6389–6398. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Zhang, M.; Yang, Z. Recent progress on boosting the perovskite film quality of all-inorganic perovskite solar cells. Coatings 2023, 13, 281. [Google Scholar] [CrossRef]
- Ekanayaka, T.K.; Richmond, D.; McCormick, M.; Nandyala, S.R.; Helfrich, H.C.; Sinitskii, A.; Pikal, J.M.; Ilie, C.C.; Dowben, P.A.; Yost, A.J. Surface versus bulk state transistions in inkjet-printed all-inorganic perovskite quantum dot films. Nanomaterials 2022, 12, 3956. [Google Scholar] [CrossRef]
- Dutta, A.; Behera, R.K.; Pal, P.; Baitalik, S.; Pradhan, N. Near-unity photoluminescence quantum efficiency for all CsPbX3 (X = Cl, Br and I) perovskite nanocrystals: A generic synthesis approach. Angew. Chem. 2019, 58, 5552–5556. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Wu, Y.; Wei, C.; Qin, Z.; Zhang, C.; Sun, Z.; Li, Y.; Wang, Y.; Zeng, H. Surface halogen compensation for robust performance enhancements of CsPbX3 perovskite quantum dots. Adv. Mater. 2019, 7, 1900276. [Google Scholar] [CrossRef]
- Gualdron-Reyes, A.; Masi, S.; Mora-Sero, I. Progress in halide-perovskite nanocrystals with near-unity photoluminescence quantum yield. Trends Chem. 2021, 3, 499–511. [Google Scholar] [CrossRef]
- Ibaceta-Jana, J.; Muydinov, R.; Rosado, P.; Mirhosseini, H.; Chug, M.; Nazarenko, O.; Dirin, D.N.; Heinrich, D.; Wagner, M.R.; Kuhne, T.D.; et al. Vibrational dynamics in lead halide hybrid perovskites investigated by Raman spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 5604–5614. [Google Scholar] [CrossRef]
- Jeong, D.-N.; Yang, J.-M.; Park, N.-G. Roadmap on halide perovskite and related devices. Nanotechnology 2020, 31, 152001. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cohen, T.A.; Sperry, B.M.; Larson, H.; Nguyen, H.A.; Homer, M.K.; Dou, F.Y.; Jacoby, L.M.; Cossairt, B.M.; Gamelin, D.R.; et al. Organic building blocks at inorganic nanomaterial interfaces. Mater. Horiz. 2022, 9, 61–87. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, N.; Sun, R.; Zheng, W.; Liu, H.; Zhang, Y. Revisiting the nanocrystal formation process of zero-dimensional perovskite. J. Mater. Chem. A 2021, 9, 4658–4663. [Google Scholar] [CrossRef]
- Huang, H.; Feil, M.W.; Fuchs, S.; Debnath, T.; Richter, A.F.; Tong, Y.; Wu, L.; Wang, Y.; Doblinger, M.; Nickel, B. Growth of perovskite CsPbBr3 nanocrystals and their formed superstructures revealed by in situ spectroscopy. Chem. Mater. 2020, 32, 8877–8884. [Google Scholar] [CrossRef]
- Udayabhaskararao, T.; Kazes, M.; Houben, L.; Lin, H.; Oron, D. Nucleation, growth and structural transformations of perovskite nanocrystals. Chem. Mater. 2017, 29, 1302–1308. [Google Scholar] [CrossRef]
- Bodnarchuk, M.I.; Boehme, S.C.; ten Brinck, S.; Bernasconi, C.; Shynkarenko, Y.; Krieg, F.; Widmer, R.; Aeschlimann, B.; Gunther, D.; Kovalenko, M.V.; et al. Rationalizing and controlling the surface structure and electronic passivation of cesium lead halide nanocrystals. ACS Energy Lett. 2019, 4, 63–74. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Kang, X.; Wang, L.; Huang, L.; Pan, D. Room temperature and gram-scale synthesis of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with 50-58% photoluminescence quantum yields. Chem. Commun. 2016, 52, 7265–7268. [Google Scholar] [CrossRef]
- Yu, W.; Wei, M.; Tang, Z.; Zou, H.; Li, L.; Zou, Y.; Yang, S.; Wang, Y.; Zhang, Y.; Li, X.; et al. Separating crystal growth from nucleation enables the in situ controllable synthesis of nanocrystals for efficient perovskite light-emitting diodes. Adv. Mater. 2023, 35, 2301114. [Google Scholar] [CrossRef]
- Abargues, R.; Navarro, J.; Rodriguez-Canto, P.; Maulu, A.; Sanchez-Royo, J.F.; Martinez-Pastor, J.P. Enhancing the photocatalytic properties of PbS QD solids: The ligand exchange approach. Nanoscale 2019, 11, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Soosaimanickam, A.; Adl, H.P.; Chirvony, V.; Rodriguez-Canto, P.J.; Martinez-Pastor, J.P.; Abargues, R. Effect of alkali metal nitrate treatment on the optical properties of CsPbBr3 nanocrystal films. Mater. Lett. 2021, 305, 130835. [Google Scholar] [CrossRef]
- Arenas, J.N.; Soosaimanickam, A.; Adl, H.P.; Abargues, R.; Boix, P.P.; Rodriguez-Canto, P.J.; Martinez-Pastor, J.P. Ligand-length modification in CsPbBr3 perovskite nanocrystals and bilayers with PbS quantum dots for improved photodetection performance. Nanomaterials 2020, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, G.C.; Thapa, S.; Yue, Y.; Zhu, H.; Zhu, P. Near unity PLQY and high stability of barium thiocyanate based all-inorganic perovskites and their applications in white light-emitting diodes. Photonics 2021, 8, 209. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.-K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.-J.; Chen, B.; Chekini, M.; Baek, S.-W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly monodisperse insulator Cs4PbX6 (X = Cl, Br, I) nanocrystals, their mixed halide compositions, and their transformation into CsPbX3 nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef]
- Almeida, G.; Goldoni, L.; Akkerman, Q.; Dang, Z.; Khan, A.H.; Marras, S.; Moreels, I.; Manna, L. Role of acid-base equilibria in the size, shape and phase control of cesium lead bromide nanocrystals. ACS Nano 2018, 12, 1704–1711. [Google Scholar] [CrossRef]
