Abstract
Metal–support interaction (MSI) is considered a key effect of electronic and geometric structures of catalysts on tuning catalytic performance. The oxygen evolution reaction (OER) is a crucial process during energy conversion and storage. However, the OER process requires the help of noble metal catalysts to reduce the reaction overpotential, enhance reactivity with intermediates, and maintain good operating stability. Carbon–supported metal catalysts have been considered candidates for noble metal catalysts for OER. MSI occurs at the interface of carbon supports and metals, affecting the catalytic performance through electronic and geometric modulation. MSI can influence the catalytic performance and change reaction pathways from charge redistribution, electron transfer, chemical coordination and bonding, and steric effect. Connecting MSI effects with the OER mechanism can provide theoretical guidance and a practical approach to the design of efficient catalysts, including the modulation of particle size, morphology, heteroatom doping, defect engineering, and coordination atom and number. Advantage can be taken of MSI modulation between metal compounds and carbon supports to provide guidance for catalyst design.
1. Introduction
The development of supported catalysts composed of carbon-based supports and metal actives is increasingly becoming an alternative to noble metal catalysts in the field of electrochemical catalysis [1,2]. Nowadays, carbon–based supported non–precious–metal catalysts are considered one of the hotspots and difficult fields of oxygen evolution reaction (OER), due to the catalysts’ difficulty in balancing low overpotential, high activity, long-term stability, and acceptable cost simultaneously [3,4,5]. When non-precious metals are deposited on the surface of carbon supports, the metal–support interaction (MSI) effect may occur, which affects the catalytic process due to electronic and geometric changes. As far as the current OER mechanism is concerned, OER is a complex electrochemical reaction, including four electron–proton transfer processes and multiple reaction intermediates, which is generally summarized as the adsorbate evolution mechanism (AEM) [6,7] and lattice oxygen evolution mechanism (LOM) [8,9]. The MSI effect mainly describes the interaction between catalysts and their supporting matrix, which influences the catalytic processes from charge transfer, electron redistribution, chemical coordination and bonding, and steric effect. Therefore, it is necessary to design, optimize, and prepare high–performance catalysts from the combination of reaction mechanisms and catalyst structure effects.
Non–precious–metal–supported carbon–based catalysts have attracted extensive attention mainly because of the structure diversity and easy surface modification of carbon–based supports, as well as the large selectivity of metal-based catalyst candidates, configurations, and functionalization. For a long time, we have paid attention to the influence of surface interface regulation on catalytic processes [10]. For instance, carbon quantum dots activated MoS2 can enhance hydrogen adsorption and accelerate catalytic kinetics due to a suitable hydrogen spillover pathway [11]. The surface hydroxyl groups on a biomass–derived carbon microtube acted as the growth sites for fixed CoP and grew in special positions [12], ensuring low overpotential and long–term stability for the hydrogen evolution reaction. The optimized geometric and electronic structure promoted the catalytic activity and electronic conductivity of MnCo2O4 by doping with transition metals (Fe, Ni, and Zn) to facilitate the OER [13]. It is increasingly recognized that the synergistic effect between multi–component and multi–dimensional structure can be tuned by surface/interface engineering to enhance photoelectrochemical catalysis [14,15,16]. Therefore, we expect to obtain good design ideas for high-performance catalysts by in–depth analysis of the relationship between the OER mechanism and the MSI effect, combined with measures such as doping, coordination, and surface/interface engineering.
Herein, based on the OER mechanism, we analyzed MSI effects on catalytic reaction pathways and catalytic performance. Charge transfer, electron redistribution, chemical coordination and bonding, and steric effect induced by MSI can influence catalytic performance and change reaction pathways. For carbon–supported catalysts, the MSI between carbon supports and metals can change electronic and geometric structures, making MSI modulation a useful method to improve catalytic performance. The modulation of MSI can be achieved by altering particle size, morphology, heteroatom doping, defect engineering, and coordination atom and number. Connecting MSI effects and the OER mechanism can provide theoretical guidance and practical approaches to design efficient and stable catalysts.
2. Fundamentals of the Oxygen Evolution Reaction
The OER is a complex electrochemical reaction engaging four electron–proton transfer processes. The reaction mechanisms of OER have been widely recognized as conventional AEM and LOM mechanisms. In AEM, H2O is firstly adsorbed on the active site (M) through a one-electron oxidation process to form M-*OH species. Further proton coupling and electron removal of M-*OH produces M-*O species (Figure 1a) [17]. The M-*O species reacts with H2O to form M-*OOH, and O2 is released via a one-electron oxidation reaction. Different from AEM, LOM indicates that the formed M-*O species couples with lattice oxygen, releasing O2 and forming oxygen vacancies (Figure 1b). The adsorption energies of intermediates for each step represent the activity of electrocatalysts. The step with largest Gibbs free energy (∆G) among the four steps is determined as the rate-determining step (RDS) for OER [18]. The ideal electrocatalyst requires the ∆G for four adsorption processes, all at the 1.23 V equilibrium potential (Figure 1c). For AEM, the linear scaling relationship of is ascribed to the minimum overpotential value of 0.37 eV [19]. When the reaction mechanism shifts from AEM to LOM, the linear relationship is broken and theoretical overpotential is lower than 0.37 V.
Carbon–supported catalysts with the MSI effect can change the OER mechanism by electronic and geometric structure changes. The covalent state of the metal–oxygen (M-O) bond can affect the reaction mechanism. With the increase of the covalent of the M-O bond, the reaction mechanism tends to transfer from AEM to the LOM mechanism [20]. LOM with higher catalytic ability is accompanied by metal dissolution and structural instability. MSI can stabilize metal compounds as well as increase the covalent state of the M-O bond, keeping a balance between the two reaction mechanisms for stable and efficient catalysts. Three strategies based on the OER mechanism have been applied to improve OER activity as follow: (1) Stabilizing the *OOH intermediate without affecting *OH adsorption. (2) Modifying the reaction pathway by introducing a proton acceptor. (3) Activating lattice O for direct O-O coupling [19].
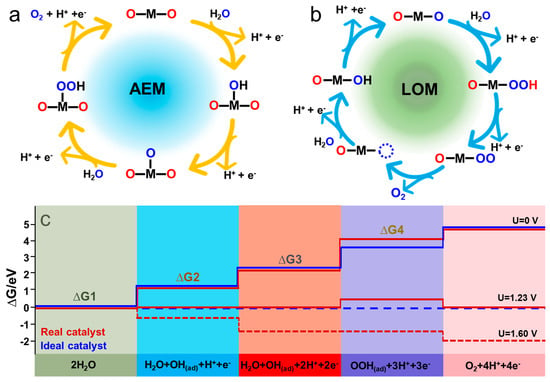
Figure 1.
Mechanisms of OER. (a) Conventional AEM mechanism; (b) LOM mechanism (M represents the active sites); (c) Gibbs free energies at U = 0 for ideal and real electrocatalysts. Reproduced with permission of [17], copyright 2021 John Wiley and Son.
3. Metal–Support Interaction Effect
MSI describes the influence of the interaction of metals and their compounds on carriers on the catalytic reaction process, especially OER mechanisms; the interrelationship between the key performance indicators, such as activity, selectivity, and stability; and the intrinsic structures of catalysts, such as electronic and geometric structures [21,22] (Figure 2). Strong interactions such as electronic interaction, chemical bond, and functional groups can stabilize metals on supports via chemical bonding and physical adsorption. Covalent states of metal active centers can be changed by MSI by charge transfer and electron redistribution, improving the intrinsic activity for OER. The MSI can modulate the O-intermediates’ adsorption energy and change the reaction pathway of OER. Modulation of MSI between the metals and supports provides guidelines for the rational design of electrocatalysts.
3.1. Stabilization Effect
The interaction between metals and carbon supports can enhance the electrocatalytic stability of metals during OER. Metal species with high surface free energy tend to aggregate to reduce surface free energy. Supporting active metals on conductive carbon supports can improve stability via MSI. A strong interaction between metal species and carbon matrix can prevent metals from aggregating, enhancing the stability of electrocatalysts. The stabilization effect is the most basic influence of MSI and has been applied in enhancing electrocatalyst stability. The stability of noble metal (Pt, Ru, Ir) electrocatalysts is of great significance for the practical application of noble metal catalysts. The degradation mechanisms of noble metals on carbon supports include particle detachment, agglomeration, carbon corrosion, noble metal dissolution, and Ostwald ripening. Anchoring the active metal phases on carbon–based supports with high stability through MSI can improve the stability of catalysts. The stability of Pt particles supported on carbon supports has been improved via strengthening the MSI [23]. A CoIr alloy supported on N–doped carbon showed high activity for OER. A strong metal–carbon interaction can optimize the properties of CoIr alloy and improve long–term stability [24]. The MSI effect was applied to prevent electrochemical degradation of CoIr alloy, and a negligible decay was observed after long-term stability measurement for OER. Transition metal selenides Ni3Se4 was supported on ultrathin carbon through a strong electronic interaction [25]. An ultrathin carbon layer protected Ni3Se4 from aggregation and corrosion, endowing electrochemical catalysts with long–term stability. The stabilization effect has been achieved in supported metal catalysts featured with MSI, making the supported catalysts stable under reaction conditions for OER.

Figure 2.
Schematic diagram of metal–support interaction effects: mechanisms, structures, and performances.
