Electromechanical Nanogenerators for Cell Modulation
Abstract
1. Introduction
2. TENGs for Cell Modulation
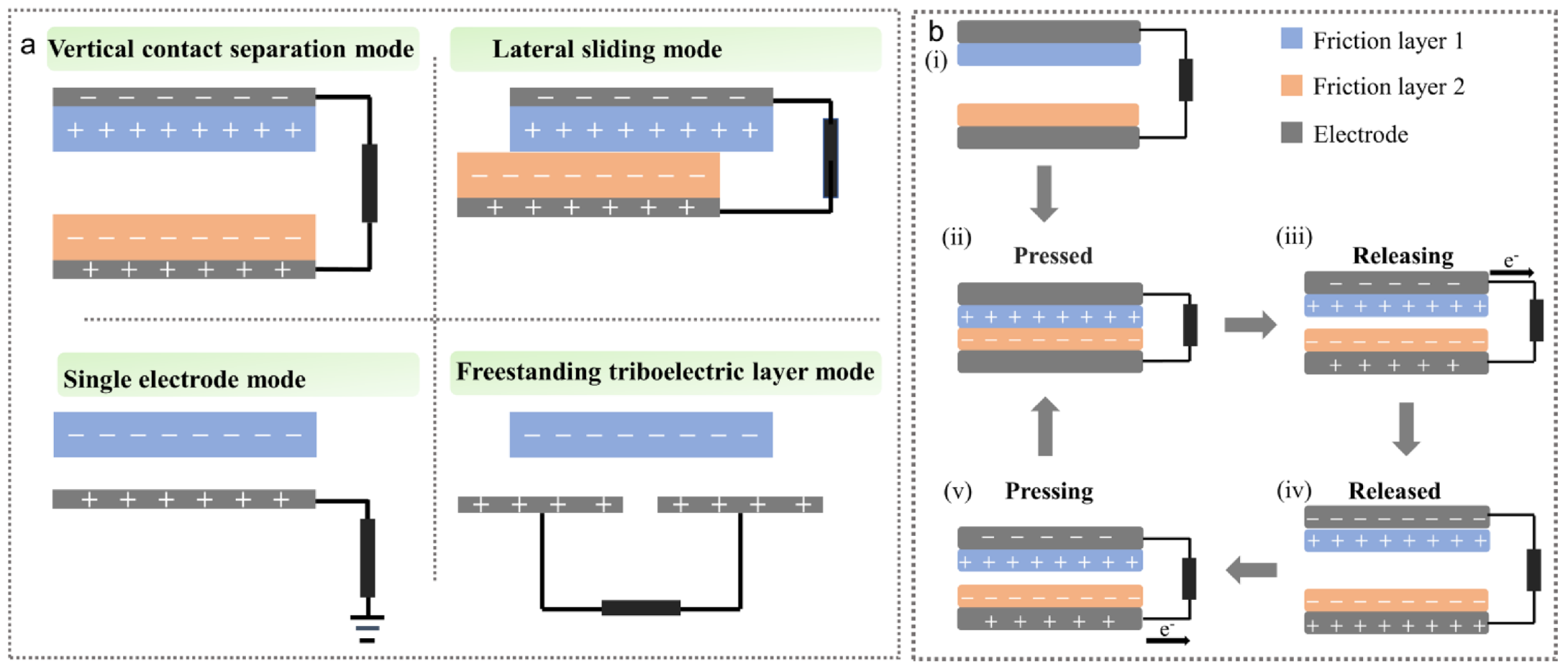
2.1. Working Mechanism of TENGs
2.2. TENGs for Cell Modulation
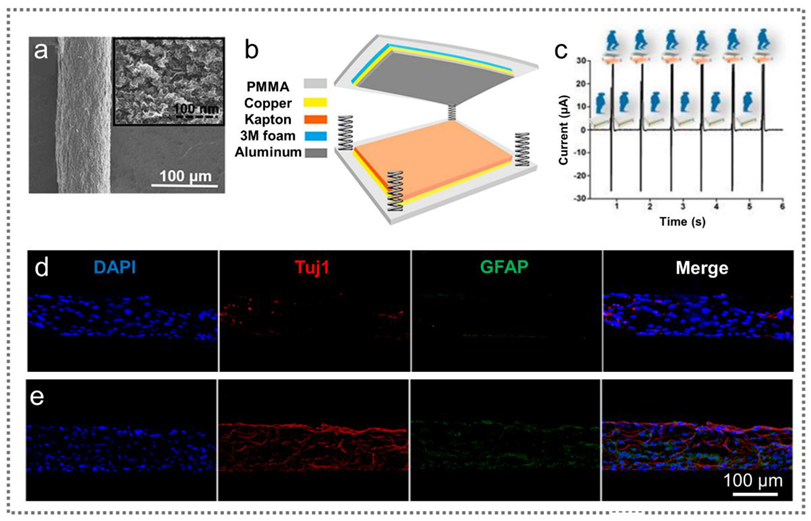
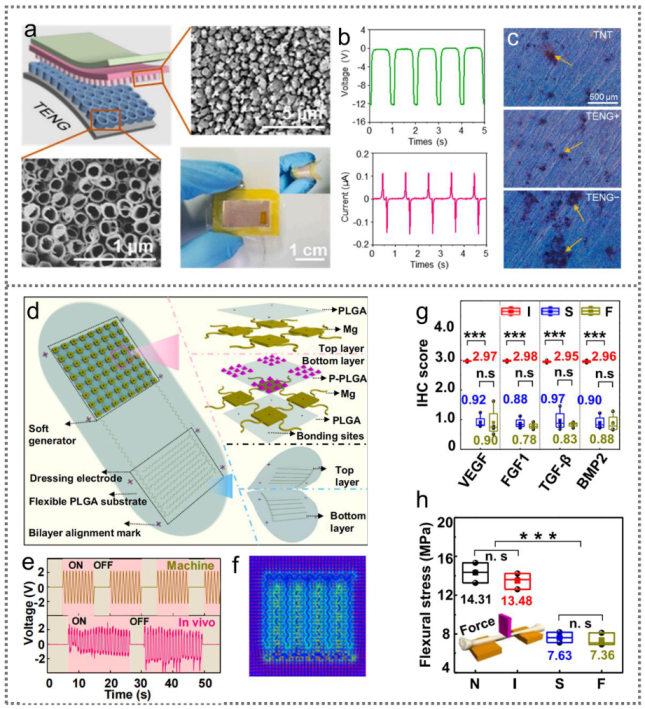
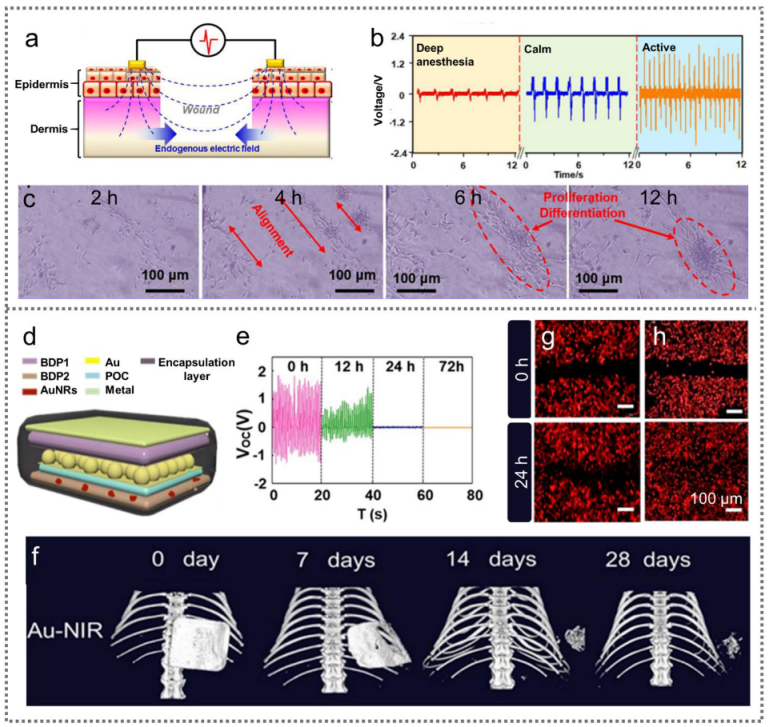
2.2.1. TENGs for Nerve Repair
2.2.2. TENG for Bone Repair
2.2.3. TENGs for Wound Healing
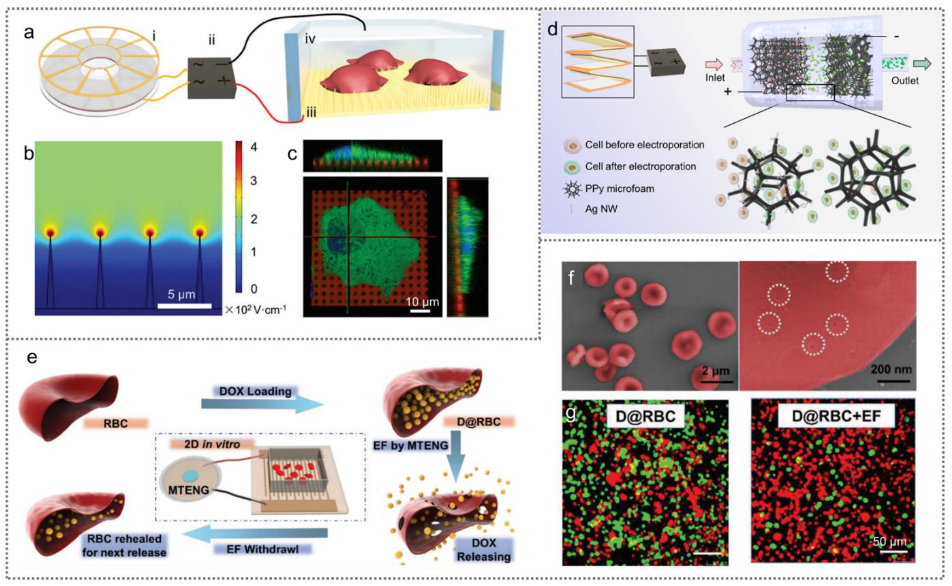
2.2.4. TENGs for Drug Delivery and Cardiac Pacing
3. PENGs for Cell Modulation
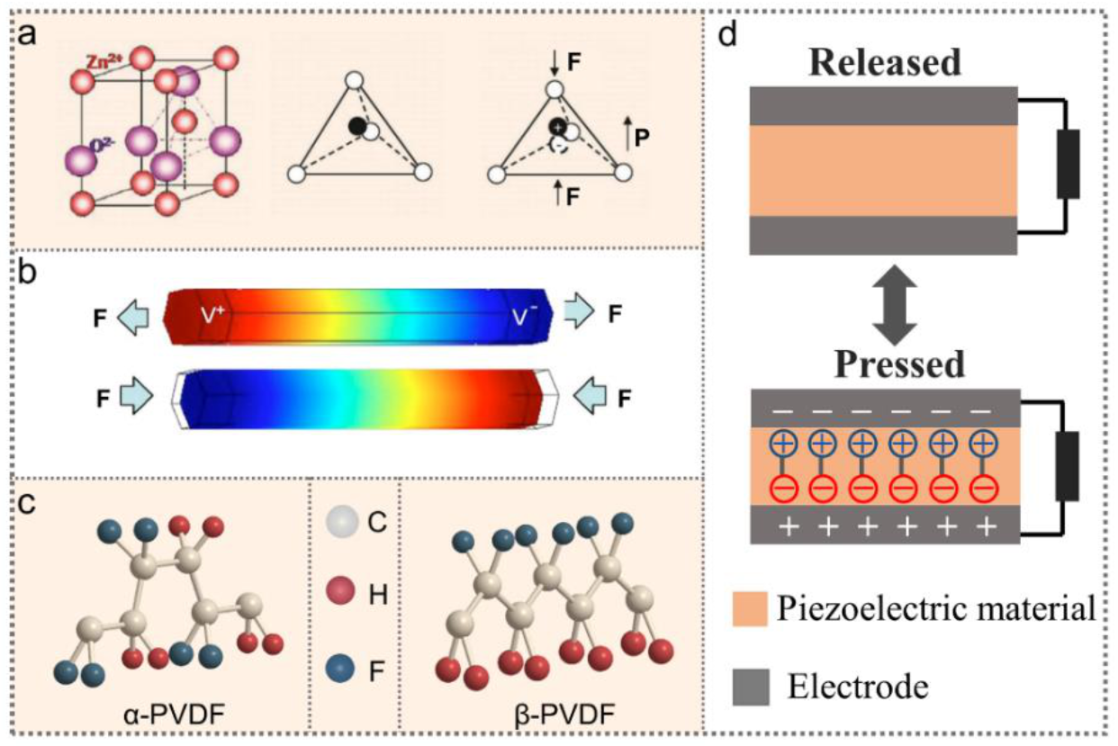
3.1. Piezoelectric Materials and Piezoelectricity
3.2. Working Mechanism of PENGs
3.3. PENGs for Cell Modulation
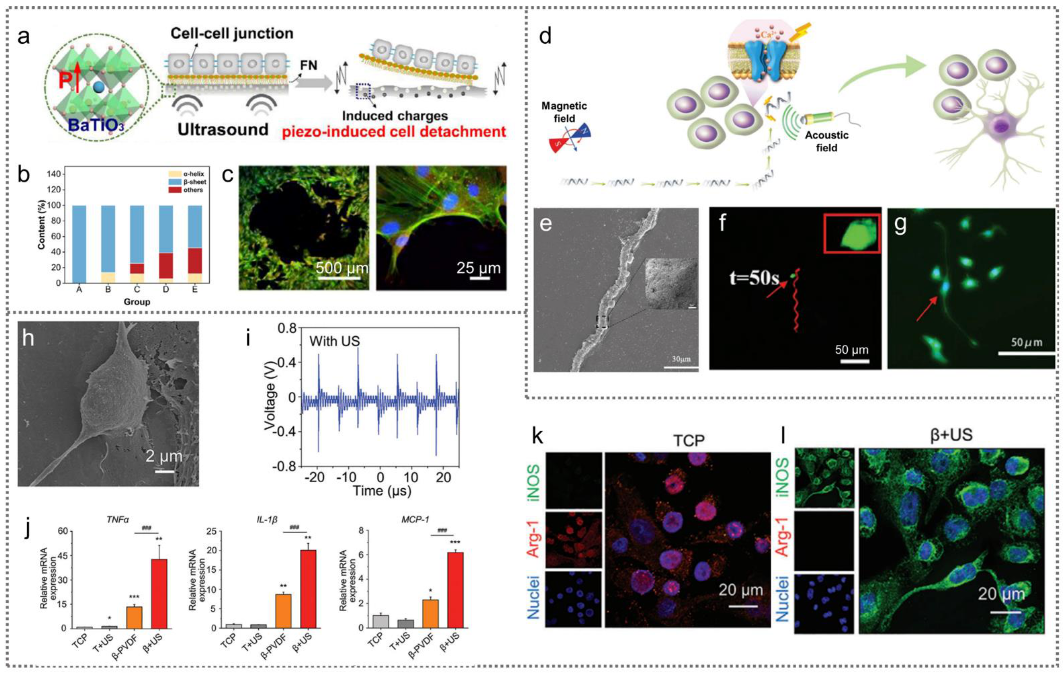
3.3.1. Acoustic Wave-Driven PENGs for Cell Modulation
3.3.2. Magnetic Field-Driven PENGs for Cell Modulation
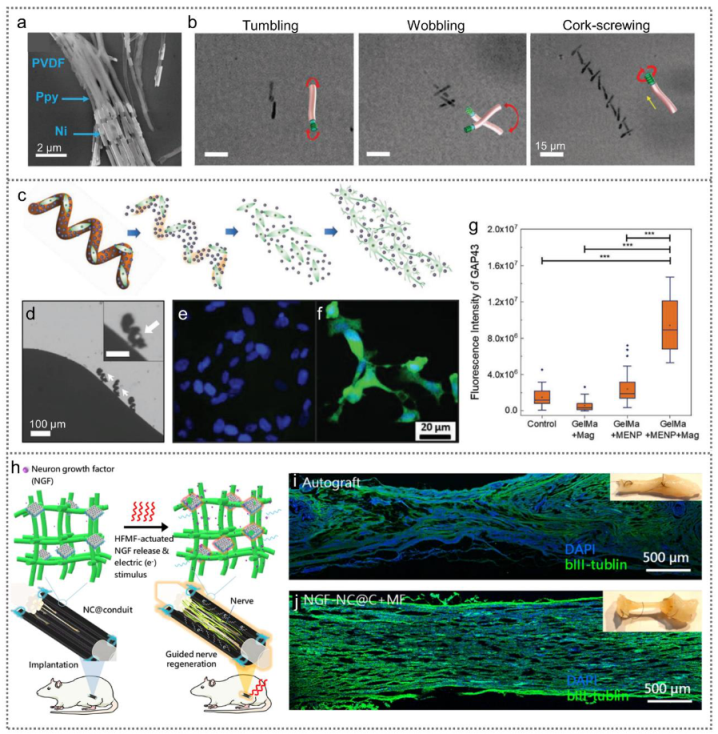
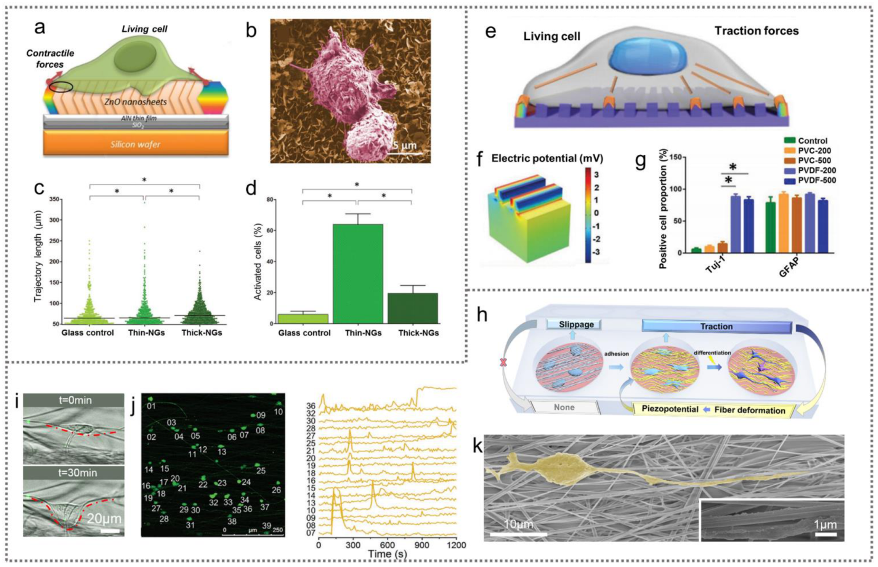
3.3.3. Cell Traction-Driven PENGs for Cell Modulation
4. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.R.; Wan, X.Y.; Wang, Z.L.; Li, L.L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, 2007429. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B—Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, B.; Ouyang, H.; Fan, Y.; Wang, Z.L.; Li, Z. Emerging Implantable Energy Harvesters and Self-Powered Implantable Medical Electronics. ACS Nano 2020, 14, 6436–6448. [Google Scholar] [CrossRef]
- Zhu, B.; Luo, S.-C.; Zhao, H.; Lin, H.-A.; Sekine, J.; Nakao, A.; Chen, C.; Yamashita, Y.; Yu, H.-h. Large enhancement in neurite outgrowth on a cell membrane-mimicking conducting polymer. Nat. Commun. 2014, 5, 4523. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, B.; Li, Z.; Wang, Z.L. Recent Progress on Piezoelectric and Triboelectric Energy Harvesters in Biomedical Systems. Adv. Sci. 2017, 4, 1700029. [Google Scholar] [CrossRef]
- Jakešová, M.; Sjöström, T.A.; Đerek, V.; Poxson, D.; Berggren, M.; Głowacki, E.D.; Simon, D.T. Wireless organic electronic ion pumps driven by photovoltaics. NPJ Flex. Electron. 2019, 3, 14997. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kato, K.; Miyake, T.; Nagamine, K.; Ofuji, T.; Yoshino, S.; Nishizawa, M. Organic Transdermal Iontophoresis Patch with Built-in Biofuel Cell. Adv. Health. Mater. 2015, 4, 506–510. [Google Scholar] [CrossRef]
- Xiao, X.X.; McGourty, K.D.; Magner, E. Enzymatic Biofuel Cells for Self-Powered, Controlled Drug Release. J. Am. Chem. Soc. 2020, 142, 11602–11609. [Google Scholar] [CrossRef]
- Sato, H.; Minamitani, Y.; Ohnishi, N.; Fujiwara, Y.; Katsukie, S. Development of a High-Frequency Burst Pulse Generator for Cancer Cell Treatment and Comparison Between a High-Power Burst Pulse and a Single Pulse. IEEE Trans. Plasma Sci. 2020, 48, 1051–1059. [Google Scholar] [CrossRef]
- Brezar, S.K.; Kranjc, M.; Cemazar, M.; Bucek, S.; Sersa, G.; Miklavcic, D. Electrotransfer of siRNA to Silence Enhanced Green Fluorescent Protein in Tumor Mediated by a High Intensity Pulsed Electromagnetic Field. Vaccines 2020, 8, 49. [Google Scholar] [CrossRef]