- Jana, A.; Meena, A.; Patil, S.A.; Jo, Y.; Cho, S.; Park, Y.; Sree, V.G.; Kim, H.; Im, H.; Taylor, R.A. Self-assembly of perovskite nanocrystals. Prog. Mater. Sci. 2022, 129, 100975. [Google Scholar] [CrossRef]
- Sanchez-Alarcon, R.I.; Noguera-Gomez, J.; Chirvony, V.S.; Pashei Adl, H.; Boix, P.P.; Alarcon-Flores, G.; Martinez-Pastor, J.P.; Abargues, R. Spray-driven halide exchange in solid-state CsPbX3 nanocrystal films. Nanoscale 2022, 14, 13214–13226. [Google Scholar] [CrossRef]
- De Roo, J.; Ibanez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Driessche, I.V.; Kovalenko, M.V.; Hens, Z. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: The role of organic acid, base, and cesium precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef] [PubMed]
- Maulu, A.; Navarro-Arenas, J.; Rodriguez-Canto, P.J.; Sanchez-Royo, J.F.; Abargues, R.; Suarez, I.; Martinez-Pastor, J.P. Charge transport in trap-sensitized infrared PbS quantum-dot-based photoconductors: Pros and cons. Nanomaterials 2018, 8, 677. [Google Scholar] [CrossRef] [PubMed]
- Maulu, A.; Rodriguez-Canto, P.J.; Navarro-Arenas, J.; Abargues, R.; Sanchez-Royo, J.F.; Garcia-Calzada, R.; Martinez-Pastor, J.P. Strongly-coupled PbS QD solids by doctor blading for IR photodetection. RSC Adv. 2016, 6, 80201–80212. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, S.; Song, D.; Xu, Z.; Qiao, B.; Song, P.; Bai, Q.; Cao, J.; Zhang, G.; Swelm, W. Improving the quality and luminescence performance of all-inorganic perovskite nanomaterials for light-emitting devices by surface engineering. Small 2020, 16, 1907089. [Google Scholar] [CrossRef]
- Toso, S.; Baranov, D.; Manna, L. Metamorphoses of cesium lead halide nanocrystals. Acc. Chem. Res. 2021, 54, 498–508. [Google Scholar] [CrossRef]
- Kumawat, N.K.; Liu, X.-K.; Kabra, D.; Gao, F. Blue perovskite light-emitting diodes: Progress, challenges and future directions. Nanoscale 2019, 11, 2109–2120. [Google Scholar] [CrossRef]
- Moyen, E.; Kanwat, A.; Cho, S.; Jun, H.; Aad, R.; Jang, J. Ligand removal and photo-activation of CsPbBr3 quantum dots for enhanced optoelectronic devices. Nanoscale 2018, 10, 8591–8599. [Google Scholar] [CrossRef]
- Zhang, Q.; Diao, F.; Xue, X.; Sheng, X.; Barba, D.; Wang, Y. Self-assembly of CsPbBr3 nanocubes into 2D nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 44777–44785. [Google Scholar] [CrossRef]
- Huang, H.; Raith, J.; Kershaw, S.V.; Kalytchuk, S.; Tomanec, O.; Jing, L.; Susha, A.S.; Rogach, A.L. Growth mechanism of strongly emitting CH3NH3PbBr3 perovskite nanocrystals with a tunable bandgap. Nat. Comm. 2017, 8, 996. [Google Scholar] [CrossRef]
- Yin, J.; Yang, H.; Gutierrez-Arzaluz, L.; Zhou, Y.; Bredas, J.-L.; Bakr, O.M.; Mohammed, O.F. Luminescence and stability enhancement of inorganic perovskite nanocrystals via selective surface ligand binding. ACS Nano 2021, 15, 17998–18005. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Nazerenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-cost synthesis of highly luminescent colloidal lead halide perovskite nanocrystals by wet ball milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Samanta, A. A facile methodology for engineering the morphology of CsPbX3 perovskite nanocrystals under ambient condition. Sci. Rep. 2016, 6, 37693. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Z.; Liu, Z.; Peng, L.; Li, Y.; Tang, A. Photoluminescence and self-assembly of cesium lead halide perovskite nanocrystals: Effects of chain length of organic amines and reaction temperature. Appl. Surf. Sci. 2017, 405, 280–288. [Google Scholar] [CrossRef]
- Stoffel, J.T.; Tsui, E.Y. Stability effects of selective anion abstraction from cesium lead halide perovskite nanocrystals. J. Phys. Chem. C 2023, 127, 2285–2293. [Google Scholar] [CrossRef]
- Dutt, V.G.V.; Akhil, S.; Mishra, N. Surface passivation strategies for improving photoluminescence and stability of cesium lead halide perovskite nanocrystals. ChemNanoMat 2020, 6, 1730–1742. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Satija, S.; Gangwar, U.; Sapra, S. Precursor-mediated synthesis of shape-controlled colloidal CsPbBr3 perovskite nanocrystals and their nanofiber directed self-assembly. Chem. Mater. 2020, 32, 721–733. [Google Scholar] [CrossRef]
- Pradhan, N. Alkylammonium halides for facet reconstruction and shape modulation in lead halide perovskite nanocrystals. Acc. Chem. Res. 2021, 54, 1200–1208. [Google Scholar] [CrossRef]
- Kazes, M.; Udayabhaskararao, T.; Dey, S.; Oron, D. Effect of surface ligands in perovskite nanocrystals: Extending in and reaching out. Acc. Chem. Res. 2021, 54, 1409–1418. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Chinh, N.D.; Sihn, M.R.; Jeon, M.-G.; Jeong, J.-R.; Kim, D.; Jang, J.H.; Choi, J. Mechanistic insight into surface defect control in perovskite nanocrystals: Ligands terminate the valence transition from Pb2+ to metallic Pb0. J. Phys. Chem. Lett. 2019, 10, 4222–4228. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Xie, Z.; Jiang, W.; Wang, L.; Jiang, W. Ionic liquid assisted preparation and modulation of the photoluminescence kinetics for highly efficient CsPbX3 nanocrystals with improved stability. Nanoscale 2020, 12, 9569–9580. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yun, R.; Liu, Y.; Zhang, X.; Yuan, M.; Zhang, L.; Li, X. Ligands in lead halide perovskite nanocrystals: From synthesis to optoelectronic applications. Small 2023, 19, 2205950. [Google Scholar] [CrossRef]