3.2. Electronic Structure Regulation
3.2.1. Metal–Carbon Electron Transfer
Electron transfer occurs on the interface of metal and carbon. The magnitude and direction of the charge transfer are driven by the EF difference between metals and carbon supports, which ultimately seeks to balance the electronic chemical potential. The equalization of EF relies on the transfer of electrons between the two materials [26]. When the EF level of metals is higher than supports, electrons are transferred from metals to supports. When the EF level of carbon supports is higher than metals, electrons transfer from carbon supports to metals until reaching an equilibrium. The electron transfer between metals and carbon supports greatly affects the OER process and mechanism by modulating the activation energies of intermediates and adsorption energies of the reactants [27]. Figure 3a shows electron transfer from metal core to a carbon shell with three graphene layers [28]. Electron transfer to graphene tunes the π electron occupancy, which can affect the catalytic ability. The binding energy of O–intermediates (∆Eads) versus an extra π electron can modulate the adsorption strength of oxygenated species. Too many or too few π electrons induces a stronger adsorption strength, and the extra π electron occupancy has more influence on the value of ∆EO*, affecting the OER mechanism (Figure 3b). For single-atom-supported catalysts, electron transfer from carbon support to metal atom is evaluated as the differential charge density of metal centers, shown in Figure 3c [29]. For nanoparticle-supported catalysts, electron transfer is across the interface between metal components and amorphous carbon. FeNi3 nanoparticles encapsulated with NC layers induced electron transfer and the interfacial electron structure analyzed via first-principles calculation, as illustrated in Figure 3d. The differential charge density of the FeNi3 and NC layers indicated electron transfer from the FeNi3 layer to the NC layer, which is attributed to the metal–carbon interaction between FeNi3 and NC. The NC layers with a charge-rich state facilitated the formation of surface catalytic active centers [30]. The more conductive platform facilitates faster electron transfer at the interface (Figure 3e). In a Sn4P3–SWCNT heterostructure, electron migration can reduce the charge density of the Sn site, minimize the O–intermediates’ Gibbs free energy, and affect the OER mechanism [31]. The magnitude and direction of electron transfer are critical in the design of efficient electrocatalysts for enhanced OER catalytic capability.
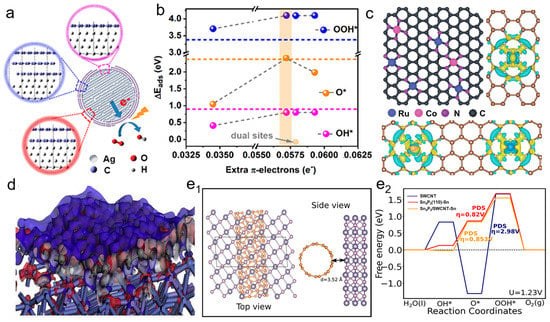
Figure 3.
(a) The interface profile of Ag core and graphene shell. Reproduced with permission of [28], copyright 2019 ACS Publishers. (b) Relationship between ΔEads and the extra π–electrons. (c) Differential charge density of Ru–N–C and Ru/Co–N–C. Reproduced with permission of [29], copyright 2022 John Wiley and Son. (d) The electron transfer between electron density difference of FeNi3 and NC. Reproduced with permission of [30], copyright 2020 Elsevier. (e1) Sn4P3–SWCNT interaction schematic diagram. (e2) Gibbs free energy of O-intermediates at Sn and P sites. Reproduced with permission of [31], copyright 2022 ACS Publishers.
3.2.2. Redistribution of Electron Density
Rearrangement of electrons between metal components and supports induces the redistribution of electron density. Redistribution of electron density can modulate the d–band center. According to the d–band center theory, the d–band center energy level indicates the degree of electron filling in an antibonding state [32]. Atoms with different radii and electronegativities lead to different degrees of electron redistribution. As shown in Figure 4a, the d-band center of the Co atom in CoNi/Co–N4–CNTs (−0.84 eV) was lower than that of Co/Co–N4–CNTs and Co–N4–CNTs [33]. The downshifted d–band center was beneficial to the desorption behavior and subsequent reactions, modulating and accelerating the OER process. The redistribution of electron density can be affected by the properties of supports. Metal oxides [34], hydroxides [35], nitrides [36], MXenes [37], and carbon materials [38] are commonly used as supports. Heteroatom doping in a carbon support can affect the charge redistribution. The charge density between Co2P2O7 and N, P co-doped carbon was confirmed by electron density difference analysis (Figure 4b). The yellow and green represent charge depletion and accumulation in the space. The electron transfer from Co2P2O7 to N, P co–doped carbon induced electron density redistribution and changed the electronic structure [39]. Redistribution of electron density of supported catalysts has proved to be a critical factor for catalytic enhancement [40]. BG and G were prepared with and without the addition of an aqueous solution of bacterial strain pandoraea sp. B-6 into graphene oxide (GO). The catalytic performance of BG@Ni/Ni3S2 has been improved due to electron density redistribution. Four-electron transfer processes for the OER mechanism were adopted to clarify the high catalytic ability (Figure 4c) [41]. BG@Ni/Ni3S2 showed the highest capacity with an overpotential of 250 mV at 20 mA/cm2 (Figure 4d). Tuning electron rearrangement and regulating MSI are feasible approaches to optimize the OER mechanism and improve catalytic performance.

Figure 4.
(a) Density of states (DOS), charge density redistributions, and d-band centers of Co−based electrocatalysts. Reproduced with permission of [33], copyright 2022 Elsevier. (b) The charge density at the interface of Co2P2O7@N, P−C. Reproduced with permission of [39], copyright 2020 Elsevier. (c) Schematics of the OER catalytic mechanism for BG@Ni/Ni3S2. (d) Linear sweep voltammetry plots of BG, BG@Ni, G@Ni, and BG@Ni/Ni3S2 in 1.0 M KOH. Reproduced with permission of [41], copyright 2020 ACS Publishers.
3.3. Geometric Structure Modulation
3.3.1. Coordination by Functional Groups
MSI can be coordinated by the functional groups on the supports. Support surface functionalization can change the characteristics of the supports, strengthening the interaction between the metals and the supports and modulating the geometric structure. Surface functionalization of carbon supports can be achieved by covalent or non-covalent interactions between supports and functional groups/molecules. Covalent interaction implies the formation of covalent bonds. Functional groups commonly covalently attached to carbon supports’ surfaces include –OH, –COOH, –NH2, and –OCH3 [42]. Non-covalent interactions are in the forms of van der Waals force, coordination bonds, hydrogen bonds, and π–π stacking interactions [43]. Functional groups on supports’ surfaces can adsorb metal precursors through electrostatic interactions, which can serve as anchoring sites for metals to attach to the supports. The metal phase connects with the functional groups by replacing the H in –OH, –COOH, –NH2, and –OCH3. The functional groups on carbon support surfaces can accelerate electron transfer, improving the electrocatalytic performance of catalysts for OER. Functional groups with various numbers and types have various effects on catalytic reactions. In our previous work, we constructed –OH functional groups on biomass-derived microtubes for surface bonding of CoP (CoP@BCMTs) [12]. The surface –OH functional groups acted as the anchoring sites for the even distribution of CoP through C–O–Co bonds (Figure 5a,b). Carbon supports with functional groups can modulate the strength of MSI, which affects the geometric structure and catalytic ability. Thiocyanate groups on carbon surfaces can alter catalytic performance by modulating the adsorption energies of intermediates. Thiocyanate groups were proposed to enhance the electrocatalytic activity for OER catalysts. As shown in Figure 5c, the thiocyanate groups firstly electrostatically interacted with Ru3+ and formed SCN covered with Ru–RuO2 nanoparticles after annealing treatment. Fourier transform infrared spectroscopy (FTIR) was applied to detect the chemical interaction of SCN and Ru-RuO2 (Figure 5d) [44]. The thiocyanate groups were beneficial to accelerate H2O and *OH dissociation, reduce the energy barrier, and decrease the overpotential for OER. Functional groups arousing strong interaction can regulate electronic and geometric structures, improving catalytic performance. Electron-withdrawing groups (–COOH and –NO2, etc.) and electron-giving groups (–OH, –NH2, –OCH3, etc.) were discussed for OER performance. Anisole, benzene, nitrobenzene, 2,5 dihydroterephthalic acid, terephthalic acid, aniline, and 2-aminoterephthalic acid acted as ligands to introduce various functional groups [45]. The zeta potentials of various ligands are presented in Figure 5e. The 2-aminoterephthalic acid with electron-withdrawing groups (-COOH) and electron-giving groups (–NH2) showed a stronger ability to bond to metal. A high density of electron-giving groups (–NH2) gave away electrons to combine H+, and electron-withdrawing groups (–COOH) absorbed electrons to combine OH−, ascribing to the highest OER electrocatalytic activity (Figure 5f). Surface functionalization can also lead to the introduction of heteroatoms in the carbon framework, including oxygen, nitrogen, phosphorus, sulfur, and so on. Heteroatom doping can regulate catalysts’ electronic structure, coordinating the MSI between metals and supports. Functional groups have adopted superaerophobic/superhydrophilic and superaerophilic/superhydrophobic surfaces into their design [46]. The superhydrophilic/superaerophobic surface facilitated electrolyte transport and bubble desorption, accelerating the OER kinetics.
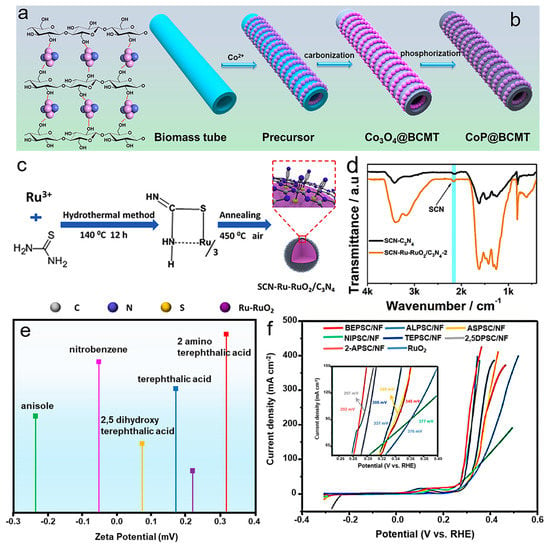
Figure 5.
(a) Schematic diagram of hydroxyl groups (C−OH) on the surface of biomass. (b) The synthesis process of CoP@BCMT. Reproduced with permission of [12], copyright 2022 Elsevier. (c) Preparation process of SCN−Ru−RuO2/C3N4. (d) The FTIR of SCN−C3N4 and SCN−Ru−RuO2/C3N4. Reproduced with permission of [44], copyright 2020 Elsevier. (e) The zeta potential of ligands (anisole, benzene, nitrobenzene, 2,5 dihydroterephthalic acid, terephthalic acid, aniline, and 2-aminoterephthalic acid) in DMF. (f) LSV curves of PSC/NF modulated with various ligands for OER. Reproduced with permission of [45], copyright 2021 Elsevier.