- Novickij, V.; Kranjc, M.; Staigvila, G.; Dermol-Cerne, J.; Melesko, J.; Novickij, J.; Miklaveic, D. High-Pulsed Electromagnetic Field Generator for Contactless Permeabilization of Cells In Vitro. IEEE Trans. Magn. 2020, 56, 5000106. [Google Scholar] [CrossRef]
- Vidal, J.V.; Slabov, V.; Kholkin, A.L.; dos Santos, M.P.S. Hybrid Triboelectric-Electromagnetic Nanogenerators for Mechanical Energy Harvesting: A Review. Nano-Micro Lett. 2021, 13, 199. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Wang, Z.L. Flexible triboelectric generator! Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Z.R.; Feng, Y.W.; Han, J.J.; Li, L.L.; An, J.; Chen, P.F.; Jiang, T.; Wang, Z.L. Spherical triboelectric nanogenerator based on spring-assisted swing structure for effective water wave energy harvesting. Nano Energy 2021, 83, 105836. [Google Scholar] [CrossRef]
- Ren, Z.W.; Wang, Z.M.; Liu, Z.R.; Wang, L.F.; Guo, H.Y.; Li, L.L.; Li, S.T.; Chen, X.Y.; Tang, W.; Wang, Z.L. Energy Harvesting from Breeze Wind (0.7-6 m s(-1)) Using Ultra-Stretchable Triboelectric Nanogenerator. Adv. Energy Mater. 2020, 10, 2001770. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.L. Emerging nanogenerators: Powering the Internet of Things by high entropy energy. iScience 2021, 24, 102358. [Google Scholar] [CrossRef]
- Luo, J.J.; Gao, W.C.; Wang, Z.L. The Triboelectric Nanogenerator as an Innovative Technology toward Intelligent Sports. Adv. Mater. 2021, 33, 2004178. [Google Scholar] [CrossRef]
- Zhao, G.R.; Zhang, Y.W.; Shi, N.; Liu, Z.R.; Zhang, X.D.; Wu, M.Q.; Pan, C.F.; Liu, H.L.; Li, L.L.; Wang, Z.L. Transparent and stretchable triboelectric nanogenerator for self-powered tactile sensing. Nano Energy 2019, 59, 302–310. [Google Scholar] [CrossRef]
- Nie, J.H.; Chen, X.Y.; Wang, Z.L. Electrically Responsive Materials and Devices Directly Driven by the High Voltage of Triboelectric Nanogenerators. Adv. Funct. Mater. 2019, 29, 1806351. [Google Scholar] [CrossRef]
- Long, Y.; Li, J.; Yang, F.; Wang, J.Y.; Wang, X.D. Wearable and Implantable Electroceuticals for Therapeutic Electrostimulations. Adv. Sci. 2021, 8, 2004023. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.T.; Xu, L.; Zhang, H.N.; Yang, Y.; Wang, Z.L. Hierarchically patterned self-powered sensors for multifunctional tactile sensing. Sci. Adv. 2020, 6, eabb9083. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.Y.; Jiang, Y.; Zhai, S.Y.; Cheng, R.W.; Liu, D.; Gao, X.P.; Wang, J.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6, eaba9624. [Google Scholar] [CrossRef]
- Sun, M.J.; Li, Z.; Yang, C.Y.; Lv, Y.J.; Yuan, L.; Shang, C.X.; Liang, S.Y.; Guo, B.W.; Liu, Y.; Li, Z.; et al. Nanogenerator-based devices for biomedical applications. Nano Energy 2021, 89, 106461. [Google Scholar] [CrossRef]
- Zou, H.Y.; Zhang, Y.; Guo, L.T.; Wang, P.H.; He, X.; Dai, G.Z.; Zheng, H.W.; Chen, C.Y.; Wang, A.C.; Xu, C.; et al. Quantifying the triboelectric series. Nat. Commun. 2019, 10, 1427. [Google Scholar] [CrossRef]
- Zou, H.Y.; Guo, L.T.; Xue, H.; Zhang, Y.; Shen, X.F.; Liu, X.T.; Wang, P.H.; He, X.; Dai, G.Z.; Jiang, P.; et al. Quantifying and understanding the triboelectric series of inorganic non-metallic materials. Nat. Commun. 2020, 11, 2093. [Google Scholar] [CrossRef]