- Yassitepe, E.; Yang, Z.; Voznyy, O.; Kim, Y.; Walters, G.; Castaneda, J.A.; Kanjanaboos, P.; Yuan, M.; Gong, X.; Fan, F.; et al. Amine-free synthesis of cesium lead halide perovskite quantum dots for efficient light-emitting diodes. Adv. Funct. Mater. 2016, 26, 8757–8763. [Google Scholar] [CrossRef]
- Smock, S.R.; Chen, Y.; Rossini, A.J.; Brutchey, R.L. The surface chemistry and structure of colloidal lead halide perovskite nanocrystals. Acc. Chem. Res. 2021, 54, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.K.; Santra, P.K.; Joshi, N.; Chugh, J.; Singh, S.K.; Rensmo, H.; Ghosh, P.; Nag, A. Origin of the substitution mechanism for the binding of organic ligands on the surface of CsPbBr3 perovskite nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. [Google Scholar] [CrossRef]
- Bertolotti, F.; Nedelcu, G.; Vivani, A.; Cervellino, A.; Masciocchi, N.; Guagliardi, A.; Kovalenko, M.V. Crystal structure, morphology and surface termination of cyan-emissive, six-monolayers-thick CsPbBr3 nanoplatelets from X-ray total scattering. ACS Nano 2019, 13, 14294–14307. [Google Scholar] [CrossRef]
- Chen, Y.; Smock, S.R.; Flintgruber, A.H.; Perras, F.A.; Brutchey, R.L.; Rossini, A.J. Surface termination of CsPbBr3 perovskite quantum dots determined by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2020, 142, 6117–6127. [Google Scholar] [CrossRef]
- Ng, C.K.; Wang, C.; Jasieniak, J.J. Synthetic evolution of colloidal metal halide perovskite nanocrystals. Langmuir 2019, 35, 11609–11628. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Cho, S.; Kim, Y.; Kim, H.; Jeong, S.; Woo, J.Y. Air-stable cesium lead bromide perovskite nanocrystals via post-synthetic treatment with oleylammonium bromides. New J. Chem. 2022, 46, 19514–19522. [Google Scholar] [CrossRef]
- Grisorio, R.; Di Clemente, M.E.; Fanizza, E.; Allegretta, I.; Altamura, D.; Striccoli, M.; Terzano, R.; Giannini, C.; Irimia-Vladu, M.; Suranna, G.P. Exploring the surface chemistry of cesium lead halide perovskite nanocrystals. Nanoscale 2019, 11, 986–999. [Google Scholar] [CrossRef]
- Qu, R.; Zou, L.; Qi, X.; Liu, C.; Zhao, W.; Yan, J.; Zhang, Z.; Wu, Y. Changing the shape and optical properties of CsPbBr3 perovskite nanocrystals with hydrohalic acids using a room-temperature synthesis process. CrystEngComm 2022, 22, 1853–1857. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Menelaou, M.; Fiuza-Maneiro, N.; Zheng, G.; Wei, S.; Perez-Juste, J.; Polavarapu, L.; Sofer, Z. Oleic acid/oleylamine ligand pair: A versatile combination in the synthesis of colloidal nanoparticles. Nanoscale Horiz. 2022, 7, 941–1015. [Google Scholar] [CrossRef]
- Amgar, D.; Stern, A.; Rotem, D.; Porath, D.; Etgar, L. Tunable length and optical properties of CsPbX3 (X = Cl, Br, I) nanowires with a few unit cells. Nano Lett. 2017, 17, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Motti, S.G.; Kandada, A.R.S.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B.A.; Miranda, L.; De Angelis, F.; et al. Solution synthesis approach to colloidal cesium lead halide perovskite nanoplatelets with monolayer-level thickness control. J. Am. Chem. Soc. 2016, 138, 1010–1016. [Google Scholar] [CrossRef]
- Do, M.; Kim, I.; Kolaczkowski, M.A.; Kang, J.; Kamat, G.A.; Yuan, Z.; Barchi, N.S.; Wang, L.-W.; Liu, Y.; Jurow, M.J.; et al. Low-dimensional perovskite nanoplatelet synthesis using in situ photophysical monitoring to establish controlled growth. Nanoscale 2019, 11, 17262–17269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Wang, S.; Long, D.; Li, M.; Wang, D.; Zhang, T. Inter-conversion between different compounds of ternary Cs-Pb-Br system. Materials 2018, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Dong, T.; Wang, P.; Matras-Postolek, K.; Yang, P. Evolution of morphology, phase composition and photoluminescence of cesium lead bromine nanocrystals with temperature and precursors. J. Phys. Chem. C 2018, 122, 28968–28976. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Yang, Z.; Ji, S.; Qiu, H. Nanowire-assisted self-assembly of one-dimensional nanocrystal superlattice chains. J. Mater. Chem. C 2019, 7, 8471–8476. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Song, B.; Dong, W.; Han, G. Suppressing growth strategy on synthesizing stable and high-quality blue-emitting CsPbBr3 quantum dots. ACS Appl. Opt. Mater. 2023, 1, 1263–1271. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, S.; Xu, Z.; Qiao, B.; Song, P.; Xu, X. Shape-controlled synthesis of all-inorganic CsPbBr3 perovskite nanocrystals with bright blue emission. ACS Appl. Mater. Interfaces 2016, 8, 28824–28830. [Google Scholar] [CrossRef]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-phase synthesis of cesium lead halide perovskite nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Hu, J.; Yao, Z.; Lu, Y.; Binks, D.; Sui, M.; Tian, J. Self-assembled perovskite nanowire clusters for high luminance red light-emitting diodes. Adv. Funct. Mater. 2020, 30, 2005990. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Ayguler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S. Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication. Angew. Chem. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Geng, C.; Shi, S.; Zhang, X.; Bi, W.; Xu, S. Rapid synthesis of quantum-confined CsPbBr3 perovskite nanowires using a microfluidic reactor. Nanoscale 2019, 11, 18790–18796. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cai, T.; Dube, L.; Hills-Kimball, K.; Chen, O. Synthesis of ultrathin perovskite nanowires via a postsynthetic transformation reaction of zero-dimensional perovskite nanocrystals. Cryst. Growth Des. 2021, 21, 1924–1930. [Google Scholar] [CrossRef]