3.3.2. Chemical Covalent Bonding
Chemical bonding is a strong interaction form between metals and supports. A strong chemical covalent bond can improve the stability and activity of electrocatalysts. Chemical covalent bonding enables uniform distribution of metals on the support substrates. Since the surface energy increases dramatically with decreasing particle size, metallic species tend to grow into larger crystals. Chemical bonding can prevent metals from growing into large crystallites and improve exposure of metal active sites [47]. Construction of covalent bonds between metals and carbon supports can endow catalysts with a unique geometric structure. The chemical covalent bonds between metals and carbon materials include metal–O–C, metal–N–C, metal–P–C, and metal–S–C bonds [48]. In situ generated Co-O-C bonds can act as an oxygen bridge between CoP nanoparticles and the carbon layer (Figure 6a) [49]. Co-O-C bonds can decrease energy barriers to improve OER activity and induce charge transfer to strengthen chemical adsorption (Figure 6b). M–N–C and M–P–C bridged the N, P co–doped carbon spheres and Co2NiOx nanoparticles to form a strong interaction between the Co2NiOx and N, P co–doped carbon. Electron transfer through covalent bonds from metal oxides to carbon supports increases the oxidation state of active metals [50]. The strategies for the formation of chemical covalent bonds between supports and metals include heteroatom doping, defect engineering, functional groups, metal-organic framework derivatization, and clicking confinement [51,52]. Click confinement based on click chemistry showed high reaction selectivity. Zinc (II) phthalocyanine and single–walled carbon nanotube (SWCNT) were represented as a metal-containing molecule and support, respectively. Zinc (II) phthalocyanine with multi–functional groups was anchored on SWCNT through azide-alkyne Huisgen cycloaddition (Figure 6c) [53]. The chemical covalent bonding of a Zn active center can improve electron transfer rate and conductivity, which can be ascribed to the high OER activity performance. For single-atom catalysts, the N–coordinated MN4 sites in carbon are deemed as candidate noble metals for electrocatalytic reactions. CoN4 sites generated through carbonization and doping are presented in Figure 6d [54]. Co hydroxide after Co2+ adsorption at room temperature decomposed to an oxide at 523 °C, shifted to CoN4 with small Co metal particles at about 700 °C, and achieved atomically dispersed CoN4 when the temperature increased to 800 °C. The Co–O and Co–OH species were converted into CoN4 sites with the temperature increase, increasing the CoN4 sites’ density and optimizing mass transport properties.
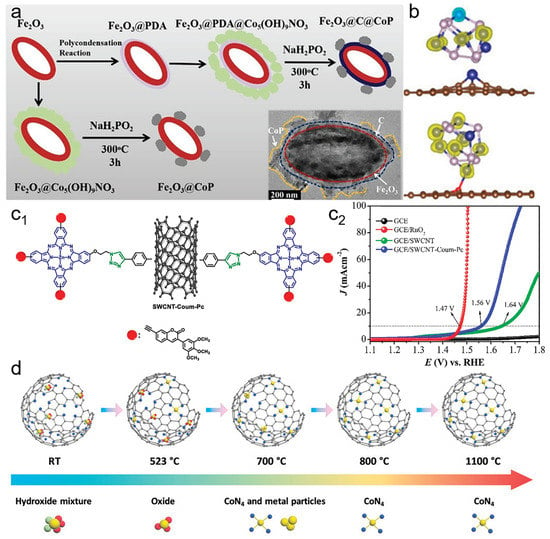
Figure 6.
(a) Scheme for the fabrication of electrocatalysts. (b) Spin density of CoP/C and Co–O–C@CoP, isovalue = 0.02 a.u. Reproduced with permission of [49], copyright 2022 John Wiley and Son. (c) Synthetic pathway of zinc (II) phthalocyanine anchored on SWCNT through click reaction and its OER performance. Reproduced with permission of [53], copyright 2021 Elsevier. (d) Dynamic structural evolution of Co ions within a nitrogen–doped carbon host with temperature changes. Reproduced with permission of [54], copyright 2022 John Wiley and Son.
4. Modulation Strategies of Metal–Support Interaction
MSI can influence catalytic performance and change reaction pathways from charge redistribution, electron transfer, chemical coordination and bonding, and steric effect. Understanding and making full use of MSI is essential to improving electrocatalytic performance. The parameters affecting MSI can be divided into three aspects, including metal properties, carbon support, and the coordination effect.
4.1. Metal Properties
Metal properties including metal species, particle size, and metal morphology can induce MSI changes, affecting the adsorption energy of intermediates and reactants. The MSI is maximized when the metal nanoparticle size is reduced to atomic level, enhancing catalytic stability and adjusting the OER mechanism. Various metal morphologies with varied crystal faces in contact with the supports modulate the charge transfer direction and adjust the reaction mechanism. The modulation of metal properties is significant in optimizing MSI for enhanced catalytic ability.
4.1.1. Particle Size
MSI can be affected by the particle size of the supported metal species. Metal supported on carbon supports can be divided into nanoparticle, nanocluster, and single atom according to particle size. Particle size depends on the MSI between metals and carbon materials by modulating the charge transfer [55]. With an increase in nanoparticle size, the work function increases, indicating the strengthened electron capture ability [56]. The nanoparticle can adsorb an electron and induce charge transfer between supports and metals, for metals with an over-oxidation valance state. The electronic structure changes from metal energy band to molecular and atomic orbitals when the particle size is reduced from a nanoparticle to a single atom (Figure 7a) [57]. Precious metals (Pt, Ir, Ru, etc.), non-precious metals (Fe, Ni, Co, Mo, etc.), and their phosphides, oxides, sulfides, nitrides, and alloys have been researched as metal compounds supported on carbon materials for OER [58,59,60]. Ru nanoparticles were anchored on N, P co–doped nanosheets, and the top sites of defected N atoms were the optimal sites for Ru nanoparticles to be anchored (Figure 7b) [61]. The Pt particle diameter ranged from 2 to 10 nm, as displayed in Figure 7c. Reducing Pt particle size can improve the utilization and dispersion of Pt. The particle size depended on shifts in oxophilicity and the electrochemically active surface area (Figure 7d) [62]. The alloy with small particle size shows high OER catalytic performance. Metal-organic framework–derived Co, Cu, Fe alloy nanospheres with diameter of 200 nm were prepared and showed high OER catalytic performance (Figure 7e) [63]. With a decrease in particle size, atom utilization increases. Recently, single-atom catalysts have been broadly used in electrocatalysis. Single-atom catalyst Ir1@NC was prepared via a π–electron-assisted strategy [64]. High–angle annular dark field (HAADF) SEM and inverse high resolution HAADF images of Ir1@NC showed a high density of isolated Ir atoms distributed on the carbon layer (Figure 7f). Ir1@NC exhibited a low energy barrier, high selectivity, and high activity for OER. A reduction in metal size increases atomic utilization and improves catalytic efficiency. MSI is maximized when the metal nanoparticle size is reduced to atomic level [65]. The modulation of metal size is significant in optimizing the MSI for enhanced catalytic ability.

Figure 7.
(a) Geometric and electronic structures of single atom, cluster, and nanoparticle. Reproduced with permission of [57], copyright 2022 John Wiley and Son. (b) Atomic structures of Ru/d-NPC and electron localization function of Ru/d–NPC. Reproduced with permission of [61], copyright 2022 Elsevier. (c) Pt particles with diameters of 2 to 10 nm and (d) the oxophilicity and electrochemically active surface area influenced by particle size. Reproduced with the permission of [62], copyright 2020 ACS Publishers. (e) Schematic illustration for the synthesis of Co, Cu, Fe alloy nanospheres with diameter of 200 nm. Reproduced with permission of [63], copyright 2022 Elsevier. (f) HAADF–STEM image of Ir1@Co/NC at high magnification and FFTI–HAADF image of Ir1@NC area. Reproduced with permission of [64], copyright 2019 John Wiley and Son.
4.1.2. Metal Morphology
Lattice mismatches can induce morphology changes in carbon-supported electrocatalysts. Metal components with various morphology and geometric changes can modulate MSI. The morphology of metal compounds includes three-dimensional (nanospheres, nanoparticles) [66], two–dimensional (nanosheets, nanofilms) [67], one–dimensional (nanowires, nanotubes) [68], and zero–dimensional structures (nanodots, quantum dots) [69]. Three–dimensional structures provide a large active surface area and make sure of sufficient contact between the electrode and electrolyte. Various phosphides species evenly distributed on the surface of hollow Co-Fe phosphide nanospheres were synthesized (Figure 8a) [70]. The Co/Fe ratio of Co–Fe phosphides can control atom migration and turn the distribution of CoP, Co2P, and FeP4 on the surface. Nanoparticles with less shape anisotropy can provide abundant edge active sites. Ni–Fe layered double hydroxide (Ni–Fe LDH) nanoparticles with ultra–small (~5 nm) size were prepared. The Ni–Fe LDH with less shape anisotropy exposed more active sites and showed high catalytic ability for OER [71]. In two–dimensional nanosheets with edge active sites at the boundary, the two–dimensional plane is beneficial to the transfer of electrons in the plane. Some hcp–Ni3Fe/C nanosheets were synthesized by the reduction of Ni3Fe–LDH via CH4 plasma under room temperature (Figure 8b) [72]. The hcp–Ni3Fe/C nanosheets exhibited high conductivity ensuring rapid electron transfer in the plane. Synergy of ultrafine wire properties ensured mass transport and active site utilization. Pseudo-periodically welded NiO with CeO2 ultrathin nanowires arrays were fabricated through the dual-metal-oxide lattice coupling strategy for high catalytic activity and fast mass transport [73]. S–doped CoWP embedded in S, N co–doped carbon matrix derived from metal–organic framework (MOF) nanowires showed outstanding catalytic performance for OER (Figure 8c) [74]. The synergic effect of multicomponents, enhanced conductivity, and increased intrinsic activity of S–CoWP@(S,N)–C was beneficial to the improvement of OER catalytic properties. Metal nanodots with small dimensions can improve metal utilization and catalytic efficiency. Monodispersed crystalline CoS2 nanodots anchored on a N, S co–doped carbon layer provided abundant active sites and excellent hydrophilic electrolyte properties, improving catalytic performance for OER [75]. Apart from the conventional morphologies, bio–inspired and biomimetic morphologies with unique structures have been obtained. Bio–inspired and biomimetic morphologies, such as flower–like [76], leaf–like [77], sea urchin–like [78], bamboo–like [79], and brush–like [80], exhibited unique properties in terms of morphological structure and catalytic performance (Figure 8d,e). Metal morphology can affect the work function of metals and influence the MSI between metals and supports. Different metal morphologies lead to changes in MSI strength; in turn, the strength of MSI can also be affected by metal morphologies. Various metal morphologies with varied crystal faces in contact with the supports modulate the charge transfer direction and adjust the reaction mechanism. Controlling crystal planes by morphology modulation provides a direction for the design and preparation of efficient electrocatalysts for OER.