- Liu, Z.R.; Liang, X.; Liu, H.H.; Wang, Z.; Jiang, T.; Cheng, Y.Y.; Wu, M.Q.; Xiang, D.L.; Li, Z.; Wang, Z.L.; et al. High-Throughput and Self-Powered Electroporation System for Drug Delivery Assisted by Microfoam Electrode. ACS Nano 2020, 14, 15458–15467. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, T.; Liu, G.X.; Feng, Y.W.; Zhang, C.; Wang, Z.L. Spherical triboelectric nanogenerator integrated with power management module for harvesting multidirectional water wave energy. Energy Environ. Sci. 2020, 13, 277–285. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Yan, Q.Y.; Liu, Z.R.; Zhao, X.Y.; Wang, Z.; Sun, J.; Wang, Z.L.; Wang, R.R.; Li, L.L. Flexible MXene composed triboelectric nanogenerator via facile vacuum-assistant filtration method for self-powered biomechanical sensing. Nano Energy 2021, 88, 106257. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Zhang, Y.L.; Liu, Z.; Shi, B.J.; Wang, X.X.; Jin, Y.M.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, e1501478. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, H.; Liu, Z.; Li, Z.; Tian, J.; Shi, B.; Zou, Y.; Ouyang, H.; Zhao, C.; Zhao, L.; et al. Fully Bioabsorbable Natural-Materials-Based Triboelectric Nanogenerators. Adv. Mater. 2018, 30, 1801895. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, H.Q.; Zheng, Q.; Li, H.; Zhao, C.C.; Ouyang, H.; Noreen, S.; Yu, M.; Su, F.; Liu, R.P.; et al. Photothermally tunable biodegradation of implantable triboelectric nanogenerators for tissue repairing. Nano Energy 2018, 54, 390–399. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerator (TENG)-Sparking an Energy and Sensor Revolution. Adv. Energy Mater. 2020, 10, 2000137. [Google Scholar] [CrossRef]
- Guo, W.B.; Zhang, X.D.; Yu, X.; Wang, S.; Qiu, J.C.; Tang, W.; Li, L.L.; Liu, H.; Wang, Z.L. Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers. ACS Nano 2016, 10, 5086–5095. [Google Scholar] [CrossRef]
- Pate, F.D. Bone chemistry and paleodiet. J. Archaeol. Method Theory 1994, 1, 161–209. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Misra, R.D.K. Electric field-mediated growth of osteoblasts—The significant impact of dynamic flow of medium. Biomater. Sci. 2016, 4, 136–144. [Google Scholar] [CrossRef][Green Version]
- Creecy, C.M.; O’Neill, C.F.; Arulanandam, B.P.; Sylvia, V.L.; Navara, C.S.; Bizios, R. Mesenchymal Stem Cell Osteodifferentiation in Response to Alternating Electric Current. Tissue Eng. Part A 2013, 19, 467–474. [Google Scholar] [CrossRef]
- Tian, J.J.; Shi, R.; Liu, Z.; Ouyang, H.; Yu, M.; Zhao, C.C.; Zou, Y.; Jiang, D.J.; Zhang, J.S.; Li, Z. Self-powered implantable electrical stimulator for osteoblasts’ proliferation and differentiation. Nano Energy 2019, 59, 705–714. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, J.S.; Tian, J.J.; Zhao, C.C.; Li, Z.; Zhang, Y.Z.; Li, Y.S.; Wu, C.G.; Tian, W.; Li, Z. An effective self-powered strategy to endow titanium implant surface with associated activity of anti-biofilm and osteogenesis. Nano Energy 2020, 77, 105201. [Google Scholar] [CrossRef]
- Yao, G.; Kang, L.; Li, C.C.; Chen, S.H.; Wang, Q.; Yang, J.Z.; Long, Y.; Li, J.; Zhao, K.N.; Xu, W.N.; et al. A self-powered implantable and bioresorbable electrostimulation device for biofeedback bone fracture healing. Proc. Natl. Acad. Sci. USA 2021, 118, e2100772118. [Google Scholar] [CrossRef]