- Fanizza, E.; Cascella, F.; Altamura, D.; Giannini, C.; Panniello, A.; Triggiani, L.; Panzarea, F.; Depalo, N.; Grisorio, R.; Suranna, G.P.; et al. Post-synthesis phase and shape evolution of CsPbBr3 colloidal nanocrystals: The role of ligands. Nano Res. 2019, 12, 1155–1166. [Google Scholar] [CrossRef]
- Paul, S.; Acharya, S. Postsynthesis transformation of halide perovskite nanocrystals. ACS Energy Lett. 2022, 7, 2136–2155. [Google Scholar] [CrossRef]
- Aamir, M.; Adhikari, T.; Sher, M.; Khan, M.D.; Akhtar, J.; Nunzi, J.-M. Cesium lead halide perovskite nanostructures: Tunable morphology and halide composition. Chem. Rec. 2018, 18, 230–238. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, D.; Xu, Y.; Lin, J.; Yu, C.; Fang, Y.; Liu, Z.; Guo, Z.; Tang, C.; Huang, Y. Surface ligand engineering of CsPbBr3 perovskite nanowires for high-performance photodetectors. J. Colloid Interface Sci. 2022, 608, 2367–2376. [Google Scholar] [CrossRef]
- Imran, M.; Di Stasio, F.; Dang, Z.; Canale, C.; Khan, A.H.; Shamsi, J.; Brescia, R.; Prato, M.; Manna, L. Colloidal synthesis of strongly fluorescent CsPbBr3 nanowires with width tunable down to the quantum confinement regime. Chem. Mater. 2016, 28, 6450–6454. [Google Scholar] [CrossRef]
- Welyab, G.; Abebe, M.; Mani, D.; Thankappan, A.; Thomas, S.; Aga, A.G.; Kim, J.Y. All-inorganic CsPbBr3 perovskite nanocrystals synthesized with olive oil and oleylamine at room temperature. Micromachines 2023, 14, 1332. [Google Scholar] [CrossRef] [PubMed]
- Ghori, A.; Midya, A.; Ray, S.K. Surfactant-induced anion exchange and morphological evolution for composition-controlled caesium lead halide perovskites with tunable optical properties. ACS Omega 2019, 4, 12948–12954. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Tang, B.; Ma, Y. Controlled synthesis of quantum confined CsPbBr3 perovskite nanocrystals under ambient conditions. Nanotechnology 2018, 29, 055601. [Google Scholar] [CrossRef] [PubMed]
- Hudait, B.; Dutta, S.K.; Patra, A.; Nasipuri, D.; Pradhan, N. Facets directed connecting perovskite nanocrystals. J. Am. Chem. Soc. 2020, 142, 7207–7217. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef]
- Xiao, X.; Li, Y.; Xie, R.-J. Blue-emitting and self-assembled thinner perovskite CsPbBr3 nanoplates: Synthesis and formation mechanism. Nanoscale 2020, 12, 9231–9239. [Google Scholar] [CrossRef]
- Pramanik, A.; Sinha, S.S.; Gates, K.; Nie, J.; Han, F.X.; Ray, P.C. Light-induced wavelength dependent self assembly process for targeted synthesis of phase stable 1D nanobelts and 2D nanoplatelets of CsPbI3 perovskites. ACS Omega 2023, 8, 13202–13212. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; Shin, Y.; Liu, X.; Chen, J.; Yao, K.X.; Pan, J.; Yang, C.; Yin, J.; Xu, L.-J.; et al. Light-induced self-assembly of cubic CsPbBr3 perovskite nanocrystals into nanowires. Chem. Mater. 2019, 31, 6642–6649. [Google Scholar] [CrossRef]
- Li, H.; Lu, W.; Zhao, G.; Song, B.; Dong, W.; Han, G. Synthesis of size-controllable self-assembled CsPbBr3 nanowires: Implications for photoemission with less recombination. ACS Appl. Nano Mater. 2022, 5, 5527–5534. [Google Scholar] [CrossRef]
- Mehetor, S.K.; Ghosh, H.; Pradhan, N. Blue-emitting CsPbBr3 perovskite quantum rods and their wide-area 2D self-assembly. ACS Energy Lett. 2019, 4, 1437–1442. [Google Scholar] [CrossRef]
- Patra, B.K.; Agrawal, H.; Zheng, J.-Y.; Zha, X.; Travesset, A.; Garnett, E.C. Close-packed ultrasmooth self-assembled monolayer of CsPbBr3 perovskite nanocubes. ACS Appl. Mater. Interfaces 2020, 12, 31764–31769. [Google Scholar] [CrossRef]
- van der Burgt, J.S.; Geuchies, J.J.; van der Meer, B.; Vanrompay, H.; Zanaga, D.; Zhang, Y.; Albrecht, W.; Petukhov, A.V.; Filion, L.; Bals, S.; et al. Cuboidal supraparticles self-assembled from cubic CsPbBr3 perovskite nanocrystals. J. Phys. Chem. C 2018, 122, 15706–15712. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Ying, Q.; Wang, C.; Jia, W.; Xing, X.; Yin, L.; Lu, Z.; Zhang, K.; Pan, Y.; et al. Self-assembly of perovskite CsPbBr3 quantum dots driven by a photo-induced alkynyl homocoupling reaction. Angew. Chem. 2020, 59, 17207–17213. [Google Scholar] [CrossRef]
- Pan, J.; Huang, H.; Zhao, W.; Lin, D.; Nie, Z.; Zhong, J.; Ma, L.; Zhang, X. Efficient and stable self-assembly blue-emitting CsPbBr3 nanoplatelets with self-repaired surface defects. ACS Appl. Nano Mater. 2022, 5, 15062–15069. [Google Scholar] [CrossRef]
- Mehetor, S.K.; Ghosh, H.; Pradhan, N. Acid-amine equilibria for formation and long-range self-organization of ultrathin CsPbBr3 perovskite platelets. J. Phys. Chem. Lett. 2019, 10, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Sygletou, M.; Brintakis, K.; Lappas, A.; Stratakis, E. Low-temperature benchtop-synthesis of all-inorganic perovskite nanowires. Nanoscale 2017, 9, 18202–18207. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, W.; Zhao, G.; Song, B.; Dong, W.; Han, G. Evolution and mechanism of cesium lead bromide nanostructures in oleylamine-rich system by hot-injection method. Adv. Mater. Interfaces 2023, 10, 2201916. [Google Scholar] [CrossRef]