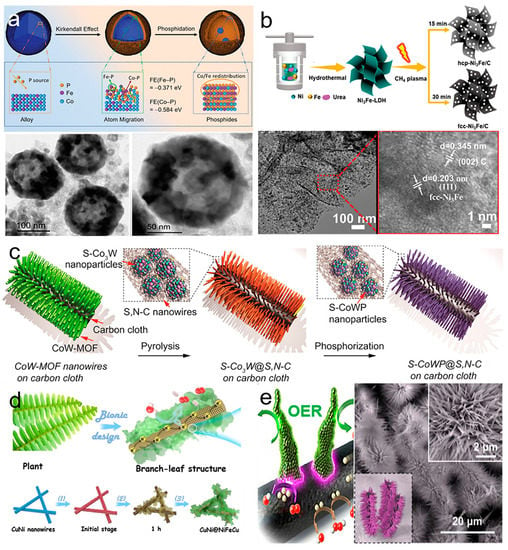
Figure 8.
(a) Schematic illustration of atom migration during the phosphorization process and TEM images of Co7Fe3 phosphides. Reproduced with permission of [70], copyright 2021 John Wiley and Son. (b) A schematic illustration for the synthesis and HRTEM images of hcp–Ni3Fe/C and fcc-Ni3Fe/C. Reproduced with permission of [72], copyright 2022 John Wiley and Son. (c) Synthesis process of S–CoWP@S,N–C nanowires. Reproduced with permission of [74], copyright 2018 ACS Publishers. (d) The preparation schematic of branch–leaf–shaped CuNi@NiFeCu. Reproduced with permission of [77], copyright 2021 Elsevier. (e) Schematic diagram and SEM image of CoOx@CoNy/NCNFs. Reproduced with permission of [80], copyright 2021 ACS Publishers.
4.2. Carbon Support
Supports can stabilize metal nanoparticles, and adjust the electronic structure and coordination environment on the surface of catalysts. Carbon materials are the most promising supports for stabilizing metal compounds and reducing agglomeration due to their large surface area and porous structure. The chemical environment and physical structure of carbon supports can alter the charge transfer between metals and carbon materials, impacting MSI. Modulation strategies for carbon supports include heteroatom doping, defect engineering, and morphography engineering.
4.2.1. Heteroatom Doping
Heteroatom doping is a prospective approach to modulate the geometric and electronic structures of carbon materials. Introducing non-metal heteroatoms (B, N, O, P, S, F, and Br) into a carbon skeleton can cause changes in the chemical/electronic environment, charge distribution, chemical bonds, and electron spin status (Table 1). Attributed to the different electronegativity and atomic radius from the host atom, the introduced heteroatoms can change the electronic structure [81,82]. The introduced heteroatom can change the chemical environment and form metal–non–metal bonds to anchor the metal compounds onto the surface. Heteroatom–doped carbon materials interact with metals, resulting in changes in interfacial electronic transfer, charge distribution, and MSI strength. High–electronegativity heteroatom (N, P, S, O, and F) doping leads to stronger interaction ascribed to the formation of covalent bonds between metals and carbon supports [83]. Covalent bonds between metals and carbon supports accelerate electron transfer and improve stability. The incorporation of heteroatoms in the carbon framework can influence the particle size of supported metals. Uniform and ultrasmall size of metals were reported to distribute on P–doped carbon with more exposed active sites and improved catalytic performance [84]. Furthermore, it has been reported that heteroatom introduction can affect the d-band center for optimized adsorption energy, further affecting the electronic structure and catalytic activity of catalysts. B, C, N, and F with varying electronegativity showed a synergistic coupling effect (Figure 9a) [85]. B–C and N–C bonds created extra electronic states of the metal center and modulated the electronic structure, improving the MSI between metals and carbon materials. Heteroatoms N, N–B, N–P, and N–S were introduced to porous carbon nanofibers to modify the d-band centers of Ni single-atom catalysts (Figure 9b) [86]. The d–band center of Ni atoms coupled with three N atoms and one P atom was regulated to moderate position for favorable binding with reaction intermediates. N was doped into the carbon skeleton in the form of pyridine-N, pyrrolic-N, and graphitic–N [87]. Different N types play various roles in catalysis reactions. Apart from the single–atom introduction, dual–atom doping into carbon supports has also been researched due to the synergistic coupling effect of dual atoms. IrO2 supported on B, N co-doped reduced graphene oxide was prepared for OER. Carbon materials doped with heteroatoms showed more pronounced acidic or basic character compared with the bare carbon, inducing the enhancement of catalyst hydrophilicity. Heteroatom doping can bring about changes to electronic structure, including band gap, density of states, charge distribution, and d–band center. Introducing heteroatoms into carbon frameworks is a promising method to modulate the interaction between metal and supports for carbon-supported electrocatalysts.

Table 1.
Non–metal heteroatom doping to carbon framework for OER electrocatalysts.
4.2.2. Defect Engineering
Defects can be divided into zero–dimensional point defects, one–dimensional line defects, two–dimensional planar defects, and three–dimensional volume defects [96]. Defects can improve conductivity and enhance the MSI between metals and supports. Defect formation strategies include plasma treatment, flame reduction, laser ablation, and electrochemical reduction [97]. Heteroatom doping can induce defects, and there exists differences in pore size according to the various heteroatoms. B and P-doping result in a slight pore size increase, while S and N–doping result in a decrease in the pore size. Defect engineering can expose more edge sites and improve electrocatalytic ability. Compared with basal plane carbon, edge carbon shows higher activity due to coordination unsaturation and a change in valence states [98]. Defective carbon materials can offer more anchor sites for active metals to interact through π–π coupling compared with bare graphene. Interaction between carbon supports and metals results in fast electron transfer and outstanding stability. Ni-Fe LDH nanosheets with positive charges were assembled with negatively charged defective graphene to form a hybrid nanocomposite (Figure 9c) [99]. Electron redistribution occurred at the interface after combining the defective graphene with Ni–Fe LDH nanoparticles, especially around the defect sites marked with blue dash frames. The electron transfer led to hole accumulation on Ni–Fe LDH, which was beneficial for the OER to shift from AEM to LOM. For single-atom catalysts, defects have an impact on the coordination environment around metal atom centers. In single-atom catalysts, M–Nx are deemed as active sites for electrocatalysis reaction. Defects near active metals can improve the intrinsic activity of the metals. A Co/N-C-800 catalyst composed of rich carbon defects and abundant Co–Nx structures was synthesized (Figure 9d) [100]. Co–Nx active sites along with carbon defects can reduce the adsorption energy of O-intermediates and improve the electron transfer ability, thus optimizing the OER process. Defect engineering modulates the electronic structure, band structure, charge distribution, and intrinsic activity of electrocatalysts, affecting the MSI between carbon materials and metals.
4.3. Coordination Effect
Tailoring the local chemistry environment of supported metal electrocatalysts for optimal electronic and geometric structures has been applied in regulating MSI and improving catalytic performance. Local chemistry environment, including coordination atoms and coordination numbers, has a great effect on catalytic performance. Changes in local chemistry environment can change reaction pathways and construct new catalytic sites [101]. Factors affecting the local chemistry environment include the location (edge or in-plane), the surrounding heteroatoms, and the surrounding ligands.
4.3.1. Coordination Atoms
Atoms adjacent to the metal centers can greatly influence the intrinsic catalytic activity of active metal sites. Various heteroatom dopants with different electronegativity and atomic radius have been introduced to alter the local chemistry environment of active metal centers. The coordination atoms can be divided into non-metal atoms and metal atoms. The non-metal heteroatoms N, O, and S have been introduced to a Mo single atom as coordination atoms to regulate the electronic structure (Figure 10a) [102]. Four S atoms surrounding one Mo atom in MoS42− and a polydopamine with N– and O–containing functional groups enabled the formation of S–Mo, C–N, and C–O bonds. Theoretical calculations indicated that the electronic structure of Mo atoms has been modulated by coordination environment engineering. Dual–metal–atom center catalysts boost the electronic and functional synergy effect between adjacent metal atoms, enhancing activities for OER. A Co–Fe bimetallic encapsulated in N-doped carbon was prepared via a “pre–constrained metal twins” strategy (Figure 10b) [103]. The contiguous metals regulated the d–band centers of metal sites according to the synergetic interaction, optimizing the free energy of the O–intermediates and affecting the OER mechanism. Coordination polymers with long range ordered and adjustable structures have also been applied as organic ligands to metals for electrocatalysis. Introducing different metals into coordination polymers is expected to fabricate highly efficient OER catalysts [104]. Catalytic activity could vary a lot for the same dopant heteroatom with various groups. N atoms are the most-used heteroatom dopant in M–N–C form. N atoms as heteroatom dopants can be divided into three types, including pyrrolic N, pyridine N, and graphitic N. Coordination N atoms with different N types can affect catalytic activity and selectivity. Co single–atom catalysts packaged in N–doped carbon with three N existing types was synthesized to investigate the N coordination environment effect on catalytic performance (Figure 10c) [105]. Coordinated N species in a particular graphitic N acted as electron donors to the Co metal center, promoting catalytic ability, kinetics, and stability of OER. Zn catalysts with C/O atoms coordination (ZnO3C) and N atom coordination (ZnN4) were derived from MOF precursors with different functional groups [106]. The modulation of the coordination environment of Zn can optimize the intermediate adsorption and change the reaction pathway. Different coordination atoms can tweak the electronic structure of the metal centers, affect reaction mechanism, and improve catalytic activity.