- Nuccitelli, R. A role for endogenous electric fields in wound healing. Curr. Top. Dev. Biol. 2003, 58, 1–26. [Google Scholar]
- Long, Y.; Wei, H.; Li, J.; Yao, G.; Yu, B.; Ni, D.L.; Gibson, A.L.F.; Lan, X.L.; Jiang, Y.D.; Cai, W.B.; et al. Effective Wound Healing Enabled by Discrete Alternative Electric Fields from Wearable Nanogenerators. ACS Nano 2018, 12, 12533–12540. [Google Scholar] [CrossRef]
- Liu, A.P.; Long, Y.; Li, J.; Gu, L.; Karim, A.; Wang, X.D.; Gibson, A.L.F. Accelerated complete human skin architecture restoration after wounding by nanogenerator-driven electrostimulation. J. Nanobiotechnol. 2021, 19, 280. [Google Scholar] [CrossRef]
- Liu, Z.R.; Nie, J.H.; Miao, B.; Li, J.D.; Cui, Y.B.; Wang, S.; Zhang, X.D.; Zhao, G.R.; Deng, Y.B.; Wu, Y.H.; et al. Self-Powered Intracellular Drug Delivery by a Biomechanical Energy-Driven Triboelectric Nanogenerator. Adv. Mater. 2019, 31, 1807795. [Google Scholar] [CrossRef]
- Yang, C.B.; Yang, G.; Ouyang, Q.L.; Kuang, S.Y.; Song, P.Y.; Xu, G.X.; Poenar, D.P.; Zhu, G.; Yong, K.T.; Wang, Z.L. Nanowire-array-based gene electro-transfection system driven by human-motion operated triboelectric nanogenerator. Nano Energy 2019, 64, 103901. [Google Scholar] [CrossRef]
- Zhao, C.C.; Feng, H.Q.; Zhang, L.J.; Li, Z.; Zou, Y.; Tan, P.C.; Ouyang, H.; Jiang, D.J.; Yu, M.; Wang, C.; et al. Highly Efficient In Vivo Cancer Therapy by an Implantable Magnet Triboelectric Nanogenerator. Adv. Funct. Mater. 2019, 29, 1808640. [Google Scholar] [CrossRef]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.J.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef]
- Liu, Z.R.; Yu, X.; Li, L.L. Piezopotential augmented photo- and photoelectro-catalysis with a built-in electric field. Chin. J. Catal. 2020, 41, 534–549. [Google Scholar] [CrossRef]
- Wang, Z.L. Progress in Piezotronics and Piezo-Phototronics. Adv. Mater. 2012, 24, 4632–4646. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, J.; Gu, Y.; Fei, P.; Hao, Y.; Bao, G.; Wang, Z.L. Effects of piezoelectric potential on the transport characteristics of metal-ZnO nanowire-metal field effect transistor. J. Appl. Phys. 2009, 105, 113707. [Google Scholar] [CrossRef]
- Shao, H.; Fang, J.; Wang, H.; Lang, C.; Lin, T. Robust Mechanical-to-Electrical Energy Conversion from Short-Distance Electrospun Poly(vinylidene fluoride) Fiber Webs. ACS Appl. Mater. Interfaces 2015, 7, 22551–22557. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, M.X.; Mao, M.Y.; Zhang, Q.H.; Wang, H.Z. Enhanced power output of an electrospun PVDF/MWCNTs-based nanogenerator by tuning its conductivity. Nanotechnology 2013, 24, 405401. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric Biomaterials for Sensors and Actuators. Adv. Mater. 2019, 31, 1802084. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J.H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Yang, R.; Wang, A.C.; Wang, Z.L. Muscle-Driven In Vivo Nanogenerator. Adv. Mater. 2010, 22, 2534–2537. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Yang, B.D.; Su, Y.; Tran, P.L.; Joe, P.; Anderson, E.; Xia, J.; Doraiswamy, V.; Dehdashti, B.; Feng, X.; et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. USA 2014, 111, 1927–1932. [Google Scholar] [CrossRef]