- Pan, J.; Li, X.; Gong, X.; Yin, J.; Zhou, D.; Sinatra, L.; Huang, R.; Liu, J.; Chen, J.; Dursun, I.; et al. Halogen vacancies enable ligand-assisted self-assembly of perovskite quantum dots into nanowires. Angew. Chem. 2019, 131, 16223–16227. [Google Scholar] [CrossRef]
- Baranov, D.; Fieramosca, A.; Yang, R.X.; Polimeno, L.; Lerario, G.; Toso, S.; Giansante, C.; De Giorgi, M.; Tan, L.Z.; Sanvitto, D.; et al. Aging of self-assembled lead halide perovskite nanocrystal superlattices: Effects on photoluminescence and energy transfer. ACS Nano 2021, 15, 650–664. [Google Scholar] [CrossRef]
- Pradhan, B.; Mushtaq, A.; Roy, D.; Sain, S.; Das, B.; Ghorai, U.K.; Pal, S.K.; Acharya, S. Postsynthesis spontaneous coalescence of mixed-halide perovskite nanocubes into phase-stable single-crystalline uniform luminescent nanowires. J. Phys. Chem. Lett. 2019, 10, 1805–1812. [Google Scholar] [CrossRef]
- Soetan, N.; Erwin, W.R.; Tonigan, A.M.; Walker, D.G.; Bardhan, R. Solvent-assisted self-assembly of CsPbBr3 perovskite nanocrystals into one-dimensional super-lattice. J. Phys. Chem. C 2017, 121, 18186–18194. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hu, Y.-H.; Ma, L.; Zhang, X.-Y.; Zhao, N.-X.; Yang, X.; Zhang, Y.-S.; Gu, Y.-L.; Xu, S.-L.; Dong, X.; et al. Self-assembled CsPbBr3 quantum dots with wavelength-tunable photoluminescence for efficient active jamming. Nanoscale 2022, 14, 17900–17907. [Google Scholar] [CrossRef]
- Leng, J.; Wang, T.; Zhao, X.; Ong, E.W.Y.; Zhu, B.; de Andrew Ng, J.; Wong, Y.-C.; Khoo, K.H.; Tamada, K.; Tan, Z.-K. Thermodynamic control in the synthesis of quantum-confined blue-emitting CsPbBr3 perovskite nanostrips. J. Phys. Chem. Lett. 2020, 11, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; He, W.; Peng, H.; Dai, Q. CsPbBr3 perovskite nanowires and their optical properties. Opt. Mater. 2020, 109, 110399. [Google Scholar] [CrossRef]
- Zhou, Z.-R.; Liao, Z.-H.; Wang, F. Shape-controlled synthesis of one-dimensional cesium lead halide perovskite nanocrystals: Methods and advances. J. Mater. Chem. C 2023, 11, 3409–3427. [Google Scholar] [CrossRef]
- Krajewska, C.J.; Kaplan, A.E.K.; Kick, M.; Berkinsky, D.B.; Zhu, H.; Sverko, T.; Voorhis, T.V.; Bawendi, M.G. Controlled assembly and anomalous thermal expansion of ultrathin cesium lead bromide nanoplatelets. Nano Lett. 2023, 23, 2148–2157. [Google Scholar] [CrossRef]
- Dang, Z.; Dhanabalan, B.; Castelli, A.; Dhall, R.; Bustillo, K.C.; Marchelli, D.; Spirito, D.; Petralanda, U.; Shamsi, J.; Manna, L.; et al. Temperature-driven transformation of CsPbBr3 nanoplatelets into mosaic nanotiles in solution through self-assembly. Nano Lett. 2020, 20, 1808–1818. [Google Scholar] [CrossRef]
- Montanarella, F.; Akkerman, Q.A.; Bonatz, D.; van der Sluijs, M.M.; van der Bok, J.C.; Prins, P.T.; Aebli, M.; Mews, A.; Vanmaekelbergh, D.; Kovalenko, M.V. Growth and self-assembly of CsPbBr3 nanocrystals in the TOPO/PbBr2 synthesis as seen with X-ray scattering. Nano Lett. 2023, 23, 667–676. [Google Scholar] [CrossRef]
- Liu, Z.; Bekenstein, Y.; Ye, X.; Nguyen, S.C.; Swabeck, J.; Zhang, D.; Lee, S.-T.; Yang, P.; Ma, W.; Alivisatos, A.P. Ligand mediated transformation of cesium lead bromide perovskite nanocrystals to lead depleted Cs4PbBr6 nanocrystals. J. Am. Chem. Soc. 2017, 139, 5309–5312. [Google Scholar] [CrossRef]
- Udayabhaskararao, T.; Houben, L.; Cohen, H.; Menahem, M.; Pinkas, I.; Avram, L.; Wolf, T.; Teitelboim, A.; Leskes, M.; Yaffe, O.; et al. A mechanistic study of phase transformation in perovskite nanocrystals driven by ligand Passivation. Chem. Mater. 2018, 30, 84–93. [Google Scholar] [CrossRef]
- Dahl, J.C.; Wang, X.; Huang, X.; Chan, E.M.; Alivisatos, A.P. Elucidating the weakly reversible Cs-Pb-Br perovskite nanocrystal reaction network with high-throughput maps and transformations. J. Am. Chem. Soc. 2020, 147, 11915. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Chiu, H.-D.; Wang, W.; Su, Q.; Yamada, T.; Chang, Y.-C.; Chen, S.; Kanemitsu, Y.; Chung, R.-J.; Liu, R.-S. Highly luminescent CsPbBr3@Cs4PbBr6 nanocrystals and their application in electroluminescent emitters. J. Phys. Chem. Lett. 2020, 11, 10196–10202. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dai, S.; Hadjiev, V.G.; Wang, C.; Xie, L.; Ni, Y.; Wu, C.; Yang, G.; Chen, S.; Deng, L.; et al. Revealing the origin of luminescence center in 0D Cs4PbBr6 perovskite. Chem. Mater. 2019, 31, 9098–9104. [Google Scholar] [CrossRef]
- Ray, A.; Maggioni, D.; Baranov, D.; Dang, Z.; Prato, M.; Akkerman, Q.A.; Goldoni, L.; Caneva, E.; Manna, L.; Abdelhady, A.L. Green-emitting powders of zero-dimensional Cs4PbBr6: Delineating the intricacies of the synthesis and the origin of photoluminescence. Chem. Mater. 2019, 31, 7761–7769. [Google Scholar] [CrossRef]
- Jing, Q.; Xu, Y.; Su, Y.; Xing, X.; Lu, Z. A systematic study of the synthesis of cesium lead halide nanocrystals: Does Cs4PbBr6 or CsPbBr3 form? Nanoscale 2019, 11, 1784–1789. [Google Scholar] [CrossRef]
- Cho, J.; Banerjee, S. Ligand-directed stabilization of ternary phases: Synthetic control of structural dimensionality in solution-grown cesium lead bromide nanocrystals. Chem. Mater. 2018, 30, 6144–6155. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xiong, Y.; Kershaw, S.V.; Rogach, A.L. Revealing the formation mechanism of CsPbBr3 perovskite nanocrystals produced via a slowed-down microwave-assisted synthesis. Angew. Chem. 2018, 57, 5833–5837. [Google Scholar] [CrossRef]