Figure 10.
(a) Preparation process of Mo single-atom catalyst with N, O, and S coordination atoms. Reproduced with permission of [102], copyright 2022 Elsevier. (b) Schematic diagram of the fabricating of Co–Fe dual–metal–atom center catalyst. Reproduced with permission of [103], copyright 2022 John Wiley and Son. (c) Illustration of the synthesis process of Co single-atom catalysts coordinated with different types of N heteroatom. Reproduced with permission of [105], copyright 2022 John Wiley and Son.
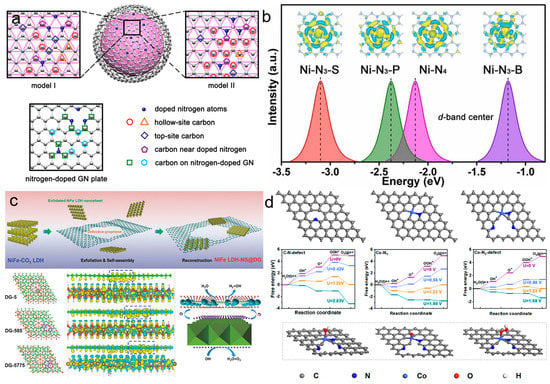
Figure 9.
(a) Schematic diagram of Co, N−doped porous hollow carbon microspheres. Reproduced with permission of [86], copyright 2020 ACS Publishers. (b) Calculated d−band centers of single−atom Ni coordinated with different atoms. Reproduced with permission of [87], copyright 2022 Elsevier. (c) DFT calculation studies of Ni−Fe LDH@DG-based composite. Reproduced with permission of [99], copyright 2017 John Wiley and Son. (d) Simulated structures of C−N1−defect, Co−N1, and Co−N1−defect. The free energies versus the reaction coordinates toward OER on C−N1−defect, Co−N1, and Co−N1−defect. Reproduced with permission of [100], copyright 2022 ACS Publishers.
4.3.2. Coordination Number
Tuning the coordination number of coordination atoms is a promising method to optimize the local chemistry environment. M–N–C catalysts are the most-reported electrocatalysts. The metal activity centers are bonded with a N coordinator. The coordination number of the N coordinator can modulate the charge distribution and metal center valance states, affecting the metal catalytic performance. MoSA–Nx–C catalysts coordinated with different Mo–N numbers were synthesized via heating ZIF-8 precursors at 800, 900, and 1000 °C (Figure 11a) [107]. The annealing temperature was found to be the key factor to determine the coordination number. X–ray absorption near edge structure (XANES) spectra were applied to analyze coordination N atom number to Mo atoms in MoSA–Nx–C catalysts (Figure 11b). The spectra indicated Mo–N3 with an oxygen molecule in the horizontal direction and a Mo–N4 structure with an axial hydroxyl. Apart from the N coordinator, an O atom with high electronegative as a coordinator can regulate the electronic properties. The replacement of a N with an O atom is beneficial to enhancing valance states of metal centers, accelerating electron transfer and modulating the adsorption free energy of O-intermediates. Noble metal Pt was grafted onto an Fe–N4 network to form a Pt1–O2–Fe–N4 dual-metal center electrocatalyst (Figure 11c) [108]. The coordination environment and chemical state of metal Ni were evaluated by FT–EXAFS. The new peak appearing in the Fe K-edge at R = 1.2 Å can be indexed to the Fe–O bond, and the peak located at R = 1.9 Å represented the adsorption bond between Pt1 and O2. The coordination number of Pt1–O2–Fe–N4 changed compared with Fe–N4. The dangling Pt1–O2– bond is promising for enhancing OER performance and reducing overpotential. The coordination number of atoms can alter the valance state of center metals, accelerating charge transfer and inducing electron redistribution. Modulating coordination atoms, coordination number, and coordination atom types are all beneficial to regulate MSI and further improve catalytic activity.

Figure 11.
(a) Schematic illustration of the synthesis of MoSA–Nx–C catalysts with different Mo–N coordination numbers. (b) XANES spectra of MoSA–Nx–C catalysts in experimental and theoretical calculation. Reproduced with permission of [107], copyright 2022 Cell Press. (c) Surface composition and valence state of Pt1@Fe–N–C and Fe–N–C. Reproduced with permission of [108], copyright 2018 John Wiley and Son.
5. Conclusions and Perspectives
In summary, the MSI effect occurring at the interface of metals and carbon supports affects the electrocatalytic kinetics and reaction pathways for OER. As far as non–precious metal-supported carbon-based catalysts, the stability, electronic, and geometric structures can be modulated by the MSI effect using charge transfer, electron redistribution, functional group coordination, and chemical covalent bonding. Integrating the OER mechanism and MSI effect together can provide theoretical guidance and a practical approach to the design of efficient catalysts, including modulations of particle size, morphology, heteroatom doping, defect engineering, and coordination atom and number. Whether these approaches can effectively improve the catalytic effect can be summed up in three aspects: the electronic structure, geometric structure, and reaction process of the catalyst. Briefly, the charge transfer and redistribution, and the central position of the d-band, are important factors affecting the catalytic process, which are directly related to the efficiency of electron directional transport in the reaction. Whether there is a reliable solid configuration and efficient charge transport between the surface functional groups of the support and the active metal species, and whether the overall configuration is conducive to mass transfer, will affect the efficiency and stability of the catalytic reaction process.
MSI modulation is a meaningful strategy for synthesis of efficient catalysts. Modulating MSI to optimize OER kinetics and pathways is still in its infancy, and some challenges need to be addressed in the future as displayed in Figure 12:
- (1)
- Improving intrinsic catalytic activity and atom utilization efficiency. Decreasing metal size from nanoparticles to single atoms can expose more active sites and improve atom utilization efficiency. Changing the coordination atoms, coordination number, and local chemical environment can regulate the intrinsic activity of active centers.
- (2)
- Controllable adjustment of MSI to change OER mechanisms. The covalent state of the M-O bond can be altered by MSI via charge transfer and electron redistribution, shifting the OER mechanism from AEM to LOM. Difficulties in controllable modulation of MSI through a simple process hampers the application of MSI in reaction mechanism optimization.
- (3)
- Enhancing the stability of electrocatalysts during the OER process. Catalytic activity and stability are mutually restricted for most reported catalysts. Carbon–supported catalysts can improve the dispersion of metal nanoparticles and modulate MSI strength of the electron coupling between carbon supports and metals for enhanced stability.
- (4)
- Developing advanced characterization techniques. Limited characterization techniques including in situ/operando experimental studies and theoretical calculations inhibit the accurate analysis of MSI across the interfaces of catalysts. The development of advanced detection methods and simulation techniques to realize in situ analysis in catalytic processes is conducive to understanding the mechanism and effects of MSI.

Figure 12.
Schematic diagrams of MSI effects and perspectives on catalyst design and preparation for OER.
Author Contributions
Writing—original draft preparation, X.Z.; resource, Y.L., X.M. and X.L.; writing—review and editing, R.Z.; supervision, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Joint Funds of NUAA-SEU (6907046031) and National Natural Science Foundation of China (61774033).