- Cheng, X.; Xue, X.; Ma, Y.; Han, M.; Zhang, W.; Xu, Z.; Zhang, H.; Zhang, H. Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: Simulated, in vitro and in vivo studies. Nano Energy 2016, 22, 453–460. [Google Scholar] [CrossRef]
- Jeong, C.K.; Han, J.H.; Palneedi, H.; Park, H.; Hwang, G.-T.; Joung, B.; Kim, S.-G.; Shin, H.J.; Kang, I.-S.; Ryu, J.; et al. Comprehensive biocompatibility of nontoxic and high-output flexible energy harvester using lead-free piezoceramic thin film. APL Mater. 2017, 5, 074102. [Google Scholar] [CrossRef]
- Kondapalli, H.; Alazzawi, Y.; Malinowski, M.; Timek, T.; Chakrabartty, S. Feasibility of Self-Powering and Energy Harvesting Using Cardiac Valvular Perturbations. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Shin, H.J.; Lee, H.; Jeong, C.K.; Park, H.; Hwang, G.-T.; Lee, H.-Y.; Joe, D.J.; Han, J.H.; Lee, S.H.; et al. In Vivo Self-Powered Wireless Transmission Using Biocompatible Flexible Energy Harvesters. Adv. Funct. Mater. 2017, 27, 1700341. [Google Scholar] [CrossRef]
- Zhai, W.C.; Zhu, L.P.; Berbille, A.; Wang, Z.L. Flexible and wearable piezoelectric nanogenerators based on P(VDF-TrFE)/SnS nanocomposite micropillar array. J. Appl. Phys. 2021, 129, 095501. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhu, L.P.; Yang, T.; Hou, X.M.; Du, Z.T.; Cao, S.; Wang, H.L.; Chou, K.C.; Wang, Z.L. Ultra-Stable and Durable Piezoelectric Nanogenerator with All-Weather Service Capability Based on N Doped 4H-SiC Nanohole Arrays. Nano-Micro Lett. 2022, 14, 30. [Google Scholar] [CrossRef]
- Dong, K.; Peng, X.; Wang, Z.L. Fiber/Fabric-Based Piezoelectric and Triboelectric Nanogenerators for Flexible/Stretchable and Wearable Electronics and Artificial Intelligence. Adv. Mater. 2020, 32, 1902549. [Google Scholar] [CrossRef]
- Song, Y.H.; Shi, Z.Q.; Hu, G.H.; Xiong, C.X.; Isogai, A.; Yang, Q.L. Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: A review. J. Mater. Chem. A 2021, 9, 1910–1937. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Lingling, X.; Liu, Z.; Cui, X.; Xiang, Z.; Bai, J.Y.; Jiang, D.J.; Xue, J.T.; Wang, C.; Lin, Y.X.; et al. Self-powered pulsed direct current stimulation system for enhancing osteogenesis in MC3T3-E1. Nano Energy 2021, 85, 106009. [Google Scholar] [CrossRef]
- Jin, F.; Li, T.; Yuan, T.; Du, L.J.; Lai, C.T.; Wu, Q.; Zhao, Y.; Sun, F.Y.; Gu, L.; Wang, T.; et al. Physiologically Self-Regulated, Fully Implantable, Battery-Free System for Peripheral Nerve Restoration. Adv. Mater. 2021, 33, 2104175. [Google Scholar] [CrossRef]
- Wang, A.C.; Liu, Z.; Hu, M.; Wang, C.C.; Zhang, X.D.; Shi, B.J.; Fan, Y.B.; Cui, Y.G.; Li, Z.; Ren, K.L. Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing. Nano Energy 2018, 43, 63–71. [Google Scholar] [CrossRef]
- Mitragotri, S. Innovation—Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Pan, X.T.; Bai, L.X.; Wang, H.; Wu, Q.Y.; Wang, H.Y.; Liu, S.; Xu, B.L.; Shi, X.H.; Liu, H.Y. Metal-Organic-Framework-Derived Carbon Nanostructure Augmented Sonodynamic Cancer Therapy. Adv. Mater. 2018, 30, 1800180. [Google Scholar] [CrossRef]