- Xiao, M.; Hao, M.; Lyu, M.; Moore, E.G.; Zhang, C.; Zhang, C.; Luo, B.; Hou, J.; Lipton-Duffin, J.; Wang, L. Surface ligands stabilized lead halide perovskite quantum dot photocatalyst for visible light-driven hydrogen generation. Adv. Funct. Mater. 2019, 29, 1905683. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tien, C.-H.; Tseng, P.-W.; Tseng, Z.-L.; Huang, W.-L.; Xu, Y.-X.; Kuo, H.-C. Effect of washing solvents on the properties of air-synthesized perovskite CsPbBr3 quantum dots for quantum dot-based light-emitting devices. IEEE Access 2020, 8, 159415–159423. [Google Scholar] [CrossRef]
- Li, F.; Lin, F.; Huang, Y.; Cai, Z.; Qiu, L.; Zhu, Y.; Wang, Y.; Chen, X. Bromobezene aliphatic nucleophilic substitution guided controllable and reproducible synthesis of high quality cesium lead bromide perovskite nanocrystals. Inorg. Chem. Front. 2019, 6, 3577–3582. [Google Scholar] [CrossRef]
- Quarta, D.; Imran, M.; Capodilupo, A.-L.; Petralanda, U.; van Beek, B.; De Angelis, F.; Manna, L.; Infante, I.; De Trizio, L.; Giansante, C. Stable ligand coordination at the surface of colloidal CsPbBr3 nanocrystals. J. Phys. Chem. Lett. 2019, 10, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, Y.; Zhang, S.; Dai, Y.; Liu, L.; Li, Y.; Chen, Q. Recent advances toward practical use of halide perovskite nanocrystals. J. Mater. Chem. A 2018, 6, 21729–21746. [Google Scholar] [CrossRef]
- Yang, D.; Cao, M.; Zhong, Q.; Li, P.; Zhang, X.; Zhang, Q. All-inorganic cesium lead halide perovskite nanocrystals: Synthesis, surface engineering and applications. J. Mater. Chem. C 2019, 7, 757–789. [Google Scholar] [CrossRef]
- McGrath, F.; Ghorpade, U.V.; Ryan, K.M. Synthesis and dimensional control of CsPbBr3 perovskite nanocrystals using phosphorous based ligands. J. Chem. Phys. 2020, 152, 174702. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Maneiro, N.; Sun, K.; Lopez-Fernandez, I.; Gomez-Grana, S.; Muller-Buschbaum, P.; Polavarapu, L. Ligand chemistry of inorganic lead halide perovskite nanocrystals. ACS Energy Lett. 2023, 8, 1152–1191. [Google Scholar] [CrossRef]
- Smock, S.R.; Williams, T.J.; Brutchey, R.L. Quantifying the thermodynamics of ligand binding to CsPbBr3 quantum dots. Angew. Chem. 2018, 57, 11711–11715. [Google Scholar] [CrossRef]
- Zaccaria, F.; Zhang, B.; Goldoni, L.; Imran, M.; Zito, J.; van Beek, B.; Lauciello, S.; De Trizio, L.; Manna, L.; Infante, I. The reactivity of CsPbBr3 nanocrystals toward acid/base ligands. ACS Nano 2022, 16, 1444–1455. [Google Scholar] [CrossRef]
- Akhil, S.; Dutt, V.; Mishra, N. Bromopropane as a novel bromine precursor for the completely amine free colloidal synthesis of ultrastable and highly luminescent green-emitting cesium lead bromide (CsPbBr3) perovskite nanocrystals. Nanoscale 2021, 13, 13142–13151. [Google Scholar] [CrossRef]
- Akhil, S.; Biswas, S.; Palabathuni, M.; Singh, R.; Mishra, N. Amine-free synthetic route: An emerging approach to making high-quality perovskite nanocrystals for futuristic applications. J. Phys. Chem. Lett. 2022, 13, 9480–9493. [Google Scholar] [CrossRef]
- Akhil, S.; Dutt, V.G.V.; Singh, R.; Mishra, N. Surface-state-mediated interfacial hole transfer dynamics between CsPbBr3 perovskite nanocrystals and phenothiazine redox couple. J. Phys. Chem. C. 2021, 125, 22133–22141. [Google Scholar] [CrossRef]
- Chen, W.-C.; Fang, Y.-H.; Chen, L.-G.; Liang, F.-C.; Yan, Z.-L.; Ebe, H.; Takahashi, Y.; Chiba, T.; Kido, J.; Kuo, C.-C. High luminescence and external quantum efficiency in perovskite quantum-dots light-emitting diodes featuring bilateral affinity to silver and short alkyl ligands. Chem. Eng. J. 2021, 414, 128866. [Google Scholar] [CrossRef]
- Zheng, W.; Wan, Q.; Liu, M.; Zhang, Q.; Zhang, C.; Yan, R.; Feng, X.; Kong, L.; Li, L. CsPbBr3 nanocrystal light-emitting diodes with efficiency up to 13.4% achieved by careful surface engineering and device engineering. J. Phys. Chem. C 2021, 125, 3110–3118. [Google Scholar] [CrossRef]
- Chiba, T.; Hoshi, K.; Pu, Y.-J.; Takeda, Y.; Hayashi, Y.; Ohisa, S.; Kawata, S.; Kido, J. High-efficiency perovskite quantum-dot light-emitting devices by effective washing process and interfacial energy level alignment. ACS Appl. Mater. Interfaces 2017, 9, 18054–18060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, G.; Wu, Y.; Chen, M.; Dai, Y.; Liu, S.; Zhao, Q.; Lin, H.; Fang, J.; Jing, C.; et al. Surface reconstruction of CsPbBr3 nanocrystals by the ligand engineering approach for achieving high quantum yield and improved stability. Langmuir 2023, 39, 6222–6230. [Google Scholar] [CrossRef]
- Abiodun, S.L.; Pellechia, P.J.; Greytak, A.B. Effective purification of CsPbBr3 nanocrystals with high quantum yield and high colloidal stability via gel permeation chromatography. J. Phys. Chem. C 2021, 125, 3463–3471. [Google Scholar] [CrossRef]
- Abiodun, S.L.; Gee, M.Y.; Greytak, A.B. Combined NMR and isothermal titration calorimetry investigation resolves conditions for ligand exchange and phase transformation in CsPbBr3 nanocrystals. J. Phys. Chem. C 2021, 125, 17897–17905. [Google Scholar] [CrossRef]
- Balakrishnan, S.K.; Kamat, P.V. Ligand assisted transformation of cubic CsPbBr3 nanocrystals into two-dimensional CsPb2Br5 nanosheets. Chem. Mater. 2018, 20, 74–78. [Google Scholar] [CrossRef]