Acknowledgments
We thank the Big Data Center of Southeast University for providing the facility support on the numerical calculations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, C.; Lu, S.; Wu, Y.; Ouyang, Y.; Zhang, Y.; Li, Q.; Ling, C.; Wang, J. A universal descriptor for complicated interfacial effects on electrochemical reduction reactions. J. Am. Chem. Soc. 2022, 144, 12874–12883. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; Qi, L.; Xing, C.; Zheng, X.; He, F.; Li, Y. Rhodium nanocrystals on porous graphdiyne for electrocatalytic hydrogen evolution from saline water. Nat. Commun. 2022, 13, 5227. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Luo, L.; Gong, S.; Wei, M.; Li, Y.; Gan, X.; Zhao, Y.; Zhu, Z.; Li, Z. Single-atom and bimetallic nanoalloy supported on nanotubes as a bifunctional electrocatalyst for ultrahigh-current-density overall water splitting. ACS Catal. 2022, 12, 1167–1179. [Google Scholar] [CrossRef]
- Li, J.-C.; Meng, Y.; Zhang, L.; Li, G.; Shi, Z.; Hou, P.-X.; Liu, C.; Cheng, H.-M.; Shao, M. Dual-phasic carbon with Co single atoms and nanoparticles as a bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Adv. Funct. Mater. 2021, 31, 2103360. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.; Cai, B.; Huang, Z.; Garcia-Esparza, A.T.; Sokaras, D.; Zhang, Y.; Giordano, L.; Akkiraju, K.; Zhu, Y.G.; et al. Tunable metal hydroxide–organic frameworks for catalysing oxygen evolution. Nat. Mater. 2022, 21, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Suo, H.; Zheng, X.; Zhang, T.; Lei, Y.; Wang, Y.-X.; Lai, W.-H.; Wang, G. Lightest metal leads to big change: Lithium-mediated metal oxides for oxygen evolution reaction. Adv. Energy Mater. 2022, 12, 2201934. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Zhao, S.-N.; Wu, Y.; Liu, C.; Huang, J.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; et al. A dual-site doping strategy for developing efficient perovskite oxide electrocatalysts towards oxygen evolution reaction. Nano Energy 2022, 99, 107344. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.-D.; Zou, J.-J. NiCo-based electrocatalysts for the alkaline oxygen evolution reaction: A review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Shi, C.; Guan, D.; Ge, L.; Hu, Z.; Chin, Y.-Y.; Lin, H.-J.; Chen, C.-T.; et al. New undisputed evidence and strategy for enhanced lattice-oxygen participation of perovskite electrocatalyst through cation deficiency manipulation. Adv. Sci. 2022, 9, 2200530. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Yang, F.; Wang, L.; Wang, Y. Surface/interface modulation in oxygen evolution reaction. Prog. Chem. 2022, 34, 547–556. [Google Scholar]
- Song, L.; Zhang, X.; Zhu, S.; Xu, Y.; Wang, Y. Hydrogen spillover effect enhanced by carbon quantum dots activated MoS2. Carbon 2022, 199, 63–69. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Tong, L.; Zeng, M.; Wang, Y. Surface bonding of CoP to biomass derived carbon microtube: Site-specific growth and high-efficiency catalysis. Chem. Eng. J. 2022, 440, 135884. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Zhu, S.; Xu, Y.; Wang, Y. Transition metal (Fe, Ni, and Zn) doping-induced modulation of geometric and electronic structures to optimize the potential-determining step of MnCo2O4 for oxygen evolution reaction. Sci. China Mater. 2022, 65, 2871–2878. [Google Scholar] [CrossRef]
- Ding, J.; Song, L.; Li, X.; Chen, L.; Li, X.; Sun, J.; Zhang, X.; Wang, Y.; Tian, X. Interfacial engineering of the platinum/molybdenum disulfide/graphitic carbon nitride composite for enhanced photocatalytic hydrogen production. ACS Appl. Energy Mater. 2022, 5, 8800–8811. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Zhang, X.; Zhu, S.; Wang, Y. Effect of substitutional and interstitial boron-doped NiCo2S4 on the electronic structure and surface adsorption: High rate and long-term stability. ACS Appl. Energy Mater. 2022, 5, 2505–2513. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Du, X.; Zhu, S.; Xu, Y.; Wang, Y. Rapid surface reconstruction strategy for oxygen evolution reaction: Chem-grafted MXene quantum dots on Ni-Co layered double hydroxide. Phys. Chem. Chem. Phys. 2022, 24, 24902–24909. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced transition metal-based OER electrocatalysts: Current status, opportunities, and challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Li, P.; Wan, X.; Su, J.; Liu, W.; Guo, Y.; Yin, H.; Wang, D. A single-phase FeCoNiMnMo high-entropy alloy oxygen evolution anode working in alkaline solution for over 1000 h. ACS Catal. 2022, 12, 11667–11674. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Xia, M.; Wu, Y.; Zhang, B.; Li, Z.; Ran, L.; Gao, J.; Zhang, X.; Fan, Z.; et al. Engineering lattice oxygen activation of iridium clusters stabilized on amorphous bimetal borides array for oxygen evolution reaction. Angew. Chem. Int. Ed. 2021, 133, 27332–27340. [Google Scholar] [CrossRef]
- Peng, M.; Huang, J.; Zhu, Y.; Zhou, H.; Hu, Z.; Liao, Y.-K.; Lai, Y.-H.; Chen, C.-T.; Chu, Y.-H.; Zhang, K.H.L.; et al. Structural anisotropy determining the oxygen evolution mechanism of strongly correlated perovskite nickelate electrocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 4262–4270. [Google Scholar] [CrossRef]
- Liao, X.; Lu, R.; Xia, L.; Liu, Q.; Wang, H.; Zhao, K.; Wang, Z.; Zhao, Y. Density functional theory for electrocatalysis. Energy Environ. Mater. 2022, 5, 157–185. [Google Scholar] [CrossRef]
- Li, C.; Liu, F.; Shi, Y.; Zheng, Y.; Fang, B.; Lin, J.; Ni, J.; Wang, X.; Lin, B.; Jiang, L. Inducing the metal-support interaction and enhancing the ammonia synthesis activity of ceria-supported ruthenium catalyst via N2H4 reduction. ACS Sustain. Chem. Eng. 2021, 9, 4885–4893. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Zeng, Y.; Spendelow, J.S.; Wu, G. Advanced nanocarbons for enhanced performance and durability of platinum catalysts in proton exchange membrane fuel cells. Small 2021, 17, 2006805. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, Y.; Gao, X.; Li, L.; Lin, Z. Simultaneous optimization of CoIr alloy nanoparticles and 2D graphitic-N doped carbon support in CoIr@CN by Ir doping for enhanced oxygen and hydrogen evolution reactions. J. Mater. Chem. A 2022, 10, 15543–15553. [Google Scholar] [CrossRef]
- Wang, R.; Liu, B.; You, S.; Li, Y.; Zhang, Y.; Wang, D.; Tang, B.; Sun, Y.; Zou, J. Three-dimensional Ni3Se4 flowers integrated with ultrathin carbon layer with strong electronic interactions for boosting oxygen reduction/evolution reactions. Chem. Eng. J. 2022, 430, 132720. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Geng, D.; Lin, L.-Y.; Meng, X. Insight into the correlation of Pt–support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Liang, Z.; Kong, N.; Yang, C.; Zhang, W.; Zheng, H.; Lin, H.; Cao, R. Highly curved nanostructure-coated Co, N-doped carbon materials for oxygen electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 12759–12764. [Google Scholar] [CrossRef]
- Huang, M.; Liu, S.; Gong, S.; Xu, P.; Yang, K.; Chen, S.; Wang, C.; Chen, Q. Silver nanoparticles encapsulated in an N-doped porous carbon matrix as high-active catalysts toward oxygen reduction reaction via electron transfer to outer graphene shells. ACS Sustain. Chem. Eng. 2019, 7, 16511–16519. [Google Scholar] [CrossRef]
- Rong, C.; Shen, X.; Wang, Y.; Thomsen, L.; Zhao, T.; Li, Y.; Lu, X.; Amal, R.; Zhao, C. Electronic structure engineering of single-atom Ru sites via Co-N4 sites for bifunctional pH-universal water splitting. Adv. Mater. 2022, 34, 2110103. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, J.; Mu, X.; Cheng, R.; Li, W.; Liu, S.; Pu, Z.; Lin, C.; Mu, S. Nitrogen-doped carbon coupled FeNi3 intermetallic compound as advanced bifunctional electrocatalyst for OER, ORR and Zn-air batteries. Appl. Catal. B Environ. 2020, 268, 118729. [Google Scholar] [CrossRef]
- Riyajuddin, S.; Pahuja, M.; Sachdeva, P.K.; Azmi, K.; Kumar, S.; Afshan, M.; Ali, F.; Sultana, J.; Maruyama, T.; Bera, C.; et al. Super-hydrophilic leaflike Sn4P3 on the porous seamless graphene-carbon nanotube heterostructure as an efficient electrocatalyst for solar-driven overall water splitting. ACS Nano 2022, 16, 4861–4875. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Wang, K.; Dai, Q.; Lei, C.; Yang, B.; Zhang, Q.; Lei, L.; Leunge, M.K.H.; Hou, Y. Tuning d-band center of tungsten carbide via Mo doping for efficient hydrogen evolution and Zn–H2O cell over a wide pH range. Nano Energy 2020, 74, 104850. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Lei, Z.; Yu, L.; Wu, W.; Wang, Z.; Cheng, N. Electronic modulation optimizes OH* intermediate adsorption on Co-Nx-C sites via coupling CoNi alloy in hollow carbon nanopolyhedron toward efficient reversible oxygen electrocatalysis. Appl. Catal. B Environ. 2022, 304, 121006. [Google Scholar] [CrossRef]
- Fu, J.; Lym, J.; Zheng, W.; Alexopoulos, K.; Mironenko, A.V.; Li, N.; Boscoboinik, J.A.; Su, D.; Weber, R.T.; Vlachos, D.G. C–O bond activation using ultralow loading of noble metal catalysts on moderately reducible oxides. Nat. Catal. 2020, 3, 446–453. [Google Scholar] [CrossRef]