- Wan, X.Y.; Zhang, X.D.; Liu, Z.R.; Zhang, J.M.; Li, Z.; Wang, Z.L.; Li, L.L. Noninvasive manipulation of cell adhesion for cell harvesting with piezoelectric composite film. Appl. Mater. Today 2021, 25, 101218. [Google Scholar] [CrossRef]
- Zhang, R.T.; Han, S.W.; Liang, L.L.; Chen, Y.K.; Sun, B.J.; Liang, N.; Feng, Z.C.; Zhou, H.X.; Sun, C.H.; Liu, H.; et al. Ultrasonic-driven electrical signal-iron ion synergistic stimulation based on piezotronics induced neural differentiation of mesenchymal stem cells on FeOOH/PVDF nanofibrous hybrid membrane. Nano Energy 2021, 87, 106192. [Google Scholar] [CrossRef]
- Liu, L.; Chen, B.; Liu, K.; Gao, J.B.; Ye, Y.C.; Wang, Z.; Qin, N.; Wilson, D.A.; Tu, Y.F.; Peng, F. Wireless Manipulation of Magnetic/Piezoelectric Micromotors for Precise Neural Stem-Like Cell Stimulation. Adv. Funct. Mater. 2020, 30, 1910108. [Google Scholar] [CrossRef]
- Chen, X.Z.; Liu, J.H.; Dong, M.; Muller, L.; Chatzipirpiridis, G.; Hu, C.Z.; Terzopoulou, A.; Torlakcik, H.; Wang, X.P.; Mushtaq, F.; et al. Magnetically driven piezoelectric soft microswimmers for neuron-like cell delivery and neuronal differentiation. Mater. Horiz. 2019, 6, 1512–1516. [Google Scholar] [CrossRef]
- Marino, A.; Arai, S.; Hou, Y.Y.; Sinibaldi, E.; Pellegrino, M.; Chang, Y.T.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation. ACS Nano 2015, 9, 7678–7689. [Google Scholar] [CrossRef]
- Ma, B.J.; Liu, F.; Li, Z.; Duan, J.Z.; Kong, Y.; Hao, M.; Ge, S.H.; Jiang, H.D.; Liu, H. Piezoelectric nylon-11 nanoparticles with ultrasound assistance for high-efficiency promotion of stem cell osteogenic differentiation. J. Mater. Chem. B 2019, 7, 1847–1854. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, F.; Ma, B.J.; Duan, J.Z.; Yuan, W.H.; Sang, Y.H.; Han, L.; Wang, S.H.; Liu, H. Wireless Localized Electrical Stimulation Generated by an Ultrasound-Driven Piezoelectric Discharge Regulates Proinflammatory Macrophage Polarization. Adv. Sci. 2021, 8, 2100962. [Google Scholar] [CrossRef]
- Kopyl, S.; Surmenev, R.; Surmeneva, M.; Fetisov, Y.; Kholkin, A. Magnetoelectric effect: Principles and applications in biology and medicine—A review. Mater. Today Bio 2021, 12, 100149. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, D.; Zhao, H.X.; Dong, X.W.; Long, L.S.; Zheng, L.S. Inorganic-Organic Hybrid Molecular Materials: From Multiferroic to Magnetoelectric. Adv. Mater. 2021, 33, 2004542. [Google Scholar] [CrossRef]
- Tokura, Y.; Kanazawa, N. Magnetic Skyrmion Materials. Chem. Rev. 2021, 121, 2857–2897. [Google Scholar] [CrossRef]
- Nair, M.; Guduru, R.; Liang, P.; Hong, J.; Sagar, V.; Khizroev, S. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun. 2013, 4, 1707. [Google Scholar] [CrossRef]
- Mushtaq, F.; Torlakcik, H.; Hoop, M.; Jang, B.J.; Carlson, F.; Grunow, T.; Laubli, N.; Ferreira, A.; Chen, X.Z.; Nelson, B.J.; et al. Motile Piezoelectric Nanoeels for Targeted Drug Delivery. Adv. Funct. Mater. 2019, 29, 1808135. [Google Scholar] [CrossRef]