- Akhil, S.; Dutt, V.G.; Singh, R.; Mishra, N. Post-synthesis treatment with lead bromide for obtaining near-unity photoluminescence quantum yield and ultra-stable amine-free CsPbBr3 perovskite nanocrystals. J. Phys. Chem. C 2022, 126, 10742–10751. [Google Scholar] [CrossRef]
- VanOrman, Z.A.; Weiss, R.; Medina, M.; Nienhaus, L. Scratching the surface: Passivating perovskite nanocrystals for future device integration. J. Phys. Chem. Lett. 2022, 13, 982–990. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; ten Brinck, S.; Aellen, P.; Suess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef]
- Wang, C.; Malinoski, A.; Yuan, J.; Hu, G. A surface engineering approach for promoting dexter energy transfer from lead halide perovskite nanocrystals. J. Phys. Chem. C 2023, 127, 1135–1144. [Google Scholar] [CrossRef]
- Mei, X.; He, K.; Zhuang, R.; Yu, M.; Hua, Y.; Zhang, X. Stabilizing dynamic surface of highly luminescent perovskite quantum dots for light-emitting diodes. Chem. Eng. J. 2023, 453, 139909. [Google Scholar] [CrossRef]
- Kreig, F.; Ong, Q.K.; Burian, M.; Raino, G.; Naumenko, D.; Amenitsch, H.; Suess, A.; Grotevent, M.J.; Krumeich, F.; Bodnarchuk, M.I.; et al. Stable ultraconcentrated and ultradilute colloids of CsPbX3 (X = Cl, Br) nanocrystals using natural lecithin as a capping ligand. J. Am. Chem. Soc. 2019, 141, 19839–19849. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Fasulo, F.; Munoz-Garcia, A.B.; Pavone, M.; Conelli, D.; Fanizza, E.; Striccoli, M.; Allegretta, I.; Terzano, R.; Margiotta, N.; et al. In situ formation of zwitterionic ligands: Changing the passivation paradigms of CsPbBr3 nanocrystals. Nano Lett. 2022, 22, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, F.; McCall, K.M.; Sakhatskyi, K.; Yakunin, S.; Trtik, P.; Bernasconi, C.; Cherniukh, I.; Mannes, D.; Bodnarchuk, M.I.; Strobl, M.; et al. Highly concentrated, zwitterionic ligand-capped Mn2+:CsPb(BrxCl1−x)3 nanocrystals as bright scintillators for fast neutron imaging. ACS Energy Lett. 2021, 6, 4365–4373. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Kim, J.; Lee, D.; Song, M.H. Efficient and stable perovskite nanocrystal light-emitting diodes with sulfobetaine-based ligand treatment. ACS Appl. Electron. Mater. 2023, 5, 5325–5331. [Google Scholar] [CrossRef]
- Guo, S.; Liu, H.; He, H.; Wang, W.; Jiang, L.; Xiong, X.; Wang, L. Eco-friendly strategy to improve durability and stability of zwitterionic capping ligand colloidal CsPbBr3 nanocrystals. Langmuir 2020, 36, 6775–6781. [Google Scholar] [CrossRef]
- Manoli, A.; Papagiorgis, P.; Sergides, M.; Bernasconi, C.; Athanasiou, M.; Pozov, S.; Choulis, S.A.; Bodnarchuk, M.I.; Kovalenko, M.V.; Othonos, A.; et al. Surface functionalization of CsPbBr3 nanocrystals for photonic applications. ACS Appl. Nano Mater. 2021, 4, 5084–5097. [Google Scholar] [CrossRef]
- Shcherbakov-Wu, W.; Sercel, P.C.; Krieg, F.; Kovalenko, M.V.; Tisdale, W.A. Temperature-independent dielectric constant in CsPbBr3 nanocrystals revealed by linear absorption spectroscopy. J. Phys. Chem. Lett. 2021, 12, 8088–8095. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.; Jin, Z.; Xin, Y.; Mattoussi, H. Enhanced stabilization and easy phase transfer of CsPbBr3 perovskite quantum dots promoted by high-affinity polyzwitterionic ligands. J. Am. Chem. Soc. 2020, 142, 12669–12680. [Google Scholar] [CrossRef]
- Jin, X.; Ma, K.; Gao, H. Tunable luminescence and enhanced polar solvent resistance of perovskite nanocrystals achieved by surface-initiated photopolymerization. J. Am. Chem. Soc. 2022, 144, 20411–20420. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Park, G.Y.; Kim, M.K.; Cha, J.; Ham, D.S.; Kim, M. Eco-friendly solvent-processible and highly luminescent perovskite nanocrystals with polymer zwitterions for air-stable optoelectronics. Chem. Eng. J. 2023, 459, 141531. [Google Scholar] [CrossRef]
- Noh, S.H.; Jeong, W.; Lee, K.H.; Yang, H.S.; Suh, E.H.; Jung, J.; Park, S.C.; Lee, D.; Jung, I.H.; Jeong, Y.J.; et al. Photocrosslinkable zwitterionic ligands for perovskite nanocrystals: Self-assembly and high-resolution direct patterning. Adv. Funct. Mater. 2023, 33, 2304004. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhao, G.; Wang, G.; Liu, P.; Li, H.; Hou, X.; Qiang, P.; Yang, Y.; Su, Q.; et al. Efficient blue CsPbBr3 perovskite nanocrystals synthesis with the assistance of zwitterionic straight chain amino acids. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131793. [Google Scholar] [CrossRef]
- Cohen, T.A.; Huang, Y.; Bricker, N.A.; Juhl, C.S.; Milstein, T.J.; MacKenzie, J.D.; Luscombe, C.K.; Gamelin, D.R. Modular zwitterion-functionalized poly(isopropyl methacrylate) polymers for hosting luminescent lead halide perovskite nanocrystals. Chem. Mater. 2021, 33, 3779–3790. [Google Scholar] [CrossRef]
- Kirsch, C.; Naujoks, T.; Haizmann, P.; Frech, P.; Peisert, H.; Chasse, T.; Brutting, W.; Scheele, M. Zwitterionic carbazole ligands enhance the stability and performance of perovskite nanocrystals in light-emitting diode. ACS Appl. Mater. Interfaces 2023, 15, 32744–32752. [Google Scholar] [CrossRef]
- Zhu, H.; Kick, M.; Ginterseder, M.; Krajewska, C.J.; Sverko, T.; Li, R.; Lu, R.; Shih, M.-C.; Voorhis, T.V.; Bawendi, M.G. Synthesis of zwitterionic CsPbBr3 nanocrystals with controlled anisotropy using surface-selective ligand pairs. Adv. Mater. 2023, 35, 2304069. [Google Scholar] [CrossRef]