- Gang, C.; Chen, J.; Chen, Q.; Chen, Y. Heterostructure of ultrafine FeOOH nanodots supported on CoAl-layered double hydroxide nanosheets as highly efficient electrocatalyst for water oxidation. J. Colloid Interf. Sci. 2021, 600, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Yang, B.; Li, A.; Liao, C.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tuning interfacial active sites over porous Mo2N-supported cobalt sulfides for efficient hydrogen evolution reactions in acid and alkaline electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 41573–41583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, M.; Wang, H.; Wei, C.; Sun, Z.; Rummeli, M.H.; Strasser, P.; Sun, J.; Yang, R. Mildly oxidized MXene (Ti3C2, Nb2C, and V2C) electrocatalyst via a generic strategy enables longevous Li-O2 Battery under a high rate. ACS Nano 2021, 15, 19640–19650. [Google Scholar] [CrossRef]
- Suryawanshi, M.P.; Ghorpade, U.V.; Shin, S.W.; Suryawanshi, U.P.; Jo, E.; Kim, J.H. Hierarchically coupled Ni: FeOOH nanosheets on 3D N-doped graphite foam as self-supported electrocatalysts for efficient and durable water oxidation. ACS Catal. 2019, 9, 5025–5034. [Google Scholar] [CrossRef]
- Liang, D.; Lian, C.; Xu, Q.; Liu, M.; Liu, H.; Jiang, H.; Li, C. Interfacial charge polarization in Co2P2O7@N, P co-doped carbon nanocages as Mott-Schottky electrocatalysts for accelerating oxygen evolution reaction. Appl. Catal. B Environ. 2020, 268, 118417. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, X.; Wang, M.; Yang, X.; Liu, S.; Chen, H.-C.; Zhuang, Z.; Zhang, Y.; Cheong, W.-C.; Zhang, C.; et al. Construction of N, P co-doped carbon frames anchored with Fe single atoms and Fe2P nanoparticles as a robust coupling catalyst for electrocatalytic oxygen reduction. Adv. Mater. 2022, 34, 2203621. [Google Scholar] [CrossRef]
- Zhang, K.; Min, X.; Zhang, T.; Si, M.; Jiang, J.; Chai, L.; Shi, Y. Biodeposited nano-CdS drives the in situ growth of highly dispersed sulfide nanoparticles during pyrolysis for enhanced oxygen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 54553–54562. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, I.; Reyes-Carmona, A.; Dupont, M.; Cavaliere, S.; Rodlert, M.; Mornaghini, F.; Larsen, M.J.; Odgaard, M.; Zajac, J.; Jones, D.J.; et al. Correlation between the surface characteristics of carbon supports and their electrochemical stability and performance in fuel cell cathode. Carbon Energy 2021, 3, 654–665. [Google Scholar] [CrossRef]
- Su, J.; Yuan, S.; Cheng, Y.-X.; Yang, Z.-M.; Zuo, J.-L. Coordination-bond-directed synthesis of hydrogen-bonded organic frameworks from metal–organic frameworks as templates. Chem. Sci. 2021, 12, 14254–14259. [Google Scholar] [CrossRef] [PubMed]
- 4Jiang, B.; Fan, X.; Dang, Q.; Liao, F.; Li, Y.; Lin, H.; Kang, Z.; Shao, M. Functionalization of metal oxides with thiocyanate groups: A general strategy for boosting oxygen evolution reaction in neutral media. Nano Energy 2020, 76, 105079. [Google Scholar]
- Zhang, Y.; Yang, J.; Yu, Z.; Hou, Y.; Jiang, R.; Huang, J.; Yang, F.; Yao, S.; Gao, L.; Tang, W. Modulating carbon-supported transition metal oxide by electron-giving and electron-absorbing functional groups towards efficient overall water splitting. Chem. Eng. J. 2021, 416, 129124. [Google Scholar] [CrossRef]
- Andaveh, R.; Darband, G.B.; Maleki, M.; Rouhaghdam, A.S. Superaerophobic/superhydrophilic surfaces as advanced electrocatalysts for the hydrogen evolution reaction: A comprehensive review. J. Mater. Chem. A 2022, 10, 5147–5173. [Google Scholar] [CrossRef]
- Yin, H.; Xia, H.; Zhao, S.; Li, K.; Zhang, J.; Mu, S. Atomic level dispersed metal-nitrogen-carbon catalyst toward oxygen reduction reaction: Synthesis strategies and chemical environmental regulation. Energy Environ. Mater. 2021, 4, 5–18. [Google Scholar] [CrossRef]
- Yin, P.; Luo, X.; Ma, Y.; Chu, S.-Q.; Chen, S.; Zheng, X.; Lu, J.; Wu, X.-J.; Liang, H.-W. Sulfur stabilizing metal nanoclusters on carbon at high temperatures. Nat. Commun. 2021, 12, 3135. [Google Scholar] [CrossRef]
- Zhong, X.; Huang, K.; Zhang, Y.; Wang, Y.; Feng, S. Constructed interfacial oxygen-bridge chemical bonding in core-shell transition metal phosphides/carbon hybrid boosting oxygen evolution reaction. ChemSusChem 2021, 14, 2188–2197. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H.C. Hybrid OER electrocatalyst combining mesoporous hollow spheres of N, P-doped carbon with ultrafine Co2NiOx. ACS Appl. Mater. Interfaces 2020, 12, 50324–50332. [Google Scholar] [CrossRef]
- Wang, X.; Raghupathy, R.K.M.; Querebillo, C.J.; Liao, Z.; Li, D.; Lin, K.; Hantusch, M.; Sofer, Z.; Li, B.; Zschech, E.; et al. Interfacial covalent bonds regulated electron-deficient 2D black phosphorus for electrocatalytic oxygen reactions. Adv. Mater. 2021, 33, 2008752. [Google Scholar] [CrossRef]
- Wang, D.; Xin, Y.; Li, X.; Ning, H.; Wang, Y.; Yao, D.; Zheng, Y.; Meng, Z.; Yang, Z.; Pan, Y.; et al. Transforming metal-organic frameworks into porous liquids via a covalent linkage strategy for CO2 capture. ACS Appl. Mater. Interfaces 2021, 13, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, D.; Şenocak, A.; Köksoy, B.; Ömeroğlu, İ.; Durmuş, M.; Demirbas, E. Coumarin bearing asymmetrical zinc (II) phthalocyanine functionalized SWCNT hybrid nanomaterial. J. Electroanal. Chem. 2021, 897, 115552. [Google Scholar] [CrossRef]
- He, Y.; Shi, Q.; Shan, W.; Li, X.; Kropf, A.J.; Wegener, E.C.; Wright, J.; Karakalos, S.; Su, D.; Cullen, D.A.; et al. Dynamically unveiling metal-nitrogen coordination during thermal activation to design high-efficient atomically dispersed CoN4 active sites. Angew. Chem. Int. Ed. 2021, 60, 9516–9526. [Google Scholar] [CrossRef]
- Lin, G.; Ju, Q.; Jin, Y.; Qi, X.; Liu, W.; Huang, F.; Wang, J. Suppressing dissolution of Pt-based electrocatalysts through the electronic metal-support interaction. Adv. Energy Mater. 2021, 11, 2101050. [Google Scholar] [CrossRef]
- TriKhoa, N.; WookKim, S.; Yoo, D.-H.; Kim, E.J.; HongHahn, S. Size-dependent work function and catalytic performance of gold nanoparticles decorated graphene oxide sheets. Appl. Catal. A Gen. 2014, 469, 159–164. [Google Scholar]
- Wu, M.; Zhang, G.; Wang, W.; Yang, H.; Rawach, D.; Chen, M.; Sun, S. Electronic metal-support interaction modulation of single-atom electrocatalysts for rechargeable zinc-air batteirs. Small Methods 2022, 6, 2100947. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S.M.; García de Arquer, F.P.; Dinh, C.T.; Li, J.; Wang, Z.; Zheng, X.; Zhang, L.; et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992. [Google Scholar] [CrossRef]
- Zhao, F.; Wen, B.; Niu, W.; Chen, Z.; Yan, C.; Selloni, A.; Tully, C.G.; Yang, X.; Koel, B.E. Increasing iridium oxide activity for the oxygen evolution reaction with hafnium modification. J. Am. Chem. Soc. 2021, 143, 15616–15623. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, K.; Hu, W.; Cheng, Z.; Chen, H.; Feng, X.; Peng, T.; Kou, Z. Monodispersed ruthenium nanoparticles interfacially bonded with defective nitrogen-and-phosphorus-doped carbon nanosheets enable pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 306, 121095. [Google Scholar] [CrossRef]
- Sandbeck, D.J.S.; Secher, N.M.; Speck, F.D.; Sørensen, J.E.; Kibsgaard, J.; Chorkendorff, I.; Cherevko, S. The particle size effect on platinum dissolution: Considerations for accelerated stability testing of fuel cell catalysts. ACS Catal. 2020, 10, 6281–6290. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Tao, B.; Wang, X.; Deng, Y.; Gu, X.; Li, L.; Xiao, W.; Li, N.; Luo, H. N-doped hollow porous carbon spheres@Co Cu Fe alloy nanospheres as novel non-precious metal electrocatalysts for HER and OER. Int. J. Hydrogen Energy 2022, 47, 5947–5960. [Google Scholar]
- Lai, W.-H.; Zhang, L.-F.; Hua, W.-B.; Indris, S.; Yan, Z.-C.; Hu, Z.; Zhang, B.; Liu, Y.; Wang, L.; Liu, M.; et al. General p-electron-assisted strategy for Ir, Pt, Ru, Pd, Fe, Ni single atom electrocatalysts with bifunctional active sites for highly efficient water splitting. Angew. Chem. Int. Ed. 2019, 58, 11868–11873. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xue, D.; Zhang, J.-N. Optimizing atomically dispersed metal electrocatalysts for hydrogen evolution: Chemical coordination effect and electronic metal support interaction. Chem. Asian J. 2022, 17, e202200319. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, Z.; Li, M.; Tian, J.; Feng, L. Surface structure regulation and evaluation of FeNi-based nanoparticles for oxygen evolution reaction. Appl. Catal. B Environ. 2021, 297, 120462. [Google Scholar] [CrossRef]
- Wei, S.; Cui, X.; Xu, Y.; Shang, B.; Zhang, Q.; Gu, L.; Fan, X.; Zheng, L.; Hou, C.; Huang, H.; et al. Iridium-triggered phase transition of MoS2 nanosheets boosts overall water splitting in alkaline media. ACS Energy Lett. 2019, 4, 368–374. [Google Scholar] [CrossRef]
- Babar, P.; Patil, K.; Karade, V.; Gour, K.; Lokhande, A.; Pawar, S.; Kim, J.H. In situ fabrication of nickel-iron oxalate catalysts for electrochemical water oxidation at high current densities. ACS Appl. Mater. Interfaces 2021, 13, 52620–52628. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Xue, Y.; He, F.; Li, Y. Acidic water oxidation on quantum dots of IrOx/graphdiyne. Adv. Energy Mater. 2021, 11, 2101138. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Liu, H.; Liang, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Preparation of hollow cobalt-iron phosphides nanospheres by controllable atom migration for enhanced water oxidation and splitting. Small 2021, 17, 2007858. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, C.; Liu, Z.; Yuan, S.; Yang, G.; Li, N. Ultra-small NiFe-layered double hydroxide nanoparticles confined in ordered mesoporous carbon as efficient electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2021, 565, 150533. [Google Scholar] [CrossRef]