- Dong, M.; Wang, X.P.; Chen, X.Z.; Mushtaq, F.; Deng, S.Y.; Zhu, C.H.; Torlakcik, H.; Terzopoulou, A.; Qin, X.H.; Xiao, X.Z.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Fang, J.H.; Hsu, H.H.; Hsu, R.S.; Peng, C.K.; Lu, Y.J.; Chen, Y.Y.; Chen, S.Y.; Hu, S.H. 4D printing of stretchable nanocookie@conduit material hosting biocues and magnetoelectric stimulation for neurite sprouting. NPG Asia Mater. 2020, 12, 61. [Google Scholar] [CrossRef]
- Shuai, C.J.; Yang, W.J.; He, C.X.; Peng, S.P.; Gao, C.D.; Yang, Y.W.; Qi, F.W.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2020, 185, 108275. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Jahanshahi, A.; Gilbert, H.B.; Yu, Y.; Erin, O.; Francisco, D.; Alosaimi, F.; Temel, Y.; Sitti, M. Nonresonant powering of injectable nanoelectrodes enables wireless deep brain stimulation in freely moving mice. Sci. Adv. 2021, 7, eabc4189. [Google Scholar] [CrossRef]
- Singer, A.; Dutta, S.; Lewis, E.; Chen, Z.Y.; Chen, J.C.; Verma, N.; Avants, B.; Feldman, A.K.; O’Malley, J.; Beierlein, M.; et al. Magnetoelectric Materials for Miniature, Wireless Neural Stimulation at Therapeutic Frequencies. Neuron 2020, 107, 631–643. [Google Scholar] [CrossRef]
- Murillo, G.; Blanquer, A.; Vargas-Estevez, C.; Barrios, L.; Ibanez, E.; Nogues, C.; Esteve, J. Electromechanical Nanogenerator-Cell Interaction Modulates Cell Activity. Adv. Mater. 2017, 29, 1605048. [Google Scholar] [CrossRef]
- Zhang, X.D.; Cui, X.; Wang, D.C.; Wang, S.; Liu, Z.R.; Zhao, G.R.; Zhang, Y.; Li, Z.; Wang, Z.L.; Li, L.L. Piezoelectric Nanotopography Induced Neuron-Like Differentiation of Stem Cells. Adv. Funct. Mater. 2019, 29, 1900372. [Google Scholar] [CrossRef]
- Li, T.; Shi, C.M.; Jin, F.; Yang, F.; Gu, L.; Wang, T.; Dong, W.; Feng, Z.Q. Cell activity modulation and its specific function maintenance by bioinspired electromechanical nanogenerator. Sci. Adv. 2021, 7, eabh2350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.R.; Cai, M.J.; Zhang, X.D.; Yu, X.; Wang, S.; Wan, X.Y.; Wang, Z.L.; Li, L.L. Cell-Traction-Triggered On-Demand Electrical Stimulation for Neuron-Like Differentiation. Adv. Mater. 2021, 33, 2106317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dzidotor, G.; Le, T.T.; Vinikoor, T.; Morgan, K.; Curry, E.J.; Das, R.; McClinton, A.; Eisenberg, E.; Apuzzo, L.N.; et al. Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Sci. Transl. Med. 2022, 14, 627. [Google Scholar] [CrossRef]
- You, J.; Moon, H.; Lee, B.Y.; Jin, J.Y.; Chang, Z.E.; Kim, S.Y.; Park, J.; Hwang, Y.S.; Kim, J. Cardiomyocyte sensor responsive to changes in physical and chemical environments. J. Biomech. 2014, 47, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Soares dos Santos, M.P.; Coutinho, J.; Marote, A.; Sousa, B.; Ramos, A.; Ferreira, J.A.F.; Bernardo, R.; Rodrigues, A.; Marques, A.T.; da Cruz e Silva, O.A.; et al. Capacitive technologies for highly controlled and personalized electrical stimulation by implantable biomedical systems. Sci. Rep. 2019, 9, 5001. [Google Scholar] [CrossRef] [PubMed]

| EMNG | Mode/Piezoelectric Scaffolds | Electrostimulation | Cells | Cell Modulation | Ref. |
|---|---|---|---|---|---|
| TENG | Implantable, contact–separation mode | 10 V mm−1, 1 Hz, 20 min/day, 5 days | Primary neurons | Cell alignment | [31] |