- Xie, Q.; Wu, D.; Wang, X.; Li, Y.; Fang, F.; Wang, Z.; Ma, Y.; Su, M.; Peng, S.; Liu, H.; et al. Branched capping ligands improve the stability of cesium lead halide (CsPbBr3) perovskite quantum dots. J. Mater. Chem. C 2019, 7, 11251–11257. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Fu, J.; Li, X.; Zhao, X.; Jiang, H.; Lv, G.; Li, P.; Li, J.; Zhang, W.-H.; et al. Hybrid ligand polymerization for weakly confined lead halide perovskite quantum dots. ACS Appl. Mater. Interfaces 2023, 15, 20208–20218. [Google Scholar] [CrossRef]
- Hou, S.; Guo, Y.; Tang, Y.; Quan, Q. Synthesis and stabilization of colloidal perovskite nanocrystals by multidentate polymer micelles. ACS Appl. Mater. Interfaces 2017, 9, 18417–18422. [Google Scholar] [CrossRef]
- Sanjayan, C.G.; Jyothi, M.S.; Sakar, M.; Balakrishna, R.G. Multidentate ligand approach for conjugation of perovskite quantum dots to biomolecules. J. Colloid Interface Sci. 2021, 603, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jia, D.; Qiu, J.; Zhuang, R.; Hua, Y.; Zhang, X. Multidentate passivation crosslinking perovskite quantum dots for efficient solar cells. Nano Energy 2022, 96, 107140. [Google Scholar] [CrossRef]
- Jin, X.; Ma, K.; Chakkamalayath, J.; Morsby, J.; Gao, H. In situ photocatalyzed polymerization to stabilize perovskite nanocrystals in protic solvents. ACS Energy Lett. 2022, 7, 610–616. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, J.; Fu, J.; Lin, Y.; Zou, C.; Di, D.; Wang, Y.; Zhang, Q.; Cao, M. Ultrahigh stability of perovskite nanocrystals by using semiconducting molecular species for displays. ACS Nano 2022, 16, 12253–12261. [Google Scholar] [CrossRef]
- Yin, W.; Li, M.; Dong, W.; Luo, Z.; Li, Y.; Qian, J.; Zhang, J.; Zhang, W.; Zhang, Y.; Kershaw, S.V.; et al. Multidentate ligand polyethyleneimine enables bright color-saturated blue light-emitting diodes based on CsPbBr3 nanoplatelets. ACS Energy Lett. 2021, 6, 477–484. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.; Donmez, S.; Xin, Y.; Mattoussi, H. Polysalt ligands achieve higher quantum yield and improved colloidal stability for CsPbBr3 quantum dots. Nanoscale 2021, 13, 16705–16718. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Donmez, S.; Xin, Y.; Mattoussi, H. Engineering highly fluorescent and colloidally stable blue-emitting CsPbBr3 nanoplatelets using polysalt/PbBr2 ligands. Chem. Mater. 2022, 34, 4924–4936. [Google Scholar] [CrossRef]
- Malinoski, A.; Hu, G.; Wang, C. Strong bidentate coordination for surface passivation and ligand-shell engineering of lead halide perovskite nanocrystals in the strongly quantum-confined regime. J. Phys. Chem. C 2021, 125, 24521–24530. [Google Scholar] [CrossRef]
- Yin, W.; Li, M.; Dong, W.; Zhang, X.; Zheng, W. Overcoming the ambient manufacturability-performance bottleneck in perovskite nanocrystal emitters for efficient light-emitting diodes. Angew. Chem. 2023, 62, e202303462. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, W.; Chen, S.; Zhang, Z.; Cai, B.; Qiu, Y.; Jiang, J.; He, Y.; Nan, M.; Chen, Y.; et al. Multidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable and efficient red-light-emitting diodes. J. Phys. Chem. Lett. 2023, 14, 6639–6646. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Bing, Q.; Xiong, Y.; Yang, F.; Liu, H.; Liu, J.-Y.; Zhang, H.; Zheng, W.; Rogach, A.L.; et al. Surface stabilization of colloidal perovskite nanocrystals via multi-amine chelating ligands. ACS Energy Lett. 2022, 7, 1963–1970. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, H.; Hills-Kimball, K.; Cai, T.; Shi, W.; Wei, Z.; Yang, H.; Candler, Y.; Wang, P.; He, J.; et al. Stereoselective C-C oxidative coupling reactions photocatalyzed by zwitterionic ligand capped CsPbBr3 perovskite quantum dots. Angew. Chem. 2020, 59, 22563–22569. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Byranvand, M.M.; Martinez, C.O.; Hoye, R.L.Z.; Saliba, M.; Polavarapu, L. Defect passivation in lead-halide perovskite nanocrystals and thin films: Toward efficient LEDs and solar cells. Angew. Chem. 2021, 133, 21804–21828. [Google Scholar] [CrossRef]
- Liu, M.; Matuhina, A.; Zhang, H.; Vivo, P. Advances in the stability of halide perovskite nanocrystals. Materials 2019, 12, 3733. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soosaimanickam, A.; Saura, A.; Farinós, N.; Abargues, R. Influence of Binary Ligands in Designing Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals-Oleic Acid and Oleylamine. Nanoenergy Adv. 2023, 3, 376-400. https://doi.org/10.3390/nanoenergyadv3040019
Soosaimanickam A, Saura A, Farinós N, Abargues R. Influence of Binary Ligands in Designing Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals-Oleic Acid and Oleylamine. Nanoenergy Advances. 2023; 3(4):376-400. https://doi.org/10.3390/nanoenergyadv3040019
Chicago/Turabian StyleSoosaimanickam, Ananthakumar, Alejandro Saura, Noemi Farinós, and Rafael Abargues. 2023. "Influence of Binary Ligands in Designing Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals-Oleic Acid and Oleylamine" Nanoenergy Advances 3, no. 4: 376-400. https://doi.org/10.3390/nanoenergyadv3040019
APA StyleSoosaimanickam, A., Saura, A., Farinós, N., & Abargues, R. (2023). Influence of Binary Ligands in Designing Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals-Oleic Acid and Oleylamine. Nanoenergy Advances, 3(4), 376-400. https://doi.org/10.3390/nanoenergyadv3040019