- Wei, G.; Shen, Y.; Zhao, X.; Wang, Y.; Zhang, W.; An, C. Hexagonal phase Ni3Fe nanosheets toward high-performance water splitting by a room-temperature methane plasma method. Adv. Funct. Mater. 2022, 32, 2109709. [Google Scholar] [CrossRef]
- Yang, H.; Dai, G.; Chen, Z.; Wu, J.; Huang, H.; Liu, Y.; Shao, M.; Kang, Z. Pseudo-periodically coupling Ni-O lattice with Ce-O lattice in ultrathin heteronanowire arrays for efficient water oxidation. Small 2021, 17, 2101727. [Google Scholar] [CrossRef]
- Weng, B.; Grice, C.R.; Meng, W.; Guan, L.; Xu, F.; Yu, Y.; Wang, C.; Zhao, D.; Yan, Y. Metal-organic framework-derived CoWP@C composite nanowire electrocatalyst for efficient water splitting. ACS Energy Lett. 2018, 3, 1434–1442. [Google Scholar] [CrossRef]
- Li, B.; Xing, R.; Mohite, S.V.; Latthe, S.S.; Fujishima, A.; Liu, S.; Zhou, Y. CoS2 nanodots anchored into heteroatom-doped carbon layer via a biomimetic strategy: Boosting the oxygen evolution and supercapacitor performance. J. Power Sources 2019, 436, 226862. [Google Scholar] [CrossRef]
- Zhang, M.; Xuan, X.; Wang, W.; Ma, C.; Lin, Z. Anode photovoltage compensation-enabled synergistic CO2 photoelectrocatalytic reduction on a flower-like graphene-decorated Cu foam cathode. Adv. Funct. Mater. 2020, 30, 2005983. [Google Scholar] [CrossRef]
- Cao, D.; Xu, H.; Cheng, D. Branch-leaf-shaped CuNi@NiFeCu nanodendrites as highly efficient electrocatalysts for overall water splitting. Appl. Catal. B Environ. 2021, 298, 120600. [Google Scholar] [CrossRef]
- Yang, B.; Huang, Z.; Wu, H.; Hu, H.; Lin, H.; Nie, M.; Li, Q. Sea urchin-like CoSe2 nanoparticles modified graphene oxide as an efficient and stable hydrogen evolution catalyst. J. Electroanal. Chem. 2022, 907, 116037. [Google Scholar] [CrossRef]
- Li, Z.; Lin, X.; Xi, W.; Shen, M.; Gao, B.; Chen, Y.; Zheng, Y.; Lin, B. Bamboo-like N, S-doped carbon nanotubes with encapsulated Co nanoparticles as high-performance electrocatalyst for liquid and flexible all-solid-state rechargeable Zn-air batteries. Appl. Surf. Sci. 2022, 593, 153446. [Google Scholar] [CrossRef]
- Yoon, K.R.; Hwang, C.-K.; Kim, S.-H.; Jung, J.-W.; Chae, J.E.; Kim, J.; Lee, K.A.; Lim, A.; Cho, S.-H.; Singh, J.P.; et al. Hierarchically assembled cobalt oxynitride nanorods and N-doped carbon nanofibers for efficient bifunctional oxygen electrocatalysis with exceptional regenerative efficiency. ACS Nano 2021, 15, 11218–11230. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Cui, X.; Jia, S.; Zhang, J.; Lin, Z. Metal–organic frameworks-derived heteroatom-doped carbon electrocatalysts for oxygen reduction reaction. Nano Energy 2021, 86, 106073. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Wang, J.; Shao, M.; Chai, J.; Wang, S.; Yang, M.; Yang, Y.; Wang, N.; Wang, S.; et al. 3D heterostructured pure and N-Doped Ni3S2/VS2 nanosheets for high efficient overall water splitting. Electrochim. Acta 2018, 269, 55–61. [Google Scholar] [CrossRef]
- Jiang, E.; Li, J.; Li, X.; Ali, A.; Wang, G.; Ma, S.; Shen, P.K.; Zhu, J. MoP-Mo2C quantum dot heterostructures uniformly hosted on a heteroatom-doped 3D porous carbon sheet network as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2022, 431, 133719. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ramakrishna, S.U.B.; Devi, B.R.; Himabindu, V. Phosphorus-doped graphene supported palladium (Pd/PG) electrocatalyst for the hydrogen evolution reaction in PEM water electrolysis. Int. J. Green Energy 2018, 15, 558–567. [Google Scholar] [CrossRef]
- Joshi, P.; Yadav, R.; Hara, M.; Inoue, T.; Motoyama, Y.; Yoshimura, M. Contribution of B, N-co-doped reduced graphene oxide as a catalyst support to the activity of iridium oxide for oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 9066–9080. [Google Scholar] [CrossRef]
- Ma, X.; Chang, C.; Zhang, Y.; Niu, P.; Liu, X.; Wang, S.; Li, L. Synthesis of Co-based prussian blue analogues/dual-doped hollow carbon microsphere hybrids as high-performance bifunctional electrocatalysts for oxygen evolution and overall water splitting. ACS Sustain. Chem. Eng. 2020, 8, 8318–8326. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Xu, C.-Y.; Li, Q.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Modulating the d-band centers by coordination environment regulation of single-atom Ni on porous carbon fibers for overall water splitting. Nano Energy 2022, 98, 107266. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Z.; Zhang, Y.; Xiao, J.; Zeng, X.; Zhang, C.; Dong, P.; Liu, T.; Zhang, Y.; Li, M. Tiny Ni nanoparticles embedded in boron- and nitrogen-co doped porous carbon nanowires for high-efficiency water splitting. ACS Appl. Mater. Interfaces 2022, 14, 24447–24461. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Z.; Wang, Y.; Yuan, D.; Tian, F.; Zhang, L. Nitrogen-doped binary spinel CuCo2O4/C nanocomposite: An efficient electrocatalyst for oxygen evolution reaction. ChemNanoMat 2020, 6, 1652–1657. [Google Scholar] [CrossRef]
- Ren, J.-T.; Ying, Y.-D.; Liu, Y.-P.; Li, W.; Yuan, Z.-Y. Charge redistribution caused by sulfur doping of bimetal FeCo phosphides supported on heteroatoms-doped graphene for Zn-air batteries with stable cycling. J. Energy Chem. 2022, 71, 619–630. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, S.; Zhang, K.A.I.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Ultrasound-triggered assembly of covalent triazine framework for synthesizing heteroatom-doped carbon nanoflowers boosting metal-free bifunctional electrocatalysis. ACS Appl. Mater. Interfaces 2021, 13, 13328–13337. [Google Scholar] [CrossRef] [PubMed]
- Saha, E.; Karthick, K.; Kundu, S.; Mitra, J. Regulating the heteroatom doping in metallogel-derived Co@dual self-doped carbon onions to maximize electrocatalytic water splitting. J. Mater. Chem. A 2021, 9, 26800–26809. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Zhang, Y.; Luo, X.; Chen, L.; Shi, Y. ZnS modified N, S dual-doped interconnected porous carbon derived from dye sludge waste as high-efficient ORR/OER catalyst for rechargeable zinc-air battery. J. Colloid Interf. Sci. 2022, 616, 659–667. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, C.; Yu, M.; Wang, W. Trifunctional Co nanoparticle confined in defect-rich nitrogen-doped graphene for rechargeable Zn-air battery with a long lifetime. Appl. Catal. B Environ. 2021, 281, 119514. [Google Scholar] [CrossRef]
- Jiang, T.; Dai, P.; Zhang, W. Fe7S8 nanoparticles encapsulated in porous N, S co-doped carbon as an efficient bifunctional electrocatalyst for Zn-air battery. Micro-Nano Lett. 2020, 15, 495–498. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect chemistry in heterogeneous catalysis: Recognition, understanding, and utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect engineering on electrode materials for rechargeable batteries. Adv. Mater. 2020, 32, 1905923. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Kang, B.; Yuan, Y.; Chen, G.; Lee, J.Y. Activating γ-graphyne nanoribbons as bifunctional electrocatalysts toward oxygen reduction and hydrogen evolution reactions by edge termination and nitrogen doping. Chem. Eng. J. 2022, 430, 133126. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Fan, X.-Z.; Du, X.; Pang, Q.-Q.; Zhang, S.; Liu, Z.-Y.; Yue, X.-Z. In situ construction of bifunctional N-doped carbon-anchored Co nanoparticles for OER and ORR. ACS Appl. Mater. Interfaces 2022, 14, 8549–8556. [Google Scholar] [CrossRef]
- Zhu, Y.; Sokolowski, J.; Song, X.; He, Y.; Mei, Y.; Wu, G. Engineering local coordination environments of atomically dispersed and heteroatom-coordinated single metal site electrocatalysts for clean energy-conversion. Adv. Energy Mater. 2020, 10, 1902844. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, H.; Wang, Y.; Liu, L.; Qin, W.; Liu, S.; Liu, J.; Qin, Y.; Zhang, D.; Chu, A.; et al. Sulfur coordination engineering of molybdenum single-atom for dual-functional oxygen reduction/evolution catalysis. Energy Storage Mater. 2022, 50, 186–195. [Google Scholar] [CrossRef]
- Liu, M.; Li, N.; Cao, S.; Wang, X.; Lu, X.; Kong, L.; Xu, Y.; Bu, X.-H. A “pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv. Mater. 2022, 34, 2107421. [Google Scholar] [CrossRef]
- Li, Q.; Lu, L.; Liu, J.; Shi, W.; Cheng, P. Two-dimensional bimetallic coordination polymers as bifunctional evolved electrocatalysts for enhanced oxygen evolution reaction and urea oxidation reaction. J. Energy Chem. 2021, 63, 230–238. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting electrocatalytic activity of single atom catalysts supported on nitrogen-doped carbon through N coordination environment engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Xue, Z.; Yang, J.; Liu, Q.; Xian, J.; Zhong, Y.; Sun, Y.; Zhang, X.; Liu, Q.; Yao, D.; et al. Tailoring the electronic structure of an atomically dispersed zinc electrocatalyst: Coordination environment regulation for high selectivity oxygen reduction. Angew. Chem. Int. Ed. 2022, 61, e202110838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, G.; Cui, X.; Zhao, X.; Zhang, Q.; Gu, L.; Zheng, L.; Li, L.H.; Wu, Q.; Singh, D.J.; et al. Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specific. Chem 2021, 7, 436–449. [Google Scholar] [CrossRef]
- Zeng, X.; Shui, J.; Liu, X.; Liu, Q.; Li, Y.; Shang, J.; Zheng, L.; Yu, R. Single-atom to single-atom grafting of Pt1 onto Fe-N4 center: Pt1@Fe-N-C multifunctional electrocatalyst with significantly enhanced properties. Adv. Energy Mater. 2018, 8, 1701345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).