| Contact–separation mode | 30 μA, 3000 pulses/day, 21 days | MSCs | Neural differentiation | [35] | |
| Implantable, contact–separation mode | 150 V cm−1, 2 Hz, 1 h/day, 18 days | Preosteoblasts MC3T3-E1 | Cell proliferation and osteogenic differentiation | [39] | |
| Contact–separation mode | 12 V, 5–21 days | MC3T3-E1 | Osteogenic differentiation | [40] | |
| Wearable, contact–separation mode | 2 V cm−1, 1 Hz, 6 h | Fibroblasts | Proliferation and differentiation into myofibroblasts | [43] | |
| Wearable, contact–separation mode | 8 V, 1 Hz, 24 h | Fibroblasts | Cell alignment | [44] | |
| Implantable, contact–separation mode | 100 mV mm−1, 24 h | Fibroblasts | Cell migration | [33] | |
| Disk, freestanding mode | 20 V, 20 Hz, 200 pluses | MCF-7, Hela, MSCs | Cell membrane permeability and drug delivery | [45] | |
| Contact–separation mode | 20 V, 5 Hz, 200 pulses | MCF-7, MSCs | Cell membrane permeability and drug delivery | [28] | |
| Contact–separation mode | 70 V, 4 kV cm−1 | RBCs | Cell membrane permeability and drug release | [47] | |
| PENG | Wearable, PVDF | 20 μA, 3 Hz, 2 h/day | MC3T3-E1 | Cell proliferation and orientation and osteogenic differentiation | [67] |
| PVDF–TrFE | d31 = 16.17 pC N−1 6 mV, 6 nA, | Fibroblasts | Cell alignment and proliferation | [69] | |
| PVDF/BaTiO3 | d33 = 15.7 pC N−1, −89.1 mV | MSCs | Regulate cell adhesion | [72] | |
| PVDF | d33 = 16.22 pC N−1 | Macrophages | Proinflammatory macrophage polarization | [78] | |
| FeOOH/PVDF | d33 = 27.2 pC N−1 | MSCs | Neural differentiation | [73] | |
| S. platensis@Fe3O4@ BaTiO3 | / | PC12 cells | Neural differentiation | [74] | |
| PVDF-TrFE/ CoFe2O4 | / | PC12 cells | Neural differentiation | [75] | |
| Nylon-11 | / | Dental pulp stem cells | Osteogenic differentiation | [77] | |
| CFO@BFO/GelMA | / | SHSY5Y cells | Neural differentiation | [84] | |
| NC@C | 28–38 μA | PC12 | Neural differentiation | [85] | |
| ZnO nanosheets | 300 µV–45 mV | Macrophages | Stimulate the motility of macrophages | [89] | |
| PVDF | 34 µV–3.4 mV | MSCs | Neural differentiation | [90] | |
| GO/PEDOT/Fe3O4/PAN | d33 = 4.5 pm V−1, 14.1 µV–1.41 mV | Hepatocytes and MSCs | Motility of primary hepatocytes and osteogenic differentiation of MSCs | [91] | |
| PVDF | d33 = 24 pC N−1, 0.73–133 mV | MSCs | Neural differentiation | [92] | |
| PENG + TENG | Contact–separation mode PVDF/ZnO | 1.5–2.7 V | Schwann cells | Cell proliferation and migration | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Z.; Li, L. Electromechanical Nanogenerators for Cell Modulation. Nanoenergy Adv. 2022, 2, 110-132. https://doi.org/10.3390/nanoenergyadv2010005
Liu Z, Wang Z, Li L. Electromechanical Nanogenerators for Cell Modulation. Nanoenergy Advances. 2022; 2(1):110-132. https://doi.org/10.3390/nanoenergyadv2010005
Chicago/Turabian StyleLiu, Zhirong, Zhuo Wang, and Linlin Li. 2022. "Electromechanical Nanogenerators for Cell Modulation" Nanoenergy Advances 2, no. 1: 110-132. https://doi.org/10.3390/nanoenergyadv2010005
APA StyleLiu, Z., Wang, Z., & Li, L. (2022). Electromechanical Nanogenerators for Cell Modulation. Nanoenergy Advances, 2(1), 110-132. https://doi.org/10.3390/nanoenergyadv2010005






